Summary
Germ cell transport across the seminiferous epithelium during the epithelial cycle is crucial to spermatogenesis, although molecular mechanism(s) that regulate these events remain unknown. Studies have shown that spatiotemporal expression of crucial regulatory proteins during the epithelial cycle represents an efficient and physiologically important mechanism to regulate spermatogenesis without involving de novo synthesis of proteins and/or expression of genes. Herein, we critically review the role of focal adhesion kinase (FAK) in coordinating the transport of spermatids and preleptotene spermatocytes across the epithelium and the blood-testis barrier (BTB), respectively, along the apical ectoplasmic specialization (ES) – blood-testis barrier – basement membrane (BM) functional axis during spermatogenesis. In the testis, p-FAK-Tyr397 and p-FAKTyr407 are spatiotemporally expressed during the epithelial cycle at the actin-rich anchoring junction known as ES, regulating cell adhesion at the Sertolispermatid (apical ES) and Sertoli cell-cell (basal ES) interface. Phosphorylated forms of FAK exert their effects by regulating the homeostasis of F-actin at the ES, mediated via their effects on actin polymerization so that microfilaments are efficiently re-organized, such as from their “bundled” to “de-bundled/branched” configuration and vice versa during the epithelial cycle to facilitate the transport of: (i) spermatids across the epithelium, and (ii) preleptotene spermatocytes across the BTB. In summary, p-FAK-Tyr407 and p-FAK-Tyr397 are important regulators of spermatogenesis which serve as molecular switches that turn “on” and “off” adhesion function at the apical ES and the basal ES/BTB, mediated via their spatiotemporal expression during the epithelial cycle. A hypothetical model depicting the role of these two molecular switches is also proposed.
Keywords: Focal adhesion kinase, Spermatogenesis, Testis, Ectoplasmic specialization, Blood-testis barrier, Seminiferous epithelial cycle
Introduction
Focal adhesion kinase (FAK, formerly called p125FAK), a 125 kDa cytosolic protein, is a substrate of c-Src (transforming protein of Rous sarcoma virus) in mammalian cells. FAK was first discovered in chicken embryo fibroblasts transformed by Rous sarcoma virus in 1992 (Schaller et al., 1992). It was also independently found in the mouse in the same year (Hanks et al., 1992). Since then, FAK has been detected in humans, designated protein tyrosine kinase 2 (PTK2), encoded by the PTK2 gene in humans (André and Becker-Andre, 1993) and is located on the long (q) arm of chromosome 8 at position 24.3 (i.e., cytogenetic location, 8q24.3) (Fiedorek and Kay, 1995). FAK is known to be involved in a wide array of cellular events, most notably cell adhesion and cell migration at the cell-extracellular matrix (ECM) interface known as focal adhesion complex (FAC, also called focal contact; note: there is no ultrastructure analogous to FAC in the mammalian testis), regulating F-actin dynamics, and it transduces signals downstream of integrin-based receptors at the FAC (Parsons, 2003; Boutros et al., 2008; Cabodi et al., 2010; Hall et al., 2011; Cheng and Mruk, 2012; Malinin et al., 2012; Wehrle-Haller, 2012; Pentassuglia and Sawyer, 2013). FAK is found virtually in all mammalian cells and/or tissues (Broussard et al., 2008; Cheng and Mruk, 2012). FAK is also the therapeutic target of a number of diseases, including fibrotic diseases (Lagares and Kapoor, 2013) and tumorigenesis (Ucar and Hochwald, 2010; Lechertier and Hodivala-Dilke, 2012; Claesson-Welsh and Welsh, 2013) since it is overexpressed in many types of cancer, which is associated with tumor cell proliferation, migration and invasion, as well as metastasis. Recent studies have shown that FAK is also involved in cell apoptosis during tumorigenesis (Wang et al., 2014) and T cell signaling function (Chapman et al., 2013). FAK is a putative substrate of c-Src, and unsurpringly, the FAK-Src dual kinase complex is a leading therapeutic target for cancer therapy (Mitra and Schlaepfer, 2006; Bolos et al., 2010; Ammoun et al., 2014). FAK also exerts its biological effects in the cell nucleus, since it can be transported to the nucleus from cell cytosol mediated by its nuclear localization signal (NLS) and nuclear export signal (NES) sequences located in its FERM (FAK N-terminal band 4.1, ezrin, radixin, moesin-homology) and also catalytic domain (Fig. 1) (Lim et al., 2008; Ossovskaya et al., 2008). This, FAK is involved in nuclear signaling and gene transcriptional regulation, such as during inflammation (Lim et al., 2008, 2012). Collectively, these findings illustrate the diverse physiological significance of FAK in cellular function in health and in disease in mammals.
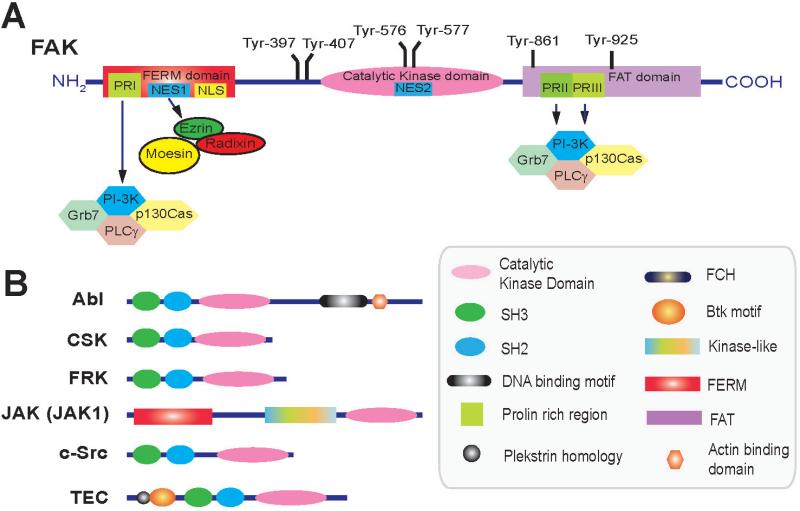
Fig. 1.
A schematic drawing that illustrates different functional domains of focal adhesion kinase (FAK) and its related non-receptor protein kinases in mammalian cells. FAK is the downstream signaling molecule that transduces signals mediated by an integrin-based receptor following its activation by various ligands (e.g., fragments of collagens and/or laminins), which usually takes place via an interaction with an integrin receptor near its N-terminus. FAK is functionally composed of three domains from its N-terminus: the FERM (band 4.1, ezrin, radixin, noesin homology) domain, the catalytic kinase domain and the FAT (focal adhesion targeting) domain near its C-terminus. There are also three proline-rich (PR) domain, PRI inside the FERM domain, and PRII and PRII within the FAT domain. It also has six putative phosphorylation sites at Tyr-397, -407, -576, -577, -861 and -925. FAK also contains the nuclear localization signal (NLS) and nuclear export signal (NES) that allows its translocation to the nucleus. FAK, unlike other non-receptor protein kinases, such as Abl, CSK, FRK, c-Src, and TEC, possess no SH2 or SH3 domain for protein-protein interactions, instead it utilizes its three PR regions to recruit a large number of signaling proteins (e.g., c-Src, PI-3K), and adaptors (e.g., vincluin, talin, p130Cas) to form a giant regulatory protein complex. Abbreviations used: AbI, Abelson leukemia virus tyrosine kinase; Btk motif, Btk-type zinc finger motif; CSK, cellular Src or C-terminal Src kinase; c-Src; a non-receptor protein tyrosine kinase in cells encoded by the Rous sarcoma virus (SRC) gene; FCH, Fes/CIP4 homology domain; FRK, Fyn-related kinase; Grb7, growth factor receptor-bound protein 7; JAK, Janus kinase also known as JAK1; PI-3K, phosphoinositide 3-kinase; p130Cas, Crk-associated substrate; PLCγ, phospholipase C γ; SH2, Src homology 2; SH3, Src homology 3; TEC, tyrosine protein kinase Tec.
The several functional domains along the primary sequence of FAK and other similar non-receptor protein kinases that share some common features of FAK are summarized in Fig. 1. FAK is composed of a FERM domain near its N-terminus, followed by the catalytic kinase domain, and a FAT (focal adhesion targeting) domain near its C-terminus (Zachary and Rozengurt, 1992; Hall et al., 2011). Except the central kinase domain that contains all the conserved motifs analogous to the catalytic domains of other protein tyrosine kinases, the two other domains, namely the FERM and FAT domains, do not display significant homology with other protein tyrosine kinases known to date. For instance, FAK does not possess motifs necessary for plasma membrane association (e.g., acylation sites), nor SH2 (Src homology 2) or SH3 domains, similar to other protein tyrosine kinases at their FERM and FAT domains (Fig. 1). Thus, there is no SH2 or SH3 domain in FAK to elicit protein-protein interaction as in other tyrosine protein kinases. To compensate for the lack of SH domains, FAK recruits interacting proteins, such as PI-3K (phosphoinositide 3-kinase), Grb7 (growth factor receptor-bound protein 7), PLCγ (phospholipase C-γ), p130Cas (Crk-associated substrate), and others via its proline-rich region 1 (Kemphues et al., 1988) located at its FERM domain, as well as its PRII and PRIII at its FAT domain (Fig. 1). Thus, FAK, besides acting as a protein tyrosine kinase following its activation by c-Src in its catalytic domain, also serves as a signaling platform by recruiting a large number of proteins, including adaptors and protein kinases to form a giant protein complex to regulate multiple cellular events.
