Abstract
Aim:
The aim of the present study was to examine the changes in the expression of T-cell activation markers, namely CD4+ CD25+ and CD8+ in patients with AITD, namely Graves’ disease and Hashimoto's thyroiditis as well as colloid nodular goitre. HLA-DR, LFA-3, and peripheral total lymphocytic count are also measured.
Materials and Methods:
We compared the expression of CD4, CD25, and CD8 surface markers in peripheral blood lymphocyte in Graves’ disease and Hashimoto's thyroiditis as autoimmune thyroid diseases, as well as colloid goitre in comparison with healthy controls. Also, LFA-3 and HLA-DR were measured in the same groups using three-color flow cytometry. Total lymphocytic count in peripheral blood, thyroid function tests, antithyroid antibodies were also included in the laboratory investigations. The total number of participants was 65. All were recruited from endocrine clinics in a tertiary care hospital in the southern region of Saudi Arabia. All participants underwent history taking, clinical examination, laboratory workup, and radiological investigations. Neck ultrasound, technecium pertechnetateψψ thyroid uptake, and fine-needle aspiration and cytology (FNAC) of the thyroid were done when indicated. The study was approved by the Hospital Research Isthics Committee and informed consents were obtained from all participants before enrollment in the study.
Results:
In comparison with thecontrol group, activation markers CD4, CD25, and CD8 were lower in the autoimmune thyroid diseases. Lymphocyte function antigen-3 (CD58) and total lymphocytic count were higher in the AIT diseases whereas HLA-DR was lower than that in the control group. The CD4/CD8 ratio was lower in the AITD compared with the healthy euthyroid subjects. No difference was found between patients with colloid nodular goitre and the healthy control in any of the study variables except for LFA-3 which was significantly higher in the colloid goitre group.
Conclusion:
Our findings indicate downregulation of CD4+ CD25+ Treg as well as CD8+ T cells in autoimmune thyroid diseases. Downregulation of suppressor T lymphocytes helps initiation, progression, and maintenance of the autoimmune thyroid diseases. Lower HLA-DR and higher CD58 in AITDs indicate their role in the expression of the autoantigen and its escape from the immune surveillance. High levels of LFA-3 in colloid goitre indicate that the autoimmune process needs interacting factors, and not only the high level of LFA-3.
Keywords: Autoimmune, CD4, CD25, CD8, Hashimoto's, HLA-DR, Graves’ disease, LFA-3, thyroid, Treg
INTRODUCTION
Autoimmune disorders include a broad spectrum of clinical entities whose pathogenesis is mediated by an immune response directed at self-antigens.[1]
The primary mechanism leading to self-tolerance has been termed as “recessive tolerance,” which is induced by the thymic deletion of autoreactive T cells.[2] However, thymic selection is incomplete, and self-reactive cells occur, even in healthy individuals. On the other hand, “dominant tolerance” is an additional mechanism for maintaining peripheral self, which is mediated by regulatory T cells actively modulating immune responses.[3] Failure of peripheral tolerance has been proposed to explain the loss of tolerance to self-antigens.[1]
Among autoimmune disorders, organ-specific autoimmune diseases constitute a subgroup in which the autoimmune response is focused upon a particular tissue or cell type.[4] Hashimoto's thyroiditis and Graves’ disease constitute one of the best-characterized groups of organ-specific autoimmune diseases, designated in general as autoimmune thyroid diseases (AITD).[5,6]
These AITD share the histological features of thyroid lymphocytic infiltration, so the probability of a similar underlying pathogenesis became established. It has become clear that a complex interaction between genetic susceptibility and environmental factors initiates the process, and that failure of immunological tolerance at multiple levels explains how this interaction operates.[7,8,9,10,11,12]
Several subtypes of regulatory T cells have been defined, each with a distinct phenotype, cytokine-production profile, and mechanism of action for suppressing immune responses. Some of these regulatory T cells are CD4; others are CD8.[13] In the CD4 regulatory T-cell compartment, detailed analysis led to identification of a subpopulation of regulatory T cells that exert their suppressive function in a contact-dependent manner and preferentially express high levels of CD25.[14]
Lymphocyte function-associated antigen 3 (LFA-3) is also known as CD58. It is a cell adhesion molecule expressed on antigen presenting cells (APC), particularly macrophages.[15] It is also expressed on about half of the circulating T and B cells. It binds to CD2 (LFA-2) on T cells and is important in strengthening the adhesion between the T cells and professional APC. This adhesion occurs as part of the transitory initial encounters between T cells and APC before T-cell activation. CD2 is expressed on all thymocytes, T cells, and NK cells.[16]
The first gene locus identified in association with the autoimmune thyroid disease was the major histocompatibility complex (MHC) region on the chromosome 6p21 which encodes human leukocyte antigens (HLAs). The HLA region comprises several immune response genes. The HLA molecule, located on antigen presenting cell (APC), binds and presents an antigenic peptide and in this way enables T-cell recognition and response to an antigen. Presumably, specific HLA alleles have a higher affinity for autoantigenic thyroidal peptides and are thus likely to contribute to the development of the autoimmune thyroid disease.[17]
Contradictory results have been published in the literature about the number of peripheral blood lymphocyte subsets in autoimmune diseases.
