Abstract
Asthma diagnoses are increasing nationally with the highest rates in the New England States. Epidemiological studies have suggested a relationship between airborne particulate matter and severity of an asthma attack. However, because particulate matter, PM, is such a complex mixture, it is difficult to isolate the exacerbating factors. In this paper we investigate the effect of NIST and Maine particulate matter and the soluble metals released from the particulate matter on the growth of human lung fibroblasts. While the NIST (National Institute of Science and Technology) particulate matter itself had the most pronounced effect on cell survival rates, solutions of metals extracted from the PM also affected cell survival. Treatment of cells with 10 ug/cm2, 50 ug/cm2, 100 ug/cm2 and 200 ug/cm2 resulted in 84 ± 13, 69 ± 15, 58 ± 14, and 58 ± 16 percent survival, respectively. Appropriate concentrations of eight acid soluble metals from NIST PM were determined and tested on cells giving 91 ± 11, 87 ± 10, 72 ± 18, and 66 ± 20 percent survival, respectively. Soluble metals from Maine particulate matter were extracted and mixtures of appropriate concentrations of these metals were used to treat cells, resulting in 88 ± 5, 81 ± 5, 79 ± 3 and 57 ± 9 survival rate. To determine which, if any, of the metals individually affected the cells, Mn, Cu, V and As were used to treat the cells. At the metal concentrations tested only As and V affected cell survival.
Keywords: particulate matter, PM10, asthma, cell culture, soluble metals
INTRODUCTION
America is in the middle of an asthma epidemic. Nationally, an estimated 26 million people are diagnosed with asthma, an increase of 82% over the last 15 years (Eggleston et al., 1999). Asthma has a significant economic and health impact on the American people, costing over $12 billion annually and accounting for about 3 million lost work days and more than 10 million lost schools days (CDC, 2001). Between 1970 and 1998 the death rate from asthma increased 55.6% even though the death rate from all causes combined decreased 18% (CDC, 2001). Of the 7 states with the highest asthma rates in the country, five are in New England with nearly a million adults in the region with asthma, and Maine and Connecticut show greater than 10% asthma prevalence among children (NEARC, 2004).
Asthma is a complex chronic inflammatory lung condition where the lung tissue is sensitized and airway remodeling occurs. Patients exhibit both acute and chronic responses from external sensitizing substances such as high molecular weight or low molecular weight allergens. These responses can be intensified or triggered by environmental substances including particulate matter (PM). In an acute attack, airway cells oversecrete mucus and smooth muscle cells in airways overcontract, sometimes blocking air passages completely, but reversibly – giving rise to symptoms ordinarily associated with asthma such as shortness of breath, wheezing and coughing (Gardner et al., 1999). Inflammatory cells, eosinophils, neutrophils and Th2 lymphocytes move into the lung tissue and surrounding fluid from the blood stream. In normal non-irritated lungs, these cells are not present. The eosinophils persist beyond the acute period. Chronic changes to the lungs in the inflamed areas include damage to and loss of epithelial cells, fibrous thickening of the basement membrane, and smooth muscle and fibroblast proliferation (Gardner et al., 1999; Oddera et al., 2002; Zagai et al., 2004; Bonner, 2007).
Epidemiological studies in Atlanta, Los Angeles, Spokane and Detroit show a correlation between hospital admissions for asthma and particulate matter (Stied et al., 2000; Tolbert et al., 2000; Ostro et al., 2001; Claiborn et al., 2002; Lin et al., 2002) A study of 6 Eastern cities, Boston, Knoxville, St. Louis, Steubenville, Madison and Topeka, found a dose-dependent linear relationship between PM2.5 (particulate matter with diameters of 2.5 microns or less) and daily deaths at PM concentrations far below the current EPA 24 hour threshold of 65 ug/m3 (Swartz et al., 2002).
Until recently there have been no studies in Maine examining the link between air quality and asthma, despite the fact that Maine is experiencing an asthma epidemic. Maine’s air quality is probably the most affected of any area in the U.S. by air pollution transported from upwind sources, including anthropogenic emissions from the mid-Atlantic states, the Midwest, eastern Canada, as well as pollution produced locally in New England and from crustal sources (Parrish et al., 1993; Moody et al., 1998; EPA 2000). Previously, we reported particulate matter components for PM10 (all particulate matter with diameters of 10 microns or less) collected by the Maine Dept. of Environmental Protection in three different Maine locations, at times when clinical asthma data showed peaks and during the summer low period (Langley-Turnbaugh et al., 2005). The PM10 and soils from the same sites were analyzed for 10 metals by acid extraction to determine total metal content and then with cell culture medium (DMEM/F12 + CCS growth medium) to determine metal biosolubility. Our results showed that Mn, Cu, Pb, As, V, Ni, and Al were present in the Maine PM samples. Vanadium, Ni, and Pb showed seasonal variation, while the others were relatively constant throughout the year. However, Pb and Al did not appear to be soluble in the biological medium. Maine citizens are exposed to these metals, as on average a person in Maine inhales about 0.2 mg of PM10 daily with highs of >2 mg daily which is calculated from the average daily exposure of 10 µg/m3 (well below the EPA limit of 65 µg/m3) and high of 100 µg/m3 respectively and adult respiration of 20 m3/day (ALAM, 2006). Due to their greater metabolic rate, children inhale more air in proportion to their body weight than adults and thus represent a vulnerable population (Bearer, 1995).
While asthma exacerbation by PM10 is not well understood, much work with particulate matter has already been done. The particles themselves are complex mixtures of soluble and insoluble substances (inorganic, organic and biological) and their composition varies from location to location (Bonner, 2007). Diesel particulates are known irritants and contain polyaromatic hydrocarbons (PAH’s) and nitrated PAH’s (Clean Air Task Force, 2005). Biological components from PM10 that have shown asthma exacerbation are endotoxins and some bacterial fragments (lipopolysaccharides) (Soukop and Becker, 2001; Becker et al., 2002). In addition, many studies have been conducted with animals and cells looking for irritation responses in the form of neutrophils, eosinophils from BAL (bronchial aveolar lavage) in animal studies or cytokines in both (Costa et al., 1997; Dreher et al., 1997; Adamson et al., 2000; Walters et al., 2001). Even so there is no agreement on whether the irritation is due to the particles themselves or a substance from the particles. For example, some studies show that when proportionate mixtures of the soluble metal components of PM as well as the particles were instilled into the lungs of various animals an irritation response was seen (Costa et al., 1997; Dreher et al., 1997; Samet et al., 1997; Adamson et al., 2000; Norwood et al., 2001; Walters et al., 2001; McGee et al., 2003). The most highly irritating metals in most studies were Ni, Zn and V (Dreher et al., 1997; Lambert et al., 1999; Adamson et al., 2000). In Baltimore, the biological activity of PM2.5, as measured by its ability to stimulate cytokine release by airway epithelial cells varied over short time intervals and seasonally, suggesting that local sources were important contributors to heath effects of ambient PM2.5 (Ondov et al., 2005). Further studies of human lung epithelial cells treated with whole particles of ROFA (residual oil fly ash), TiO2, Fe2O3 or α-silica (model for asbestos) for cell irritation showed that ROFA and α-silica particles produced dose-dependent responses in cells weakened with tumor necrosis factor-α. (Stringer and Kobzik, 1997)
When a challenge to the lungs does occur, the first point of contact is the epithelial layer, although in damaged areas other types of cells such as the fibroblasts may be exposed. Following such a challenge fibroblast cell populations increase rapidly and consequently play a major role in tissue remodeling resulting in further lung susceptibility. As yet, the factors that stimulate the fibroblast responses are not well understood, but may include stimulation by or production of cytokines or eosinophil binding (Doucet et al., 1998; Oddera et al., 2002; Morishita et al., 2004; Zagai et al., 2004). Furthermore, we have found no literature which examines the relation between fibroblasts and direct contact with environmental particulate matter, particularly the soluble fraction which could easily reach the fibroblasts via the interstitial fluid.