Actin-based cytoskeleton and regulatory proteins in the testis
Background
In the testis, the seminiferous tubule is the functional unit that produces spermatozoa. Each man produces ~400 million sperms per day from puberty at ~12-13 years of age through his entire adulthood versus ~70 million in rats by 45 days of age postpartum (testis weight is ~32 gm vs. 3.6 gm per pair testes in adult men and rats, respectively) (Robb et al., 1978; Amann and Howards, 1980; Johnson et al., 1980) via spermato-genesis, which takes place exclusively in the seminiferous epithelium of the tubule, comprised of only Sertoli and germ cells (Fig. 2). This enormous output of sperm production thus illustrates extensive restructuring at the Sertoli-Sertoli and Sertoli-germ cell interface, requiring rapid “adhesion” and “de-adhesion” to accommodate the rapid changes in germ cell morphology during spermatogenesis, in particular spermiogenesis, as well as germ cell transport across the seminiferous epithelium from the basal to the adluminal (apical) compartment, traversing the blood-testis barrier (BTB) (Fig. 2) during the epithelial cycle of spermatogenesis. Furthermore, unlike other motile cells (e.g., fibroblasts, macrophages, lymphocytes, and metastatic tumor cells) that require the coordination of integrins, FAK, Rac GTPase, adaptor proteins (e.g., talin, vinculin, paxillin) (Lechertier and Hodivala-Dilke, 2012; Wehrle-Haller, 2012), spermatocytes and spermatids are not motile cells per se. Instead, preleptotene spermatocytes are being transported by Sertoli cells across the BTB from the basal to the adluminal compartment at stage VIII of the epithelial cycle to prepare for meiosis I/II. Furthermore, spermatids derived from meiosis are also being transported back and forth across the adluminal compartment during the epithelial cycle while differentiating from step I-19 spermatids, until all step 19 spermatids line-up properly at the edge of the seminiferous tubule lumen at stage VIII of the epithelial cycle, transformed to spermatozoa shortly before the release of sperms at spermiation (Mruk and Cheng, 2004; Mruk et al., 2008; O'Donnell et al., 2011) (Fig. 2). As such, it is conceivable that there are extensive changes in the F-actin organization during the epithelial cycle. However, the molecular mechanism(s) that regulate cytoskeletal dynamics remains unknown. Recent studies, however, have demonstrated that F-actin organization in the mammalian testis is regulated by two classes of actin regulatory proteins, namely actin-bundling and branched actin-polymerization proteins, which work in concert to confer plasticity of the actin-based cytoskeleton to accommodate changes in cell shape and to elicit germ cell transport during spermatogenesis. Some limited studies have also demonstrated the role of FAK in these events, and these findings will be evaluated herein. The role of tubulin-based cytoskeleton and the involvement of FAK in microtubule dynamics in the testis during the epithelial cycle is largely unknown (Tang et al., 2013). As such, the topic on tubulin-based cytoskeletal dynamics is not discussed herein.
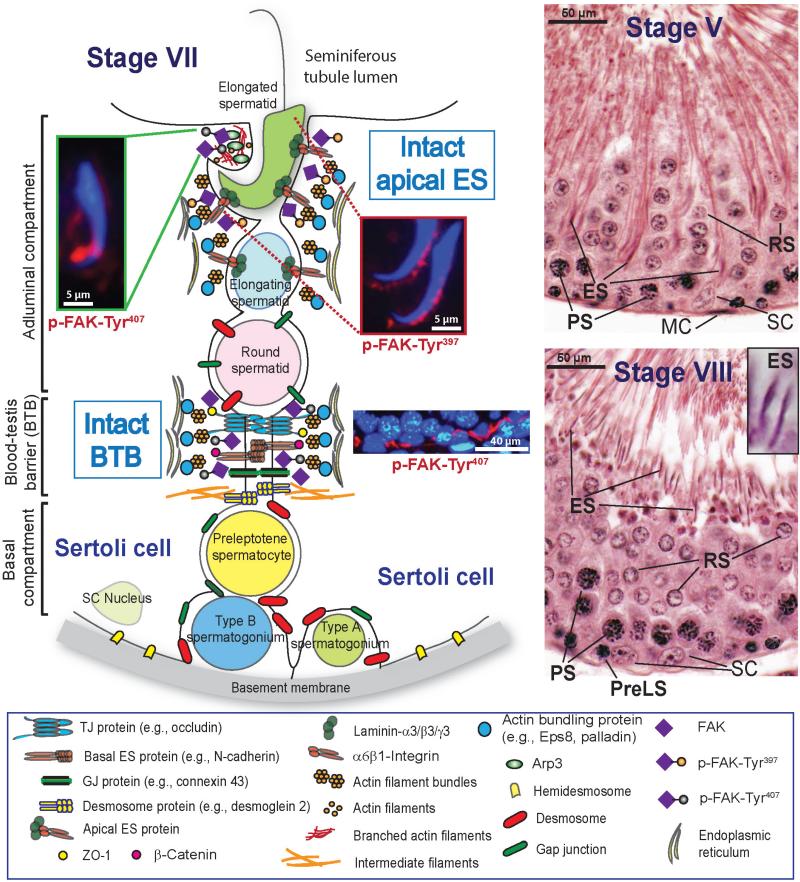
Fig. 2.
A schematic drawing illustrating the anatomical features of the seminiferous epithelium in adult rat testes. The BTB physically divides the seminiferous epithelium into the basal and the adluminal (apical) compartment in the mammalian testis. This drawing highlights the actin-rich ultrastructure known as ectoplasmic specialization (ES) in the seminiferous epithelium. ES is present at the Sertoli cell-cell interface at the BTB called basal ES, it is also found at the Sertoli-spermatid interface and designated apical ES. ES is typified by the presence of an array of actin filament bundles that line perpendicular to the Sertoli cell plasma membrane. These actin filament bundles are sandwiched in-between cisternae of endoplasmic reticulum and the Sertoli cell plasma membrane, and these bundles of actin filaments thus confer unusual adhesive strength to the basal and the apical ES. These morphological features are virtually identical between the apical and the basal ES. Since the array of actin filament bundles is only found in Sertoli cells, so that a single array of actin filament bundles is present at the apical ES versus two arrays of microfilament bundles at the basal ES, and the constituent proteins of the apical and the basal ES are also somewhat different (Cheng and Mruk, 2002, 2010). During spermiogenesis, apical ES appears in step 8 spermatids, once it forms, the apical ES replaces desmosome and gap junction, and remains to be the only anchoring device until step 19 spermatids prior to spermiation that occurs at stage VIII of the epithelial cycle (see micrograph on the lower right panel). Due to the unique cellular associations between different germ cells, such as developing spermatids, and the Sertoli cell in the seminiferous epithelium, the epithelium can be divided into 14, 12 and 6 stages in the rat, mouse and man, respectively, and known as the epithelial cycle of spermatogenesis. Herein, two stages of the seminiferous tubule at V and VIII of the epithelial cycle are shown. At stage V, elongating spermatids (ES) (step 17) are transported back to the basal compartment with the spermatid head almost touches the Sertoli cell (SC) nucleus. In stage VIII, preleptotene spermatocytes (PreLS) transformed from type B spermatogonia are transported across the BTB which is made possible via BTB restructuring, step 19 ES also line-up near the lumen of the seminiferous tubule so that spermiation, the release of sperm, can take place in late stage VIII of the cycle. Thus, it is conceivable that the basal and the apical ES undergo extensive restructuring in stage VIII tubules. The schematic drawing illustrates features of the apical and basal ES at stage VII of the epithelial cycle when these junctions remain intact. p-FAK-Tyr397 and -Tyr407 are highly expressed at the apical ES, but restricted to different sites with p-FAK-Tyr397 (red fluorescence) restricted to the convex (dorsal) side of the spermatid head, while p-FAK-Tyr407 (red fluorescence) is most prominent on the concave (ventral) side of the spermatid head. At the BTB, p-FAK-Tyr407 (red fluorescence), but not p-FAK-Tyr397, is highly expressed. It is likely that these two phosphorylated forms of FAK regulate the dynamic organization of actin microfilament bundles at the ES via its effects on actin filament bundling proteins Eps8 and palladin versus branched actin polymerization-inducing proteins Arp2/3 complex/N-WASP and filamin A, depending on the stage of the epithelial cycle.
