Abstract
The use of an in vitro system based on primary cultures of Sertoli cells isolated from rat testes has greatly facilitated the study of the blood-testis barrier in recent years. Herein, we summarize the detailed procedures on the isolation of undifferentiated Sertoli cells from 20-day-old rat testes, the culture of these cells as a monolayer on Matrigel-coated bicameral units, the characterization of these cultured cells, and the use of the Sertoli cell epithelium for monitoring the integrity of the Sertoli cell blood-testis barrier. This information is based on the routine use of this system in our laboratory to study the Sertoli cell blood-testis barrier in the past two decades, which should be helpful for investigators in the field.
Keywords: Testis, Sertoli cell, Blood-testis barrier, Tight junction, Ectoplasmic specialization, Anchoring junction, Spermatogenesis
1. Introduction
The concept of the blood-testis barrier (BTB) was described more than a century ago when dyes injected into animals were found to stain all organs except the brain and seminiferous tubules in the testis (1, 2), illustrating the presence of a blood–tissue barrier in these two organs. Subsequent studies showed that the BTB, unlike other blood–tissue barrier [e.g., blood–brain barrier (BBB) and blood–retina barrier (BRB, also known as the blood-ocular barrier)] which are composed of tight junctions (TJs) between endothelial cells, is constituted almost exclusively by adjacent Sertoli cells whose function is to create an immunological barrier located near the basement membrane in the seminiferous epithelium (2–5). In other words, TJs between endothelial cells found in the interstitium between seminiferous tubules play a relatively insignificant role in maintaining BTB function. Equally important, the BTB is not only constituted by TJs, but also by coexisting basal ectoplasmic specializations [basal ES, a testis-specific atypical adherens junction (AJ)], gap junctions, and desmosome junctions present between Sertoli cells (6–8) so that in this respect the BTB is a unique ultrastructure worthy of study.
In recent years, there has been increasing interest in using Sertoli cells as a model to study BTB dynamics for a number of important reasons. First, it has been known since the 1980s that Sertoli cells cultured at high density in vitro have the ability to form a functional epithelium that closely mimics the BTB in vivo both structurally and functionally (9, 10). For instance, these Sertoli cells became polarized when seeded on extracellular matrix (e.g., Matrigel™) (9, 10) and created a functional TJ-permeability barrier (11–14). Moreover, ultrastructures corresponding to TJs, basal ES, gap junctions, and desmosome junctions that closely mimic the BTB in vivo were visible between adjacent Sertoli cells by electron microscopy (15, 16). Second, even though the BTB is one of the tightest blood–tissue barriers known to exist in mammals, surprisingly it is extremely susceptible to damage by environmental toxicants (e.g., cadmium) with its disruption often occurring before damage to the endothelial TJ-barrier is detected (17, 18). In fact, a recent study has shown that the developing BTB in immature rats is even more sensitive to toxicants, such as bisphenol A, than the animal as a whole which exhibited no signs of overt toxicity (19). Thus, this in vitro system provides a simple but suitable model that can be used in place of in vivo models [e.g., BTB integrity assay in which a dye is injected via the jugular vein to monitor barrier function similar to studies published in the 1970s (1, 20)] to assess the effects of different compounds on BTB function (21–23). Indeed, this in vitro system was recently used to examine the role of focal adhesion kinase (FAK) in BTB dynamics when the association of FAK with the occludin-ZO-1 protein complex was found to increase following cadmium treatment just prior to the disruption of the BTB (24, 25). It was shown that such an increase in association between FAK and the occludin-ZO-1 protein complex led to “unwanted” phosphorylation of occludin, causing occludin's dissociation from ZO-1 (24), thereby disrupting cell adhesion at the BTB. These findings also suggest that cadmium-induced disruption of the BTB may be “kept in check” if FAK in the testis can be targeted therapeutically (24). Third, because of coexisting TJs, basal ES, gap junctions, and desmosome junctions which collectively contribute to the unique nature of the BTB, this in vitro system provides an interesting model to study the roles of these junctions in immunological barrier function. For instance, it was recently shown that gap junctions (26) and desmosomes (27) are crucial to maintaining the integrity of the BTB in that they mediate crosstalk not only among themselves but also between basal ES and TJs. Finally, the BTB is a very important structure in the seminiferous epithelium that sequesters meiosis I and II, and all subsequent events of postmeiotic germ cell development (e.g., spermiogenesis) in a specialized microenvironment known as the apical (or adluminal) compartment so that the host's immune system cannot develop antibodies against sperm-specific antigens, some of which arise transiently during meiosis and spermiogenesis. If this were to occur, infertility would result. Thus, culturing Sertoli cells at high density in vitro provides a good system to study immunological barrier function.
Herein, we provide a detailed protocol for the isolation of highly pure Sertoli cells from rat pups, as well as a protocol for the measurement of transepithelial resistance as a reliable means to assess barrier function in vitro. The protocol that we have been using for the past three decades to isolate Sertoli cells was adopted from Dr. Jennie Mather (28, 29) and published previously (30) with minor modifications (31), whereas the procedure that we have been using to lyse germ cells that may have inadvertently contaminated Sertoli cell cultures (it involves the use of a hypo-tonic buffer) was originally published by Dr. Mario Sefanini's laboratory (32). We generally isolate Sertoli cells from 20-day-old rats for three important reasons. First, Sertoli cells isolated from rats at this age had almost negligible somatic (i.e., Leydig and peritubular myoid cells) and germ cell contamination with a reported Sertoli cell purity of >98% (33, 34) when cultures were hypotonically treated and cell purity was monitored by RT-PCR and/or immunoblotting using markers specific for different cell types in the testis (34). Second, Sertoli cell yield (~180– 200 × 106 Sertoli cells) has been shown to be excellent when ten rat pups at 20 days of age were used. Third, Sertoli cells isolated from 20-day-old rat testes are differentiated, and they have ceased to divide by this time (35). As such, they share similar physiological characteristics with adult Sertoli cells as reported in earlier studies (36, 37). It is also worth noting that a procedure is available to isolate Sertoli cells from adult rat testes using BSA gradients (38). However, the procedure for isolating Sertoli cells from adult rat testes is very tedious, time consuming, and expensive. Moreover, the purity of adult Sertoli cells isolated using this technique stands at ~85% owing to the fact that cultures are mostly contaminated with elongating/elongated spermatids, and multiple treatments with hypotonic buffer do not appear to increase cell purity to a more acceptable level (36, 37). This being the case, we suggest using 20-day-old rat testes for the isolation of Sertoli cells for most in vitro experiments.
