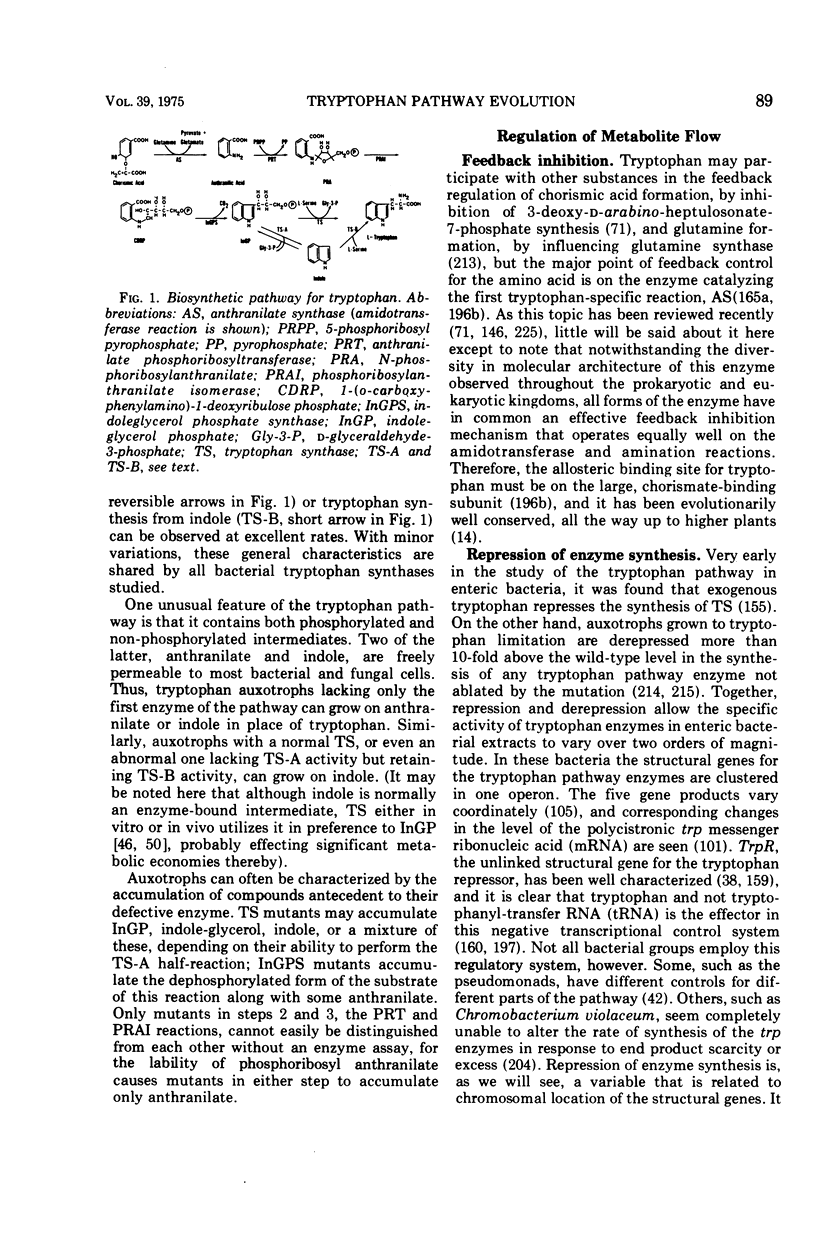
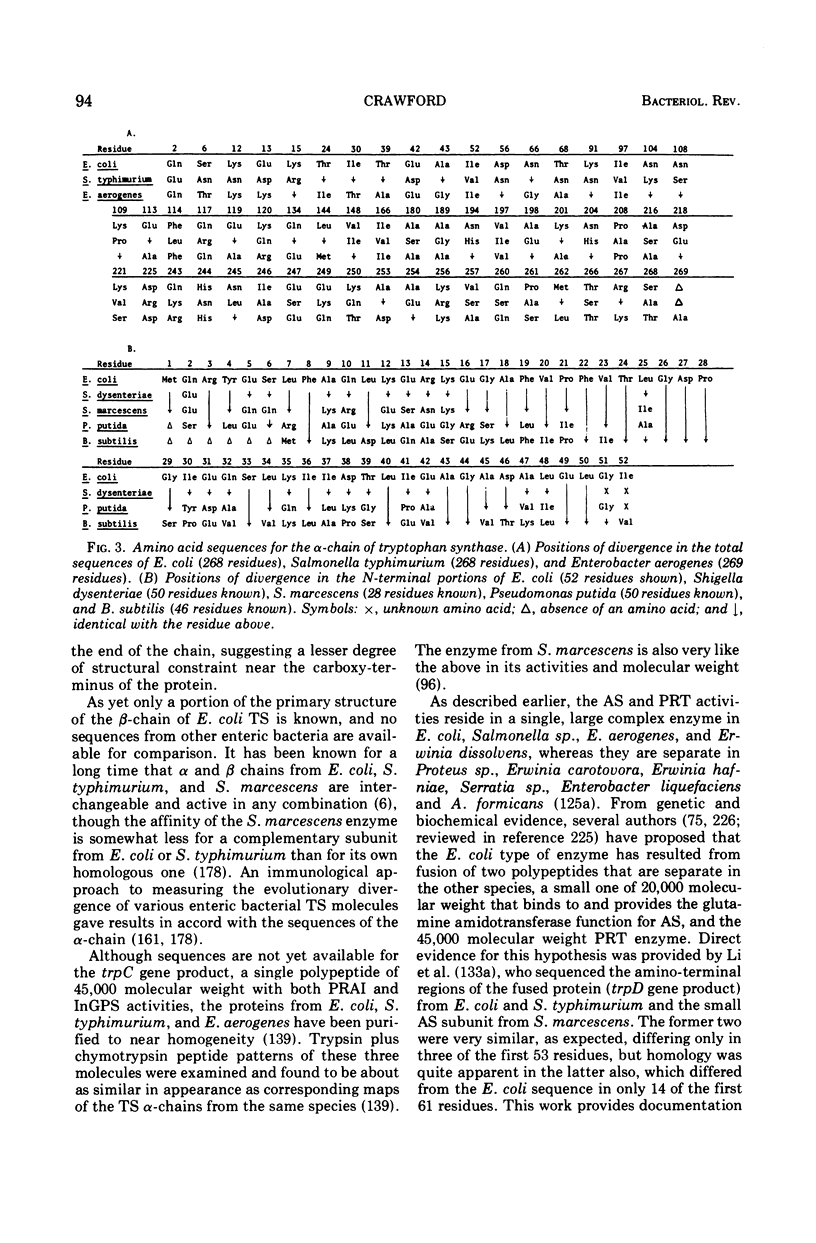
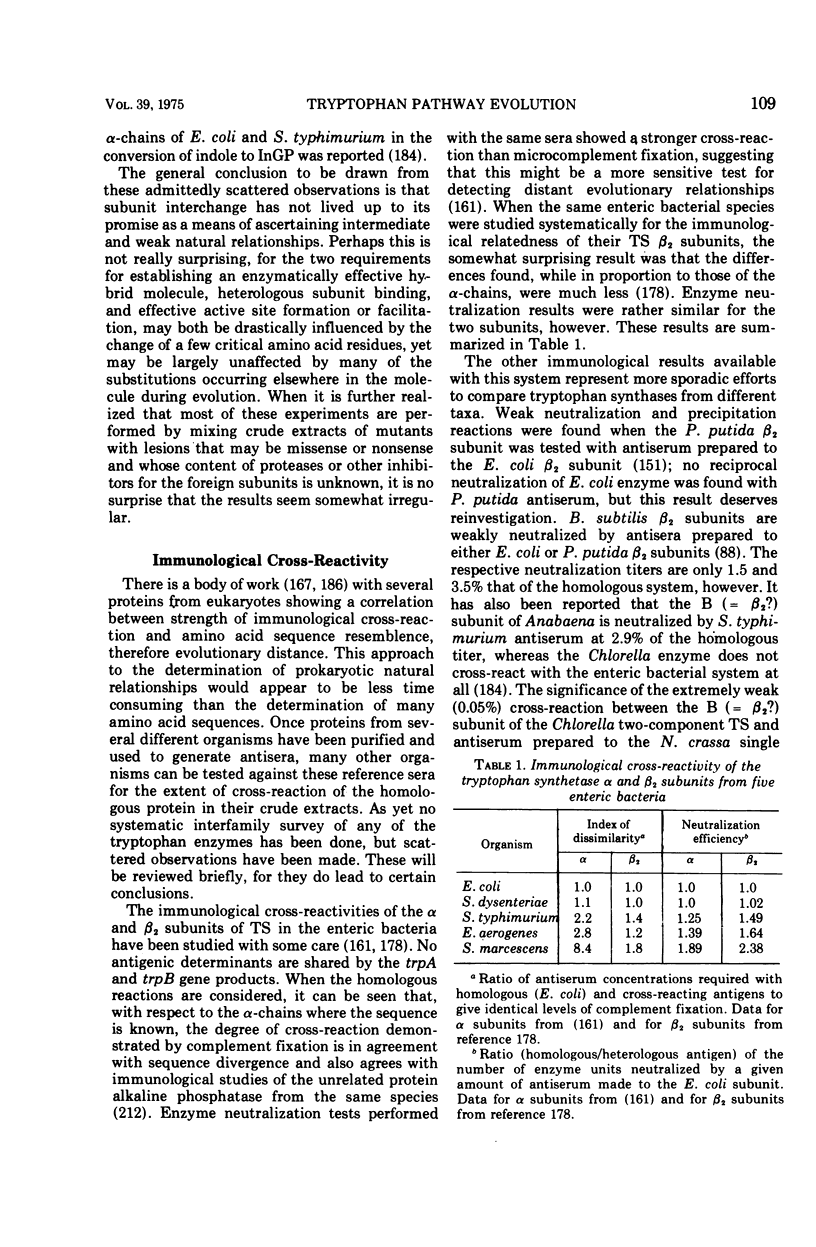
Full text
PDF






























Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANAGNOSTOPOULOS C., CRAWFORD I. P. Transformation studies on the linkage of markers in the tryptophan pathway in Bacillus subtilis. Proc Natl Acad Sci U S A. 1961 Mar 15;47:378–390. doi: 10.1073/pnas.47.3.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adachi O., Kohn L. D., Miles E. W. Crystalline alpha2 beta2 complexes of tryptophan synthetase of Escherichia coli. A comparison between the native complex and the reconstituted complex. J Biol Chem. 1974 Dec 25;249(24):7756–7763. [PubMed] [Google Scholar]
- Adachi O., Miles E. W. A rapid method for preparing crystalline beta 2 subunit of tryptophan synthetase of Escherichia coli in high yield. J Biol Chem. 1974 Sep 10;249(17):5430–5434. [PubMed] [Google Scholar]
- Ahmad M., Mozmadar A., Hendler S. A new locus in the tryptophan pathway of Neurospora crassa. Genet Res. 1968 Oct;12(2):103–107. doi: 10.1017/s001667230001171x. [DOI] [PubMed] [Google Scholar]
- Anagnostopoulos C., Crawford I. P. Le groupe des gènes régissant la biosynthèse du tryptophane chez Bacillus subtilis. C R Acad Sci Hebd Seances Acad Sci D. 1967 Jul 3;265(1):93–96. [PubMed] [Google Scholar]
- BOVRE K., HENRIKSEN S. D. An approach to transformation studies in Moraxella. Acta Pathol Microbiol Scand. 1962;56:223–228. doi: 10.1111/j.1699-0463.1962.tb04183.x. [DOI] [PubMed] [Google Scholar]
- Balbinder E., Blume A. J., Weber A., Tamaki H. Polar and antipolar mutants in the tryptophan operon of Salmonella typhimurium. J Bacteriol. 1968 Jun;95(6):2217–2229. doi: 10.1128/jb.95.6.2217-2229.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balbinder E., Callahan R., 3rd, McCann P. P., Cordaro J. C., Weber A. R., Smith A. M., Angelosanto F. Regulatory mutants of the tryptophan operon of Salmonella typhimurium. Genetics. 1970 Sep;66(1):31–53. doi: 10.1093/genetics/66.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baskerville E. N., Twarog R. Regulation of the tryptophan synthetic enzymes in Clostridium butyricum. J Bacteriol. 1972 Oct;112(1):304–314. doi: 10.1128/jb.112.1.304-314.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauerle R. H., Margolin P. A multifunctional enzyme complex in the tryptophan pathway of Salmonella typhimurium: comparison of polarity and pseudopolarity mutations. Cold Spring Harb Symp Quant Biol. 1966;31:203–214. doi: 10.1101/sqb.1966.031.01.028. [DOI] [PubMed] [Google Scholar]
- Bauerle R. H., Margolin P. The functional organization of the tryptophan gene cluster in Salmonella typhimurium. Proc Natl Acad Sci U S A. 1966 Jul;56(1):111–118. doi: 10.1073/pnas.56.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann P., Doudoroff M., Stanier R. Y. A study of the Moraxella group. II. Oxidative-negative species (genus Acinetobacter). J Bacteriol. 1968 May;95(5):1520–1541. doi: 10.1128/jb.95.5.1520-1541.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann P., Doudoroff M., Stanier R. Y. Study of the Moraxella group. I. Genus Moraxella and the Neisseria catarrhalis group. J Bacteriol. 1968 Jan;95(1):58–73. doi: 10.1128/jb.95.1.58-73.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belser W. L., Murphy J. B., Delmer D. P., Mills S. E. End product control of tryptophan biosynthesis in extracts and intact cells of the higher plant Nicotiana tabacum var. Wisconsin 38. Biochim Biophys Acta. 1971 Apr 20;237(1):1–10. doi: 10.1016/0304-4165(71)90023-7. [DOI] [PubMed] [Google Scholar]
- Berlyn M. B., Ahmed S. I., Giles N. H. Organization of polyaromatic biosynthetic enzymes in a variety of photosynthetic organisms. J Bacteriol. 1970 Nov;104(2):768–774. doi: 10.1128/jb.104.2.768-774.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berlyn M. B., Giles N. H. Organization of interrelated aromatic metabolic pathway enzymes in Acinetobacter calco-aceticus. J Gen Microbiol. 1973 Feb;74(2):337–341. doi: 10.1099/00221287-74-2-337. [DOI] [PubMed] [Google Scholar]
- Blume A. J., Weber A., Balbinder E. Analysis of polar and nonpolar tryptophan mutants by derepression kinetics. J Bacteriol. 1968 Jun;95(6):2230–2241. doi: 10.1128/jb.95.6.2230-2241.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brammar W. J. Independent expression of the A gene of the tryptophan operon of Escherichia coli during tryptophan starvation. J Gen Microbiol. 1973 Jun;76(2):395–405. doi: 10.1099/00221287-76-2-395. [DOI] [PubMed] [Google Scholar]
- Bronson M. J., Squires C., Yanofsky C. Nucleotide sequences from tryptophan messenger RNA of Escherichia coli: the sequence corresponding to the amino-terminal region of the first polypeptide specified by the operon. Proc Natl Acad Sci U S A. 1973 Aug;70(8):2335–2339. doi: 10.1073/pnas.70.8.2335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CARSIOTIS M., LACY A. M. INCREASED ACTIVITY OF TRYPTOPHAN BIOSYNTHETIC ENZYMES IN HISTIDINE MUTANTS OF NEUROSPORA CRASSA. J Bacteriol. 1965 Jun;89:1472–1477. doi: 10.1128/jb.89.6.1472-1477.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CATLIN B. W., CUNNINGHAM L. S. Transforming activities and base contents of deoxyribonucleate preparations from various Neisseriae. J Gen Microbiol. 1961 Oct;26:303–312. doi: 10.1099/00221287-26-2-303. [DOI] [PubMed] [Google Scholar]
- CATLIN B. W. RECIPROCAL GENETIC TRANSFORMATION BETWEEN NEISSERIA CATARRHALIS AND MORAXELLA NONLIQUEFACIENS. J Gen Microbiol. 1964 Dec;37:369–379. doi: 10.1099/00221287-37-3-369. [DOI] [PubMed] [Google Scholar]