Because particulate matter (PM) appears to play an important role in the severity of asthma episodes, it is important to study PM effects in human lung cells including both fibroblasts and epithelial cells to better understand the response of these cells to PM, mixtures of soluble metals from the PM and the individual soluble metals themselves. Consequently, as a first step, we focused on human lung fibroblasts as our test cell population using cytotoxicity as our initial screen as the measure of effectiveness of component(s). We compared PM10 and its soluble metals using NIST (National Institute of Standards and Technology) urban particulate matter and soluble metals from Maine particulate matter.
MATERIALS AND METHODS
Cells and Cell Culture
The effects of various metals were studied using WTHBF-6 cells, a clonal cell line derived from human bronchial fibroblasts with reconstituted telomerase activity. The detailed isolation and characterization of these cells are described elsewhere (Wise et al., 2004). These cells retain a response to particulates that is similar to young primary human lung cells (Wise et al., 2004). Previously, we have used these cells to determine the physicochemical mechanism of particulate chromate (PbCrO4) in human lung cells (Wise et al., 2004; Xie et al., 2004; Holmes et al., 2006). In asthma, epithelial cells are a primary target, but fibroblasts are also known to be a target of particulate toxicity (DeFlora et al., 1990). Growing evidence indicates that damaged fibroblasts produce an unhealthy microenvironment that contributes to effects in epithelial cells (Hussain et al., 2000; Kurose et al., 2001; Rubin, 2001). Furthermore, soluble vanadium treatment of bronchial epithelial cells causes secretion of substances into the cell culture medium which then stimulates fibroblast growth (Zhang et al.,2001). Interestingly, a recent study of particulate chromate-induced tumors indicated that fibroblasts and not the epithelial cells bioaccumulated the metal (Kondo et al., 2003).
WTHBF-6 cells were cultured in a 50:50 mix of Dublecco’s minimal essential medium and Ham’s F-12 (DMEM/F-12) with 15% Cosmic calf serum (CCS), 1% L-glutamine, 1% penicillin/streptomycin, and 0.1% sodium pyruvate. Cells were maintained as adherent subconfluent monolayers by feeding at least twice weekly and subculturing at least once a week using 0.25% trypsin/1 mM EDTA solution.
Preparation of Soluble Metal Media
Reagent grade soluble metal salts were used to prepare stock solutions of metal ions in DI water. These metal stock solutions were then diluted 1 mL to 50 mL using the cell culture medium (above) to make the treatment solutions. The treatment solutions were cold sterile filtered and then used to replace a calculated amount of the cell culture medium (for 10 µg/cm2 0.021 mL (equivalent to 10 µg/m3 exposure over 24 hours for an adult), for 50 µg/cm2 0.11 mL, for 100 µg/cm2 0.0.21mL and for 200 µg/cm2 0.42 mL). The treatment solution for the acid soluble metals contained (in µg/mL): Mn 6.0, Cd 0.7, V 1.0, Ni 1.0, Cu 5.4, Zn 49 and Pb 50 – the biosoluble metals solution contained (in µg/mL): Mn 0.6, Cd 0.14, V 0.2, Ni 0.2, Cu 1.1, Zn 9.8 and Pb 1.1 – the Maine metals solution contained (in µg/mL): As 1, Mn 3, V 5, Ni 2, and Cu 12.
Cytotoxicity Assays
Cytotoxicity was determined by a clonogenic assay measuring reduction in plating efficiency in treated groups relative to controls based on our published methods (Wise et al., 2002). Two hundred thousand cells were seeded in 5 mL of medium on a 60 mm tissue-culture dish and allowed to grow for 48 h. The cultures were then treated for 24 h with aliquots of either NIST Standard Reference Material (SRM) 1648 Urban Particulate Matter (UPM) at 10 mg/mL suspended in culture medium, a solution of metals based on the metals in solution after acid extraction or cell culture medium extraction of NIST SRM 1648, a Maine metals solution, or an individual metal solution (Cu, Mn, or V). After 24 h the treatment medium was collected (to include any loosely adherent cells); the cells were rinsed with phosphate-buffered saline (PBS); then removed from the dish with 0.25% trypsin/ 1 mM EDTA solution. The collected media was then added to the trypsinized cells to stop the trypsin and centrifuged at 1000 rpm for 5 min at 4°C. The resulting pellet was resuspended in 5 mL of medium then counted with a Coulter Multisizer. The cells were reseeded at colony forming density (1000 cells per dish) on a 100 mm tissue-culture dish in 5 mL of medium. The colonies were allowed to grow for an average of 8 days; fixed with 100% methanol; stained with crystal violet; and the colonies counted. There were 4 replicates per treatment group and each experiment was repeated at least 3 times.
RESULTS
In order to determine whether PM10 has an effect on cells, we first tested the effect of standard particulate matter obtained from the National Institute of Standards and Technology, SRM 1648 (NIST UPM). As reported previously, we extracted this material for metals according to EPA Compendium Method (CM) IO-3 (acid extract) and then using a similar procedure with cell culture medium (biological extract) (Langley-Turnbaugh et al., 2005). The results of our extractions of this material and a comparison to the known analysis are shown in Table 1.
Table 1.
Comparison of Acid Soluble and Medium Soluble Metals (ug/g) in NIST UPM (SRM 1648)
| Sample treatment |
Mn | Cd | Cu | Cr | V | Ni | Zn | Pb | As |
|---|---|---|---|---|---|---|---|---|---|
| NIST analysis | 786 (±17) | 75 (±7) | 609 (±27) | 403 (±12)* | 127 (±7)* | 82 (±3) | 4760 (±140) | 6550 (±80) | 115 (±10)* |
| CM IO-3.11 (acid) | 636 | 67 | 541 | 76 | 113 | 63 | 4187 | 5708 | |
| Biological Extract2 | 58 | 21 | 140** | 4.9 | 17 | 17 | 663** | 117 |
NIST analyzed by neutron activation not by AA
Zn and Cu corrected for amounts of these metals in the medium
Average of three determinations
Average of 6 determinations
The results showed that the acid extractable components analyzed for comprised less than 2% of the particle weight, while the bioextractable components were less than 0.1% particle weight.