In this study, we investigated the state of CD4+ CD8+ Treg, CD8+ T cells the expression of HLA-DR, LFA-3 on peripheral blood cells and total lymphocytic count in AITD, subjects with colloid nodular goitre and in healthy euthyroid volunteers as a control group.
MATERIALS AND METHODS
We recruited 65 subjects from the endocrine clinics in a tertiary care hospital in the southern region of Saudi Arabia. The study was approved by the Hospital Research Ethics Committee. Informed consents were taken from all participants. The study population was categorized into four groups; group 1: 20 healthy volunteers, group 2: 15 subjects with colloid nodular goitre, group 3: 15 patients with Hashimoto's thyroiditis, and group 4: 15 patients with Graves’ disease. Complete physical examination and history taking were done for all participants. Laboratory investigations included full blood count, thyroid function tests, antithyroid peroxidase (antiTPO), antithyroglobulin antibodies (antiTG), as well as antiTSH antibodies when needed. Radiological studies included neck ultrasound, Doppler flow in the inferior thyroid artery, and Tcm99 thyroid uptake each according to its indication. Fine-needle aspiration and cytology of the thyroid were also performed when indicated.
Classification of the study population was based on clinical, laboratory, and radiological data.
Healthy control persons did not have goitre or history of any abnormality of thyroid function. They had normal thyroid function tests and their blood tests were negative for the thyroid autoantibodies at time of the study.
Persons with colloid nodular goitre did not have any abnormality of thyroid function or any of the antithyroid antibodies. Ultrasound imaging and FNAC which were requested when needed to confirm the diagnosis were also continued with the diagnosis of colloid nodular goitre.
Patients with Hashimoto's thyroiditis had high or suppressed TSH, positive tests for antiTPO and antiTG. Patients with Graves’ disease had suppressed TSH with high T4 and T3 and positive antiTSH when the test was indicated to confirm the diagnosis. Ultrasound imaging, Doppler blood flow in the inferior thyroid artery, and Technecium99 pertechnetate scanning of the thyroid were used to confirm the diagnosis of AITD whenever needed.
All patients did not receive any medication for their underlying thyroid dysfunction at the time of investigation.
Exclusion criteria: evidence of infection or inflammation, intake of immunomodulatory drugs or drugs known to interfere with thyroid function, intake of any thyroid medication at any time, and any disease that may affect the result of any of the measured variables.
Methods
Venous blood was obtained from all patients and control subjects in the morning after an overnight fasting. Measurement of free tetraiodothyronine (FT4), free triiodothyronine (FT3), thyrotropin (TSH), antithyroid peroxidase (antiTPO) and anti-thyroglobulin (antiTG) antibodies were measured by Advia centaur autoanalyzer (Siemens) using commercially available kits. Measurement was based on solid-phase chemiluminescent immunoassays.[18,19] Normal ranges for all parameters: TSH: 0.27-4.2 μIU/mL, FT4: 12-22 pmol/L, FT3: 3.9-6.8 pmol/L.[20]
Fluorescence-activated cell sorter analysis
For detection of surface antigens, the following antihuman monoclonal antibodies (mAb) were used: fluorescein isothiocyanate (FITC), phycoerythrin (PE), and peridinin chlorophyll protein (PerCP). Control g1/g2a (anti-isotype control mAb was used as a negative control), CD4-FITC, CD4-PerCP, CD8-PE, CD8-FITC. The flow cytometer was calibrated prior to performing every single assay.
The adhesion molecule LFA-3 was assessed by using CD58 -FITC (BD Bioscience, 555920), while the expression of T-reg cells was dependent on the number of helper cells CD4 with CD25 (FITC), HLA-DR allophycocyanin (APC) B.D Bioscience—San Jose, California, USA, HLA-DR (FITC 35298).
The collected samples were mixed and incubated with monoclonal antibodies and incubated in a dark room at 4-8°C. Then FACS lysing solution (Becton Dickinson) was added to lyse the red cells. After incubation in the dark room, the tubes were centrifuged at 3000 rounds/minutes (r.p.m.) for 10 minutes, supernatant was discarded and the cells were washed with phosphate-buffered saline PBS with 0.1% azide and centrifuged again. A second wash was performed with aspiration of the supernatant and 0.5 mL of phosphate buffer solution (PBS) was added to the tube, ready for specimen analysis. Using a FACS caliber flowcytometer (Becton Dickinson) and Cell Quest soft ware (Becton Dickinson), the population of lymphocytes was identified from forward and side scatter characteristics on dot plot profiles, in more was carrying CD 45 and analyzed for fluorescence intensity using defined gates. Data collected were reported as either percentage of positive cells or mean fluorescence intensity (MFI) values.[21]
Statistical analysis
The statistical Package for Social Sciences (version 21.0) was used for data entry and analysis. Descriptive statistics (i.e. mean and standard deviation) were calculated. Student's t-test was applied to assess the significance of differences between two study groups. P < 0.05 were considered as statistically significant.
