Abstract
Background
Curcumin is a polyphenol, found in the spice turmeric, that has promising anticancer properties, but previous studies suggest that absorption of curcumin may be limited.
Methods
This study examined the pharmacokinetics of a curcumin preparation in healthy human volunteers 0.25-72 hours after a single oral dose. Curcumin was administered at doses of 10 g (N=6) and 12 g (N=6). Subjects were randomly allocated to dose level for a total of 6 subjects at each dose level. Serum samples were assayed for free curcumin, for its glucuronide and for it sulfate conjugate. The data were fit to a one-compartment absorption and elimination model.
Results
Using an HPLC assay with a limit of detection of 50 ng/mL, only one subject had detectable free curcumin at any of the 14 time points assayed, but curcumin glucuronides and sulfates were detected in all subjects. Based on the PK model, AUC for the 10g and 12 g doses was estimated (mean±standard error) to be 35.33±3.78 and 26.57±2.97 μg/mL × hr, respectively, while Cmax was 2.30±0.26 and 1.73±0.19 μg/mL. The tmax and t1/2 were estimated to be 3.29±0.43 hr and 6.77±0.83 hr. The ratio of glucuronide:sulfate was 1.92:1. The curcumin conjugates were present as either glucuronide or sulfate, not mixed conjugates.
Conclusion
Curcumin is absorbed after oral dosing in humans and can be detected as glucuronide and sulfate conjugates in plasma.
Keywords: Chemoprevention, Pharmacokinetics, Curcumin, Metabolism, Clinical trial
Introduction
Wide arrays of phenolic substances, especially those present in dietary and medicinal plants, have been reported to possess substantial antioxidant, anti-inflammatory, anticarcinogenic and antimutagenic effects (1-3). Curcumin is a yellow, naturally occurring polyphenolic phytochemical derived from turmeric, the powdered rhizome of the herb, Curcuma longa Linn. The spice turmeric is used in curries as a coloring and flavoring agent in various parts of the world, especially in the Indian subcontinent, an area that has a low incidence of colorectal cancer(4).
Several animal model studies have shown that curcumin suppresses carcinogenesis in skin (5-7), stomach (8, 9), colon (10, 11), breast (12-14) and liver (15). Curcumin is reported to induce apoptosis in a wide variety of tumor cells, including B- and T-cell leukemias (16, 17), colon (18) and breast carcinoma (19, 20). Chemopreventive activities of curcumin are thought to involve up-regulation of carcinogen detoxifying enzymes (21-23) antioxidants (24, 25), suppression of cyclooxygense -2 (COX-2) expression (26-30) and inhibition of nuclear factor-κB (NF-κB) release (30-32). Inhibition of NF-κB release by curcumin also leads to the down regulation of various proinflammatory cytokines (e.g., tumor necrosis factor, interleukins) and inhibition of the mRNA expression of several proinflammatory enzymes (e.g., COX, lipoxygenases, metalloproteinases, and nitric oxide synthase) (33).
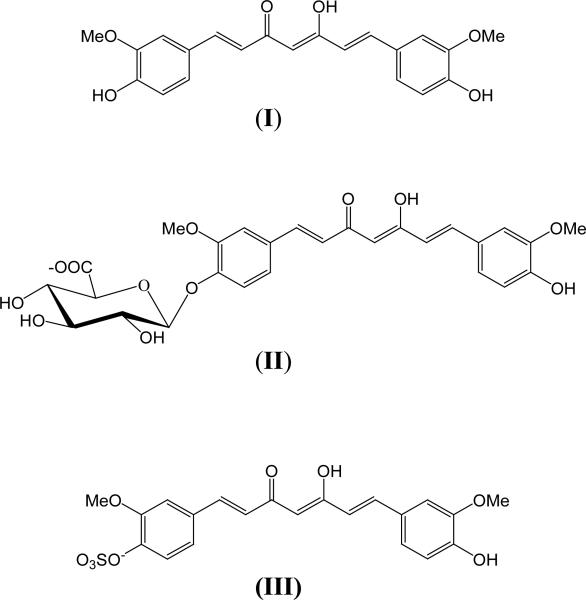
In animal studies, curcumin undergoes rapid metabolic reduction and conjugation, resulting in poor systemic bioavailability after oral administration (34-36). For example, an oral dose of 0.1 g/kg administered to mice yielded a peak plasma concentration of free curcumin that was only 2.25 μg/mL (35). In rats, curcumin completely disappeared from plasma within 1 hour after a 40 mg/kg intravenous dose (34). When given orally at a 500 mg/kg dose, peak concentrations of 1.8 ng/mL of free curcumin were detected in plasma (34). The major metabolites of curcumin (Fig.1) identified in rat plasma were curcumin glucuronide and curcumin sulfate, based on enzymatic hydrolysis studies. Hexahydrocurcumin, hexahydrocurcuminol and hexahydrocurcumin glucuronide were also present in minor amounts (34).
Fig. 1.
Structures of curcumin (I), curcumin glucuronide (II) and curcumin sulfate (III).
Data on the pharmacokinetic properties and metabolism of curcumin in humans are very limited. In a human study conducted in 25 patients with precancerous lesions, free curcumin concentrations (mean ±SD) in plasma after taking 4, 6 and 8 g of curcumin per day for three months were 0.19, 0.20 and 0.60 μg/mL, respectively (37). None of the curcumin conjugates or metabolites of curcumin were reported in that study. A study of six patients with advanced colorectal cancer dosed with 3.6 g of curcumin daily for up to three months yielded 4.3, 5.8, and 3.3 ng/mL mean plasma concentrations of curcumin, curcumin glucuronide, and curcumin sulfate respectively, 1 h post administration (38).
