Abstract
Polychlorinated biphenyls (PCBs) are pervasive environmental contaminants that can have damaging effects on physiologic, motoric and cognitive function. Results from studies on PCBs and behavior have shown that exposure can alter learning and memory processes and that these shifts in cognitive abilities can be related to changes in hormonal and neural function. Little experimentation has been done on the impact of exposure to PCBs on social and emotional development. Previous work has shown that exposure to PCBs in children can alter play behavior. Importantly, exposure to PCBs has been found to change aspects of maternal–offspring interactions in rodents. The present study examined the impact of PCBs on maternal odor conditioning in rat pups 12–14 days of age. A modified version of the conditioned place preference paradigm was used that incorporated a maternal-associated odor cue (lemon scent) as the conditioned stimulus. PCBs significantly depressed the preference for the maternal-associated cue but did not impair discrimination for a novel odor. These effects could arise due to changes in the social dynamics between the dam and offspring after co-exposure to PCBs. For example, dams exposed to PCBs during gestation have been found to show elevated grooming directed towards pups exposed to PCBs. This change in maternal care can have dramatic effects on behavioral and hormonal systems in the developing rat pup. In conclusion, perinatal PCBs alter important social behaviors of both the mother and pup, and these alterations could have long-lasting effects on behavioral, cognitive and emotional development.
Keywords: Endocrine disruptor, Social behavior, Attachment, Development, Toxicant
1. Introduction
Polychlorinated biphenyls (PCBs) are environmental endocrine disruptors that can have significant impact on the development of diverse physiological and psychological functions [1–3]. Manufacturing of PCBs was terminated in the USA in 1977 but they persist in the global environment [4–6]. Humans and other animals are continuously exposed to low levels of PCBs from soil, air and food sources [7,8]. In populations in which there are large amounts of fish consumption, PCBs can bioaccumulate and noticeably alter physical and motor development [9,10]. Studies on natural animal populations consistently find moderate-to-high levels of PCBs in many diverse animal groups [range in concentration from 3 to 500 ng/g; [11–13]] and it is principally unknown as to how this exposure influences development of psychological function. Lower, continuous exposure could be leading to less noticeable insults that impact more complex behaviors.
Persistence of PCBs can be well appreciated from a recent report by the United Nations Environment Programme and the World Health Organization [14]. In this report, PCBs are recognized as a major threat to human health with exposure occurring from ingestion of contaminated food substances and in lesser amounts from air or water sources. Adverse effects include lower birth weights in children, shorter gestational periods, impaired autonomic function, weak reflexes and attentional deficits [1–3, 15]. Developing fetuses and newborns are the most vulnerable, obtaining more concentrated PCBs from the placenta or from breast milk during suckling [16,17].
Previous studies in humans exposed to PCBs following accidental spills have found significant alterations in motor, sensory and cognitive abilities [15,18–20]. Animal models have supported these findings. Exposure to PCBs alters general motor function in mice and rats [21,22]. Other work has focused on the cognitive impairments caused by exposure to PCBs [2,23,24]. Meserve and colleagues [25–27] have focused on the relationship between cognition and PCB-induced hormonal and neural alterations. Studies found that animals exposed to PCBs have depressed choline acetyltransferase activity [25] and deficits in working and spatial memory as revealed by performance in the radial arm maze and Morris water maze tasks [26,27]. Several of these cognitive behavioral alterations have been found to be most prominent in juvenile animals (15–30 days of age) compared to young adulthood (~60 days of age).
Few studies have been completed that solely focus on social functional alterations after chemical exposure. Simons and colleagues (2005) found that administration of PCB to pregnant dams altered maternal care directed to the rat pups [28]. An elegant follow-up study by Cummings and colleagues (2005) used cross-fostering to tease apart the maternal and pup contributions to the altered care [29]. The pups’ exposure to PCBs led mainly to the alteration in nursing bouts and maternal autogrooming while increases in time on the nest and allogrooming were mainly due to the dynamic interaction of PCB exposure to both pups and mom. Related human/clinical work examines a host of social behaviors in different settings such as the home or school environment [30,31]. Other social-related research has focused on the effects of PCBs on reproductive behavior and fertility [32,33]. One of the recent reports on social behavior described the effects of PCBs on social play interactions in children in a Dutch cohort [34]. This study revealed that boys with a higher level of PCBs scored higher on the feminine scale and lower on the masculine scale of a questionnaire indirectly gauging play activity. For girls the opposite relationship was observed with individuals with higher levels of PCBs showing more masculine behaviors. The findings have led to publication of several critiques [35,36]. Several problems exist when examining natural, human populations including exposure to a variety of pollutants (PCBs, dioxin and others) and the usage of an indirect measure of social behavior. The present study reports on early mother–offspring interactions that may facilitate later social behavior such as play interaction and aid in the production of normal cognitive and emotional development. One innovative aspect is inclusion of the determination of the impact of toxicants on pup behavior and not maternal care per se. There are few studies that have examined early social tendencies or behaviors of the developing offspring this early in the lifespan [37–40]. By using an animal model to explore this issue, we can control for the type and concentration of exposure to PCBs and make comparisons between groups treated identically except for toxicant exposure.
2. Methods
2.1. Animals
All animals (adults and pups) were housed in 12 hour light/dark cycle at 24 °C. Breeding rats were obtained from Harlan–Sprague–Dawley distributors (Indianapolis, IN, USA). Female rats weighing 225–275 g were mated to males of the same strain. Females were determined to be pregnant as confirmed by a sperm positive vaginal smear. After parturition, rat pups were housed together with the dam in their natal group.
2.2. Exposure to PCBs
From the first day of pregnancy, pregnant females were housed individually and fed either standard laboratory chow or chow with PCB mix of 47/77 added at either 12.5 ppm or 25 ppm (w/w). PCB 47 (2,2′, 4,4′-tetrachlorobiphenyl) and PCB 77 (3,3′ 4,4′-tetrachlorobiphenyl) were obtained from AccuStandard, Inc., New Haven, CT., USA. This mixture of PCBs was used because it has been found in previous work to significantly alter thyroid hormone function [41], and it mimics possible combinations of ortho-substituted and non-ortho-substituted congeners found in nature and in exposed animal populations. Stock PCB mixture was dissolved in absolute ethanol, mixed with 100 g of rat chow (Mowlan Teklad, Madison WI, USA), and ethanol was allowed to evaporate. Equal amounts of PCBs 47- and 77-containing diet were mixed together and formulation of 12.5 ppm and 25 ppm doses was completed by adding the appropriate weight of this concentrated mixture to sufficient unaltered diet to give a weight of 1000 g, then thoroughly mixed by prolonged tumbling in a sealed container. Food consumption of each animal was determined by weighing the remaining food daily. This protocol has been used in several of our (L.A.M.) previous studies and we are confident that the animals ingest the food at normal rates compared to normal laboratory chow intake (see supporting data below). Control animals were continued on standard rat chow after conception.
