Abstract
Arteriovenous malformations (AVMs) of the brain are rare, complex, vascular lesions that can result in significant morbidity and mortality. Modern treatment of brain AVMs is a multimodality endeavor, requiring a multidisciplinary team with expertise in cerebrovascular neurosurgery, endovascular intervention, and radiation therapy in order to provide all therapeutic options and determine the most appropriate treatment regimen depending on patient characteristics and AVM morphology. Current therapeutic options include microsurgical resection, radiosurgery (focused radiation), and endovascular embolization. Endovascular embolization is primarily used as a preoperative adjuvant before microsurgery or radiosurgery. Palliative embolization has been used successfully to reduce the risk of hemorrhage, alleviate clinical symptoms, and preserve or improve neurological function in inoperable or nonradiosurgical AVMs. Less frequently, embolization is used as ‘primary therapy’ particularly for smaller, surgically difficult lesions. Current embolic agents used to treat brain AVMs include both solid and liquid agents. Liquid agents including N-butyl cyanoacrylate and Onyx are the most commonly used agents. As newer embolic agents become available and as microcatheter technology improves, the role of endovascular treatment for brain AVMs will likely expand.
Key Words : Arteriovenous malformations, Embolization, Endovascular treatment, Microcatheters, Multimodality treatment
Introduction
Arteriovenous malformations (AVMs) of the brain are rare, complex, vascular lesions that can result in significant morbidity and mortality. Current therapeutic modalities include microsurgical resection, radiosurgery (focused radiation), and endovascular embolization [1]. Endovascular embolization is primarily used as a preoperative adjuvant before microsurgery or radiosurgery. Endovascular treatment is directed at particularly ‘weak points’ within the AVM, including flow-related aneurysms and high-flow fistulae, with the aim to decrease lesion volume and establish more normal blood flow patterns within surrounding brain parenchyma [2]. In cases of nonsurgical or nonradiosurgical AVMs, palliative embolization has been used successfully to reduce the risk of hemorrhage, alleviate clinical symptoms, and preserve or improve neurological function [3,4,5,6,7,8]. Less frequently, embolization is used as ‘primary therapy’ particularly for smaller, surgically difficult lesions. Treatment planning for AVMs requires a multidisciplinary team with expertise in cerebrovascular neurosurgery, endovascular intervention, and radiation therapy in order to provide all therapeutic options and determine the most appropriate treatment regimen according to patient characteristics and AVM morphology.
Definition/Pathogenesis
Brain AVMs are focal, intraparenchymal conglomerations of dilated arteries and veins which lack normal vascular organization at the subarteriolar level as well as a normal capillary bed resulting in direct connections between the cerebral arterial and venous systems [9,10]. The nidus, the intervening network of vessels between the distal aspects of the arterial feeders and the proximal aspects of the draining veins, is the primary target of embolization. On angiography, brain AVMs demonstrate arteriovenous (AV) shunting resulting in early opacification of the draining vein(s) and shortened AV transit time. Brain AVMs are classified as superficial or deep types. Superficial AVMs are further divided into sulcal, gyral, or mixed, while deep types, which are relatively rare, are subdivided into subarachnoid, deep parenchymal, plexal, and mixed types [11]. Brain AVMs are likely congenital vascular lesions that result from the failure of capillary formation during embryonic development [12]. However, cases of both de novo development in an adult and recurrent disease in children have been reported [13,14].
Epidemiology/Clinical Manifestations/Natural History/Grading Systems
Brain AVMs are relatively uncommon with a prevalence <1% and an incidence between 0.01 and 0.001% [15,16,17]. The overwhelming majority of brain AVMs are sporadic, although familial occurrence has been described [18]. An increased prevalence of brain AVMs is seen with certain conditions including Osler-Weber-Rendu disease and Sturge-Weber syndrome [19,20].
The morbidity and mortality of brain AVMs are primarily related to intracranial hemorrhage, mainly intraparenchymal hemorrhage, and, to a lesser degree, subarachnoid and intraventricular hemorrhage [21]. Approximately 45-70% of brain AVMs present with intracranial hemorrhage. Mortality rates after the first hemorrhage range from 10 to 30% and morbidity rates from 25 to 60% [15]. Other less ominous presentations of brain AVMs include seizures, headache, and focal neurological deficits. Venous hypertension, arterial steal, and hydrocephalus are known complications of brain AVMs [17,22].
Brain AVMs are extremely heterogeneous with respect to their angioarchitecture and biological behavior (fig. 1). Hemorrhage risk is related to both angiographic appearance as well as other clinical features. Brain AVMs carry an annual risk of hemorrhage of 1-3% and an annual mortality rate of 1% [16,23]. The risk of rebleeding during the 1st year following initial rupture is as high as 6-17%, declining to baseline thereafter [23,24]. Prognosis is particularly poor for patients with posterior fossa AVMs, with mortality rates of up to 66.7% after the first hemorrhage approaching 100% with recurrent hemorrhage. Risk factors for hemorrhage include previous hemorrhage, deep venous drainage, intraventricular or periventricular location, intranidal aneurysms (fig. 2), feeding by perforators, feeding by the vertebrobasilar system, location in the basal ganglia, occlusive changes in the venous channel(s), and male gender (table 1) [23,25,26,27,28,29]. Pregnancy does not increase the risk of hemorrhage [30]. By all accounts, there is no evidence to suggest that a substantial undiagnosed reservoir of nonsymptomatic brain AVMs exits in the general population. On the contrary, the great majority of brain AVMs will become symptomatic during a patient's lifetime, and the majority will bleed (fig. 3). The risk of hemorrhage is lifelong and rises with age [31].
Fig. 1.
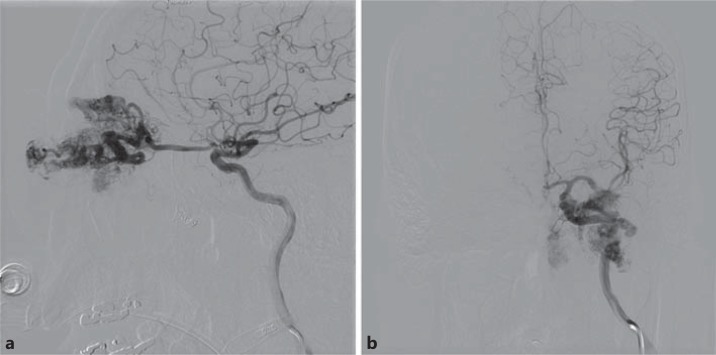
Lateral (a) and anteroposterior view (b) of a 56-year-old male with left eye pain, erythema, and signs of venous congestion secondary to a left ophthalmic artery AVM.
Fig. 2.
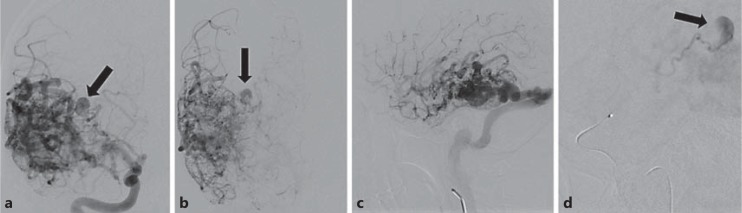
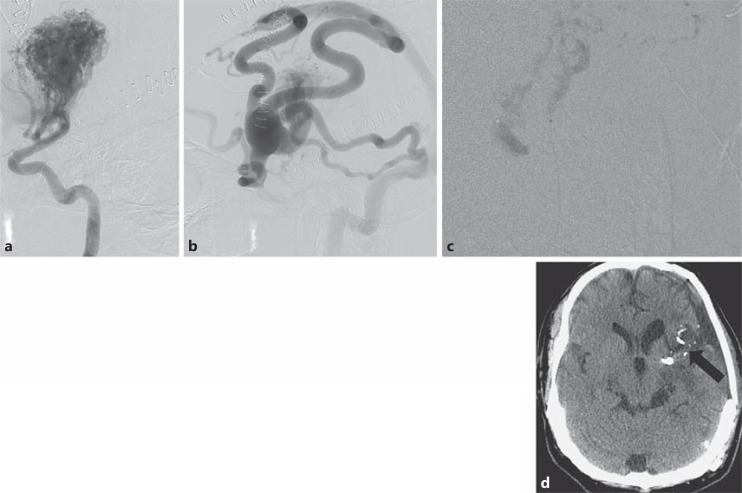
A 28-year-old female with diffuse intraventricular hemorrhage and hydrocephalus secondary to rupture of a posterior right temporal lobe AVM. Intranidal anterior choroidal artery aneurysm (arrows) was identified and felt to be the source of bleeding. a Anteroposterior view, arterial phase. b Anteroposterior view, late arterial phase. c Lateral view. d Superselective angiogram of the anterior choroidal artery.