In animal models no toxicity has been reported to date. Similarly, in humans to date, few adverse events due to curcumin even at very high doses have been reported (7). Whether the low toxicity is merely a function of lack of bioavailability is an open question. We have recently reported a trial escalating curcuminoid dose from 0.5 g to 10 g in healthy humans that demonstrated few adverse events, but bioavailability of curcumin was limited (39). The present clinical study assessed the complete pharmacokinetic profile of curcumin and its conjugate metabolites in healthy human subjects administered either a 10 or a 12 g single dose, the highest doses given to humans. The aims of this study were to 1) determine if curcumin, in capsule formulation, is absorbed and biotransformed in humans and 2) assess the human pharmacokinetics of curcumin and its conjugate metabolites.
Materials and Methods
Curcuminoids were provided in a capsule form as a powder extract obtained from Alleppey finger turmeric (C3 Complex™, Sabinsa Corporation) in a standardized, Good Manufacturing Practice formulation. The mixture contained curcumin (75%), demethoxycurcumin (23%) and bisdemethoxycurcumin (2%) (verified by HPLC assay in our laboratory). β-17-estradiol acetate, and the enzymes β-glucuronidase (Type IX-A from E.coli) and sulfatase (Type H-1 from Helix pomatia), were purchased from Sigma- Aldrich Inc (St. Louis, MO). Sodium Phosphate and Sodium acetate (ACS certified) were purchased from Fisher Scientfic (Fair Lawn, NJ). HPLC grade ethyl acetate and methanol were purchased from Burdick and Jackson (Honeywell International Inc., Muskegon, MI).
Clinical Trial Design
Twelve healthy human subjects solicited by advertisement or word of mouth, 18 years of age or older, with normal organ function were recruited. Subjects included five males, seven females, all Caucasian, ages 18-65 years, BMI range 20-29. Subjects were not taking any medications chronically. They were educated on food sources of curcumin and asked to avoid all foods containing high concentrations of curcumin within the 14 days prior to drug administration. Subjects completed a food checklist to verify that they were not consuming any curcumin-rich foods prior to dosing. A standardized, high-fat breakfast with 750 calories and 35 grams of total fat (42% fat), designed to slow gastric emptying time was fed to all subjects to allow maximum absorption of curcumin, which is highly lipophilic. Then, curcumin was administered at a dose of 10 g (N=6) or 12 g (N=6). Subjects were randomly allocated to dose level for a total of 6 subjects at each dose level. Curcumin was formulated as Sabinsa C3 complex in 250 mg capsules (containing approximately 187 mg curcumin, 58 mg demethoxycurcumin, and 5 mg bisdemethoxycurcumin) and administered with 16 to 32 oz of water over a maximum of 30 minutes. Blood samples (10 mL) were collected in heparinized tubes prior to dosing and at 0.25, 0.5, 0.75, 1, 2, 4, 6, 8, 10, 24, 36, 48, and 72 hours after the last capsule was taken. All protocol procedures were performed in the University of Michigan Medical Center General Clinical Research Center. The plasma fraction was separated from blood immediately, and kept at −20°C until assayed. Adverse events were graded based on National Cancer Institute Common Toxicity Criteria version 2.0 at 4, 24, 48 and 72 hours after curcumin administration. The study protocol and the comprehensive written informed consent used in this study protocol were approved by IRBMED, University of Michigan Medical School's Human Subject Review Board prior to the start of the study.
Extraction of Curcumin from Plasma
The extraction procedure was based on the previously validated method of Heath et al. (40). An aliquot of plasma (200 μL) was mixed with water (80 μL), and β-17-estradiol acetate (internal standard, 20 μL), and vortex mixed for 30 seconds. The solution was then extracted three times with 1 ml of ethyl acetate: methanol (95:5; v/v) as an extracting reagent and vortex mixed for 3 minutes. The samples were centrifuged at 3000 rpm for 15 minutes at 4°C. The upper organic layer was collected. The organic phases from the three extractions were pooled and evaporated under a stream of argon at room temperature. All the extraction procedures were performed under dim light to prevent the degradation of curcumin. The extracts were then reconstituted in methanol (100 μL) before HPLC analysis.
Enzymatic Hydrolysis of Curcumin Conjugates
Samples were also assayed for curcumin and its conjugates, after incubating the plasma samples with the enzymes β-glucuronidase and sulfatase using the method of Asai et al. (36). For these assays, plasma samples (200 μL) were mixed with water (80 μL), and internal standard (20 μL), and vortexed for 30 seconds. The samples were then mixed with β-glucuronidase (50 μL, 446 units) in 0.1 M phosphate buffer (pH 6.8), and sulfatase (45 μL, 52 units) in 0.1M sodium acetate buffer (pH 5.0), and incubated at 37°C for 3.5 hours. The incubation time was determined by a test run, where samples were incubated with enzymes for 1, 2, 3, 4, 5, 6 and 15 hours demonstrating that the optimal incubation time was 3.5 hours. The samples were extracted three times with 1 ml of ethyl acetate:methanol (95:5, v/v) using the extraction procedure mentioned above. The extracts were then reconstituted in methanol (100 μL) before HPLC analysis. To determine the amount of glucuronide and sulfate conjugates in plasma, samples were incubated separately with β-glucuronidase and sulfatase enzymes prior to extraction.
Quantitation of Curcumin in Plasma
Reverse-phase HPLC was used to quantify curcumin in plasma. The separations were performed on a μBondapak, C18 column (250 × 4.6mm i.d.; 10 μm particle size; Waters, Millford, MA) at room temperature. The mobile phase consisted of 0.1% acetic acid /65% methanol/35% water (v/v/v; A) and 100% methanol (B). The extracted sample was eluted on a gradient mobile phase starting 100% of A at zero time to 100% B in 15 min in a linear gradient and then to 100% A in 2 min at a flow rate of 2 mL/min. The injection volume for all samples was 20 μL and detection was performed at 420 nm.