The following text is divided into two main sections. In the first section, we provide the protocol for the isolation of Sertoli cells from 20-day-old rat testes. In the second section, we describe the technique used to assess the integrity of the TJ-permeability barrier.
2. Material
2.1. Isolation and Culturing of Sertoli Cells
The isolation of Sertoli cells from rat testes is a relatively straightforward procedure involving several enzymatic digestion and washing steps.
Sterile, disposable 50 ml and 15 ml conicals (15 ml conicals must contain graduations from 0.1 to 1 ml which are needed to determine cell pack volume).
Sterile, glass 125 ml Erlenmeyer flasks with caps.
Sterile Pasteur pipettes.
Sterile, individually wrapped, disposable 10 ml pipettes. Tissue culture-treated 100-mm dishes, multiwell plates (Corning, Corning NY) and/or glass coverslips (Thomas Scientific, Swedesboro, NJ).
Matrigel™ (BD Biosciences, Bedford, MA).
Approximately 3L of F12/DMEM prepared according to the specification sheet using Milli-Q-grade water (e.g., Millipore Advantage A10 or Siemens UltraGenetic Pure Lab water systems) with a conductivity of 18.2 MΩ cm. Media must be sterilized by filtration through a 0.2-μm filtering unit inside a certified cell/ tissue culture hood.
Standard laboratory microscope.
Soybean trypsin inhibitor (STI) Type IIS – (Cat. No. T9128 Sigma-Aldrich, St. Louis, MO); Prepare 1% (i.e., 1 gm/100 ml) in PBS (10 mM sodium phosphate, pH 7.4 at pH 22°C containing 0.15 M NaCl); Store in 0.4 ml aliquots at −20°C.
Trypsin (from bovine pancreas); Type I – (Cat. No. T8003 Sigma-Aldrich); Store in 40 mg/0.4 ml aliquots at −20°C.
Collagenase; Type I – (Cat. No. C0130 Sigma-Aldrich); Store in 20 mg/0.2 ml and 40 mg/0.4 ml aliquots containing 0.1% STI at –20°C.
Hyaluronidase (from bovine testes); Type IS (Cat. No. H3506 Sigma-Aldrich); Store in 40 mg/0.4 ml aliquots containing 0.1% STI at −20°C.
DNase (from bovine pancreas) (Cat. No. DN25 Sigma-Aldrich); Prepare 2 mg/ml in F12/DMEM.
EGF 10 μg/ml stock.
Human transferrin 10 mg/ml stock.
Bovine insulin 20 mg/ml stock.
Bacitracin 10 mg/ml stock.
All enzymes and reagents listed above should be prepared in PBS unless otherwise specified and filtered through a 0.2-μm low protein binding filter (e.g., 0.2-μm Nalgene syringe filters, Thermo Scientific, Cat. No. 190-2520) to sterilize.
Dulbecco's Modified Eagle's Medium Ham's F12 Nutrient Mixture (F12/DMEM) (Cat. No. D2906 Sigma-Aldrich) supplemented with sodium bicarbonate (1.2 gm/L), gentamicin (20 mg/L), and phenol red (0.00863 gm/L) as instructed by the vendor.
2.2. To Assess Sertoli Cell TJ-Permeability Barrier Function
2.2.1. Materials
Millicell-HA culture plate inserts (Millipore, Billerica, MA) (e.g., Cat. #: PIHA01250, 12-mm diameter; ~1.1304 cm2 surface area).
Millicell ERS system (Millipore, Cat. #:MERS 000 01): meter and Ag/AgCl electrodes (Millipore, Cat. #:MERSSTX01).
3. Methods
3.1. Pre-isolation set-up
Use sterile scissors and forceps, or place them into 70% ethanol on the morning of the cell isolation.
On the morning of the cell isolation, prepare 1 M glycine, 2 mM EDTA in F12/DMEM, pH 7.4 (~100 ml), sterilized by filtration through a 0.2-μm filter.
Media must be warmed to 37°C.
A centrifuge set at room temperature (~20°C), shaking water bath set at 37°C, and cell culture hood should be reserved for ~5–6 h of usage.
Matrigel™-coated dishes, plates, and/or bicameral units should be prepared 24 h prior to cell plating by diluting Matrigel™ 1:7 in F12/DMEM. These should be incubated in a humidified CO2 incubator at 35°C in order to allow the Matrigel™ to completely dry. (Note: MatrigelTM should be removed from –20°C and kept at 4°C overnight for its liquefaction prior to its use for dilution in F12/DMEM for plating on culture dishes, bicameral units, and/or microscopic slides.
3.2. Sertoli Cell Isolation
Ten male Sprague–Dawley rats (Charles River Laboratories, Kingston, NY) to be 20 days of age on the day of use. One can anticipate routinely isolating ~180–200 × 106 Sertoli cells from ten rat pups. Euthanize animals by asphyxiation using CO2 from a carbon dioxide tank with the regulator set at ~20 psi (~1.4 bars) for about 2–2.5 min using a setup similar to the one commercially available from Braintree Scientific, Inc. (Braintree, MA).
Rinse the scrotal area with 70% ethanol.