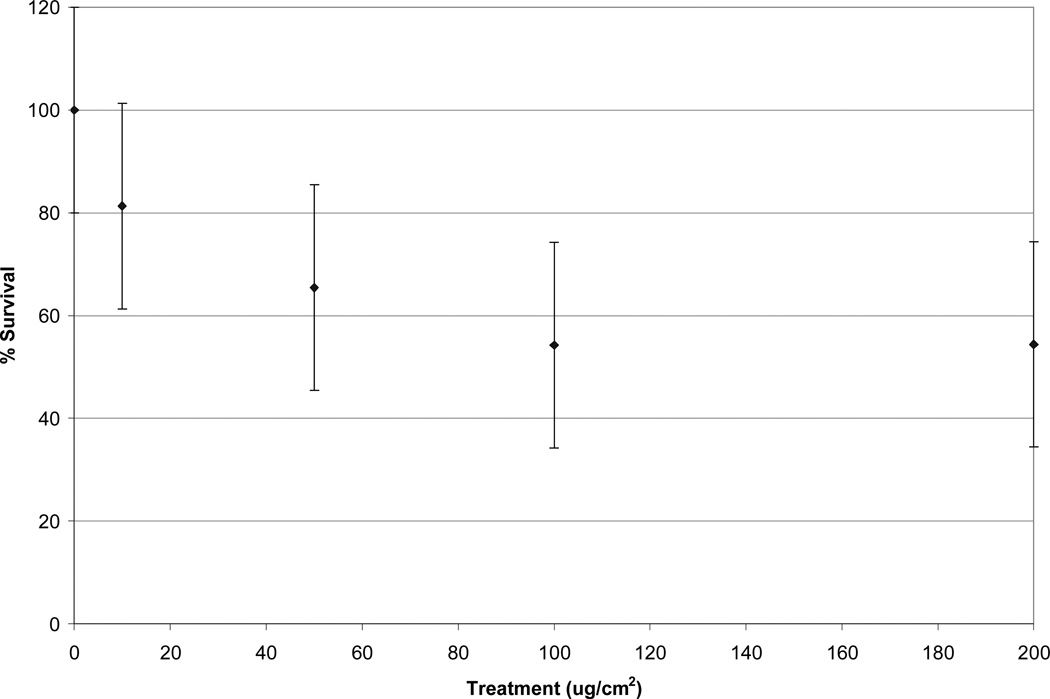
Initially, cells were treated with a solution containing NIST UPM at 10, 50, 100 and 200 ug/cm2 which in a 60 mm culture dish (5 mL of medium) results in 0.21 mg (average daily dose in Maine), 1.05 mg, 2.1 mg and 4.2 mg (20 times the average daily dose in Maine, but only twice the highest daily dose) respectively. Cytotoxicity was found for all treatments (Figure 1). Survival rates were 84 ± 13 %, 69 ± 15%, 58 ± 14% and 58 ± 16% respectively.
Figure 1.
Cytotoxicity of SRM 1648 (NIST UPM) in WTHBF-6 cells. All treatments were significantly different (p < 0.05) from one another except versus 100 ug/cm2. Data represent an average of three or more experiments ± standard error of the mean.
To compare the cytotoxicity of the particles with the cytotoxicity of the metals extracted from the particles, the cells were treated with metal ion solutions (Table 2). The concentrations used were equal to the solution concentrations expected after treatment with the specified amounts of solid.
Table 2.
Medium Concentrations in NIST metal solutions (ug/ml)
| Mn | Cd | V | Ni | Cu | Zn | Pb | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Treatment | acid | bio | acid | bio | acid | bio | acid | bio | acid | bio | acid | bio | acid | bio |
| 10 µg/cm2 | 0.0252 | 0.0025 | 0.003 | 0.0006 | 0.0042 | 0.0008 | 0.0042 | 0.0008 | 0.023 | 0.0046 | 0.21 | 0.041 | 0.21 | 0.0046 |
| 50 µg/cm2 | 0.126 | 0.013 | 0.015 | 0.003 | 0.021 | 0.0042 | 0.021 | 0.0042 | 0.12 | 0.023 | 1 | 0.21 | 1 | 0.023 |
| 100 µg/cm2 | 0.252 | 0.025 | 0.029 | 0.006 | 0.042 | 0.0084 | 0.042 | 0.0084 | 0.23 | 0.046 | 2 | 0.41 | 2.1 | 0.046 |
| 200 µg/cm2 | 0.504 | 0.05 | 0.058 | 0.012 | 0.084 | 0.0168 | 0.084 | 0.0168 | 0.46 | 0.092 | 4.1 | 0.82 | 4.2 | 0.092 |
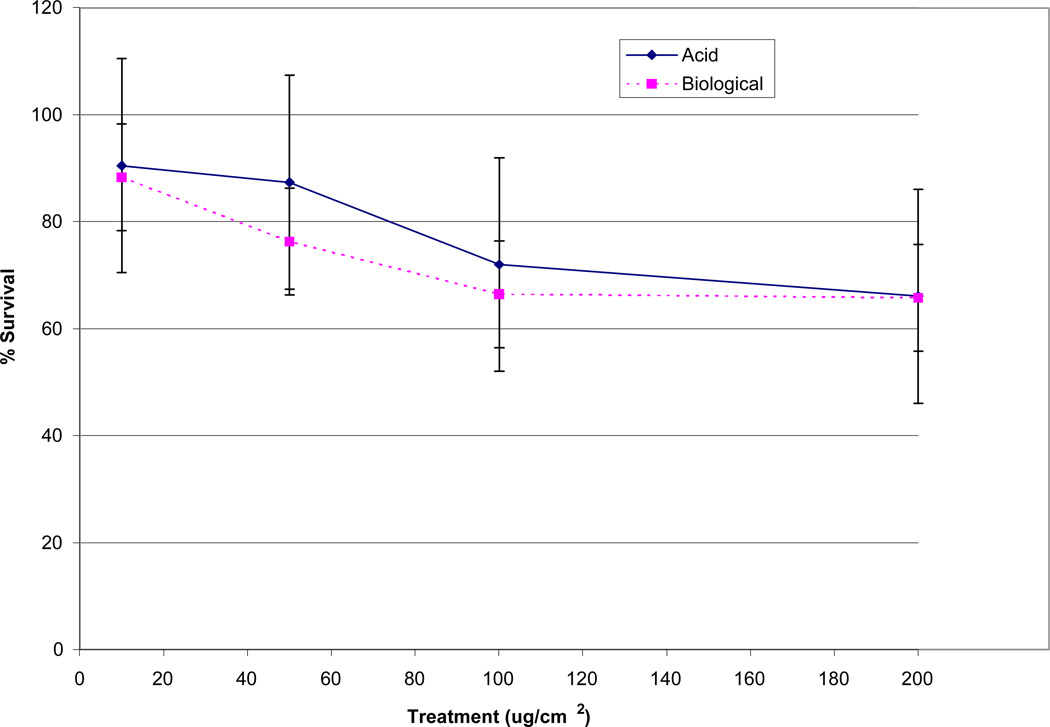
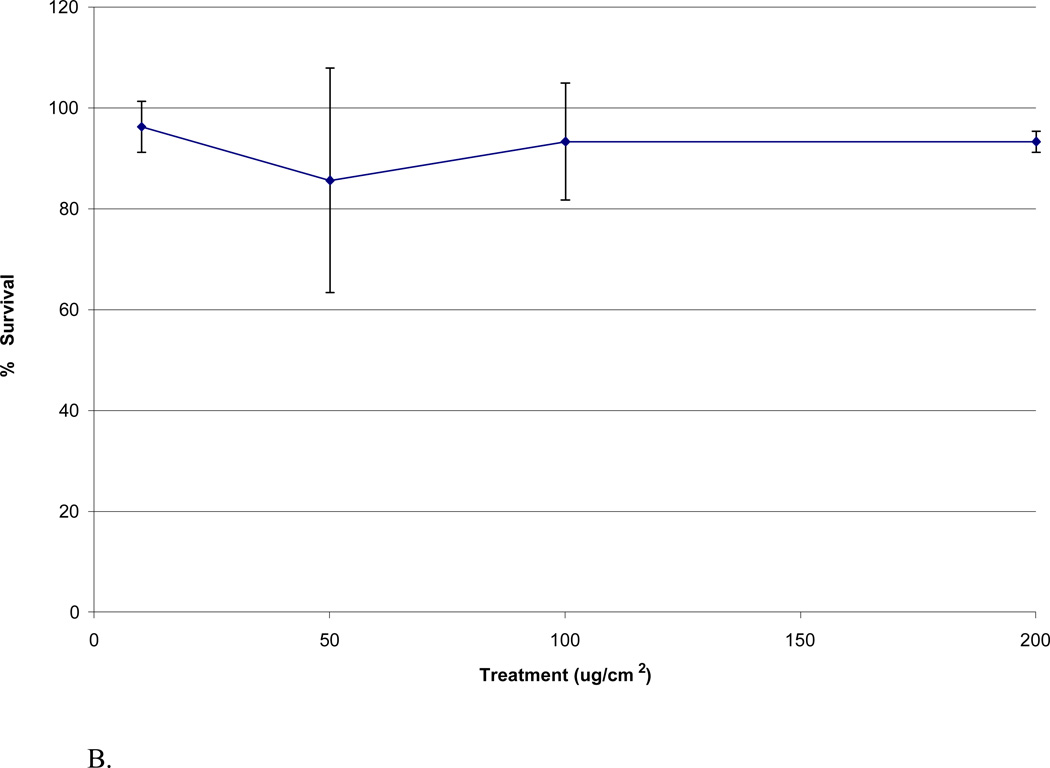
