Abstract
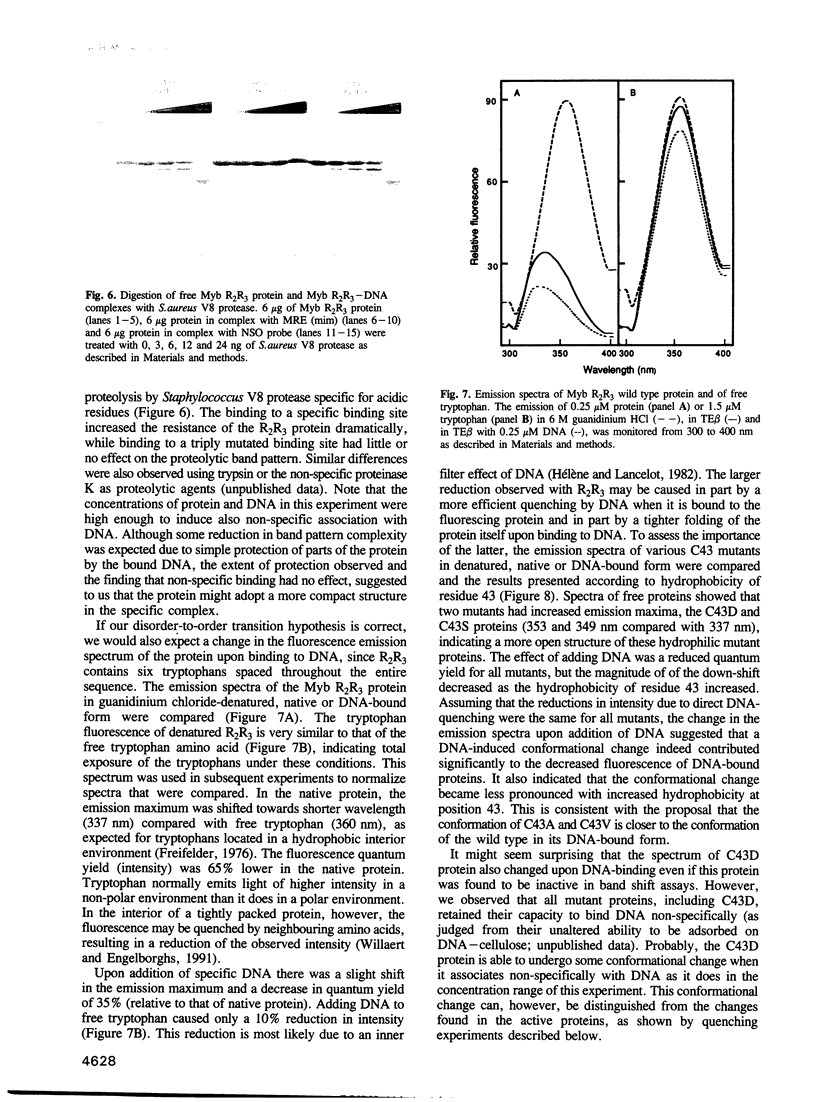
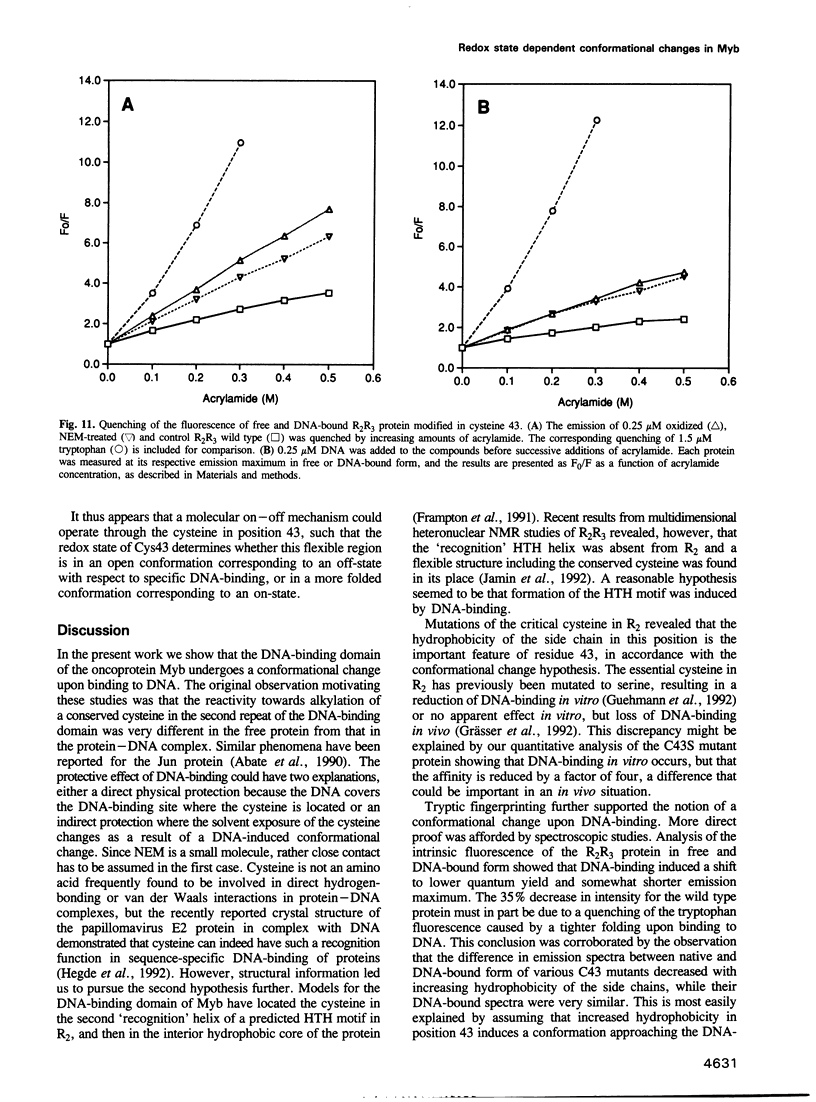
The DNA-binding domain of the oncoprotein Myb comprises three imperfect repeats, R1, R2 and R3. Only R2 and R3 are required for sequence-specific DNA-binding. Both are assumed to contain helix-turn-helix (HTH)-related motifs, but multidimensional heteronuclear NMR spectroscopy revealed a disordered structure in R2 where the second HTH helix was predicted [Jamin et al. (1993) Eur. J. Biochem., 216, 147-154]. We propose that the disordered region folds into a 'recognition' helix and generates a full HTH-related motif upon binding to DNA. This would move Cys43 into the hydrophobic core of R2. We observed that Cys43 was accessible to N-ethylmaleimide alkylation in the free protein, but inaccessible in the DNA complex. Mutant proteins with charged (C43D) or polar (C43S) side chains in position 43 bound DNA with reduced affinity, while hydrophobic replacements (C43A, C43V and C43I) gave unaltered or improved DNA-binding. Specific DNA-binding enhanced protease resistance dramatically. Fluorescence emission spectra and quenching experiments supported a DNA-induced conformational change. Moreover, reversible oxidation of Cys43 had an effect similar to the inactivating C43D mutation. The highly oxidizable Cys43 could function as a molecular sensor for a redox regulatory mechanism turning specific DNA-binding on or off by controlling the DNA-induced conformational change in R2.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abate C., Patel L., Rauscher F. J., 3rd, Curran T. Redox regulation of fos and jun DNA-binding activity in vitro. Science. 1990 Sep 7;249(4973):1157–1161. doi: 10.1126/science.2118682. [DOI] [PubMed] [Google Scholar]
- Bannister A. J., Cook A., Kouzarides T. In vitro DNA binding activity of Fos/Jun and BZLF1 but not C/EBP is affected by redox changes. Oncogene. 1991 Jul;6(7):1243–1250. [PubMed] [Google Scholar]
- Carr M. D., Mott R. F. The transcriptional control proteins c-Myb and v-Myb contain a basic region DNA binding motif. FEBS Lett. 1991 May 6;282(2):293–294. doi: 10.1016/0014-5793(91)80498-r. [DOI] [PubMed] [Google Scholar]
- Dasgupta P., Reddy C. D., Saikumar P., Reddy E. P. The cellular proto-oncogene product Myb acts as transcriptional activator of the long terminal repeat of human T-lymphotropic virus type I. J Virol. 1992 Jan;66(1):270–276. doi: 10.1128/jvi.66.1.270-276.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dasgupta P., Saikumar P., Reddy C. D., Reddy E. P. Myb protein binds to human immunodeficiency virus 1 long terminal repeat (LTR) sequences and transactivates LTR-mediated transcription. Proc Natl Acad Sci U S A. 1990 Oct;87(20):8090–8094. doi: 10.1073/pnas.87.20.8090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eftink M. R., Ghiron C. A. Exposure of tryptophanyl residues in proteins. Quantitative determination by fluorescence quenching studies. Biochemistry. 1976 Feb 10;15(3):672–680. doi: 10.1021/bi00648a035. [DOI] [PubMed] [Google Scholar]