Remove testes, decapsulate to remove the tunica albuginea, and place all 20 testes into ~10 ml F12/DMEM in a 100-mm dish. From this step onward, all remaining culture steps will be performed inside a ventilated, certified cell culture hood with proper air flow located inside a specialized tissue culture room at room temperature unless otherwise specified.
Transfer all testes (20 total) into another 100-mm dish containing ~10 ml F12/DMEM. Cut testes into ~1-mm pieces with surgical-grade, curved scissors, such as from Miltex.
Transfer testes into a 50-ml conical tube and add F12/ DMEM up to 50 ml. Wash testes by gentle agitation to remove contaminating blood cells and centrifuge at 800 × g for 2 min at room temperature.
Aspirate F12/DMEM with a Pasteur pipette. Add F12/ DMEM up to 50 ml again and wash until media is clear, usually two times.
Resuspend testes in 40 ml F12/DMEM containing 40 mg of trypsin and 0.8 mg DNase. Transfer testes into a sterile 125 ml Erlenmeyer flask with cap. Place in a shaking water bath at 60–90 osc/min for 30 min. This step will release Leydig cells and other interstitial cells (e.g., fibroblasts).
Transfer seminiferous tubules into a 50-ml conical. Add F12/ DMEM up to 50 ml and centrifuge at 800 × g for 2 min.
Aspirate F12/DMEM with a Pasteur pipette.
Resuspend seminiferous tubules in 40 ml of 1 M glycine, 2 mM EDTA, pH 7.4 containing 0.01% STI, and 0.8 mg DNase. Incubate at room temperature for 10 min with periodic gentle agitation. This step will lyse Leydig cells.
Transfer seminiferous tubules into a 50-ml conical. Add F12/ DMEM up to 50 ml and centrifuge at 800 × g for 2 min.
Aspirate F12/DMEM with a Pasteur pipette.
Wash cells by centrifugation at 800 × g two times or until F12/DMEM is clear.
Resuspend cells by gentle pipetting (~20–25 times) with a Pasteur pipette in 40 ml F12/DMEM containing 20 mg of collagenase and 0.2 mg DNase. Transfer cells into an Erlenmeyer flask and place in a shaking water bath at 60–90 osc/min for 5 min.
Transfer cells into a 50 ml conical. Add F12/DMEM up to 50 ml and centrifuge at 800 × g for 2 min.
Resuspend cells by gentle pipetting (as described above) with a Pasteur pipette in 40 ml F12/DMEM containing 40 mg of collagenase and 0.2 mg DNase. Transfer cells into an Erlenmeyer flask and place in a shaking water bath at 60–90 osc/min for 30 min. These two consecutive steps will remove peritubular myoid cells.
Transfer cells into a 50-ml conical. Add F12/DMEM up to 50 ml and centrifuge at 800 × g for 2 min.
Wash cells by centrifugation at 800 × g three times (with gentle resuspension of cells in fresh F12/DMEM between centrifugations).
Resuspend cells by gentle pipetting (as described above) with a Pasteur pipette in 40 ml F12/DMEM containing 40 mg of hyaluronidase and 0.2 mg DNase. Transfer cells into an Erlenmeyer flask and place in the shaking water bath at 60–90 osc/min for 30 min. This step will break down hyaluronic acid, a major component of the extracellular matrix.
Transfer cells into a 50-ml conical. Add F12/DMEM up to 50 ml and centrifuge at 800 × g for 2 min.
Wash cells by centrifugation at 800 × g five times.
- Transfer cells into a 15-ml conical. Add F12/DMEM up to 15 ml and centrifuge at 800 × g for 2 min. Depending on the cell pack volume and on experimental design, resuspend cells as follows (to obtain 0.5 × 106 cells/ml):
Cell pack (ml) Number of cells (106) Volume of media (ml) 0.3 108 216 0.4 144 288 0.5 180 360 0.6 216 432 0.7 252 504 0.8 288 576 Resuspend cells in F12/DMEM containing 10 μg/ml insulin, 5 μg/ml human transferrin, 2.5 ng/ml EGF and 5 μg/ml bacitracin. Plate cells at low (5 × 104 cells/cm2) or high (0.5 × 106 cells/cm2) density. For high density, 100-mm dishes, multiwell plates, glass coverslips, or culture plate inserts should be coated with Matrigel™ to improve cell viability. To prepare cells for quantifying TJ permeability barrier function, a cell density of 1–1.2 × 106 cells/cm2 should be used.
Incubate cells in culture dishes/bicameral units in a humidified CO2 incubator at 35°C with 95% air and 5% CO2 (v/v). Sertoli cells will be in small clusters of five to ten cells as it is impossible to isolate single Sertoli cells. Once these cell clusters attach to the substratum, cells will quickly spread out to form an epithelium.
3.3. Hypotonic Treatment
This step is performed 36–48 h after plating Sertoli cells on 100-mm dishes, multiwell dishes or glass coverslips to remove residual germ cells from Sertoli cells, so that these Sertoli cells will be contaminated with negligible germ cells and Leydig cells (see Note 4.1). For hypotonic treatment:
Prepare 20 mM Tris, pH 7.4 (100–500 ml), depending on the number of dishes/plates that need to be treated.
Filter the above buffer to sterilize.
- Buffer must be sterilized by filtration through a 0.2-μm filtering unit.
- Aspirate media with a Pasteur pipette.
- Add an appropriate volume of the above buffer per dish (e.g., ~10 ml per 100-mm dish; ~ 2 ml per well in a six-well dish) for exactly 2.5 min to lyse contaminating germ cells.
- Aspirate buffer with a Pasteur pipette.
- Wash two times with F12/DMEM to remove residual Tris buffer.
- Add F12/DMEM supplemented with four factors as described above.
- Incubate cells in a humidified CO2 incubator at 35°C with 95% air and 5% CO2 (v/v).
- Feed cells every 3–4 days (low cell density) or every 1–2 days (high cell density) with growth factor supplemented-F12/DMEM.
