Abstract
A growing body of research has examined the regulation of negative emotions. However, little is known about the physiological processes underlying the regulation of positive emotions, such as when amusement is enhanced during periods of stress, or attenuated in the pursuit of social goals. The aim of this study was to examine the psychophysiological consequences of the cognitive up- and down-regulation of amusement. To address this goal, participants viewed brief, amusing film clips while measurements of experience, behavior, and peripheral physiology were collected. Using an event-related design, participants viewed each film under the instructions either to a) watch, b) use cognitive reappraisal to increase amusement, or c) use cognitive reappraisal to decrease amusement. Findings indicated that emotion experience, emotion-expressive behavior, and autonomic physiology (including heart rate, respiration, and sympathetic nervous system activation) were enhanced and diminished in accordance with regulation instructions. This finding is a critical extension of the growing literature on the voluntary regulation of emotion, and has the potential to help us better understand how people use humor in the service of coping and social goals.
Keywords: emotion regulation, amusement, reappraisal, psychophysiology, positive emotion
The ability to regulate negative and positive emotions in a context-sensitive manner is a hallmark of successful human functioning. In the past decade, research on emotion regulation has developed rapidly (Gross, 2007). However, studies have focused nearly exclusively on negative emotion. In particular, much work has focused on the down-regulation of emotions like disgust and sadness (Levesque et al., 2003; Ochsner et al., 2004), perhaps because of the clinical significance of the mis- and dys-regulation of negative emotion (Taylor & Liberzon, 2007).
Despite this emphasis on negative emotion, there is a growing awareness of the important role played by positive emotions in a variety of life outcomes (Ryff & Singer, 1998). One important emotion in this regard is amusement, which is a frequent target of regulation, such as when we down-regulate it by shifting our attention to avoid inappropriate laughter, or up-regulate it by focusing on a humorous aspect of a negative situation to reduce stress.
Indeed, the up-regulation of amusement may be particularly important to well-being, as correlations have been documented between increased humor and psychological resilience (Thorson, Powell, Sarmany-Schuller, & Hampes, 1997), immune functioning (Dowling, Hockenberry, & Gregory, 2003) and cardiovascular health (Taylor, Bagozzi, & Gaither, 2005). Therefore, as little research exists on this powerful coping technique, this study seeks to extend prior research on the regulation of negative emotion to the cognitive up- and down-regulation of amusement. Before presenting this study, we first review the cognitive regulation of negative emotion, and then consider further why it is important to extend this analysis to positive emotion.
Using Reappraisal to Regulate Negative Emotion
One prominent form of cognitive emotion regulation is reappraisal, which involves changing how we think in order to change the way we respond emotionally (Giuliani & Gross, in press). A large number of studies have shown that reappraisal is an effective means of minimizing the impact of a negative situation. Recent models of reappraisal have built on the extensive literature concerning cognitive control, positing that increased activation of control mechanisms during reappraisal modulates emotion-related activation (Ochsner & Gross, 2007). The physiological and neural substrates of these reappraisal-related mechanisms have been shown to be distinguishable in the context of negative emotion by the divergent consequences of up- and down-regulation for emotional outcomes (Jackson, Malmstadt, Larson & Davidson, 2000; Kunzmann, Kupperbusch, & Levenson, 2005; Ochsner et al., 2004).
Extending the Study of Emotion Regulation to Positive Emotion
One limitation of the literature on reappraisal is the relative lack of attention to positive emotion. This is an important omission because the association between positive emotions and health outcomes may be attributable to enhanced coping responses (Fredrickson, Tugade, Waugh, & Larkin, 2003; Tugade, Fredrickson, & Barrett, 2004). One coping response that has been of particular interest in this context is the purposeful engagement of humor during trying times. According to the Diagnostic and Statistical Manual of the American Psychiatric Association (DSM-IV-TR), humor may be defined as a coping mechanism whereby “the individual deals with emotional conflict or external stressors by emphasizing the amusing or ironic aspects of the conflict or stressor” (p. 812, APA, 2000).
It has been shown that inducing amusement (e.g., via films) elicits elevated levels of smiling behavior, somatic activity, skin conductance, respiratory activation, and sympathetic activation of the cardiovascular system (Gross & Levenson, 1997). It is not known, however, whether these behavioral and physiological consequences of amusement are all magnified when amusement is cognitively enhanced. Similarly, it is also not known whether these behavioral and physiological consequences of amusement are all reduced when amusement is cognitively diminished (as when one is trying to curb situationally appropriate amusement responses).