Both the acid and biological solutions induced concentration dependent cytotoxicity in WTHBF-6 cells (Figure 2). The acid metal solutions corresponding to the 10, 50, 100, and 200 µg/cm2 treatments induced 91 ± 11%, 87 ± 10%, 72 ± 18%, and 66 ± 20% relative survival, respectively. The biological metal solutions corresponding to the same amounts of NIST UPM induced 88 ± 14%, 76 ± 14%, 66 ± 13%, and 65 ± 15% relative survival, respectively (Fig 2).
Fig 2.
Cytotoxicity of acid and biological solutions in WTHBF-6 cells. Data represent an average of three or more experiments ± standard error of the mean.
Maine Metals Cytotoxicity
Detailed metal analysis results for Maine particulate matter (PM10) are reported in Langley-Turnbaugh et al., (2005). In that study we showed that lead was not soluble in the cell culture medium, cadmium levels were low, and we were unable to analyze the Maine particulate matter for zinc. Consequently in this study, we focused on mixtures containing the five metals we could measure, As, Mn, Cu, Ni, and V. In the Maine particles these metals are almost equally soluble in both the acid and biological extracting media, and combined they comprise less than 0.3% of the particle weight. The amounts of metals used for treatment are those that would be found in solution from an average winter particle in Portland (Table 3). Composition of the average winter particle would be As 100 µg/g, Mn 300 µg/g, Cu 1200 µg/g, Ni 200 µg/g and V 500 µg/g.
Table 3.
Soluble metal treatment from Maine PM10 (ug/mL) (medium concentrations)
| Particle treatment |
As | Mn | Cu | Ni | V |
|---|---|---|---|---|---|
| 10 ug/cm2 | 0.004 | 0.01 | 0.05 | 0.01 | 0.02 |
| 50 ug/cm2 | 0.02 | 0.07 | 0.26 | 0.04 | 0.11 |
| 100 ug/cm2 | 0.04 | 0.13 | 0.53 | 0.09 | 0.22 |
| 200 ug/cm2 | 0.09 | 0.26 | 1.06 | 0.18 | 0.44 |
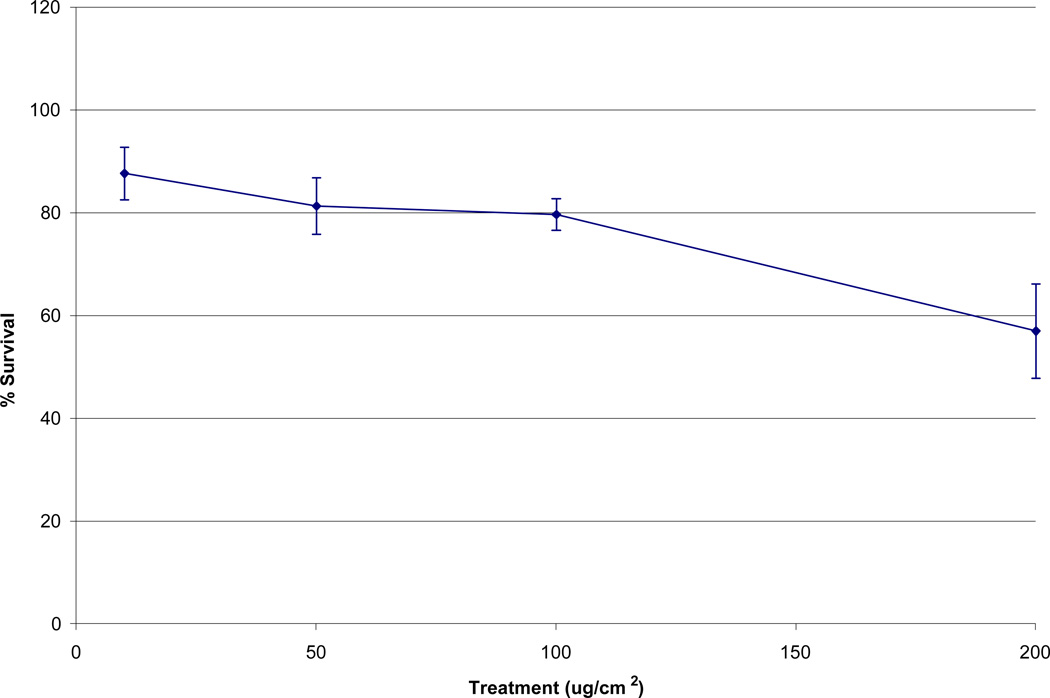
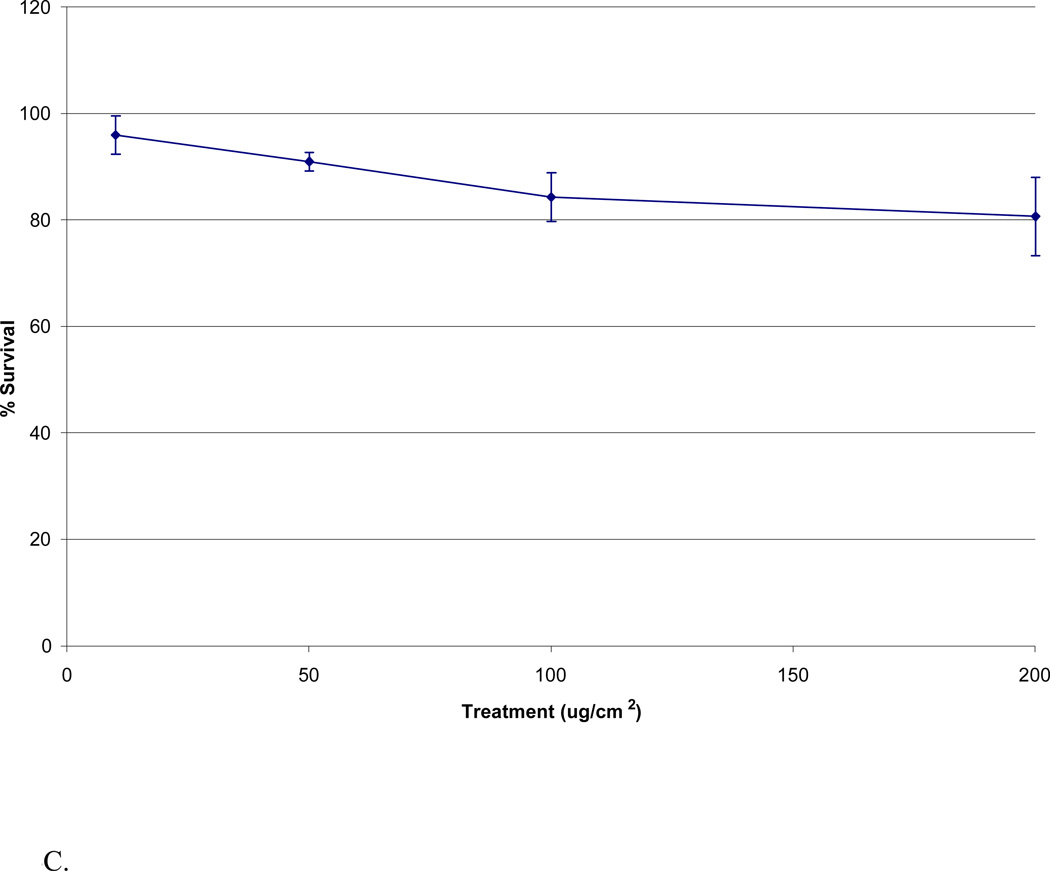
The Maine metals solution induced concentration-dependent cytotoxicity in WTHBF-6 cells. Maine metals solutions which correspond to treatment 10, 50, 100, and 200 ug/cm2 of particulate matter induced 88% ± 5, 81% ± 5, 79% ± 3, and 57% ± 9, relative survival, respectively (Fig 3).
Fig 3.