Standard curves were constructed using plasma spiked with curcumin. Plasma samples with no detectable curcumin (200 μL) were spiked with varying amount of a standard solution of curcumin to yield final curcumin concentrations of 0, 0.025, 0.05, 0.075, 0.10, 0.125, 0.25, 0.5, 1.25, 2.5, 5 and 10 μg /mL, respectively. Spiked samples were extracted as described above. Calibration curves were obtained by plotting the area ratios of curcumin to internal standard, β-17-estradiol, against concentrations of curcumin in spiked plasma. Each sample was analyzed in duplicate.
Analytical Assessment/Quality Control
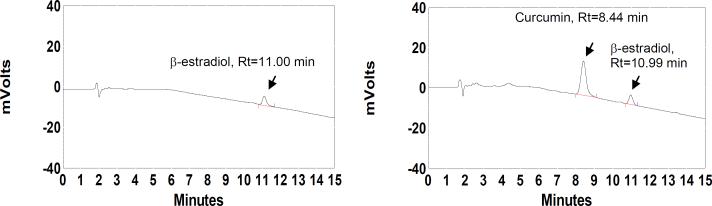
Curcumin and internal standards were well resolved by HPLC. Retention time of curcumin was 8.4 and internal standard 11.0 minutes, respectively (Fig. 2). A linear relationship between curcumin plasma concentration and peak area response was found in the concentration ranges 0.075 -10 μg/mL. Inter-day coefficients of determination (R2) from 0.9979 to 0.9999 were observed over four days with curcumin spiked plasma. The lower limit of quantitation of curcumin in plasma based on this method was 0.075 μg/mL. The extraction efficiency of curcumin at 1.25 and 2.5 μg/mL levels was 98.29% and 98.49%, respectively. The corresponding coefficients of variation (CV) were 1.8% and 1.2% respectively. At 0.025 μg/mL, the internal standard β-17-estradiol acetate showed 95.01% extraction efficiency with CV 4%. The percentage recovery of curcumin at 1 μg/mL was 95.14% with a CV of 2.7%. The lower limit of detection was 0.050 μg/mL.
Fig. 2.
HPLC chromatograms of extracted plasma 1 hour after a 12 g dose of curcumin. The plasma was analyzed without incubation (A) and after incubation with β-glucuronidase (440 units) and aryl sulfatase (50 units) for 3.5 h (B).
We assayed the samples in random order, mixing samples from subjects receiving 10g and 12g doses in assay batches. We matched the subject identification number on the plasma tube to the dose administered, raw HPLC chromatogram data sets, and to the pharmacokinetic study case report form to verify that samples were not misidentified in the assay procedure and correlate dose administered to each chromatogram after analysis was complete.
Statistical Methods and Pharmacokinetic (PK) analysis
Curcumin and curcumin conjugates were assessed in samples collected up to 72 hours after dosing. The conjugate concentration of subject i at time t, cit, was modeled as a single compartment pharmacokinetic process, with first-order absorption and elimination:
where di is the dose of subject i, kai and kei are the absorption and elimination parameters of the ith subject, vi is the lumped bioavailability/volume parameter, λ is the lag time for absorption and eit is an additive normal error term with variance (σE(cit))2, where E is the expectation operator. To consider inter-subject variation, kei and vi were modeled as exponential functions of independent, normally distributed random variables:
The population means of kei, vi and kai respectively, were estimated by eβ1, eβ2, and eβ3, and the standard errors of those population means were calculated by means of the delta method. These primary model parameters were estimated using SAS PROC NLINMIXED (SAS Institute, Cary, NC), from which estimates of AUC, tmax, cmax and t1/2 were derived; their standard errors and 95% confidence intervals, which describe the precision of the estimates, were calculated by means of the delta method (41). Goodness of fit was evaluated by means of plots of observed versus predicted responses and plots of residuals versus time. Prediction intervals, which describe the distribution of values in the population, were estimated by parametric bootstrap (42). It was observed from the raw data and model parameters that the concentration profiles did not linearly increase with dose. The model above was rerun, with di=10 or di=10+δ, for subjects in the 10 g and 12 g arms, respectively.
Results
Subjects and Adverse Events
Twelve healthy Caucasian subjects are shown in Table 1, five males, seven females, mean age 35 years (range 18-65 years), BMI 20-29, recruited from July, 2003 through September, 2003. Adverse events reported were all Grade 1 by NCI Common Toxicity Criteria (v. 2.0). No adverse events greater than Grade 1 were reported. The major adverse events included one subject with headache and one with sore arms hours after dosing, likely unrelated to treatment and 2 subjects with yellow, loose stool the day after dosing, most likely due to the lack of curcumin absorption in the GI tract. One subject consumed curcumin containing foods 4 times weekly, but had no detectable curcumin or conjugates at baseline. Interestingly, this subject had the lowest area under the curve for curcumin glucuronide and curcumin sulfate compared to all other subjects.
Table 1.
Description of Study Subjects
| Dose (g) | Sex | Age (years) | BMI |
|---|---|---|---|
| 10g | female | 26 | 22.2 |
| 10g | female | 40 | 21.3 |
| 10g | female | 33 | 28.8 |
| 10g | female | 65 | 27.4 |
| 10g | male | 18 | 21.4 |
| 10g | male | 20 | 20.8 |
| Mean (Range) | 4 females | 33.6 (18-65) | 24.38 (20.8-28.8) |
| 2 males | |||
| 12g | female | 30 | 23.0 |
| 12g | female | 19 | 25.6 |
| 12g | female | 19 | 26.8 |
| 12g | male | 55 | 21.1 |
| 12g | male | 51 | 20.4 |
| 12g | male | 37 | 29.4 |
| Mean (Range) | 3 males | 35.16 (19-55) | 23.65 (20.4-29.4) |
| 3 females |
Detection of Curcumin and Conjugate Metabolites in Plasma Samples
Plasma samples of all subjects were analyzed with and without incubation with deconjugating enzymes using a slightly acidic pH since curcumin is not stable at pH 7.4. Fig. 2 shows HPLC chromatograms of plasma with and without hydrolysis of conjugates. Free curcumin was detected in the plasma of only one subject without incubation, 30 minutes after ingestion of a 10 g dose. No free curcumin was detected in any other plasma samples from any other subject. Subsequent results refer exclusively to curcumin conjugates.