2.3. Conditioned odor preference
2.3.1. Apparatus
(Postnatal Days (PND) 12–14) A two compartment conditioned place preference (CPP) apparatus was used and composed of a Plexiglas chamber (8×18 cm; width × length) with a floor of parallel metal rods that allowed for air flow from below where two glass containers were located at each end. The chamber was arranged such that a 3 cm strip separated the regions over the two glass containers. One cottonball was placed inside each glass container. The cottonball in one of the containers was sprayed with 1 ml pure lemon extract. The cottonball of the second glass container was sprayed with 1 ml of water to control the humidity between compartments.
2.3.2. Habituation phase
Prior to the conditioning sessions, an exploration/habituation trial was completed. In this trial each rat pup (PND 12) was observed as they explored the environment of the conditioned place preference apparatus. The exploration/habituation period lasted for 1 min.
2.3.3. Conditioning
Conditioning trials took place on PND 13. Three thirty minute odor-conditioning trials were completed for each litter. Each conditioning session was preceded by 3 h of maternal deprivation in which pups were placed along with their littermates in a 30×20×10 cm plastic tub located inside a temperature and humidity regulated environmental chamber. Temperature for the pups during isolation was maintained at 35 °C and relative humidity was maintained at approximately 50%.
Conditioning sessions consisted of placing the pups into one of two different conditioning groups. Each PCB-exposed litter and control litter was divided into the 2 different conditioning subgroups. Animals within each experimental (PCBs) or control litter were randomly chosen from the group to be placed into either:
Group 1. Dam-odor group. This group was placed with the mother in a 40×23×13 plastic tub. The mother was sprayed with lemon extract (1 ml) on the ventral surface prior to placing her in the tub.
Group 2. Odor-alone group. This group was placed into the same size tub with four cottonballs each sprayed with 0.25 ml of the same lemon extract.
At the end of the third conditioning session, the ventral surface of the mother was well washed using soap and water and she was returned along with the pups to the home cage.
2.3.4. Testing
(PND 14): Test days immediately followed the conditioning day. Each testing session consisted of giving individual rat pups equal access to both sides of the CPP chamber for a 5 minute session. All test sessions were recorded on DVDs for future slow motion action scoring by observers blind to the experimental conditions. Rats were run in counterbalanced order so that half of the animals experienced the lemon odor over the left-side glass containers and half of the animals experienced the lemon odor over the right-side glass containers.
2.4. Novel odor approach
Immediately after the conditioned odor preference test, each rat was tested in the novel odor approach test. Novel odor approach was completed using a 37×7.5 cm (length × width) straight alley with start and goal ends. Pups were tested individually in the straight alley and placed in the start end of the alley at the beginning of each trial. At the goal end of the alley, a small jar contained a cottonball that had been sprayed with either 1 ml of lemon extract or with 1 ml of peppermint extract. The small bottle faced the start end of the alley in each trial. The alley was covered with a strip of Plexiglas to prevent the rat pups from escaping and to reduce the dispersion of the odor from the apparatus. At the beginning of the trial, the rat pup was placed in the start end facing the goal end. The time to reach the goal end was recorded for each subject. The animal was considered to have made it to the goal box after its nose crossed the plane of the goal box. If the pup did not reach the goal box within 60 s, then 60 s was recorded as the score for that trial. All pups received a trial for the lemon and for the peppermint extract odors. Separate alleys were used for the two different odors. Exposure order to the two odors was counterbalanced for each litter tested.
2.5. Behavioral scoring
Rat pup behavior from the conditioned odor preference test was scored from DVDs using a computer behavioral scoring program (Rockwell Instruments). The scoring software allowed the viewing of the behavior and the accurate measuring of both the time spent in the left and right compartments and the number of compartment entries the rat pups made into the individual locations. Rats were deemed to have entered into one of the compartments when their snout and two forelimbs crossed the plane into the compartment.
2.6. Statistics
We completed a series of analyses of variances (ANOVAs) on the data. For the maternal and pup weight data, we performed a mixed design (3×2×2×3) ANOVA with the within subjects factor of week of measurement (Weeks 1–3) and between subjects factors of gender, level of exposure to PCBs and littersize. In order to use littersize as a factor, we divided the litters (range from 8 to 18) into three categories small, medium and large. The small range=8–11 pups; medium range=12–14 pups and large range=15–18 pups. The two extremes have a larger range due to the fewer litters within that range. We completed an ANOVA on maternal food intake using these same factors. For the conditioned odor preference we completed a four factor ANOVA on the time duration and compartment entries using the within subjects factors of lemon and no-odor compartments and the between subject factors of exposure to PCBs, conditioning experience, and gender. Finally, we performed an ANOVA on the conditioned approach data using the between subject variables of exposure to PCBs (3 levels). All ANOVA analyses that reached significance (p<0.05) were followed with pairwise t-tests to examine the differences between the different groups and within-subject factors.
3. Results
3.1. Body weights
Data were obtained from seven control litters, six PCB 12.5ppm litters and eight PCB 25ppm litters. Litter sizes ranged from 11 to 18 pups in the control group (mean littersize =15.4), 11–17 pups in the PCB 12.5ppm group (mean=13.3) and 8–15 pups in the PCB 25ppm group (mean=12). There was no significant difference in numbers of pups/litter among the different groups (p=0.28). We inspected weights of the dams from all three groups both prenatally and postnatally during ingestion of PCBs. We analyzed maternal body weight gain over time with a three factor ANOVA (PCB and littersize as between group factors and time as the repeated measures factor). We did find a major impact of time on weight in that there was a significant difference in percent weight gain of the dam from Week 1 to Week 3 gestation (F(2,32)=18.54, p<0.001) (Fig. 1A). There were no significant differences dependent upon the factors of toxicant exposure (p=0.29) or littersize (p=0.28). In addition, no interaction effects were obtained between the different factors (Fig. 1A; p=0.13–0.65). Postnatally, we found similar results for maternal weights (Fig. 1B). There was a significant increase in weight from Week 1 to Week 3 after parturition (F(2,34)=6.09, p<0.01) but no significant differences among the different conditions (p =0.11) or between litters of different sizes (p=0.24) (see Fig. 1B).
Fig. 1.
