Abstract
Processing the self-relevance of information facilitates recall. Similarly, processing close-other-related information facilitates recall to a lesser degree than processing self-relevant information. This memory advantage may be viewed as an index of the degree to which the representation of self is differentiated from representations of close others. To test developmental hypotheses concerning the development of self, we examined the relation of memory for self- and mother- referentially processed information in participants age 7–13. Memory for words encoded with reference to oneself increases with age, relative to memory for words encoded with reference to one’s mother. When used as an individual difference measure, the difference in self versus mother memory correlates with regions of the rACC associated with affective salience.
Social cognitive theory and research suggests that the cognitive representation of one’s self develops and individuates from the representation of parents in childhood and adolescence, as cognitive capacity and interpersonal experience increase (Baldwin, 1895; Blos, 1979; Damon & Hart, 1988; Erikson, 1968; Harter, 2003). One approach to observing such individuation is through the cognitive representation of self and its distinction from one’s parents. Little is known about the developing cognitive self representation as it individuates from the cognitive representation of one’s parents. Moreover, little is known about changes in brain function that underlie the maturation of individuated self-representation within childhood.
The Self in Adulthood
As a highly elaborated mental construct, an adult’s self plays an essential role in organizing and prioritizing information (Klein & Loftus, 1988; Sarbin, 1962). This is reflected in the "self-reference effect" (SRE; Rogers, Kuiper & Kirker, 1977): Adults exhibit better memory for information that they evaluate with reference to themselves than for information evaluated along other semantic dimensions like valence (Bower & Gilligan, 1979; Ferguson, Rule, & Carlson, 1983; Rogers, Kuiper & Kirker, 1977; see Symons & Johnson, 1997 for a review). The extensive elaboration and organization of the self representation is thought to be the basis for preferential memory for and facilitated processing of information referenced to the self (Klein & Loftus, 1988; Kihlstrom & Klein, 1994). Greater elaboration facilitates memory by providing a large web of semantic associations into which new information can be integrated.
Cognitive representations of close others (such as parents, spouses, and friends) are also well elaborated and may strengthen memory (Klein & Loftus, 1988; Kihlstrom & Klein, 1994). In some studies with adults, processing information with reference to an intimate other, such as one’s partner, has produced superior memory equal to that found with self-referential encoding (“close-other effect”) (Aron, Aron, Tudor & Nelson, 1991; Bower & Gilligan, 1979; Maki & McCaul, 1985). In most cases, however, memory for information encoded about close others is inferior to memory for information encoded about oneself (Lord, 1980; Ferguson et al, 1983; Ray, et al, submitted). Similarly, a meta-analysis of self-referential processing studies found that the effect size of the self-reference effect surpasses that for close others (Symons & Johnson, 1997). Thus, as indexed by memory formation, adults' cognitive representation of self is most elaborated, and the cognitive representations of close others are less elaborated, though still more elaborated than many other kinds of representations that underlie memory encoding.
Neuroimaging studies of self-referential processing in adults have shown that regions of medial prefrontal cortex (MPFC) and posterior cingulate (PCC) are commonly recruited for judgments about oneself (Craik et al., 1999; D’Argembeau et al., 2007; Kelley et al., 2002, Kircher et al., 2002; Johnson et al, 2002; Ochsner et al, 2005). Although there is some general agreement as to the neural bases of self-referential processing, the results have been mixed as to whether the representation of one's self is unique in the recruitment of these regions (Gillihan & Farah, 2005). Some studies demonstrate that judgments about oneself, a best friend, or a relative yielded equivalent MPFC and PCC activations (Lou et al., 2004; Ochsner et al., 2005; Schmitz et al., 2004). In other studies, the MPFC and rostral anterior cingulate (rACC) regions were more activated when making judgments about oneself than a close other (Heatherton et al., 2006; Vanderwal, et al., 2008). Studies of individual and cultural differences suggest that attachment and cultural differences may account for differences in activation between self and close other-referential processing in the rACC (Ray et al, submitted; Zhu, Zhang, Fan, & Han, 2007).
The Development of Self
In the developmental literature, several decades of research suggest that the cognitive representation of one’s self develops in childhood and adolescence (Baldwin, 1895; Blos, 1979; Damon & Hart, 1988; Erikson, 1968; Harter, 2003). Studies of the self-reference effect in children suggest that some form of preferential memory for self encoded items appears as early as 8 years of age and that adolescents demonstrate a self-reference effect commensurate with adults (Hammen & Zupan, 1984; Haplin, Puff, Mason & Marston, 1984; Pullyblank, Bisanz, Scott, & Champion, 1985). More specifically, the self-reference effect appears to increase from ages 6 to 8 and reaches adult levels by 10 years of age. However, there has not been a study of the “close-other effect” in children. Therefore, the developmental growth of the close-other effect is unknown, as is the growth of the self-reference effect relative to the close-other effect.
Only one imaging study has compared self-referential processing in children and adults while processing statements of social and academic competence about themselves and an imaginary social other, Harry Potter (Pfeifer, Lieberman & Dapretto, 2007). Similar to adults, children showed greater activation in MPFC for judgments about oneself relative to a fictional other. However, no imaging study has compared self-referential and close-other referential processing in children, leaving unanswered questions about the differentiation of self representation from the representation of close others.
The Present Investigation
The goal of this investigation was to examine the development of self representation in children. One objective measure of the development of self representation is the difference other is the closest possible, the child’s mother. This difference may be interpreted as an index of individuation, with a larger difference associated with greater individuation. The present investigation, therefore, used both behavioral and neuroimaging methods to examine the development of the self-reference versus close other effect and the neural correlates of its differential growth.