- COHEN G., JACOB F. Sur la répression de la synthèse des enzymes intervenant dans la formation du tryptophane chez Escherichia coll. C R Hebd Seances Acad Sci. 1959 Jun 15;248(24):3490–3492. [PubMed] [Google Scholar]
- Calhoun D. H., Pierson D. L., Jensen R. A. The regulation of tryptophan biosynthesis in Pseudomonas aeruginosa. Mol Gen Genet. 1973 Mar 1;121(2):117–132. doi: 10.1007/BF00277526. [DOI] [PubMed] [Google Scholar]
- Callahan R., 3rd, Balbinder E. Tryptophan operon: structural gene mutation creating a "promoter" and leading to 5-methyltryptophan dependence. Science. 1970 Jun 26;168(3939):1586–1589. doi: 10.1126/science.168.3939.1586. [DOI] [PubMed] [Google Scholar]
- Carlton B. C., Whitt D. D. The isolation and genetic characterization of mutants of the tryptophan system of Bacillus subtilis. Genetics. 1969 Jul;62(3):445–460. doi: 10.1093/genetics/62.3.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carsiotis M., Jones R. F. Cross-pathway regulation: tryptophan-mediated control of histidine and arginine biosynthetic enzymes in Neurospora crassa. J Bacteriol. 1974 Sep;119(3):889–892. doi: 10.1128/jb.119.3.889-892.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carsiotis M., Jones R. F., Lacy A. M., Cleary T. J., Fankhauser D. B. Histidine-mediated control of tryptophan biosynthetic enzymes in Neurospora crassa. J Bacteriol. 1970 Oct;104(1):98–106. doi: 10.1128/jb.104.1.98-106.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carsiotis M., Jones R. F., Wesseling A. C. Cross-pathway regulation: histidine-mediated control of histidine, tryptophan, and arginine biosynthetic enzymes in Neurospora crassa. J Bacteriol. 1974 Sep;119(3):893–898. doi: 10.1128/jb.119.3.893-898.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casse F., Pascal M. C., Chippaux M. Comparison between the chromosomal maps of Escherichia coli and Salmonella typhimurium. Length of the inverted segment in the trp region. Mol Gen Genet. 1973 Aug 17;124(3):253–257. doi: 10.1007/BF00293096. [DOI] [PubMed] [Google Scholar]
- Catena A., DeMoss R. D. Physiological and kinetic studies with anthranilate synthetase of Bacillus alvei. J Bacteriol. 1970 Feb;101(2):476–482. doi: 10.1128/jb.101.2.476-482.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chilton M. D., McCarthy B. J. Genetic and base sequence homologies in bacillus. Genetics. 1969 Jul;62(3):697–710. [PMC free article] [PubMed] [Google Scholar]
- Colwell R. R. Genetic and phenetic classification of bacteria. Adv Appl Microbiol. 1973;16:137–175. doi: 10.1016/s0065-2164(08)70026-1. [DOI] [PubMed] [Google Scholar]
- Crawford I. P., Gunsalus I. C. Inducibility of tryptophan synthetase in Pseudomonas putida. Proc Natl Acad Sci U S A. 1966 Aug;56(2):717–724. doi: 10.1073/pnas.56.2.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford I. P., Sikes S., Melhorn D. K. The natural relationships of Aeromonas formicans. Arch Mikrobiol. 1967;59(1):72–81. doi: 10.1007/BF00406318. [DOI] [PubMed] [Google Scholar]
- Crawford I. P., Yanofsky C. Pseudomonas putida tryptophan synthetase: partial sequence of the subunit. J Bacteriol. 1971 Oct;108(1):248–253. doi: 10.1128/jb.108.1.248-253.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creighton T. E. A steady-state kinetic investigation of the reaction mechanism of the tryptophan synthetase of Escherichia coli. Eur J Biochem. 1970 Mar 1;13(1):1–10. doi: 10.1111/j.1432-1033.1970.tb00892.x. [DOI] [PubMed] [Google Scholar]
- Creighton T. E. N-(5'-phosphoribosyl)anthranilate isomerase-indol-3-ylglycerol phosphate synthetase of tryptophan biosynthesis. Relationship between the two activities of the enzyme from Escherichia coli. Biochem J. 1970 Dec;120(4):699–707. doi: 10.1042/bj1200699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEMOSS J. A. Studies on the mechanism of the tryptophan synthetase reaction. Biochim Biophys Acta. 1962 Aug 13;62:279–293. doi: 10.1016/0006-3002(62)90041-0. [DOI] [PubMed] [Google Scholar]
- DEMOSS J. A. THE CONVERSION OF SHIKIMIC ACID TO ANTHRANILIC ACID BY EXTRACTS OF NEUROSPORA CRASSA. J Biol Chem. 1965 Mar;240:1231–1235. [PubMed] [Google Scholar]
- DEMOSS R. D., EVANS N. R. Incorporation of C14-labeled substrates into violacein. J Bacteriol. 1960 May;79:729–733. doi: 10.1128/jb.79.5.729-733.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeMoss J. A., Jackson R. W., Chalmers J. H., Jr Genetic control of the structure and activity of an enzyme aggregate in the tryptophan pathway of Neurospora crassa. Genetics. 1967 Jul;56(3):413–424. doi: 10.1093/genetics/56.3.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delmer D. P., Mills S. E. Tryptophan Biosynthesis in Cell Cultures of Nicotiana tabacum. Plant Physiol. 1968 Jan;43(1):81–87. doi: 10.1104/pp.43.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delmer D. P., Mills S. Tryptophan synthase from Nicotiana tabacum. Biochim Biophys Acta. 1968 Oct 8;167(2):431–443. doi: 10.1016/0005-2744(68)90223-4. [DOI] [PubMed] [Google Scholar]
- Denney R. M., Yanofsky C. Detection of tryptophan messenger RNA in several bacterial species and examination of the properties of heterologous DNA-RNA hybrids. J Mol Biol. 1972 Mar 14;64(2):319–339. doi: 10.1016/0022-2836(72)90501-3. [DOI] [PubMed] [Google Scholar]
- Doolittle W. F., Yanofsky C. Mutants of Escherichia coli with an altered tryptophanyl-transfer ribonucleic acid synthetase. J Bacteriol. 1968 Apr;95(4):1283–1294. doi: 10.1128/jb.95.4.1283-1294.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doy C. H., Cooper J. M. Aromatic biosynthesis in yeast. I. The synthesis of tryptophan and the regulation of this pathway. Biochim Biophys Acta. 1966 Oct 31;127(2):302–316. [PubMed] [Google Scholar]