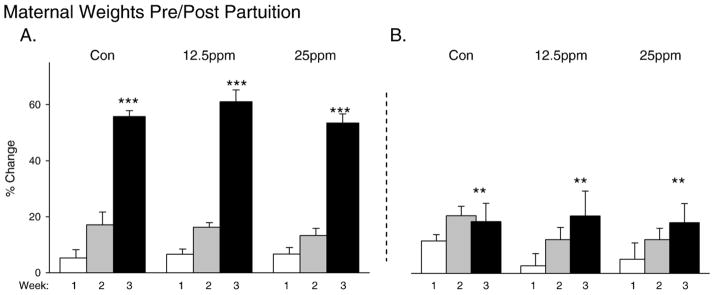
Weights of dams during the 3 week periods before (A) and after (B) parturition. There was a significant weight gain over the 3 week period but no significant effects of exposure to PCBs. *p<0.05, **p<0.001 and ***p<0.001 for this and all following figures within this manuscript.
Pups were weighed as a group (litter) for the first 3 weeks after birth (see Fig. 2). There was a significant gain in the litter weight during the first 3 weeks after birth (F(2,34)=273.6, p<0.001). This weight gain was not significantly different among the litters from the different conditions (p=0.17) or among the different litters based on size (p=0.13). We also examined the weights of the pups individually at a juvenile time period (PN Day 25). A significant effect of condition was found between PCBs-exposed and control animals (F(2,210)=5.4, p<0.01). Surprisingly, the difference reflects an actual larger weight for the PCBs-exposed animals (mean=58 g) compared to the control animals at this time point (mean=50.5 g). In addition we found no significant difference between male and female animals at this age and a lack of an interaction between exposure to PCBs and gender. There was no significant difference for the littersize factor for the pups at this later age (p=0.53).
Fig. 2.
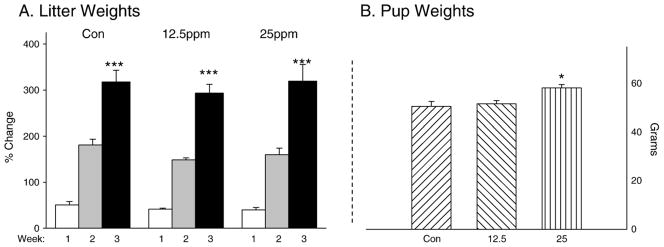
A. Litter weight after birth for all groups. Again a significant effect of time was apparent but no significant effect for the ingestion of PCBs. B. Juvenile weights showed that pups exposed to PCBs were actually heavier than controls.
3.2. Ingestion of PCBs
We recorded daily intake of normal chow and chow laced with PCBs for the dams prior to parturition (see Fig. 3A) and the dam while nursing the pups postnatally (see Fig. 3B). We converted scores to percentage change in intake on Days 4, 10 and 18, as compared to Day 1 either following conception or after parturition. There was a significant increase in chow intake from the first to the last time point sampled (dam alone, F(2,34)= 19.05, p<0.01 and dam+pups (F(2,34)=26.39, p<0.01). There was no significant effect for exposure to PCBs on food intake and no significant interaction between intake over time and PCBs-exposure condition.
Fig. 3.
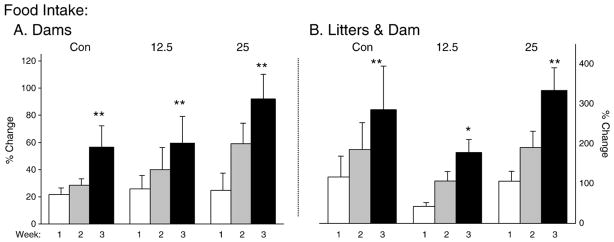
Ingestion of PCBs before (A) and after (B) parturition. Rat chow was ingested at an equal rate in both control and PCB groups. Rat chow in the PCB-exposed groups was laced with PCB mix 47/77 at either 12.5 or 25 ppm (w/w).
3.3. Conditioned odor preference: time duration
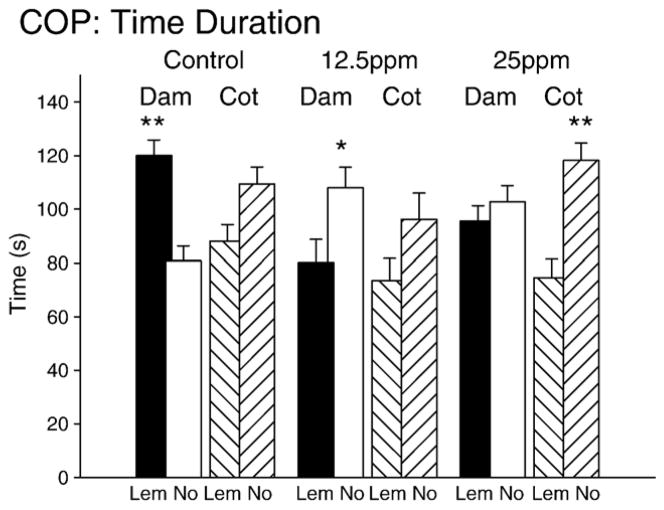
For the analysis of conditioned odor preference, we examined both the duration of time spent by the pups in either lemon-scented or the non-scented compartments of the test apparatus and the number of entries the animal made into either of these compartments (Figs. 4 and 5). Our analysis obtained significant effects for the time duration as a within-subjects factor. Overall, animals spent significantly more time in the lemon-scented compartment (F(1,249)=8.3, p<0.01). Importantly, the statistical results revealed a main effect for exposure to PCBs supporting a difference between the various groups (F(2,249)=3.39, p<0.05). No significant differences were found for the gender factor (p=0.33) so all future analyses combined data from the male and female rat pups within a given treatment condition. Significant interaction effects were found for time duration and ingestion of PCBs (F(2,249)=6.0, p<0.01) and time duration and associative history (dam or cottonball) (F(2,249)=9.7, p<0.01) as well as a three way interaction for time duration, exposure to PCBs and associative conditioning history (dam or cottonball) (F(2,249)= 4.27, p<0.05). These results signify that the animals demonstrated a specific preference for the lemon-scented location but that preference depended upon both the exposure to PCBs and the associative learning history. Paired t-tests within each group identified a significant difference between lemon and no-odor time in the control group paired with the dam (t(49)=4.04, p<0.01) but not the subgroup paired with cotton. These animals displayed a significantly longer time period in the scented compartment compared to the no-odor side (see Fig. 4). Interestingly, the group exposed to low dose PCBs (12.5 ppm) had a significant difference as well but in the inverse direction (see Fig. 4; t(34)= −2.39, p<0.05). No other pairwise comparisons were significant except the PCB 25ppm group exposed to cottonball and they showed an elevated time duration in the no-odor compartment compared to the lemon-scented side (t(47)= −3.59, p<0.01).
Fig. 4.
Conditioned odor preference results for time spent in the scented or unscented compartments for each group. The bars are divided into 2 groups for each PCB exposure level. These include the subgroup paired with the dam during the conditioning session (Dam) and the subgroup in the control setting (Cot for scented cottonballs). Within these subgroups, animals were either spending time in the lemon-scented compartment (Lem) or the non-scented compartment (No).