3.4. Measurement of TER to Assess Barrier Function In Vitro
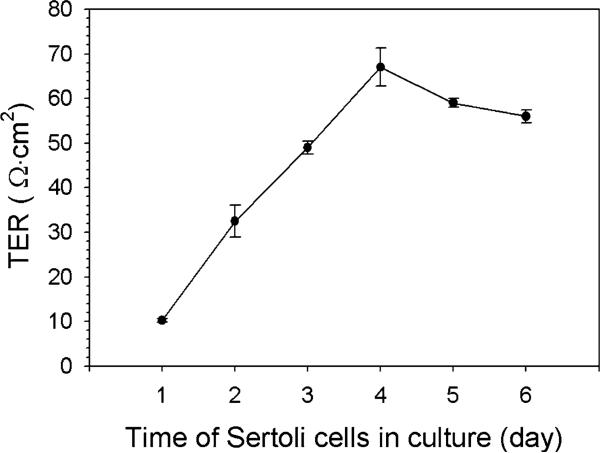
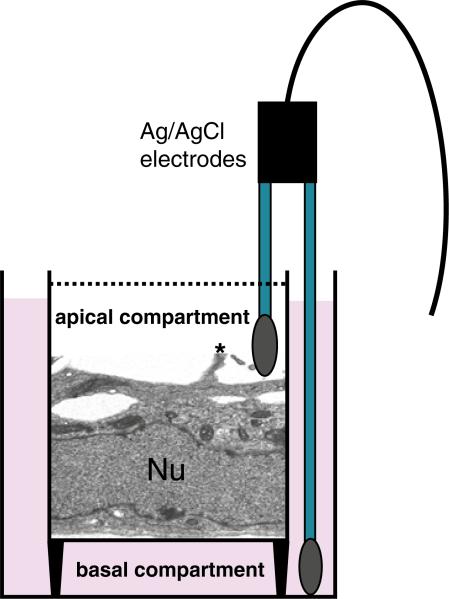
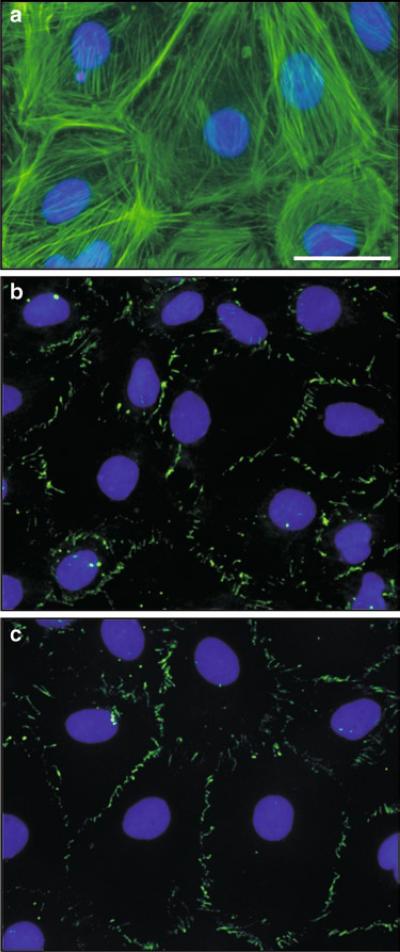
When Sertoli cells are plated on Matrigel™-coated bicameral culture units at 0.5–1.2 × 106 cells/cm2, they are capable of forming an intact epithelium with a functional TJ-permeability barrier, and these cells can resist the passage of an electrical current (i.e., ~2 s pulse of ~20 μA) sent across the epithelium (Fig. 1) with a Millipore Millicell-ERS meter with two electrodes which are placed in the apical and basal chambers of the bicameral unit (Fig. 2) (see also Note 4.2). TJ-barrier function is recorded in ohms (Ω) (i.e., the resistance of the cell epithelium to the flow of current across the epithelium), and this value is multiplied by the surface area of the bicameral unit (cm2) to yield transepithelial electrical resistance (TER) (Fig. 1) (see Note 4.2 for precaution-ary notes to obtain reproducible and reliable data from this experiment). The TJ-barrier in Sertoli cells is formed by day 3 at which time TER values reach a peak (~50–70 Ω cm2), followed by a plateau (Fig. 1). The assembly of functional junctions between Sertoli cells can be further characterized by fluorescence microscopy, which in this case was used to visualize actin filaments (Fig. 3a), as well as occludin (a TJ-integral membrane protein) (Fig. 3b) and ZO-1 (a TJ-associated adaptor known to form a 1:1 stoichiometric ratio with occludin) (Fig. 3c) between adjacent Sertoli cells.
Fig. 1.
A typical experiment assessing the assembly of the TJ-permeability barrier in Sertoli cells cultured on Matrigel™-coated culture units by quantifying the TER across the epithelium. TER was recorded from quadruplet bicameral culture chambers (see Fig. 2A) in which Sertoli cells were plated at a density of 1 × 106 cells/cm2 as described in the text.
Fig. 2.
A schematic drawing illustrating Sertoli cells (Nu, nucleus of a Sertoli cell) forming an intact epithelium after being plated on bicameral culture units and used for recording TER. One chopstick (i.e., the longer end) of the Millicell ERS electrode is inserted into the basal compartment, whereas the other chopstick (i.e., the shorter end) is placed in the apical compartment of culture units. A short pulse of current is then sent across the Sertoli cell epithelium and resistance (in Ω) is recorded. This technique monitors the integrity of the TJ-permeability barrier. A cytoplasmic process on the apical side of the Sertoli cell is clearly visible (see asterisk) which is a typical characteristic of Sertoli cells when cultured in vitro.
Fig. 3.