- Evans J. L., Moore T. L., Kuehl W. M., Bender T., Ting J. P. Functional analysis of c-Myb protein in T-lymphocytic cell lines shows that it trans-activates the c-myc promoter. Mol Cell Biol. 1990 Nov;10(11):5747–5752. doi: 10.1128/mcb.10.11.5747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frampton J., Gibson T. J., Ness S. A., Döderlein G., Graf T. Proposed structure for the DNA-binding domain of the Myb oncoprotein based on model building and mutational analysis. Protein Eng. 1991 Dec;4(8):891–901. doi: 10.1093/protein/4.8.891. [DOI] [PubMed] [Google Scholar]
- Fried M. G. Measurement of protein-DNA interaction parameters by electrophoresis mobility shift assay. Electrophoresis. 1989 May-Jun;10(5-6):366–376. doi: 10.1002/elps.1150100515. [DOI] [PubMed] [Google Scholar]
- Furukawa Y., Piwnica-Worms H., Ernst T. J., Kanakura Y., Griffin J. D. cdc2 gene expression at the G1 to S transition in human T lymphocytes. Science. 1990 Nov 9;250(4982):805–808. doi: 10.1126/science.2237430. [DOI] [PubMed] [Google Scholar]
- Gabrielsen O. S., Sentenac A., Fromageot P. Specific DNA binding by c-Myb: evidence for a double helix-turn-helix-related motif. Science. 1991 Sep 6;253(5024):1140–1143. doi: 10.1126/science.1887237. [DOI] [PubMed] [Google Scholar]
- Grässer F. A., LaMontagne K., Whittaker L., Stohr S., Lipsick J. S. A highly conserved cysteine in the v-Myb DNA-binding domain is essential for transformation and transcriptional trans-activation. Oncogene. 1992 May;7(5):1005–1009. [PubMed] [Google Scholar]
- Guehmann S., Vorbrueggen G., Kalkbrenner F., Moelling K. Reduction of a conserved Cys is essential for Myb DNA-binding. Nucleic Acids Res. 1992 May 11;20(9):2279–2286. doi: 10.1093/nar/20.9.2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halazonetis T. D., Davis L. J., Kandil A. N. Wild-type p53 adopts a 'mutant'-like conformation when bound to DNA. EMBO J. 1993 Mar;12(3):1021–1028. doi: 10.1002/j.1460-2075.1993.tb05743.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hegde R. S., Grossman S. R., Laimins L. A., Sigler P. B. Crystal structure at 1.7 A of the bovine papillomavirus-1 E2 DNA-binding domain bound to its DNA target. Nature. 1992 Oct 8;359(6395):505–512. doi: 10.1038/359505a0. [DOI] [PubMed] [Google Scholar]