Fig. 5.
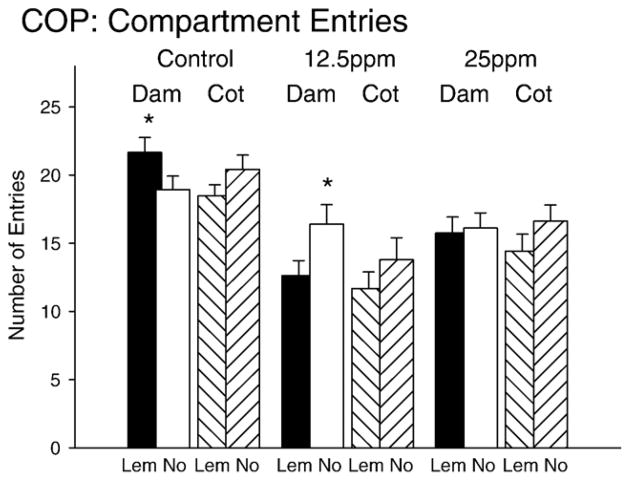
Conditioned odor preference results for number for entries into the scented (Lem) or non-scented (No) compartment. Subgroups are represented in a similar fashion as Fig. 4.
3.4. Conditioned odor preference: compartment entries
The ANOVA on entries in to the odor or non-odor sides revealed a significant difference between the number of entries into the two sides (F(1,249)=5.27, p<0.05) and a 3 way interaction between entries, exposure to PCBs and associative learning history (F(2,249)=3.30, p<0.05). Main effects for the between group factors of associative learning history and gender were significant (associative learning: F(2,249)=19.8, p<0.001 and gender: F(1,249)=5.75, p <0.05). Pairwise t-tests identified differences within each condition. Control animals paired with the dam had significantly more lemon entries (see Fig. 5; t(49)=2.5, p<0.05) while low dose PCB-exposed animal (12.5ppm) paired with the dam showed the opposite result with higher entries for the no-odor side (t(33)= −2.31, p<0.05).
3.5. Novel odor approach
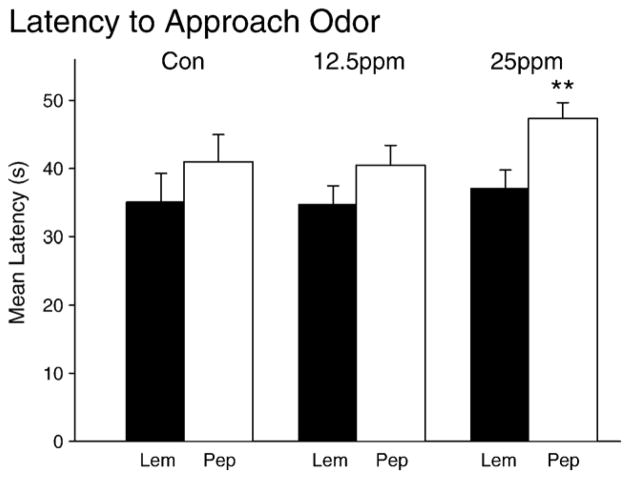
The ANOVA revealed a significant main effect for the odor (F(1,150)=12.09, p<0.01) but no main effect for exposure to PCBs (p > 0.2) and no interaction effects between the scent and exposure to PCBs factors (p>0.5). Pairwise tests revealed a significant difference in the approach latencies only for the PCB 25ppm group (see Fig. 6; t(70)=−3.68, p<0.01) but the latencies for the other two groups displayed trends in a similar direction (p<0.15).
Fig. 6.
Novel odor approach data depicting the time latencies to approach the odor stimulus for each group. Animals from each group were faster to approach the familiar odor compared to the novel odor.
4. Discussion
The majority of studies examining the influence of toxicants on behavior test adult organisms or if they explore early development, they focus on general sensory or motor processing as a dependent variable [19,42,43]. If social behavior is investigated, it has been heavily weighted towards an examination of the caregiver and the changes in parental care directed towards the offspring during neonatal or juvenile stages [44–46]. Direct examination of the psychological abilities of offspring like the rat pup has been widely neglected. We used a maternal odor-conditioning paradigm to inspect whether or not exposure to PCBs to rat pups would change a preference for an olfactory cue previously paired with mother. The test is very similar to the well-established conditioned place preference (CPP) paradigm used to study basic reward processing that can become impaired in eating disorders, drug addiction and mental illness [47,48]. The CPP test has been used frequently because of its reliability and validity in measuring the reward value of stimuli and outcomes over time and across different situations [49]. In the present work, our intent was to evaluate the incentive value of the maternal cue for the young rat pups.
Our results suggest that exposure to PCBs decreases the preference for the maternal cue. Pups exposed to perinatal PCBs did not remain in the cue-associated location longer relative to the non-cue related location and for the lower dose of PCB (12.5 ppm) the pups actually spent significantly longer time in the non-cue location. These shifts in maternal cue preference were observed without significant changes in body weight, feeding or olfactory function. We tested olfaction in a limited manner in the present study and future work is necessary in order to conclude that these doses of PCB do not alter this sensory ability. To understand the alterations completely, a more global view on how PCBs alter early social interactions must be discussed.
Recent work has documented that exposure to PCBs can alter maternal care [28,29]. Surprisingly, some aspects of maternal care are enhanced in dams exposed to PCBs and some of the changes in behaviors rely on both direct exposure of the dam and exposure to the pups to PCB, as was used in the present experiment. For example, dams exposed to PCBs spent more time on the nest and more time grooming (and licking) the pups compared to dams not exposed to PCBs. This maternal effect was greatest when pups were exposed to PCBs as well [29]. The authors interpreted these findings as a maternal strategy of the dams to overcome the deleterious effects of the PCBs on their offspring. Increases in licking and grooming by mothers have been shown to reduce harmful effects of early stressors and exposure to contaminants [50]. The influence of the quality of care has been examined in great detail in a variety of animal groups including humans [51–54]. Clinical work on developing children has documented that quality of early care can influence individual variability in terms of exposure and expression of stress-induced illness [55]. Children who have experienced severe neglect or abuse display deficits in cognitive and emotional functions that endure for an extended period [56,57].
Early experiences can also be beneficial to developing organisms. If social interactions and attachment are appropriate or even enhanced, then early care can actually lead to a better ability of the developing organism to overcome stress and show high resiliency over time [58]. This ‘stress inoculation’ can arise following nurturing behaviors within the social organization of the family with reductions in stress-induced illness and, on the preventive side, enhanced resistance to the harmful effects of stress [59,60].