Actin-bundling proteins
Actin filaments found in the testis-specific adherens junction ES are tightly bundled and lie perpendicular to the Sertoli cell plasma membrane, sandwiched in-between cisternae of endoplasmic reticulum and the plasma membrane (Fig. 2). These actin-based filament bundles are maintained via the combined action of Eps8 (epidermal growth factor receptor pathway substrate 8, an actin filament barbed end capping and bundling protein) (Di Fiore and Scita, 2002; Lie et al., 2009), palladin (an actin cross-linking and bundling protein) (Qian et al., 2013a,b) and espin (Bartles et al., 1996; Chen et al., 1999). These proteins display highly stage-specific and restrictive spatiotemporal expression at the apical and/or basal ES during the epithelial cycle of spermatogenesis. Studies have shown that these proteins are working in concert with actin branching and polymerization proteins to confer plasticity of the actin filament bundles at the ES during spermatogenesis, such as at spermiogenesis when developing spermatids are transported across the seminiferous epithelium allowing the rapid reorganization of actin filaments from their “bundled” to “unbundled” configuration and vice versa. However, the precise molecular mechanism(s) that regulates these events remained elusive until recently, which is likely mediated by the spatiotemporal expression of FAK, in particular its activated/ phosphorylated forms, such as p-FAK-Tyr397 and -Tyr407, during the epithelial cycle as recently shown (Lie et al., 2012), which will be critically reviewed herein.
Branched actin polymerization-inducing proteins
Actin filament bundles at the ES can become unbundled and branched via the action of barbed end actin polymerization mediated by the Arp2/3 (actin-related protein 2/3) complex. In order for the Arp2/3 complex to induce actin nucleation, it is required to be initially activated by N-WASP (neuronal Wiskott-Aldrich syndrome protein). Following activation, this protein complex binds to an existing filament to induce branched actin polymerization, effectively forming a branched F-actin network, thereby converting actin filament bundles into a branched network. This thus destabilizes ES, facilitating both endocytic vesicle-mediated protein trafficking and de-adhesion of spermatids onto the Sertoli cell in the seminiferous epithelium, a crucial step for the release of sperms at spermiation and the recycling of apical ES proteins (e.g., ß1-integrin, nectin-3, JAM-C, ICAM-2) to assemble newly formed apical ES at the interface of step 8 spermatids and Sertoli cells, all of which take place at stage VIII of the epithelial cycle. Similar events also take place at the basal ES of the BTB, so that BTB integral membrane proteins (e.g., occludin, claudin-11, JAM-A) at the “old” BTB site can be endocytosed and transcytosed from the apical region of the transiting preleptotene spermatocytes to the basal region, with these proteins effectively recycled to assemble “new” BTB, thereby maintaining the integrity of the immunological barrier at stage VIII of the epithelial cycle. Recent studies have shown that Arp3 indeed is expressed stage-specifically and spatiotemporally (Lie et al., 2010), which may also work in concert with filamin A, which is an actin cross-linking protein that facilitates the formation of branched actin network by favoring perpendicular branching of F-actin (Su et al., 2012a,b).
FAK and the testis
As briefly summarized above, FAK is likely to be involved in the events of Sertoli-Sertoli and Sertoli-germ cell adhesion, as well as germ cell transport during spermatogenesis because of active restructuring at the cell junctions. In this context, it is of interest to note that there is neither functional FAC (or focal contact), nor ultrastructures analogous to FAC in the seminiferous epithelium of the mammalian testis, such as at the Sertoli-basement membrane (BM is a modified ECM in the testis) (Dym, 1994; Mruk and Cheng, 2004; Siu and Cheng, 2004b; Cheng and Mruk, 2010) interface. Thus, when FAK was first detected in the rat testis in 2003, we were surprised to observe that FAK was not localized to the Sertoli-BM interface in the seminiferous epithelium; instead, it was expressed mostly at the basal compartment, at or near the BTB at the Sertoli cell-cell interface (Siu et al., 2003, 2009a). However, its two phosphorylated (activated) forms, p-FAK-Tyr397 and -Tyr576 are restricted to the Sertoli-elongating/elongated spermatid interface in an ultrastructure known as apical ectoplasmic specialization (apical ES, a testis-specific adherens junction (AJ), apical ES first appears at the interface of step 8 spermatids and the Sertoli cell, and it is the only anchoring device that persist from step 8 through 19 spermatids. Apical ES also confers spermatid polarity besides adhesion, replacing desmosome and gap junction once it appears). It is noted that these two phosphorylated forms of FAK are absent from the BTB, displaying a spatiotemporal pattern of expression during the epithelial cycle, and it is tightly associated with ß1- integrin at the apical ES (Siu et al., 2003). Subsequent studies have confirmed these earlier findings that p-FAK-Tyr397 is indeed an integrated component of the ß1-integrin-based adhesion complex at the apical ES and it is a crucial molecule involved in spermiation (Beardsley et al., 2006; O'Donnell et al., 2011). On the other hand, p-FAK-Tyr407 is highly expressed at the apical ES and most prominently at stage VII to early VIII, but it was considerably down-regulated and subsided to a level almost non-detectable by late VIII, when the release of sperm occurs at spermiation (Lie et al., 2012). Unlike p-FAK-Tyr397 and -Tyr576, p-FAK-Tyr407 is also highly expressed at the Sertoli cell-cell interface at the BTB in virtually all stages of the epithelial cycle including late stage VIII (Lie et al., 2012). Additionally, FAK forms an integrated protein complex with occludin and ZO-1 (Siu et al., 2009c), and it regulates the phosphorylation status of occludin, thereby conferring the adhesive function of the occludin-ZO-1 complex at the BTB (Siu et al., 2009b). Also, a knockdown of FAK in Sertoli cells by RNAi perturbs the Sertoli cell TJ-permeability barrier (Siu et al., 2009b), suggesting FAK is a crucial regulator of BTB dynamics during spermatogenesis. Collectively, these findings illustrate that FAK may be involved in junction restructuring events pertinent to germ cell transport at these sites. Since these earlier studies, additional findings are available in the literature regarding the functional significance of FAK, in particular its several phosphorylated forms, in spermatogenesis. Herein, we provide a brief update on these findings. Furthermore, we provide a critical discussion on the role of FAK in spermatogenesis, highlighting its function in coordinating cellular events that occur across the seminiferous epithelium during the epithelial cycle. Based on these recent findings, we also provide a hypothetical model regarding the involvement of FAK and its phosphorylated forms in discrete cellular events in the seminiferous epithelium during spermatogenesis (Fig. 3).
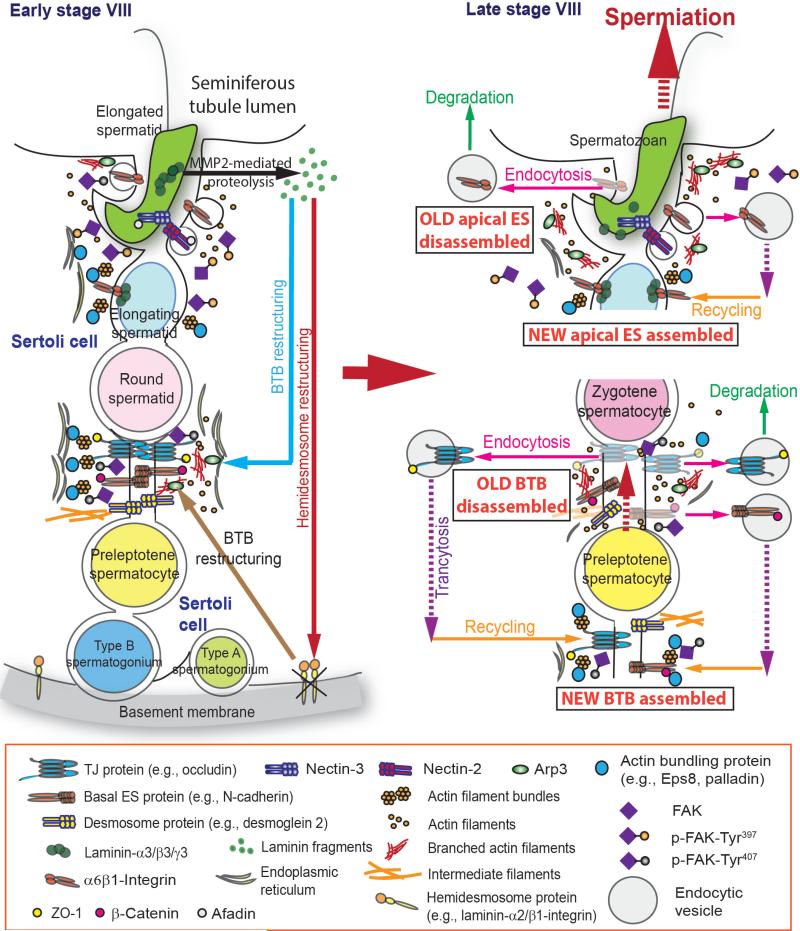
Fig. 3.