Experiment 1: Development of Self- and Close Other Referential Effect
In Experiment 1, we examined the growth of memory effects related to a close other (one’s mother) in children ages 7–13 and related that to the growth of the self-reference effect. We hypothesized that as children individuated with age, the self-reference effect would grow relative to the close-other effect. Further, we hypothesized that this differential development of the self-reference and close-other reference effects would occur for psychological traits, which directly tap self- and close-other representations, and not for physical descriptors, which have superficial relations to self- and close-other representations. As control conditions, we included a semantic encoding condition (valence decisions) and an orthographic, non-semantic condition (decisions on whether words were or were not outlined).
Method
Participants
Thirty seven male children between the ages of 7 and 13 years of age (M = 10.5, SD = 2.1) were recruited with fliers from the community, in compliance with Stanford University’s human subjects guidelines, to participate in a study about language processing. Participants were compensated $25 for their time. Only males were recruited for this initial study to hold constant the gender relationship of the mother to the child.
Materials
A depth of processing task was employed similar to the one that has been used in previous studies of self-referential processing in adults (Roger, Kuiper & Kirker, 1977). Two lists were constructed with 60 psychological trait words (Anderson, 1968; e.g., "kind") and 60 physical trait words (e.g., "tall").The two lists were presented in orders counterbalanced across subjects. Words were positive in valence and chosen both for their frequency of occurrence in the English language as well as for readability by 2nd graders. Stimulus presentation and behavioral response recording were controlled using Psyscope software (Cohen, MacWhinney, Flatt, & Provost, 1993).
Procedure
Each child was instructed in the task and given a short practice trial. Using a block design, each child was randomly presented with one of four instruction types designed to prompt either orthographic, valence, self, or close-other processing (respectively, “Is this word outlined?”, “Is this a nice word?”, “Is this word like you?”, “Is this word like Mom?”). After a one second inter-stimulus interval, each question was followed by the sequential presentation of five randomly selected words from the list. Participants were directed to respond to each word with either “Yes” or “No” by pressing the buttons on the button box. Each word was presented for three seconds with a one second interstimulus interval. Participants saw three repetitions of each block type (orthographic, valence, self, close-other). After twelve blocks (three each of four types, or 60 words), the participant was administered a recall task in which he was asked to recall as many words as he could.
Results
Recall was scored as the proportion (out of 15 words) remembered for each of the four encoding conditions. A 4 X 2 repeated measures ANOVA was performed, with encoding condition (orthographic, valence, self, or close-other processing) and list type (physical and psychological) as within subjects variables. There was a main effect of list, F(1,36)= 33.78, p <.001, with physical words being recalled better than psychological words (Table 1). There was also a main effect of encoding condition, (F(3,108)=15.86, p<.001). Memory was superior for words encoded in the self versus the valence condition (t(36)=2.87, p =.007) and for the valence versus the outline condition (t(36)= 4.41, p<.001). Memory for words encoded in the mother condition was numerically in between the self and valence conditions, and did not differ reliably from the self (t(36)= 0.87, p=.39), but tended towards being superior relative to the valence condition (t(36)= 1.89, p=.067). There was superior memory for physical relative to psychological trait words in the self, mother, and valence conditions (ps<.002) but not in the orthographic condition (p=.47, Figure 1). Finally, there was an interaction of encoding condition and list, (F(3,108)= 2.78, p=.045).
Table 1.
Experiment 1: Proportion of Words Recalled (Standard Deviations) and Correlation with Age
| Encoding Condition | Physical Words | Psychological Words | ||
|---|---|---|---|---|
| Proportion | Age Correlation | Proportion | Age Correlation | |
| Self | 22.0% (12.3%)a | .29† | 15.1% (13.5%)b | .46* |
| Mother | 21.6% (15.9%)a | .36* | 12.8% (10.8%) b,d | .17 |
| Nice | 19.1% (14.1%)a | .29† | 8.8% (10.9%)d,c | .50* |
| Outline | 9.0% (9.0%)c | .33* | 7.6% (8.2%)c | .09 |
p <.05,
p<.1
Figure 1.

Experiment 1 histogram of proportion of words recalled in the self, mother, valence and orthographic conditions from physical and psychological lists.
To examine age-related changes in recall, we correlated recall and age separately for physical and psychological words. For the physical words, recall improved significantly with age for words encoded in mother (r(36) = .36, p = 028) and outline (r(36) = .33, p = .047) conditions, and also tended towards significance in the self (r(36) = .29, p = .08) and valence (r(36) = .29, p = .07) conditions. Correlations with age for psychological words showed a different pattern. Recall improved significantly with age for the self (r (36) = .42, p = .01) and valence (r(36) = .50, p = .002), conditions, but did not change with age for the mother and outline conditions (ps >.31). To test our hypothesis that the self-reference effect would grow relative to the close-other effect for psychological traits but not for physical descriptors, we created a difference score by subtracting the proportion of mother words from the proportion of self words recalled. As hypothesized, this difference increased with age, r(36) = .29, p = .04 (Figure 2) for the psychological words, but not the physical words (r(36)= −.16, p=.17.
Figure 2.

Experiment 1 scatterplot of the difference between proportion of self versus mother psychological words recalled across age. Positive values on the y-axis represent better memory for self than mother encoded words, and negative values represent better memory for mother than self encoded words.