C max, t1/2 and AUC of total curcumin conjugates calculated from the raw data are presented in Table 2. One subject on the 10g dose level had a Tmax of 10 hr, but no curcumin conjugates were detected in this subject before 2 hr and after 48 hours of drug intake. When the Tmax was within the range of 1 to 4 hr, curcumin conjugates were completely eliminated from plasma at the 24 hr time point. The concentrations (mean ±SD) of curcumin glucuronide and curcumin sulfate at Tmax at the 10 g dose level were 2.04±0.31 (range 1.57-2.39) μg/mL and 1.06±0.40 (range 0.44-1.62) μg/mL, respectively, and at the 12 g dose level were 1.40±0.74 (range 0.61-2.56) μg/mL and 0.87±0.44 (range 0.41-1.65) μg/mL, respectively (Table 3).
Table 2.
Curcumin conjugate pharmacokinetic parameters estimated from the raw data. AUC was determined by the trapezoid rule, and t1/2 by means of linear interpolation
| Dose=10 g (n=6) | Dose=12 g (n=6) | |||
|---|---|---|---|---|
| Mean±S.D. | (Min,Max) | Mean±S.D. | (Min,Max) | |
| AUC | 39.8±7.6 | (30.8,47.8) | 26.8±10.9 | (8.2,40.3) |
| C | 3.2±0.56 | (2.4,3.8) | 2.1±0.73 | (1.3,3.2) |
| t1/2 | 10.3±4.4 | (6.0,18.8) | 8.8±2.7 | (5.2,14.2) |
| tmax | 4.33±3.2 | (2,10) | 3.67±2.0 | (2,6) |
Table 3.
The fraction of curcumin glucuronide and curcumin sulfate in plasma at C max after administration of 10 and 12 g curcumin based on enzymatic hydrolysis performed in duplicate. Total curcumin in plasma was assayed after incubating plasma with enzymes β-glucuronidase and sulfatase separately (Separate Assay) and combined (Mixed Assay).
| Total Curcumin | ||||
|---|---|---|---|---|
| Dose (g) | Curcumin glucuronide (μg/mL) | Curcumin sulfate (μg/mL) | Separate Assay (μg/mL) | Mixed Assay (μg/mL) |
| 10 | 2.04±0.31* | 1.06±0.40 | 3.10±0.60 | 3.09±0.53 |
| 12 | 1.40±0.74 | 0.87±0.44 | 2.27±1.17 | 2.03±0.74 |
mean ±SD of six subjects
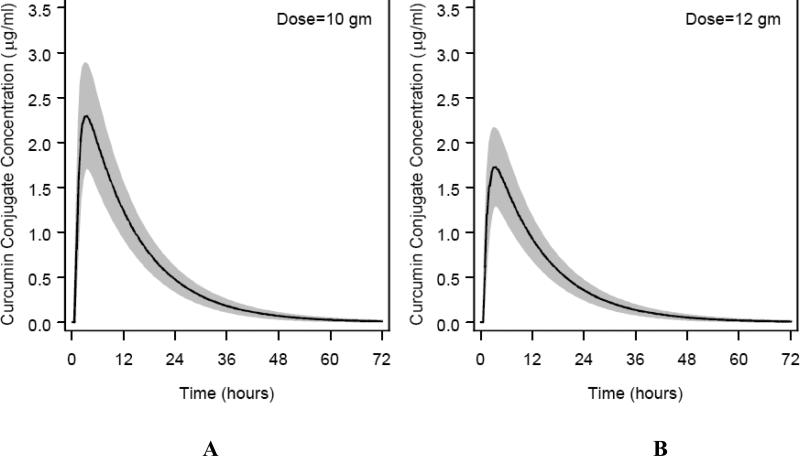
The kinetic parameters of the total curcumin conjugates from the biexponential model are shown in Table 4. A typical subject has an absorption rate constant (ka) of 1.05±0.26 hr-1(mean ±SE), elimination rate constant (k)of 0.08±0.0.005 hr−1 (mean ±SE), and half life (t1/2) of 6.77± 0.83 hr (mean ±SE). Based on the PK model, AUC (mean±SE) for the 10 and 12 g doses were 35.33±3.78 and 26.57±2.97 μg/mL × hr, respectively. The Cmax (mean±SE) for the 10 and 12 doses were 2.30±0.26 and 1.73±0.19 μg/mL, respectively. Note that the modeled Cmaxand AUC are, like the direct estimates from the raw concentrations in Table 2, greater for the 10 g than for the 12 g subjects. This difference was not due to a single outlier.
Table 4.
Pharmacokinetic parameters derived from PK model of curcumin conjugates: Area Under the Curve (AUC); peak concentration (Cmax); time of peak concentration (tmax); half life (t1/2 ); absorption constant (ka); excretion constant (ke); bioavailability/volume (v); absorption lag (lag ). The mean±standard error of the estimate, 95% confidence intervals for the parameter estimates and 95% prediction intervals for the population are presented.
