Abstract
Purpose
We conducted a pediatric phase I study to estimate the maximum tolerated dose (MTD), dose-limiting toxicities (DLT), and pharmacokinetic properties of vorinostat, a histone deacetylase (HDAC) inhibitor, when given in combination with temozolomide in children with refractory or recurrent CNS malignancies.
Patients and Methods
Vorinostat, followed by temozolomide approximately one hour later, was orally administered, once daily, for 5 consecutive days every 28 days at 3 dose levels using the rolling 6 design. Studies of histone accumulation in peripheral blood mononuclear cells were performed on day 1 at 0, 6, and 24 h after vorinostat dosing. Vorinostat pharmacokinetics (PK) and serum MGMT promoter status were also assessed
Results
Nineteen eligible patients were enrolled and eighteen patients were evaluable for toxicity. There were no DLTs observed at dose level 1 or 2. DLTs occurred in 4 patients at dose level 3: thrombocytopenia (4), neutropenia (3), and leucopenia (1). Non-dose limiting grade 3 or 4 toxicities related to protocol therapy were also hematologic and included neutropenia, lymphopenia, thrombocytopenia, anemia, and leucopenia. Three patients exhibited stable disease and one patient had a partial response. There was no clear relationship between vorinostat dosage and drug exposure over the dose range studied. Accumulation of acetylated H3 histone in PBMC was observed after administration of vorinostat.
Conclusion
Five-day cycles of vorinostat in combination with temozolomide are well tolerated in children with recurrent CNS malignancies with myelosuppression as the DLT. The recommended phase II combination doses are vorinostat, 300 mg/m2/day and temozolomide,150 mg/m2/day.
Keywords: vorinostat, temozolomide phase I trial, pediatric cancer, Children’s Oncology Group
INTRODUCTION
Epigenetic alterations, including changes in structure of chromatin by histone modification, play an important role in tumorigenesis by altering gene expression and subsequently affecting viability and growth of neoplastic cells.[1] Modification of core histones, H3 and H4, by phosphorylation, methylation or acetylation is relevant as excessive deacetylation by histone deacetylases (HDAC) and the subsequent silencing of gene expression can be seen in malignancies.[2–4] HDAC inhibitors are a class of agents that have been designed to relax DNA to allow increased active transcription of genes which may have been silenced during tumor development.[5].
Vorinostat (VOR), (suberoylanilide hydroxamic acid, SAHA), is an oral HDAC inhibitor which inhibits HDAC activity by inserting into the active site of the enzyme, resulting in arrest of cell cycle transition at G1 and G2M and inducing p53-independent apoptosis.[6] The build -up of acetylated histones leads to increased transcription of approximately 2% of all expressed genes, with a predilection for reactivating genes associated with differentiation or apoptosis.[7] VOR has successfully induced differentiation, growth arrest, and apoptosis in medulloblastoma cell lines and primary tumor cell cultures at clinically achievable concentrations.[8–10] Systemically administered VOR crosses the blood brain barrier[10, 11] and causes apoptotic cell death in transgenic mouse models of medulloblastoma.[10] Histone deacetylation in blood and tumor tissue is seen in a dose-dependent fashion, and can be used as a surrogate marker of biological activity.[7, 8] VOR has activity against glioblastoma cell lines and systemic administration significantly prolonged survival in mice bearing intracranial glioblastoma xenografts.[7] VOR has also been shown to induce cell cycle arrest in glioma cells with an associated increase in p21 levels and reduced cyclin B1.[12]
Temozolomide (TEM) is an oral imidazotetrazine prodrug that undergoes spontaneous hydrolysis to the active metabolite MTIC, which methylates DNA at O6-guanine and other sites. TEM has been shown to be effective against xenograft models of glioblastoma, medulloblastoma, and ependymomas.[13] Although TEM used during and following radiotherapy is the current standard of care for adults with newly diagnosed high-grade glioma, single-agent activity of TEM for pediatric high grade glioma has been modest at best.[14–16] Other tumor types may be more responsive, as evidenced by the COG Phase II trial of TEM in children with relapsed brain tumors that demonstrated complete or partial responses in 4 of 25 evaluable patients with medulloblastoma/PNETs.[17] This study confirmed five other clinical reports of responses in patients with relapsed medulloblastoma following treatment with TEM.[18–22] TEM may also be active in patients with low-grade glioma. Adult Phase II studies have demonstrated response rates of as high as 50–60% with single-agent temozolomide, with another 30% of patients having prolonged stable disease.[23, 24] The median progression-free survival (PFS) ranges from 22–28 months in these trials, which is consistent in published pediatric studies.[17, 25–27].
