Abstract
Background
Chronic alcohol intoxication suppresses immune function and increases osteoporosis risk suggesting bone tissue cytotoxicity. Human immunodeficiency virus (HIV) infection leads to similar impairments. This study investigated the effects of chronic alcohol administration during the early stage of simian immunodeficiency virus (SIV) infection on hematopoietic stem and progenitor cells and their differentiated progeny in the bone marrow and peripheral blood of rhesus macaques.
Methods
Rhesus macaques were administered alcohol or sucrose daily for a period of 3 months prior to intrarectal inoculation with 250 TCID50 of SIVmac251. Bone marrow aspirates and blood samples were taken prior to and 2 weeks after SIV infection. Bone marrow cells (BMCs) were assessed using flow cytometric phenotyping for upstream hematopoietic stem and progenitor cells (HSPCs) and for differentiated cells of the monocyte-granulocyte lineages. Likewise, cells were quantitated in peripheral blood.
Results
Of the bone marrow HSPCs, only the common lymphoid progenitor (CLP) was altered by alcohol administration pre-SIV (38±9.4 / 106 BMCs v 226±64.1 / 106 BMCs, sucrose v alcohol). Post-SIV, the frequency of CLPs in the bone marrow of alcohol-administered macaques decreased compared to the sucrose-administered macaques (107±47.6 / 106 BMCs v 43±16.3 / 106 BMCs). However, marrow mature cells of the monocyte lineage, specifically macrophages and osteoclast progenitors, were increased by both chronic alcohol administration and SIV infection (287% and 662%, respectively). As expected, mature cells such as granulocytes (polymorphonuclear cells (PMN)), B cells, and CD4+ T cells in the peripheral blood were decreased by SIV infection (37-62% decline from pre-infection), but not affected after three months of chronic alcohol administration.
Conclusions
Chronic alcohol administration disrupts myelomonocytic development in the bone marrow during the early period of SIV infection promoting macrophage and osteoclast lineages. We predict this shift in CLP:macrophage/osteoclast balance creates an environment that favors bone resorption and immunosuppression.
Keywords: hematopoiesis, alcohol, immune cells, SIV, bone marrow
Introduction
Alcohol abuse and human immunodeficiency virus (HIV) infection are significant public health problems in the United States and frequently co-occur in the same individual (Lefevre et al., 1995; Samet et al., 2004). Nearly two-thirds of U.S. adults were classified as current drinkers, and over 20% were classified as moderate to heavy drinkers in a 2013 survey (Schoenborn et al., 2013). Of note, up to 50% of HIV-infected individuals have a history of alcohol use disorders (Lefevre et al., 1995; Samet et al., 2004). HIV infection and chronic alcohol abuse independently cause immunosuppression (Shan et al., 2007; Becker, 1995; Happel and Nelson, 2005; Szabo, 1999). However, few studies have looked at the combined effects on immune function despite the frequent occurrence of alcohol use disorders in HIV infected patients.
Cells of the immune system arise from hematopoietic stem and progenitor cells (HSPCs), the majority of which reside in the bone marrow. Experimentally determining the direct effects of alcohol abuse on bone marrow HSPCs or their differentiated progeny during HIV infection in humans is technically challenging. We therefore utilized a rhesus macaque model of simian immunodeficiency virus (SIV) infection during chronic intragastric alcohol or isocaloric sucrose administration. SIV closely mimics HIV infection in both cell tropism and disease course. Rhesus macaques are not natural reservoirs for SIV. Therefore they progress to end-stage disease similar to acquired immunodeficiency syndrome (AIDS) in humans (Hirsch and Johnson, 1994; Bagby et al., 2003). Other advantages of the macaque model include the ability to sample important body compartments, such as bone marrow, to control the exact timing and route of viral infection, and to tightly regulate alcohol administration while controlling for confounding variables, such as polysubstance abuse and nutrition.
In previous studies from our laboratories and others, chronic alcohol administration disrupted normal functioning of numerous immune cell populations in both the bone marrow and systemic circulation (Szabo, 1999; Siggins et al., 2011; Melvan et al., 2011; Verma et al., 2008; Choudhry et al., 2000). Alcohol interferes with immune cell development, homing, and function (Melvan et al., 2011; Parlet and Schlueter, 2013; Szabo and Mandrekar, 2008). This negatively affects both the innate and acquired immune systems. Previously we found that chronic alcohol administration to rhesus macaques impairs dendropoiesis (Siggins et al., 2009). In other preclinical models of chronic alcohol administration, the HSPC response to systemic infections is severely attenuated leading to increased mortality (Zhang et al., 2009). Overall, most experimental models of chronic alcohol administration and infectious disease result in increased morbidity and mortality due to the pathophysiological effects of alcohol on multiple levels of the immune system.
In the current study, we sought to determine the effects of acute SIV infection with and without chronic alcohol administration on bone marrow HSPCs and mature cell population phenotypes. Additionally we examined circulating HSPCs and progeny in rhesus. We hypothesized that both chronic alcohol administration and SIV infection independently and in combination would lead to impaired myelopoiesis. Our results show that chronic alcohol administration leads to impaired immunity beginning at the level of the bone marrow. We predict this results in dysregulated peripheral immunity during the early phase SIV infection.