A hypothetical model illustrating the physiological role of p-FAK-Tyr397 and p-FAK-Tyr407 in coordinating the apical and the basal ES disruption to facilitate spermiation and BTB restructuring at stage VIII of the epithelial cycle. At stage VIII of the epithelial cycle, spermiation and BTB restructuring that facilitate the release of sperm and the transport of preleptotene spermatocytes across the BTB take place simultaneously, but at opposite ends of the seminiferous epithelium. As detailed in the text, p-FAK-Tyr397 and -Tyr407 play crucial roles in coordinating these cellular events. Shown on the left panel, biologically active fragments of laminin chains released via the action of MMP-2 during the degeneration of apical ES induce BTB restructuring possibly via the action of p-FAK-Tyr407, which likely induces an activation and the recruitment of Arp2/3 protein complex to induce branched actin polymerization, effectively converting actin filaments from a “bundled” to a “branched/de-bundled” configuration, this thus enhances endocytic vesicle-mediated protein trafficking including transcytosis and recycling so that a “new” BTB is assembled at the basal region of the preleptotene spermatocyte being transported across the BTB prior to the disassembly of the “old” BTB above the spermatocyte (see bottom right panel). On the other hand, p-FAK-Tyr397 at the apical ES also recruits the Arp2/3 complex but re-localizes actin bundling proteins Eps8 and palladin away from the apical ES, the net results thus favors the “branched/de-bundled” actin filaments at the apical ES, facilitating endocytic vesicle-mediated protein degradation and/or transcytosis and recycling to assemble “new” apical ES for the newly formed step 8 spermatids at stage VIII of the epithelial cycle. This hypothesis is supported by recent findings in the field as discussed in the text.
FAK and its phosphorylated/activated forms p-FAK-Tyr397, -Tyr407, and -Tyr576in the rat testis
As noted in Fig. 1, there are six putative phosphorylation sites in FAK at Tyr-397, -407, -576, -577, -861 and -925. To date, only p-FAK-Tyr397, -Tyr407, and -Tyr576 have been studied in the rat testis, which are equivalent to p-FAK-Tyr428, -Tyr438, and -Tyr614, respectively, in the mouse testis. All three phosphorylated forms of FAK display spatiotemporal and stage-specific expression in the seminiferous epithelium during the epithelial cycle in the rat testis. For instance, p-FAK-Tyr397 and -Tyr576 are highly expressed at the apical ES, virtually undetectable at the basal ES, but limited mostly to stage VII through early VIII tubules with their expression considerably down-regulated by late stage VIII when spermiation occurs (Siu et al., 2003; Lie et al., 2012). These findings imply that these FAK forms may be necessary to maintain the integrity of the apical ES to confer spermatid adhesion in the seminiferous epithelium during spermiogenesis until the release of sperm at spermiation. On the other hand, p-FAK-Tyr407 is expressed at both the apical and basal ES (Lie et al., 2012). However, the expression of p-FAK-Tyr407 is considerably diminished to an almost non-detectable level at the apical ES at late stage VIII, coinciding with the release of sperm at spermiation (Lie et al., 2012). Yet its expression remains relatively high at the basal ES (Lie et al., 2012), illustrating that p-FAKTyr407 may be involved in regulating Sertoli cell adhesion function at the BTB besides its effects at the apical ES. In this context, it is of interest to note that the apical ES is an F-actin-rich anchoring junction, uniquely found in the testis, characterized by the presence of actin filament bundles aligned perpendicular to the Sertoli cell plasma membrane and sandwiched in-between the cisternae of endoplasmic reticulum and the apposing spermatid-Sertoli cell plasma membrane (Fig. 2) (Russell, 1977; Cheng and Mruk, 2002; Vogl et al., 2008; Cheng and Mruk, 2010). Even though p-FAKTyr397 and –Tyr407 are both expressed at the apical ES, they display differential spatial expression at the site in which p-FAK-Tyr397 is limited to the convex (or dorsal) side of the spermatid head, whereas p-FAK-Tyr407 is predominantly found at the concave (or ventral) side as illustrated in Fig. 2, supporting the notion that these two activated forms of FAK play different roles around the spermatid head which anchor onto the Sertoli cell and to maintain spermatid polarity during spermiogenesis. For instance, it is known that apical ES at the concave side of the spermatid head is the site where endocytic vesicle-mediated protein trafficking initially takes place before it spreads to the convex side to create an ultrastructure known as apical tubulobulbar complex (Vogl et al., 2013), so that “old” apical ES proteins can be transcytosed and recycled to assemble “new” apical ES in newly formed step 8 spermatids (Fig. 3). For the basal ES, the ultrastructural feature is the same as the apical ES except that the network of actin filament bundles is found on both sides of the adjacent Sertoli cells (Pelletier, 2011; Cheng and Mruk, 2012; Franca et al., 2012). Another major difference between the apical and basal ES is that once the former appears at the interface of the Sertoli cell and the step 8 spermatid, it persists until step 19 spermatid, replacing both gap junction and desmosome, making it the only anchoring device that supports spermatid adhesion, confers its polarity and facilitates spermatid transport across the seminiferous epithelium during the epithelial cycle. However, basal ES never exists alone, it either coexists with the actin-based tight junction or gap junction (Vogl et al., 2008; Pelletier, 2011; Cheng and Mruk, 2012; Franca et al., 2012), and these junctions together with the intermediate filament-based desmosome effectively create the BTB.
FAK and spermatid adhesion in the testis
Background
The ES is an actin-based testis-specific adherens junction (AJ) that is found at the Sertoli-spermatid or the Sertoli cell-cell interface known as the apical and the basal ES, respectively. Once the apical ES appears at the interface of step 8 spermatids and the Sertoli cell, it is the only anchoring device that confers spermatid polarity and adhesion, replacing desmosome and gap junction, which persist from step 8 through 19 spermatids. There is no conventional AJ found in the seminiferous epithelium. However, basal ES coexists with TJ and GJ, which together with desmosome constitute the BTB. Apical and basal ES share virtually identical ultrastructural features in which tightly packed actin filament bundles that lie perpendicular to the Sertoli cell plasma membrane are sandwiched in-between cisternae of endoplasmic reticulum and the apposing plasma membranes of Sertoli-spermatid or Sertoli-Sertoli cells, except that there is no visible or comparable ultrastructures of actin filaments found in spermatids in apical ES. Due to the presence of these unique actin filament bundles at the ES that anchor adhesion protein complexes at the site to confer spermatid adhesion to the Sertoli cell, the ES has been shown to be a stronger adhesive junction when compared to desmosome (Wolski et al., 2005).
FAK regulates spermatid adhesion via its effects on apical ES
The notion that p-FAK-Tyr397 may be crucial to confer spermatid adhesion is supported by findings that it is highly expressed at stage VI through early VIII tubules until late stage VIII, when spermiation takes place (Siu et al., 2003; Beardsley et al., 2006), suggesting it may be used to maintain apical ES integrity (O'Donnell et al., 2011). This possibility is supported by recent findings in which overexpression of p-FAKTyr397 via the use of a phosphomimetic mutant of FAK Y397E in rat testes in vivo leads to a delay in spermiation, in which step 19 spermatids are trapped within the seminiferous epithelium, sometimes near the basal compartment (Wan et al., 2013). In short, these observations suggest that step 19 spermatids fail to be transported to the luminal edge of the tubule lumen to undergo spermiation properly due to defects of the apical ES, which in turn perturbs proper spermatid transport across the epithelium. The stage IX tubules in these mice appear to be a hybrid stage VIII and IX tubule type since they were shown to contain step 9 spermatids transformed from step 8, but also step 19 spermatids which should have been eliminated at stage VIII via spermiation (Wan et al., 2013). This defect in spermatogenesis following the overexpression of constitutively active p-FAK-Tyr397 in rat testes apparently is mediated by a delay in the timely restructuring of F-actin at the apical ES in stage VIII. For instance, the actin filament bundles at the apical ES should have been converted to a “unbundled/branched” configuration to facilitate the release of sperm at spermiation, instead, actin filaments continued to assume their bundled configuration in late stage VIII, analogous to stage VII tubules (Wan et al., 2013). More importantly, this persistence of F-actin at the apical ES in late stage VIII tubules is shown to be assisted by the up-regulation of an actin barbed end capping and bundling protein Eps8 (epidermal growth factor receptor pathway substrate 8) (Lie et al., 2009) and also an actin cross-linking and bundling protein palladin (Qian et al., 2013a), so that actin filament bundles are maintained following overexpression of p-FAK-Tyr397 in stage VIII tubules when they should have been re-organized to “branched/un-bundled” configuration (Wan et al., 2013). This, in turn, leads to the persistent presence of nectin-3, a spermatid-specific integral membrane adhesion molecule (Ozaki-Kuroda et al., 2002) at the apical ES to anchor spermatids onto the epithelium long after spermiation has taken place (Wan et al., 2013). Collectively, these findings prompt us to propose a possible role for p-FAK-Tyr397 in which it acts as an “on” and “off” switch in conferring the rapid reorganization of actin filament bundles at the apical ES during spermiogenesis by coordinating the spatiotemporal expression of actin filament bundling proteins Eps8 and palladin (Fig. 3). This, in turn, affects the timely restructuring of the actin filament bundles at the apical ES, and thereby perturbing the transport of spermatids in the adluminal compartment of the epithelium. Much work is needed to understand the underlying mechanism(s) by which the spatiotemporal expression of p-FAK-Tyr397 is regulated at the apical ES. Is this mediated by changes in the androgen or cytokine (e.g., TNFα, TGF-ß2, TGF-ß3, and IL-1α) gradient in the microenvironment, since these molecules are known to modulate apical ES function (Lui and Cheng, 2007; Li et al., 2009; Wang et al., 2009; Cheng and Mruk, 2010). Studies have shown that the biologically active laminin fragments released at the apical ES, likely mediated by MMP-2 at spermiation (Siu and Cheng, 2004a; Yan et al., 2008), regulate the spatiotemporal expression of p-FAK-Tyr407, causing down-regulation of p-FAK-Tyr407 at the apical ES as well as its mis-localization (Su et al., 2012c). These recent findings thus illustrate the presence of a local regulatory feedback loop in which the initial break-down of the apical ES leads to the release of biologically active laminin fragments that potentiate the further degeneration of apical ES adhesion to facilitate spermiation. Another question that remains to be addressed is the signaling cascade by which these laminin fragments activate spermiation. Does this involve integrin-based receptors? What is the identity of the integrin receptor?