We report the results of a phase I trial of VOR in combination with TEM in children with recurrent or refractory brain or spinal cord tumors. The primary objectives were to estimate the maximum tolerated dose (MTD) and/or recommended Phase II dose of VOR that can be administered orally in combination with TEM (daily x 5 days), every 28 days, to children with relapsed or refractory primary central nervous system tumors and to define and describe the toxicities of VOR administered on this schedule in combination with TEM.
PATIENTS AND METHODS
Patient Eligibility
Patients older than 12 months and ≤21 years with refractory or recurrent primary brain or spinal cord tumors for which there was no known curative therapy were eligible for this trial. Tumors had to measurable or evaluable by imaging and histological verification of malignancy was required except for patients with diffuse intrinsic brain stem tumors, optic pathway gliomas or patients with pineal tumors and elevations of CSF or serum tumor markers including alpha-fetoprotein or beta-HCG. Other eligibility criteria included: a Lansky or Karnofsky score ≥50; recovery from the acute toxic effects of prior therapy; and at least 7 days from prior growth factor therapy; 3 weeks from myelosuppressive chemotherapy (6 weeks if prior nitrosourea), 6 months from craniospinal or total body irradiation, 2 weeks sinc local palliative radiotherapy, and 2 weeks since prior therapy with valproic acid (another HDAC inhibitor). Patients were excluded if they were pregnant, lactating women, had an uncontrolled infection, were receiving enzyme-inducing anticonvulsants, had a QTc > 450 msec or were previously treated with vorinistat. Patients who were on corticosteroids must be taking a stable or decreasing dose for 7 days prior to enrollment. Prior treatment with TEM was allowed, provided there was no disease progression during or within one month after that treatment.
Other requirements included adequate bone marrow function (peripheral absolute neutrophil count ≥1000/microliter, platelet count ≥100,000/microliter, transfusion independent, and hemoglobin ≥8.0 gm/dL), renal function (age-adjusted normal serum creatinine or GFR ≥70 ml/min/1.73m2), liver function (total bilirubin ≤1.5 × institutional upper limit of normal for age, ALT ≤ 110 U/L and albumin ≥2 g/dL).
Institutional review board approval was obtained at participating institutions. Informed consent was obtained from patients aged ≥18 years or from parents/legal guardians of children age less than 18 years (with child assent in patients ≥7 years of age as established by National Commission for Protection of Human Subjects of Biomedical and Behavioral Research), according to individual institutional policies.
Drug Administration and Study Design
Vorinostat was supplied by Merck and Co., Inc. (Whitehouse Station, NJ) and distributed by the Cancer Therapy Evaluation Program (National Cancer Institute, Bethesda, MD) as a white, opaque gelatin, size 3 capsule, containing 100 mg of vorinostat. A dosing nomogram was used to minimize interpatient dosing variability. Patients with BSA < 1.25 m2 received the vorinostat suspension rounded to the nearest 5 mg. The suspension was prepared locally by investigational pharmacist by adding 20 mL of Suspensol S or OraPlus with the contents of twenty 100 mg (2000 mg) vorinostat capsules in a 4 ounce amber or clear glass bottle. After shaking for up to 3 minutes to disperse, an additional 20mL of OraSweet was added to achieve a total volume of 40 mL and a final concentration of 50 mg/mL. The suspension was stored at room temperature and based on manufacturer’s recommendation was stable for a maximum of 2 weeks. Vorinostat was administered orally each day, preferably with food.
Temozolomide was commercially available in 5 mg, 20 mg, 100 mg, 140 mg, 180 mg, 250 mg capsules, stored at room temperature. A dosing nomogram was used to minimize interpatient dosing variability. For patients unable to swallow the capsules whole, the oral capsules were formulated into a suspension at a final concentration of 10 mg/mL. The suspension was packaged in an amber plastic prescription bottle and was stable for 7 days at room temperature or 60 days in the refrigerator.
The starting dose (dose level 1) was 230 mg/m2/day on days 1–5 for VOR and 150 mg/m2/day on days 1–5 for TEM. The VOR dose was increased to 300 mg/m2/day at dose level 2 while the TEM remained at 150 mg/m2/day. Dose level 3 VOR dose was static at 300 mg/m2/day while the TEM increased to 200 mg/m2/day. No intrapatient dose escalation allowed. Each treatment cycle lasted 28 days. Vorinostat was given approximately one hour prior to temozolomide. In the absence of disease progression, and if laboratory parameters as defined in the eligibility section were met, each 28 day cycle could be repeated for up to 13 cycles.