RESULTS
Hashimoto's thyroiditis as well as Graves’ disease patients showed lower expression of CD4, CD25, and CD8 on T-cells in comparison with the control group. Higher expression of LFA-3 and lower presentation of HLA-DR were also found in the two groups of AITDs. Peripheral lymphocytic count was higher in AITDs in comparison with the control group [Tables 1 and 2].
Table 1.
Measured variables in Hashimoto's thyroiditis in comparison with the healthy control
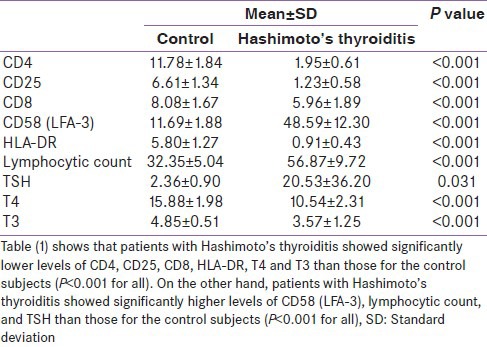
Table 2.
Measured variables in Graves’ diseasn comparison with the healthy control
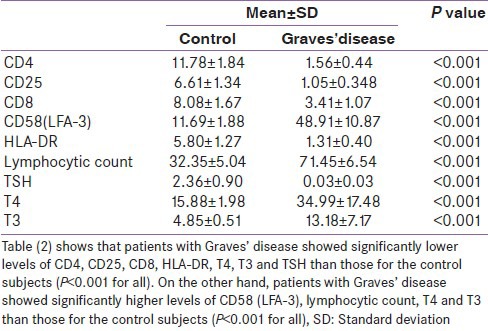
Subjects with colloid nodular goitre did not show significant difference from the control in any of the measured parameters except for CD58 which was higher in the colloid goitre group (P < 0.001) [Table 3].
Table 3.
Measured variables in colloid goitre in comparison with the healthy control
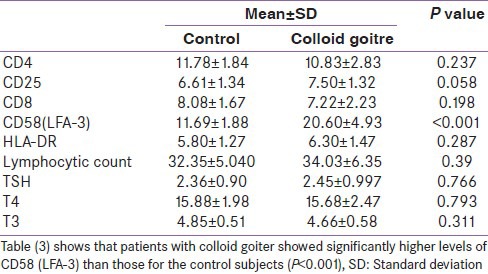
Graves’ disease showed lower CD8+ and higher HLA-DR in comparison with Hashimoto's thyroiditis [Table 4].
Table 4.
Measured variables in Hashimoto's thyroiditis in comparison with Graves’ disease
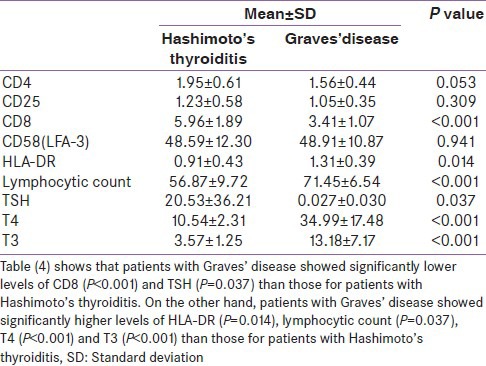
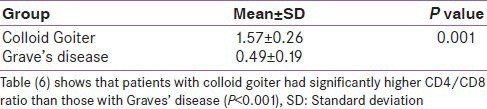
CD4/CD8 ratio was lower in AITD than in the euthyroid healthy control and the colloid goitre group as well. A cut-off value of CD4/CD8 ratio of 1 was calculated to differentiate AITD from colloid nodular goitre. Values more than 1 indicate colloid goitre, and values less than 1 indicate AITD. No difference in the CD4/CD8 ratio between colloid goitre and healthy control subjects was found [Tables 4-7].
Table 7.
Comparing CD4/CD8 ratio in Hashimoto's thyroiditisand Graves’ disease

Table 5.
Comparing CD4/CD8 ratio in Hashimoto's thyroiditis, Graves’ disease and colloid goitre with the healthy control group

Table 6.