FAK and BTB dynamics in the testis
Background
The BTB is an anatomical ultrastructure in the seminiferous tubule, which physically divides the seminiferous epithelium into the basal and the adluminal (apical) compartment (Fig. 2) (Pelletier, 2011; Cheng and Mruk, 2012; Franca et al., 2012). Spermatogonial stem cells (SSC) at the stem cell niche, and all spermatogonia (both A and B types) reside outside the BTB, adjacent to the basement membrane of the tunica propria, and preleptotene spermatocytes differentiated from type B spermatogonia are the only germ cells that are transported across the BTB at stage VII-VIII of the epithelial cycle, whereas other spermatocytes and all post-meiotic spermatids reside behind the BTB in the adluminal compartment (Fig. 2). BTB is constituted by actin-based tight junctions (TJ) and gap junctions (GJ) as well as the intermediate filament-based desmosomes between adjacent Sertoli cells. The TJ and GJ at the BTB are further reinforced by an array of tightly packed actin filament bundles that lie perpendicular to the Sertoli cell plasma membrane, and are sandwiched in-between cisternae of endoplasmic reticulum and the plasma membrane known as basal ES (Vogl et al., 2008, 2013). In the rat testis, BTB begins its assembly by 15-day postpartum (dpp), complete by 20-dpp, and becomes fully functional by 25-dpp, coinciding with the onset of meiosis and the appearance of haploid spermatids (Mok et al., 2011). Permanent disruption of BTB, such as via an acute dose of cadmium (Setchell and Waites, 1970; Hew et al., 1993; Siu et al., 2009a), glycerol (Wiebe and Barr, 1984; Wiebe et al., 2000) or adjudin (Mok et al., 2012), leads to irreversible infertility in rats, illustrating the significance of the BTB in spermatogenesis. The BTB was thought to contribute to the maintenance of the immune privilege status of the testis by sequestering post-meiotic spermatid development behind the BTB in a specialized microenvironment so that spermatid-specific antigens, many of which expressed transiently and known as cancer-testis (CT) antigens which are oncogenes (Cheng et al., 2011), can be “sealed off” from the host immune system to avoid the production of anti-sperm antibodies. However, recent studies seem to challenge this concept since spermatogonia and preleptotene spermatocytes also express many CT antigens (Cheng et al., 2011). It is likely that Sertoli cells actively secrete immunosuppressive biomolecules that are more crucial than the immunological barrier granted by the BTB to confer the testis its immune-privilege status (Selawry and Cameron, 1993; Shamekh et al., 2006; Dufour et al., 2008). The BTB may also be used to protect SSC and spermatogonia from potentially harmful biomolecules that are released from post-meiotic germ cells during spermiogenesis, as well as maintaining the homeostasis of the stem cell niche.
FAK regulates BTB function
FAK is expressed in the seminiferous epithelium of adult rat testes, but it is localized mostly to the basal compartment, near the basement membrane of the tunica propria (Siu et al., 2003). Thus, unlike p-FAK-Tyr397 and -Tyr576 which are expressed exclusively at the apical ES and structurally interact with the ß1-integrin-based adhesion protein complex, such as its peripheral adaptors (e.g., vinculin, paxillin) (Siu et al., 2003, 2011; Beardsley et al., 2006), FAK is predominantly expressed at the BTB (Siu et al., 2003, 2009b). Subsequent studies have shown that FAK structurally interacts with the occludin-ZO-1 adhesion protein complex in the rat testis (Siu et al., 2009c). This finding is also consistent with an earlier study reporting that FAK structurally interacts with occludin in human embryonic kidney, 293 cells (Stewart et al., 2002). Studies have shown that whole body (Ilic et al., 1995) or endothelial cell-specific (Shen et al., 2005; Braren et al., 2006) knockout (KO) of FAK leads to embryonic lethality with vascular defects. Interestingly, using a tamoxifen-inducible, Cre-mediated FAK deletion model to inactivate FAK from endothelium of blood vessels in adult mice, it was noted that the loss of FAK function is not lethal due to the functional compensation of Pyk2 (FAK-related protein proline-rich tyrosine kinase 2) in which endothelial cells in blood vessels in adult mice, it was noted that the loss of FAK function or cultured endothelial cells have the adaptive capacity of switching to Pyk2-dependent signaling to maintain the lost FAK function (Weis et al., 2008). Using a Sertoli cell cultured system in vitro that mimics the BTB in vivo, a knockdown of FAK by siRNA duplexes was found to induce a disruption of the Sertoli cell TJ-permeability barrier, which is likely mediated via changes in the phosphorylation status of occludin of the occludin-ZO-1 adhesion protein complex, altering the affinity of occludin and ZO-1 (Siu et al., 2009b). These findings thus illustrate that FAK is crucial to BTB function. Subsequent studies have shown that p-FAK-Tyr407, unlike p-FAK-Tyr397 and -Tyr576, is highly expressed at the BTB but also at the apical ES, displaying a stage-specific pattern of expression (Lie et al., 2012) (Fig. 2). More importantly, overexpression of p-FAK-Tyr407 via the use of a phosphomimetic mutant of FAK Y407E (but not FAK Y407F, a nonphorphorylatable mutant) in Sertoli cells tightens the Sertoli cell TJ-permeability barrier (Lie et al., 2012), illustrating that p-FAK-Tyr407 promotes BTB integrity. In short, p-FAK-Tyr397 and -Tyr407 promote anchoring junction integrity at the apical and basal ES/BTB, respectively. These findings also illustrate that these phosphorylated forms of FAK serve as molecular switches that can turn “on” and/or “off” adhesion function at the apical and basal ES, depending on their spatiotemporal expression during the epithelial cycle. Two recent studies have reported that the biologically active fragment, designated F5-peptide, released from laminin-γ3 chains at the apical ES during spermiation induces Sertoli cell TJ-permeability barrier function (Yan et al., 2008; Su et al., 2012), and the disruptive effect of this F5 fragment can be blocked via an overexpression of p-FAK-Tyr407 using the phosphomimetic Y407E mutant (Su et al., 2012a). These findings thus illustrate the presence of a tight regulatory loop in the seminiferous epithelium during the epithelial cycle regarding BTB restructuring, in which F5-peptide released at the apical ES during spermiation, possibly mediated by MMP-2 (Siu and Cheng, 2004a), at stage VIII of the cycle induces BTB “opening” to facilitate the transport of preleptotene spermatocytes across the BTB that also takes place at stage VIII (Fig. 3). However, this event of F5 peptide-induced BTB restructuring can be “turned off” by an increase in spatiotemporal expression p-FAK-Tyr407 in the microenvironment, such as at stage IX of the epithelial cycle. These findings thus illustrate that there is a local mechanism in the seminiferous epithelium in which the events that take place at the apical and the basal ES at stage VIII of the epithelial cycle, namely spermiation and BTB restructuring, are tightly coordinated via endogenously produced biologically active peptides. In fact, this concept is supported by the stage-specific and spatiotemporal expression of p-FAK-Tyr407 at the BTB during the epithelial cycle of spermatogenesis (Lie et al., 2012). Fig. 3 depicts a hypothetical model in which FAK, in particular p-FAK-Tyr397 and -Tyr407, regulates restructuring of the F-actin network at the apical and the basal ES/BTB to facilitate the transport of: (i) spermatids in the seminiferous epithelium to line-up at the luminal edge to prepare for spermiation, and (ii) preleptotene spermatocytes across the BTB, both take place at stage VIII of the epithelial cycle.
Concluding remarks and future perspectives
It is increasingly clear that the cellular events that take place in the seminiferous epithelium are tightly regulated. Earlier studies have shown that hormones produced either locally (e.g., estradiol-17ß or testosterone) in the testis or systemically (e.g., FSH, LH) via the hypothalamic-pituitary-testicular axis are major players in these events (Sharpe, 1994; O'Donnell et al., 2001, 2006; Winters and Moore, 2007; Carreau and Hess, 2010). Studies in the last decade have shown that cytokines produced in the microenvironment of the epithelium also play a role in regulating these events (Mruk and Cheng, 2004; O'Bryan and Hedger, 2008; Li et al., 2009; Cheng and Mruk, 2012). As discussed herein, biologically active peptides are also produced locally, such as at the apical ES during spermiation, and serve as autocrine and paracrine factors that regulate apical ES as well as basal ES/BTB restructuring, respectively, which, in turn, is regulated via the spatiotemporal expression of p-FAK-Tyr397 and -Tyr407. Future studies should include a careful examination of Pyk2 and the involvement of this FAK-related protein kinase in cellular events during the epithelial cycle in functional experiments. Since a recent report has shown that the spatiotemporal expression of FAK, in particular its phosphorylated form, is regulated by miRNAs (Wan et al., 2014), which are found mostly in germ cells, such as spermatocytes and spermatids, it is important to determine how these small regulatory RNAs are being “transported” from germ cells to Sertoli cells to confer the spatiotemporal expression of p-FAK-Tyr397 and -Tyr407, and perhaps other non-receptor protein tyrosine kinases, such as c-Yes, which also has been recently shown to play a role in the expression of p-FAK-Tyr407 in the rat testis (Xiao et al., 2013). Once this information is known, these findings should provide not just a better understanding of the biology and regulation of spermatogenesis, but also may unravel some new targets, which can serve as the candidates for contraceptive development and/or treatment of unexplained infertility, such as non-obstructive azoospermia in men.