Cytotoxicity of Maine metals solution in WTHBF-6 cells. Data represent an average of three or more experiments ± standard error of the mean.
Individual Metal Cytotoxicities
To determine which metal or metals are responsible for the cytotoxicity the individual metals were tested at the levels shown for the individual metal in Table 3. As arsenic cytotoxicity was determined in another study with these cells, the results are not shown here (L. Savery, personal communication). Work with nickel with similar cells had shown that much higher concentrations were needed for an effect and its cytotoxicity was not determined in this work.
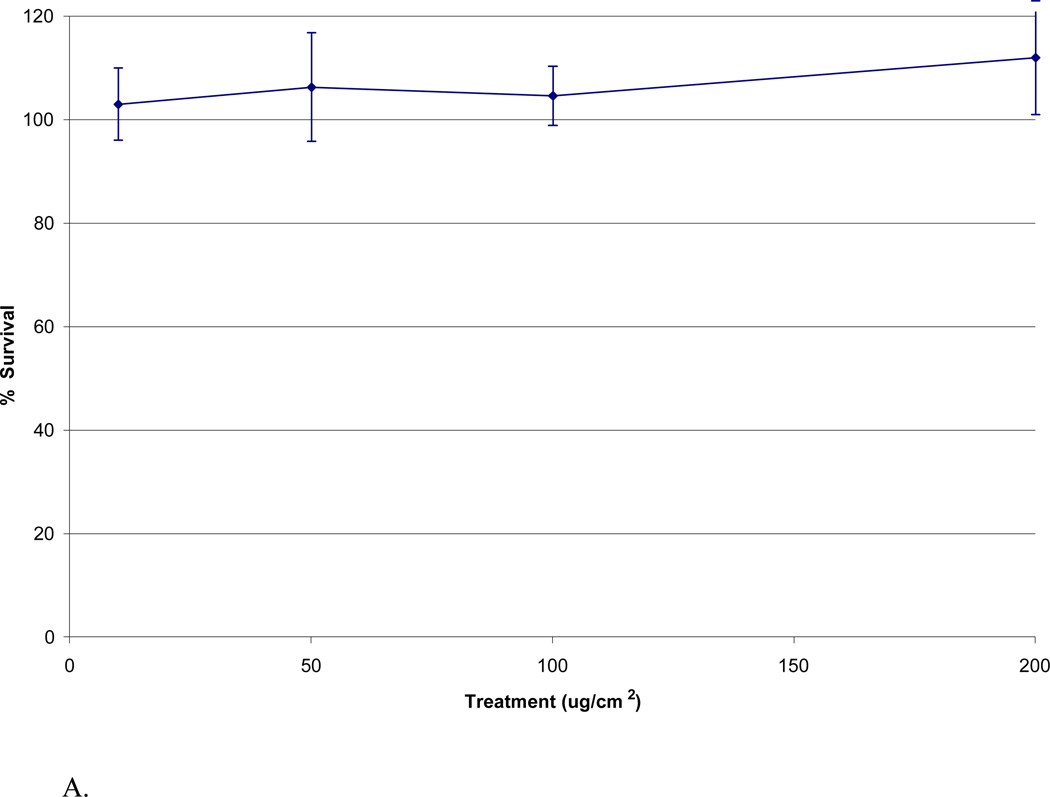
Copper did not induce cytotoxicity; instead it increased survival in a nearly concentration-dependent manner in WTHBF-6 cells. Copper concentrations in solution corresponding to treatments of 10, 50, 100, and 200 ug/cm2 particulate matter (.05, .26, .53, and 1.06 µg/mL respectively) induced 103% ± 7, 106% ± 11, 104% ± 6 and 112% ± 11 relative survival, respectively (Fig 4A).
Fig 4.
Cytotoxicities of Cu, Mn, and V in WTHBF-6 cells. WTHBF-6 cells were treated with solutions of individual metals for 24 h. Data represents an average of three or more experiments ± standard error of the mean. A: Cu induced cytotoxicity in WTHBF-6 cells. B: Mn induced cytotoxicity in WTHBF-6 cells. C: V induced cytotoxicity in WTHBF-6 cells.
Manganese induced a slight cytotoxicity which was not concentration-dependent in WTHBF-6 cells. Manganese concentrations in solution corresponding to treatments of 10, 50, 100, and 200 ug/cm2 particulate matter (.01, .07, .13 and .26 µg/mL respectively) induced 96% ± 5, 86% ± 22, 93% ± 12, and 93% ± 2 relative survival, respectively (Fig 4B).
Vanadium induced concentration-dependent cytotoxicity in WTHBF-6 cells compared to controls. Vanadium concentrations in solution corresponding to treatments of 10, 50, 100, and 200 ug/cm2 particulate matter (.02, .11, .22, and .44 µg/mL respectively) induced 94% ± 5, 88% ± 2, 81% ± 5, and 77% ± 3 relative survival, respectively (Fig 4C).