Materials and Methods
Animals, Gastric Catheter Implantation, Alcohol Administration Protocol, and SIV Inoculation
This study was conducted at the Tulane National Primate Research Center (TNPRC) in Covington, Louisiana, on male rhesus monkeys (Macaca mulatta) of Indian origin that were 4 to 6 years of age. Institutional Animal Care and Use Committees at both TNPRC and LSU Health Sciences Center in New Orleans approved experimental procedures on these animals. Data from a total of 24 animals are included in these analyses. Animals were fed a commercial primate chow, supplemented with fruit, and received alcohol or isocaloric sucrose daily (30% alcohol as a 0.5 hour infusion) via a permanently indwelling intragastric catheter (17 gauge; Access Technologies, Skokie, IL) that was attached to a cage-mounted swivel via a tether (Lomir Biomedical, Malone, NY) as previously described (Bagby et al., 2006). A blood sample was obtained weekly 2 hours after starting alcohol delivery in order to adjust infusion rates so that peak plasma alcohol concentrations were between 50 and 60 mM. Following 3 months of alcohol or sucrose administration, all macaques were intrarectally inoculated with 250 TCID50 of SIVmac251.
Plasma SIV Viral Load Measurements
Plasma viral loads were determined as previously described with minor modifications (Bagby et al., 2003). Briefly, 500 μL of plasma is centrifuged at 16,000 × g and 4°C for 1 hour. The pellet was decanted and dissolved in 1 mL of Trizol reagent (Life Technologies, Grand Island, NY). Samples were stored at -80°C for batched RNA extraction according to manufacturer's protocol. Final isolated RNA was resuspended in 25 μL of RNase-free water. Two-step RT PCR was performed using the TaqMan Reverse Transcriptase Reagent Kit (Applied Biosystems, Grand Island, NY) for first strand synthesis, followed by qPCR with IQ Supermix (BioRad, Hercules, CA). Primers and probe to SIVgag were: forward GCG TCA TTT GGT GCA TTC AC, reverse TCC ACC ACT AGA TGT CTC TGC ACT AT, and FAM-labeled probe with Tamra quencher TGT TTT GCT TCC TCA GTA TGT TTC ACT TTC TCT TCT G. Quantification was determined by comparing sample Cp value to a standard curve of 101 to 107 gag copies, and calculated to copies / mL of plasma. Attempts to measure virus in bone marrow samples were unsuccessful as SIV RNA was below our assay's detection limit.
Bone Marrow and Peripheral Blood Isolation
Peripheral blood and bone (humeri) marrow samples were obtained 3 months after chronic alcohol or sucrose administration to investigate the impact of chronic alcohol consumption alone, and 2 weeks after SIV infection to examine the combined impact of alcohol and SIV on the selected outcome measures. Samples were collected from anesthetized animals following an overnight fast in the absence of detectable blood alcohol levels. Peripheral blood was collected in 1 mL of 5 mM EDTA. Bone marrow was centrifuged at 500 × g for 5 minutes and the bone marrow cells (BMCs) were incubated in 20 mL respectively of RBC Lysis Solution (Qiagen, Valencia, CA) for 10 min at room temperature (RT). RBC lysis was stopped by washing in an equal volume of RPMI-1640 + 10% fetal bovine serum (FBS). Nucleated BMCs were filtered through a 70 micron nylon mesh and resuspended to a final concentration of 2 × 107 cells/mL in RPMI-1640 + 10% FBS. Bone marrow aspirates contain variable amounts of peripheral blood that can lead to variations in phenotypic analysis. Therefore aspirates were assessed for peripheral blood via cell cycle analysis. Samples having greater than 90% frequency of G0/G1 cells were eliminated from analysis.
Flow Cytometric Analysis
Cells isolated from the bone marrow and peripheral blood (2 × 106 cells/sample) were stained with three distinct antibody panels (or appropriate isotype control panels) containing 1 μg each of the following conjugated antibodies to analyze (1) BM HSPCs, (2) BM mature myeloid cells, and (3) peripheral blood HSPC and mature cells. BMCs were first incubated for 10 min at RT with Fc Block (BioLegend, San Diego, CA). The BM HSPC flow panel included a biotin-conjugated lineage panel containing CD8, CD14 (BioLegend, San Diego, CA), CD20 (Thermo Fisher Scientific, Waltham, MA), CD3 (LifeSpan BioSciences, Inc., Seattle, WA), CD66 (Miltenyi, Auburn, CA), and CD56 (BD Biosciences, San Jose, CA). Following 30 min of incubation on ice, the BMCs were washed with RPMI-1640 + 10% FCS and resuspended in an antibody panel containing FITC-CD8 (StemCell Technologies, Vancouver, BC, Canada), APC-CD10 (BioLegend, San Diego, CA), PE-CD34, PerCP-Cy5.5-CD123, PE-Cy7-CD45RA, PE-Cy5-CD90, and PE-TxRed-Streptavidin (All BD Biosciences, San Jose, CA). After an additional 30 min incubation on ice, the cells were washed and resuspended in 300 μL of PBS + 1% paraformaldehydye (PFA) for flow cytometric analysis. Cell staining for mature BMC phenotypes was performed in a similar manner. Biotin-CD1c was first added to the cells, followed by an antibody panel containing FITC-CD66, APC-CD33, PerCP-CD14 (all Miltenyi, Auburn, CA), PerCP-Cy5.5-HLA-DR, PE-Cy7-CD11c (BioLegend, San Diego, CA), PE-RANK (Imgenex, San Diego, CA), APC-CD13 (Beckman Coulter, Indianapolis, IN), APC-Cy7-αVβ3 (EMD Millipore, Billerica, MA), V450-CD16, and PE-TxRed-Strptavidin (BD Biosciences). Cells were washed and resuspended in PBS + 1% PFA for phenotypic analysis as aforementioned. For phenotypic analysis of peripheral blood, 100 μL of whole blood was added to an antibody panel containing the following or appropriate isotype controls: PerCP-CD3, PacBlue-CD14, AlexaFluor 700-CD56, PE-CD34, PerCP-Cy5.5-CD123, (all from BD Biosciences, San Jose, CA), ECD-CD20 (BeckmannCoulter, Indianapolis, IN), FITC-CD38 (StemCell Technologies, Vancouver, BC, Canada), APC-CD66 (Miltenyi Biotec, Auburn, CA), PE-Cy7-CD16, APC-Cy7-CD4 (BioLegend, San Diego, CA), Qdot655-CD8, and AmCyan-Live/Dead (Invitrogen, Grand Island, NY). The cell suspension was mixed and incubated at RT for 30 min. Samples were then mixed with 3 mL of FACS lysis buffer (BD Biosciences, San Jose, CA) for 10 min at RT, washed twice with PBS in and finally resuspended in 300 μL of PBS + 1% PFA for phenotypic analysis. All phenotypic analyses of cells were performed on a BD LSRII flow cytometer using BD FACSDiva software (BD Biosciences, San Jose, CA). See Table 1 for definitions of cell types based on surface phenotypes.