- Howe K. M., Reakes C. F., Watson R. J. Characterization of the sequence-specific interaction of mouse c-myb protein with DNA. EMBO J. 1990 Jan;9(1):161–169. doi: 10.1002/j.1460-2075.1990.tb08092.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchison K. A., Matić G., Meshinchi S., Bresnick E. H., Pratt W. B. Redox manipulation of DNA binding activity and BuGR epitope reactivity of the glucocorticoid receptor. J Biol Chem. 1991 Jun 5;266(16):10505–10509. [PubMed] [Google Scholar]
- Hélène C., Lancelot G. Interactions between functional groups in protein-nucleic acid associations. Prog Biophys Mol Biol. 1982;39(1):1–68. doi: 10.1016/0079-6107(83)90013-5. [DOI] [PubMed] [Google Scholar]
- Introna M., Golay J., Frampton J., Nakano T., Ness S. A., Graf T. Mutations in v-myb alter the differentiation of myelomonocytic cells transformed by the oncogene. Cell. 1990 Dec 21;63(6):1289–1297. doi: 10.1016/0092-8674(90)90424-d. [DOI] [PubMed] [Google Scholar]
- Jamin N., Gabrielsen O. S., Gilles N., Lirsac P. N., Toma F. Secondary structure of the DNA-binding domain of the c-Myb oncoprotein in solution. A multidimensional double and triple heteronuclear NMR study. Eur J Biochem. 1993 Aug 15;216(1):147–154. doi: 10.1111/j.1432-1033.1993.tb18126.x. [DOI] [PubMed] [Google Scholar]
- Ku D. H., Wen S. C., Engelhard A., Nicolaides N. C., Lipson K. E., Marino T. A., Calabretta B. c-myb transactivates cdc2 expression via Myb binding sites in the 5'-flanking region of the human cdc2 gene. J Biol Chem. 1993 Jan 25;268(3):2255–2259. [PubMed] [Google Scholar]
- Kumar S., Rabson A. B., Gélinas C. The RxxRxRxxC motif conserved in all Rel/kappa B proteins is essential for the DNA-binding activity and redox regulation of the v-Rel oncoprotein. Mol Cell Biol. 1992 Jul;12(7):3094–3106. doi: 10.1128/mcb.12.7.3094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunkel T. A., Roberts J. D., Zakour R. A. Rapid and efficient site-specific mutagenesis without phenotypic selection. Methods Enzymol. 1987;154:367–382. doi: 10.1016/0076-6879(87)54085-x. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lüscher B., Christenson E., Litchfield D. W., Krebs E. G., Eisenman R. N. Myb DNA binding inhibited by phosphorylation at a site deleted during oncogenic activation. Nature. 1990 Apr 5;344(6266):517–522. doi: 10.1038/344517a0. [DOI] [PubMed] [Google Scholar]
- Lüscher B., Eisenman R. N. New light on Myc and Myb. Part II. Myb. Genes Dev. 1990 Dec;4(12B):2235–2241. doi: 10.1101/gad.4.12b.2235. [DOI] [PubMed] [Google Scholar]