(a–c) The functional characteristics of the Sertoli cell TJ-barrier are assessed by fluorescence microscopy. Sertoli cells were cultured at 0.025 × 106 cells/cm2 on Matrigel™-coated glass coverslips in order to visualize actin using FITC-conjugated phalloidin (a, Molecular Probes, Eugene, OR), occludin using an anti-occludin antibody (b, Invitrogen, San Francisco, CA) and ZO-1 using an anti-ZO-1 antibody (c, Invitrogen). For (b) and (c), occludin and ZO-1 were visualized using a secondary antibody conjugated to FITC. Sertoli cell nuclei were stained with DAPI. Bar = 10 μm in (a), which applies to (b) and (c).
Prior to their use, electrodes to be used to quantify the TER across the Sertoli cell epithelium (see below) should be placed into 70% ethanol (~5 ml) on the morning of the isolation, and then into F12/DMEM (~5 ml) 3 h before recording TER.
Sertoli cells being used for TER experiments must be first plated on Matrigel™-coated culture units at a density of 0.5–1.2 × 106 cells/cm before remaining Sertoli cells are plated on other substrates (i.e., 100-mm dishes, multiwell plates, or glass coverslips). Each experiment must also include Matrigel™-coated culture units without cultured Sertoli cells (i.e., blank) for background subtraction.
When plating cells, caution should be taken to avoid trapping air bubbles on the inner side of culture units (i.e., where the nitrocellulose membrane meets the plastic). If this occurs, use a Gilson pipette with 1 ml attached pipette tip to resuspend Sertoli cells, in the process dislodging the air bubble.
Each bicameral culture unit is placed into one well of a 24 multiwell plate. Each culture unit should be positioned in the center of the 24 well. The maximum volume of media in each culture unit compartment should be 500 μl (both basal and apical chambers). Growth factor supplemented-F12/DMEM should be replaced daily.
It is important that Sertoli cells be plated uniformly on culture units. After plating Sertoli cells, allow the 24-well plate containing culture units to sit in the hood at room temperature for ~5–10 min before placing it into the CO2 incubator. Placing the plate into the incubator immediately after seeding tends to cluster cells in the center of the plate/ well/culture unit, leading to variations in TER within one culture unit.
The Millicell ERS meter, as well as the electrodes, should be first tested as instructed by the vendor. The range should be set to 2,000 Ω, and the mode should be set to RESISTANCE on the meter.
The first TER measurement should be ~24 h after plating cells and daily thereafter by placing one electrode in the apical chamber and the other electrode in the basal chamber. It is noted that one cannot obtain reliable TER readings immediately after plating Sertoli cells on culture units so that cells must be cultured for 24 h prior to obtaining the first reading. A total of four readings should be recorded at 12, 3, 6, and 9 o’clock positions for each bicameral culture unit, and these should be averaged into a single TER reading.
Multiwell plates containing culture units must be at room temperature before recording TER to avoid fluctuations in TER readings. Remove plates from the incubator and allow them to sit at room temperature for 20 min before recording TER.
When recording TER, Millicell ERS electrodes should stand upright in the well, and the culture unit should sit in the center of the 24-well. A ~2 s pulse of current (~20 μA) should be passed through the cell epithelium, and this number should be recorded.
F12/DMEM should be replaced after (NOT before) recording TER.
It is not necessary to rinse electrodes in between culture units. However, electrodes can be dipped into a well containing F12/DMEM to rinse the electrodes if the experiment involved treating Sertoli cells with different chemicals/compounds. Each treatment or control group should contain at least four replicate bicameral units to obtain sufficient data for statistical analysis. Each experiment should be repeated at least three times to assess reproducibility.
TER is recorded as follows: Resistanceunknown − Resistanceblank = Resistancecalculated × Effective surface area = Ω cm2.
4. Notes
4.1. Preparation of Sertoli Cell Cultures
It is noted that Sertoli cells isolated from 20-day-old rats and cultured in vitro are differentiated cells (35) since they cease to divide by postnatal day 15–17 at the time the blood-testis barrier is established in vivo. Furthermore, these cells establish the ultra-structures of both TJ, basal ectoplasmic specialization [a testisspecific atypical adherens junction type (39)], gap junction and desmosome junction when examined by electron microscopy within 48 h (15) which mimic the ultrastructural features of BTB in vivo. These morphological observations are also consistent with physiological data shown in Fig. 1, illustrating the establishment of a TJ-barrier within ~24–48 h after plating Sertoli cells onto the Matrigel-coated bicameral units. Listed below are a few precautionary notes for a successful experiment to monitor the Sertoli cell TJ-permeability barrier function.
A proper Sertoli cell density plated on Matrigel-coated bicameral units is critical to obtain reliable and reproducible TER reading since the entire surface area of the bicameral units must be covered with Sertoli cells to prevent a “leaky” TJ-barrier. We noted that using a Sertoli cell density at a range of 0.75–1.2 × 106 Sertoli cells/cm2 yielded the best data for this type of experiment as reported earlier (40) since at this cell density range, the cell epithelium on the bicameral unit was composed a single columnar-shaped Sertoli cell layer (41–43) similar to the Sertoli cells observed in vivo. However, when a higher Sertoli cell density was used, such as at 1.5–2 × 106 cells/ cm2, Sertoli cells began to stack into multiple layers (41). Even though this occurred in just a few patched areas on the bicameral units, necrosis was detected in these high cell density cultures (41), which in turn led to cell lysis, generating a small area without healthy Sertoli cells, causing the TJ-barrier to become “leaky” (22, 40). To prepare Sertoli cells for immunoblotting to assess changes of steady-state protein levels in treatment groups versus controls, we recommend the use of cell density at 0.5 × 106 cells/cm2, which would yield sufficient protein lysates for probing different marker proteins.