Table 1.
Phenotypic markers for Cell Discrimination. Lineage negative = Lin-; Lineage markers include CD3, CD14, CD20, CD66, and CD56.
| Cell Type | Phenotypic Markers |
|---|---|
| Hematopoietic Stem Cell (HSC) | Lin-CD34+CD38- |
| Hematopoietic Progenitor Cell (HPC) | Lin-CD34+CD38+ |
| Common Myeloid Progenitor (CMP) | Lin-CD34+CD38+CD123loCD45RA- |
| Common Lymphoid Progenitor (CLP) | Lin-CD34+CD38+CD10+ |
| Granulocyte/Monocyte Progenitor (GMP) | Lin-CD34+CD38+CD123+CD45RA- |
| Granulocyte (PMN) | HLA-DR-CD66+ |
| Macrophage (Mac) | CD13+/CD33+HLA-DR+CD14+CD16+ |
| Type 1 Myeloid Dendritic Cell (mDC1) | CD13+/CD33+HLA-DR+CD16+CD14+/-CD11c+CD1c+ |
| Osteoclast Progenitor (OCP) | CD13+/CD33+HLA-DR+/-CD16-CD14+/-CD11c+/-CD1c-/RANK+ |
| Differentiating Osteoclast (diff OC) | CD13+/CD33+HLA-DR-CD66-CD16-CD14-CD11c-CD1c-/RANK+αVβ3+ |
| B Cell | CD3-CD20+ |
| CD4 T Cell | CD3+CD8-CD4+ |
| CD8 T Cell | CD3+CD8+CD4- |
Statistical Analysis
Data are presented as mean ± standard error of the mean (SEM). The sample size is indicated in the legend of each figure. Statistical analyses of data were performed using GraphPad Prism 5 (GraphPad Software, Inc. La Jolla, CA) conducted by unpaired Student's t-test (for comparison between 2 groups) or repeated measures two-way analysis of variance followed by Bonferroni posttest (for comparisons among multiple groups). Differences were considered statistically significant at p < 0.05.
Results
Plasma Viral Load at 2 Weeks Post-SIV Infection
Plasma from sucrose- and alcohol-administered animals was assessed for viral load. Similar to our previous findings (Bagby et al., 2006), we did not find a difference in viral load at time that peak viral load would be expected between the sucrose-and alcohol-administered animals (data not shown).
Chronic Alcohol Administration and Early Period SIV Infection are not associated with marked alterations in Hematopoietic Stem and Progenitor Cells
Analysis of hematopoietic stem cells (HPCs, Fig. 1A), total hematopoietic progenitor cells (HPCs, Fig. 1B), and common myeloid progenitors (CMPs, Fig. 1C) from the bone marrow of sucrose- and alcohol-administered rhesus macaques showed little variation after 3 months of alcohol administration or following 2 weeks of SIV infection. Notably, 3 months of chronic alcohol administration significantly increased common lymphoid progenitors (CLPs, Fig. 1D), which were significantly reduced following SIV infection. CLPs give rise to both B cells and T cells, and the observed alterations in the frequency of this population may be due to changes in cell turnover, maturation, or migration.
Figure 1. Hematopoietic Stem and Progenitor Cell Frequency in the Bone Marrow.
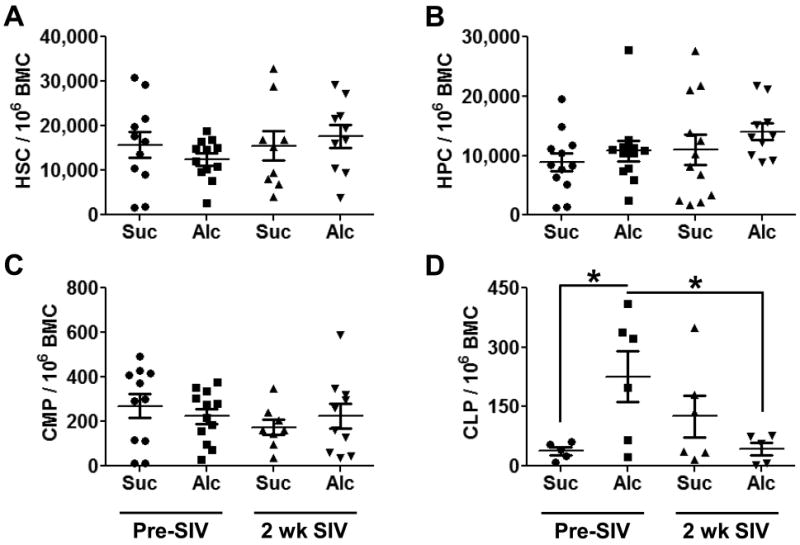
Chronic alcohol administration and SIV infection did not alter hematopoietic stem cell (HSC; A), hematopoietic progenitor cell (HPC, B), or common myeloid progenitor (CMP, C) cell frequency compared to sucrose-administered macaques. Common lymphoid progenitor (CLP, D) frequency was enhanced by 3 months of chronic alcohol administration and greatly decreased after 2 weeks of SIV infection. N = 5-12 for each group. * = p<0.05.