Acknowledgements
We are grateful to former and current members of our laboratory who have provided helpful and critical discussions during the preparation and writing of this review article.
Authors’ roles. C.Y.C. conceived the idea of preparing this review article; N.E.G.O., H.T.W., E.W.P.W., P.P.Y.L. and C.Y.C. performed the literature search, research on the topic and critically evaluated findings in the field; N.E.G.O., D.D.M., H.T.W., E.W.P.W., C.C.O., P.P.Y.L. and C.Y.C. critically discussed and evaluated the latest research on different aspects of FAK and testis function; D.D.M. and C.Y.C. provided the first draft of the hypothetical model; N.E.G.O. and C.Y.C. prepared the figures; C.Y.C. wrote the paper, and C.Y.C. performed the final editing of the manuscript.
Funding. This work was supported by grants from the National Institutes of Health (NICHD, R01 HD056034 to C.Y.C., and U54 HD029990, Project 5 to C.Y.C.); Akdeniz University Research Foundation (2013.03.0122.011 to N.E.G.O.); and International Joint Doctorate Fellowship Program of The Scientific and Technological Research Council of Turkey (2214/B to N.E.G.O.).
Footnotes
Conflict of interest. The authors have nothing to declare.
References
- Amann RP, Howards SS. Daily spermatozoal production and epididymal spermatozoal reserves of the human male. J. Urol. 1980;124:211–215. doi: 10.1016/s0022-5347(17)55377-x. [DOI] [PubMed] [Google Scholar]
- Ammoun S, Provenzano L, Zhou L, Barczyk M, Evans K, Hilton DA, Hafizi S, Hanemann CO. Axl/gas6/nfÎb signalling in schwannoma pathological proliferation, adhesion and survival. Oncogene. 2014;33:336–346. doi: 10.1038/onc.2012.587. [DOI] [PubMed] [Google Scholar]
- André E, Becker-Andre M. Expression of an N-terminally truncated form of human focal adhesion kinase in brain. Biochem. Biophys. Res. Commun. 1993;190:140–147. doi: 10.1006/bbrc.1993.1022. [DOI] [PubMed] [Google Scholar]
- Bartles J, Wierda A, Zheng L. Identification and characterization of espin, an actin-binding protein localized to the f-actin-rich junctional plaques of sertoli cell ectoplasmic specializations. J. Cell Sci. 1996;109:1229–1239. doi: 10.1242/jcs.109.6.1229. [DOI] [PubMed] [Google Scholar]
- Beardsley A, Robertson DM, O'Donnell L. A complex containing α6β1-integrin and phosphorylated focal adhesion kinase between sertoli cells and elongated spermatids during spermatid release from the seminiferous epithelium. J. Endocrinol. 2006;190:759–770. doi: 10.1677/joe.1.06867. [DOI] [PubMed] [Google Scholar]
- Bolos V, Gasent JM, Lopez-Tarruella S, Grande E. The dual kinase complex fak-src as a promising therapeutic target in cancer. Onco. Targets Ther. 2010;3:83–97. doi: 10.2147/ott.s6909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boutros T, Chevet E, Metrakos P. Mitogen-activated protein (MAP) kinase/MAP kinase phosphatase regulation: Roles in cell growth, death, and cancer. Pharmacol. Rev. 2008;60:261–310. doi: 10.1124/pr.107.00106. [DOI] [PubMed] [Google Scholar]
- Braren R, Hu H, Kim YH, Beggs HE, Reichardt LF, Wang R. Endothelial fak is essential for vascular network stability, cell survival, and lamellipodial formation. J. Cell Biol. 2006;172:151–162. doi: 10.1083/jcb.200506184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broussard JA, Webb DJ, Kaverina I. Asymmetric focal adhesion disassembly in motile cells. Curr. Opin. Cell Biol. 2008;20:85–90. doi: 10.1016/j.ceb.2007.10.009. [DOI] [PubMed] [Google Scholar]
- Cabodi S, del Pilar Camacho-Leal M, Di Stefano P, DeFilippi P. Integrin signalling adaptor: Not only figurants in the cancer story. Nat. Rev. Cancer. 2010;10:858–870. doi: 10.1038/nrc2967. [DOI] [PubMed] [Google Scholar]
- Carreau S, Hess RA. Oestrogens and spermatogenesis. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2010;365:1517–1535. doi: 10.1098/rstb.2009.0235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman NM, Connolly SF, Reinl EL, Houtman JC. Focal adhesion kinase negatively regulates lck function downstream of the t cell antigen receptor. J. Immunol. 2013;191:6208–6221. doi: 10.4049/jimmunol.1301587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen B, Li A, Wang D, Wang M, Zheng L, Bartles J. Espin contains an additional actin-binding site in its n-terminus and is a major actin-bundling protein of the sertoli cell-spermatid ectoplasmic specialization junctional plaque. Mol. Biol. Cell. 1999;10:4327–4339. doi: 10.1091/mbc.10.12.4327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng CY, Mruk DD. Cell junction dynamics in the testis: Sertoli-germ cell interactions and male contraceptive development. Physiol. Rev. 2002;82:825–874. doi: 10.1152/physrev.00009.2002. [DOI] [PubMed] [Google Scholar]
- Cheng CY, Mruk DD. A local autocrine axis in the testes that regulates spermatogenesis. Nature Rev. Endocrinol. 2010;6:380–395. doi: 10.1038/nrendo.2010.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng CY, Mruk DD. The blood-testis barrier and its implication in male contraception. Pharmacol. Rev. 2012;64:16–64. doi: 10.1124/pr.110.002790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng YH, Wong EWP, Cheng CY. Cancer/testis (ct) antigens, carcinogenesis and spermatogenesis. Spermatogenesis. 2011;1:209–220. doi: 10.4161/spmg.1.3.17990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claesson-Welsh L, Welsh M. VEGFA and tumour angiogenesis. J. Intern. Med. 2013;273:114–127. doi: 10.1111/joim.12019. [DOI] [PubMed] [Google Scholar]
- Di Fiore PP, Scita G. Eps8 in the midst of gtpases. Int. J. Biochem. Cell Biol. 2002;34:1178–1183. doi: 10.1016/s1357-2725(02)00064-x. [DOI] [PubMed] [Google Scholar]
- Dufour JM, Dass B, Halley KR, Korbutt GS, Dixon DE, Rajotte RV. Sertoli cell line lacks the immunoprotective properties associated with primary sertoli cells. Cell Transplant. 2008;17:525–534. doi: 10.3727/096368908785096033. [DOI] [PubMed] [Google Scholar]
- Dym M. Basement membrane regulation of sertoli cells. Endocr. Rev. 1994;15:102–115. doi: 10.1210/edrv-15-1-102. [DOI] [PubMed] [Google Scholar]
- Fiedorek FTJ, Kay ES. Mapping of the focal adhesion kinase (FAK) gene to mouse chromosome 15 and human chromosome 8. Mammalian Genome. 1995;6:123–126. doi: 10.1007/BF00303256. [DOI] [PubMed] [Google Scholar]
- Franca LR, Auharek SA, Hess RA, Dufour JM, Hinton BT. Blood-tissue barriers: Morphofunctional and immunological aspects of the blood-testis and blood-epididymal barriers. Adv. Exp. Med. Biol. 2012;763:237–259. [PubMed] [Google Scholar]
- Hall JE, Fu W, Schaller MD. Focal adhesion kinase: Exploring FAK structure to gain insight into function. Int. Rev. Cell Mol. Biol. 2011;288:185–225. doi: 10.1016/B978-0-12-386041-5.00005-4. [DOI] [PubMed] [Google Scholar]
- Hanks SK, Calalb MB, Harper MC, Patel SK. Focal adhesion protein-tyrosine kinase phosphorylated in response to cell attachment to fibronectin. Proc. Natl. Acad. Sci. USA. 1992;89:8487–8491. doi: 10.1073/pnas.89.18.8487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hew KW, Heath GL, Jiwa AH, Welsh MJ. Cadmium in vivo causes disruption of tight junction-associated microfilaments in rat sertoli cells. Biol. Reprod. 1993;49:840–849. doi: 10.1095/biolreprod49.4.840. [DOI] [PubMed] [Google Scholar]
- Ilic D, Furuta Y, Kanazawa S, Takeda N, Sobue K, Nakatsuli N, Nomura S, Fujimoto J, Okada M, Yamamoto T. Reduced cell motility and enhanced focal adhesion contact formation in cells from fak-deficient mice. Nature. 1995;377:539–544. doi: 10.1038/377539a0. [DOI] [PubMed] [Google Scholar]
- Johnson L, Petty CS, Neaves WB. A comparative study of daily sperm production and testicular composition in humans and rats. Biol. Reprod. 1980;22:1233–1243. doi: 10.1093/biolreprod/22.5.1233. [DOI] [PubMed] [Google Scholar]