Dose escalation in this study utilized a rolling six design [28]. Tumor response was reported using the Response Evaluation Criteria in Solid Tumors (RECIST).[29] Toxicities were graded according to the Common Terminology Criteria for Adverse Events version 4.0. Hematologic DLT was defined as Grade 4 neutropenia for > 7 days, platelet count < 20,000/microliters on 2 separate days or requiring a platelet transfusion on 2 separate days (within a 7 day period), or myelosuppression that causes a delay of ≥ 14 days between treatment cycles. Nonhematologic DLT was defined as any toxicity that precludes administration of at least 80% of the planned dose intensity of VOR or TEM during a given cycle, non-hematological toxicity that causes a delay of 14 days between treatment cycles, and any Grade 3 or Grade 4 non-hematological toxicity attributable to VOR or TEM with the specific exclusion of: grade 3 nausea and vomiting < 3 days duration, grade 3 ALT/AST elevation that returns to grade ≤1 or baseline prior to the next treatment cycle, grade 3 fever, grade 3 infection, grade 3 hypophosphatemia, hypokalemia, hypocalcemia or hypomagnesemia responsive to oral supplementation, grade 3 fatigue for ≤3 days duration. The observation period for the purposes of dose-escalation was the first cycle of therapy.
Pretreatment evaluations included a history, physical examination, CBC, electrolytes, renal and liver function tests, serum protein and albumin, urinalysis, and EKG. CBCs were obtained twice weekly during the first cycle and weekly thereafter. History, physical examinations, and laboratory studies were obtained weekly in cycle 1 and before each subsequent cycle. Disease evaluations were obtained at baseline, at the end of cycle 1 and after every other cycle.
Pharmacokinetic Studies
Blood samples (1.5 mL) were collected in red-top Vacutainer tubes before VOR administration and 0.25, 0.5, 1, 2, 4, 6, 8, and 24 hours after the administration of VOR in consenting patients. The serum concentrations of VOR and its metabolites, 4-anilino-4-oxobutanoic acid (VA) VOR-glucuronide (VG) were measured using a previously described validated liquid chromatography, tandem mass spectrometry (LC/MS/MS) method.[30] The lower limits of quantitation and linear range for VOR, VA and VG were 1 ng/ml and 1 – 1000 ng/ml, respectively. The within-day and between-day precision (coefficient of variation) and accuracy values for the three analytes met standard assay validation criteria.[31] VOR, VA, and VG serum concentration-time data were analyzed using noncompartmental methods with the program WinNonlin Professional, version 5.3 (Pharsight Corporation, Mountain View, CA).
Histone Accumulation in Peripheral Blood Mononuclear Cells
PBMC protein lysates were isolated as described previously [32] from whole blood (3–4 ml) collected at 0, 6, and 24 hours after the initial dose of VOR in Cycle 1 in consenting patients. For patients less than 10 kg in size, samples were collected at 0 and 24 hours after the first dose of VOR. Sixty micrograms of protein extracted from WBCs along with a loading control for normalization were analyzed via Western Blot. Intensities of the proteins in the Western blots were quantified along with loading control for normalization using Image J processing..
MGMT Promoter Status
A single blood sample (5 mL if ≥10 kg or 3 mL if < 10 kg) was collected in an in ACDA or preservative-free heparinized tubes within 2 weeks prior to the initial dose of VOR. MGMT promoter methylation was determined as previously described.[33]
RESULTS
Patient Characteristics
Nineteen eligible patients were enrolled on study between June 2010 and November 2010. The distribution of age, gender, and diagnoses is summarized in Table I. Eighteen patients were evaluable for toxicity as one patient at dose level 3 was not evaluable for hematologic and non-hematologic toxicity as the patient took less than 80% of the total number of doses of temozomolide The median number of cycles administered was 2 (range, 1–13).
Table I.