Table 1.
Risk factors for AVM hemorrhage
| (1) Previous hemorrhage |
| (2) Intranidal aneurysms |
| (3) Intraventricular or periventricular location |
| (4) Deep venous drainage |
| (5) Feeding by perforators |
| (6) Feeding by the vertebrobasilar system |
| (7) Location in the basal ganglia |
| (8) Occlusive changes in the venous channel(s) |
| (9) Male gender |
Fig. 3.
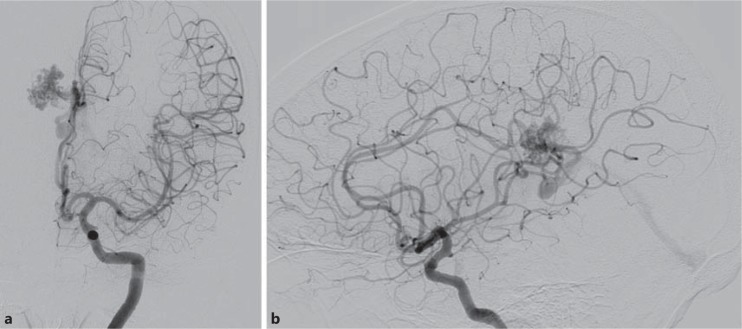
A 65-year-old male presented with intraventricular hemorrhage secondary to right pericallosal splenial region parasagittal AVM. a Anteroposterior view, left internal carotid artery injection. b Lateral view, left internal carotid artery injection. The patient went on to have radiosurgery with complete AVM resolution (images not shown).
The ability to estimate the treatment risk for an individual patient with a brain AVM is extremely difficult as the variability in the complexity of AVMs is so heterogeneous. As a result, numerous grading systems have been developed in order to correlate specific AVM characteristics (anatomic, hemodynamic, and physiological properties) with surgical out-comes in order to define the degree of difficulty involved in safely removing AVMs surgically. The most widely utilized system used to perform a relative surgical risk analysis was reported by Spetzler and Martin [32] in 1986. Their grading system is based on three criteria: AVM size [small (<3 cm), medium (3-6 cm), or large (>6 cm)], pattern of venous drainage (superficial or deep), and neurological eloquence of adjacent brain regions (sensorimotor, language, and visual cortex; hypothalamus and thalamus; internal capsule; brain stem; cerebellar peduncles, and deep cerebellar nuclei are considered eloquent). Points are assigned for each of the aforementioned variables and added for a total score of 1-5 (table 2). Grade I and II lesions are generally considered safe surgical lesions with very low incidence of surgically induced neurological deficits, while grade IV and V lesions are frequently accompanied by significant surgically induced neurological deficits. Grade VI lesions are considered inoperable [32]. A prospective evaluation of the Spetzler-Martin grading system examining 120 patients accurately correlated both new-temporary and new-permanent neurological deficits. Permanent major neurological deficits for grades I-III were 0%, increasing to 21.9 and 16.7% in patients with grade IV and V lesions, respectively [33].
Table 2.
Spetzler-Martin grading system for brain AVMs
| Graded feature | Points assigned |
|---|---|
| Size of AVM | |
| Small (<3 cm) | 1 |
| Medium (3–6 cm) | 2 |
| Large (>6 cm) | 3 |
| Eloquence of adjacent brain | |
| Non-eloquent | 0 |
| Eloquent | 1 |
| Pattern of venous drainage | |
| Superficial only | 0 |
| Deep | 1 |
Total grade = size + eloquence + venous drainage.
Embolization of Brain AVMs
Background
Therapeutic embolization of a brain AVM was first described in 1960 by Luessenhop and Spence [34] utilizing flow-directed steel spheres covered with methyl methacrylate injected directly into a surgically accessed cervical internal carotid artery. This technique relied on the proportionately greater degree of blood flow to the AVM compared with normal cerebral branches to direct the embolic agents into the AVM nidus. Accidental embolization of normal cerebral vessels resulting in cerebral infarction was a potential problem. Additionally, occlusion of proximal arterial feeders could prevent entry into the nidus, leaving the nidus unoccluded and active. This often leads to AVM recruitment of deep perforators making surgery ultimately more challenging [35,36]. In 1974, Serbinenko [37] reported on the use of a detachable balloon attached to a flexible flow-directed catheter. Similar to the problem seen with Silastic spheres, the detachable balloons sometimes occluded the proximal feeder pedicles inducing the nidus to recruit deep perforators making surgical resection more challenging. In 1976, Kerber [38] described the use of a microcatheter with a calibrated-leak balloon to superselectively catheterize the cerebral vasculature. This novel device overcame the problems seen with the use of the diagnostic catheters, i.e. the difficulty in placing the catheter precisely in the desired area and controlling the infusion of the occluding agent. In addition, Kerber used a liquid embolic agent, isobutyl-2-cyanoacrylate. Over the last several decades, the development of smaller microcatheters, microguidewires, and novel embolic materials has led to technical achievements in AVM embolization and solidified its role as an important tool in the treatment of brain AVMs.
Embolization Indications
Although surgical resection remains the standard for the definitive eradication of most brain AVMs, endovascular embolization has the potential to enhance the safety and efficacy of AVM treatment when applied as adjuvant therapy before microsurgery (table 3) [4,5,6,7,8]. Preoperative embolization of AVMs has been shown to reduce operation time and intraoperative blood loss, with no difference in surgical complications or long-term neurological outcome [39]. Pasqualin et al. [40] demonstrated that patients treated with embolization prior to surgery experienced fewer postoperative neurological deficits and fewer deaths, and had a lower incidence of postoperative epilepsy when compared with patients who had surgery alone. Embolization is also capable of converting high Spetzler-Martin grade lesions to lower-grade lesions, thus turning potentially inoperable lesions into operable lesions [41]. Embolization improves surgical outcomes through several mechanisms including the elimination of deep feeding arteries such as the anterior/posterior perforating vessels, choroidal vessels, and posterior cerebral vessels [42], decreasing the size of the active nidus, and the elimination of the feeding pedicle or nidal aneurysms that have either bled or are at risk of rupture [43,44,45]. Presurgical embolization decreases the risk of postsurgical hemorrhage caused by changes in hemodynamics within the surrounding normal brain, and can act as a surgical roadmap as embolized vessels are easily identifiable. Preoperative embolization in conjunction with surgery has also been shown to be cost-effective when compared with surgery alone (fig. 4) [46].
Table 3.
Indications for AVM embolization
| (1)Adjuvant therapy prior to microsurgery |
| (2) Adjuvant therapy prior to radiosurgery |
| (3) Palliative therapy for inoperable lesions |
| (4) Primary therapy for smaller, surgically difficult lesions |
Fig. 4.