Despite the importance of positive emotions, only two studies have examined the cognitive up- and down-regulation of positive emotion (Beauregard, Levesque, & Bourgouin, 2001; Kim & Hamann, 2007). While these are important demonstrations of the power of cognition to regulate positive emotion, they have significant limitations. First, neither of these studies focused on amusement, which is one of the most frequently regulated positive emotions (Gross, Richards, & John, 2006) and plays a special role in coping (Dowling, Hockenberry, & Gregory, 2003). Second, each has methodological limitations. One study used erotic films, which have limited generalizability, and employed a block-design to compare regulated and unregulated conditions, which makes it difficult to discern the effect of condition order (Beauregard, Levesque, & Bourgouin, 2001). The second study used an event-related design, which is conducive to drawing strong conclusions about the differences between conditions, but used static images that targeted positive emotion more generally, which may not be effective at eliciting moderate to high levels of targeted positive affect (Kim & Hamann, 2007).
The Present Study
The present study aimed to examine the experiential, behavioral, and physiological consequences of up- and down-regulating amusement. Using short, amusing film clips, we tested the hypothesis that reappraising to increase and decrease amusement would lead to respective increases and decreases in 1) amusement experience, 2) amusement-related facial behavior, and 3) associated autonomic responses.
Method
Participants
Sixteen female undergraduates participated in this study (mean age: 18.8 years, SD 0.8; ethnic composition: 9 Caucasian, 4 mixed race, 2 Hispanic, 1 Asian) in exchange for class credit. Only women were recruited due to sex differences in emotional responsivity (Bradley, Codispoti, Sabatinelli, & Lang, 2001).
Materials
Amusing film clips consisted of 105 10–20s segments from Spike TV’s “Most Extreme Elimination Challenge,” previously found to elicit a moderate level of amusement (between 3 and 6 on the 8-point rating scale, mean 3.9) with low variation across subjects (standard deviation below 2.0, mean SD 1.6). Neutral stimuli consisted of 35 10-20s clips from the film “On the Edge,” which contained many of the features of the amusing clips, including rapid biological motion, outdoor setting, and audible speech.
Procedure
Participants were invited to the Stanford Psychophysiology Laboratory for an individual session. Room, monitor, physiological sensor and videotape setup followed Gross (1998). Each participant saw the same stimuli in the same order, and viewed each film once. Amusing film clips were presented with each of three regulation cues (‘look,’ ‘increase,’ or ‘decrease’) an approximately equal number of times across participants. For all three amusement conditions, instruction order was randomized with the limitation that no more than two consecutive presentations of a particular cue was allowed. Trials were divided into five runs of 28 trials each in an event-related design. Timing for each trial was as follows: 1s instructional cue (‘increase,’ ‘look,’ or ‘decrease’), 10–20s amusing or neutral film clip, 2s affect rating, 2s relaxation period indicated by an asterisk.
A total of 140 intermixed trials were shown, 35 for each of the four trial types: ‘look neutral’ (LN), ‘decrease amuse’ (DA), ‘look amuse’ (LA), and ‘increase amuse’ (IA). Neutral film clips were only included in the ‘look’ instruction condition. In the ‘look’ trial condition, participants were instructed to let their responses to the film clips unfold naturally. In the ‘increase’ condition, participants were instructed to reappraise the clip in order to maximize their amusement (i.e. to imagine that the man tripping was not actually a game-show contestant, but instead a personally relevant figure who takes himself very seriously). In the ‘decrease’ condition, participants were instructed to reappraise the situation in order to minimize their amusement response to the clip (i.e. to imagine how painful it was for the contestant to fall off of the rope swing into the mud). Before beginning the experiment, participants were carefully trained in strategies for each instruction type. With feedback, the experimenter (N.G.) helped shape reappraisals so that they involved the reinterpretation or recontextualization of the clips, as opposed to distraction or the use of another non-cognitive strategy.
Measures
Experience
After viewing each film segment, participants rated how amused they had felt during the film. Ratings of amusement were obtained using an 8-point Likert scale (1 = not amused, 8 = very amused) via a keyboard. Participants could take as much time as they needed to make this rating (mean = 188.8ms, SD = 68.9).