Chronic Alcohol Administration and Early Period SIV Infection Does Not Impair the Granulocyte Lineage in the Bone Marrow
Examination of bone marrow granulocyte-monocyte progenitors (GMPs) showed no significant effects of 3 months chronic alcohol administration or SIV infection (Fig. 2A). Similarly, bone marrow polymorphonuclear cells (PMN, Fig. 2B) were not altered at the analyzed time points. The other most common progeny from the GMP, monocytes, were not affected by either treatment in our model (data not shown).
Figure 2. Granulocyte Monocyte Progenitor (GMP) Cells and Polymorphonuclear (PMN) Cells in the Bone Marrow.
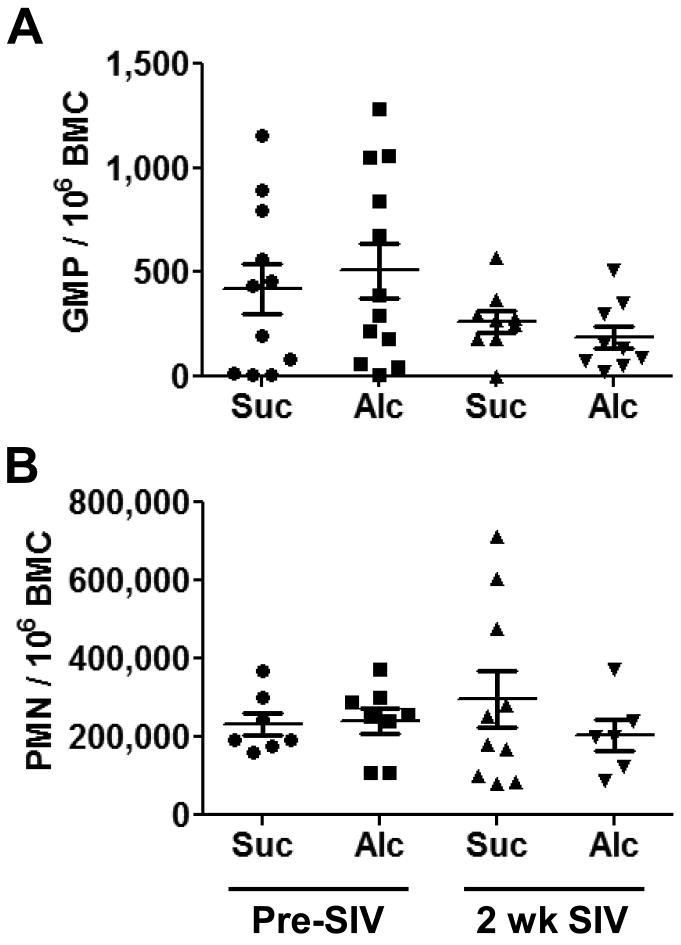
Chronic alcohol administration and SIV infection did not change the frequency of the upstream progenitor (GMP, A) to PMNs (granulocytes or neutrophils), or to the PMN population (B). N = 6-12 for each group.
Chronic Alcohol Administration and Early Period SIV Infection Preferentially alters Cells of the Common Pathway for Myeloid Dendritic Cell and Osteoclast Development
We analyzed the more differentiated progeny of the GMP lineage and found an enhanced frequency of bone marrow macrophages after 2 weeks of SIV infection in the alcohol-administered macaques (Fig. 3A). No differences were observed in the bone marrow macrophages from sucrose-administered animals at these time points. Two less frequently studied cell lineages arising from the GMP were also affected by either alcohol administration or SIV infection. Type 1 myeloid dendritic cells (mDC1) were decreased in bone marrow from sucrose-administered animals following SIV infection; a similar decrease in mDC1after SIV was observed in alcohol-administered animals. However, this change was not significant (Fig. 3B). Osteoclast progenitor cells (OCPs) and more mature differentiating osteoclasts (Diff OC) were significantly increased in alcohol-administered animals after 2 weeks of SIV infection (Fig. 3C-D).
Figure 3. Differentiating and Mature Myelomonocytic Cells in the Bone Marrow.
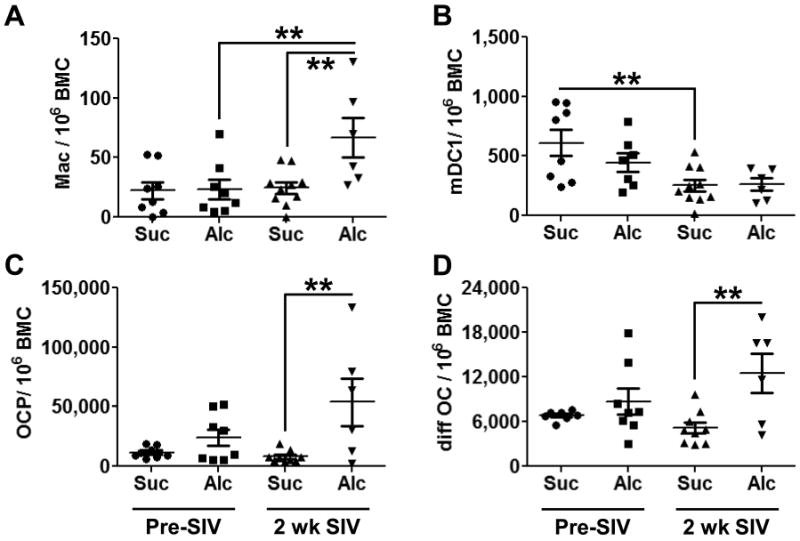
Macrophages (A) were significantly increased compared to both time-matched controls and the pre-SIV time point in the bone marrow of alcohol-administered macaques following 2 weeks of SIV infection. SIV infection decreased Type 1 mDC (mDC1) in sucrose-administered animals (B). Chronic alcohol administration after 2 weeks of SIV infection increased osteoclast progenitor cells (OCP, C) and differentiating osteoclast (diff OC, D) compared to the time match sucrose-administered controls. N = 6-10 for each group. * = p<0.05; ** = p<0.01.