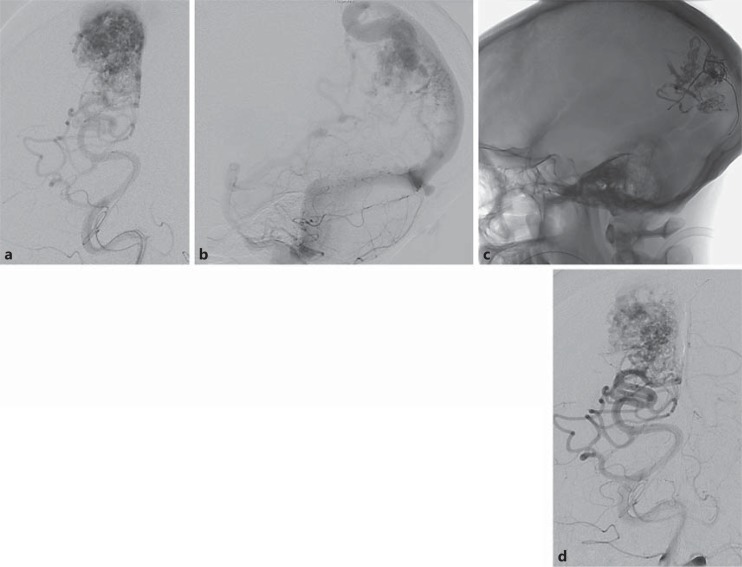
A 39-year-old male presented with SAH related to a large AVM involving the left frontal lobe, left basal ganglia and left temporal lobe. a Lateral view, left ICA injection – arterial phase. b Left ICA injection – venous phase. c Subtracted microcatheter injection of feeding pedicle. d NBCA in nidus (arrow) on unenhanced CT image.
Another potential role of embolization is adjuvant therapy before radiotherapy. This is particularly useful for medium- and large-size AVMs. Embolization in this setting has several theoretical benefits [47]. First, it has been shown to reduce AVM size so that the residual nidus is a smaller target that can be irradiated with a better cure rate and fewer side effects. The cure rate increases with decreasing AVM volume, and smaller lesions (<3 cm in diameter) carry lower rates of morbidity [48]. Preradiosurgical embolization may also be used to occlude arterial feeder or intranidal aneurysms to reduce the risk of bleeding while awaiting the delayed action of radiosurgery, as well as target large high-flow AV fistulas which are less sensitive to radiosurgery [49,50,51]. The risk-benefit ratio must be carefully considered in these cases. Untoward complications of embolization could precipitate the need for surgical extirpation of an inoperable AVM. There is also some evidence that embolization prior to radiosurgery may reduce the efficacy of radiotherapy to cure AVMs. Although the causes are not entirely clear, experienced authors have argued that radiopaque embolic material may ‘shield’ the nidus from adequate radiation absorption or alternatively may activate certain vascular growth factors [52,53]. Embolization has also been shown to be safe and effective in treating residual lesions that persist after radiosurgery. Marks et al. [54] reported on 6 patients who underwent endovascular treatment of their brain AVMs after failing radiosurgery. One patient was cured with embolization alone, 3 patients underwent surgical resection for cure after embolization, and 2 patients went on to have successful repeat radiosurgery following embolization.
Palliative embolization of brain AVMs is typically employed in patients with large, symptomatic but inoperable lesions. The indication for palliative embolization is usually acute hemorrhage. Embolization under these circumstances should be used to treat specific high-risk AVM angioarchitectural features such as aneurysms, or to alleviate specific clinical signs and symptoms related to vascular steal and/or mechanical compression [4,55,56]. Partial embolization may also be beneficial in patients with medically refractory seizures or with progressive neurological deficits thought to be secondary to venous hypertension or arterial ‘steal’ phenomenon causing ischemia. Palliative embolization is not recommended as a broad treatment strategy for inoperable lesions as it does not appear to produce better clinical results than medical management and there is no evidence to suggest that partial AVM embolization alters long-term hemorrhagic risk [4,57,58]. On the contrary, partial embolization has been shown to worsen the clinical course of the patient compared with the natural history of an untreated lesion [59].
Less frequently, embolization is used as ‘primary therapy’, particularly for smaller, surgically difficult AVMs that contain few arterial feeders. Published cure rates in the literature vary considerably secondary to selection bias, goals of treatment, and technique. Although cure rates of up to 40% have been reported in some series [60,61], delayed recanalization seems to be a problem even for smaller AVMs. Furthermore, the AVMs that would have a high probability of cure with embolization are typically amenable to complete surgical resection or radiosurgical cure without the associated risks of embolization [62,63,64]. Deep central lesions with limited feeders are exceptions where embolization can play an important role in cure [65].
Embolic Agents
Current embolic materials used to treat brain AVMs can be divided into solid and liquid agents. Solid agents consist of polyvinyl alcohol (PVA) particles, fibers, coils, and balloons. Liquid agents, which are more commonly used, consist of cyanoacrylate monomers such as N-butyl cyanoacrylate (NBCA), as well as polymeric precipitates in solutions such as ethylene co-vinyl alcohol. Absolute (100% anhydrous) ethanol is an additional liquid embolic agent that is not commonly used now. The principal agents currently in use for treating brain AVMs include NBCA (Trufil, Codman Inc.), Onyx (Covidien, eV3 Neurovascular, Irvine, Calif., USA), a copolymer of ethylene vinyl, and, to a lesser extent, PVA particles and coils. NBCA and Onyx are the most commonly used agents and will be discussed individually.
NBCA
Embolization with cyanoacrylate has evolved tremendously since its introduction nearly 30 years ago because of the development of different adhesive formulations as well as the advancement of catheter and guidewire technologies. Isobutyl-2-cyanoacrylate has been replaced by NBCA, which was approved by the Food and Drug Administration for brain embolization in 2000 because of the more predicable polymerization qualities, ease of surgical resection, and lack of toxicity of NBCA [66]. The use of NBCA for the treatment of brain AVMs is predicted on its liquid character, which allows it to penetrate nidal vessels where it ultimately polymerizes to a solid state, and causes thrombosis and vessel occlusion [67]. NBCA polymerizes into an adhesive, nonbiodegradable solid material upon contact with blood and endothelial cells via an anionic mechanism. It induces an inflammatory response within the walls of embolized vessels that is believed to play an important role in the permanence of the occlusion created with this agent [68].
NBCA is not radiopaque and, thus, must be opacified to monitor its flow during injection (fig. 5). Ethiodol and, to a lesser extent, tantalum powder are mixed with NBCA to make the solution radiopaque. Ethiodol also acts as a retarding agent to slow the polymerization rate as pure NBCA polymerizes almost immediately at the catheter tip. The goal is to create a mixture of glue/ethiodol that will prevent early polymerization within the feeding artery but also late polymerization within the draining vein(s). Concentrations of 25% glue and higher are typically used to achieve nidal penetration. Concentrations below 25% will usually poly-merize too slowly [67]. Due to the importance that mastery of this process represents, there is a learning curve to assure its safe and proper use.
Fig. 5.
A 40-year-old male with a left occipital AVM. a AP view, left vertebral artery injection – arterial phase. b Lateral view, left vertebral artery injection – venous phase. c NBCA in nidus on unsubtracted lateral image. d Final digital subtraction angiography after embolization. The AVM was embolized using NBCA with satisfactory reduction in size and flow. The patient went on to have complete surgical resection.
Embolization with NBCA facilitates surgical resection by helping identify the embolized vessels, differentiating them from normal vessels, as well as providing a distinct boundary between the AVM and normal brain parenchyma. Vessels embolized with NBCA are compressible and easily cut with microscissors [39].
Onyx
Onyx is one of the newest nonadhesive liquid embolic agents available for treatment of brain AVMs [69]. It consists of an ethyl-vinyl alcohol copolymer dissolved in dimethyl sulfoxide. Tantalum powder is added for radiopaque visualization. Onyx comes available in 1.5-ml ready-to-use vials in 3 different viscosities: Onyx 18, 20, and 34. The concentrations of ethyl-vinyl alcohol copolymer are 6, 6.5, and 8% for Onyx 18, 20, and 34, respectively. The Onyx vials must be shaken for at least 20 min prior to use in order to obtain a homogeneous solution consisting of the embolic component and the tantalum powder [70].