Comparing CD4/CD8 ratio in colloid goitre and Graves’ disease
DISCUSSION
Regulatory CD4+ CD25+ T cells are natural controllers of self-reactive T cells and their deficiency produces autoimmune disease.[22] The high surface expression of CD25 is generally considered as a characteristic feature of the majority of human Tregs, and regulatory activity is enriched in CD4+ T cells expressing the highest levels of CD25. Thus, CD25 expression can be used as an indicator of the number of activated lymphocytes.[23,24,25]
In our study, the presence of lower levels of CD4+ CD25+ in patients with AITD does not necessarily reflect a deficit of Tregs, but rather indicates an increased shift of TREGs from the CD25+ Treg with high expression of CD25 into memory/effector T-cell phenotype with low expression of CD25, associated with an enhanced traffic toward inflamed tissue, where they exert their suppressive function in the target organ. This is in agreement with reports of Christian et al.[26]
Another report that may explain our finding is that of Chaoming et al., who demonstrated that the APC mainly the dendritic cell subset, which is found in the peripheral blood and lymphoid organs, was polarized in untreated Graves’ disease patients, leading to a reduction in the number and regulatory capacity of Treg cells through induction of apoptosis.[27]
Supporting our findings, the severe inflammation and autoimmunity occur in individuals who suffer from immunodysregulation, polyendocrinopathy, enteropathy, X-linked syndrome (IPEX). These individuals suffer from depletion of CD25+ CD4+ T cell. They also develop a broad range of autoantibodies.[28,29,30]
Experimental in vivo studies have demonstrated that the absence of CD4+ CD25+ Treg cells allows organ and nonorgan-specific autoimmune diseases such as thyroiditis, gastritis, rheumatoid arthritis, and systemic lupus erythematosus to occur, while the addition of this T-cell population can prevent or delay these diseases.[31]
Treg cell dysfunction in autoimmune disease may be due to a defect in one of the many mechanisms through which TReg cells function.[32] This could occur through inadequate expression of cell surface molecules that are known to be involved in contact-dependent suppression or as a result of failure to produce the soluble factors that are involved in some aspects of suppression. Underlying genetic factors may influence these mechanisms. In addition, the composition of the local milieu, including the types of antigen-presenting cells and cytokines,[33] can Influence Treg cell function.[34]
In our study population, patients of AITDs and those having colloid nodular goitre showed significantly higher CD58 levels than the healthy control group.
Initiation of an autoimmune process requires the interaction between LFA-3 and CD2. Without this, the immune reaction is not established.
The heterotypic interaction between CD2 and its major ligand LFA-3 enhances T-cell antigen recognition. CD2 engagement by LFA-3 expressed on an antigen presenting cell (APC) increases intercellular adhesion and delivers costimulatory signals leading to T-cell proliferation and differentiation.[35]
High level in AITD indicates the active presentation of autoantigen to lymphocytes with stimulation of the autoimmune process. High values of CD58 in colloid goitre indicates that development of an autoimmune thyroid disease requires not only the expression of LFA-3 on the APC but also requires co-stimulatory factors which are not present in colloid goiter.[36]
An interesting potential consequence of T lymphocytic adherence to thyroid cells via LFA-3 and other adhesion molecule interaction, is the stimulation of thyroid cell proliferation, which could lead to goiter formation.[37,38]
Besides CD4+ CD25+ T cells, CD8+ T cells contribute to the regulation of several pathogenic autoimmune responses. Autoreactive CD8+ T cells can downregulate autoimmune responses.[39]
It has been recognized that the proportion and number of CD8+ T cells in the peripheral blood are decreased in patients with autoimmune diseases including Graves’ disease[40,41] and Hashimoto's.[42,43] This is in consistence with our results. We found that CD8+ lymphocytes are significantly lower in Hashimoto's thyroiditis and Graves’ disease compared with the healthy control group.
Although some studies have not found CD8+ T-cell deficiency in patients with autoimmune diseases[44] or have attributed the deficiency to hormonal factors[45] CD8+ T-cell deficiency would appear to be a general feature of human chronic autoimmune diseases.[46]
This finding may be explained by a decrease in suppressor CD8+ T cells leading to disinhibition of autoimmune responses.[47,48,49,50,51] or may be attributed to sequestration of CD8+ T cells in the target organ.[52,53,54]
Covas et al., found a significant decrease in CD8 lymphocytes in Graves’ disease hyperthyroid patients.[55]
In our study the CD4/CD8 ratio was lower in AITDs than colloid goiter and control healthy participants. A cut-off value for differentiation between AITDs and colloid goiter was 1. Higher values indicate colloid goiter while lower values indicate AITDs.
Genetic factors play a role in determination of the CD4/CD8 T-cell ratio in humans[56] with at least some of the responsible genes being located in the HLA complex.[57]
The significant decrease in the CD4/CD8 T-cell ratio in Hashimoto's thyroiditis, reported by Covas et al., is in consistence with our results, but the report of an increased ratio in Graves’ disease is contrary to our findings.[55]
Contradictory results have been published in the literature about the number of peripheral blood lymphocyte subsets in autoimmune diseases.
In his study, Ehler found that the absolute lymphocytic count was significantly lower in Hashimoto's thyroiditis (HT) patients compared to control.[58] In the same study Hashimoto's thyroiditis patients showed significantly lower proportions of CD8+ T cells than healthy control.[58] Some authors have demonstrated a significant decrease in all populations of T-lymphocytes in Graves’ disease hyperthyroid patients.[55] These reports are contradictory to our finding of increased peripheral total lymphocytic count in AITDs.
Similar to our study, some studies reported a significant increase in total peripheral lymphocytic counts in patients with thyrotoxic Graves’ disease and Hashimoto's thyroiditis.[59] We found the total lymphcytic count to be higher in AITD than healthy control and colloid goiter subjects.