Facial behavior
Working with videotapes of subjects’ facial behavior recorded during the task, expressions of amusement (number of smiles, laughs) during each film presentation period were rated by two independent coders blind to hypotheses and experimental condition. Average inter-rater reliability was satisfactory, with Cronbach’s alphas of 0.76 for smiles and 0.66 for laughs (p<0.001 for all). The coders’ ratings were averaged to create one smile and one laugh rating for each participant for each film.
Physiology
During the experimental session, physiological channels previously found to be related to the experience and/or regulation of emotion (Gross, 1998; Gross & Levenson, 1997; Mauss, Wilhelm, & Gross, 2003) were sampled continuously at 400 Hz using laboratory software. Details of these measurements can be found in Mauss, et al (2003). Briefly, heart rate was calculated from RR intervals in the electrocardiogram, and values from abnormal beats were deleted and replaced by linearly interpolated values. Mean arterial blood pressure was obtained from the third finger of the non-dominant hand by means of the Finapres™ 2300 (Ohmeda, Madison, WI) system, and beat-to-beat stroke volume was measured using Wesseling’s validated pulse-contour analysis method. Skin conductance response amplitude was derived from a signal using a constant-voltage device to pass 0.5 V between mini-electrodes attached to the palmar surface of the middle phalanges of the first and second fingers of the nondominant hand. Respiratory rate was measured using an inductive plethysmography device (Respitrace Corporation, Ardsley, NY) connected to bands insulated wire coils placed around the abdomen and chest. Respiratory rate was calculated breath-by-breath using customized programs. Somatic activity was measured by a piezo-electric device attached to the subject’s chair, which generates an electrical signal proportional to the subject’s overall body movement. Finger temperature was obtained using a small thermometer attached to the participant’s last distal phalange. Finger pulse amplitude was assessed using a plethysmograph transducer on the tip of the participant’s second finger, and finger pulse transit time was indexed by the time (ms) between the closest previous R-wave and the upstroke of the peripheral pulse at the finger. Ear pulse transit time was determined similarly using a UFI plethysmograph transducer on participant’s left ear.
Data Reduction and Analysis
After data collection, customized analysis software (Wilhelm, Grossman, & Roth, 1999) was applied to physiological data reduction, artifact control, and computation of average physiological scores for each participant for each of the four experimental conditions (LN, DA, LA, IA). For the present analyses, we averaged across the entire film clip presentation to obtain a mean value for each measure. Each neutral film clip had an average value for the ‘look’ condition, and each amusing film clip had an average value for the ‘decrease,’ ‘look,’ and ‘increase’ conditions, which were then averaged by condition across clips. To assess sympathetic activation of the cardiovascular system, we employed a method used by previous researchers to create a composite of reverse and z-scored finger pulse amplitude, finger pulse transit time, ear pulse transit time and finger temperature (Gross & Levenson, 1997; Hagemann, Levenson, & Gross, 2006). Cronbach’s alpha for these measures was satisfactory (0.69). These measures were chosen as a collection of cardiovascular measures thought to be principally sympathetically mediated (Berntson, Quigley & Lozano, 2007). Repeated measures general linear models (GLMs) were used to evaluate the effects of film type and instruction.
As a manipulation check, we analyzed emotional reactivity by looking at changes from LN to LA for each measure. To test our hypotheses, we analyzed up-regulation by assessing changes in each measure from LA to IA, and analyzed down-regulation by assessing changes from LA to DA.