- DuBuy B., Weissman S. M. Nucleotide sequence of Pseudomonas fluorescens 5 S ribonucleic acid. J Biol Chem. 1971 Feb 10;246(3):747–761. [PubMed] [Google Scholar]
- Dubnau D., Smith I., Morell P., Marmur J. Gene conservation in Bacillus species. I. Conserved genetic and nucleic acid base sequence homologies. Proc Natl Acad Sci U S A. 1965 Aug;54(2):491–498. doi: 10.1073/pnas.54.2.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn N. W., Gunsalus I. C. Transmissible plasmid coding early enzymes of naphthalene oxidation in Pseudomonas putida. J Bacteriol. 1973 Jun;114(3):974–979. doi: 10.1128/jb.114.3.974-979.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EISENSTEIN R. B., YANOFSKY C. Tryptophan synthetase levels in Escherichia coli, Shigella dysenteriae, and transduction hybrids. J Bacteriol. 1962 Jan;83:193–204. doi: 10.1128/jb.83.1.193-204.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enatsu T., Crawford I. P. Enzymes of the tryptophan synthetic pathway in Pseudomonas putida. J Bacteriol. 1968 Jan;95(1):107–112. doi: 10.1128/jb.95.1.107-112.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FARGIE B., HOLLOWAY B. W. ABSENCE OF CLUSTERING OF FUNCTIONALLY RELATED GENES IN PSEUDOMONAS AERUGINOSA. Genet Res. 1965 Jul;6:284–299. doi: 10.1017/s0016672300004158. [DOI] [PubMed] [Google Scholar]
- Fink G. R., Martin R. G. Translation and polarity in the histidine operon. II. Polarity in the histidine operon. J Mol Biol. 1967 Nov 28;30(1):97–107. doi: 10.1016/0022-2836(67)90246-x. [DOI] [PubMed] [Google Scholar]
- Fitch W. M., Margoliash E. Construction of phylogenetic trees. Science. 1967 Jan 20;155(3760):279–284. doi: 10.1126/science.155.3760.279. [DOI] [PubMed] [Google Scholar]
- Gaertner F. H., DeMoss J. A. Purification and characterization of a multienzyme complex in the tryptophan pathway of Neurospora crassa. J Biol Chem. 1969 May 25;244(10):2716–2725. [PubMed] [Google Scholar]
- Gaertner F. H., Ericson M. C., DeMoss J. A. Catalytic facilitation in vitro by two multienyzme complexes from Neurospora crassa. J Biol Chem. 1970 Feb 10;245(3):595–600. [PubMed] [Google Scholar]
- Gibson F., Pittard J. Pathways of biosynthesis of aromatic amino acids and vitamins and their control in microorganisms. Bacteriol Rev. 1968 Dec;32(4 Pt 2):465–492. [PMC free article] [PubMed] [Google Scholar]
- Gibson F., Pittard J., Reich E. Ammonium ions as the source of nitrogen for tryptophan biosynthesis in whole cells of Escherichia coli. Biochim Biophys Acta. 1967 Apr 25;136(3):573–576. doi: 10.1016/0304-4165(67)90020-7. [DOI] [PubMed] [Google Scholar]
- Gibson M. I., Gibson F. Preliminary studies on the isolation and metabolism of an intermediate in aromatic biosynthesis: chorismic acid. Biochem J. 1964 Feb;90(2):248–256. doi: 10.1042/bj0900248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberger R. F. Autogenous regulation of gene expression. Science. 1974 Mar 1;183(4127):810–816. doi: 10.1126/science.183.4127.810. [DOI] [PubMed] [Google Scholar]
- Guest J. R., Drapeau G. R., Carlton B. C., Yanofsky C. The amino acid sequence of the A protein (alpha subunit) of the tryptophan synthetase of Escherichia coli. J Biol Chem. 1967 Nov 25;242(22):5442–5446. [PubMed] [Google Scholar]
- Gunsalus C., Gunsalus C. F., Chakrabarty A. M., Sikes S., Crawford I. P. Fine structure mapping of the tryptophan genes in Pseudomonas putida. Genetics. 1968 Nov;60(3):419–435. doi: 10.1093/genetics/60.3.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helinski D. R. Plasmid determined resistance to antibiotics: molecular properties of R factors. Annu Rev Microbiol. 1973;27:437–470. doi: 10.1146/annurev.mi.27.100173.002253. [DOI] [PubMed] [Google Scholar]
- Henriksen S. D. Moraxella, Acinetobacter, and the Mimeae. Bacteriol Rev. 1973 Dec;37(4):522–561. doi: 10.1128/br.37.4.522-561.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman N. J., Juni E. Isolation and characterization of a generalized transducing bacteriophage for Acinetobacter. J Virol. 1974 Jan;13(1):46–52. doi: 10.1128/jvi.13.1.46-52.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hershfield V., Boyer H. W., Yanofsky C., Lovett M. A., Helinski D. R. Plasmid ColEl as a molecular vehicle for cloning and amplification of DNA. Proc Natl Acad Sci U S A. 1974 Sep;71(9):3455–3459. doi: 10.1073/pnas.71.9.3455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiraga S., Ito K., Matsuyama T., Ozaki H., Yura T. 5-methyltryptophan-resistant mutations lniked with the arginine G marker in Escherichia coli. J Bacteriol. 1968 Nov;96(5):1880–1881. doi: 10.1128/jb.96.5.1880-1881.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiraga S. Operator mutants of the tryptophan operon in Escherichia coli. J Mol Biol. 1969 Jan 14;39(1):159–179. doi: 10.1016/0022-2836(69)90340-4. [DOI] [PubMed] [Google Scholar]
- Hiraga S., Yanofsky C. Hyper-labile messenger RNA in polar mutants of the tryptophan operon of Escherichia coli. J Mol Biol. 1972 Dec 14;72(1):103–110. doi: 10.1016/0022-2836(72)90072-1. [DOI] [PubMed] [Google Scholar]
- Hoch S. O., Anagnostopoulos C., Crawford I. P. Enzymes of the tryptophan operon of Bacillus subtilis. Biochem Biophys Res Commun. 1969 Jun 27;35(6):838–844. doi: 10.1016/0006-291x(69)90700-1. [DOI] [PubMed] [Google Scholar]