Human leucocyte antigen-DR are maturation and costimulatory markers expressed on the surface of mature dendritic cells activated by various stimuli. We found that, patients with AITD whether Graves's disease or Hashimoto's thyroiditis, have lower HLA-DR compared with that in the control group. This may be explained by the fact that defective APC function is associated with impaired HLA expression and lack of costimulatory molecules. This is perceived to be one of the primary mechanisms by which immune surveillance is evaded.[60]
Consistent with our findings are reports of Norio Yoshikawa. He measured the expression of HLA-DR on the surfaces of peripheral blood mononuclear cells, by flow cytometric analysis in 10 patients with Graves’ disease and 11 with Hashimoto's thyroiditis in comparison with healthy controls. Human Leucocyte antigen + T cells were significantly lower in both AITD (P < 0.05 to 0.01 respectively). This suggests deficient or decreased transduction between IL-2R and HLA-DR expression on the cell surface of T cells in AITDs. This may relate to a possible role of IL-2 as a nonspecific stimulatory factor in the pathogenesis of organ-specific autoimmune diseases.[61]
Gess et al. demonstrated that patients with Hashimoto's thyroiditis, but not those with Graves’ disease, expressed increased amounts of HLA-DR antigen compared with healthy subjects on T cells in peripheral blood using three-color flow cytometry.[62]
In our study, CD25 and CD8 are significantly lower, while HLA-DR is significantly higher in Graves’ disease than Hashimoto's thyroiditis. This may be attributed to the nature of the immunological attack in Hashimoto's thyroiditis and Graves’ disease, whether destructive or stimulatory. More analysis of the subtypes of HLA-DR on peripheral blood cells in both diseases may be required in further studies. Long-term follow-up of cases of AITD to find out the relation between findings at time of diagnosis of the disease and the natural history in the study group may also be beneficial in similar but extended studies.
In the light of our study and other available studies, targeting Treg cells should be intensively investigated, and many therapeutic modalities that were proven to increase suppressive abilities of Treg cells should be applied in the treatment of many autoimmune diseases.[63]
CONCLUSION
In autoimune thyroid diseases, namely Hashimoto's thyroiditis and Graves’ disease, CD4+ CD25+ Treg cells as well as CD8+ T lymphocytes, which are concerned with control of autoimmune processes, are down regulated. Leucocyte function antigen-3 is upregulated indicating its role in antigen presentation in such AITD. The lower expression of HLA-DR may indicate its role in the escape of autoantigen from immune surveillance.
ACKNOWLEDGMENT
The authors thank the laboratory team, radiology team, nursing staff, and everybody who participated in the elaboration of this study.
Footnotes
Source of Support: This research did not receive funding from any authority
Conflict of Interest: None declared
REFERENCES
- 1.Armengol MP, Sabater L, Fernandez M, Ruiz M, Alonso N, Otero MJ, et al. Influx of recent thymic emigrants into autoimmune thyroid disease glands in humans. Clin Exp Immunol. 2008;153:338–50. doi: 10.1111/j.1365-2249.2008.03706.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fontenot JD, Rudensky AY. A well adapted regulatory contrivance: regulatory T cell development and the forkhead family transcription factor Foxp3. Nat Immunol. 2005;6:331–7. doi: 10.1038/ni1179. [DOI] [PubMed] [Google Scholar]
- 3.Graca L, Chen TC, Le Moine A, Cobbold SP, Howie D, Waldmann H. Dominant tolerance: Activation thresholds for peripheral generation of regulatory T cells. Trends Immunol. 2005;26:130–5. doi: 10.1016/j.it.2004.12.007. [DOI] [PubMed] [Google Scholar]
- 4.Davidson A, Diamond B. General features of autoimmune diseases. In: Rose E, Mackay R, editors. The autoimmune diseases. St Louis, MO: Academic Press; 2006. pp. 25–36. [Google Scholar]
- 5.Rapoport B, McLachlan SM. Thyroid autoimmunity. J Clin Invest. 2001;108:1253–9. doi: 10.1172/JCI14321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Weetman P, Rose R. Thyroid disease. In: Rose E, Mackay R, editors. The autoimmune diseases. St Louis, MO: Academic Press; 2006. pp. 467–82. [Google Scholar]
- 7.Tait KF, Gough SC. The genetics of autoimmune endocrine disease. Clin Endocrinol. 2003;59:1–11. doi: 10.1046/j.1365-2265.2003.01723.x. [DOI] [PubMed] [Google Scholar]