Results
Manipulation Check
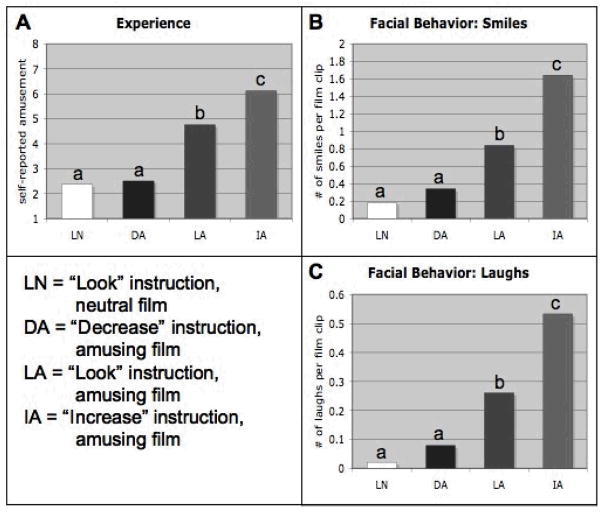
To confirm that participants responded differently to amusing and neutral films, paired t- tests were performed to compare self-reported amusement experience, facial behavior, and peripheral physiology in LN and LA trials. As expected, participants reported greater levels of amusement, showed more amusement-related facial behavior, and had stronger respiratory and sympathetic activation responses to amusing versus neutral films: self-reported amusement, t(15)=4.93, p<0.001; coded number of smiles per film clip, t(15)=5.08, p<0.001; coded number of laughs per film clip, t(15)=3.49, p=0.003; respiration rate, t(15)=6.24, p<0.001; cardiovascular sympathetic activation composite, t(15)=2.27, p=0.04). There were no significant differences between LN and LA for heart rate (p=0.12), mean blood pressure (p=0.19), skin conductance response amplitude (p=0.86) and somatic activity (p=0.25). Means for all conditions are presented in Figures 1 and 2.
Figure 1.
Mean Self-Reported Experience and Expressive Behavior During Film Presentation
For all panels, means are for the film period; means that do not share a superscript differ at p < .05. LN = “Look” instruction, neutral film; DA = “Decrease” instruction, amusing film; LA = “Look” instruction, amusing film; IA = “Increase” instruction, amusing film. Panel A: Mean self-reported experience (Mean square error [MSE] = 1.104). Panel B: Mean smiling behavior (MSE = 0.100). Panel C: Mean laughing behavior (MSE = 0.039).
Figure 2.
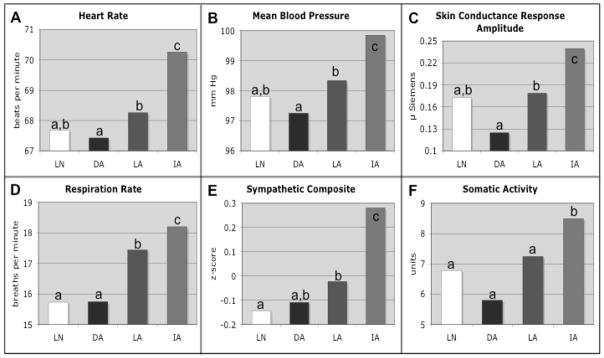
Mean Physiological Responses During Film Presentation
For all panels, means are for the film period; means that do not share a superscript differ at p < .05. LN = “Look” instruction, neutral film; DA = “Decrease” instruction, amusing film; LA = “Look” instruction, amusing film; IA = “Increase” instruction, amusing film. Panel A: Mean heart rate (Mean square error [MSE] = 1.498). Panel B: Mean blood pressure (MSE = 1.345). Panel C: Mean skin conductance response amplitude (MSE = 0.009). Panel D: Mean respiration rate (MSE = 0.862). Panel E: Mean composite of sympathetic activation of the cardiovascular system (MSE = 0.029). Panel F: Mean somatic activity (MSE = 0.054).
Effects of Reappraisal
Experience
A repeated-measures ANOVA of LA, IA and DA instruction conditions revealed a significant effect of reappraisal condition on experience, F(2,14)=65.1, p<0.001. As shown in Figure 1a, planned comparisons revealed that cognitive up- and down-regulation significantly modulated amusement experience as compared to passive viewing. Amusing films seen in the ‘increase’ instruction condition were rated as significantly more amusing than amusing films seen in the ‘look’ condition, F(1,15)=54.5, p<0.001. Amusing films seen in the ‘decrease’ condition were rated as significantly less amusing than those seen in the ‘look’ condition, F(1,15)=108.7, p<0.001. A post-hoc comparison between DA and LN revealed that the amusement elicited by amusing films seen in the ‘decrease’ condition was not significantly different than neutral films in the ‘look’ condition, p=0.75.
Behavior
ANOVAs revealed significant effects of reappraisal on both measures of amusement-related facial behavior (Figure 1b and 1c). For coded smiles, F(2,14)=56.1, p<0.001, planned comparisons demonstrated that cognitive up-regulation produced a greater number of smiles than passive viewing, F(2,14)=77.0, p<0.001. Cognitive down-regulation produced a lesser number of smiles than passive viewing, F(2,14)=26.7, p<0.001. This pattern was also seen in laughs, F(2,14)=13.5, p=0.001; both contrasts p<0.001. For smiles and laughs, the number of coded facial behaviors elicited during the DA condition was only significantly different than the number of smiles elicited by the LN condition at the trend level (smiles: p=0.12, laughs: p=0.09).