- Hoch S. O., Crawford I. P. Enzymes of the tryptophan pathway in three Bacillus species. J Bacteriol. 1973 Nov;116(2):685–693. doi: 10.1128/jb.116.2.685-693.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoch S. O. Mapping of the 5-methyltryptophan resistance locus in Bacillus subtilis. J Bacteriol. 1974 Jan;117(1):315–317. doi: 10.1128/jb.117.1.315-317.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoch S. O., Roth C. W., Crawford I. P., Nester E. W. Control of tryptophan biosynthesis by the methyltryptophan resistance gene in Bacillus subtilis. J Bacteriol. 1971 Jan;105(1):38–45. doi: 10.1128/jb.105.1.38-45.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoch S. O. Tryptophan synthetase from Bacillus subtilis. Purification and characterization of the component. J Biol Chem. 1973 May 10;248(9):2999–3003. [PubMed] [Google Scholar]
- Holloway B. W., Krishnapillai V., Stanisich V. Pseudomonas genetics. Annu Rev Genet. 1971;5:425–446. doi: 10.1146/annurev.ge.05.120171.002233. [DOI] [PubMed] [Google Scholar]
- Hopwood D. A., Chater K. F., Dowding J. E., Vivian A. Advances in Streptomyces coelicolor genetics. Bacteriol Rev. 1973 Sep;37(3):371–405. doi: 10.1128/br.37.3.371-405.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchinson M. A., Belser W. L. Enzymes of tryptophan biosynthesis in Serratia marcescens. J Bacteriol. 1969 Apr;98(1):109–115. doi: 10.1128/jb.98.1.109-115.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hütter R., DeMoss J. A. Enzyme analysis of the tryptophan pathway in Aspergillus nidulans. Genetics. 1967 Feb;55(2):241–247. doi: 10.1093/genetics/55.2.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hütter R., DeMoss J. A. Organization of the tryptophan pathway: a phylogenetic study of the fungi. J Bacteriol. 1967 Dec;94(6):1896–1907. doi: 10.1128/jb.94.6.1896-1907.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imamoto F. Translation and transcription of the tryptophan operon. Prog Nucleic Acid Res Mol Biol. 1973;13:339–407. doi: 10.1016/s0079-6603(08)60107-5. [DOI] [PubMed] [Google Scholar]
- Ingram L. O., Pierson D., Kane J. F., Van Baalen C., Jensen R. A. Documentation of auxotrophic mutation in blue-green bacteria: characterization of a tryptophan auxotroph in Agmenellum quadruplicatum. J Bacteriol. 1972 Jul;111(1):112–118. doi: 10.1128/jb.111.1.112-118.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito J., Crawford I. P. Regulation of the enzymes of the tryptophan pathway in Escherichia coli. Genetics. 1965 Dec;52(6):1303–1316. doi: 10.1093/genetics/52.6.1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito J. Effect of tryptophan starvation on the rate of translation of the tryptophan operon in Escherichia coli. Biochim Biophys Acta. 1970 Apr 15;204(2):624–626. doi: 10.1016/0005-2787(70)90183-8. [DOI] [PubMed] [Google Scholar]
- Ito J. Hybrid anthranilate synthetase molecules from enterobacterial sources. Nature. 1969 Jul 5;223(5201):57–59. doi: 10.1038/223057a0. [DOI] [PubMed] [Google Scholar]
- Ito J., Yanofsky C. Anthranilate synthetase, an enzyme specified by the tryptophan operon of Escherichia coli: Comparative studies on the complex and the subunits. J Bacteriol. 1969 Feb;97(2):734–742. doi: 10.1128/jb.97.2.734-742.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito K. Regulatory mechanism of the tryptophan operon in Escherichia coli: possible interaction between trpR and trpS gene products. Mol Gen Genet. 1972;115(4):349–363. doi: 10.1007/BF00333173. [DOI] [PubMed] [Google Scholar]
- JOHNSON R. B., MAYS C. G. A strain of Shigella dysenteriae. I. Requiring proline. J Bacteriol. 1954 May;67(5):542–544. doi: 10.1128/jb.67.5.542-544.1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson E. N., Yanofsky C. Duplication-translocations of tryptophan operon genes in Escherichia coli. J Bacteriol. 1973 Oct;116(1):33–40. doi: 10.1128/jb.116.1.33-40.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson E. N., Yanofsky C. Internal promoter of the tryptophan operon of Escherichia coli is located in a structural gene. J Mol Biol. 1972 Aug 21;69(2):307–313. doi: 10.1016/0022-2836(72)90232-x. [DOI] [PubMed] [Google Scholar]
- Jackson E. N., Yanofsky C. Thr region between the operator and first structural gene of the tryptophan operon of Escherichia coli may have a regulatory function. J Mol Biol. 1973 May 5;76(1):89–101. doi: 10.1016/0022-2836(73)90082-x. [DOI] [PubMed] [Google Scholar]
- Jensen R. A., Nasser D. S. Comparative regulation of isoenzymic 3-deoxy-D-arabino-heptulosonate 7-phosphate synthetases in microorganisms. J Bacteriol. 1968 Jan;95(1):188–196. doi: 10.1128/jb.95.1.188-196.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson J. L., Anderson R. S., Ordal E. J. Nucleic acid homologies among oxidase-negative Moraxella species. J Bacteriol. 1970 Feb;101(2):568–573. doi: 10.1128/jb.101.2.568-573.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juni E. Interspecies transformation of Acinetobacter: genetic evidence for a ubiquitous genus. J Bacteriol. 1972 Nov;112(2):917–931. doi: 10.1128/jb.112.2.917-931.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juni E., Janik A. Transformation of Acinetobacter calco-aceticus (Bacterium anitratum). J Bacteriol. 1969 Apr;98(1):281–288. doi: 10.1128/jb.98.1.281-288.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KAPLAN S., MILLS S. E., ENSIGN S., BONNER D. M. GENETIC DETERMINATION OF THE ANTIGENIC SPECIFICITY OF TRYPTOPHAN SYNTHETASE. J Mol Biol. 1964 Jun;8:801–813. doi: 10.1016/s0022-2836(64)80161-3. [DOI] [PubMed] [Google Scholar]
- Kane J. F., Holmes W. M., Jensen R. A. Metabolic interlock. The dual function of a folate pathway gene as an extra-operonic gene of tryptophan biosynthesis. J Biol Chem. 1972 Mar 10;247(5):1587–1596. [PubMed] [Google Scholar]
- Kane J. F., Jensen R. A. The molecular aggregation of anthranilate synthase in Bacillus subtilis. Biochem Biophys Res Commun. 1970 Oct 23;41(2):328–333. doi: 10.1016/0006-291x(70)90507-3. [DOI] [PubMed] [Google Scholar]