Animal work in this field has identified key indicators of harmful and helpful early parental care as well as uncovered the particular behaviors of dams that are effective. Maternal licking (and grooming) is one of the most effective actions that the dams can direct toward young offspring [61,62]. This behavior can have dramatic effects on stress hormone levels [61], brain mechanisms related to anxiety [62] and fear reactivity in aversive situations [63].
Stress can have significant effects on conditioned place preferences in rats. Footshock-induced stress enhances CPP for the location paired with opiate drug morphine [64]. Aversive social interactions (social defeat) reinstate an extinguished place preference in mice [65]. Glucocorticoids seem to play a large role in the enhanced preferences during the CPP test seen in stressed rats because alteration of the adrenal output that decreases GC levels, significantly reduces this effect [66,67].
It could be that in non-PCB-exposed rat pups stress of maternal separation during conditioning regulates the conditioned preference for the maternal odor location. Pups exposed to PCBs and reared by PCB-exposed dams might express a lessened effect of this regulation. This reduced effect of the stress of separation could lead to a reduced maternal preference during the test session day. In some ways, the pups reared by mothers exposed to PCBs have been ‘inoculated’ to the stressor of the conditioning paradigm. This stress protection could be even more potent if the direct effects of PCB exposure might reduce baseline or stress-induced corticostreone levels. An examination of corticosterone after exposure to PCBs has documented this type of relationship. Several studies have found that exposure to PCBs reduces stress hormone levels in a variety of animals in the wild [68] and in the laboratory setting [69–71]. Meserve and colleagues systematically examined the relationship in 15 day old rat pups [72]. They administered PCBs (Aroclor 1254, 250 ppm) via the maternal diet from conception and on PND 15 found decreased basal corticosterone levels in PCB-exposed pups. In addition, the group found a reduced stress-induced release of corticosterone using an ether-stress paradigm [72]. Overall these effects could combine and lead to a hyporesponsive rat in terms of the responding to or seeking out a cue that had been previously paired with mother.
Another important hormone that might be involved in the present results is the nonapeptide, oxytocin. Exposure to PCBs alters oxytocin function [72,73]. To our knowledge, the sole previous study that has examined maternal–pup bonding using this similar task actually found decreased cue preference after administration of oxytocin antagonists [37]. This study used the same age pups and identical conditioning procedures as the present study. It could be that exposure to PCBs alters the important temporal dynamics of oxytocin synthesis or release that fosters the attachment between mother and offspring [74]. It is clear that PCBs influence multiple hormones and neuro-chemicals during development. Our study points to significant effects after low dose exposure. An important implication of this work is that these alterations in early development can have extended effects on behavior and general coping style [75]. Links between alterations in HPA axis sensitivity and coping style [76–78] and PCBs might clarify how influences can be so diverse and long-lasting. To understand the overall effects, it is essential to combine information from physiology and behavior at different levels. Our work extends this trend and builds on previous related studies.
Acknowledgments
We would like to thank Dr. Jaak Panksepp for his support and guidance in using the maternal odor preference test. This work would not have been possible without the support from the Sponsored Programs and Research Office at Bowling Green State University (Research Incentive Grant to H.C.C.) and the Graduate College research funds (to L.A.M.) used for animal housing.
References
- 1.Ulbrich B, Stahlmann R. Developmental toxicity of polychlorinated biphenyls (PCBs): a systematic review of experimental data. Arch Toxicol. 2004;78:252–68. doi: 10.1007/s00204-003-0519-y. [DOI] [PubMed] [Google Scholar]
- 2.Schantz SL. Developmental neurotoxicity of PCBs in humans: what do we know and where do we go from here? Neurotoxicol Teratol. 1996;18:217–27. doi: 10.1016/s0892-0362(96)90001-x. discussion 229–76. [DOI] [PubMed] [Google Scholar]
- 3.Faroon OM, Keith S, Jones D, de Rosa C. Effects of polychlorinated biphenyls on development and reproduction. Toxicol Ind Health. 2001;17:63–93. doi: 10.1191/0748233701th097oa. [DOI] [PubMed] [Google Scholar]
- 4.Jacobson JL, Jacobson SW. Evidence for PCBs as neurodevelopmental toxicants in humans. Neurotoxicology. 1997;18:415–24. [PubMed] [Google Scholar]
- 5.Gioia R, Steinnes E, Thomas GO, Mejier SN, Jones KC. Persistent organic pollutants in European background air: derivation of temporal and latitudinal trends. J Environ Monit. 2006;8:700–10. doi: 10.1039/b604821h. [DOI] [PubMed] [Google Scholar]
- 6.Wurl O, Potter JR, Obbard JP, Durville C. Persistent organic pollutants in the equatorial atmosphere over the open Indian Ocean. Environ Sci Technol. 2006;40:1454–61. doi: 10.1021/es052163z. [DOI] [PubMed] [Google Scholar]
- 7.Shen L, Wania F, Lei YD, Teixeira C, Muir DC, Xiao H. Polychlorinated biphenyls and polybrominated diphenyl ethers in the North American atmosphere. Environ Pollut. 2006;144(2):434–44. doi: 10.1016/j.envpol.2005.12.054. [DOI] [PubMed] [Google Scholar]
- 8.Carlson DL, Hites RA. Polychlorinated biphenyls in salmon and salmon feed: global differences and bioaccumulation. Environ Sci Technol. 2005;39:7389–95. doi: 10.1021/es048023r. [DOI] [PubMed] [Google Scholar]