If one intends to use these Sertoli cell cultures to assess the effects of different treatments and/or drugs/chemicals/ reagents on the Sertoli cell TJ-barrier morphologically, such as the use of dual-labeled immunofluorescence analysis with fluorescence or confocal microscopy, a cell density at ~0.02– 0.05 × 106 cells/cm2 is recommended (27, 44–46) (see Fig. 3). If a higher cell density is used, Sertoli cells become too closely packed when nuclei were visualized by DAPI staining, making it difficult to visualize changes in protein localization, in particular protein redistribution, at the Sertoli–Sertoli cell interface. However, at this lower cell density, a few selected areas on the culture dish, glass slide, or coverslip may not be covered by Sertoli cells, but since the analysis is restricted on the use of fluorescent or confocal microscopy to assess changes in protein localization and/or redistribution at the Sertoli–Sertoli cell interface, this will neither affect data analysis nor the purpose of the experiment.
It is also important that from time to time, such as every 2–3 months, Sertoli cells from a given investigator who uses these Sertoli cell cultures for his/her experiments routinely, should be monitored for cell purity such as for contamination of germ cells and/or Leydig cells by RT-PCR using specific markers for spermatogonia, spermatocytes, spermatids, and Leydig cells as described earlier (33, 34). It is also necessary to monitor the presence of ultrastructures of TJ, basal ES, desmosome-like junction by electron microscopy, as well as the establishment of a functional TJ-permeability barrier by physiological techniques to assess TER across the Sertoli cell epithelium as described (15, 47, 48).
4.2. Assessing the Sertoli Cell TJ-Permeability Barrier by Recording TER Across the Sertoli Cell Epithelium
Below are some additional precautionary notes to be taken in order to obtain reproducible and reliable TER readings in a given experiment in order to assess Sertoli cell TJ-permeability barrier function.
As described above, a Sertoli cell density at 0.75–1.0 × 106cells/ cm2 is recommended to assess the Sertoli cell TJ-permeability barrier. At this range, the entire surface area of the Matrigel-coated bicameral unit will be covered with a layer of columnar-shaped Sertoli cells. This will yield stable and steady TER readings across the cell epithelium. Caution should be taken to allow the Matrigel-coated units to dry overnight (at least ~8–10 h) before use to provide a scaffolding support to the Sertoli cell epithelium.
If a hypotonic treatment (32) is used to obtain Sertoli cell cultures with >98% purity, it is important that the cell epithelium on the Matrigel-coated bicameral unit be handled gently during the washing step with F12/DMEM to avoid perturbing the cell epithelium to create an artificial “leak,” causing a loss of TER across the Sertoli cell epithelium. We had also used collagen gel-coated bicameral units to substitute Matrigel, but these experiments did not yield results comparable to the use of Matrigel since the TER readings are less stable.
Since the Millipore ERS system will be used for several days (at least 5–7 days), it is important to check if the system is properly calibrated, the battery level is optimal, and the pair of electrodes is functioning properly. This is important since any unwanted changes to the ERS system and the electrodes will cause an artificial shift in the baseline TER reading, causing fluctuation in the TER readings between different time points within an experiment.
It is also important to have at least a triplicate set of bicameral units for each treatment group versus control. If an experiment is being handled by a less experienced investigator, one can consider to use five replicates per treatment group including control.
References
- 1.Setchell BP, Waites GMB. The blood-testis barrier. In: Hamilton DW, Greep RO, editors. The Handbook of Physiology. Section 7, Vol. V. Male Reproductive System. American Physiological Society; Washington, D.C.: 1975. pp. 143–172. [Google Scholar]
- 2.Setchell BP. Blood-testis barrier, junctional and transport proteins and spermato-genesis. In: Cheng CY, Austin TX, editors. Molecular Mechanisms in Spermatogenesis. Landes Bioscience/Springer Science + Business Media, LLC; 2008. pp. 212–233. [Google Scholar]
- 3.Wong CH, Cheng CY. The blood-testis barrier: Its biology, regulation and physiological role in spermatogenesis. Curr Topics Dev Biol. 2005;71:263–296. doi: 10.1016/S0070-2153(05)71008-5. [DOI] [PubMed] [Google Scholar]
- 4.Cheng CY, Mruk DD. Cell junction dynamics in the testis: Sertoli-germ cell interactions and male contraceptive development. Physiol Rev. 2002;82:825–874. doi: 10.1152/physrev.00009.2002. [DOI] [PubMed] [Google Scholar]
- 5.Mruk DD, Silvestrini B, Cheng CY. Anchoring junctions as drug targets: Role in contraceptive development. Pharmacol Rev. 2008;60:146–180. doi: 10.1124/pr.107.07105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Russell LD, Peterson RN. Sertoli cell junctions: morphological and functional correlates. Int Rev Cytol. 1985;94:177–211. doi: 10.1016/s0074-7696(08)60397-6. [DOI] [PubMed] [Google Scholar]
- 7.Yan HHN, Mruk DD, Cheng CY. Junction restructuring and spermatogenesis: The biology, regulation, and implication in male contraceptive development. Curr Top Dev Biol. 2008;80:57–92. doi: 10.1016/S0070-2153(07)80002-0. [DOI] [PubMed] [Google Scholar]