Patient Characteristics for eligible Patients (n=19)
| Characteristic | Number (%) |
|---|---|
|
| |
| Age (years) | |
| Median | 8.3 |
| Range | 2.1–20.8 |
|
| |
| Sex | |
| Male | 12 (63.2) |
| Female | 7 (36.8) |
|
| |
| Race | |
| White | 15 (78.9) |
| Other | 2 (10.5) |
| Asian India, Pakistani | 1 (5.3) |
| Unknown | 1 (5.3) |
|
| |
| Ethnicity | |
| Non-Hispanic | 15 (78.9) |
| Mexican (including Chicano) | 2 (10.5) |
| Hispanic NOS | 2 (10.5) |
|
| |
| Diagnosis | |
|
| |
| Choroid plexus carcinoma | 1 (5.3) |
|
| |
| PNET | 2 (10.5) |
|
| |
| High grade glioma | 7 (36.9) |
|
| |
| Ependymoma | 4 (21.1) |
|
| |
| Medulloblastoma | 2 (10.5) |
|
| |
| Ganglioglioma | 1 (5.3) |
|
| |
| Atypical teratoid/rhabdoid tumor | 2 (10.5) |
|
| |
| Prior Therapy | |
|
| |
| Chemotherapy Regimens | |
|
| |
| Median | 1 |
| Range | 0–7 |
|
| |
| Number of Patients with Prior Radiation Therapy | 17 |
Toxicity
The observed DLTs are summarized in Table II. There were no DLTs observed at dose level 1 (n=6) or 2 (n=6). DLTs occurred in 4 patients at dose level 3: thrombocytopenia (4), neutropenia (3), and leucopenia (1) thus defining the pediatric MTD and recommended phase II dosing for the combination of VOR and TEM as 300 mg/m2/day and 150 mg/m2/day, respectively. Table III summarizes all adverse events at least possibly attributable to VOR or TEM observed in more than 10 percent of the 18 toxicity evaluable patients. The majority of hematological toxicities occurred in cycles 1 and 2. There were no grade 3 or 4 non-hematologic toxicities experienced during the first or subsequent cycles of therapy and all non-hematological toxicities were ≤ grade 2.
Table II.
DLTs Summary
| Dose Level | No. of Patients Entered | No. of Patients Evaluable | No. of Patients with DLT | DLT Detail (n) |
|---|---|---|---|---|
| 230 mg/m2 Vorinostat and 150 mg/m2 Temozolomide | 6 | 6 | 0 | |
| 300 mg/m2 Vorinostat and 150 mg/m2 Temozolomide | 7 | 6 | 0 | |
| 300 mg/m2 Vorinostat and 200 mg/m2 Temozolomide | 6 | 6 | 4 | Neutropenia (3) Thrombocytopenia (4) Leucopenia (1) |
DLT – Dose limiting toxicity
Table III.
Non-dose limiting toxicities related to protocol therapy and observed in more than 10 percent of evaluable patients.
| Toxicity Type | Maximum grade of toxicity across cycle 1 (total, 18 cycles) | Maximum grade of toxicity across cycles 2–13 (total, 45 cycles) | ||||||
|---|---|---|---|---|---|---|---|---|
| Grade 1 | Grade 2 | Grade 3 | Grade 4 | Grade 1 | Grade 2 | Grade 3 | Grade 4 | |
| Hematologic Toxicities | ||||||||
| Anemia | 2 | 5 | 1 | 2 | 2 | 1 | ||
| Neutrophil count decreased | 5 | 1 | 2 | 2 | ||||
| Platelet count decreased | 5 | 1 | 1 | 1 | 4 | 1 | 1 | 2 |
| White blood cell decreased | 3 | 2 | 4 | 1 | 3 | 3 | 1 | |
| Lymphocyte count decreased | 3 | 4 | 3 | 1 | 2 | 2 | 3 | 1 |
| Non-Hematologic Toxicities | ||||||||
| Anorexia | 2 | 2 | 1 | 1 | ||||
| Alanine aminotransferase increased | 2 | 1 | 1 | |||||
| Aspartate aminotransferase increased | 2 | 1 | ||||||
| Constipation | 1 | 2 | ||||||
| Diarrhea | 3 | 1 | 2 | 1 | ||||
| Fatigue | 3 | 1 | 1 | 1 | ||||
| Fever | 2 | 1 | ||||||
| Headache | 1 | 1 | 1 | |||||
| Hyperglycemia | 4 | 1 | ||||||
| Hypermagnesemia | 3 | 1 | ||||||
| Hypernatremia | 2 | 1 | ||||||
| Hypoalbuminemia | 2 | 2 | ||||||
| Hypocalcemia | 3 | 1 | ||||||
| Hypokalemia | 2 | 3 | ||||||
| Hyponatremia | 3 | |||||||
| Nausea | 7 | 2 | 2 | 1 | ||||
| Stomach pain | 2 | |||||||
| Vomiting | 5 | 2 | 2 | |||||
| Weight loss | 2 | 1 | ||||||
Responses
Of the 19 patients enrolled on study, 16 patients were evaluable for response. One patient was not evaluable as they came off therapy after cycle 1 due to adverse effects. Two patients were not evaluable due to withdrawal from therapy. Stable disease was observed in 3 patients (ependymoma, ganglioglioma, high grade glioma) with one patient completing all 13 cycles on protocol therapy. One patient with ependymoma received 13 cycles of protocol therapy and had a partial response confirmed after 13 cycles. The remaining 12 patients have had progressive disease.
Pharmacokinetics
Sixteen patients consented to participate in pharmacokinetic studies after the first VOR dose on day 1, cycle 1. The pharmacokinetic data for each dose level is summarized in Table IV. While Cmax and AUC values appeared to be higher for the 300 mg/m2 dose level as compared to the 230 mg/m2 dose level, there was substantial variability in drug disposition at each dose level such that there was no clear relationship between dose and drug exposure over the two dose levels that were evaluated.