Discussion
Our findings replicate and extend prior research on memory and the development of self concept. As expected from prior findings, we found that (1) memory performance showed the self-reference effect, (2) memory performance was superior for concrete (physical descriptors) relative to abstract (psychological trait descriptors) words and for semantically encoded words relative to non-semantically encoded words, (3) memory performance improved with age, and (4) memory for semantic encoding of psychological trait words increased with age, whereas memory for orthographic encoding of psychological trait words did not increase with age.
A novel contribution of this study is that it examined children’s memory for words encoded in reference to a close other, in this case one’s mother. Consistent with adult findings, children’s memory for words encoded in reference to a close other fell numerically between self-reference and impersonal semantic encoding conditions. Importantly, age moderated the relation between memory for words encoded in self- versus close-other conditions. Memory for self-encoded trait words increased with age, whereas memory for mother-encoded trait words did not. Therefore, the difference between memory for self- and mother-encoded trait words grew significantly with age. Whereas younger children often recalled more words encoded in relation to their mothers than themselves, older children often recalled more words encoded in relation to themselves than to their mothers. One unexpected finding was that memory for mother-encoded words did not increase with age, but that memory for valence-encoded words did increase with age. Given that trait judgments about one’s mother involve semantic processing, it is noteworthy that there were not commensurate age-related increases in memory for mother-encoded and valence-encoded words. Overall, this study suggests that children ages 7–13 exhibit a growth of individuation as the self-reference effect grows disproportionately relative to the close-other effect.
Experiment 2: Neural Bases of Development of Self-Referential Processing
The goal of Experiment 2 was to elucidate the neural correlates of the individuation of self-representation that was found in Experiment 1, as indexed by the memory difference in reference to oneself versus to one’s mother. In adults, judgments about the self-relevance of trait descriptors have been shown to recruit a network of brain regions involving midline structures, including the medial prefrontal cortex (MPFC), posterior cingulate regions (PCC) and rostral anterior cingulate (rACC) (Craik et al., 1999; D’Argembeau et al., 2007; Kelley et al., 2002, Kircher et al., 2002; Johnson et al, 2002; Ochsner et al, 2005).
There is evidence that rACC activation may be especially sensitive to individual differences in the relationship of self to close other. For adult participants from a culture characterized as emphasizing personal independence (Western), activations in the rACC are greater for oneself than one’s mother, but not so for adult participants from a culture characterized as emphasizing interdependence (East Asian) (Zhu, Zhang, Fan & Han, 2007). Similarly, among American participants, activation of the rACC is greater for words encoded in relation to oneself than in relation to one’s mother, as a function of maternal attachment—greater attachment is correlated with a smaller activation difference between self- and mother-encoding conditions (Ray et al, submitted). These imaging findings suggest that adults' rACC activation is related to individuation of oneself relative to one’s mother. Therefore, we hypothesized that developmental changes in individuation may be related to differential rACC activation for self versus close-other judgments.
Experiment 2 examined activation in children performing the same kinds of judgments as in Experiment 1 (orthographic, valence, self, close-other), but for only psychological trait words because only trait words showed a difference between self and mother with age. Experiment 2 assessed memory by recognition rather than free recall, due to the need to use a larger number of stimuli for an imaging study. The key question was whether we could identify a brain region that is associated with the growth of psychological individuation, as manifested behaviorally in the difference between memory for words remembered from the self and mother conditions.
Method
Participants
Fourteen healthy male children between ages 7 and 10 were recruited (M age = 9.26 years, SD = 0.86) from the community in compliance with Stanford University’s human subjects informed consent guidelines and paid $75 in compensation for their time. All participants read and signed assent forms while their parents read and signed consent forms. All participants were righted handed, native English speakers, reading at or above grade level and having no history of neurological problems. Only males were recruited to hold constant the gender relationship of the mother to the child. One participant was dropped for failing to comply with instructions.
Procedure
Scanning was conducted at the Lucas Center at Stanford University. Each scanning session consisted of a short practice session with the task, a high resolution three dimensional anatomy scan, an in-plane anatomy scan, the four functional runs, and ended with a surprise recognition memory task outside the scanner.
Materials
One hundred sixty psychological trait adjectives selected for their high frequency in the English language and early elementary reading level (e.g., kind, funny; Anderson, 1968) were divided into four lists, one for each scanning run. The word lists were equated for valence, frequency, and reading level. Each participant viewed all four lists in a modified Latin square presentation order. Stimulus presentation and behavioral response recording was controlled using E-Prime software (Psychology Software Tools, Inc., Pittsburgh, PA). An LCD projector projected the image to a mirror attached to the head coil, reflecting the stimulus image onto a screen in the participant’s visual field. A dental impression bite bar was used to decrease head movement, and a button box placed in the participant’s right hand collected behavioral data.
Behavioral Task
During the encoding portion of the study, four different conditions were used: an orthographic baseline, valence judgments, self-reference, and close-other-reference. Respectively, these conditions were operationalized by the questions: “Are the next words lower case?”, “Are the next words nice words?”, “Are the next words like you?”, and “Are the next words like your mother?” These conditions were presented in blocks beginning with a three second presentation of the question. After a one second interstimulus interval, the question was followed by the sequential presentation of five randomly selected words without replacement. Each word was presented for three seconds with a one second interstimulus interval. Participants were directed to respond either “Yes” or “No” by pressing the buttons on the button box. They saw eight repetitions of each block type in random order. The encoding portion was broken up into four, 3 minute 14 second imaging runs.