- 9.Dellinger JA. Exposure assessment and initial intervention regarding fish consumption of tribal members of the Upper Great Lakes Region in the United States. Environ Res. 2004;95:325–40. doi: 10.1016/j.envres.2003.07.012. [DOI] [PubMed] [Google Scholar]
- 10.Fitzgerald EF, Hwang SA, Lambert G, Gomez M, Tarbell A. PCB exposure and in vivo CYP1A2 activity among Native Americans. Environ Health Perspect. 2005;113:272–7. doi: 10.1289/ehp.7370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gillilland CD, Summer CL, Gillilland MG, Kannan K, Villeneuve DL, Coady KK, et al. Organochlorine insecticides, polychlorinated biphenyls, and metals in water, sediment, and green frogs from southwestern Michigan. Chemosphere. 2001;44:327–39. doi: 10.1016/s0045-6535(00)00335-0. [DOI] [PubMed] [Google Scholar]
- 12.Hela DG, Konstantinou IK, Sakellarides TM, Lambropoulou DA, Akriotis T, Albanis TA. Persistent organochlorine contaminants in liver and fat of birds of prey from Greece. Arch Environ Contam Toxicol. 2006;50(4):603–13. doi: 10.1007/s00244-005-0101-0. [DOI] [PubMed] [Google Scholar]
- 13.Reiser DW, Greenberg ES, Helser TE, Branton M, Jenkins KD. In situ reproduction, abundance, and growth of young-of-year and adult large-mouth bass in a population exposed to polychlorinated biphenyls. Environ Toxicol Chem. 2004;23(7):1762–73. doi: 10.1897/03-323. [DOI] [PubMed] [Google Scholar]
- 14.Faroon OM, Keith SL, Smith-Simon C, De Rosa CT. Polychlorinated biphenyls: human health aspects. Geneva: World Health Organization; 2003. pp. 1–64. [Google Scholar]
- 15.Lai TJ, Guo YL, Guo NW, Hsu CC. Effect of prenatal exposure to polychlorinated biphenyls on cognitive development in children: a longitudinal study in Taiwan. Br J Psychiatry Suppl. 2001;40:s49–52. doi: 10.1192/bjp.178.40.s49. [DOI] [PubMed] [Google Scholar]
- 16.Ribas-Fito N, Sala M, Kogevinas M, Sunyer J. Polychlorinated biphenyls (PCBs) and neurological development in children: a systematic review. J Epidemiol Community Health. 2001;55:537–46. doi: 10.1136/jech.55.8.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Przyrembel H, Heinrich-Hirsch B, Vieth B. Exposition to and health effects of residues in human milk. Adv Exp Med Biol. 2000;478:307–25. doi: 10.1007/0-306-46830-1_27. [DOI] [PubMed] [Google Scholar]
- 18.Aoki Y. Polychlorinated biphenyls, polychlorinated dibenzo-p-dioxins, and polychlorinated dibenzofurans as endocrine disrupters—what we have learned from Yusho disease. Environ Res. 2001;86:2–11. doi: 10.1006/enrs.2001.4244. [DOI] [PubMed] [Google Scholar]
- 19.Roegge CS, Schantz SL. Motor function following developmental exposure to PCBs and/or MEHG. Neurotoxicol Teratol. 2006;28:260–77. doi: 10.1016/j.ntt.2005.12.009. [DOI] [PubMed] [Google Scholar]
- 20.Grandjean P, Weihe P, Burse VW, Needham LL, Storr-Hansen E, Heinzow B, et al. Neurobehavioral deficits associated with PCBs in 7-year-old children prenatally exposed to seafood neurotoxicants. Neurotoxicol Teratol. 2001;23:305–17. doi: 10.1016/s0892-0362(01)00155-6. [DOI] [PubMed] [Google Scholar]
- 21.Bushnell PJ, Moser VC, MacPhail RC, Oshiro WM, Derr-Yellin EC, Phillips PM, et al. Neurobehavioral assessments of rats perinatally exposed to a commercial mixture of polychlorinated biphenyls. Toxicol Sci. 2002;68:109–20. doi: 10.1093/toxsci/68.1.109. [DOI] [PubMed] [Google Scholar]
- 22.Hany J, Lilienthal H, Roth-Harer A, Ostendorp G, Heinzow B, Winneke G. Behavioral effects following single and combined maternal exposure to PCB 77 (3,4,3′,4′-tetrachlorobiphenyl) and PCB 47 (2,4,2′,4′-tetrachlorobiphenyl) in rats. Neurotoxicol Teratol. 1999;21:147–56. doi: 10.1016/s0892-0362(98)00038-5. [DOI] [PubMed] [Google Scholar]
- 23.Schantz SL, Bowman RE. Learning in monkeys exposed perinatally to 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) Neurotoxicol Teratol. 1989;11:13–9. doi: 10.1016/0892-0362(89)90080-9. [DOI] [PubMed] [Google Scholar]
- 24.Vreugdenhil HJ, Lanting CI, Mulder PG, Boersma ER, Weisglas-Kuperus N. Effects of prenatal PCBs and dioxin background exposure on cognitive and motor abilities in Dutch children at school age. J Pediatr. 2002;140:48–56. doi: 10.1067/mpd.2002.119625. [DOI] [PubMed] [Google Scholar]
- 25.Juarez de Ku LM, Sharma-Stokkermans M, Meserve LA. Thyroxine normalizes polychlorinated biphenyl (PCB) dose-related depression of choline acetyltransferase (ChAT) activity in hippocampus and basal forebrain of 15-day-old rats. Toxicology. 1994;94:19–30. doi: 10.1016/0300-483x(94)90025-6. [DOI] [PubMed] [Google Scholar]
- 26.Corey DA, Juarez de Ku LM, Bingman VP, Meserve LA. Effects of exposure to polychlorinated biphenyl (PCB) from conception on growth, and development of endocrine, neurochemical, and cognitive measures in 60 day old rats. Growth Dev Aging. 1996;60:131–43. [PubMed] [Google Scholar]
- 27.Provost TL, Juarez de Ku LM, Zender C, Meserve LA. Dose- and age-dependent alterations in choline acetyltransferase (ChAT) activity, learning and memory, and thyroid hormones in 15- and 30-day old rats exposed to 1.25 or 12. 5 PPM polychlorinated biphenyl (PCB) beginning at conception. Prog Neuropsychopharmacol Biol Psychiatry. 1999;23:915–28. doi: 10.1016/s0278-5846(99)00035-4. [DOI] [PubMed] [Google Scholar]
- 28.Simmons SL, Cummings JA, Clemens LG, Nunez AA. Exposure to PCB 77 affects the maternal behavior of rats. Physiol Behav. 2005;84:81–6. doi: 10.1016/j.physbeh.2004.10.022. [DOI] [PubMed] [Google Scholar]
- 29.Cummings JA, Nunez AA, Clemens LG. A cross-fostering analysis of the effects of PCB 77 on the maternal behavior of rats. Physiol Behav. 2005;85:83–91. doi: 10.1016/j.physbeh.2005.04.001. [DOI] [PubMed] [Google Scholar]
- 30.Nakayama K, Oshima Y, Hiramatsu K, Shimasaki Y, Honjo T. Effects of polychlorinated biphenyls on the schooling behavior of Japanese medaka (Oryzias latipes) Environ Toxicol Chem. 2005;24:2588–93. doi: 10.1897/04-518r2.1. [DOI] [PubMed] [Google Scholar]