Unlike NBCA which polymerizes almost immediately, Onyx has a very slow solidification rate which allows for a more prolonged and controlled injection. This, in theory, enables larger parts of the AVM to be occluded with each microcatheterization. The slow polymerization rate and lack of adherence allows for prolonged and repeated injections from the same point, resulting in deeper penetration into a larger part of the nidus [70,71,72]. However, it should be noted that these are theoretical advantages as there is currently no published evidence confirming this.
One disadvantage or limitation of Onyx is its high radiopacity. It may be difficult or impossible to visualize the distribution of the material one is injecting in large AVMs previously treated with Onyx because of overprojection. This can potentially result in untoward injection of collateral arteries or the venous outflow system with catastrophic results. Another disadvantage of Onyx is its poor visualization during reflux in very small vessels [72]. Additionally, some peer-reviewed publications describe longer fluoroscopy times and higher complication rates with Onyx compared with NBCA [73].
Risks/Outcomes/Complications
Embolization of brain AVMs is not without risk, with large clinical series reporting morbidity rates of up to 16% and mortality rates of up to 4% [3,8,63,67,70,71,72,74,75,76,77,78,79,80,81,82,83]. Rates of morbidity and mortality vary greatly as they are dependent on many factors, including patient selection, embolic agent(s) used, goals of embolization, time of outcome assessment, and preexisting neurological morbidity [8]. In the following, the largest and most recent clinical series to date regarding AVM embolization outcomes are summarized (table 4).
Table 4.
Summary of modern embolization series1
| Reference | Cases n | Mortality rate, % | Morbidity rate, % | Embolic agent |
|---|---|---|---|---|
| Debrun et al. [67], 1997 | 54 | 3.7 | 5.6 | NBCA |
| Hartmann et al. [74], 2002 | 233 | 1 | 2 | NBCA |
| Taylor et al. [3], 2004 | 201 | 2 | 9 | NBCA, PVA particles, coils |
| Haw et al. [63], 2006 | 306 | 2.6 | 8.7 | NBCA |
| Starke et al. [8], 2009 | 202 | NA | 2.5 | NBCA |
| Sahlein et al. [78], 2012 | 130 | 0.8 | 0.8 | NBCA |
| Pierot et al. [81], 2009 | 48 | 2.1 | 10.4 | Onyx |
| Katsaridis et al. [72], 2008 | 101 | 3.0 | 8 | Onyx |
| Panagiotopoulos et al. [70], 2009 | 82 | 2.4 | 7.3 | Onyx |
Modified from Sahlein et al. [78]. NA = Not available.
In 1995, Frizzel and Fisher [83] published data on 1,246 patients from 32 brain AVM embolization series performed over a 35-year period from 1969 to 1993. Temporary morbidity from embolization was 10%, and permanent morbidity was 8%. Overall mortality was 1%. Embolization resulted in cure in 5% of AVM. They found no difference in these rates prior to or after 1990.
Debrun et al. [67] reported on 54 brain AVMs treated with NBCA from April 1994 to December 1995. A mortality rate of 3.7% and morbidity rate of 5.6% (2 minor and 1 severe permanent neurological deficit) were seen; 5.6% of the patients (3) were cured with embolization alone while 11 patients were cured after surgical resection. Three patients underwent radiosurgery, with 1 cure after 1 year.
Hartmann et al. [74] evaluated 233 consecutive patients who underwent 545 embolization procedures between 1991 and 1998. Thirty-three patients (14%) showed treatment-related neurological deficits, including 5 with disabling deficits (2%). There were 2 deaths (both secondary to parenchymal hemorrhage with ventricular extension). The 5 patients with disabling complications had treatment-induced ischemic strokes. Factors statistically associated with new deficits included increasing age, number of embolizations, and normal neurological status at baseline. Neither the total score of the Spetzler-Martin grading system nor any of its 3 components predicted treatment complications.
Taylor et al. [3] reviewed 339 AVM embolization procedures performed in 201 patients from June 1992 to May 2003. A variety of embolic agents was used, including PVA particles (260 procedures; 80.2%), NBCA (43 procedures; 13.3%), detachable coils (30 procedures; 9.3%), and Onyx (5 procedures 1.5%). Four patients died (2%), 18 patients developed permanent neurological deficits (9%), 7 patients had temporary neurological deficits (3.5%), and 18 patients had vascular complications without neurological compromise (9%). Analysis of numerous variables including patient age, sex, AVM location (supra- or infratentorial), and the type of embolic agent used was performed. None of the variables tested was a statistically significant predictor of poor outcome, although there was a trend toward poorer outcome with increasing patient age [3].
In 2006, Haw et al. [63] reported on 306 patients who underwent 513 brain AVM embolizations. Sixty-two complications occurred in 56 patients. Eight deaths were reported, 6 secondary to hemorrhage and 2 secondary to ischemic stroke. Location of the AVM in an eloquent part of the brain, presence of a fistula, and venous deposition of glue cast were all statistically associated with complications.
In 2009, Starke et al. [8] reported the results of 377 embolizations in 202 patients. There were 29 new clinical deficits after embolization (8% of procedures; 14% of patients), 19 of which were moderate or significant. Interestingly, on long-term follow-up, 43.3 ± 34.6 months after embolization, clinical deficits resolved in a significant number of patients, with only 5 patients having persistent neurological deficits after embolization, of which 4 were moderate and 1 was significant. Factors statistically associated with new deficits were staged embolization using more than 1 procedure, AVM diameter <3 cm, AVM diameter >6 cm, deep venous drainage, and eloquent location. Patients with medium-sized lesions were less likely to develop postprocedural deficits. Similar to prior reports, there were 10 periprocedural hemorrhages (2.6% of procedures; 4% of patients). Six cerebral infarctions were also reported [8].
More recently, Sahlein et al. [78] reported a retrospective review of 130 patients with 131 brain AVMs spanning over 8 years from January 1997 to December 2006. Their report is novel in that they largely used a single-stage approach to embolization as opposed to a multiple-stage embolization strategy typically employed at most institutions. An average of 1.28 embolization sessions per AVM were used with 105 lesions treated with a single embolization (80%). The reduction in embolization sessions per patient occurred without an increase in untoward events or compromise in the degree of devascularization. Permanent and/or significant morbidity and mortality rates were 0.8%. Additionally, a higher rate of angiographically complete occlusion (33%) compared with that reported in most other similarly sized studies was seen [3,8,63,67,70,71,72,74,75,76,77,78,79,80,81,82,83].
Studies using Onyx have shown similar rates of morbidity and mortality. Panagiotopoulos et al. [70] retrospectively reviewed 82 consecutive patients with brain AVMs treated with Onyx between July 2002 and January 2008. One hundred and nineteen embolization procedures were performed (1.45/patient). The authors report complete obliteration in 20/82 patients (24.4%). Sixteen of the 82 patients developed new neurological deficits following embolization; 10 patients experienced nondisabling neurologic deficits, and 6 patients experienced disabling neurologic deficits. The causes of periprocedural deficits included intracerebral hemorrhage (6 patients), intraventricular hemorrhage (1 patient), subarachnoid hemorrhage (3 patients), and infarcts/perfusion deficits (3 patients). In 3 cases, the cause was not identified. Two patients died secondary to intraparenchymal hemorrhage.
Pierot et al. [81] prospectively studied 50 patients from May 2003 to March 2005 to evaluate the efficacy and safety of Onyx in treating brain AVMs. One hundred and forty-nine embolization procedures were performed (mean 3.0 sessions/patient). One hundred and sixteen sessions (77.9%) were performed with Onyx. The remaining embolizations were performed with a combination of Onyx and glue. At 1 month, morbidity and mortality after treatment were 8 and 2%, respectively. AVM cure was reported in 8.3% of cases.