In very preliminary work the WTHBF-6 cells were again treated with the mixture of Maine metals for 24 hours and the growth medium analyzed for cytokines using a RayBio Human Cytokine Antibody Array Kit. Results indicated a cytokine response but further work is needed.
DISCUSSION
The WTHBF-6 cells used for treatment with the NIST UPM and showed cytotoxicity for all treatments with levels which range from the average daily exposure of a Maine resident to 2 times the highest exposure level. However, because the particles had not been prewashed, we do not know if the effect was due to the particles, a soluble component from the particles or some combination of both. An earlier study had shown that washed particles from Baltimore particulate matter produced an adverse effect in mouse lungs but the leachate did not (Walters et al., 2001). However, in addition to the difference between cell culture and mouse lungs, the particle composition in the Baltimore study was quite different from the NIST UPM and Maine particulate matter (PM10) (Table 4). Other studies in rats showed adverse responses from soluble metal mixtures alone (Dreher et al., 1997; Lambert et al., 1999; Campen et al., 2002)
Table 4.
Comparison of DI soluble metals from PM (ug/g)
| Source | Cu | Ni | As | V | Pb | Mn |
|---|---|---|---|---|---|---|
| World Trade Center(1) | 6.4 | 4.1 | 1.1 | 0.7 | 1.8 | |
| ROFA(1) | 573 | 17030 | 1.7 | 1749 | 17.9 | 365 |
| ROFA(2) | 230 | 35000 | 35000 | |||
| SRM 1648(1) | 83.6 | 32.2 | 13.5 | |||
| SRM 1648(2) | 120 | 40 | 30 | |||
| SRM 1648(3) | 140 | 17 | 17 | 117 | 58 | |
| Portland(3) | 1200 | 200 | 100 | 500 | *ns | 300 |
| Baltimore(4) | 1444 | 68 | 7 | 84 | 231 | 1220 |
| Dusseldorf(2) | 84 | 120 | 60 | |||
| Ottawa(2) | 160 | 90 | 200 | |||
| SRM 1649(2) | 40 | 20 | 150 |
not soluble
this work (medium extracted)
In this work when soluble metal mixtures of both NIST UPM acid extract and the biological extracts were used to treat the WTHBF-6 cells there was a cytotoxic effect but less cytotoxicity than when treated with particles. However, there was little difference in survival rates between the two types of metal solutions although all metals in the acid metals solution are at least a factor of 5 more concentrated than those in the biological metals solution. The only individual metal from this mixture which had been previously tested on these cells was lead and the lead treatment levels that showed a cytotoxic effect occurred at 10 µg/mL which is much higher than the levels used in this study (4.2 µg/mL highest) (Holmes et al., 2005). Lead was a substantial fraction of the acid metal extract, but was still below the effective level so it is likely other metals or the combination of lead with other metals are causing the effect. Thus, even though the soluble metal fraction from the NIST UPM was very small it appears to have an important effect on the survival of the WTHBF-6 cells.
In general, the metals in the Maine particles are more soluble than those from particulate matter from other places, but again these metals constitute a very small fraction <0.3%, of the particle weight (Table 4). The Maine particles had concentrations of vanadium and nickel that were much higher than most locations but several orders of magnitude less than those found in the ROFA (residual oil fly ash) extracts. Comparison between the NIST UPM acid metal solution and the Maine metal solution shows that Mn in the solutions was at similar levels, Cu and Ni were lower in the NIST UPM solution and the highest V level in the acid NIST UPM treatment was equivalent to the 50 µg/cm2 level in the Maine metal solution (Tables 2 and 3). There was no As in the NIST UPM solutions and no Pb, Zn or Cd in the Maine metals. No further work was done with Cd or Pb as they were not found in significant amounts in Maine particulate matter and furthermore, the lead in the Maine particulate matter was not soluble in cell culture medium, and we were unable to obtain meaningful results for Zn in the Maine particulate analyses (Langley-Turnbaugh et al., 2006).
Our work showed that copper appeared to enhance the cell growth, manganese had no effect, but vanadium had a cytotoxic effect at all treatment levels. The vanadium results agree with cytotoxicity results found with human lung epithelial cells where cells were treated with V2O5 at 10, 50 and 100 µg/cm2. The solid V2O5 treatments of 50 and 100 µg/cm2 dissolved to give levels of soluble vanadium that were higher than any of our treatments (Zhang et al., 2001). These effects of V are contradictory to the study in mice lungs (Walters et al., 2001), but V levels were much higher in the V2O5 studies and ours. In ROFA studies vanadium levels were higher still and toxic results were seen in whole animals. (Dreher et al., 1997; Lambert et al., 1999; Campen et al., 2002) The cytotoxicity of arsenic has been studied with WTHBF-6 cells and results showed about an 80% survival rate at the highest treatment levels for our study (L. Savery, personal communication). Ni had been studied with human lung epithelial cells and again levels which produced an effect were much higher (at least 5 mg/mL) compared to those used in our studies (0.18 µg/mL highest) (Patierno 1993, Karaczyn 2005). Based on our results, it would appear that the V and As were the soluble metals that adversely affected the cells. However, neither metal by itself was as cytotoxic as the Maine metals solution, and we have no information on their combined effects or effects in combination with any other metals in this study. Cytotoxicity of vanadium is not surprising as vanadium has been reported to form a protein tyrosine phosphatase inhibitor which alters gene expression patterns leading to apoptosis. It is also known to stimulate other cell types to cause fibroblast growth, but it is not know if the vanadium reaches the fibroblasts as well as the signaling molecules from other vanadium exposed cell types (Bonner, 2007). But, vanadium has been found in the lungs of rats exposed to concentrated urban particulate matter (Morishita et al., 2004). Arsenic, a well known poison, tends to react with thiol containing molecules and may also be a phosphokinase inhibitor (Chen 2002). Both metals affect the immune system (Cassarett and Doull, 2001).
Although a cytotoxic effect in WTHBF-6 cells is an indicator that these metals might exacerbate an asthma episode, the substance or mixture of substances does not necessarily need to kill a lung cell, but only irritate it to produce an adverse effect. Studies have shown the predictive ability of cytotoxicity in fibroblasts for generalized lung, skin and mucous membrane irritation by the same substance (Reinhardt 1985) Cells which are treated or exposed to an irritant often produce cytokines as signals to the immune system that there is a foreign potentially damaging substance present. Our brief work with the RayBio Human Cytokine Antibody Array Kit suggests that the Maine metal solution may be an irritant.
CONCLUSIONS
NIST UPM particles produce a cytotoxic effect in WTHBF-6 cells, and soluble metals from the particles seem to be a major contributor to the effect. Furthermore, a cytotoxic effect was found at all treatment levels for the Maine metals solution, and vanadium and arsenic seem to be the most likely metals to cause this effect. However, further work will involve combination experiments and growth experiments as well as cell culture using human epithelial cells. Cellular irritation by soluble metals also seems likely but further work on cytokine detection is needed.
ACKNOWLEDGEMENTS
This research was supported, in part, by a grant from the Maine Space Grant Consortium and NIEHS Grant number ES010838. D.A., G.V. and A.J. wish to thank the Center for Toxicology and Environmental Health at USM for the assistance with cell culture.