- 8.Yan HHN, Mruk DD, Lee WM, Cheng CY. Ectoplasmic specialization: a friend or a foe of spermatogenesis? BioEssays. 2007;29:36–48. doi: 10.1002/bies.20513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Byers S, Hadley MA, Djakiew D, Dym M. Growth and characterization of epididymal epithelial cells and Sertoli cells in dual environment culture chambers. J Androl. 1986;7:59–68. doi: 10.1002/j.1939-4640.1986.tb00871.x. [DOI] [PubMed] [Google Scholar]
- 10.Janecki A, Steinberger A. Polarized Sertoli cell functions in a new two-compartment culture system. J Androl. 1986;7:69–71. doi: 10.1002/j.1939-4640.1986.tb00873.x. [DOI] [PubMed] [Google Scholar]
- 11.Janecki A, Jakubowiak A, Steinberger A. Regulation of transepithelial electrical resistance in two-compartment Sertoli cell cultures: in vitro model of the blood-testis barrier. Endocrinology. 1991;129:1489–1496. doi: 10.1210/endo-129-3-1489. [DOI] [PubMed] [Google Scholar]
- 12.Janecki A, Jakubowiak A, Steinberger A. Effects of cyclic AMP and phorbol ester on transepithelial electrical resistance of Sertoli cell monolayers in two-compartment culture. Mol Cell Endocrinol. 1991;82:61–69. doi: 10.1016/0303-7207(91)90009-h. [DOI] [PubMed] [Google Scholar]
- 13.Okanlawon A, Dym M. Effect of chloroquine on the formation of tight junctions in cultured immature rat Sertoli cells. J Androl. 1996;17:249–255. [PubMed] [Google Scholar]
- 14.Grima J, Pineau C, Bardin CW, Cheng CY. Rat Sertoli cell clusterin, α2-macro-globulin, and testins: biosynthesis and differential regulation by germ cells. Mol Cell Endocrinol. 1992;89:127–140. doi: 10.1016/0303-7207(92)90219-v. [DOI] [PubMed] [Google Scholar]
- 15.Siu MKY, Wong CH, Lee WM, Cheng CY. Sertoli-germ cell anchoring junction dynamics in the testis are regulated by an interplay of lipid and protein kinases. J Biol Chem. 2005;280:25029–25047. doi: 10.1074/jbc.M501049200. [DOI] [PubMed] [Google Scholar]
- 16.Byers S, Pelletier RM, Suarez-Quain C. Sertoli cell junctions and the seminiferous epithelium barrier. In: Russell LD, Griswold MD, editors. The Sertoli Cell. Clearwater. Cache River Press; 1993. pp. 431–446. [Google Scholar]
- 17.Setchell BP, Waites GMH. Changes in the permeability of the testicular capillaries and of the “blood-testis barrier” after injection of cadmium chloride in the rat. J Endocrinol. 1970;47:81–86. doi: 10.1677/joe.0.0470081. [DOI] [PubMed] [Google Scholar]
- 18.Wong CH, Mruk DD, Siu MKY, Cheng CY. Blood-testis barrier dynamics are regulated by α2-macroglobulin via the c-Jun N-terminal protein kinase pathway. Endocrinology. 2005;146:1893–1908. doi: 10.1210/en.2004-1464. [DOI] [PubMed] [Google Scholar]
- 19.Li MWM, Mruk DD, Lee WM, Cheng CY. Disruption of the blood-testis barrier integrity by bisphenol A in vitro: Is this a suitable model for studying blood-testis barrier dynamics? Int J Biochem Cell Biol. 2009;41:2302–2314. doi: 10.1016/j.biocel.2009.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li MWM, Xia W, Mruk DD, Wang CQF, Yan HHY, Siu MKY, Lui WY, Lee WM, Cheng CY. TNFα reversibly disrupts the blood-testis barrier and impairs Sertoli-germ cell adhesion in the seminiferous epithelium of adult rat testes. J Endocrinol. 2006;190:313–329. doi: 10.1677/joe.1.06781. [DOI] [PubMed] [Google Scholar]
- 21.Janecki A, Jakubowiak A, Steinberger A. Effect of cadmium chloride on transepithelial electrical resistance of Sertoli cell monolayers in two-compartment cultures – a new model for toxicological investigations of the “blood-testis” barrier in vitro. Toxicol Appl Pharmacol. 1992;112:51–57. doi: 10.1016/0041-008x(92)90278-z. [DOI] [PubMed] [Google Scholar]
- 22.Chung NPY, Cheng CY. Is cadmium chloride-induced inter-Sertoli tight junction permeability barrier disruption a suitable in vitro model to study the events of junction disassembly during spermatogenesis in the rat testis? Endocrinology. 2001;142:1878–1888. doi: 10.1210/endo.142.5.8145. [DOI] [PubMed] [Google Scholar]
- 23.Siu ER, Mruk DD, Porto CS, Cheng CY. Cadmium-induced testicular injury. Toxicol Appl Pharmacol. 2009;238:240–249. doi: 10.1016/j.taap.2009.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Siu ER, Wong EWP, Mruk DD, Porto CS, Cheng CY. Focal adhesion kinase is a blood-testis barrier regulator. Proc Natl Acad Sci USA. 2009;106:9298–9303. doi: 10.1073/pnas.0813113106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Siu ER, Wong EWP, Mruk DD, Sze KL, Porto CS, Cheng CY. An occludin-focal adhesion kinase protein complex at the blood-testis barrier: a study using the cadmium model. Endocrinology. 2009;150:3336–3344. doi: 10.1210/en.2008-1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li MWM, Mruk DD, Lee WM, Cheng CY. Connexin 43 and plakophilin-2 as a protein complex that regulates blood-testis barrier dynamics. Proc Natl Acad Sci USA. 2009;106:10213–10218. doi: 10.1073/pnas.0901700106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lie PPY, Cheng CY, Mruk DD. Crosstalk between desmoglein-2/desmocollin-2/Src kinase and coxsackie and adenovirus receptor/ZO-1 protein complexes, regulates blood-testis barrier dynamics. Int J Biochem Cell Biol. 2010;42:975–986. doi: 10.1016/j.biocel.2010.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mather JP. Establishment and characterization of two distinct mouse testicular epithelial cell lines. Biol Reprod. 1980;23:243–252. doi: 10.1095/biolreprod23.1.243. [DOI] [PubMed] [Google Scholar]