| Mean±S.E. | 95% Confidence Interval | 95% Prediction Interval | |
|---|---|---|---|
| AUC (μg·hr/ml) 10 gm dose | 35.33±3.78 | (26.90,43.75) | (10.62,117.01) |
| AUC (μg·hr/ml) 12 gm dose | 26.57±2.97 | (19.96,33.18) | (7.86,89.46) |
| Cmax (μg/ml) 10 gm dose | 2.30±0.26 | (1.72,2.88) | (0.71,7.04) |
| Cmax (μg/ml) 12 gm dose | 1.73±0.19 | (1.30,2.16) | (0.52,5.41) |
| tmax (hr) | 3.29±0.43 | (2.34,4.24) | (1.56,7.37) |
| t1/2 (hr) | 6.77±0.83 | (4.82,8.71) | (3.19,14.46) |
| ka(hr−1) | 1.05±0.26 | (0.47,1.64)) | n.a. |
| ke (hr−1) | 0.08±0.0050 | (0.069,0.091) | n.a. |
| v | 3.54±0.41 | (2.63,4.46) | n.a. |
| lag (hr) | 0.68±0.016 | (0.64,0.71) | n.a. |
| dose (gm) | −2.48±0.98 | (−4.66,−0.30) | n.a. |
Table 4 also presents estimates of the variation in AUC, Cmax, tmax and t1/2 in the subjects. After a 10 gm dose, 95% of the population would be expected to demonstrate Cmax between 0.71 and 7.04 μg/mL, with half-life between 3.19 and 14.46 hours.
The subjects in the 10 g dose group were similar in BMI or mean age compared to the 12g dose group (Table 1). PK parameters were analyzed by sex, age, and BMI but were not found to differ significantly based on these variables; however, this study was not powered to detect such differences.
The amount of glucuronide conjugate assessed at each participant's observed T max ranged between 20% and 67% higher than the concentration of sulfate conjugate (Table 3). Total curcumin released after incubating plasma with β-glucuronidase and sulfatase enzymes separately was almost equal to the curcumin released after incubating plasma with β-glucuronidase and sulfatase enzymes together (Table 3). These results indicate that most of the curcumin conjugates are either glucuronides or sulfates and not mixed conjugates.
Discussion
Prior clinical reports at lower doses have suggested that orally administered curcumin is poorly bioavailable. Gram doses were required to detect curcumin in blood, using 90-99% pure curcumin preparations, and the highest dose tested, 8 g, resulted in less than 1 μg/mL curcumin (37, 38, 43). A dose escalation study detected free curcumin concentrations of 0.03-0.06 μg/mL 1-4 hours after a 10 or 12 g oral dose of curcuminoids in only one of three subjects at each dose level (39). In the present single-dose pharmacokinetic study, we also failed to detect free curcumin in all but one subject, using the Sabinsa curcuminoid (C3) mixture that was 75% curcumin. Repetitive daily administration of curcumin may cause accumulation of curcumin at the gastrointestinal mucosal interface, resulting in more free curcumin delivered to systemic sites after multiple dosing, compared to a single dose (37, 38, 43). Alternatively, at the extremely high doses we used, curcumin transport may be saturated at the gut epithelium while conjugation is not. Curcumin absorption may not be limited. Rather, it may induce its own biotransformation (both conjugation and CYP reduction enzymes) in the gut epithelium or in the liver. In this scenario, one should detect proportionately higher conjugate concentrations at higher doses unless the excess conjugates are eliminated via the gut.
Data to date do not support the concept that curcumin serves as a prodrug (34, 44, 45). For example, data suggest that tetrahydrocurcumin, a major reduction metabolite, is less potent an inhibitor of NF-κB, cyclooxygenase-1, 5-lipoxygenase and proliferation at equivalent concentrations as curcumin (45, 46). The potential biological impact of the high concentrations and long systemic exposure to conjugates remain unclear. Nevertheless, our findings that the majority of curcumin is biotransformed prior to measurement at the systemic (peripheral venous) site are consistent with the very low concentrations of free curcumin detected by both Cheng et al. (37) and Sharma et al. (39).
In this present study, a single, high dose of curcumin resulted in rapid appearance of curcumin conjugates in human plasma with a lag of 0.68 hrs post dose with Tmax of approximately 4 hrs as shown in Table 4. In the rat, curcumin glucuronide and curcumin sulfate were the predominant metabolites (35, 36). In a previously published human study, using four months of oral dosing of 3.6 g of curcumin daily, both glucuronide and sulfate conjugates were detected in plasma, but at very low levels (3-6 ng/mL) similar to that of free curcumin (34). Curcumin conjugates in our study were present at levels that were about 1000-fold higher (1-3 μg/mL) when extracting curcumin after hydrolysis of conjugates. The present study suggests that curcumin was rapidly biotransformed at anatomic sites, potentially gastric mucosa, liver, or both, prior to peripheral venous circulation.
Surprisingly, the Cmax and AUC of curcumin conjugates in the 10g arm were significantly higher than in the 12g arm (p<0.01) (Table 4). Our review did not find bias in the sample handling, processing, or analytical procedures.
This outcome could be explained by saturation of a transport mechanism, resulting in no incremental curcumin absorption at higher doses, induction of metabolism at higher doses and other mechanisms discussed earlier. Since the subjects took large numbers of capsules (40 capsules for the 10g dose, 48 capsules for the 12g dose), variation in the time required to swallow all of these capsules might have affected the absorption. The potentially longer time required to swallow the 48 capsule dose may have resulted in a lower Cmax and lower absorption compared to the 40 capsule dose.
In this trial, no serious adverse events were reported after curcumin dosing. All adverse events reported were Grade 1. While the small size of this trial precludes any formal safety endpoint analysis and statistical certainty of safety, the safety profile observed here is consistent with previous clinical and preclinical data (7, 37-39).