In 2008, Katsaridis et al. [72] reported on 101 patients who underwent a total of 219 embolization sessions with Onyx. There were 3 deaths, and 8 patients had permanent neurological deficits. Complete AVM obliteration was reported in 28 patients.
As with all neurointerventional procedures, any step in the embolization process from gaining arterial access to achieving hemostasis involves risks to the patient, including groin complications (hematoma or dissection), contrast reactions, and nephrotoxicity. Periprocedural complications that are particularly relevant to brain AVM embolization include intracerebral hemorrhage and ischemia/infarction leading to transient or permanent neurological deficits.
Both technical factors and physiological changes brought about by embolization can result in periprocedural hemorrhage [63,84]. Technical factors include arterial perforation/dissection by the microwire or microcatheter, rupture of an aneurysm as well as vascular injuries during retrieval of the catheter after glue injection [85]. Periprocedural hemorrhage can also be seen with inadvertent compromise of outflow veins and subsequent elevation of intranidal pressure. Although proximal arterial pressure elevation after nidus embolization has been raised as a potential cause of periprocedural hemorrhage, the changes are very small and unlikely to result in hemorrhagic complications [86].
However, the risk of a normal perfusion breakthrough phenomenon [63], or the occurrence of postprocedural edema and hemorrhage in cerebral tissue adjacent to the AVM nidus, is more compelling as a cause of periprocedural hemorrhage. High flow through the nidus of AVMs elevates venous pressures resulting in chronically low cerebral perfusion pressure that can impair cerebral autoregulation within the brain parenchyma surrounding an AVM. Originally described by Spetzler et al. [84], the idea of normal perfusion breakthrough argues that the sudden increase in perfusion pressure resulting from embolization of the nidus disrupts the chronically ischemic capillary bed within the surrounding brain parenchyma. Without normal autoregulatory mechanisms in place this can result in cerebral edema and hemorrhage. This phenomenon can be minimized by staging endovascular procedures [43,87].
Causes of acute cerebral ischemia/infarction include arterial dissection and thromboembolic disease-related catheter or guidewire manipulation, reflux of embolic material into normal cerebral vessels, and showering of glue droplets during retrieval of the microcatheter. Retrograde thrombosis of feeding arteries is another potential cause of cerebral infarction reported in the literature [88] and venous infarction can result from delayed venous thrombosis [89].
The decision to perform embolization of a brain AVM should take into consideration the Spetzler-Martin grade of the lesion as well as inherent risks of embolization. In general, Spetzler-Martin grade II or III lesions may be embolized before surgery or radiosurgery. Grade IV or V lesions should not be embolized unless this is to be done in conjunction with other treatment modalities (surgery or radiosurgery) for the goal of complete care. The only exception to this may be in a patient with a grade IV or V lesion with venous outflow obstruction, in whom embolization is used to reduce arterial inflow to control edema, or in a patient with true ‘steal’, in whom embolization is used to relieve the amount of shunt through the AVM.
Conclusion
Modern treatment of brain AVMs is a multimodality endeavor, requiring a multidisciplinary approach with a team consisting of physicians with expertise in cerebrovascular neurosurgery, endovascular intervention, and radiation therapy. Transarterial embolization has become a major component of AVM management, either as a stand-alone curative method or more commonly as an adjunct to microsurgery or radiosurgery. As newer embolic agents become available and as microcatheter technology improves the role of endovascular treatment for brain AVMs will likely expand.
References
- 1.Richling B, Killer M, Al-Schameri AR, Ritter L, Agic R, Krenn M. Therapy of brain arteriovenous malformations: multimodality treatment from a balanced standpoint. Neurosurgery. 2006;59(suppl 3):S148–S157. doi: 10.1227/01.NEU.0000237408.95785.64. [DOI] [PubMed] [Google Scholar]
- 2.Morgan MK, Zurin AA, Harrington T, Little N. Changing role for preoperative embolisation in the management of arteriovenous malformations of the brain. J Clin Neurosci. 2000;7:527–530. doi: 10.1054/jocn.2000.0759. [DOI] [PubMed] [Google Scholar]
- 3.Taylor CL, Dutton K, Rappard G, Pride GL, Replogle R, Purdy PD, White J, Giller C, Kopitnik TA, Jr, Samson DS. Complications of preoperative embolization of cerebral arteriovenous malformations. J Neurosurg. 2004;100:810–812. doi: 10.3171/jns.2004.100.5.0810. [DOI] [PubMed] [Google Scholar]
- 4.Ogilvy CS, Stieg PE, Awad I, Brown RD, Jr, Kondziolka D, Rosenwasser R, Young WL, Hademenos G, Special Writing Group of the Stroke Council, American Stroke Association AHA Scientific Statement. Recommendations for the management of intracranial arteriovenous malformations: a statement for healthcare professionals from a special writing group of the Stroke Council, American Stroke Association. Stroke. 2001;32:1458–1471. doi: 10.1161/01.str.32.6.1458. [DOI] [PubMed] [Google Scholar]
- 5.Deruty R, Pelissou-Guyotat I, Mottolese C, Bascoulergue Y, Amat D. The combined management of cerebral arteriovenous malformations. Experience with 100 cases and review of the literature. Acta Neurochir (Wien) 1993;123:101–112. doi: 10.1007/BF01401864. [DOI] [PubMed] [Google Scholar]
- 6.Deruty R, Pelissou-Guyotat I, Amat D, Mottolese C, Bascoulergue Y, Turjman F, Gerard JP. Multidisciplinary treatment of cerebral arteriovenous malformations. Neurol Res. 1995;17:169–177. doi: 10.1080/01616412.1995.11740307. [DOI] [PubMed] [Google Scholar]
- 7.Hartmann A, Mast H, Mohr JP, Pile-Spellman J, Connolly ES, Sciacca RR, Khaw A, Stapf C. Determinants of staged endovascular and surgical treatment outcome of brain arteriovenous malformations. Stroke. 2005;36:2431–2435. doi: 10.1161/01.STR.0000185723.98111.75. [DOI] [PubMed] [Google Scholar]
- 8.Starke RM, Komotar RJ, Otten ML, Hahn DK, Fischer LE, Hwang BY, Garrett MC, Sciacca RR, Sisti MB, Solomon RA, Lavine SD, Connolly ES, Meyers PM. Adjuvant embolization with N-butyl cyanoacrylate in the treatment of cerebral arteriovenous malformations: outcomes, complications, and predictors of neurologic deficits. Stroke. 2009;40:2783–2790. doi: 10.1161/STROKEAHA.108.539775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Friedlander RM. Clinical practice. Arteriovenous malformations of the brain. N Engl J Med. 2007;356:2704–2712. doi: 10.1056/NEJMcp067192. [DOI] [PubMed] [Google Scholar]