REFERENCES
- Adamson IYR, Prieditis H, Hedgecock C, Vincent R. Zinc is the toxic factor in the Lung Response to an Atmospheric Particulate Sample. Toxicol. App. Pharmocol. 2000;166:111–119. doi: 10.1006/taap.2000.8955. [DOI] [PubMed] [Google Scholar]
- American Lung Association of Maine. Asthma; Introduction, Outdoor Air Quality. 2006 http://www.mainelung.org/ [Google Scholar]
- Batra V, Musani AL, Hastie AT, Khurana S, Carpenter KA, Zangrilli JG, Peters SP. Bronchoalveolar lavage fluid concentrations of transforming growth factor (TGF) –β1, TGF – β2, interleukin (IL)-4 and IL-13 after segmental allergen challenge and their effects on ά-smooth muscle actin and collagen III synthesis by primary human lung fibroblasts. Clin Exp Allergy. 2004;34:437–444. doi: 10.1111/j.1365-2222.2004.01885.x. [DOI] [PubMed] [Google Scholar]
- Becker S, Fenton MJ, Soukup JM. Involvement of Microbial Components and Toll-like Receptors 2 and 4 in Cytokine Responses to Air Pollution Particles. Am J. Respir. Cell Mol. Biol. 2002;27:611–618. doi: 10.1165/rcmb.4868. [DOI] [PubMed] [Google Scholar]
- Bearer C. How are Children Different From Adults? Env. Health Pers. Supp. 1995;103(6):7–13. doi: 10.1289/ehp.95103s67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner J. Lung fibrotic responses to particle exposure. Toxicologic Pathology. 2007;35:148–153. doi: 10.1080/01926230601060009. [DOI] [PubMed] [Google Scholar]
- Campen MJ, Nolan JP, Schladweiler MCJ, Kodavanti UP, Costa DL, Watkinson WP. Cardiac and Thermoregulatory Effects of Instilled Particulate Matter-associated Transition Metals in Healthy and Cardiopulmonary-compromised Rats. J. Tox. Environ. Health. 2002;65:1615–1631. doi: 10.1080/00984100290071694. [DOI] [PubMed] [Google Scholar]
- CDC (Center for Disease Control) Trends in Asthma Morbidity and Mortality. 2001 [Google Scholar]
- Chen F, Shi X. Intracellular signal transduction of cell in response to carcinogenic metals. Crit. Rev. Onc /Hematology. 2000;42:105–121. doi: 10.1016/s1040-8428(01)00211-6. [DOI] [PubMed] [Google Scholar]
- Claiborn CS, Larson T, Sheppard L. Testing the Metals Hypothesis in Spokane, Washington. Environ. Health Perspect. 2002;110(supp 4):547–552. doi: 10.1289/ehp.02110s4547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa DL, Dreher KL. Bioavailable Transition Metals in Particulate Matter Mediate Cardiopulmonary Injury in Healthy and Compromised Animal Models. Environ. Health Perspec. 1997;105(supp 5):1053–1060. doi: 10.1289/ehp.97105s51053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeFlora S, Bagnasco M, Serra D, Zanacchi P. Genotoxicity of chromium compounds. A review. Mutat Res. 1990;238:99–172. doi: 10.1016/0165-1110(90)90007-x. [DOI] [PubMed] [Google Scholar]
- Doucet C, Brouty-Boye D, Pottin-Clemenceau C, Canonica GW, Jasmin C, Azzarone B. Interleukin (IL) 4 and IL-13 act on Human Lung Fibroblasts, Implication in Asthma. J. Clin Invest. 1998;101:2129–2139. doi: 10.1172/JCI741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreher KL, Jaskot RH, Lehmann JR, Richards JH, Ghio AJ, Costa DL. Soluble Transition Metals Mediate Residual Oil Fly Ash Induced Acute Lung Injury. J. Toxicol. Environ. Health. 1997;50:285–305. [PubMed] [Google Scholar]
- Eggleston PA, Buckley TJ, Breysse PN, Wills-Karp M, Kleeberger SR, Jaakola JJK. The Environment and Asthma in US Inner Cities. Environ. Health Perspect. 1999;107:439–450. doi: 10.1289/ehp.99107s3439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EPA. Office of Air Quality, Planning and Standards. North Carolina: Research Triangle Park; 2000. National Air Pollution Emission Trends 1900–1998. EPA-454/R00-002. [Google Scholar]
- EPA. Washington, D.C.: Office of Research and Development; 2004. Particulate Matter Research Program – 5 years of progress. http://www.epa.gov/pmresearch/pm_research_accomplishments/pdf/pm_research_program_five_years_of_progress.pdf. [Google Scholar]
- Gardner DE, Crapo JD, McClellan RO. Toxicology of the Lung. 3rd Ed. Taylor and Francis: 1999. [Google Scholar]
- Holmes AL, Wise SS, Xie H, Gordon N, Thompson WD, Wise JP., Sr Lead Ions Do Not Cause Human Lung Cells to Escape Chromate-Induced Cytotoxicity. Toxicology and Applied Pharmacology. 2005;203:167–176. doi: 10.1016/j.taap.2004.08.006. [DOI] [PubMed] [Google Scholar]
- Holmes AL, Wise SS, Sandwick SJ, Lingle WL, Negron VC, Thompson WD, Wise JP., Sr Chronic exposure to lead chromate causes centrosome abnormalities and aneuploidy in human lung cells. Cancer Research. 2006;66(8):4041–4048. doi: 10.1158/0008-5472.CAN-05-3312. [DOI] [PubMed] [Google Scholar]
- Hussain SP, Amstad P, Raja K, Ambs S, Nagashima M, Bennett WP, Shields PG, Ham A-J, Swenberg JA, Marrogi AJ, Harris CC. Increased p53 mutation load in noncancerous colon tissue from ulcerative colitis: A cancer-prone chronic inflammatory disease. Cancer Res. 2000;60:3333–3337. [PubMed] [Google Scholar]
- Karaczyn A, Golebiowske F, Kasprzak KS. Truncation, Demaidation and Oxidation of Histone H2B in Cells Cultured with Nickel(II) Chem. Res. Toxicol. 2005;18:1934–1942. doi: 10.1021/tx050122a. [DOI] [PubMed] [Google Scholar]
- Kondo K, Takahashi Y, Ishikawa S, Uchihara H, Hirose Y, Yoshizawa K, Tsuyuguchi M, Takizawa H, Miyoshi T, Sakiyama S, Monden Y. Microscopic analysis of chromium accumulation in the bronchi and lung of chromate workers. Cancer. 2003;98:2420–2429. doi: 10.1002/cncr.11818. [DOI] [PubMed] [Google Scholar]
- Lambert AL, Dong W, Winsett DW, Selgrade MJK, Gilmour MI. Residual Oil Fly Ash Exposure Enhances Allergic Sensitization to House Dust Mite. Toxicol. Appl. Pharmacol. 1999;158:269–277. doi: 10.1006/taap.1999.8709. [DOI] [PubMed] [Google Scholar]
- Langley-Turnbaugh SJ, Gordon NR, Lambert T. Airborne Particulates and Asthma: A Maine Case Study. Toxicology and Industrial Health. 2005;21:1–18. doi: 10.1191/0748233705th218oa. [DOI] [PubMed] [Google Scholar]