A high rate of curcumin conjugation, through glucuronidation and sulfation, may explain low concentrations of free curcumin when administered orally in previous studies (34, 37, 38). The potential activation of curcumin conjugates by intracellular glucuronidases or sulfatases should be explored further. Ireson et al reported that curcumin metabolites, specifically, hexahydrocurcumin, tetrahydrocurcumin and curcumin sulfate, had very low activity for inhibition of phorbol ester-induced prostaglandin E2 (PGE2) production in human colonic epithelial cells compared to the parent compound (34). If, however, conjugates are deconjugated to free curcumin, as occurs in inflammation (47, 48), one can speculate that the curcumin conjugate concentrations detected here are sufficient to modulate key curcumin-associated intracellular targets. In vitro, concentrations in the range of 3.6-36 μg/mL of curcumin were necessary to modulate cellular targets (e.g. cyclooxygenase-2) in human colonic cells (30, 34). The concentrations of curcumin conjugates observed in human plasma after consumption of 10 or 12 g single doses described here resulted in plasma concentrations sufficient to elicit pharmacologic activity in vitro, if de-conjugated. Alternatively, curcumin conjugates have recently been shown to have interesting biological properties. Conjugation, therefore, may not necessarily be an inactivation pathway (49, 50). Preliminary data in rodents have demonstrated modulation of carcinogenesis targets at systemic sites after curcumin administration (10, 34, 51).
The results of this study permit the following two conclusions to be drawn regarding oral curcumin in humans: (1) consumption of 10 or 12g curcumin single doses generates mainly curcumin glucuronide and curcumin sulfate; (2) glucuronide conjugate predominates over sulfate conjugate in plasma with no evidence of a mixed conjugate being formed. These results indicate that curcumin is absorbed after oral administration and could be transported to sites other than the intestinal tract.
Fig. 3.
Modeled time concentration plots of curcumin conjugates detected in plasma from 12 human subjects treated with a single 10 g (A) or 12 g (B) dose with confidence interval depicted in gray.
Acknowledgments
Grant support: AICR-06A035; NCI CN-55124; and University of Michigan General Clinical Research Center M01-RR00024.
We thank the volunteers who participated in this study and Bill C Frame for his contributions to the pharmacokinetic analysis.
REFERENCES
- 1.Cragg GM, Newman DJ, Snader KM. Natural products in drug discovery and development. J Nat Prod. 1997 Jan;60(1):52–60. doi: 10.1021/np9604893. [DOI] [PubMed] [Google Scholar]
- 2.Newman DJ, Cragg GM, Holbeck S, Sausville EA. Natural products and derivatives as leads to cell cycle pathway targets in cancer chemotherapy. Curr Cancer Drug Targets. 2002 Dec;2(4):279–308. doi: 10.2174/1568009023333791. [DOI] [PubMed] [Google Scholar]
- 3.Newman DJ, Cragg GM, Snader KM. The influence of natural products upon drug discovery. Nat Prod Rep. 2000 Jun;17(3):215–34. doi: 10.1039/a902202c. [DOI] [PubMed] [Google Scholar]
- 4.Jemal A, Murray T, Ward E, et al. Cancer statistics, 2005. CA: A Cancer Journal for Clinicians. 2005 Jan-Feb;55(1):10–30. doi: 10.3322/canjclin.55.1.10. [DOI] [PubMed] [Google Scholar]
- 5.Conney AH, Lysz T, Ferraro T, et al. Inhibitory effect of curcumin and some related dietary compounds on tumor promotion and arachidonic acid metabolism in mouse skin. Adv Enzyme Regul. 1991;31:385–96. doi: 10.1016/0065-2571(91)90025-h. [DOI] [PubMed] [Google Scholar]
- 6.Lu YP, Chang RL, Lou YR, et al. Effect of curcumin on 12-O-tetradecanoylphorbol-13-acetate- and ultraviolet B light-induced expression of c-Jun and c-Fos in JB6 cells and in mouse epidermis. Carcinogenesis. 1994;15(10):2363–70. doi: 10.1093/carcin/15.10.2363. [DOI] [PubMed] [Google Scholar]
- 7.Sharma RA, Gescher AJ, Steward WP. Curcumin: the story so far. Eur J Cancer. 2005 Sep;41(13):1955–68. doi: 10.1016/j.ejca.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 8.Huang MT, Lou YR, Ma W, Newmark HL, Reuhl KR, Conney AH. Inhibitory effects of dietary curcumin on forestomach, duodenal, and colon carcinogenesis in mice. Cancer Res. 1994 Nov 15;54(22):5841–7. [PubMed] [Google Scholar]
- 9.Singh SV, Hu X, Srivastava SK, et al. Mechanism of inhibition of benzo[a]pyrene-induced forestomach cancer in mice by dietary curcumin. Carcinogenesis. 1998 Aug;19(8):1357–60. doi: 10.1093/carcin/19.8.1357. [DOI] [PubMed] [Google Scholar]
- 10.Kawamori T, Lubet R, Steele VE, et al. Chemopreventive effect of curcumin, a naturally occurring anti-inflammatory agent, during the promotion/progression stages of colon cancer. Cancer Res. 1999 Feb 1;59(3):597–601. [PubMed] [Google Scholar]
- 11.Kim J, Araki S, Kim D, et al. Chemopreventive effects of carotenoids and curcumins on mouse colon carcinogenesis after 1,2-demethylhydrazine initiation. Carcinogenesis. 1998;19:81–5. doi: 10.1093/carcin/19.1.81. [DOI] [PubMed] [Google Scholar]