Circulating Hematopoietic Stem and Progenitor Cells are Increased by Early Period SIV Infection
While we did not observe changes in HSCs or HPCs in bone marrow samples, circulating HSCs and HPCs were markedly enhanced in the peripheral blood of both sucrose- and alcohol-administered, SIV-infected animals (Fig. 4A-B). There was no observed alcohol-related effect.
Figure 4. Hematopoietic Stem and Progenitor Cell Frequency in the Peripheral Blood.
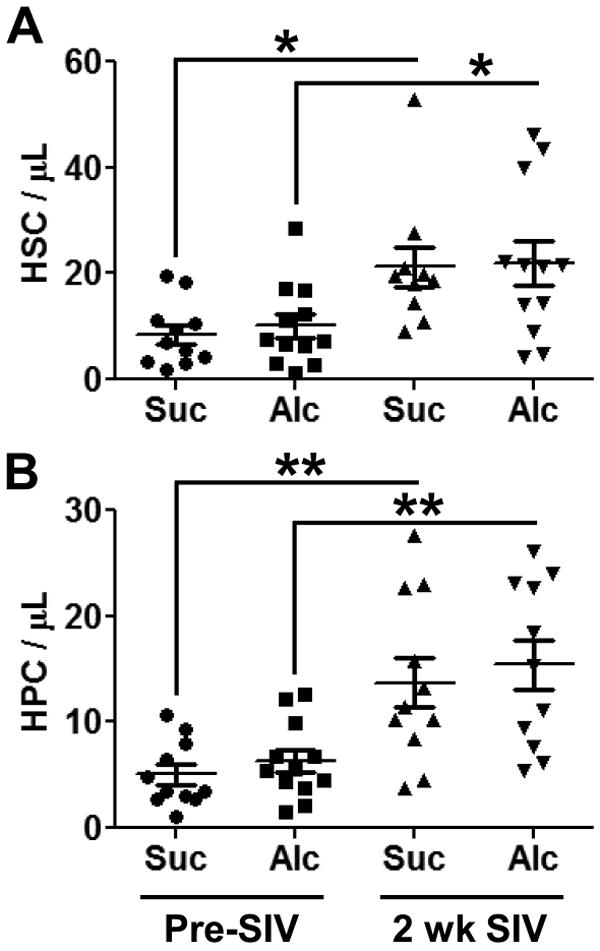
Three months of chronic alcohol administration did not alter hematopoietic stem cell (HSC; A) or hematopoietic progenitor cell (HPC, B) numbers in the peripheral blood. Two weeks after SIV infection, both HSCs and HPCs were significantly increased in the circulation regardless of pretreatment. N = 10-12 for each group. * = p<0.05. ** = p<0.01.
Early Period SIV Infection is Characterized by Decreases in Specific Mature Circulating Blood Cell Lineages
We examined the majority of mature blood cell phenotypes after 3 months of sucrose and alcohol administration and after 2 weeks of SIV infection. While most examined phenotypes showed no changes with treatments, we did observe an SIV-induced relative granulocytopenia (Fig. 5A). Alcohol administration showed a trend for an additive effect with SIV, yet this result was not statistically significant. Analysis of circulating B cells showed similar results as the PMN population (Fig. 5B). As expected, SIV decreased total numbers of circulating CD4 in both the sucrose- and alcohol-administered animals (Fig. 5C). Analysis of the CD4 to CD8 ratio showed a similar finding, though alcohol-administered macaques had a trend for a higher ratio following 2 weeks of SIV infection (Fig. 5D).
Figure 5. Mature Immune Cells in the Peripheral Blood.
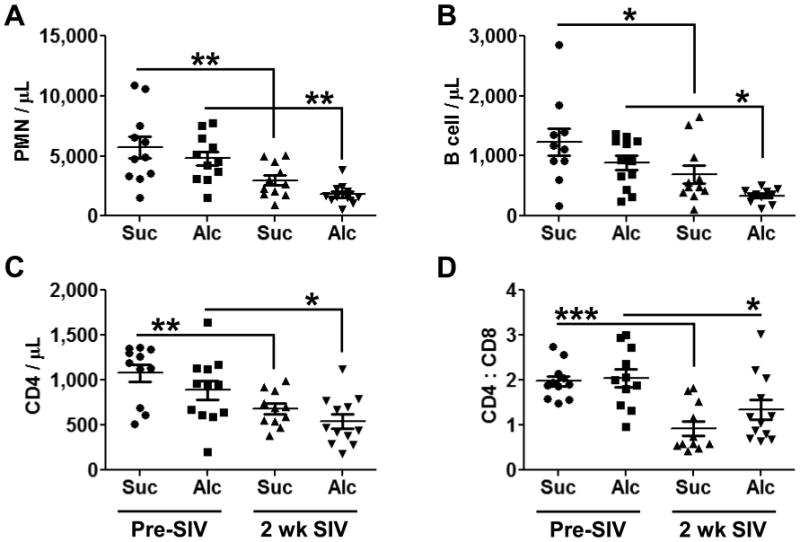
Three months of chronic alcohol administration did not change the number of the immune cells that we examined. After 2 weeks of SIV infection, PMNs (A), B cells (B), CD4+ T cells (C), and the CD4 : CD8 ratio (D) were all decreased regardless of pretreatment. N = 10-12 for each group. * = p<0.05; ** = p<0.01; ***=p<0.001.