Physiology
ANOVAs for five of the six physiological measures revealed significant effects of reappraisal (Figure 2). Activation of the following measures was significantly increased during the cognitive up-regulation of amusement: Heart rate [F(2,14)=10.9, p=0.01], mean blood pressure [F(2,14)=16.3, p<0.001], skin conductance response amplitude [F(2,14)=8.3, p=0.004], respiration rate [F(2,14)=11.9, p=0.003], and sympathetic activation of the cardiovascular system [F(2,14)=13.6, p=0.001]. For all measures except skin conductance response amplitude (p=0.07), planned comparisons between LA and IA were significant at p<0.05. In addition, although the omnibus test for somatic activity did not meet the threshold of significance [F(2,14)=3.1, p=0.08], the planned comparison between LA and IA was significant (IA > LA, p=0.022).
These measures were also significantly decreased by the down-regulation of amusement as compared to passive viewing. Planned comparisons between LA and DA revealed that heart rate (p=0.029), mean blood pressure (p<0.001), skin conductance response amplitude (p=0.012), and respiration rate (p=0.002) were all significantly greater in the LA than the DA condition. In addition, sympathetic activation of the cardiovascular system trended towards significance in the hypothesized direction, p=0.08. For none of the above measures was DA significantly different than LN (all p>0.1).
In view of the known links between somatic activity and autonomic responding (Obrist, 1981), we conducted secondary analyses in which somatic activity was entered as a covariate. These ANCOVAs yielded the same pattern of findings reported here. This finding is important because it suggests that alterations in somatic activity were not responsible for the regulation-related changes in autonomic responses observed in this study1.
Discussion
Prior studies have demonstrated that reappraisal of negative emotion-eliciting stimuli modulates the experiential, behavioral, physiological, and neural components of emotion in accordance with the regulatory goal. Although two studies have previously examined the regulation of positive emotion, no study to date has demonstrated that experiential, behavioral, and peripheral physiological responses associated with amusement are subject to cognitive regulation. The facial behavior and autonomic physiology measures serve as essential confirmation the modulation of amusement represented by the experiential self-report (Mauss, Levenson, McCarter, Wilhelm, & Gross, 2005) due to the strong demand characteristics of this type of emotion regulation task.
In accord with previous studies on the autonomic physiology of amusement, measures of respiration rate (Gross & Levenson, 1997), sympathetic activation (Gross & Levenson, 1997) and skin conductance (Christie & Friedman, 2004) were all found to be significantly related to amusement reactivity (LA > LN). In addition, amusement-related facial behavior and autonomic responses followed the cognitively driven changes in self-reported amusement experience during the two regulation conditions. This coherence among amusement experience, behavior and physiology supports the view that cognitive regulation changes emotion as a whole, and not just subjective experience.
These data strongly support the idea that purposefully up-regulating a positive emotion like amusement increases the same beneficial outcomes as naturally experiencing it. If, during times of prolonged negative emotion and/or stress, one is able to identify a potentially amusing aspect of the situation and cognitively up-regulate it, these data show that the individual would experience the same physical and experiential consequences as if the amusement had been generated in the absence of regulatory efforts. This provides the first experimental evidence of the mechanisms underlying the many documented links between humor and increased physical and psychological health. Consequently, this work has implications for the treatment and prevention of stress-related illness, as these cognitive coping techniques may be easily taught so that those who do not naturally laugh in the face of stress may also reap the benefits.
To place these results in the context of previous work done on the reappraisal of positive emotion more generally, we compared the pattern of self-report ratings to those found by Kim and Hamman (2007). In both studies, up-regulation resulted in significantly increased experienced positive emotion, and down-regulation resulted in significantly decreased emotion reports (Kim & Hamann, 2007). However, the extent of down-regulation observed was different across experiments. Reappraising to decrease amusement brought amusement ratings down to the same level as watching a neutral film, but reappraising the positive pictures used by Kim and Hamann left levels of positive emotion significantly elevated above neutral. This may be a function of the positive and neutral stimuli chosen each study (dynamic vs. static), or an effect specific to amusement.