- Kemphues KJ, Priess JR, Morton DG, Cheng NS. Identification of genes required for cytoplasmic localization in early C. Elegans embryos. Cell. 1988;52:311–320. doi: 10.1016/s0092-8674(88)80024-2. [DOI] [PubMed] [Google Scholar]
- Lagares D, Kapoor M. Targeting focal adhesion kinase in fibrotic diseases. BioDrugs. 2013;27:15–23. doi: 10.1007/s40259-012-0003-4. [DOI] [PubMed] [Google Scholar]
- Lechertier T, Hodivala-Dilke K. Focal adhesion kinase and tumour angiogenesis. J. Pathol. 2012;226:404–412. doi: 10.1002/path.3018. [DOI] [PubMed] [Google Scholar]
- Li MWM, Mruk DD, Lee WM, Cheng CY. Cytokines and junction restructuring events during spermatogenesis in the testis: An emerging concept of regulation. Cytokine Growth Factor Rev. 2009;20:329–338. doi: 10.1016/j.cytogfr.2009.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lie PPY, Mruk DD, Lee WM, Cheng CY. Epidermal growth factor receptor pathway substrate 8 (Eps8) is a novel regulator of cell adhesion and the blood-testis barrier integrity in the seminiferous epithelium. FASEB J. 2009;23:2555–2567. doi: 10.1096/fj.06-070573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lie PPY, Chan AYN, Mruk DD, Lee WM, Cheng CY. Restricted Arp3 expression in the testis prevents blood-testis barrier disruption during junction restructuring at spermatogenesis. Proc. Natl. Acad. Sci. USA. 2010;107:11411–11416. doi: 10.1073/pnas.1001823107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lie PPY, Mruk DD, Mok KW, Su L, Lee WM, Cheng CY. Focal adhesion kinase-Tyr407 and -Tyr397 exhibit antagonistic effects on blood-testis barrier dynamics in the rat. Proc. Natl. Acad. Sci. USA. 2012;109:12562–12567. doi: 10.1073/pnas.1202316109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim ST, Chen XL, Lim Y, Hanson DA, Vo TT, Howerton K, Larocque N, Fisher SJ, Schlaepfer DD, Ilic D. Nuclear fak promotes cell proliferation and survival through ferm-enhanced p53 degradation. Mol. Cell. 2008;29:9–22. doi: 10.1016/j.molcel.2007.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim ST, Miller NL, Chen XL, Tancioni I, Walsh CT, Lawson C, Uryu S, Weis SM, Cheresh DA, Schlaepfer DD. Nuclear-localized focal adhesion kinase regulates inflammatory vcam-1 expression. J. Cell Biol. 2012;197:907–919. doi: 10.1083/jcb.201109067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lui WY, Cheng CY. Regulation of cell junction dynamics by cytokines in the testis - a molecular and biochemical perspective. Cytokine Growth Factor Rev. 2007;18:299–311. doi: 10.1016/j.cytogfr.2007.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malinin NL, Pluskota E, Byzova TV. Integrin signaling in vascular function. Curr. Opin. Hematol. 2012;19:206–211. doi: 10.1097/MOH.0b013e3283523df0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitra SK, Schlaepfer DD. Integrin-regulated fak-src singaling in normal and cancer cells. Curr. Opin. Cell Biol. 2006;18:516–523. doi: 10.1016/j.ceb.2006.08.011. [DOI] [PubMed] [Google Scholar]
- Mok KW, Mruk DD, Lee WM, Cheng CY. A study to assess the assembly of a functional blood-testis barrier in developing rat testes. Spermatogenesis. 2011;1:270–280. doi: 10.4161/spmg.1.3.17998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mok KW, Mruk DD, Lee WM, Cheng CY. Spermatogonial stem cells alone are not sufficient to re-initiate spermatogenesis in the rat testis following adjudin-induced infertility. Int. J. Androl. 2012;35:86–101. doi: 10.1111/j.1365-2605.2011.01183.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mruk DD, Cheng CY. Sertoli-sertoli and sertoli-germ cell interactions and their significance in germ cell movement in the seminiferous epithelium during spermatogenesis. Endocr. Rev. 2004;25:747–806. doi: 10.1210/er.2003-0022. [DOI] [PubMed] [Google Scholar]
- Mruk DD, Silvestrini B, Cheng CY. Anchoring junctions as drug targets: Role in contraceptive development. Pharmacol. Rev. 2008;60:146–180. doi: 10.1124/pr.107.07105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Bryan MK, Hedger MP. Inflammatory networks in the control of spermatogenesis: Chronic inflammation in an immunologically privileged tissues? Adv. Exp. Med. Biol. 2008;636:92–114. doi: 10.1007/978-0-387-09597-4_6. [DOI] [PubMed] [Google Scholar]
- O'Donnell L, Robertson KM, Jones ME, Simpson ER. Estrogen and spermatogenesis. Endocr. Rev. 2001;22:289–318. doi: 10.1210/edrv.22.3.0431. [DOI] [PubMed] [Google Scholar]
- O'Donnell L, Meachem SJ, Stanton PG, McLachlan RI. Endocrine regulation of spermatogenesis. In: Neill JD, editor. Physiology of reproduction. 3rd edition Elsevier; Amsterdam: 2006. pp. 1017–1069. [Google Scholar]
- O'Donnell L, Nicholls PK, O'Bryan MK, McLachlan RI, Stanton PG. Spermiation: The process of sperm release. Spermatogenesis. 2011;1:14–35. doi: 10.4161/spmg.1.1.14525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ossovskaya V, Lim ST, Ota N, Schlaepfer DD, Ilic D. FAK nuclear export signal sequences. FEBS Lett. 2008;582:2402–2406. doi: 10.1016/j.febslet.2008.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozaki-Kuroda K, Nakanishi H, Ohta H, Tanaka H, Kurihara H, Mueller S, Irie K, Ikeda W, Sakai T, Wimmer E, Nishimune Y, Takai Y. Nectin couples cell-cell adhesion and the actin scaffold at heterotypic testicular junctions. Curr. Biol. 2002;12:1145–1150. doi: 10.1016/s0960-9822(02)00922-3. [DOI] [PubMed] [Google Scholar]
- Parsons JT. Focal adhesion kinase: The first ten years. J. Cell Sci. 2003;116:1409–1416. doi: 10.1242/jcs.00373. [DOI] [PubMed] [Google Scholar]
- Pelletier RM. The blood-testis barrier: The junctional permeability, the proteins and the lipids. Prog. Histochem. Cytochem. 2011;46:49–127. doi: 10.1016/j.proghi.2011.05.001. [DOI] [PubMed] [Google Scholar]
- Pentassuglia L, Sawyer DB. Erbb/integrin singaling interactions in regulation of myocardial cell-cell and cell-matrix interactions. Biochim. Biophys. Acta. 2013;1833:909–916. doi: 10.1016/j.bbamcr.2012.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian X, Mruk DD, Cheng YH, Cheng CY. Actin cross-linking protein palladin and spermatogenesis. Spermatogenesis. 2013a;3:e23473. doi: 10.4161/spmg.23473. (DOI:10.4161.spmg.23473) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian X, Mruk DD, Wong EWP, Lie PPY, Cheng CY. Palladin is a regulator of actin filament bundles at the ectoplasmic specialization in the rat testis. Endocrinology. 2013b;154:1907–1920. doi: 10.1210/en.2012-2269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robb GW, Amann RP, Killian GJ. Daily sperm production and epididymal sperm reserves of pubertal and ault rats. J. Reprod. Fertil. 1978;54:103–107. doi: 10.1530/jrf.0.0540103. [DOI] [PubMed] [Google Scholar]
- Russell LD. Observations on rat sertoli ectoplasmic (‘junctional’) specializations in their association with germ cells of the rat testis. Tissue Cell. 1977;9:475–498. doi: 10.1016/0040-8166(77)90007-6. [DOI] [PubMed] [Google Scholar]
- Schaller MD, Borgman CA, Cobb BS, Vines RR, Reynolds AB, Parsons JT. Pp125FAK, a structurally distinctive protein-tyrosine kdinase associated with focal adhesions. Proc. Natl. Acad. Sci. USA. 1992;89:5192–5196. doi: 10.1073/pnas.89.11.5192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selawry HP, Cameron DF. Sertoli cell-enriched fractions in successful islet cell transplantation. Cell Transplant. 1993;2:123–129. doi: 10.1177/096368979300200206. [DOI] [PubMed] [Google Scholar]
- Setchell BP, Waites GMH. Changes in the permeability of the testicular capillaries and of the “blood-testis barrier” after injection of cadmium chloride in the rat. J. Endocrinol. 1970;47:81–86. doi: 10.1677/joe.0.0470081. [DOI] [PubMed] [Google Scholar]