- 12.Huang MT, Lou YR, Xie JG, et al. Effect of dietary curcumin and dibenzoylmethane on formation of 7,12-dimethylbenz[a]anthracene-induced mammary tumors and lymphomas/leukemias in Sencar mice. Carcinogenesis. 1998 Sep;19(9):1697–700. doi: 10.1093/carcin/19.9.1697. [DOI] [PubMed] [Google Scholar]
- 13.Inano H, Onoda M, Inafuku N, et al. Chemoprevention by curcumin during the promotion stage of tumorigenesis of mammary gland in rats irradiated with gamma-rays. Carcinogenesis. 1999 Jun;20(6):1011–8. doi: 10.1093/carcin/20.6.1011. [DOI] [PubMed] [Google Scholar]
- 14.Inano H, Onoda M, Inafuku N, et al. Potent preventive action of curcumin on radiation-induced initiation of mammary tumorigenesis in rats. Carcinogenesis. 2000 Oct;21(10):1835–41. doi: 10.1093/carcin/21.10.1835. [DOI] [PubMed] [Google Scholar]
- 15.Chuang SE, Kuo ML, Hsu CH, et al. Curcumin-containing diet inhibits diethylnitrosamine-induced murine hepatocarcinogenesis. Carcinogenesis. 2000 Feb;21(2):331–5. doi: 10.1093/carcin/21.2.331. [DOI] [PubMed] [Google Scholar]
- 16.Han SS, Chung ST, Robertson DA, Ranjan D, Bondada S. Curcumin causes the growth arrest and apoptosis of B cell lymphoma by downregulation of egr-1, c-myc, bcl-XL, NF-kappa B, and p53. Clin Immunol. 1999 Nov;93(2):152–61. doi: 10.1006/clim.1999.4769. [DOI] [PubMed] [Google Scholar]
- 17.Piwocka K, Zablocki K, Wieckowski MR, et al. A novel apoptosis-like pathway, independent of mitochondria and caspases, induced by curcumin in human lymphoblastoid T (Jurkat) cells. Exp Cell Res. 1999 Jun 15;249(2):299–307. doi: 10.1006/excr.1999.4480. [DOI] [PubMed] [Google Scholar]
- 18.Chen H, Zhang ZS, Zhang YL, Zhou DY. Curcumin inhibits cell proliferation by interfering with the cell cycle and inducing apoptosis in colon carcinoma cells. Anticancer Res. 1999 Sep-Oct;19(5A):3675–80. [PubMed] [Google Scholar]
- 19.Simon A, Allais DP, Duroux JL, Basly JP, Durand-Fontanier S, Delage C. Inhibitory effect of curcuminoids on MCF-7 cell proliferation and structure-activity relationships. Cancer Lett. 1998 Jul 3;129(1):111–6. doi: 10.1016/s0304-3835(98)00092-5. [DOI] [PubMed] [Google Scholar]
- 20.Ramachandran C, You W. Differential sensitivity of human mammary epithelial and breast carcinoma cell lines to curcumin. Breast Cancer Res Treat. 1999 Apr;54(3):269–78. doi: 10.1023/a:1006170224414. [DOI] [PubMed] [Google Scholar]
- 21.Sharma RA, Ireson CR, Verschoyle RD, et al. Effects of dietary curcumin on glutathione S-transferase and malondialdehyde-DNA adducts in rat liver and colon mucosa: relationship with drug levels. Clin Cancer Res. 2001 May;7(5):1452–8. [PubMed] [Google Scholar]
- 22.Holder GM, Lummer JL, Ryan AJ. The metabolism and excretion of curcumin (1,7-bis-(4-Hydroxy-3-methoxyphenyl)-1,6-heptadiene-3,5-dione) in the rat. Xenobiotica. 1978;8:761–8. doi: 10.3109/00498257809069589. [DOI] [PubMed] [Google Scholar]
- 23.Susan M, Rao MN. Induction of glutathione S-transferase activity by curcumin in mice. Arzneimittelforschung. 1992;42(7):962–4. [PubMed] [Google Scholar]
- 24.Subramanian M, Sreejayan-Rao MNA, Devasagayam TPA, Singh BB. Diminution of singlet oxygen induced DNA damage by curcumin and related antioxidants. Mutat Res. 1994;311:249–55. doi: 10.1016/0027-5107(94)90183-x. [DOI] [PubMed] [Google Scholar]
- 25.Reddy AC, Lokesh BR. Studies on the inhibitory effects of curcumin and eugenol on the formation of reactive oxygen species and the oxidation of ferrous iron. Mol Cell Biochem. 1994 Aug 17;137(1):1–8. doi: 10.1007/BF00926033. [DOI] [PubMed] [Google Scholar]
- 26.Goel A, Boland CR, Chauhan DP. Specific inhibition of cyclooxygenase-2 (COX-2) expression by dietary curcumin in HT-29 human colon cancer cells. Cancer Lett. 2001 Oct 30;172(2):111–8. doi: 10.1016/s0304-3835(01)00655-3. [DOI] [PubMed] [Google Scholar]
- 27.Huang MT, Lysz T, Ferraro T, Abidi TF, Laskin JD, Conney AH. Inhibitory effects of curcumin on in vitro lipoxygenase and cyclooxygenase activities in mouse epidermis. Cancer Res. 1991 Feb 1;51(3):813–9. [PubMed] [Google Scholar]
- 28.Huang MT, Newmark HL, Frenkel K. Inhibitory effects of curcumin on tumorigenesis in mice. J Cell Biochem Suppl. 1997;27:26–34. [PubMed] [Google Scholar]
- 29.Atsumi T, Murakami Y, Shibuya K, Tonosaki K, Fujisawa S. Induction of cytotoxicity and apoptosis and inhibition of cyclooxygenase-2 gene expression, by curcumin and its analog, alpha-diisoeugenol. Anticancer Res. 2005 Nov-Dec;25(6B):4029–36. [PubMed] [Google Scholar]
- 30.Plummer SM, Holloway KA, Manson MM, et al. Inhibition of cyclo-oxygenase 2 expression in colon cells by the chemopreventive agent curcumin involves inhibition of NF-kappaB activation via the NIK/IKK signalling complex. Oncogene. 1999 Oct 28;18(44):6013–20. doi: 10.1038/sj.onc.1202980. [DOI] [PubMed] [Google Scholar]