After all four encoding runs were completed, each participant was given an unexpected recognition memory test outside of the scanner. Participants saw all 160 words again with an equal number of foils and made judgments whether they had seen the word during encoding using a one to four scale. Participants answered “1” if they believed that they definitely had not seen the word, “2” if they might not have seen the word, but were not sure, “3” if they might have seen the word, but were not sure, and “4” if they definitely remembered seeing the word.
Behavioral Data Analysis
For each participant, response latencies and accuracy were calculated for each encoding condition and for the foils. Hits were defined as those previously seen words that were recognized with high and low confidence combined whereas misses were defined as those previously seen words that were not recognized with either low or high confidence. False alarms were defined as foils that were reported as recognized with high or low confidence, and correct rejections were those foils that were not recognized with high or low confidence. Accuracy was quantified by calculating d’ for each condition (z-score(probability of hit)- z-score(probability of false alarm)).
Neuroimaging Data Acquisition and Analysis
Functional magnetic imaging was performed on a 1.5 Tesla General Electric Medical Echospeed LX CV/I MR scanner. Twenty five axial slices (3.75 mm thick with a 0.5 mm skip) were recorded during the functional scans using a T2* - sensitive two dimensional gradient echo spiral in/out pulse sequence (40 ms TE, 2000 ms TR, 90° flip angle, 24 cm field of view, 64 x 64 data acquisition matrix). T2 –weighted flow-compensated spin echo anatomical scans were acquired using the same slice prescription (3000 TR, 68 TE, 24 cm field of view, 256 x 256 data acquisition matrix). The three dimensional high resolution structural scan was acquired using a fast SPGR EDR IrP (15° flip angle, 24 cm field of view, 1.2 mm thick slices, 256 x 256 data acquisition matrix).
Imaging data was preprocessed and analyzed using SPM2 (Wellcome Department of Cognitive Neurology, Institute of Neurology, London, UK). Functional images were corrected for slice time acquisition and corrected for motion using a sinc interpolation and resampled into 2mm3 voxels. Anatomical images were coregistered to the mean functional image and both the anatomical and functional images were normalized to the Montreal Neurologic Institute (MNI) template brain and smoothed with a 6 mm full-width at half maximum (FWHM) isotropic Gaussian kernel. A 128 s high-pass filter was applied to exclude low-frequency artifacts such as scanner drift. Each participant’s data was modeled using a regressor function for the four levels of condition (case, valence, self, mother), in which each word was modeled as a 3 second epoch using a box car regressor convolved with the canonical hemodynamic response function.1 We performed a whole-brain correlation (thresholded at p < .001, minimum 5 voxel cluster) between (a) the difference in memory for words encoded under self versus mother conditions and (b) regions showing a difference in activation for self versus mother activation.
Results
Behavioral Results
A repeated measures ANOVA was performed with follow-up paired t-tests using Bonferroni correction. Based on a priori hypotheses, only the self and mother conditions are reported. For d’ sensitivity, we found that there was a trend for better memory for words encoded in the self than mother conditions (self d’ M = 1.37 (.79), mother d’ M =1.23 (.69), t(12)=-2.06, p = .062; Table 2). A correlation between age and recognition memory for words encoded in the self versus mother conditions was not significant (r(13)=.10, p=.75). Nonetheless, as we report below, despite the reduced age range and reduced number of participants by comparison with Experiment 1, there was sufficient variability to use this difference as a regressor in the functional imaging analyses, with participants who recognized more words in the mother condition and participants who recognized more words in the self condition.
Table 2.
Recognition in Experiment 2
| Encoding Response Latency | Hits | Misses | |
|---|---|---|---|
| Self | 1565.4 (250.3) | 76.2% (11.3%) | 23.8% (11.3%) |
| Mother | 1634.3 (163.0) | 72.7% (12.1%) | 27.3% (12.1%) |
|
| |||
| False Alarm | Correct Rejection | ||
|
| |||
| Foils | 32.7% (22.1%) | 67.3% (22.1%) | |
Response latency for words in the self (M = 1565.4 + 250.3) and mother conditions (M = 1634.3 + 163.0 did not differ reliably t(12) = 1.02, p=.33). Moreover, response latency did not correlate with response accuracy (ps > .144); therefore, any activation differences are not likely due to differences in the processing duration of words in the two encoding conditions.
Imaging Results
There was a significant and positive correlation between the self versus mother d’ and the self versus mother activation in the rACC (r(13) = .66, p = .015, Figure 3). As participants showed greater rACC activation for self than for mother encoding during scanning, they subsequently showed a greater memory advantage for self over mother. Conversely, as participants showed greater rACC activation for mother than for self encoding during scanning, they subsequently showed a greater memory advantage for mother over self. Similar correlations between self vs. mother encoding activation and subsequent self vs. mother memory were observed in other regions, including additional regions of the anterior and subgenual cingulate and medial orbital frontal cortex (OFC; Table 3), a region of the right inferior frontal gyrus near the insula, two regions in the left inferior frontal gyrus, and a region of the right head of the caudate.
Figure 3.