- 29.Mather JP, Sato GH. The use of hormone-supplemented serum-free media in primary cultures. Exp Cell Res. 1979;124:215–221. doi: 10.1016/0014-4827(79)90271-4. [DOI] [PubMed] [Google Scholar]
- 30.Cheng CY, Mather JP, Byer AL, Bardin CW. Identification of hormonally responsive proteins in primary Sertoli cell culture medium by anion-exchange high performance liquid chromatography. Endocrinology. 1986;118:480–488. doi: 10.1210/endo-118-2-480. [DOI] [PubMed] [Google Scholar]
- 31.Mruk DD, Siu MKY, Conway AM, Lee NPY, Lau ASN, Cheng CY. Role of tissue inhibitor of metalloproteases-1 in junction dynamics in the testis. J Androl. 2003;24:510–523. doi: 10.1002/j.1939-4640.2003.tb02703.x. [DOI] [PubMed] [Google Scholar]
- 32.Galdieri M, Ziparo E, Palombi F, Russo MA, Stefanini M. Pure Sertoli cell cultures: a new model for the study of somatic-germ cell interactions. J Androl. 1981;5:249–259. [Google Scholar]
- 33.Lee NPY, Mruk DD, Lee WM, Cheng CY. Is the cadherin/catenin complex a functional unit of cell-cell-actin-based adherens junctions (AJ) in the rat testis? Biol Reprod. 2003;68:489–508. doi: 10.1095/biolreprod.102.005793. [DOI] [PubMed] [Google Scholar]
- 34.Lee NPY, Mruk DD, Conway AM, Cheng CY. Zyxin, axin, and Wiskott-Aldrich syndrome protein are adaptors that link the cadherin/catenin protein complex to the cytoskeleton at adherens junctions in the seminiferous epithelium of the rat testis. J Androl. 2004;25:200–215. doi: 10.1002/j.1939-4640.2004.tb02780.x. [DOI] [PubMed] [Google Scholar]
- 35.Orth JM. Proliferation of Sertoli cells in fetal and postnatal rats: A quantitative auto-radiographic study. Anat Rec. 1982;203:485–492. doi: 10.1002/ar.1092030408. [DOI] [PubMed] [Google Scholar]
- 36.Li JCH, Lee WM, Mruk DD, Cheng CY. Regulation of Sertoli cell myotubularin (rMTM) expression by germ cells in vitro. J Androl. 2001;22:266–277. [PubMed] [Google Scholar]
- 37.Lui WY, Lee WM, Cheng CY. Transforming growth factor-β3 regulates the dynamics of Sertoli cell tight junctions via the p38 mitogen-activated protein kinase pathway. Biol Reprod. 2003;68:1597–1612. doi: 10.1095/biolreprod.102.011387. [DOI] [PubMed] [Google Scholar]
- 38.Wright WW, Zabludoff SD, Erickson-Lawrence M, Karzai AW. Germ cell-Sertoli cell interactions. Studies of cyclic protein-2 in the seminiferous tubule. Ann N Y Acad Sci. 1989;564:173–185. doi: 10.1111/j.1749-6632.1989.tb25896.x. [DOI] [PubMed] [Google Scholar]
- 39.Wong EWP, Mruk DD, Cheng CY. Biology and regulation of ectoplasmic specialization, an atypical adherens junction type, in the testis. Biochem Biophys Acta. 2008;1778:692–708. doi: 10.1016/j.bbamem.2007.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chung NPY, Mruk DD, Mo MY, Lee WM, Cheng CY. A 22-amino acid synthetic peptide corresponding to the second extracellular loop of rat occludin perturbs the blood-testis barrier and disrupts spermato-genesis reversibly in vivo. Biol Reprod. 2001;65:1340–1351. doi: 10.1095/biolreprod65.5.1340. [DOI] [PubMed] [Google Scholar]
- 41.Wong CCS, Chung SSW, Grima J, Zhu LJ, Mruk DD, Lee WM, Cheng CY. Changes in the expression of junctional and nonjunctional complex component genes when inter-Sertoli tight junctions are formed in vitro. J Androl. 2000;21:227–237. [PubMed] [Google Scholar]
- 42.Mruk DD, Zhu LJ, Silvestrini B, Lee WM, Cheng CY. Interactions of proteases and protease inhibitors in Sertoli-germ cell cocultures preceding the formation of specialized Sertoli-germ cell junctions in vitro. J Androl. 1997;18:612–622. [PubMed] [Google Scholar]
- 43.Lee NPY, Cheng CY. Regulation of Sertoli cell tight junction dynamics in the rat testis via the nitric oxide synthase/soluble guanylate cyclase/3′,5′-cyclic guanosine monophosphate/protein kinase G signaling pathway: an in vitro study. Endocrinology. 2003;144:3114–3129. doi: 10.1210/en.2002-0167. [DOI] [PubMed] [Google Scholar]
- 44.Lie PPY, Chan AYN, Mruk DD, Lee WM, Cheng CY. Restricted Arp3 expression in the testis prevents blood-testis barrier disruption during junction restructuring at spermatogenesis. Proc Natl Acad Sci USA. 2010;107:11411–11416. doi: 10.1073/pnas.1001823107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wong EWP, Mruk DD, Lee WM, Cheng CY. Regulation of blood-testis barrier dynamics by TGF-β3 is a Cdc42-dependent protein trafficking event. Proc Natl Acad Sci USA. 2010;107:11399–11404. doi: 10.1073/pnas.1001077107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wong EWP, Sun S, Li MWM, Lee WM, Cheng CY. 14-3-3 protein regulates cell adhesion in the seminiferous epithelium of rat testes. Endocrinology. 2009;150:4713–4723. doi: 10.1210/en.2009-0427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lui WY, Lee WM, Cheng CY. Transforming growth factor-β3 perturbs the inter-Sertoli tight junction permeability barrier in vitro possibly mediated via its effects on occludin, zonula occludens-1, and claudin-11. Endocrinology. 2001;142:1865–1877. doi: 10.1210/endo.142.5.8116. [DOI] [PubMed] [Google Scholar]
- 48.Lui WY, Wong CH, Mruk DD, Cheng CY. TGF-β3 regulates the blood-testis barrier dynamics via the p38 mitogen activated protein (MAP) kinase pathway: an in vivo study. Endocrinology. 2003;144:1139–1142. doi: 10.1210/en.2002-0211. [DOI] [PubMed] [Google Scholar]