Table IV.
Summary of Vorinostat pharmacokinetic parameters*
| Dose Level† | 230/150 (N=6) | 300/150 (N=5) | 300/200 (N=5) | |
|---|---|---|---|---|
|
| ||||
| VOR | Tmax (hrs) | 2.00 (0.25 – 2.05) | 2.08 (0.53 – 4.25) | 1.02 (0.50 – 4.07) |
| Cmax(ng/ml) | 390 (221 – 655) | 422 (178 – 663) | 578 (200 – 821) | |
| Half-life(hrs) | 2.61 (0.99 – 11.1) | 2.62 (1.12 – 5.30) | 2.80 (2.23 – 5.90) | |
| AUC0-∞ (ng/ml* hr) | 1420 (547 – 2310) | 1790 (1100 – 2180) | 2070 (1280 – 5430) | |
| V/F(L/m2) | 424 (222 – 7050) | 529 (289 – 1460) | 759 (207 – 1260) | |
| Cl/F(L/hr/m2) | 161 (90.9 – 439) | 175 (138 – 296) | 148 (62.6 – 236) | |
|
| ||||
| 4-anilino-4- oxobutanoic acid | Tmax (hrs) | 3.00 (1.92 – 4.08) | 4.00 (1.00 – 4.25) | 2.00 (0.88 – 6.00) |
| Cmax(ng/ml) | 490 (160 – 884) | 814 (588 – 832) | 750 (559 – 984) | |
| Half-life(hrs) | 10.4 (1.81 – 216) | 9.18 (5.56 – 13.5) | 9.19 (6.49 – 11.6) | |
| AUC0-∞ (ng/ml* hr) | 6900 (2460 – 19100) | 7490 (6410 – 9330) | 9590 (7150 – 16600) | |
|
| ||||
| VOR- glucuronide | Tmax (hrs) | 2.00 (0.45 – 2.05) | 2.08 (1.00 – 4.25) | 2.00 (0.88 – 4.07) |
| Cmax(ng/ml) | 1700 (503 – 2720) | 1870 (1310 – 2620) | 1910 (1570 – 3860) | |
| Half-life(hrs) | 2.53 (1.05 – 49.5) | 2.69 (2.04 – 5.18) | 3.25 (2.12 – 4.62) | |
| AUC0-∞ (ng/ml* hr) | 6810 (4260 – 11000) | 6210 (5630 – 14800) | 13200 (5460 – 16200) | |
Median values with range in parentheses.
SAHA/TEM (mg/m2).
Histone Accumulation in Peripheral Blood Mononuclear Cells
Adequate samples for assessment of histone accumulation were available from 8 patients. Patients at all dose levels showed evidence of acetyl-H3 accumulation in PBMC. There was an increase in the accumulation of acetyl-H3 in those patients receiving 300 mg/m2/day of VOR in combination with 150 mg/m2/day of TEM compared to patients receiving 230 mg/m2/day of VOR in combination with 150 mg/m2/day of TEM, however, this did not reach significance (p=0.3).
MGMT Promoter Status
Fifteen samples were evaluated for DNA for methylation of the MGMT promoter in plasma. A total 4 of the 14 patients had evidence of promoter methylation in plasma. This did not appear to correlate with response or disease stabilization.
DISCUSSION
It has been hypothesized that the looser chromatin structure and cell cycle arrest seen following treatment with HDAC inhibitors may render cells more sensitive to drugs targeting DNA or enzymes acting on DNA. Supporting this hypothesis are demonstrations that pretreatment with clinically achievable concentrations of VOR markedly augments the cytotoxicity of etoposide, but not a mitotic tubule inhibitor (vincristine) in medulloblastoma cell lines.[9] Similar synergy has been demonstrated in other cancer cell lines when HDAC inhibitors are used prior to treatment with cisplatin and doxorubicin, but not with the antimetabolite 5-fluorouracil.[34] Consistent with the proposed mechanism of action, beneficial effects were only seen when the HDAC inhibitor preceded the DNA damaging agent.