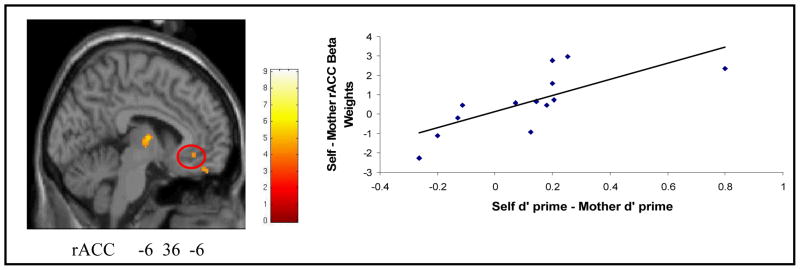
Experiment 2: Sagital view of the statistical parametric map from the regression of self d’ versus mother d’ on the contrast of self greater than mother. The scatterplot is a graphical display of the results of the whole brain regression at the peak of the rACC. The graph demonstrates the relationship between the self d’ versus mother d’ (high confidence hit – false alarm for self – mother), and the average beta weight extracted from the peak of the activation in the rostral anterior cingulate (BA 32, -6 36 6) from the Self > Mother contrast. The map meets a p<.001 uncorrected threshold with a cluster threshold of 5 voxels.
Table 3.
Regions in Regression of Self – Mother Memory on Self > Mother Contrast
| MNI Coordinates | ||||||
|---|---|---|---|---|---|---|
| Region | BA | x | y | z | # Voxels | T-value |
| Anterior Cingulate Gyrus | L 32/10 | −6 | 36 | −6 | 7 | 4.23 |
| Anterior Cingulate Gyrus | L 24 | −4 | 30 | 0 | 11 | 5.20 |
| Anterior Cingulate Gyrus | L 24 | −16 | −8 | 50 | 10 | 4.90 |
| Subgenual Cingulate Gyrus | L 25 | −2 | 28 | −20 | 11 | 5.59 |
| Medial Orbital Frontal Cortex | L 14 | −2 | 28 | −22 | ||
| Medial Orbital Frontal Gyrus | L 11 | −2 | 52 | −18 | 22 | 4.61 |
| Inferior Frontal Gyrus | L 45/47 | −56 | 32 | 6 | 13 | 5.27 |
| Inferior Frontal Gyrus | L 47 | −40 | 22 | −12 | 10 | 5.58 |
| Inferior Frontal Gyrus | R 47 | 30 | 30 | −8 | 49 | 8.96 |
| Medial Frontal Gyrus | R 6 | 14 | −16 | 52 | 11 | 4.90 |
| Caudate Head | R | 12 | 16 | −2 | 85 | 6.91 |
| Ventral Thalamic Nucleus | R | 16 | −12 | 14 | 50 | 5.70 |
| Anterior Thalamic Nucleus | L | −6 | −6 | 8 | 63 | 5.57 |
Note: Contrast threshold of p <.001 with cluster threshold of k=5
Discussion
The present study was the first to investigate the neural bases of the individuation of the self-representation of self from the representation of one’s mother in children, as measured by superior memory for self versus mother encoded information. The critical new finding was that the difference in self and mother recognition memory correlated positively with the difference in self and mother encoding activations in the scanner in regions of the rACC, subgenual cingulate, and medial and lateral OFC. Participants showed marginally significant superior recognition memory for psychological trait words encoded about themselves than about their mothers, similar to the recall findings in Experiment 1, but the age correlation with that memory difference evident in Experiment 1 was not observed in Experiment 2. The two experiments were similar in two ways—psychological trait words were presented in blocks and in identical encoding conditions.
The two experiments also differed in several ways, including recall versus recognition memory measures, age ranges ( 7–13 years in Experiment 1, 7–10 years in Experiment 2), and numbers of participants (37 in Experiment 1, 14 in Experiment 2). In general, recall memory measures are known to be more sensitive than recognition memory measures for the self-reference effect (Symons & Johnson, 1997). Also, the constricted age range and reduced power of Experiment 2 may have influenced the lack of a measurable age effect in Experiment 2.
Despite these limitations, there were significant correlations between the self versus mother encoding activation in multiple regions, and the subsequent self versus mother memory difference. Most notable among these activations, the rACC region has previously shown activation differences in adults, in relation to cultural influences on self and mother referential processing (Zhu, Zhang, Fan, & Han, 2007), as well as in relation to degree of maternal attachment (Ray et al, submitted). Furthermore, both this region of the rACC as well as the regions of bilateral inferior frontal cortex that we observed have been associated with processing the valence of self relevant items (Moran et al, 2006), suggesting that affective processing may contribute to individual differences in memory for mother- or self-encoded information. Finally, differential activations in bilateral inferior prefrontal cortex as well as right caudate head have been previously associated with the interaction of affect and memory (Adcock, Thangavel, Whitfield-Gabrieli, Knutson & Gabrieli, 2006; Longe, Senior & Rippon, 2009; Wittmann, Schott, Guderian, Frey, Heinze & Düzel, 2005). These findings suggest a role for affect to influence differences in memory for self versus mother encoded information.
General Discussion
The present study is the first to examine children’s behavioral and brain responses while processing information about both the self and a close other (their mother), and the first to find an association between differential patterns of neural activation and an index of the individuation process. These findings offer a first view of the individuation of self-concept, defined as a separation of self from close-other, both in memory performance and in brain function.
Experiment 1 demonstrated a shift, across late childhood and into early adolescence, from preferential memory for mother-encoded information to self-encoded information. Memory for self-referentially encoded items increased with age, whereas memory for information encoded with reference to one’s mother showed little to no increase as overall memory improved.
Experiment 2 used neuroimaging methods to leverage individual differences in memory for self- versus mother-encoded items, and identified regions that were more active when encoding the type of word for which the child subsequently exhibited better memory. Of particular note is the pattern of activation in the ventral portions of the rACC, which have been associated with affectively valenced components of self-referentially processed information (Moran et al., 2006). These regions were recruited more when judging information about oneself than one’s mother, by children who subsequently showed better memory for self than mother items. Conversely, for those children who subsequently remembered more mother encoded items than self encoded items, these same regions were recruited more when making judgments about their mother than about themselves.