- 10.Berman MF, Sciacca RR, Pile-Spellman J, Stapf C, Connolly ES, Jr, Mohr JP, Young WL. The epidemiology of brain arteriovenous malformations. Neurosurgery. 2000;47:389–396. doi: 10.1097/00006123-200008000-00023. [DOI] [PubMed] [Google Scholar]
- 11.Valavanis A. The role of angiography in the evaluation of cerebral vascular malformations. Neuroimaging Clin N Am. 1996;6:679–704. [PubMed] [Google Scholar]
- 12.Mullan S, Mojtahedi S, Johnson DL, Macdonald RL. Embryological basis of some aspects of cerebral vascular fistulas and malformations. J Neurosurg. 1996;85:1–8. doi: 10.3171/jns.1996.85.1.0001. [DOI] [PubMed] [Google Scholar]
- 13.Friedman JA, Pollock BE, Nichols DA. Development of a cerebral arteriovenous malformation documented in an adult by serial angiography. Case report. J Neurosurg. 2000;93:1058–1061. doi: 10.3171/jns.2000.93.6.1058. [DOI] [PubMed] [Google Scholar]
- 14.Kader A, Goodrich JT, Sonstein WJ, Stein BM, Carmel PW, Michelsen WJ. Recurrent cerebral arteriovenous malformations after negative postoperative angiograms. J Neurosurg. 1996;85:14–18. doi: 10.3171/jns.1996.85.1.0014. [DOI] [PubMed] [Google Scholar]
- 15.Ondra SL, Troupp H, George ED, Schwab K. The natural history of symptomatic arteriovenous malformations of the brain: a 24-year follow-up assessment. J Neurosurg. 1990;73:387–391. doi: 10.3171/jns.1990.73.3.0387. [DOI] [PubMed] [Google Scholar]
- 16.Crawford PM, West CR, Chadwick DW, Shaw MD. Arteriovenous malformations of the brain: natural history in unoperated patients. J Neurol Neurosurg Psychiatry. 1986;49:1–10. doi: 10.1136/jnnp.49.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Laakso A, Hernesniemi J. Arteriovenous malformations: epidemiology and clinical presentation. Neurosurg Clin N Am. 2012;23:1–6. doi: 10.1016/j.nec.2011.09.012. [DOI] [PubMed] [Google Scholar]
- 18.Van Beijnum J, Van der Worp HB, Schippers HM, Van Nieuwenhuizen O, Kappelle LJ, Rinkel GJ, Berkelbach van der Sprenkel JW, Klijn CJ. Familial occurrence of brain arteriovenous malformations: a systematic review. J Neurol Neurosurg Psychiatry. 2007;78:1213–1217. doi: 10.1136/jnnp.2006.112227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kikuchi K, Kowada M, Sasajima H. Vascular malformations of the brain in hereditary hemorrhagic telangiectasia (Rendu-Osler-Weber disease) Surg Neurol. 1994;41:374–380. doi: 10.1016/0090-3019(94)90030-2. [DOI] [PubMed] [Google Scholar]
- 20.Laufer L, Cohen A. Sturge-Weber syndrome associated with a large left hemispheric arteriovenous malformation. Pediatr Radiol. 1994;24:272–273. doi: 10.1007/BF02015455. [DOI] [PubMed] [Google Scholar]
- 21.Mast H, Young WL, Koennecke HC, Sciacca RR, Osipov A, Pile-Spellman J, Hacein-Bey L, Duong H, Stein BM, Mohr JP. Risk of spontaneous haemorrhage after diagnosis of cerebral arteriovenous malformation. Lancet. 1997;350:1065–1068. doi: 10.1016/s0140-6736(97)05390-7. [DOI] [PubMed] [Google Scholar]
- 22.Graf CJ, Perret GE, Torner JC. Bleeding from cerebral arteriovenous malformations as part of their natural history. J Neurosurg. 1983;58:331–337. doi: 10.3171/jns.1983.58.3.0331. [DOI] [PubMed] [Google Scholar]
- 23.Fults D, Kelly DL., Jr Natural history of arteriovenous malformations of the brain: a clinical study. Neurosurgery. 1984;15:658–662. doi: 10.1227/00006123-198411000-00003. [DOI] [PubMed] [Google Scholar]
- 24.Zacharia BE, Vaughan KA, Jacoby A, Hickman ZL, Bodmer D, Connolly ES., Jr Management of ruptured brain arteriovenous malformations. Curr Atheroscler Rep. 20126;14:335–342. doi: 10.1007/s11883-012-0257-9. [DOI] [PubMed] [Google Scholar]
- 25.Kader A, Young WL, Pile-Spellman J, Mast H, Sciacca RR, Mohr JP, Stein BM. The influence of hemodynamic and anatomic factors on hemorrhage from cerebral arteriovenous malformations. Neurosurgery. 1994;34:801–807. doi: 10.1227/00006123-199405000-00003. [DOI] [PubMed] [Google Scholar]
- 26.Marks MP, Lane B, Steinberg GK, Chang PJ. Hemorrhage in intracerebral arteriovenous malformations: angiographic determinants. Radiology. 1990;176:807–813. doi: 10.1148/radiology.176.3.2389040. [DOI] [PubMed] [Google Scholar]
- 27.Turjman F, Massoud TF, Viñuela F, Sayre JW, Guglielmi G, Duckwiler G. Correlation of the angioarchitectural features of cerebral arteriovenous malformations with clinical presentation of hemorrhage. Neurosurgery. 1995;37:856–860. doi: 10.1227/00006123-199511000-00002. discussion 860-862. [DOI] [PubMed] [Google Scholar]
- 28.Viñuela F, Nombela L, Roach MR, Fox AJ, Pelz DM. Stenotic and occlusive disease of the venous drainage system of deep brain AVM's. J Neurosurg. 1985;63:180–184. doi: 10.3171/jns.1985.63.2.0180. [DOI] [PubMed] [Google Scholar]
- 29.Mansmann U, Meisel J, Brock M, Rodesch G, Alvarez H, Lasjaunias P. Factors associated with intracranial hemorrhage in cases of cerebral arteriovenous malformation. Neurosurgery. 2000;46:272–279. doi: 10.1097/00006123-200002000-00004. [DOI] [PubMed] [Google Scholar]
- 30.Horton JC, Chambers WA, Lyons SL, Adams RD, Kjellberg RN. Pregnancy and the risk of hemorrhage from cerebral arteriovenous malformations. Neurosurgery. 1990;27:867–871. doi: 10.1097/00006123-199012000-00002. discussion 871-872. [DOI] [PubMed] [Google Scholar]
- 31.ApSimon HT, Reef H, Phadke RV, Popovic EA. A population-based study of brain arteriovenous malformation: long-term treatment outcomes. Stroke. 2002;33:2794–2800. doi: 10.1161/01.str.0000043674.99741.9b. [DOI] [PubMed] [Google Scholar]
- 32.Spetzler RF, Martin NA. A proposed grading system for arteriovenous malformations. J Neurosurg. 1986;65:476–483. doi: 10.3171/jns.1986.65.4.0476. [DOI] [PubMed] [Google Scholar]
- 33.Hamilton MG, Spetzler RF. The prospective application of a grading system for arteriovenous malformations. Neurosurgery. 1994;34:2–6. discussion 6-7. [PubMed] [Google Scholar]
- 34.Luessenhop AJ, Spence WT. Artificial embolization of cerebral arteries. Report of use in a case of arteriovenous malformation. JAMA. 1960;172:1153–1155. doi: 10.1001/jama.1960.63020110001009. [DOI] [PubMed] [Google Scholar]
- 35.Wolpert SM, Stein BM. Catheter embolization of intracranial arteriovenous malformations as an aid to surgical excision. Neuroradiology. 1975;10:73–85. doi: 10.1007/BF00338550. [DOI] [PubMed] [Google Scholar]
- 36.Luessenhop AJ, Rosa L. Cerebral arteriovenous malformations. Indications for and results of surgery, and the role of intravascular techniques. J Neurosurg. 1984;60:14–22. doi: 10.3171/jns.1984.60.1.0014. [DOI] [PubMed] [Google Scholar]
- 37.Serbinenko FA. Balloon catheterization and occlusion of major cerebral vessels. J Neurosurg. 1974;41:125–145. doi: 10.3171/jns.1974.41.2.0125. [DOI] [PubMed] [Google Scholar]