- McBride A. A., Klausner R. D., Howley P. M. Conserved cysteine residue in the DNA-binding domain of the bovine papillomavirus type 1 E2 protein confers redox regulation of the DNA-binding activity in vitro. Proc Natl Acad Sci U S A. 1992 Aug 15;89(16):7531–7535. doi: 10.1073/pnas.89.16.7531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ness S. A., Marknell A., Graf T. The v-myb oncogene product binds to and activates the promyelocyte-specific mim-1 gene. Cell. 1989 Dec 22;59(6):1115–1125. doi: 10.1016/0092-8674(89)90767-8. [DOI] [PubMed] [Google Scholar]
- Nicolaides N. C., Gualdi R., Casadevall C., Manzella L., Calabretta B. Positive autoregulation of c-myb expression via Myb binding sites in the 5' flanking region of the human c-myb gene. Mol Cell Biol. 1991 Dec;11(12):6166–6176. doi: 10.1128/mcb.11.12.6166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Neil K. T., Shuman J. D., Ampe C., DeGrado W. F. DNA-induced increase in the alpha-helical content of C/EBP and GCN4. Biochemistry. 1991 Sep 17;30(37):9030–9034. doi: 10.1021/bi00101a017. [DOI] [PubMed] [Google Scholar]
- Ogata K., Hojo H., Aimoto S., Nakai T., Nakamura H., Sarai A., Ishii S., Nishimura Y. Solution structure of a DNA-binding unit of Myb: a helix-turn-helix-related motif with conserved tryptophans forming a hydrophobic core. Proc Natl Acad Sci U S A. 1992 Jul 15;89(14):6428–6432. doi: 10.1073/pnas.89.14.6428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel L., Abate C., Curran T. Altered protein conformation on DNA binding by Fos and Jun. Nature. 1990 Oct 11;347(6293):572–575. doi: 10.1038/347572a0. [DOI] [PubMed] [Google Scholar]
- Sakura H., Kanei-Ishii C., Nagase T., Nakagoshi H., Gonda T. J., Ishii S. Delineation of three functional domains of the transcriptional activator encoded by the c-myb protooncogene. Proc Natl Acad Sci U S A. 1989 Aug;86(15):5758–5762. doi: 10.1073/pnas.86.15.5758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen-Ong G. L. The myb oncogene. Biochim Biophys Acta. 1990 Jun 1;1032(1):39–52. doi: 10.1016/0304-419x(90)90011-o. [DOI] [PubMed] [Google Scholar]
- Simons M., Edelman E. R., DeKeyser J. L., Langer R., Rosenberg R. D. Antisense c-myb oligonucleotides inhibit intimal arterial smooth muscle cell accumulation in vivo. Nature. 1992 Sep 3;359(6390):67–70. doi: 10.1038/359067a0. [DOI] [PubMed] [Google Scholar]
- Siu G., Wurster A. L., Lipsick J. S., Hedrick S. M. Expression of the CD4 gene requires a Myb transcription factor. Mol Cell Biol. 1992 Apr;12(4):1592–1604. doi: 10.1128/mcb.12.4.1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stober-Grässer U., Brydolf B., Bin X., Grässer F., Firtel R. A., Lipsick J. S. The Myb DNA-binding domain is highly conserved in Dictyostelium discoideum. Oncogene. 1992 Mar;7(3):589–596. [PubMed] [Google Scholar]
- Studier F. W., Rosenberg A. H., Dunn J. J., Dubendorff J. W. Use of T7 RNA polymerase to direct expression of cloned genes. Methods Enzymol. 1990;185:60–89. doi: 10.1016/0076-6879(90)85008-c. [DOI] [PubMed] [Google Scholar]