Prior studies investigating the relationship between self- and mother-referential processing in adults have identified the rACC as being sensitive to relational differences. For example, the rACC was more active when making judgments about oneself than about one’s mother for participants from Western cultures; however, no difference was found for those from an East Asian culture (Zhu, Zhang, Fan, & Han, 2007). Also, individual differences in attachment to one’s mother predicted less difference in activation of the rACC for self and mother judgments in adults (Ray et al, submitted). The present findings converge well with these studies, suggesting that greater individuation from mother corresponds to greater activation in the rACC when making judgments about oneself, but not in other areas commonly activated by self-reference.
These findings provide support for theories of the social construction of self representation which suggest that, as children’s social processing develops through elementary and middle school (6–11 years), the representations of the self and of social others become more complex (Damon & Hart 1988; Harter, 1999; Harter, 2003; Rosenberg, 1979). In the transition from late childhood to early adolescence, due to increasing self-awareness, children shift from a parent-dominated view of themselves to a self system in which the parental perspective is less influential (Mazer & Enright, 1982). Instead of parents narrating the salient or emotional contents of children’s lives, adolescents orient more towards their peers or their internal states to determine the importance and emotional significance of events (Allen & Land, 1999; Berndt & Perry, 1990; Hazan & Zeifman, 1994; Nickerson & Nagle, 2005; Steinberg & Silverberg, 1986). Our results are also broadly consistent with many psychoanalytic theories in which, starting from early infancy, a child's individuation is understood to be, in part, a progressive waning of an initial, undifferentiated state, in which one’s identity is inextricably bound-up with one's mother (e.g., Mahler, 1968; Winnicott, 1964). Furthermore, these results complement the literature on peer relationships, which suggests that during early adolescence, parents begin to play a less influential role in children’s lives and peers take on a more influential role (Allen & Land, 1999; Berndt & Perry, 1990; Hazan & Zeifman, 1994; Nickerson & Nagle, 2005). Our own study offers an objective cognitive experimental index of such differentiation, and initial evidence about the neural basis of that individuation.
Even though this study provides new information for our understanding of the development and individuation of self representations, it has important limitations. This was a first study, in which we simplified the problems of gender by examining the memory processes in children of one sex, boys, in relation to the parent of the opposite sex, their mothers. Future investigations will need to consider how the gender of the child and the gender of the parent affect the observed behavioral and brain patterns. For example, it is possible that boys and girls individuate from their parents at different ages, or that boys and girls differentiate from the opposite sex parent at one age but the same sex parent at a different age. How children utilize their neurobiological resources in the service of memory may differ as a function of the differing skill level such age differences could produce.
Children’s development into autonomously functioning adults is predicated upon the construction of a self-concept that acts as a reference point to filter and to organize incoming information. This study indexed individuation in participants between 7 and 13, in terms of differences in memory for self-encoded information versus mother-encoded information. The individual differences found in memory map on to differential activation of neural mechanisms. Notable are the differential patterns of activation observed in a region associated with cultural differences in inclusion of the other in the self, with individual differences in maternal attachment, and with processing affective valenced components of self-referential information. The present investigation provides behavioral grounding for a child's representation of self and of close other, and it identifies a specific neural basis for a mechanism and for that mechanism's changing role in processing information about self and close other, as a child individuates.
Acknowledgments
The first author was supported by the MH58147 and MH20006 grant during data collection and the MH18921 grant during manuscript preparation. These studies were completed as a part of the first author’s dissertation. This paper is dedicated to our children.
Footnotes
The data were also modeled as 1 second events and produced the same results only slightly weaker.
References
- Adcock RA, Thangavel A, Whitfield-Gabrieli S, Knutson B, Gabrieli JDE. Reward-motivated learning: Mesolimbic activation precedes memory formation. Neuron. 2006;50:507–517. doi: 10.1016/j.neuron.2006.03.036. [DOI] [PubMed] [Google Scholar]
- Allan JP, Land D. Attachment in adolescence. In: Cassidy J, Shaver PR, editors. Handbook of attachment: Theory, research, and clinical applications. New York: Guilford; 1999. pp. 319–335. [Google Scholar]