- Shamekh R, El-Badri NS, Saporta S, Pascual C, Sanberg PR, Cameron DF. Sertoli cells induce systemic donor-specific tolerance in xenogenic transplantation model. Cell Transplant. 2006;15:45–53. doi: 10.3727/000000006783982205. [DOI] [PubMed] [Google Scholar]
- Sharpe RM. Regulation of spermatogenesis. In: Knobil E, Neill JD, editors. The Physiology of Reproduction. Raven Press; New York: 1994. pp. 1363–1434. [Google Scholar]
- Shen TL, Park AYJ, Alcaraz A, Peng X, Jang I, Koni P, Flavell RA, Gu H, Guan JL. Conditional knockout of focal adhesion kinase in endothelial cells reveals its role in angiogenesis and vascular development in late embryogenesis. J. Cell Biol. 2005;169:941–952. doi: 10.1083/jcb.200411155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siu MKY, Cheng CY. Interactions of proteases, protease inhibitors, and the β1 integrin/laminin γ3 protein complex in the regulation of ectoplasmic specialization dynamics in the rat testis. Biol. Reprod. 2004a;70:945–964. doi: 10.1095/biolreprod.103.023606. [DOI] [PubMed] [Google Scholar]
- Siu MKY, Cheng CY. Dynamic cross-talk between cells and the extracellular matrix in the testis. BioEssays. 2004b;26:978–992. doi: 10.1002/bies.20099. [DOI] [PubMed] [Google Scholar]
- Siu MKY, Mruk DD, Lee WM, Cheng CY. Adhering junction dynamics in the testis are regulated by an interplay of β1-integrin and focal adhesion complex (FAC)-associated proteins. Endocrinology. 2003;144:2141–2163. doi: 10.1210/en.2002-221035. [DOI] [PubMed] [Google Scholar]
- Siu ER, Mruk DD, Porto CS, Cheng CY. Cadmium-induced testicular injury. Toxicol. Appl. Pharmacol. 2009a;238:240–249. doi: 10.1016/j.taap.2009.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siu ER, Wong EWP, Mruk DD, Porto CS, Cheng CY. Focal adhesion kinase is a blood-testis barrier regulator. Proc. Natl. Acad. Sci. USA. 2009b;106:9298–9303. doi: 10.1073/pnas.0813113106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siu ER, Wong EWP, Mruk DD, Sze KL, Porto CS, Cheng CY. An occludin-focal adhesion kinase protein complex at the blood-testis barrier: A study using the cadmium model. Endocrinology. 2009c;150:3336–3344. doi: 10.1210/en.2008-1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siu MKY, Wong CH, Xia W, Mruk DD, Lee WM, Cheng CY. The β1-integrin-p-FAK-p130cas-DOCK180-RhoA-vinculin is a novel regulatory protein complex at the apical ectoplasmic specialization in adult rat testes. Spermatogenesis. 2011;1:73–86. doi: 10.4161/spmg.1.1.15452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart A, Ham C, Zachary I. The focal adhesion kinase amino-terminal domain localises to nuclei and intercellular junctions in Hek293 and mdck cells independently of tyrosine 397 and the carboxy-terminal domain. Biochem. Biophys. Res. Commun. 2002;299:62–73. doi: 10.1016/s0006-291x(02)02547-0. [DOI] [PubMed] [Google Scholar]
- Su L, Mruk DD, Lie PPY, Silvestrini B, Cheng CY. A peptide derived from laminin-γ3 reversibly impairs spermatogenesis in rats. Nat. Communs. 2012a;3:1185. doi: 10.1038/ncomms2171. (doi:1110.1038/ncomms2171) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su WH, Mruk DD, Cheng CY. Filamin A: A regulator of blood-testis barrier assembly during post-natal development. Spermatogenesis. 2012b;2:73–78. doi: 10.4161/spmg.20223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su WH, Mruk DD, Lie PPY, Lui WY, Cheng CY. Filamin A is a regulator of blood-testis barrier assembly during postnatal development in the rat testis. Endocrinology. 2012c;153:5023–5035. doi: 10.1210/en.2012-1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang EI, Mruk DD, Cheng CY. Map/microtubule affinity-regulating kinases, microtubule dynamics, and spermatogenesis. J. Endocrinol. 2013;217:R13–R23. doi: 10.1530/JOE-12-0586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ucar DA, Hochwald SN. FAK and interacting proteins as therapeutic targets in pancreatic cancer. Anticancer Agents Med. Chem. 2010;10:742–746. doi: 10.2174/187152010794728675. [DOI] [PubMed] [Google Scholar]
- Vogl AW, Vaid KS, Guttman JA. The Sertoli cell cytoskeleton. Adv. Exp. Med. Biol. 2008;636:186–211. doi: 10.1007/978-0-387-09597-4_11. [DOI] [PubMed] [Google Scholar]
- Vogl AW, Young JS, Du M. New insights into roles of tubulobulbar complexes in sperm release and turnover of blood-testis barrier. Int. Rev. Cell Mol. Biol. 2013;303:319–355. doi: 10.1016/B978-0-12-407697-6.00008-8. [DOI] [PubMed] [Google Scholar]
- Wan HT, Mruk DD, Wong CKC, Cheng CY. Perfluorooctanesulfonate (PFOS) perturbs male rat Sertoli cell blood-testis barrier function by affecting F-actin organization via p-FAK-Tyr407 - and in vitro study. Endocrinology. 2014;155:249–262. doi: 10.1210/en.2013-1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan HT, Mruk DD, Li SYT, Mok KW, Lee WM, Wong CKC, Cheng CY. p-Fak-Tyr397 regulates spermatid adhesion in the rat testis via its effects on F-actin organization at the ectoplasmic specialization. Am. J. Physiol. Endocrinol. Metab. 2013;305:E687–E699. doi: 10.1152/ajpendo.00254.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang RS, Yeh S, Tzeng CR, Chang C. Androgen receptor roles in spermatogenesis and fertility: Lessons from testicular cell-specific androgen receptor knockout mice. Endocr. Rev. 2009;30:119–132. doi: 10.1210/er.2008-0025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Zu J, Xu G, Zhao W, Yan J. Inhibition of focal adhesion kinase induces apoptosis in human osteosarcoma Saos-2 cells. Tumour Biol. 2014;35:1551–1556. doi: 10.1007/s13277-013-1214-0. [DOI] [PubMed] [Google Scholar]
- Wehrle-Haller B. Assembly and disassembly of cell matrix adhesions. Curr. Opin. Cell Biol. 2012;24:569–581. doi: 10.1016/j.ceb.2012.06.010. [DOI] [PubMed] [Google Scholar]
- Weis SM, Lim ST, Lutu-, Futu-Fuga KM, Barnes LA, Chen XL, Gothert JR, Shen TL, Guan JL, Schlaepfer DD, Cheresh DA. Compensatory role for Pyk2 during angiogenesis in adult mice lacking endothelial cell FAK. J. Cell Biol. 2008;181:43–50. doi: 10.1083/jcb.200710038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiebe J, Barr K. The control of male fertility by 1,2,3-trihydroxypropane (THP; glycerol): Rapid arrest of spermatogenesis without altering libido, accessory organs, gonadal steroidogenesis, and serum testosterone, LH, and FSH. Contraception. 1984;29:291–302. doi: 10.1016/s0010-7824(84)80009-8. [DOI] [PubMed] [Google Scholar]
- Wiebe J, Kowalik A, Gallardi R, Egeler O, Clubb B. Glycerol disrupts tight junction-associated actin microfilaments, occludin, and microtubules in sertoli cells. J. Androl. 2000;21:625–635. [PubMed] [Google Scholar]
- Winters SJ, Moore JP. Paracrine control of gonadotrophs. Semin. Reprod. Med. 2007;25:379–387. doi: 10.1055/s-2007-984744. [DOI] [PubMed] [Google Scholar]
- Wolski KM, Perrault C, Tran-Son-Tay R, Cameron DF. Strength measurement of the sertoli-spermatid junctional complex. J. Androl. 2005;26:354–359. doi: 10.2164/jandrol.04142. [DOI] [PubMed] [Google Scholar]
- Xiao X, Mruk DD, Cheng CY. C-yes regulates cell adhesion at the apical ectoplasmic specialization-blood-testis barrier axis via its effects on protein recruitment and distribution. Am. J. Physiol. Endocrinol. Metab. 2013;304:E145–E159. doi: 10.1152/ajpendo.00422.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan HHN, Mruk DD, Wong EWP, Lee WM, Cheng CY. An autocrine axis in the testis that coordinates spermiation and blood-testis barrier restructuring during spermatogenesis. Proc. Natl. Acad. Sci. USA. 2008;105:8950–8955. doi: 10.1073/pnas.0711264105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zachary I, Rozengurt E. Focal adhesion kinase (p125FAK): A point of convergence in the action of neuropeptides, integrins, and oncogenes. Cell. 1992;71:891–894. doi: 10.1016/0092-8674(92)90385-p. [DOI] [PubMed] [Google Scholar]