- Kloos W. E., Rose N. E. Transformation mapping of tryptophan loci in Micrococcus luteus. Genetics. 1970 Dec;66(4):595–605. doi: 10.1093/genetics/66.4.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuwano M., Schlessinger D., Morse D. E. Loss of dispensable endonuclease activity in relief of polarity by suA. Nat New Biol. 1971 Jun 16;231(24):214–217. doi: 10.1038/newbio231214a0. [DOI] [PubMed] [Google Scholar]
- LESTER G. Some aspects of tryptophan synthetase formation in Neurospora crassa. J Bacteriol. 1961 Jun;81:964–973. doi: 10.1128/jb.81.6.964-973.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lara J. C., Mills S. E. Tryptophan synthetase in Euglena gracilis strain G. J Bacteriol. 1972 Jun;110(3):1100–1106. doi: 10.1128/jb.110.3.1100-1106.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Largen M., Belser W. L. Tryptophan biosynthetic pathway in the Enterobacteriaceae: some physical properties of the enzymes. J Bacteriol. 1975 Jan;121(1):239–249. doi: 10.1128/jb.121.1.239-249.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Largen M., Belser W. The apparent conservation of the internal low efficiency promoter of the tryptophan operons of several species of Enterobacteriaceae. Genetics. 1973 Sep;75(1):19–22. doi: 10.1093/genetics/75.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leidigh B. J., Wheelis M. L. The clustering on the Pseudomonas putida chromosome of genes specifying dissimilatory functions. J Mol Evol. 1973 Nov 27;2(4):235–242. doi: 10.1007/BF01654092. [DOI] [PubMed] [Google Scholar]
- Lester G. In vivo regulation of intermediate reactions in the pathway of tryptophan biosynthesis in Neurospora crassa. J Bacteriol. 1968 Nov;96(5):1768–1773. doi: 10.1128/jb.96.5.1768-1773.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lester G. Regulation of tryptophan biosynthetic enzymes in Neurospora crassa. J Bacteriol. 1971 Jul;107(1):193–202. doi: 10.1128/jb.107.1.193-202.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S. L., Denney R. M., Yanofsky C. Nucleotide sequence divergence in the -chain-structural genes of tryptophan synthetase from Escherichia coli, Salmonella typhimurium, and Aerobacter aerogenes. Proc Natl Acad Sci U S A. 1973 Apr;70(4):1112–1116. doi: 10.1073/pnas.70.4.1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S. L., Drapeau G. R., Yanofsky C. Amino terminal sequence of the tryptophan synthetase alpha chain of Serratia marcescens. J Bacteriol. 1973 Mar;113(3):1507–1508. doi: 10.1128/jb.113.3.1507-1508.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S. L., Hanlon J., Yanofsky C. Structural homology of the glutamine amidotransferase subunits of the anthranilate synthetases of Escherichia coli, Salmonella typhimurium and Serratia marcescens. Nature. 1974 Mar 1;248(5443):48–50. doi: 10.1038/248048a0. [DOI] [PubMed] [Google Scholar]
- Li S. L., Hoch S. O. Amino-terminal sequence of the tryptophan synthetase alpha chain of Bacillus subtilis. J Bacteriol. 1974 Apr;118(1):187–191. doi: 10.1128/jb.118.1.187-191.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S. L., Yanofsky C. Amino acid sequence studies with the tryptophan synthetase chain of Aerobacter aerogenes. J Biol Chem. 1973 Mar 10;248(5):1837–1843. [PubMed] [Google Scholar]
- Li S. L., Yanofsky C. Amino acid sequence studies with the tryptophan synthetase chain of Salmonella typhimurium. J Biol Chem. 1973 Mar 10;248(5):1830–1836. [PubMed] [Google Scholar]
- Li S. L., Yanofsky C. Amino acid sequences of fifty residues from the amino termini of the tryptophan synthetase chains of several enterobacteria. J Biol Chem. 1972 Feb 25;247(4):1031–1037. [PubMed] [Google Scholar]
- Lorence J. H., Nester E. W. Multiple molecular forms of chorismate mutase in Bacillus subtillis. Biochemistry. 1967 May;6(5):1541–1553. doi: 10.1021/bi00857a041. [DOI] [PubMed] [Google Scholar]
- MATSUSHIRO A., SATO K., ITO J., KIDA S., IMAMOTO F. ON THE TRANSCRIPTION OF THE TRYTOPHAN OPERON IN ESCHERICHIA COLI. I. THE TRYPTOPHAN OPERATOR. J Mol Biol. 1965 Jan;11:54–63. doi: 10.1016/s0022-2836(65)80170-x. [DOI] [PubMed] [Google Scholar]
- MONOD J., COHEN-BAZIRE G. L'effet d'inhibition spécifique dans la biosynthèse de la tryptophane-desmase chez Aerobacter aerogenes. C R Hebd Seances Acad Sci. 1953 Feb 2;236(5):530–532. [PubMed] [Google Scholar]
- Mandel M. New approaches to bacterial taxonomy: perspective and prospects. Annu Rev Microbiol. 1969;23:239–274. doi: 10.1146/annurev.mi.23.100169.001323. [DOI] [PubMed] [Google Scholar]
- Manney T. R., Duntze W., Janosko N., Salazar J. Genetic and biochemical studies of partially active tryptophan synthetase mutants of Saccharomyces cerevisiae. J Bacteriol. 1969 Aug;99(2):590–596. doi: 10.1128/jb.99.2.590-596.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manney T. R. Evidence for chain termination by super-suppressible mutants in yeast. Genetics. 1968 Dec;60(4):719–733. doi: 10.1093/genetics/60.4.719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manney T. R. Regulation of factors that influence the in vitro stability of tryptophan synthetase from yeast. J Bacteriol. 1968 Aug;96(2):403–408. doi: 10.1128/jb.96.2.403-408.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marinus M. G., Loutit J. S. Regulation of isoleucine-valine biosynthesis in Pseudomonas aeruginosa. II. Regulation of enzyme activity and synthesis. Genetics. 1969 Nov;63(3):557–567. doi: 10.1093/genetics/63.3.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matchett W. H., DeMoss J. A. The subunit structure of tryptophan synthase from Neurospora crassa. J Biol Chem. 1975 Apr 25;250(8):2941–2946. [PubMed] [Google Scholar]