This pediatric phase I trial established the MTD of concurrent 5 day administration of VOR in combination with TEM as 300 mg/m2/day and 150 mg/m2/day administered orally in patients with recurrent or refractory central nervous system tumors. DLTs for this combination were thrombocytopenia, neutropenia, and leucopenia. These DLTs are similar to those observed in the adult phase 1 combination study with the notable exception of fatigue which was not a DLT in this pediatric trial.[35] We note that pediatric phase 1 trial of single-agent oral VOR found the MTD to be 230 mg/m2/day given continuously as a single daily dose with one of six patients having a DLT (e.g. deep vein thrombosis). At the higher dose of 300 mg/m2/day, reversible hypokalemia, neutropenia, and thrombocytopenia were dose-limiting, similar toxicities to what were seen in this combination study.[36] We also note that there were no grade 3 or 4 non-hematologic toxicities reported for this combination at any dose level unlike the adult single agent phase 1 trials of VOR in which diarrhea, dehydration, fatigue, and thrombocytopenia were seen as DLTs.[37]
The disposition of VOR administered 1 hour before TEM in children was similar to that observed in children [36] and adults [37] when VOR was administered as a single agent. The parent drug is absorbed rapidly with a time to maximum concentration of 2 hours (range, 0.25 – 4 hours). There was also substantial variability in the pharmacokinetics of the inactive VOR metabolites, 4-anilino-4-oxobutanoic acid and VOR- glucuronide. There was not an association between pharmacokinetic, pharmacodynamic, toxicities, or response data.
Accumulation of acetyl H3 histones in PBMCs was detected in patients receiving the all dosages of VOR in combination with TEM. There was no statistical difference in the accumulation of acetyl-H3 in those patients at the MTD compared to patients receiving study drug either one dose level above or below the MTD. There was no association of peak accumulation of acetyl-H3 with radiographic responses.
Immunoblot analyses confirmed that methylation of free DNA can be detected in the plasma of patients with brain tumors. In this study 4 of the 14 patients had evidence of MGMT promoter methylation. While expression of the MGMT DNA repair gene is one of the primary mechanism of resistance to temozolomide,[38] none of the patients with promoter methylation exhibited stable disease or response to treatment.
Overall, this combination of VOR and TEM was well tolerated in children. VOR disposition was/was not altered when TEM was given concurrently. One objective response was seen on this trial. Three additional patients exhibited stable disease with one patient currently in extended treatment cycles. Notably only one low grade CNS neoplasm enrolled on this phase 1 trial (ganglioglioma). This may explain the lack of responses given the track record with TEM in pediatric CNS high grade gliomas since TEM has been shown to be a viable option for treating refractory low grade neoplasms[25–27] and one may have seen more responses with TEM combined with VOR in low grade neoplasms. An additional factor that could account for the lack of responses in this patient cohort was that these patients were heavily pretreated (median number of prior treatments was 3) and, as such, did not tolerate extended cycles of this combination necessary to induce a response.
In conclusion, this combination at the recommended Phase 2 dose (vorinostat at 300 mg/m2/day and temozolomide at 150 mg/m2/day) is tolerable but demonstrated limited clinical activity in a heterogenous population of CNS tumors.
Figure 1. O6- Methylguanine-DNA methyltransferase (MGMT) promoter methylation in patient plasma.
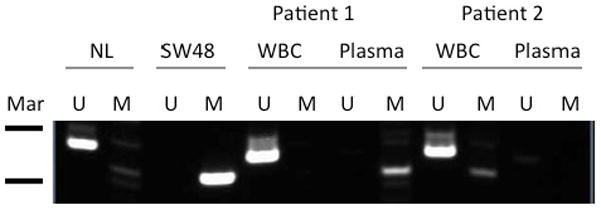
Genomic DNA was extracted before TMZ/SAHA treatment and MGMT promoter methylation was determined by methylation-specific polymerase chain reaction (PCR). Peripheral blood monouclear cells (WBC) from each patient served as an internal control, and was unmethylated in all patients. Markers (Mar): 100bp (upper) and 75bp (lower). U, unmethylated, M, methylated. NL = WBC from individual without cancer (unmethylated control), SA48-colon cancer (methylated control).
Acknowledgments
Support: Supported by the Phase I/Pilot Consortium Grant U01 CA97452 of the Children’s Oncology Group from the NCI, NIH, Bethesda, MD, USA.