- Sureau A., Soret J., Vellard M., Crochet J., Perbal B. The PR264/c-myb connection: expression of a splicing factor modulated by a nuclear protooncogene. Proc Natl Acad Sci U S A. 1992 Dec 15;89(24):11683–11687. doi: 10.1073/pnas.89.24.11683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toledano M. B., Leonard W. J. Modulation of transcription factor NF-kappa B binding activity by oxidation-reduction in vitro. Proc Natl Acad Sci U S A. 1991 May 15;88(10):4328–4332. doi: 10.1073/pnas.88.10.4328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travali S., Reiss K., Ferber A., Petralia S., Mercer W. E., Calabretta B., Baserga R. Constitutively expressed c-myb abrogates the requirement for insulinlike growth factor 1 in 3T3 fibroblasts. Mol Cell Biol. 1991 Feb;11(2):731–736. doi: 10.1128/mcb.11.2.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullrich S. J., Anderson C. W., Mercer W. E., Appella E. The p53 tumor suppressor protein, a modulator of cell proliferation. J Biol Chem. 1992 Aug 5;267(22):15259–15262. [PubMed] [Google Scholar]
- Venturelli D., Travali S., Calabretta B. Inhibition of T-cell proliferation by a MYB antisense oligomer is accompanied by selective down-regulation of DNA polymerase alpha expression. Proc Natl Acad Sci U S A. 1990 Aug;87(15):5963–5967. doi: 10.1073/pnas.87.15.5963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasylyk C., Wasylyk B. Oncogenic conversion of Ets affects redox regulation in-vivo and in-vitro. Nucleic Acids Res. 1993 Feb 11;21(3):523–529. doi: 10.1093/nar/21.3.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss M. A., Ellenberger T., Wobbe C. R., Lee J. P., Harrison S. C., Struhl K. Folding transition in the DNA-binding domain of GCN4 on specific binding to DNA. Nature. 1990 Oct 11;347(6293):575–578. doi: 10.1038/347575a0. [DOI] [PubMed] [Google Scholar]
- Xanthoudakis S., Curran T. Identification and characterization of Ref-1, a nuclear protein that facilitates AP-1 DNA-binding activity. EMBO J. 1992 Feb;11(2):653–665. doi: 10.1002/j.1460-2075.1992.tb05097.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xanthoudakis S., Miao G., Wang F., Pan Y. C., Curran T. Redox activation of Fos-Jun DNA binding activity is mediated by a DNA repair enzyme. EMBO J. 1992 Sep;11(9):3323–3335. doi: 10.1002/j.1460-2075.1992.tb05411.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zobel A., Kalkbrenner F., Guehmann S., Nawrath M., Vorbrueggen G., Moelling K. Interaction of the v-and c-Myb proteins with regulatory sequences of the human c-myc gene. Oncogene. 1991 Aug;6(8):1397–1407. [PubMed] [Google Scholar]