- 8.Weetman P. Cellular immune responses in autoimmune thyroid disease. Clin Endocrinol. 2004;61:405–13. doi: 10.1111/j.1365-2265.2004.02085.x. [DOI] [PubMed] [Google Scholar]
- 9.Duntas LH. Environmental factors and autoimmune thyroiditis. Nat Clin Pract Endocrinol Metab. 2008;4:454–60. doi: 10.1038/ncpendmet0896. [DOI] [PubMed] [Google Scholar]
- 10.Tomer Y. Genetic susceptibility to autoimmune thyroid disease: Past, present, and future. Thyroid. 2010;20:715–25. doi: 10.1089/thy.2010.1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brix TH, Kyvik KO, Hegedus L. A population-based study of chronic autoimmune hypothyroidism in Danish twins. J Clin Endocrinol Metab. 2000;85:536–9. doi: 10.1210/jcem.85.2.6385. [DOI] [PubMed] [Google Scholar]
- 12.Barbesino G, Chiovato L. The genetics of Hashimoto's disease. Endocrinol Metab Clin North Am. 2000;29:357–74. doi: 10.1016/s0889-8529(05)70136-5. [DOI] [PubMed] [Google Scholar]
- 13.Sarantopoulos S, Lu L, Cantor H. Qa-1 restriction of CD8+suppressor T cells. J Clin Invest. 2004;114:1218–21. doi: 10.1172/JCI23152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Itoh M, Takahashi T, Sakaguchi N, Kuniyasu Y, Shimizu J, Otsuka F, et al. Thymus and autoimmunity: Production of CD25+CD4+naturally anergic and suppressive T cells as a key function of the thymus in maintaining immunologic self-tolerance. J Immunol. 1999;162:5317–26. [PubMed] [Google Scholar]
- 15.Wallich R, Brenner C, Brand Y, Roux M, Reister M, Meuer S. Gene structure, promoter characterization, and basis for alternative mRNA splicing of the human CD58 gene. J Immunol. 1998;160:2862–71. [PubMed] [Google Scholar]
- 16.Wang JH, Smolyar A, Tan K, Liu JH, Kim M, Sun ZY, et al. Structure of a heterophilic adhesion complex between the human CD2 and CD58 (LFA-3) counterreceptors. Cell. 1999;97:791–803. doi: 10.1016/s0092-8674(00)80790-4. [DOI] [PubMed] [Google Scholar]
- 17.Jacobson EM, Huber A, Tomer Y. The HLA gene complex in thyroid autoimmunity: From epidemiology to etiology. J Autoimmun. 2008;30:58–62. doi: 10.1016/j.jaut.2007.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Clinical and Laboratory Standards Institute (formerly NCCLS) 3rd ed. Wayne, PA: Clinical and Laboratory Standards Institute; 2004. Procedures for the Handling and Processing of Blood Specimens; Approved Guideline. NCCLS Document H18-A3. [Google Scholar]
- 19.Kaplan MM. Mono: Thyroid Testing; Chiron Diagnostics Corporation; 1996. Thyroid function testing in patients with thyroid and non-thyroid diseases. [Google Scholar]
- 20.Kaplan MM. Mono: Thyroid Testing; Chiron Diagnostics Corporation; 1996. Thyroid function testing in patients with thyroid and non-thyroid diseases. [Google Scholar]
- 21.Holmes KL, Otten G, Yokoyama WM. Flow cytometry analysis using the Becton Dickinson FACS Calibur. Curr Protoc Immunol. 2002;(Chapter 5: Unit 5.4) doi: 10.1002/0471142735.im0504s49. [DOI] [PubMed] [Google Scholar]
- 22.Ganesh BB, Bhattacharya P, Gopisetty A, Prabhakar BS. Role of cytokines in the pathogenesis and suppression of thyroid autoimmunity. J Interferon Cytokine Res. 2011;31:721–31. doi: 10.1089/jir.2011.0049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Baecher-Allan C, Brown JA, Freeman GJ, Hafler DA. CD4+CD25high regulatory cells in human peripheral blood. J Immunol. 2001;167:1245–53. doi: 10.4049/jimmunol.167.3.1245. [DOI] [PubMed] [Google Scholar]
- 24.de Kleer IM, Wedderburn LR, Taams LS, Patel A, Varsani H, Klein M, et al. CD4+CD25bright regulatory T cells actively regulate inflammation in the joints of patients with the remitting form of juvenile idiopathic arthritis. J Immunol. 2004;172:6435–43. doi: 10.4049/jimmunol.172.10.6435. [DOI] [PubMed] [Google Scholar]
- 25.Baecher-Allan C, Wolf E, Hafler DA. Functional analysis of highly defined, FACS-isolated populations of human regulatory CD4+CD25+T cells. Clin Immunol. 2005;115:10–8. doi: 10.1016/j.clim.2005.02.018. [DOI] [PubMed] [Google Scholar]
- 26.Dejaco C, Duftner C, Grubeck-Loebenstein B, Schirmer M. Imbalance of regulatory T cells in human autoimmune diseases. Immunology. 2006;117:289–300. doi: 10.1111/j.1365-2567.2005.02317.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mao C, Wang S, Xiao Y, Xu J, Jiang Q, Jin M, et al. Impairment of regulatory capacity of CD4+CD25+Regulatory T cells mediated by dendritic cell polarization and hyperthyroidism in Graves’ disease. J Immunol. 2011;186:4734–43. doi: 10.4049/jimmunol.0904135. [DOI] [PubMed] [Google Scholar]