Footnotes
Prior Presentations: Presented in part at the American Society of Clinical Oncology, Chicago, IL, June 2011
ClinicalTrials.gov Identifier: NCT01076530
References
- 1.Jones PA, Baylin SB. The epigenomics of cancer. Cell. 2007;128(4):683–92. doi: 10.1016/j.cell.2007.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Marks P, et al. Histone deacetylases and cancer: causes and therapies. Nat Rev Cancer. 2001;1(3):194–202. doi: 10.1038/35106079. [DOI] [PubMed] [Google Scholar]
- 3.Marks PA. Discovery and development of SAHA as an anticancer agent. Oncogene. 2007;26(9):1351–6. doi: 10.1038/sj.onc.1210204. [DOI] [PubMed] [Google Scholar]
- 4.Weidle UH, Grossmann A. Inhibition of histone deacetylases: a new strategy to target epigenetic modifications for anticancer treatment. Anticancer Res. 2000;20(3A):1471–85. [PubMed] [Google Scholar]
- 5.Johnstone RW. Histone-deacetylase inhibitors: novel drugs for the treatment of cancer. Nat Rev Drug Discov. 2002;1(4):287–99. doi: 10.1038/nrd772. [DOI] [PubMed] [Google Scholar]
- 6.Xu WS, Parmigiani RB, Marks PA. Histone deacetylase inhibitors: molecular mechanisms of action. Oncogene. 2007;26(37):5541–52. doi: 10.1038/sj.onc.1210620. [DOI] [PubMed] [Google Scholar]
- 7.Yin D, et al. Suberoylanilide hydroxamic acid, a histone deacetylase inhibitor: effects on gene expression and growth of glioma cells in vitro and in vivo. Clinical Cancer Research. 2007;13(3):1045–52. doi: 10.1158/1078-0432.CCR-06-1261. [DOI] [PubMed] [Google Scholar]
- 8.Furchert SE, et al. Inhibitors of histone deacetylases as potential therapeutic tools for high-risk embryonal tumors of the nervous system of childhood. International Journal of Cancer. 2007;120(8):1787–94. doi: 10.1002/ijc.22401. [DOI] [PubMed] [Google Scholar]
- 9.Sonnemann J, et al. Histone deacetylase inhibitors induce cell death and enhance the susceptibility to ionizing radiation, etoposide, and TRAIL in medulloblastoma cells. International Journal of Oncology. 2006;28(3):755–66. [PubMed] [Google Scholar]
- 10.Spiller SE, et al. Suberoylanilide hydroxamic acid is effective in preclinical studies of medulloblastoma. Journal of Neuro-Oncology. 2006;79(3):259–70. doi: 10.1007/s11060-006-9142-0. [DOI] [PubMed] [Google Scholar]
- 11.Hockly E, et al. Suberoylanilide hydroxamic acid, a histone deacetylase inhibitor, ameliorates motor deficits in a mouse model of Huntington’s disease. Proc Natl Acad Sci U S A. 2003;100(4):2041–6. doi: 10.1073/pnas.0437870100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xu J, et al. Vorinostat modulates cell cycle regulatory proteins in glioma cells and human glioma slice cultures. J Neurooncol. 2011 doi: 10.1007/s11060-011-0604-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Friedman HS, et al. Activity of temozolomide in the treatment of central nervous system tumor xenografts. Cancer Res. 1995;55(13):2853–7. [PubMed] [Google Scholar]
- 14.Broniscer A, et al. Temozolomide after radiotherapy for newly diagnosed high-grade glioma and unfavorable low-grade glioma in children. Journal of Neuro-Oncology. 2006;76(3):313–9. doi: 10.1007/s11060-005-7409-5. [DOI] [PubMed] [Google Scholar]
- 15.Cohen KJ, et al. Temozolomide in the treatment of children with newly diagnosed diffuse intrinsic pontine gliomas: a report from the Children’s Oncology Group. Neuro Oncol. 2011;13(4):410–6. doi: 10.1093/neuonc/noq205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lashford LS, et al. Temozolomide in malignant gliomas of childhood: a United Kingdom Children’s Cancer Study Group and French Society for Pediatric Oncology Intergroup Study. Journal of Clinical Oncology. 2002;20(24):4684–91. doi: 10.1200/JCO.2002.08.141. [DOI] [PubMed] [Google Scholar]
- 17.Nicholson HS, et al. Phase 2 study of temozolomide in children and adolescents with recurrent central nervous system tumors: a report from the Children’s Oncology Group. Cancer. 2007;110(7):1542–50. doi: 10.1002/cncr.22961. [DOI] [PubMed] [Google Scholar]
- 18.Barone G, et al. Role of temozolomide in pediatric brain tumors. Childs Nervous System. 2006;22(7):652–61. doi: 10.1007/s00381-006-0081-z. [DOI] [PubMed] [Google Scholar]
- 19.Baruchel S, et al. Safety and pharmacokinetics of temozolomide using a dose-escalation, metronomic schedule in recurrent paediatric brain tumours. European Journal of Cancer. 2006;42(14):2335–42. doi: 10.1016/j.ejca.2006.03.023. [DOI] [PubMed] [Google Scholar]