- Leikauf GD. Hazardous Air Pollutants and Asthma. Environ. Health Perspect. 2002;110:505–526. doi: 10.1289/ehp.02110s4505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin M, Chen Y, Burnett RT, Villeneuve PJ, Krewski D. The Influence of Ambient Coarse Particulate Matter on Asthma Hospitalization in Children: Case-crossover and Time-series Analysis. Environ. Health Perspec. 2002;110:575–581. doi: 10.1289/ehp.02110575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGee JK, Chen LC, Cohen MD, Chee GR, Prophete CM, Haykal-Coates N, Wasson SJ, Conner TL, Costa DL, Gavett SH. Chemical Analysis of World Trade Center Fine Particulate Matter for Use in Toxicologic Assessment. Env. Health Persp. 2003;111:1–11. doi: 10.1289/ehp.5930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moody JL, Munger J, Goldstein A, Jacob D, Wofsy S. Harvard Forest Regional Scale Air Mass Composition by Patterns in Atmospheric Transport History (PATH) J. Geophys. Res. 1998;103:13181–13194. [Google Scholar]
- Morishita M, Keeler GJ, Wagner JG, Marsik FJ, Timm EJ, Dvonch JT, Harkema Pulmonary retention of particulate matter is associated with airway inflammation in allergic rats exposed to air pollution in urban Detroit. Inhalation Toxicology. 2004;16:663–674. doi: 10.1080/08958370490476550. [DOI] [PubMed] [Google Scholar]
- NEARC. Asthma in New England Special Report. 2004 http://www.asthmaregionalcouncil.org/documents/AsthmainNewEngland_000.DOC. [Google Scholar]
- Oddera S, Cagnoni F, Mangraviti S, Giron-Michel J, Popova O, Canonica GW. Effects of Triamcinolone Acetonide on Adult Human Lung Fibroblasts: Decrease in Proliferation, Surface Molecule Expression and Mediator Release. Int. Arch Allergy Immunol. 2002;129:152–159. doi: 10.1159/000065877. [DOI] [PubMed] [Google Scholar]
- Ondov JM, Buckley TJ, Hopke PK, Parlange MB, Rogge WF, Squibb KS, Wexler AS. Baltimore Supersite: Highly Time and Size Resolved Concentrations of Urban PM2.5 and its Constituents for Resolution of Sources and Immune Responses. 2005 http://www.epa.gov/ttn/amtic/files/ambient/super/baltfin.pdf.
- Ostro B, Lipsett M, Mann J, Braxton-Owens H, White M. Air Pollution and Exacerbation of Asthma in African-American Children in Los Angeles. Epidemiology. 2001;12:200–208. doi: 10.1097/00001648-200103000-00012. [DOI] [PubMed] [Google Scholar]
- Patierno SA, Dirscherl LA, Xu J. Transformation of rat tracheal epithelial cells to immortal growth variants by particulate and soluble nickel compounds. Mut. Res. 1993;300:179–193. doi: 10.1016/0165-1218(93)90049-j. [DOI] [PubMed] [Google Scholar]
- Reinhardt CA, Pelli DA, Zbinden G. Interpretation of cell toxicity data for the estimation of potential irritation. Food Chem. Toxicol. 1985;23:247–252. doi: 10.1016/0278-6915(85)90024-9. [DOI] [PubMed] [Google Scholar]
- Rubin H. Selected cell and selective microenvironment in neoplastic development. Cancer Res. 2001;61:799–807. [PubMed] [Google Scholar]
- Schwartz J, Laden F, Zanobetti A. The Concentration-Response between PM2.5 and Daily Deaths. Env. Health Persp. 2002;110:1–8. doi: 10.1289/ehp.021101025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soukup JM, Becker S. Human Alveolar Macrophange Responses to Air Pollution Particulates are Associated with Insoluble Components of Coarse Material, Including Particulate Endotoxin. Toxicol. Appl. Pharmacol. 2001;171:20–26. doi: 10.1006/taap.2000.9096. [DOI] [PubMed] [Google Scholar]
- Stringer B, Kobzik L. Environmental Particulate Mediated Cytokine Production in Lung Epithelial Cells (A549): Role of Preexisting Inflammation and Oxidant Stress. J Toxicol. Environ. Health. 1998;55:31–44. doi: 10.1080/009841098158601. [DOI] [PubMed] [Google Scholar]
- Stringer B, Imrich A, Kabzik L. Lung Epithelial Cell (A549) Interaction with Unopsonized Environmental Particulates: Quantitation of Particle-Specific Binding and IL-8 Production. Exp. Lung Res. 1996;22:495–508. doi: 10.3109/01902149609046038. [DOI] [PubMed] [Google Scholar]
- Tolbert E, Klein M, Metzger KB, Peel J, Flanders WD, Todd K, Mulholland JA, Ryan PB, Frumkin H. Interim Results of the Study of Particulates and Health in Atlanta (SOPHIA) J. Expo. Anal. Environ. Epidemiol. 2000;10:446–446. doi: 10.1038/sj.jea.7500106. [DOI] [PubMed] [Google Scholar]
- Walters DM, Breyesse PN, Wills-Karp M. Ambient Urban Baltimore Particulate-induced Airway Hyperresponsiveness and Inflammation in Mice. Am. J. Respir. Crit. Care Med. 2001;164:1438–1443. doi: 10.1164/ajrccm.164.8.2007121. [DOI] [PubMed] [Google Scholar]
- Wise JP, Sr, Wise SS, Little JE. The cytotoxicity and genotoxocity of particulate and soluble hexavalent chromium in human lung cells. Mutat. Res. 2002;517:221–229. doi: 10.1016/s1383-5718(02)00071-2. [DOI] [PubMed] [Google Scholar]
- Wise SS, Elmore LW, Holt SE, Little JE, Antonucci PG, Bryant BH, Wise JP., Sr Telomerase-mediated lifespan extension of human bronchial cells does not affect hexavalent chromium-induced cytotoxicity or genotoxicity. Mol. Cell. Biochem. 2004;255:103–111. doi: 10.1023/b:mcbi.0000007266.82705.d9. [DOI] [PubMed] [Google Scholar]
- Xie H, Holmes AL, Wise SS, Gordon N, Wise JP., Sr Lead chromate-induced chromosome damage requires extracellular dissolution to liberate chromium ions but does not require particle internalization or intracellular dissolution. Chem. Res. Toxicol. 2004;17(10):1362–1367. doi: 10.1021/tx0498509. [DOI] [PubMed] [Google Scholar]
- Zagai U, Skold CM, Trulson A, Venge P, Lundahl J. The effect of eosinophils on collagen gel contraction and implications for tissue remodeling. Clin. Exp. Immunol. 2004;135:427–433. doi: 10.1111/j.1365-2249.2004.02396.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Rice AB, Adler K, Sannes P, Martin L, Gladwell W, Koo JS, Gray TE, Bonner JC. Vanadium Stimulates Human Bronchial Epithelial Cells to Produce Heparin-Binding Dpidermal Growth Factor-Like Growth Factor: A Mitogen for Lung Fibroblasts. Am J Respir. Cell Mol Biol. 2001;24:123–131. doi: 10.1165/ajrcmb.24.2.4096. [DOI] [PubMed] [Google Scholar]