- 31.Liebl B, Schettgen T, Kerscher G, Broding HC, Otto A, Angerer J, et al. Evidence for increased internal exposure to lower chlorinated polychlorinated biphenyls (PCB) in pupils attending a contaminated school. Int J Hyg Environ Health. 2004;207:315–24. doi: 10.1078/1438-4639-00296. [DOI] [PubMed] [Google Scholar]
- 32.Khanjani N, Sim MR. Maternal contamination with PCBs and reproductive outcomes in an Australian population. J Expo Sci Environ Epidemiol. 2006;17(2):191–5. doi: 10.1038/sj.jes.7500495. [DOI] [PubMed] [Google Scholar]
- 33.McLachlan JA, Simpson E, Martin M. Endocrine disrupters and female reproductive health. Best Pract Res Clin Endocrinol Metab. 2006;20:63–75. doi: 10.1016/j.beem.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 34.Vreugdenhil HJ, Slijper FM, Mulder PG, Weisglas-Kuperus N. Effects of perinatal exposure to PCBs and dioxins on play behavior in Dutch children at school age. Environ Health Perspect. 2002;110:A593–8. doi: 10.1289/ehp.021100593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cicchetti DV. Dutch girls and boys, PCB levels, and play behavior: what do the data really tell us? Environ Health Perspect. 2003;111:A381–2. doi: 10.1289/ehp.111-a381. author reply A382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kaufman AS. Critique of Vreugdenhil et al ’s study linking PCBs to the play behaviors of Dutch girls and boys. Environ Health Perspect. 2003;111:A380. doi: 10.1289/ehp.111-a380a. author reply A380–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nelson E, Panksepp J. Oxytocin mediates acquisition of maternally associated odor preferences in preweanling rat pups. Behav Neurosci. 1996;110:583–92. doi: 10.1037//0735-7044.110.3.583. [DOI] [PubMed] [Google Scholar]
- 38.Zimmerberg B, Rosenthal AJ, Stark AC. Neonatal social isolation alters both maternal and pup behaviors in rats. Dev Psychobiol. 2003;42:52–63. doi: 10.1002/dev.10086. [DOI] [PubMed] [Google Scholar]
- 39.Sokoloff G, Blumberg MS. Competition and cooperation among huddling infant rats. Dev Psychobiol. 2001;39:65–75. doi: 10.1002/dev.1030. [DOI] [PubMed] [Google Scholar]
- 40.Polan HJ, Milano D, Eljuga L, Hofer MA. Development of rats’ maternally directed orienting behaviors from birth to day 2. Dev Psychobiol. 2002;40:81–103. [PubMed] [Google Scholar]
- 41.Donahue DA, Dougherty EJ, Meserve LA. Influence of a combination of two tetrachlorobiphenyl congeners (PCB 47; PCB 77) on thyroid status, choline acetyltransferase (ChAT) activity, and short- and long-term memory in 30-day-old Sprague–Dawley rats. Toxicology. 2004;203:99–107. doi: 10.1016/j.tox.2004.06.011. [DOI] [PubMed] [Google Scholar]
- 42.Goldey ES, Crofton KM. Thyroxine replacement attenuates hypothyroxinemia, hearing loss, and motor deficits following developmental exposure to Aroclor 1254 in rats. Toxicol Sci. 1998;45:94–105. doi: 10.1006/toxs.1998.2495. [DOI] [PubMed] [Google Scholar]
- 43.Walkowiak J, Wiener JA, Fastabend A, Heinzow B, Kramer U, Schmidt E, et al. Environmental exposure to polychlorinated biphenyls and quality of the home environment: effects on psychodevelopment in early childhood. Lancet. 2001;358:1602–7. doi: 10.1016/S0140-6736(01)06654-5. [DOI] [PubMed] [Google Scholar]
- 44.Lugo JN, Jr, Marino MD, Cronise K, Kelly SJ. Effects of alcohol exposure during development on social behavior in rats. Physiol Behav. 2003;78:185–94. doi: 10.1016/s0031-9384(02)00971-x. [DOI] [PubMed] [Google Scholar]
- 45.Busidan Y, Dow-Edwards DL. Neurobehavioral effects of perinatal AZT exposure in Sprague–Dawley weaning rats. Pharmacol Biochem Behav. 1999;64:479–85. doi: 10.1016/s0091-3057(99)00157-4. [DOI] [PubMed] [Google Scholar]
- 46.Wiggins RC, Ruiz B. Development under the influence of cocaine. II. Comparison of the effects of maternal cocaine and associated undernutrition on brain myelin development in the offspring. Metab Brain Dis. 1990;5:101–9. doi: 10.1007/BF01001050. [DOI] [PubMed] [Google Scholar]
- 47.Bardo MT, Bevins RA. Conditioned place preference: what does it add to our preclinical understanding of drug reward? Psychopharmacology (Berl) 2000;153:31–43. doi: 10.1007/s002130000569. [DOI] [PubMed] [Google Scholar]
- 48.Mitchell JM, Bergren LJ, Chen KS, Fields HL. Cholecystokinin is necessary for the expression of morphine conditioned place preference. Pharmacol Biochem Behav. 2006;85(4):787–95. doi: 10.1016/j.pbb.2006.11.014. [DOI] [PubMed] [Google Scholar]
- 49.Tzschentke TM. Measuring reward with the conditioned place preference paradigm: a comprehensive review of drug effects, recent progress and new issues. Prog Neurobiol. 1998;56:613–72. doi: 10.1016/s0301-0082(98)00060-4. [DOI] [PubMed] [Google Scholar]
- 50.Champagne FA, Francis DD, Mar A, Meaney MJ. Variations in maternal care in the rat as a mediating influence for the effects of environment on development. Physiol Behav. 2003;79:359–71. doi: 10.1016/s0031-9384(03)00149-5. [DOI] [PubMed] [Google Scholar]
- 51.Meaney MJ. Maternal care, gene expression, and the transmission of individual differences in stress reactivity across generations. Annu Rev Neurosci. 2001;24:1161–92. doi: 10.1146/annurev.neuro.24.1.1161. [DOI] [PubMed] [Google Scholar]
- 52.Felitti VJ, Anda RF, Nordenberg D, Williamson DF, Spitz AM, Edwards V, et al. Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults. The Adverse Childhood Experiences (ACE) Study. Am J Prev Med. 1998;14:245–58. doi: 10.1016/s0749-3797(98)00017-8. [DOI] [PubMed] [Google Scholar]
- 53.Holub CK, Kershaw TS, Ethier KA, Lewis JB, Milan S, Ickovics JR. Prenatal and parenting stress on adolescent maternal adjustment: identifying a high-risk subgroup. Matern Child Health J. 2006;11(2):153–9. doi: 10.1007/s10995-006-0159-y. [DOI] [PubMed] [Google Scholar]
- 54.Sanchez MM. The impact of early adverse care on HPA axis development: nonhuman primate models. Horm Behav. 2006;50:623–31. doi: 10.1016/j.yhbeh.2006.06.012. [DOI] [PubMed] [Google Scholar]