- 38.Kerber C. Balloon catheter with a calibrated leak. A new system for superselective angiography and occlusive catheter therapy. Radiology. 1976;120:547–550. doi: 10.1148/120.3.547. [DOI] [PubMed] [Google Scholar]
- 39.Jafar JJ, Davis AJ, Berenstein A, Choi IS, Kupersmith MJ. The effect of embolization with N-butyl cyanoacrylate prior to surgical resection of cerebral arteriovenous malformations. J Neurosurg. 1993;78:60–69. doi: 10.3171/jns.1993.78.1.0060. [DOI] [PubMed] [Google Scholar]
- 40.Pasqualin A, Scienza R, Cioffi F, Barone G, Benati A, Beltramello A, Da Pian R. Treatment of cerebral arteriovenous malformations with a combination of preoperative embolization and surgery. Neurosurgery. 1991;29:358–368. doi: 10.1097/00006123-199109000-00004. [DOI] [PubMed] [Google Scholar]
- 41.Spetzler RF, Martin NA, Carter LP, Flom RA, Raudzens PA, Wilkinson E. Surgical management of large AVM's by staged embolization and operative excision. J Neurosurg. 1987;67:17–28. doi: 10.3171/jns.1987.67.1.0017. [DOI] [PubMed] [Google Scholar]
- 42.Debrun G, Vinuela F, Fox A, Drake CG. Embolization of cerebral arteriovenous malformations with bucrylate. J Neurosurg. 1982;56:615–627. doi: 10.3171/jns.1982.56.5.0615. [DOI] [PubMed] [Google Scholar]
- 43.Martin NA, Khanna R, Doberstein C, Bentson J. Therapeutic embolization of arteriovenous malformations: the case for and against. Clin Neurosurg. 2000;46:295–318. [PubMed] [Google Scholar]
- 44.Rosenblatt S, Lewis AI, Tew JM. Combined interventional and surgical treatment of arteriovenous malformations. Neuroimaging Clin N Am. 1998;8:469–482. [PubMed] [Google Scholar]
- 45.Perata HJ, Tomsick TA, Tew JM., Jr Feeding artery pedicle aneurysms: association with parenchymal hemorrhage and arteriovenous malformation in the brain. J Neurosurg. 1994;80:631–634. doi: 10.3171/jns.1994.80.4.0631. [DOI] [PubMed] [Google Scholar]
- 46.Jordan JE, Marks MP, Lane B, Steinberg GK. Cost-effectiveness of endovascular therapy in the surgical management of cerebral arteriovenous malformations. AJNR Am J Neuroradiol. 1996;17:247–254. [PMC free article] [PubMed] [Google Scholar]
- 47.Dion JE, Mathis JM. Cranial arteriovenous malformations. The role of embolization and stereotactic surgery. Neurosurg Clin N Am. 1994;5:459–474. [PubMed] [Google Scholar]
- 48.Deruty R, Pelissou-Guyotat I, Amat D, Mottolese C, Bascoulergue Y, Turjman F, Gerard JP. Complications after multidisciplinary treatment of cerebral arteriovenous malformations. Acta Neurochir (Wien) 1996;138:119–131. doi: 10.1007/BF01411350. [DOI] [PubMed] [Google Scholar]
- 49.Pollock BE, Flickinger JC, Lunsford LD, Maitz A, Kondziolka D. Factors associated with successful arteriovenous malformation radiosurgery. Neurosurgery. 1998;42:1239–1244. doi: 10.1097/00006123-199806000-00020. [DOI] [PubMed] [Google Scholar]
- 50.Gobin YP, Laurent A, Merienne L, Schlienger M, Aymard A, Houdart E, Casasco A, Lefkopoulos D, George B, Merland JJ. Treatment of brain arteriovenous malformations by embolization and radiosurgery. J Neurosurg. 1996;85:19–28. doi: 10.3171/jns.1996.85.1.0019. [DOI] [PubMed] [Google Scholar]
- 51.Friedman WA, Bova FJ. Radiosurgery for arteriovenous malformations. Neurol Res. 2011;33:803–819. doi: 10.1179/1743132811Y.0000000043. [DOI] [PubMed] [Google Scholar]
- 52.Mamalui-Hunter M, Jiang T, Rich KM, Derdeyn CP, Drzymala RE. Effect of liquid embolic agents on Gamma Knife surgery dosimetry for arteriovenous malformations. Clinical article. J Neurosurg. 2011;115:364–370. doi: 10.3171/2011.3.JNS10717. [DOI] [PubMed] [Google Scholar]
- 53.Kano H, Kondziolka D, Flickinger JC, Park KJ, Iyer A, Yang HC, Liu X, Monaco EA, 3rd, Niranjan A, Lunsford LD. Stereotactic radiosurgery for arteriovenous malformations after embolization: a case-control study. J Neurosurg. 2012;117:265–275. doi: 10.3171/2012.4.JNS111935. [DOI] [PubMed] [Google Scholar]
- 54.Marks MP, Lane B, Steinberg GK, Fabrikant JI, Levy RP, Frankel KA, Phillips MH. Endovascular treatment of cerebral arteriovenous malformations following radiosurgery. AJNR Am J Neuroradiol. 1993;14:297–303. [PMC free article] [PubMed] [Google Scholar]
- 55.Konan AV, Roy D, Raymond J. Endovascular treatment of hemifacial spasm associated with a cerebral arteriovenous malformation using transvenous embolization: case report. Neurosurgery. 1999;44:663–666. doi: 10.1097/00006123-199903000-00130. [DOI] [PubMed] [Google Scholar]
- 56.Sugita M, Takahashi A, Ogawa A, Yoshimoto T. Improvement of cerebral blood flow and clinical symptoms associated with embolization of a large arteriovenous malformation: case report. Neurosurgery. 1993;33:748–751. doi: 10.1227/00006123-199310000-00030. [DOI] [PubMed] [Google Scholar]
- 57.Kwon OK, Han DH, Han MH, Chung YS. Palliatively treated cerebral arteriovenous malformations: follow-up results. J Clin Neurosci. 2000;7(suppl 1):69–72. doi: 10.1054/jocn.2000.0715. [DOI] [PubMed] [Google Scholar]
- 58.Lv X, Wu Z, Li Y, Yang X, Jiang C. Hemorrhage risk after partial endovascular NBCA and ONYX embolization for brain arteriovenous malformation. Neurol Res. 2012;34:552–556. doi: 10.1179/1743132812Y.0000000044. [DOI] [PubMed] [Google Scholar]
- 59.Miyamoto S, Hashimoto N, Nagata I, Nozaki K, Morimoto M, Taki W, Kikuchi H. Posttreatment sequelae of palliatively treated cerebral arteriovenous malformations. Neurosurgery. 2000;46:589–594. doi: 10.1097/00006123-200003000-00013. discussion 594-595. [DOI] [PubMed] [Google Scholar]
- 60.Fournier D, TerBrugge KG, Willinsky R, Lasjaunias P, Montanera W. Endovascular treatment of intracerebral arteriovenous malformations: experience in 49 cases. J Neurosurg. 1991;75:228–233. doi: 10.3171/jns.1991.75.2.0228. [DOI] [PubMed] [Google Scholar]
- 61.Valavanis A, Yaşargil MG. The endovascular treatment of brain arteriovenous malformations. Adv Tech Stand Neurosurg. 1998;24:131–214. doi: 10.1007/978-3-7091-6504-1_4. [DOI] [PubMed] [Google Scholar]
- 62.Schaller C, Schramm J. Microsurgical results for small arteriovenous malformations accessible for radiosurgical or embolization treatment. Neurosurgery. 1997;40:664–672. doi: 10.1097/00006123-199704000-00003. [DOI] [PubMed] [Google Scholar]
- 63.Haw CS, TerBrugge K, Willinsky R, Tomlinson G. Complications of embolization of arteriovenous malformations of the brain. J Neurosurg. 2006;104:226–232. doi: 10.3171/jns.2006.104.2.226. [DOI] [PubMed] [Google Scholar]
- 64.Willinsky R, Goyal M, Terbrugge K, Montanera W, Wallace MC, Tymianski M. Embolisation of small (<3 cm) brain arteriovenous malformations. Correlation of angiographic results to a proposed angioarchitecture grading system. Interv Neuroradiol. 2001;7:19–27. doi: 10.1177/159101990100700102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hurst RW, Berenstein A, Kupersmith MJ, Madrid M, Flamm ES. Deep central arteriovenous malformations of the brain: the role of endovascular treatment. J Neurosurg. 1995;82:190–195. doi: 10.3171/jns.1995.82.2.0190. [DOI] [PubMed] [Google Scholar]