- Matchett W. H. Inhibition of tryptophan synthetase by indoleacrylic acid. J Bacteriol. 1972 Apr;110(1):146–154. doi: 10.1128/jb.110.1.146-154.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurer R., Crawford I. P. New regulatory mutation affecting some of the tryptophan genes in Pseudomonas putida. J Bacteriol. 1971 May;106(2):331–338. doi: 10.1128/jb.106.2.331-338.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurer R., Crawford I. P. Properties and subunit structure of the B component of Pseudomonas putida tryptophan synthetase. Arch Biochem Biophys. 1971 May;144(1):193–203. doi: 10.1016/0003-9861(71)90468-1. [DOI] [PubMed] [Google Scholar]
- McQuade J. F., 3rd, Creighton T. E. Purification and comparison of the N-(5'-phosphoribosyl)anthranilic acid isomerase-indole-3-glycerol phosphate synthetase of tryptophan biosynthesis from three species of Enterobacteriaceae. Eur J Biochem. 1970 Oct;16(2):199–207. doi: 10.1111/j.1432-1033.1970.tb01072.x. [DOI] [PubMed] [Google Scholar]
- Meduski J. W., Zamenhof S. Studies on tryptophan synthetases from various strains of Bacillus subtilis. Biochem J. 1969 Apr;112(3):285–292. doi: 10.1042/bj1120285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metzenberg R. L. Genetic regulatory systems in Neurospora. Annu Rev Genet. 1972;6:111–132. doi: 10.1146/annurev.ge.06.120172.000551. [DOI] [PubMed] [Google Scholar]
- Morishita T., Fukada T., Shirota M., Yura T. Genetic basis of nutritional requirements in Lactobacillus casei. J Bacteriol. 1974 Dec;120(3):1078–1084. doi: 10.1128/jb.120.3.1078-1084.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morse D. E., Yanofsky C. A transcription-initiating mutation within a structural gene of the tryptophan operon. J Mol Biol. 1969 May 14;41(3):317–328. doi: 10.1016/0022-2836(69)90278-2. [DOI] [PubMed] [Google Scholar]
- Morse D. E., Yanofsky C. Amber mutants of the trpR regulatory gene. J Mol Biol. 1969 Aug 28;44(1):185–193. doi: 10.1016/0022-2836(69)90413-6. [DOI] [PubMed] [Google Scholar]
- Morse D. E., Yanofsky C. The internal low-efficiency promoter of the tryptophan operon of Escherichia coli. J Mol Biol. 1968 Dec;38(3):447–451. doi: 10.1016/0022-2836(68)90401-4. [DOI] [PubMed] [Google Scholar]
- Murgola E. J., Yanofsky C. Selection for new amino acids at position 211 of the tryptophan synthetase alpha chain of Escherichia coli. J Mol Biol. 1974 Jul 15;86(4):775–784. doi: 10.1016/0022-2836(74)90353-2. [DOI] [PubMed] [Google Scholar]
- Murphy T. M., Mills S. E. Immunochemical and enzymatic comparisons of the tryptophan synthase alpha subunits from five species of Enterobacteriaceae. J Bacteriol. 1969 Mar;97(3):1310–1320. doi: 10.1128/jb.97.3.1310-1320.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagao R. T., Moore T. C. Partial purification and properties of tryptophan synthase of pea plants. Arch Biochem Biophys. 1972 Apr;149(2):402–413. doi: 10.1016/0003-9861(72)90338-4. [DOI] [PubMed] [Google Scholar]
- Nazario M., Kinsey J. A., Ahmad M. Neurospora mutant deficient in tryptophanyl-transfer ribonucleic acid synthetase activity. J Bacteriol. 1971 Jan;105(1):121–126. doi: 10.1128/jb.105.1.121-126.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nester E W, Schafer M, Lederberg J. Gene Linkage in DNA Transfer: A Cluster of Genes Concerned with Aromatic Biosynthesis in Bacillus Subtilis. Genetics. 1963 Apr;48(4):529–551. doi: 10.1093/genetics/48.4.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nester E. W., Jensen R. A. Control of aromatic acid biosynthesis in Bacillus subtilis: sequenial feedback inhibition. J Bacteriol. 1966 Apr;91(4):1594–1598. doi: 10.1128/jb.91.4.1594-1598.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel N., Holmes W. M., Kane J. F. Homologous and hybrid complexes of anthranilate synthase from Bacillus species. J Bacteriol. 1974 Jul;119(1):220–227. doi: 10.1128/jb.119.1.220-227.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel N., Holmes W. M., Kane J. F. Intergeneric complementation of anthranilate synthase subunits. J Bacteriol. 1973 May;114(2):600–602. doi: 10.1128/jb.114.2.600-602.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinel J. P., Chorover S. L. Inhibition by arousal of epilepsy induced by chlorambucil in rats. Nature. 1972 Mar 31;236(5344):232–234. doi: 10.1038/236232a0. [DOI] [PubMed] [Google Scholar]
- Prager E. M., Wilson A. C. The dependence of immunological cross-reactivity upon sequence resemblance among lysozymes. I. Micro-complement fixation studies. J Biol Chem. 1971 Oct 10;246(19):5978–5989. [PubMed] [Google Scholar]
- Proctor A. R., Crawford I. P. Autogenous regulation of the inducible tryptophan synthase of Pseudomonas putida. Proc Natl Acad Sci U S A. 1975 Apr;72(4):1249–1253. doi: 10.1073/pnas.72.4.1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proctor A. R., Kloos W. E. The tryptophan gene cluster of Staphylococcus aureus. J Gen Microbiol. 1970 Dec;64(3):319–327. doi: 10.1099/00221287-64-3-319. [DOI] [PubMed] [Google Scholar]
- Proctor A. R., Kloos W. E. Tryptophan biosynthetic enzymes of Staphylococcus aureus. J Bacteriol. 1973 Apr;114(1):169–177. doi: 10.1128/jb.114.1.169-177.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Queener S. F., Gunsalus I. C. Anthranilate synthase enzyme system and complementation in Pseudomonas species. Proc Natl Acad Sci U S A. 1970 Nov;67(3):1225–1232. doi: 10.1073/pnas.67.3.1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Queener S. W., Queener S. F., Meeks J. R., Gunsalus I. C. Anthranilate synthase from Pseudomonas putida. Purification and properties of a two-component enzyme. J Biol Chem. 1973 Jan 10;248(1):151–161. [PubMed] [Google Scholar]