- 28.Gambineri E, Torgerson T, Ochs H. Immune dysregulation, polyendocrinopathy, enteropathy, and X-linked inheritance (IPEX), a syndrome of systemic autoimmunity caused by mutations of FOXP3, a critical regulator of T-cell homeostasis. Curr Opin Rheumatol. 2003;15:430–5. doi: 10.1097/00002281-200307000-00010. [DOI] [PubMed] [Google Scholar]
- 29.Wildin RS, Smyk-Pearson S, Filipovich AH. Clinical and molecular features of the immunodysregulation, polyendocrinopathy, enteropathy, X linked (IPEX) syndrome. J Med Genet. 2002;39:537–45. doi: 10.1136/jmg.39.8.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wing K, Sakaguchi S. Regulatory T cells exert checks and balances on self tolerance and autoimmunity. Nat Immunol. 2010;11:7–13. doi: 10.1038/ni.1818. [DOI] [PubMed] [Google Scholar]
- 31.Toubi E. Targeting T regulatory cells in autoimmune diseases. Isr Med Assoc J. 2008;10:73–6. [PubMed] [Google Scholar]
- 32.Vignali DA, Collison LW, Workman CJ. How regulatory T cells work. Nat Rev Immunol. 2008;8:523–32. doi: 10.1038/nri2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Korn T, Reddy J, Gao W, Bettelli E, Awasthi A, Petersen TR, et al. Myelin-specific regulatory T cells accumulate in the CNS but fail to control autoimmune inflammation. Nat Med. 2007;13:423–31. doi: 10.1038/nm1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Buckner JH. Mechanisms of impaired regulation by CD4+CD25+FOXP3+regulatory T cells in human autoimmune diseases. Nat Rev Immunol. 2010;10:849–59. doi: 10.1038/nri2889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Virella G, editor. 6th ed. Charleston, South Carolina, New York, USA: Medical University of South Carolina; 2007. Medical Immunology. [Google Scholar]
- 36.Iwona BS, Jadwiga SS, Elzbieta K, Leszek S. Lymphocytes in peripheral blood and thyroid tissue in children with Graves’ disease. Pediatrics. 2008;121:S103–4. doi: 10.1007/s12519-008-0050-6. [DOI] [PubMed] [Google Scholar]
- 37.Arao T, Morimoto I, Kakinuma A, Ishida O, Zeki K, Tanaka Y, et al. Thyrocyte proliferation by cellular adhesion to infiltrating lymphocytes through the intercellular adhesion molecule-1/lymphocyte function-associated antigen-1 pathway in Graves’ disease. J Clin Endocrinol Metab. 2000;85:382–9. doi: 10.1210/jcem.85.1.6320. [DOI] [PubMed] [Google Scholar]
- 38.Tandon N, Makgoba MW, Gahmberg CG, Weetman AP. The expression and role in T cell adhesion of LFA-3 and ICAM-2 on human thyroid cells. J Clin Endocrinol Metab. 1983;56:2. doi: 10.1016/0090-1229(92)90056-t. [DOI] [PubMed] [Google Scholar]
- 39.Walter U, Santamaria P. CD8+T cells in autoimmunity. Curr Opin Immunol. 2005;17:624–31. doi: 10.1016/j.coi.2005.09.014. [DOI] [PubMed] [Google Scholar]
- 40.Thielemans C, Vanhaelst L, De Waele M. Autoimmune thyroiditis: A condition related to a decrease in T-suppressor cells. Clin Endocrinol. 1981;15:259–63. doi: 10.1111/j.1365-2265.1981.tb00664.x. [DOI] [PubMed] [Google Scholar]
- 41.Xia N, Zhou S, Liang Y, Xiao C, Shen H, Pan H, et al. CD4+T cells and the Th1/Th2 imbalance are implicated in the pathogenesis of Graves’ ophthalmopathy. Int J Mol Med. 2006;17:911–6. [PubMed] [Google Scholar]
- 42.Thielemans C, Vanhaelst L, De Waele M. Autoimmune thyroiditis: A condition related to a decrease in T-suppressor cells. Clin Endocrinol. 1981;15:259–63. doi: 10.1111/j.1365-2265.1981.tb00664.x. [DOI] [PubMed] [Google Scholar]
- 43.Iwatani Y, Amino N, Hidaka Y, Kaneda T, Ichihara K, Tamaki H, et al. Decreases in αβ T cell receptor negative T cells and CD8 cells, and an increase in CD4+CD8+cells in active Hashimoto's disease and subacute thyroiditis. Clin Exp Immunol. 1992;87:444–9. doi: 10.1111/j.1365-2249.1992.tb03017.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Iwatani Y, Amino N, Mori H, Asari S, Izumiguchi Y, Kumahara Y, et al. T lymphocyte subsets in autoimmune thyroid diseases and subacute thyroiditis detected with monoclonal antibodies. J Clin Endocrinol Metab. 1983;56:251–4. doi: 10.1210/jcem-56-2-251. [DOI] [PubMed] [Google Scholar]
- 45.Bonnyns M, Bentin J, Devetter G, Duchateau J. Heterogeneity of immunoregulatory T cells in human thyroid autoimmunity: Influence of thyroid status. Clin Exp Immunol. 1983;52:629–34. [PMC free article] [PubMed] [Google Scholar]