- 31.Singh S, Aggarwal BB. Activation of transcription factor NF-kappa B is suppressed by curcumin (diferuloylmethane) [corrected]. J Biol Chem. 1995 Oct 20;270(42):24995–5000. doi: 10.1074/jbc.270.42.24995. [DOI] [PubMed] [Google Scholar]
- 32.Aggarwal BB, Shishodia S, Takada Y, et al. Curcumin suppresses the paclitaxel-induced nuclear factor-kappaB pathway in breast cancer cells and inhibits lung metastasis of human breast cancer in nude mice. Clin Cancer Res. 2005 Oct 15;11(20):7490–8. doi: 10.1158/1078-0432.CCR-05-1192. [DOI] [PubMed] [Google Scholar]
- 33.Jagetia GC, Aggarwal BB. “Spicing up” of the immune system by curcumin. J Clin Immunol. 2007 Jan;27(1):19–35. doi: 10.1007/s10875-006-9066-7. [DOI] [PubMed] [Google Scholar]
- 34.Ireson C, Orr S, Jones DJ, et al. Characterization of metabolites of the chemopreventive agent curcumin in human and rat hepatocytes and in the rat in vivo, and evaluation of their ability to inhibit phorbol ester-induced prostaglandin E2 production. Cancer Res. 2001 Feb 1;61(3):1058–64. [PubMed] [Google Scholar]
- 35.Pan MH, Huang TM, Lin JK. Biotransformation of curcumin through reduction and glucuronidation in mice. Drug Metab Dispos. 1999 Apr;27(4):486–94. [PubMed] [Google Scholar]
- 36.Asai A, Miyazawa T. Occurrence of orally administered curcuminoid as glucuronide and glucuronide/sulfate conjugates in rat plasma. Life Sci. 2000 Oct 27;67(23):2785–93. doi: 10.1016/s0024-3205(00)00868-7. [DOI] [PubMed] [Google Scholar]
- 37.Cheng AL, Hsu CH, Lin JK, et al. Phase I clinical trial of curcumin, a chemopreventive agent, in patients with high-risk or pre-malignant lesions. Anticancer Res. 2001 Jul-Aug;21(4B):2895–900. [PubMed] [Google Scholar]
- 38.Sharma RA, Euden SA, Platton SL, et al. Phase I clinical trial of oral curcumin: biomarkers of systemic activity and compliance. Clin Cancer Res. 2004 Oct 15;10(20):6847–54. doi: 10.1158/1078-0432.CCR-04-0744. [DOI] [PubMed] [Google Scholar]
- 39.Lao CD, Ruffin MT, Normolle D, et al. Dose escalation of a curcuminoid formulation. BMC Complement Altern Med. 2006;6:10. doi: 10.1186/1472-6882-6-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Heath DD, Pruitt MA, Brenner DE, Rock CL. Curcumin in plasma and urine: quantitation by high-performance liquid chromatography. J Chromatogr B Analyt Technol Biomed Life Sci. 2003 Jan 5;783(1):287–95. doi: 10.1016/s1570-0232(02)00714-6. [DOI] [PubMed] [Google Scholar]
- 41.Casella G, Berger RL. Statistical inference. 2nd ed. Thomson Learning; Australia ; Pacific Grove, CA: 2002. [Google Scholar]
- 42.Davison AC, Hinkley DV, Canty AJ. Bootstrap methods and their application. Cambridge University Press; Cambridge ; New York, NY, USA: 1997. [Google Scholar]
- 43.Sharma RA, McLelland HR, Hill KA, et al. Pharmacodynamic and pharmacokinetic study of oral Curcuma extract in patients with colorectal cancer. Clin Cancer Res. 2001 Jul;7(7):1894–900. [PubMed] [Google Scholar]
- 44.Kawamori T, Lubet R, Steele V, et al. Chemopreventive effect of curcumin, a naturally occurring anti-inflammatory agent, during the promotion/progression stages of colon cancer. Cancer Res. 1999;59:597–601. [PubMed] [Google Scholar]
- 45.Sandur SK, Pandey MK, Sung B, et al. Curcumin, demethoxycurcumin, bisdemethoxycurcumin, tetrahydrocurcumin and turmerones differentially regulate anti-inflammatory and anti-proliferative responses through a ROS-independent mechanism. Carcinogenesis. 2007 Aug;28(8):1765–73. doi: 10.1093/carcin/bgm123. [DOI] [PubMed] [Google Scholar]
- 46.Hong J, Bose M, Ju J, et al. Modulation of arachidonic acid metabolism by curcumin and related beta-diketone derivatives: effects on cytosolic phospholipase A(2), cyclooxygenases and 5-lipoxygenase. Carcinogenesis. 2004 Sep;25(9):1671–9. doi: 10.1093/carcin/bgh165. [DOI] [PubMed] [Google Scholar]
- 47.Shimoi K, Nakayama T. Glucuronidase deconjugation in inflammation. Methods Enzymol. 2005;400:263–72. doi: 10.1016/S0076-6879(05)00015-7. [DOI] [PubMed] [Google Scholar]
- 48.Shimoi K, Okada H, Furugori M, et al. Intestinal absorption of luteolin and luteolin 7-O-beta-glucoside in rats and humans. FEBS Lett. 1998 Nov 6;438(3):220–4. doi: 10.1016/s0014-5793(98)01304-0. [DOI] [PubMed] [Google Scholar]
- 49.Hoehle SI, Pfeiffer E, Metzler M. Glucuronidation of curcuminoids by human microsomal and recombinant UDP-glucuronosyltransferases. Mol Nutr Food Res. 2007 Aug;51(8):932–8. doi: 10.1002/mnfr.200600283. [DOI] [PubMed] [Google Scholar]
- 50.Pfeiffer E, Hoehle SI, Walch SG, Riess A, Solyom AM, Metzler M. Curcuminoids form reactive glucuronides in vitro. J Agric Food Chem. 2007 Jan 24;55(2):538–44. doi: 10.1021/jf0623283. [DOI] [PubMed] [Google Scholar]
- 51.Perkins S, Verschoyle RD, Hill K, et al. Chemopreventive efficacy and pharmacokinetics of curcumin in the min/+ mouse, a model of familial adenomatous polyposis. Cancer Epidemiol Biomarkers Prev. 2002 Jun;11(6):535–40. [PubMed] [Google Scholar]