- Rebello J. L., Jensen R. A. Metabolic interlock. The multi-metabolite control of prephenate dehydratase activity in Bacillus subtilis. J Biol Chem. 1970 Aug 10;245(15):3738–3744. [PubMed] [Google Scholar]
- Rheinwald J. G., Chakrabarty A. M., Gunsalus I. C. A transmissible plasmid controlling camphor oxidation in Pseudomonas putida. Proc Natl Acad Sci U S A. 1973 Mar;70(3):885–889. doi: 10.1073/pnas.70.3.885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robb F., Belser W. L. Anthranilate synthetase. In vitro complementation between Serratia marcescens and Escherichia coli subunits. Biochim Biophys Acta. 1972 Nov 28;285(1):243–252. [PubMed] [Google Scholar]
- Roberts C. F. Complementation analysis of the tryptophan pathway in Aspergillus nidulans. Genetics. 1967 Feb;55(2):233–239. doi: 10.1093/genetics/55.2.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocha V., Crawford I. P., Mills S. E. Comparative immunological and enzymatic study of the tryptophan synthetase beta 2 subunit in the Enterobacteriaceae. J Bacteriol. 1972 Jul;111(1):163–168. doi: 10.1128/jb.111.1.163-168.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose J. K., Squires C. L., Yanofsky C., Yang H. L., Zubay G. Regulation of in vitro transcription of the tryptophan operon by purified RNA polymerase in the presence of partially purified repressor and tryptophan. Nat New Biol. 1973 Oct 3;245(144):133–137. doi: 10.1038/newbio245133a0. [DOI] [PubMed] [Google Scholar]
- Rose J. K., Yanofsky C. Transcription of the operator proximal and distal ends of the tryptophan operon: evidence that trpE and trpA are the delimiting structural genes. J Bacteriol. 1971 Oct;108(1):615–618. doi: 10.1128/jb.108.1.615-618.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg S. L., Hegeman G. D. Clustering of functionally related genes in Pseudomonas aeruginosa. J Bacteriol. 1969 Jul;99(1):353–355. doi: 10.1128/jb.99.1.353-355.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth C. W., Nester E. W. Co-ordinate control of tryptophan, histidine and tyrosine enzyme synthesis in Bacillus subtilis. J Mol Biol. 1971 Dec 28;62(3):577–589. doi: 10.1016/0022-2836(71)90157-4. [DOI] [PubMed] [Google Scholar]
- SCHWARTZ A. K., BONNER D. M. TRYPTOPHAN SYNTHETASE IN BACILLUS SUBTILIS: EFFECTS OF HIGH POTASSIUM ION CONCENTRATION ON A TWO COMPONENT ENZYME. Biochim Biophys Acta. 1964 Aug 26;89:337–347. doi: 10.1016/0926-6569(64)90223-8. [DOI] [PubMed] [Google Scholar]
- SOMERVILLE R. L., YANOFSKY C. ON THE TRANSLATION OF THE A GENE REGION OF TRYPTOPHAN MESSENGER RNA. J Mol Biol. 1964 Apr;8:616–619. doi: 10.1016/s0022-2836(64)80019-x. [DOI] [PubMed] [Google Scholar]
- SOMERVILLE R. L., YANOFSKY C. STUDIES ON THE REGULATION OF TRYPTOPHAN BIOSYNTHESIS IN ESCHERICHIA COLI. J Mol Biol. 1965 Apr;11:747–759. doi: 10.1016/s0022-2836(65)80032-8. [DOI] [PubMed] [Google Scholar]
- Saheki T., Holzer H. Comparisons of the tryptophan synthase inactivating enzymes with proteinases from yeast. Eur J Biochem. 1974 Mar 1;42(2):621–626. doi: 10.1111/j.1432-1033.1974.tb03377.x. [DOI] [PubMed] [Google Scholar]
- Sakaguchi K. The similarity of tryptophan synthetases of Anabaena variabilis and Chlorella ellipsoidea with that of bacteria. Biochim Biophys Acta. 1970 Dec 16;220(3):580–593. doi: 10.1016/0005-2744(70)90288-3. [DOI] [PubMed] [Google Scholar]
- Sanderson K. E., Hall C. A. F-prime factors of Salmonella typhimurium and an inversion between S. typhimurium and Escherichia coli. Genetics. 1970 Feb;64(2):215–228. doi: 10.1093/genetics/64.2.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarich V. M., Wilson A. C. Immunological time scale for hominid evolution. Science. 1967 Dec 1;158(3805):1200–1203. doi: 10.1126/science.158.3805.1200. [DOI] [PubMed] [Google Scholar]
- Sawula R. V., Crawford I. P. Anthranilate synthetase of Acinetobacter calcoaceticus. Separation and partial characterization of subunits. J Biol Chem. 1973 May 25;248(10):3573–3581. [PubMed] [Google Scholar]
- Sawula R. V., Crawford I. P. Mapping of the tryptophan genes of Acinetobacter calcoaceticus by transformation. J Bacteriol. 1972 Nov;112(2):797–805. doi: 10.1128/jb.112.2.797-805.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scaife J., Beckwith J. R. Mutational alteration of the maximal level of Lac operon expression. Cold Spring Harb Symp Quant Biol. 1966;31:403–408. doi: 10.1101/sqb.1966.031.01.052. [DOI] [PubMed] [Google Scholar]
- Schulz G. E., Creighton T. E. Preliminary x-ray diffraction study of the wild-type and a mutationally-altered tryptophan synthetase alpha-subunit. Eur J Biochem. 1969 Sep;10(2):195–197. doi: 10.1111/j.1432-1033.1969.tb00673.x. [DOI] [PubMed] [Google Scholar]
- Schweingruber M. E., Dietrich R. Gene-enzyme relationships in the tryptophan pathway of Schizosaccharomyces pombe. Experientia. 1973 Sep 15;29(9):1152–1154. doi: 10.1007/BF01946778. [DOI] [PubMed] [Google Scholar]
- Schürch A., Miozzari J., Hütter R. Regulation of tryptophan biosynthesis in Saccharomyces cerevisiae: mode of action of 5-methyl-tryptophan and 5-methyl-tryptophan-sensitive mutants. J Bacteriol. 1974 Mar;117(3):1131–1140. doi: 10.1128/jb.117.3.1131-1140.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu Y., Shimizu N., Hayashi M. In vitro repression of transcription of the tryptophan operon by trp repressor. Proc Natl Acad Sci U S A. 1973 Jul;70(7):1990–1994. doi: 10.1073/pnas.70.7.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith O. H. Structure of the trpC cistron specifying indoleglycerol phosphate synthetase, and its localization in the tryptophan operon of Escherichia coli. Genetics. 1967 Sep;57(1):95–105. doi: 10.1093/genetics/57.1.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smithers C. M., Engel P. P. Gene-enzyme relations of tryptophan mutants in Streptomyces coelicolor A3(2). Genetics. 1974 Nov;78(3):799–808. doi: 10.1093/genetics/78.3.799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squires C. L., Rose J. K., Yanofsky C., Yang H. L., Zubay G. Tryptophanyl-tRNA and tryptophanyl-tRNA synthetase are not required for in vitro repression of the tryptophan operon. Nat New Biol. 1973 Oct 3;245(144):131–133. doi: 10.1038/newbio245131a0. [DOI] [PubMed] [Google Scholar]