- 46.Pender MP. CD8+T-Cell Deficiency, Epstein-Barr Virus Infection, Vitamin D Deficiency, and Steps to Autoimmunity: A Unifying Hypothesis. Autoimmune Dis 2012. 2012:189096. doi: 10.1155/2012/189096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Reinherz EL, Weiner HL, Hauser SL. Loss of suppressor T cells in active multiple sclerosis. Analysis with monoclonal antibodies. N Engl J Med. 1980;303:125–9. doi: 10.1056/NEJM198007173030303. [DOI] [PubMed] [Google Scholar]
- 48.Veys EM, Hermanns P, Goldstein G, Kung P, Schindler J, Van Wauwe J. Determination of T lymphocyte subpopulations by monoclonal antibodies in rheumatoid arthritis. Influence of immunomodulating agents. Int J Immunopharmacol. 1981;3:313–9. doi: 10.1016/0192-0561(81)90025-4. [DOI] [PubMed] [Google Scholar]
- 49.Morimoto C, Reinherz EL, Schlossman SF, Schur PH, Mills JA, Steinberg AD. Alterations in immunoregulatory T cell subsets in active systemic lupus erythematosus. J Clin Investig. 1980;66:1171–4. doi: 10.1172/JCI109948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Thielemans C, Vanhaelst L, De Waele M. Autoimmune thyroiditis: A condition related to a decrease in T-suppressor cells. Clin Endocrinol. 1981;15:259–63. doi: 10.1111/j.1365-2265.1981.tb00664.x. [DOI] [PubMed] [Google Scholar]
- 51.Berrih S, Gaud C, Bach MA, Le Brigand H, Binet JP, Bach JF. Evaluation of T cell subsets in myasthenia gravis using anti-T cell monoclonal antibodies. Clin Exp Immunol. 1981;45:1–8. [PMC free article] [PubMed] [Google Scholar]
- 52.Kreuzfelder E, Shen G, Bittorf M, Scheiermann N, Thraenhart O, Seidel D, et al. Enumeration of T, B and natural killer peripheral blood cells of patients with multiple sclerosis and controls. Eur Neurol. 1992;32:190–4. doi: 10.1159/000116820. [DOI] [PubMed] [Google Scholar]
- 53.Fox RI, Fong S, Sabharwal N, Carstens SA, Kung PC, Vaughan JH. Synovial fluid lymphocytes differ from peripheral blood lymphocytes in patients with rheumatoid arthritis. J Immunol. 1982;128:351–4. [PubMed] [Google Scholar]
- 54.Moreno-Otero R, Civeira MP, Suou T, Kanof ME, James SP, Jones EA. Reduced numbers of CD8+T cells and B cell expression of Leu-8 antigen in peripheral blood of patients with primary biliary cirrhosis. Hepatogastroenterology. 1994;41:239–43. [PubMed] [Google Scholar]
- 55.Covas MI, Esquerda A, García-Rico A, Mahy N. Peripheral blood T-lymphocyte subsets in autoimmune thyroid disease. J Investig Allergol Clin Immunol. 1992;2:131–5. [PubMed] [Google Scholar]
- 56.Clementi M, Forabosco P, Amadori A, Zamarchi R, De Silvestro G, Di Gianantonio E, et al. Genetic control of the CD4/CD8 T-cell ratio in humans. Nat Med. 1995;1:1279–83. doi: 10.1038/nm1295-1279. [DOI] [PubMed] [Google Scholar]
- 57.Ferreira MA, Hottenga JJ, Warrington NM, Medland SE, Willemsen G, Lawrence RW, et al. Quantitative trait loci for CD4:CD8 lymphocyte ratio are associated with risk of type 1 diabetes and HIV-1 immune control. Am J Hum Genet. 2010;86:88–92. doi: 10.1016/j.ajhg.2009.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ehlers M, Thiel A, Bernecker C, Porwol D, Papewalis C, Willenberg HS, et al. Evidence of a combinedcytotoxic thyroglobulin and thyroperoxidase epitope-specific cellular immunity in Hashimoto's Thyroiditis. J Clin Endocrinol Metab. 2012;97:1347–54. doi: 10.1210/jc.2011-2178. [DOI] [PubMed] [Google Scholar]
- 59.Brown RS. Autoimmune thyroiditis in childhood. J Clin Res Pediatr Endocrinol. 2013;5:45–9. doi: 10.4274/Jcrpe.855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Iwatani Y, Amino N, Mori H, Asari S, Izumiguchi Y, Kumahara Y, et al. lymphocyte subsets in autoimmune thyroid diseases and subacute thyroiditis detected with monoclonal antibodies. J Investig Allergol Clin Immunol. 1992;2:131–5. doi: 10.1210/jcem-56-2-251. [DOI] [PubMed] [Google Scholar]
- 61.Yoshikawa N, Morita T, Arreaza G, Resetkova E, Mukuta T, Volpé R. The effect of interleukin-2 on suppressor T lymphocytes in autoimmune thyroid disease. Clin Invest Med. 1995;18:91–8. [PubMed] [Google Scholar]
- 62.Gessl A, Waldhäusl W. Elevated CD69 expression on naive peripheral blood T-cells in hyperthyroid Graves’ disease and autoimmune thyroiditis: Discordant effect of methimazole on HLA-DR and CD69. Clin Immunol Immunopathol. 1998;87:168–75. doi: 10.1006/clin.1998.4524. [DOI] [PubMed] [Google Scholar]
- 63.Toubi E. Targeting T regulatory cells in autoimmune diseases MD. Clin Immunol Immunopathol. 1992;64:30–5. [Google Scholar]