- 55.Bale TL. Sensitivity to stress: dysregulation of CRF pathways and disease development. Horm Behav. 2005;48:1–10. doi: 10.1016/j.yhbeh.2005.01.009. [DOI] [PubMed] [Google Scholar]
- 56.McCauley J, Kern DE, Kolodner K, Dill L, Schroeder AF, DeChant HK, et al. Clinical characteristics of women with a history of childhood abuse: unhealed wounds. Jama. 1997;277:1362–8. [PubMed] [Google Scholar]
- 57.Gottman JM. Psychology and the study of marital processes. Annu Rev Psychol. 1998;49:169–97. doi: 10.1146/annurev.psych.49.1.169. [DOI] [PubMed] [Google Scholar]
- 58.Kaufman J, Yang BZ, Douglas-Palumberi H, Houshyar S, Lipschitz D, Krystal JH, et al. Social supports and serotonin transporter gene moderate depression in maltreated children. Proc Natl Acad Sci U S A. 2004;101:17316–21. doi: 10.1073/pnas.0404376101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rutter M. Protective factors in children’s responses to stress and disadvantage. Ann Acad Med Singapore. 1979;8:324–38. [PubMed] [Google Scholar]
- 60.Smith J, Prior M. Temperament and stress resilience in school-age children: a within-families study. J Am Acad Child Adolesc Psychiatry. 1995;34:168–79. doi: 10.1097/00004583-199502000-00012. [DOI] [PubMed] [Google Scholar]
- 61.Liu D, Diorio J, Day JC, Francis DD, Meaney MJ. Maternal care, hippocampal synaptogenesis and cognitive development in rats. Nat Neurosci. 2000;3:799–806. doi: 10.1038/77702. [DOI] [PubMed] [Google Scholar]
- 62.Caldji C, Diorio J, Meaney MJ. Variations in maternal care in infancy regulate the development of stress reactivity. Biol Psychiatry. 2000;48:1164–74. doi: 10.1016/s0006-3223(00)01084-2. [DOI] [PubMed] [Google Scholar]
- 63.Caldji C, Diorio J, Meaney MJ. Variations in maternal care alter GABA(A) receptor subunit expression in brain regions associated with fear. Neuropsychopharmacology. 2003;28:1950–9. doi: 10.1038/sj.npp.1300237. [DOI] [PubMed] [Google Scholar]
- 64.Menard JL, Champagne DL, Meaney MJ. Variations of maternal care differentially influence ‘fear’ reactivity and regional patterns of cFos immunoreactivity in response to the shock-probe burying test. Neuroscience. 2004;129:297–308. doi: 10.1016/j.neuroscience.2004.08.009. [DOI] [PubMed] [Google Scholar]
- 65.Dai Z, Kang L, Wang L, Ma L. Different roles of dopamine receptor subtypes in footshock stress-induced enhancement of morphine conditioned place preference. Neurosci Lett. 2006;409:52–6. doi: 10.1016/j.neulet.2006.09.012. [DOI] [PubMed] [Google Scholar]
- 66.Ribeiro Do Couto B, Aguilar MA, Manzanedo C, Rodriguez-Arias M, Armario A, Minarro J. Social stress is as effective as physical stress in reinstating morphine-induced place preference in mice. Psychopharmacology (Berl) 2006;185:459–70. doi: 10.1007/s00213-006-0345-z. [DOI] [PubMed] [Google Scholar]
- 67.Der-Avakian A, Bland ST, Schmid MJ, Watkins LR, Spencer RL, Maier SF. The role of glucocorticoids in the uncontrollable stress-induced potentiation of nucleus accumbens shell dopamine and conditioned place preference responses to morphine. Psychoneuroendocrinology. 2006;31:653–63. doi: 10.1016/j.psyneuen.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 68.Der-Avakian A, Will MJ, Bland ST, Deak T, Nguyen KT, Schmid MJ, et al. Surgical and pharmacological suppression of glucocorticoids prevents the enhancement of morphine conditioned place preference by uncontrollable stress in rats. Psychopharmacology (Berl) 2005;179:409–17. doi: 10.1007/s00213-004-2041-1. [DOI] [PubMed] [Google Scholar]
- 69.Hontela A, Rasmussen JB, Audet C, Chevalier G. Impaired cortisol stress response in fish from environments polluted by PAHs, PCBs, and mercury. Arch Environ Contam Toxicol. 1992;22:278–83. doi: 10.1007/BF00212086. [DOI] [PubMed] [Google Scholar]
- 70.Miller DB, Gray LE, Jr, Andrews JE, Luebke RW, Smialowicz RJ. Repeated exposure to the polychlorinated biphenyl (Aroclor 1254) elevates the basal serum levels of corticosterone but does not affect the stress-induced rise. Toxicology. 1993;81:217–22. doi: 10.1016/0300-483x(93)90014-j. [DOI] [PubMed] [Google Scholar]
- 71.Love OP, Shutt LJ, Silfies JS, Bortolotti GR, Smits JE, Bird DM. Effects of dietary PCB exposure on adrenocortical function in captive American kestrels (Falco sparverius) Ecotoxicology. 2003;12:199–208. doi: 10.1023/a:1022502826800. [DOI] [PubMed] [Google Scholar]
- 72.Meserve LA, Murray BA, Landis JA. Influence of maternal ingestion of Aroclor 1254 (PCB) or FireMaster BP-6 (PBB) on unstimulated and stimulated corticosterone levels in young rats. Bull Environ Contam Toxicol. 1992;48:715–20. doi: 10.1007/BF00195992. [DOI] [PubMed] [Google Scholar]
- 73.Wrobel M, Kotwica J. Effect of polychlorinated biphenyls (PCBs) on basal and OT-stimulated calcium concentrations in myometrial cells in cows. Reprod Biol. 2005;5:321–30. [PubMed] [Google Scholar]
- 74.Mlynarczuk J, Kotwica J. Influence of polychlorinated biphenyls on LH-stimulated secretion of progestereone and oxytocin from bovine luteal cells. Pol J Vet Sci. 2006;9:101–8. [PubMed] [Google Scholar]
- 75.Carter CS. The chemistry of child neglect: do oxytocin and vasopressin mediate the effects of early experience? Proc Natl Acad Sci U S A. 2005;102:18247–8. doi: 10.1073/pnas.0509376102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Koolhaas JM, Korte SM, De Boer SF, Van Der Vegt BJ, Van Reenen CG, Hopster H, et al. Coping styles in animals: current status in behavior and stress-physiology. Neurosci Biobehav Rev. 1999;23:925–35. doi: 10.1016/s0149-7634(99)00026-3. [DOI] [PubMed] [Google Scholar]
- 77.Korte SM, Buwalda B, Bouws GA, Koolhaas JM, Maes FW, Bohus B. Conditioned neuroendocrine and cardiovascular stress responsiveness accompanying behavioral passivity and activity in aged and in young rats. Physiol Behav. 1992;51:815–22. doi: 10.1016/0031-9384(92)90120-q. [DOI] [PubMed] [Google Scholar]
- 78.Weiss JM. Effects of coping responses on stress. J Comp Physiol Psychol. 1968;65:251–60. doi: 10.1037/h0025562. [DOI] [PubMed] [Google Scholar]