One noteworthy limitation of this study is that we chose to limit our sample to female participants in light of previously documented sex differences in affective responding to emotional stimuli. Consequently, it is unknown whether similar results would be observed in a male sample. The results of this study demonstrate that prior work on the psychophysiology of cognitive regulation of negative emotion can be extended to positive emotion. Reappraising while watching these brief film clips significantly modulated the experience, facial behavior, and peripheral physiology associated with amusement. These results contribute to our growing understanding of the cognitive regulation of all emotions, both negative and positive.
Acknowledgments
Preparation of this manuscript was supported by NIH grant R01 MH58147.
We thank the members of the Stanford Psychophysiology Laboratory for their help with this project, with particular thanks to Allison Brian, Nathaniel Nakashima, Thomas Nguyen, Ephraim Trahktenberg, and Brian Urbon.
Footnotes
In addition, we assessed the relations among all dependent measures reported in Figures 1 and 2. To do this, we correlated our measures of changes from LA to IA and LA to DA. The change in amusement experience from LA to IA was significantly correlated with mean blood pressure (r=0.56, p=0.003). The change in smile behavior from LA to DA was significantly correlated with sympathetic activation of the cardiovascular system (r=0.67, p=0.005) and skin conductance response amplitude (r=0.66, p=0.006).
References
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders (DSM_IV) 4. Washington, D.C: American Psychiatric Press; 1994. [Google Scholar]
- Beauregard M, Levesque J, Bourgouin P. Neural correlates of conscious self-regulation of emotion. Journal of Neuroscience. 2001;21(18):RC165. doi: 10.1523/JNEUROSCI.21-18-j0001.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernston GG, Quigley KS, Lozano D. Cardiovascular Psychophysiology. In: Cacioppo JT, Tassinary LG, Bernston G, editors. Handbook of Psychophysiology. New York, NY: Cambridge University Press; 2007. pp. 159–181. [Google Scholar]
- Bradley MM, Codispoti M, Sabatinelli D, Lang PJ. Emotion and motivation II: sex differences in picture processing. Emotion. 2001;1(3):300–319. [PubMed] [Google Scholar]
- Christie IC, Friedman BH. Autonomic specificity of discrete emotion and dimensions of affective space: a multivariate approach. International Journal of Psychophysiology. 2004;51(2):143–153. doi: 10.1016/j.ijpsycho.2003.08.002. [DOI] [PubMed] [Google Scholar]
- Dowling JS, Hockenberry M, Gregory RL. Sense of humor, childhood cancer stressors, and outcomes of psychosocial adjustment, immune function, and infection. Journal of Pediatric Oncology Nursing. 2003;20(6):271–292. doi: 10.1177/1043454203254046. [DOI] [PubMed] [Google Scholar]
- Fredrickson BL, Tugade MM, Waugh CE, Larkin GR. What good are positive emotions in crises? A prospective study of resilience and emotions following the terrorist attacks on the United States on September 11th, 2001. Journal of Personality and Social Psychology. 2003;84(2):365–376. doi: 10.1037//0022-3514.84.2.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giuliani NR, Gross JJ. Reappraisal. In: Ellsworth P, editor. Oxford Companion to the Affective Sciences. Oxford: Oxford University Press; in press. [Google Scholar]
- Gross JJ. Antecedent- and response-focused emotion regulation: divergent consequences for experience, expression, and physiology. Journal of Personality and Social Psychology. 1998;74(1):224–237. doi: 10.1037//0022-3514.74.1.224. [DOI] [PubMed] [Google Scholar]
- Gross JJ, editor. Handbook of emotion regulation. New York, NY: Guilford Press; 2007. [Google Scholar]
- Gross JJ, Levenson RW. Emotional suppression: physiology, self-report, and expressive behavior. Journal of Personality and Social Psychology. 1993;64(6):970–986. doi: 10.1037//0022-3514.64.6.970. [DOI] [PubMed] [Google Scholar]