Discussion
This study investigated the effects of acute SIV infection with and without chronic alcohol administration on bone marrow HSPCs and mature cell population phenotypes. Additionally we examined circulating HSPCs and progeny in macaques after 3 months of sucrose or alcohol administration and in the first two weeks of SIV infection. We found that chronic alcohol administration increased the frequency of CLPs and that early stage SIV infection caused a decreased frequency in CLPs when compared to the sucrose-administered macaques. Chronic alcohol administration and SIV infection resulted in increased macrophages and osteoclast progenitors. As expected, mature cells such as PMNs, B cells, and CD4+ T cells in the peripheral blood were decreased by SIV infection, and chronic alcohol administration did not have a great effect during this early stage of SIV infection.
The effects of alcohol abuse on early HIV disease are virtually impossible to determine in human studies. This is due to the fact that time from infection to diagnosis with HIV averages between 37-53 months precluding the study of the events that transpire during the early period of infection (Ndawinz et al., 2011). Therefore, this study is both timely and informative to raise the awareness of the rapid changes that occur in both bone marrow and the peripheral hematopoietic system during the early period of infection. This knowledge can serve as a foundation for future research to bolster the immune response and enhance outcomes in HIV-infected individuals, with and without alcohol use disorders.
We first examined bone marrow hematopoietic stem and progenitor cells. We saw no change in HSC, HPC, or CMP frequency between sucrose- and alcohol-administered macaques following 3 months of chronic exposure. Additionally, SIV infection did not lead to any significant changes in these cell types. The lack of statistically significant changes in the frequency of these cell populations could be attributed to the variability that is inherent in samples obtained from allogeneic rhesus macaques. We attempted to control for this variability using 5-12 animals per group and by controlling for peripheral blood cell contamination. Our examination of CLPs showed a robust increase in the frequency of these progenitors in the alcohol-administered animals after 3 months. Studies in mice have demonstrated that in vitro alcohol can inhibit B cell differentiation at the stage of the CLP (Wang et al., 2009). Our results suggest that the CLP differentiation is inhibited by alcohol administration, leading to an accumulation of this progenitor. However, this is only one potential mechanism for alcohol-induced increased CLP frequency that requires further investigation. Notably, SIV infection preferentially decreased the CLP frequency in the alcohol-administered animals compared to sucrose-administered controls.
Previous studies from our group have demonstrated that chronic alcohol administration to rhesus macaques decreased total myeloid dendritic cells (mDCs) in bone marrow (Siggins et al., 2009). Myeloid DCs are required to bridge innate and adaptive immunity. To expand on our original analysis, we examined two subsets of mDCs: type 1 (mDC1) and type 2 (mDC2). While we did not see a statistically significant decrease in either subset in the bone marrow after chronic alcohol administration, we did find that early stage SIV infection significantly reduced mDC1 in bone marrow of sucrose-administered animals. A similar decrease was observed in the alcohol-administered animals after infection, yet this group had a slightly decreased frequency prior to SIV infection. mDC1 function is typically considered to be classical antigen presentation; however in certain disease states, mDC1 have been shown to produce IL-10, the archetypal anti-inflammatory cytokine (Johnson et al., 2011). While we did not measure IL-10, the uncertainty in mDC1 and IL-10 function requires further examination in our model of SIV infection and chronic alcohol administration.
The role of macrophages in the bone marrow is gaining increased research focus with discoveries of macrophage functional diversity. A recent study revealed that α-smooth muscle actin positive macrophages prevent hematopoietic stem cell activation (Ludin et al., 2012). Other reports have identified a central role for bone marrow macrophages in regulating homing and egress from the marrow (Chow et al., 2011; Winkler et al., 2010). We observed that the early phase of SIV infection greatly enhanced macrophage frequency in the alcohol-administered macaques. Due to the longitudinal nature of our study, we are not able to determine functional consequences of this increase, but additional studies examining the macrophage subtype may yield therapeutic targets for preservation of bone marrow function in the alcoholic host.
Our studies show that chronic alcohol administration with SIV infection enhanced Osteoclast Progenitor Cells (OCP) commitment. The combination of chronic alcohol administration and SIV infection led to the enhancement of the osteoclastogenic differentiation pathway, suggesting that chronic alcohol may prime the bone marrow to favor osteoclast production when a secondary inflammatory insult is present, such as SIV infection. In previous unpublished work, we found that a longer duration of alcohol administration (13 months) in the absence of SIV infection statistically increased osteoclast progenitor cells in the bone marrow. A similar enhanced tissue inflammation has been observed in skeletal muscle of animals subjected to similar protocols (LeCapitaine et al, 2011) Moreover, chronic alcoholics are typically immunosuppressed and have an increased incidence of osteoporosis supporting the possibility that the shift in the balance between CLP and macrophage/osteoclast progenitors is an important mechanism underlying this defect (Bikle, 1988). The mature downstream progeny of OCPs, the osteoclasts, have been shown to liberate HSPCs from the bone marrow to the circulation through their physiological and pathophysiological functions (Kollet et al., 2006; Kollet et al., 2007). This has been suggested to provide HSPCs a role in immune surveillance. The increases in circulating HSPCs after SIV infection may be due to the increased frequency in bone marrow cells of the macrophage and osteoclast lineage. However, determining the reason for the enhanced frequency of HSPCs in the circulation is beyond the scope of this study.