- Steinberg W. Temperature-induced derepression of tryptophan biosynthesis in a tryptophanyl-transfer ribonucleic acid synthetase mutant of Bacillus subtilis. J Bacteriol. 1974 Mar;117(3):1023–1034. doi: 10.1128/jb.117.3.1023-1034.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenmark S. L., Pierson D. L., Jensen R. A., Glover G. I. Blue-green bacteria synthesise L-tyrosine by the pretyrosine pathway. Nature. 1974 Feb 1;247(5439):290–292. doi: 10.1038/247290a0. [DOI] [PubMed] [Google Scholar]
- Tatum E. L., Bonner D. Indole and Serine in the Biosynthesis and Breakdown of Tryptophane. Proc Natl Acad Sci U S A. 1944 Feb 15;30(2):30–37. doi: 10.1073/pnas.30.2.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai H., Yang C. Y., Tsai J. H. The subunit structure of Neurospora tryptophan synthase: a reappraisal. Biochem Biophys Res Commun. 1974 Dec 23;61(4):1332–1339. doi: 10.1016/s0006-291x(74)80430-4. [DOI] [PubMed] [Google Scholar]
- Turner J. R., Matchett W. H. Alteration of tryptophan-mediated regulation in Neurospora crassa by indoleglycerol phosphate. J Bacteriol. 1968 May;95(5):1608–1614. doi: 10.1128/jb.95.5.1608-1614.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twarog R., Liggins G. L. Enzymes of the tryptophan pathway in Acinetobacter calco-aceticus. J Bacteriol. 1970 Oct;104(1):254–263. doi: 10.1128/jb.104.1.254-263.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wegman J., Crawford I. P. Tryptophan synthetic pathway and its regulation in Chromobacterium violaceum. J Bacteriol. 1968 Jun;95(6):2325–2335. doi: 10.1128/jb.95.6.2325-2335.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitt D. D., Carlton B. C. Non-coordinate regulation in 5-methyl tryptophan-resistant mutants of Bacillus subtilis. Biochem Biophys Res Commun. 1968 Nov 25;33(4):636–642. doi: 10.1016/0006-291x(68)90343-4. [DOI] [PubMed] [Google Scholar]
- Widholm J. M. Anthranilate synthetase from 5-methyltryptophan-susceptible and -resistant cultured Daucus carota cells. Biochim Biophys Acta. 1972 Aug 18;279(1):48–57. doi: 10.1016/0304-4165(72)90240-1. [DOI] [PubMed] [Google Scholar]
- Widholm J. M. Measurement of the five enzymes which convert chorismate to tryptophan in cultured Daucus carota cell extracts. Biochim Biophys Acta. 1973 Sep 14;320(2):217–226. doi: 10.1016/0304-4165(73)90301-2. [DOI] [PubMed] [Google Scholar]
- Widholm J. M. Tryptophan biosynthesis in Nicotiana tabacum and Daucus carota cell cultures: site of action of inhibitory tryptophan analogs. Biochim Biophys Acta. 1972 Jan 28;261(1):44–51. doi: 10.1016/0304-4165(72)90311-x. [DOI] [PubMed] [Google Scholar]
- Woese C. R., Sogin M. L., Sutton L. A. Procaryote phylogeny. I. Concerning the relatedness of Aerobacter aerogenes to Escherichia coli. J Mol Evol. 1974;3(4):293–299. doi: 10.1007/BF01796044. [DOI] [PubMed] [Google Scholar]
- Wuesthoff G., Bauerle R. H. Mutations creating internal promoter elements in the tryptophan operon of Salmonella typhimurium. J Mol Biol. 1970 Apr 14;49(1):171–196. doi: 10.1016/0022-2836(70)90384-0. [DOI] [PubMed] [Google Scholar]
- YANOFSKY C., LENNOX E. S. Transduction and recombination study of linkage relationships among the genes controlling tryptophan synthesis in Escherichia coli. Virology. 1959 Aug;8:425–447. doi: 10.1016/0042-6822(59)90046-7. [DOI] [PubMed] [Google Scholar]
- YANOFSKY C. The enzymatic conversion of anthranilic acid to indole. J Biol Chem. 1956 Nov;223(1):171–184. [PubMed] [Google Scholar]
- Yanofsky C., Crawford I. P. THE EFFECTS OF DELETIONS, POINT MUTATIONS, REVERSIONS AND SUPPRESSOR MUTATIONS ON THE TWO COMPONENTS OF THE TRYPTOPHAN SYNTHETASE OF ESCHERICHIA COLI. Proc Natl Acad Sci U S A. 1959 Jul;45(7):1016–1026. doi: 10.1073/pnas.45.7.1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanofsky C., Horn V., Bonner M., Stasiowski S. Polarity and enzyme functions in mutants of the first three genes of the tryptophan operon of Escherichia coli. Genetics. 1971 Dec;69(4):409–433. doi: 10.1093/genetics/69.4.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanofsky C., Horn V. Tryptophan synthetase chain positions affected by mutations near the ends of the genetic map of trpA of Escherichia coli. J Biol Chem. 1972 Jul 25;247(14):4494–4498. [PubMed] [Google Scholar]
- Yanofsky C., Ito J. Nonsense codons and polarity in the tryptophan operon. J Mol Biol. 1966 Nov 14;21(2):313–334. doi: 10.1016/0022-2836(66)90102-1. [DOI] [PubMed] [Google Scholar]
- Yourno J., Kohno T., Roth J. R. Enzyme evolution: generation of a bifunctional enzyme by fusion of adjacent genes. Nature. 1970 Nov 28;228(5274):820–824. doi: 10.1038/228820a0. [DOI] [PubMed] [Google Scholar]
- Yu P. H., Kula M. R., Tsai H. Studies on the apparent instability of Neurospora tryptophan synthase. Evidence for protease. Eur J Biochem. 1973 Jan 3;32(1):129–135. doi: 10.1111/j.1432-1033.1973.tb02588.x. [DOI] [PubMed] [Google Scholar]
- Zalkin H. Anthranilate synthetase. Adv Enzymol Relat Areas Mol Biol. 1973;38:1–39. doi: 10.1002/9780470122839.ch1. [DOI] [PubMed] [Google Scholar]