- Gross JJ, Levenson RW. Hiding feelings: the acute effects of inhibiting negative and positive emotion. Journal of Abnormal Psychology. 1997;106(1):95–103. doi: 10.1037//0021-843x.106.1.95. [DOI] [PubMed] [Google Scholar]
- Gross JJ, Richards JM, John OP. Emotion regulation in everyday life. In: Snyder DA, Simpson JA, Hughes JN, editors. Emotion regulation in families: Pathways to dysfunction and health. Washington DC: American Psychological Association; 2006. pp. 13–35. [Google Scholar]
- Gross JJ, Thompson RA. Emotion regulation: Conceptual foundations. In: Gross JJ, editor. Handbook of emotion regulation. New York: Guilford Press; 2007. pp. 3–24. [Google Scholar]
- Hagemann T, Levenson RW, Gross JJ. Expressive suppression during an acoustic startle. Psychophysiology. 2006;43(1):104–112. doi: 10.1111/j.1469-8986.2006.00382.x. [DOI] [PubMed] [Google Scholar]
- Jackson DC, Malmstadt JR, Larson CL, Davidson RJ. Suppression and enhancement of emotional responses to unpleasant pictures. Psychophysiology. 2000;37(4):515–522. [PubMed] [Google Scholar]
- Kim SH, Hamann S. Neural correlates of positive and negative emotion regulation. Journal of Cognitive Neuroscience. 2007;19(5):776–798. doi: 10.1162/jocn.2007.19.5.776. [DOI] [PubMed] [Google Scholar]
- Kunzmann U, Kupperbusch CS, Levenson RW. Behavioral inhibition and amplification during emotional arousal: a comparison of two age groups. Psychology and Aging. 2005;20(1):144–158. doi: 10.1037/0882-7974.20.1.144. [DOI] [PubMed] [Google Scholar]
- Levesque J, Eugene F, Joanette Y, Paquette V, Mensour B, Beaudoin G, et al. Neural circuitry underlying voluntary suppression of sadness. Biological Psychiatry. 2003;53(6):502–510. doi: 10.1016/s0006-3223(02)01817-6. [DOI] [PubMed] [Google Scholar]
- Mauss IB, Levenson RW, McCarter L, Wilhelm FH, Gross JJ. The tie that binds? Coherence among emotion experience, behavior, and physiology. Emotion. 2005;5(2):175–190. doi: 10.1037/1528-3542.5.2.175. [DOI] [PubMed] [Google Scholar]
- Mauss IB, Wilhelm FH, Gross JJ. Autonomic recovery and habituation in social anxiety. Psychophysiology. 2003;40(4):648–653. doi: 10.1111/1469-8986.00066. [DOI] [PubMed] [Google Scholar]
- Obrist PA. Cardiovascular psychophysiology: A perspective. New York: Plenum Press; 1981. [Google Scholar]
- Ochsner KN, Gross JJ. Cognitive emotion regulation: Insights from social cognitive and affective neuroscience. Current Directions in Psychological Science. doi: 10.1111/j.1467-8721.2008.00566.x. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochsner KN, Ray RD, Cooper JC, Robertson ER, Chopra S, Gabrieli JD, et al. For better or for worse: neural systems supporting the cognitive down- and up-regulation of negative emotion. Neuroimage. 2004;23(2):483–499. doi: 10.1016/j.neuroimage.2004.06.030. [DOI] [PubMed] [Google Scholar]
- Ryff CD, Singer B. The contours of positive human health. Psychological Inquiry. 1998;9(1):1–28. [Google Scholar]
- Taylor SD, Bagozzi RP, Gaither CA. Decision making and effort in the self-regulation of hypertension: testing two competing theories. British Journal of Health Psychology. 2005;10(Pt 4):505–530. doi: 10.1348/135910704X22376. [DOI] [PubMed] [Google Scholar]
- Taylor SF, Liberzon I. Neural correlates of emotion regulation in psychopathology. Trends in Cognitive Science. 2007;11:413–418. doi: 10.1016/j.tics.2007.08.006. [DOI] [PubMed] [Google Scholar]
- Thorson JA, Powell FC, Sarmany-Schuller I, Hampes WP. Psychological health and sense of humor. Journal of Clinical Psychology. 1997;53(6):605–619. doi: 10.1002/(sici)1097-4679(199710)53:6<605::aid-jclp9>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- Tugade MM, Fredrickson BL, Barrett LF. Psychological resilience and positive emotional granularity: examining the benefits of positive emotions on coping and health. Journal of Personality. 2004;72(6):1161–1190. doi: 10.1111/j.1467-6494.2004.00294.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilhelm FH, Grossman P, Roth WT. Analysis of cardiovascular regulation. Biomedical Sciences Instrumentation. 1999;35:135–140. [PubMed] [Google Scholar]