- Anderson NH. Likableness ratings of 555 personality-trait words. Journal of Personality and Social Psychology. 1968;9:272–279. doi: 10.1037/h0025907. [DOI] [PubMed] [Google Scholar]
- Aron A, Aron EN, Tudor M, Nelson G. Close relationships as including other in the self. Journal of Personality and Social Psychology. 1991;60:241–253. [Google Scholar]
- Baldwin JM. Mental development of the dhild and the race: Methods and processes. New York: Macmillan; 1895. [Google Scholar]
- Berndt TJ, Perry TB. Distinctive feature and effects of early adolescent friendships. In: Montemayor R, Adams GR, Gullotta TP, editors. From childhood to adolescence: A transitional period? Newbury Park, CA: Sage; 1990. pp. 269–287. [Google Scholar]
- Blos P. The adolescent passage. New York: International Universities Press; 1979. [Google Scholar]
- Bower GH, Gilligan SG. Remembering information related to oneself. Journal of Research in Personality. 1979;13:420–32. [Google Scholar]
- Bruner J. The transactional self. In: Bruner J, Haste H, editors. Marking sense: The child’s construction of the world. London: Methuen; 1987. pp. 81–96. [Google Scholar]
- Cohen JD, MacWhinney B, Flatt M, Provost J. PsyScope: A new graphic interactive environment for designing psychology experiments. Behavioral Research Methods, Instruments, and Computers. 1993;25(2):257–271. [Google Scholar]
- Craik FIM, Moroz TM, Moscovitch M, Stuss DT, Winocur G, Tulving E, Kapur S. In search of the self: a positron emission tomography study. Psychological Science. 1999;10:26–34. [Google Scholar]
- Damon W, Hart D. Self-understanding in childhood and adolescence. New York: Cambridge University Press; 1988. [Google Scholar]
- D’Argembeau A, Ruby P, Collette F, Degueldre C, Balteau E, Luxen A, Maquet P, Salmon E. Distinct regions of the medial prefrontal cortex are associated with self-referential processing and perspective taking. Journal of Cognitive Neuroscience. 2007;19:935–944. doi: 10.1162/jocn.2007.19.6.935. [DOI] [PubMed] [Google Scholar]
- Erikson EH. Identity, youth, and crisis. New York: Norton; 1968. [Google Scholar]
- Ferguson TJ, Rule GR, Carlson D. Memory for personally relevant information. Journal of Personality and Social Psychology. 1983;44:251–261. [Google Scholar]
- Fivush R. Scripts and categories: Interrelationships in development. In: Neisser U, editor. Concepts and conceptual development: Ecological and intellectual factors in categorization. Cambridge, UK: Cambridge University Press; 1987. [Google Scholar]
- Gillihan SJ, Farah MJ. Is self special? A critical review of evidence from experimental psychology and cognitive neuroscience. Psychological Bulletin. 2005;131:76–97. doi: 10.1037/0033-2909.131.1.76. [DOI] [PubMed] [Google Scholar]
- Halpin JA, Puff CR, Mason HF, Marston SP. Self-reference encoding and incidental recall by children. Bulletin of the Psychonomic Society. 1984;22:87–89. [Google Scholar]
- Hammen C, Zupan BA. Self-schemas, depression, and the processing of personal information in children. Journal of Experimental Child Psychology. 1984;37:598–608. doi: 10.1016/0022-0965(84)90079-1. [DOI] [PubMed] [Google Scholar]
- Harter S. In: The construction of self: A developmental perspective. Fischer KW, Higgins ET, editors. New York, NY: Guilford Publications, Inc; 1999. [Google Scholar]
- Harter S. The development of self-representations during childhood and adolescence. In: Leary MR, Tangney JP, editors. Handbook of Self and Identy. New York, New York: The Guilford Press; 2003. pp. 610–642. [Google Scholar]
- Hazan C, Zeifman . Sex and the psychological tether. In: Bartholomew K, Perlman D, editors. Attachment processes in adulthood. Advances in personal relationships. Vol. 5. London: Jessica Kingsley; 1994. pp. 151–177. [Google Scholar]
- Heatherton TF, Wyland CL, Macrae CN, Demos KE, Denny BT, Kelley MW. Medial prefrontal activity differentiates self from close others. Social Cognitive and Affective Neuroscience. 2006;1:18–25. doi: 10.1093/scan/nsl001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SC, Baxter LC, Wilder LS, Pipe JG, Heiserman JE, Prigatano GP. Neural correlates of self-reflection. Brain. 2002;125:1808–1814. doi: 10.1093/brain/awf181. [DOI] [PubMed] [Google Scholar]
- Kelley WM, Macrae CN, Wyland CL, Caglar S, Inati S, Heatherton TF. Finding the Self? An Event-Related fMRI Study. Journal of Cognitive Neuroscience. 2002;14:785–795. doi: 10.1162/08989290260138672. [DOI] [PubMed] [Google Scholar]
- Kircher TT, Brammer M, Bullmmore E, Simmons A, Bartels M, David AS. The neural correlates of intentional and incidental self processing. Neuropsychologia. 2002;40:683–692. doi: 10.1016/s0028-3932(01)00138-5. [DOI] [PubMed] [Google Scholar]
- Klein SB, Loftus J. The nature of self-referent encoding: The contributions of elaborative and organizational processes. Journal of Personality and Social Psychology. 1988;55:5–11. [Google Scholar]
- Kihlstrom JF, Klein SB. The self as a knowledge structure. In: Wyer RS, Srull TK, editors. Handbook of Social Cognition: Basic Processes. Vol. 1. Hillsdale, NJ: Lawrence Erlbaum Associates; 1994. pp. 153–208. [Google Scholar]
- Lane RD, Reiman EM, Axelrod B, Yun L, Holmes A, Schwartz GE. Neural correlates of levels of emotional awareness: Evidence of an interaction between emotion and attention in the anterior cingulate cortex. Journal of Cognitive Neuroscience. 1998;10:525–535. doi: 10.1162/089892998562924. [DOI] [PubMed] [Google Scholar]