Circulating PMNs, B cells, and CD4+ T cells were all decreased following SIV infection. Others have shown that SIV and HIV infection decreases these circulating immune cells, but few studies have examined the impact of chronic alcohol and acute SIV infection on circulating immune cells (Jaresko, 1999; Steger et al., 1998). As we have previously reported, chronic alcohol intoxication during early SIV infection may create a host environment susceptible to subsequent bacterial and viral infections (Bagby et al., 2003). Our results show that chronic alcohol administration resulted in a modest, yet not statistically significant decrease in PMNs, B cells and CD4+T cells. One could speculate that the immunosuppressive effects of chronic alcohol administration may manifest in the later stages of SIV disease progression. Studies are currently underway to examine that possibility.
In summary, we found that chronic alcohol administration during early SIV infection most notably dysregulates the bone marrow monocytic lineage including macrophages, mDCs, and osteoclasts. Enhanced osteoclastogenesis may be one mechanism by which both alcohol and SIV/HIV cause increased bone loss. Future studies are needed to determine the functional role of the different subtypes of macrophages and mDCs in the bone marrow. However, we propose that preventing dysregulated late-stage myelopoiesis during early HIV infection may enhance immune function by preserving normal bone marrow activity.
Acknowledgments
We thank Amy B. Weinberg, Joseph S. Soblosky, PhD, Rhonda R. Martinez, Jean Carnal, and Jane A. Schexnayder for their expert technical assistance. The authors also thank Connie P. Porretta for her expert assistance with flow cytometric analyses. We thank Meredith Booth for her assistance in preparing figures and data management. This work was supported by NIH grants AA020312, AA09803, and AA021097.
Funding Sources: NIH grants AA020312, AA09803, and AA021097.
References
- Bagby GJ, Stoltz DA, Zhang P, Kolls JK, Brown J, Bohm RP, Jr, Rockar R, Purcell J, Murphey-Corb M, Nelson S. The effect of chronic binge ethanol consumption on the primary stage of SIV infection in rhesus macaques. Alcohol Clin Exp Res. 2003;27:495–502. doi: 10.1097/01.ALC.0000057947.57330.BE. [DOI] [PubMed] [Google Scholar]
- Bagby GJ, Zhang P, Purcell JE, Didier PJ, Nelson S. Chronic binge ethanol consumption accelerates progression of simian immunodeficiency virus disease. Alcohol Clin Exp Res. 2006;30:1781–1790. doi: 10.1111/j.1530-0277.2006.00211.x. [DOI] [PubMed] [Google Scholar]
- Becker Y. Retrovirus and filovirus “immunosuppressive motif” and the evolution of virus pathogenicity in HIV-1, HIV-2, and Ebola viruses. Virus Genes. 1995;11:191–195. doi: 10.1007/BF01728658. [DOI] [PubMed] [Google Scholar]
- Bikle DD. Effects of alcohol abuse on bone. Compr Ther. 1988;14:16–20. [PubMed] [Google Scholar]
- Choudhry MA, Messingham KA, Namak S, Colantoni A, Fontanilla CV, Duffner LA, Sayeed MM, Kovacs EJ. Ethanol exacerbates T cell dysfunction after thermal injury. Alcohol. 2000;21:239–243. doi: 10.1016/s0741-8329(00)00093-8. [DOI] [PubMed] [Google Scholar]
- Chow A, Lucas D, Hidalgo A, Mendez-Ferrer S, Hashimoto D, Scheiermann C, Battista M, Leboeuf M, Prophete C, van Rooijen N, Tanaka M, Merad M, Frenette PS. Bone marrow CD169+ macrophages promote the retention of hematopoietic stem and progenitor cells in the mesenchymal stem cell niche. J Exp Med. 2011;208:261–271. doi: 10.1084/jem.20101688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Happel KI, Nelson S. Alcohol, immunosuppression, and the lung. Proc Am Thorac Soc. 2005;2:428–432. doi: 10.1513/pats.200507-065JS. [DOI] [PubMed] [Google Scholar]
- Hirsch VM, Johnson PR. Pathogenic diversity of simian immunodeficiency viruses. Virus Res. 1994;32:183–203. doi: 10.1016/0168-1702(94)90041-8. [DOI] [PubMed] [Google Scholar]
- Jaresko GS. Etiology of neutropenia in HIV-infected patients. Am J Health Syst Pharm. 1999;56(Suppl 5):S5–8. doi: 10.1093/ajhp/56.suppl_5.S5. [DOI] [PubMed] [Google Scholar]
- Johnson TR, Johnson CN, Corbett KS, Edwards GC, Graham BS. Primary human mDC1, mDC2, and pDC dendritic cells are differentially infected and activated by respiratory syncytial virus. PLoS One. 2011;6:e16458. doi: 10.1371/journal.pone.0016458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kollet O, Dar A, Lapidot T. The multiple roles of osteoclasts in host defense: bone remodeling and hematopoietic stem cell mobilization. Annu Rev Immunol. 2007;25:51–69. doi: 10.1146/annurev.immunol.25.022106.141631. [DOI] [PubMed] [Google Scholar]
- Kollet O, Dar A, Shivtiel S, Kalinkovich A, Lapid K, Sztainberg Y, Tesio M, Samstein RM, Goichberg P, Spiegel A, Elson A, Lapidot T. Osteoclasts degrade endosteal components and promote mobilization of hematopoietic progenitor cells. Nat Med. 2006;12:657–664. doi: 10.1038/nm1417. [DOI] [PubMed] [Google Scholar]