- 66.Brothers MF, Kaufmann JC, Fox AJ, Deveikis JP. n-Butyl 2-cyanoacrylate – substitute for IBCA in interventional neuroradiology: histopathologic and polymerization time studies. AJNR Am J Neuroradiol. 1989;10:777–786. [PMC free article] [PubMed] [Google Scholar]
- 67.Debrun GM, Aletich V, Ausman JI, Charbel F, Dujovny M. Embolization of the nidus of brain arteriovenous malformations with n-butyl cyanoacrylate. Neurosurgery. 1997;40:112–120. discussion 120-121. [PubMed] [Google Scholar]
- 68.Vinters HV, Lundie MJ, Kaufmann JC. Long-term pathological follow-up of cerebral arteriovenous malformations treated by embolization with bucrylate. N Engl J Med. 1986;314:477–483. doi: 10.1056/NEJM198602203140804. [DOI] [PubMed] [Google Scholar]
- 69.Taki W, Yonekawa Y, Iwata H, Uno A, Yamashita K, Amemiya H. A new liquid material for embolization of arteriovenous malformations. AJNR Am J Neuroradiol. 1990;11:163–168. [PMC free article] [PubMed] [Google Scholar]
- 70.Panagiotopoulos V, Gizewski E, Asgari S, Regel J, Forsting M, Wanke I. Embolization of intracranial arteriovenous malformations with ethylene-vinyl alcohol copolymer (Onyx) AJNR Am J Neuroradiol. 2009;30:99–106. doi: 10.3174/ajnr.A1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Weber W, Kis B, Siekmann R, Kuehne D. Endovascular treatment of intracranial arteriovenous malformations with onyx: technical aspects. AJNR Am J Neuroradiol. 2007;28:371–377. [PMC free article] [PubMed] [Google Scholar]
- 72.Katsaridis V, Papagiannaki C, Aimar E. Curative embolization of cerebral arteriovenous malformations (AVMs) with Onyx in 101 patients. Neuroradiology. 2008;50:589–597. doi: 10.1007/s00234-008-0382-x. [DOI] [PubMed] [Google Scholar]
- 73.Velat GJ, Reavey-Cantwell JF, Sistrom C, Smullen D, Fautheree GL, Whiting J, Lewis SB, Mericle RA, Firment CS, Hoh BL. Comparison of N-butyl cyanoacrylate and onyx for the embolization of intracranial arteriovenous malformations: analysis of fluoroscopy and procedure times. Neurosurgery. 2008;63(suppl 1):ONS73–ONS78. doi: 10.1227/01.neu.0000335015.83616.12. discussion ONS78-ONS80. [DOI] [PubMed] [Google Scholar]
- 74.Hartmann A, Pile-Spellman J, Stapf C, Sciacca RR, Faulstich A, Mohr JP, Schumacher HC, Mast H. Risk of endovascular treatment of brain arteriovenous malformations. Stroke. 2002;33:1816–1820. doi: 10.1161/01.str.0000020123.80940.b2. [DOI] [PubMed] [Google Scholar]
- 75.Meisel HJ, Mansmann U, Alvarez H, Rodesch G, Brock M, Lasjaunias P. Effect of partial targeted N-butyl-cyano-acrylate embolization in brain AVM. Acta Neurochir (Wien) 2002;144:879–887. doi: 10.1007/s00701-002-0978-6. discussion 888. [DOI] [PubMed] [Google Scholar]
- 76.Jayaraman MV, Marcellus ML, Hamilton S, Do HM, Campbell D, Chang SD, Steinberg GK, Marks MP. Neurologic complications of arteriovenous malformation embolization using liquid embolic agents. AJNR Am J Neuroradiol. 2008;29:242–246. doi: 10.3174/ajnr.A0793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ledezma CJ, Hoh BL, Carter BS, Pryor JC, Putman CM, Ogilvy CS. Complications of cerebral arteriovenous malformation embolization: multivariate analysis of predictive factors. Neurosurgery. 2006;58:602–611. doi: 10.1227/01.NEU.0000204103.91793.77. discussion 602-611. [DOI] [PubMed] [Google Scholar]
- 78.Sahlein DH, Mora P, Becske T, Nelson PK. Nidal embolization of brain arteriovenous malformations: rates of cure, partial embolization, and clinical outcome. J Neurosurg. 2012;117:65–77. doi: 10.3171/2012.3.JNS111405. [DOI] [PubMed] [Google Scholar]
- 79.n-BCA Trail Investigators N-butyl cyanoacrylate embolization of cerebral arteriovenous malformations: results of a prospective, randomized, multi-center trial. AJNR Am J Neuroradiol. 2002;23:748–755. [PMC free article] [PubMed] [Google Scholar]
- 80.Mounayer C, Hammami N, Piotin M, Spelle L, Benndorf G, Kessler I, Moret J. Nidal embolization of brain arteriovenous malformations using Onyx in 94 patients. AJNR Am J Neuroradiol. 2007;28:518–523. [PMC free article] [PubMed] [Google Scholar]
- 81.Pierot L, Januel AC, Herbreteau D, Barreau X, Drouineau J, Berge J, Sourour N, Cognard C. Endovascular treatment of brain arteriovenous malformations using onyx: results of a prospective, multicenter study. J Neuroradiol. 2009;36:147–152. doi: 10.1016/j.neurad.2008.11.005. [DOI] [PubMed] [Google Scholar]
- 82.van Rooij WJ, Jacobs S, Sluzewski M, van der Pol B, Beute GN, Sprengers ME. Curative embolization of brain arteriovenous malformations with onyx: patient selection, embolization technique, and results. AJNR Am J Neuroradiol. 2012;33:1299–1304. doi: 10.3174/ajnr.A2947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Frizzel RT, Fisher WS III. Cure, morbidity, and mortality associated with embolization of brain arteriovenous malformations: a review of 1,246 patients in 32 series over a 35-year period. Neurosurgery. 1995;37:1031–1039. doi: 10.1227/00006123-199512000-00001. discussion 1039-1040. [DOI] [PubMed] [Google Scholar]
- 84.Spetzler RF, Wilson CB, Weinstein P, Mehdorn M, Townsend J, Telles D. Normal perfusion pressure breakthrough theory. Clin Neurosurg. 1978;25:651–672. doi: 10.1093/neurosurgery/25.cn_suppl_1.651. [DOI] [PubMed] [Google Scholar]
- 85.Purdy PD, Batjer HH, Samson D. Management of hemorrhagic complications from preoperative embolization of arteriovenous malformations. J Neurosurg. 1991;74:205–211. doi: 10.3171/jns.1991.74.2.0205. [DOI] [PubMed] [Google Scholar]
- 86.Henkes H, Gotwald TF, Brew S, Kaemmerer F, Miloslavski E, Kuehne D. Pressure measurements in arterial feeders of brain arteriovenous malformations before and after endovascular embolization. Neuroradiology. 2004;46:673–677. doi: 10.1007/s00234-004-1229-8. [DOI] [PubMed] [Google Scholar]
- 87.Alexander MD, Connolly ES, Meyers PM. Revisiting normal perfusion pressure breakthrough in light of hemorrhage-induced vasospasm. World J Radiol. 2010;2:230–232. doi: 10.4329/wjr.v2.i6.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Sipos EP, Kirsch JR, Nauta HJ, Debrun G, Ulatowski JA, Bell WR. Intra-arterial urokinase for treatment of retrograde thrombosis following resection of an arteriovenous malformation. Case report. J Neurosurg. 1992;76:1004–1007. doi: 10.3171/jns.1992.76.6.1004. [DOI] [PubMed] [Google Scholar]
- 89.Duckwiler GR, Dion JE, Viñuela F, Reichman A. Delayed venous occlusion following embolotherapy of vascular malformations in the brain. AJNR Am J Neuroradiol. 1992;13:1571–1579. [PMC free article] [PubMed] [Google Scholar]