- Longe O, Senior C, Rippon G. The lateral prefrontal cortex and the ventromedial prefrontal cortex work as a dynamic integrated system: Evidence from functional magnetic resonance imaging connectivity analysis. Journal of Cognitive Neuroscience. 2009;21:1–14. doi: 10.1162/jocn.2009.21012. [DOI] [PubMed] [Google Scholar]
- Lord CG. Schemas and images as memory aids: Two modes of processing social information. Journal of Personality and Social Personality. 1980;38:257–269. [Google Scholar]
- Lou HC, Luber B, Crupain M, Keenan JP, Nowak M, Kjaer TW, Sackeim HA, Lisanby SH. Parietal cortex and representation of the mental Self. Proceedings of the National Academy of Science. 2004;101:6827–32. doi: 10.1073/pnas.0400049101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macrae CN, Moran JM, Heatherton TF, Banfield JF, Kelley WM. Medial prefrontal activity predicts memory for self. Cerebral Cortex. 2004;14:647–654. doi: 10.1093/cercor/bhh025. [DOI] [PubMed] [Google Scholar]
- Mahler MS. On human symbiosis and the vicissitudes of individuation. New York, NY, USA: International Universities Press; 1968. [DOI] [PubMed] [Google Scholar]
- Maki RH, McCaul KD. The effects of self-reference versus other reference on the recall of traits and nouns. Bulletin of the Psychonomic Society. 1985;23:169–172. [Google Scholar]
- Markus HR. Self-schemata and processing information about the self. Journal of Personality and Social Psychology. 1977;35:63–78. [Google Scholar]
- Mazer A, Enright RD. The development of the individuation process from a social-cognitive perspective. Journal of Adolescence. 1982;11:29–47. doi: 10.1016/s0140-1971(88)80021-6. [DOI] [PubMed] [Google Scholar]
- Mitchell JP, Macrae CN, Banaji MR. Dissociable medial prefrontal contributions to judgments of similar and dissimilar others. Neuron. 2006;50:655–63. doi: 10.1016/j.neuron.2006.03.040. [DOI] [PubMed] [Google Scholar]
- Moran JM, Macrae CN, Heatherton TF, Wyland CL, Kelley WM. Neuroanatomical evidence for distinct cognitive and affective components of self. Journal of Cognitive Neuroscience. 2006;18:1586–1594. doi: 10.1162/jocn.2006.18.9.1586. [DOI] [PubMed] [Google Scholar]
- Nelson K. Event knowledge: Structure and function in development. Hillsdale, NJ: Erlbaum; 1986. [Google Scholar]
- Nelson K. The psychological and social origins of autobiographical memory. Psychological Science. 1993;4:7–14. [Google Scholar]
- Nickerson AB, Nagle RJ. Parent and peer attachment in late childhood and early adolescence. Journal of Early Adolescence. 2005;25:223–249. [Google Scholar]
- Ochsner KN, Beer JS, Robertson ER, Cooper JC, Gabrieli JD, Kihsltrom JF, D'Esposito M. The neural correlates of direct and reflected self-knowledge. NeurIimage. 2005;28:797–814. doi: 10.1016/j.neuroimage.2005.06.069. [DOI] [PubMed] [Google Scholar]
- Pfeifer JH, Lieberman MD, Dapretto M. “I know you are but what am I!?” Neural bases of self- and social knowledge bases in children and adults. Journal of Cognitive Neuroscience. 2007;19:1323–1337. doi: 10.1162/jocn.2007.19.8.1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pullyblank J, Bisanz J, Scott C, Champion MA. Developmental invariance in the effects of functional self-knowledge on memory. Child Development. 1985;56:1447–1454. [Google Scholar]
- Ray RD, Hollon NG, Shelton A, Gross JJ, Gabrieli JDE. Our mothers, ourselves: Overlapping neural representations of self and mother submitted. [Google Scholar]
- Rogers TB, Kuiper NA, Kirker WS. Self-reference and the encoding of personal information. Journal of Personality and Social Psychology. 1977;35:677–688. doi: 10.1037//0022-3514.35.9.677. [DOI] [PubMed] [Google Scholar]
- Rosenberg M. Conceiving the Self. New York: Basic Books; 1979. [Google Scholar]
- Sarbin TR. A preface to a psychological analysis of the self. Psychological Review. 1962;59:11–22. doi: 10.1037/h0058279. [DOI] [PubMed] [Google Scholar]
- Schmitz TW, Kawahara-Baccus TN, Johnson SC. Metacognitive evaluation, self-relevance, and the right prefrontal cortex. Neuroimage. 2004;22:941–7. doi: 10.1016/j.neuroimage.2004.02.018. [DOI] [PubMed] [Google Scholar]
- Snow K. Building memories: The ontogeny of autobiography. In: Cicchetti D, Beeghly M, editors. The self in transition: Infancy to childhood. Chicago: University of Chicago Press; 1990. pp. 213–242. [Google Scholar]
- Symons CS, Johnson BT. The self-reference effect in memory: A meta-analysis. Psychological Bulletin. 1997;121:371–394. doi: 10.1037/0033-2909.121.3.371. [DOI] [PubMed] [Google Scholar]
- Vanderwal T, Hunyadi E, Grupe DW, Connors CM, Schultz RT. Self, mother and abastract other: An fMRI study of reflective social processing. NeuroImage. 2008;41:1437–1446. doi: 10.1016/j.neuroimage.2008.03.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsh-Ross MK, Fasig LG, Farrar MF. Predictors of preschoolers’ self-knowledge: Reference to emotion and mental states in mother-child conversations about past events. Cognitive Development. 1999;14:401–422. [Google Scholar]
- Winnicott DW. The child, the family and the outside world. Harmondsworth, UK: Penguin Books; 1964. [Google Scholar]
- Wittmann BC, Schott BH, Guderian S, Frey JU, Heinze H, Düzel E. Neuron. 2005;45:459–467. doi: 10.1016/j.neuron.2005.01.010. [DOI] [PubMed] [Google Scholar]
- Zhu Y, Zhang L, Fan J, Han S. Neural basis of cultural influence on self- representation. NeuroImage. 2007;34:1310–1316. doi: 10.1016/j.neuroimage.2006.08.047. [DOI] [PubMed] [Google Scholar]