- 20.Durando X, et al. Temozolomide treatment of an adult with a relapsing medulloblastoma. Cancer Investigation. 2007;25(6):470–5. doi: 10.1080/07357900701518164. [DOI] [PubMed] [Google Scholar]
- 21.Hongeng S, et al. Treatment of leptomeningeal relapse of medulloblastoma with temozolomide. Journal of Pediatric Hematology/Oncology. 2002;24(7):591–3. doi: 10.1097/00043426-200210000-00024. [DOI] [PubMed] [Google Scholar]
- 22.O’Reilly SM, et al. Temozolomide: a new oral cytotoxic chemotherapeutic agent with promising activity against primary brain tumours.[erratum appears in Eur J Cancer 1993;29A(10):1500] European Journal of Cancer. 1993;29A(7):940–2. doi: 10.1016/s0959-8049(05)80198-4. [DOI] [PubMed] [Google Scholar]
- 23.Kaloshi G, et al. Temozolomide for low-grade gliomas: predictive impact of 1p/19q loss on response and outcome.[see comment] Neurology. 2007;68(21):1831–6. doi: 10.1212/01.wnl.0000262034.26310.a2. [DOI] [PubMed] [Google Scholar]
- 24.Quinn JA, et al. Phase II trial of temozolomide in patients with progressive low-grade glioma.[see comment] Journal of Clinical Oncology. 2003;21(4):646–51. doi: 10.1200/JCO.2003.01.009. [DOI] [PubMed] [Google Scholar]
- 25.Gururangan S, et al. Temozolomide in children with progressive low-grade glioma. Neuro-Oncology. 2007;9(2):161–8. doi: 10.1215/15228517-2006-030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Khaw SL, et al. Temozolomide in pediatric low-grade glioma. Pediatric Blood & Cancer. 2007;49(6):808–11. doi: 10.1002/pbc.21270. [DOI] [PubMed] [Google Scholar]
- 27.Kuo DJ, et al. Temozolomide is active in childhood, progressive, unresectable, low-grade gliomas. Journal of Pediatric Hematology/Oncology. 2003;25(5):372–8. doi: 10.1097/00043426-200305000-00005. [DOI] [PubMed] [Google Scholar]
- 28.Skolnik JM, et al. Shortening the timeline of pediatric phase I trials: the rolling six design. J Clin Oncol. 2008;26(2):190–5. doi: 10.1200/JCO.2007.12.7712. [DOI] [PubMed] [Google Scholar]
- 29.Therasse P, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92(3):205–16. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 30.Parise RA, et al. A liquid chromatography-electrospray ionization tandem mass spectrometric assay for quantitation of the histone deacetylase inhibitor, vorinostat (suberoylanilide hydroxamicacid, SAHA), and its metabolites in human serum. J Chromatogr B Analyt Technol Biomed Life Sci. 2006;840(2):108–15. doi: 10.1016/j.jchromb.2006.04.044. [DOI] [PubMed] [Google Scholar]
- 31.Shah VP, et al. Bioanalytical method validation--a revisit with a decade of progress. Pharm Res. 2000;17(12):1551–7. doi: 10.1023/a:1007669411738. [DOI] [PubMed] [Google Scholar]
- 32.Fouladi M, et al. Phase I study of everolimus in pediatric patients with refractory solid tumors. J Clin Oncol. 2007;25(30):4806–12. doi: 10.1200/JCO.2007.11.4017. [DOI] [PubMed] [Google Scholar]
- 33.Horton TM, et al. Phase I pharmacokinetic and pharmacodynamic study of temozolomide in pediatric patients with refractory or recurrent leukemia: a Children’s Oncology Group Study. Journal of Clinical Oncology. 2007;25(31):4922–8. doi: 10.1200/JCO.2007.12.0667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim MS, et al. Inhibition of histone deacetylase increases cytotoxicity to anticancer drugs targeting DNA. Cancer Research. 2003;63(21):7291–300. [PubMed] [Google Scholar]
- 35.Wen PY, PJ, Kuhn J, et al. Phase I study of vorinostat (suberoylanilide hydroxamic acid) in combination with temozolomide in patients with malignant gliomas. J Clin Oncol. 2007;25:84s. (abstract 2039) [Google Scholar]
- 36.Fouladi M, et al. Pediatric phase I trial and pharmacokinetic study of vorinostat: a Children’s Oncology Group phase I consortium report. J Clin Oncol. 2010;28(22):3623–9. doi: 10.1200/JCO.2009.25.9119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kelly WK, et al. Phase I study of an oral histone deacetylase inhibitor, suberoylanilide hydroxamic acid, in patients with advanced cancer. Journal of Clinical Oncology. 2005;23(17):3923–31. doi: 10.1200/JCO.2005.14.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Middlemas DS, et al. Biochemical correlates of temozolomide sensitivity in pediatric solid tumor xenograft models. Clinical Cancer Research. 2000;6(3):998–1007. [PubMed] [Google Scholar]