- LeCapitaine NJ, Wang ZQ, Dufour JP, Potter BJ, Bagby GJ, Nelson S, Cefalu WT, Molina PE. Disrupted anabolic and catabolic processes may contribute to alcohol-accentuated SAIDS-associated wasting. J Infect Dis. 2011;204:1246–1255. doi: 10.1093/infdis/jir508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefevre F, O'Leary B, Moran M, Mossar M, Yarnold PR, Martin GJ, Glassroth J. Alcohol consumption among HIV-infected patients. J Gen Intern Med. 1995;10:458–460. doi: 10.1007/BF02599920. [DOI] [PubMed] [Google Scholar]
- Ludin A, Itkin T, Gur-Cohen S, Mildner A, Shezen E, Golan K, Kollet O, Kalinkovich A, Porat Z, D'Uva G, Schajnovitz A, Voronov E, Brenner DA, Apte RN, Jung S, Lapidot T. Monocytes-macrophages that express alpha-smooth muscle actin preserve primitive hematopoietic cells in the bone marrow. Nat Immunol. 2012;13:1072–1082. doi: 10.1038/ni.2408. [DOI] [PubMed] [Google Scholar]
- Melvan JN, Siggins RW, Bagby GJ, Stanford WL, Welsh DA, Nelson S, Zhang P. Suppression of the stem cell antigen-1 response and granulocyte lineage expansion by alcohol during septicemia. Crit Care Med. 2011;39:2121–2130. doi: 10.1097/CCM.0b013e31821e89dc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ndawinz JD, Costagliola D, Supervie V. New method for estimating HIV incidence and time from infection to diagnosis using HIV surveillance data: results for France. AIDS. 2011;25:1905–1913. doi: 10.1097/QAD.0b013e32834af619. [DOI] [PubMed] [Google Scholar]
- Parlet CP, Schlueter AJ. Mechanisms by which chronic ethanol feeding impairs the migratory capacity of cutaneous dendritic cells. Alcohol Clin Exp Res. 2013;37:2098–2107. doi: 10.1111/acer.12201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samet JH, Phillips SJ, Horton NJ, Traphagen ET, Freedberg KA. Detecting alcohol problems in HIV-infected patients: use of the CAGE questionnaire. AIDS Res Hum Retroviruses. 2004;20:151–155. doi: 10.1089/088922204773004860. [DOI] [PubMed] [Google Scholar]
- Schoenborn CA, Adams PF, Peregoy JA. Health behaviors of adults: United States, 2008–2010. Vital and Health Statistics. 2013;10(257):1–171. [PubMed] [Google Scholar]
- Shan M, Klasse PJ, Banerjee K, Dey AK, Iyer SP, Dionisio R, Charles D, Campbell-Gardener L, Olson WC, Sanders RW, Moore JP. HIV-1 gp120 mannoses induce immunosuppressive responses from dendritic cells. PLoS Pathog. 2007;3:e169. doi: 10.1371/journal.ppat.0030169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siggins RW, Bagby GJ, Molina P, Dufour J, Nelson S, Zhang P. Alcohol exposure impairs myeloid dendritic cell function in rhesus macaques. Alcohol Clin Exp Res. 2009;33:1524–1531. doi: 10.1111/j.1530-0277.2009.00980.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siggins RW, Melvan JN, Welsh DA, Bagby GJ, Nelson S, Zhang P. Alcohol suppresses the granulopoietic response to pulmonary Streptococcus pneumoniae infection with enhancement of STAT3 signaling. J Immunol. 2011;186:4306–4313. doi: 10.4049/jimmunol.1002885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steger KK, Dykhuizen M, Mitchen JL, Hinds PW, Preuninger BL, Wallace M, Thomson J, Montefiori DC, Lu Y, Pauza CD. CD4+-T-cell and CD20+-B-cell changes predict rapid disease progression after simian-human immunodeficiency virus infection in macaques. J Virol. 1998;72:1600–1605. doi: 10.1128/jvi.72.2.1600-1605.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabo G. Consequences of alcohol consumption on host defence. Alcohol Alcohol. 1999;34:830–841. doi: 10.1093/alcalc/34.6.830. [DOI] [PubMed] [Google Scholar]
- Szabo G, Mandrekar P. Human monocytes, macrophages, and dendritic cells: alcohol treatment methods. Methods Mol Biol. 2008;447:113–124. doi: 10.1007/978-1-59745-242-7_9. [DOI] [PubMed] [Google Scholar]
- Verma S, Alexander CM, Carlson MJ, Tygrett LT, Waldschmidt TJ. B-cell studies in chronic ethanol mice. Methods Mol Biol. 2008;447:295–323. doi: 10.1007/978-1-59745-242-7_20. [DOI] [PubMed] [Google Scholar]
- Wang H, Zhou H, Chervenak R, Moscatello KM, Brunson LE, Chervenak DC, Wolcott RM. Ethanol exhibits specificity in its effects on differentiation of hematopoietic progenitors. Cell Immunol. 2009;255:1–7. doi: 10.1016/j.cellimm.2008.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler IG, Sims NA, Pettit AR, Barbier V, Nowlan B, Helwani F, Poulton IJ, van Rooijen N, Alexander KA, Raggatt LJ, Levesque JP. Bone marrow macrophages maintain hematopoietic stem cell (HSC) niches and their depletion mobilizes HSCs. Blood. 2010;116:4815–4828. doi: 10.1182/blood-2009-11-253534. [DOI] [PubMed] [Google Scholar]
- Zhang P, Welsh DA, Siggins RW, 2nd, Bagby GJ, Raasch CE, Happel KI, Nelson S. Acute alcohol intoxication inhibits the lineage- c-kit+ Sca-1+ cell response to Escherichia coli bacteremia. J Immunol. 2009;182:1568–1576. doi: 10.4049/jimmunol.182.3.1568. [DOI] [PMC free article] [PubMed] [Google Scholar]


