Abstract
Perinatal exposure to polychlorinated biphenyls (PCBs) leads to significant alterations of neural and hormonal systems. These alterations have been shown to impair motor and sensory development. Less is known about the influence of PCB exposure on developing emotional and motivational systems involved in social interactions and social learning. The present study examined the impact of perinatal PCB exposure (mixture of congeners 47 and 77) on social recognition in juvenile animals, conspecific-directed investigation in adults and on neural and hormonal systems involved in social functions. We used a standard habituation–dishabituation paradigm to evaluate juvenile recognition and a social port paradigm to monitor adult social investigation. Areal measures of the periventricular nucleus (PVN) of the hypothalamus were obtained to provide correlations with related hormone and brain systems. PCB exposed rats were significantly impaired in social recognition as indicated by persistent conspecific-directed exploration by juvenile animals regardless of social experience. As adults, PCB exposure led to a dampening of the isolation-induced enhancement of social investigation. There was not a concomitant alteration of social investigation in pair-housed PCB exposed animals at this stage of development. Interestingly, PVN area was significantly decreased in juvenile animals exposed to PCB during the perinatal period. Shifts in hypothalamic regulation of hormones involved in social behavior and stress could be involved in the behavioral changes observed. Overall, the results suggest that PCB exposure impairs context or experience-dependent modulation of social approach and investigation. These types of social-context deficits are similar to behavioral deficits observed in social disorders such as autism and other pervasive developmental disorders.
Keywords: Affect, Emotion, Development, Hormones, Motivation, Toxicant
1. Introduction
Polychlorinated biphenyls (PCBs) are environmental toxicants that remain pervasive in the soil, water and air (Beyer and Biziuk, 2009; Shields, 2006). Exposure in work environments has been reduced since the industrial use of PCBs was banned in 1976; however, the threat of exposure persists particularly from ingestion of certain food items (i.e., fish and other marine organisms) and breast milk (Schecter et al., 2010; La Rocca and Mantovani, 2006; Domingo and Bocio, 2007). Exposure early in development has been shown to have the most potential to alter important neural and endocrine systems (Carpenter, 2006). Impairments in basic functions related to growth, metabolism and hormone function have been characterized and replicated in basic animal research and human health surveys (Patisaul and Adewale, 2009). PCB is a powerful endocrine disruptor that alters thyroid status (Donahue et al., 2004), sex hormone functions (Jonsson et al., 1975; Steinberg et al., 2008) and other neuroendocrine processes (Khan et al., 2002; Corey et al., 1996; Meserve et al., 1992). These effects can lead to significant and long-lasting changes in neurochemistry and brain morphology (Faroon et al., 2001; Tilson and Kodavanti, 1998). Several brain regions including areas of the hypothalamus (Colciago et al., 2009; Gore, 2001) and the basal ganglia (Seegal et al., 2005) have been found to be deleteriously impacted by PCB exposure during development.
Behavioral work examining PCB effects has focused on reflex integrity, sensorimotor processing and motor learning (Roegge and Schantz, 2006). More recent work has examined effects on learning and memory using established measures of retention, consolidation and spatial map representation (Stewart et al., 2008; Widholm et al., 2004; Provost et al., 1999). Less work has been done on the effects of PCB on social and affective behaviors. A series of studies have documented effects of early PCB exposure on reproductive behavior and maternal care (Chung et al., 2001; Wang et al., 2002; Steinberg et al., 2007). Clemens and colleagues found a significant effect on female receptive behavior and alteration in anogenital distance suggesting a shift in androgen/estrogen activity and partial masculinization (Wang et al., 2002). A recent paper examining both sexual behavior and partner preference in adult females following perinatal PCB exposure found that direct exposure to PCB caused impairment in partner preference but did not lead to significant alteration of reproductive strategies (Cummings et al., 2008). Interestingly, basic alterations in these social behaviors such as partner preference could be explained partially by impairments in social memory and appetitive social investigation (Lee et al., 2008; Wang et al., 1999).
The present study extends this work and explores the possible underlying neural and hormonal basis for these behavioral alterations. We have used a standard habituation–dishabituation paradigm (Thor and Holloway, 1982) to examine social memories in the rat. This paradigm uses a series of conspecific presentations to the test animal to monitor social approach. Typically, animals reduce approach over repeated exposures to the same animal (habituation phase) and then return to higher levels of social exploration when a novel conspecific is presented (dishabituation). The test has been extremely useful in delineating important genetic, neural and hormonal mechanisms of pair bonding and other attachment behaviors (Thor and Holloway, 1981, 1986; Sekiguchi et al., 1991; Spiteri and Agmo, 2009). We have focused on male juvenile animals because previous work has shown that these animals express robust social memories using short inter-trial intervals (Thor and Holloway, 1982) and it removes the confounding influence of estrus cycling in the female animals. A second test was done on adult animals to monitor social investigation and to explore the impact of social isolation. This social investigation test uses two holes or ports into which the test animal can poke its nose, one port is labeled the ‘social port’ and is adjacent to a cage containing another animal while the other port is labeled the ‘non-social port’, facing an empty area in space. This test is useful in evaluating social appetitive behavior in the absence of inconsistent, direct contact between animals. Finally, we examined the area of sectioned periventricular nucleus (PVN) in the hypothalamus of the juvenile animals exposed to perinatal PCB. If social memories and investigation are perturbed, then one would expect that elements of the social brain systems could be altered structurally at one or several levels.
2. Materials and methods
2.1. Animals and PCB exposure
Maintenance and procedures were performed in accordance with the Bowling Green State University and NIH Guide for the Care and Use of Laboratory Animals, and were approved by the BGSU Institutional Animal Care and Use Committee (Protocol #04-015). Male rats used in these procedures were bred in the animal facilities at BGSU. Females weighing 225–275 g were mated to males (Sprague–Dawley, Harlan, Indianapolis, IN), and pregnancy was determined by the presence of sperm in a vaginal smear, which was designated gestational day 1. These pregnant females were caged separately and fed either control or PCB containing diet from the first day of pregnancy until pup weaning. From weaning onward, all animals were fed control diet. Exposure to PCB was provided to the rat pups through the maternal diet during gestation and lactation. Litters were standardized to 10 pups (5 males/5 females) on day 3 of age. Only male rats were used for the present measurements, with the females assigned to another project. Two PCB congeners, PCB 47 (ortho substituted; 2,2′,4,4′-tetrachlorobiphenyl) and PCB 77 (non-ortho substituted; 3,3′,4,4′-tetrachlorobiphenyl), were obtained from AccuStandard, Inc., New Haven, CT. These two congeners were originally chosen because their degree of halogenation resembles that of the thyroid hormone thyroxine. This mixture has been used successfully by our research group to examine physiological (Donahue et al., 2004) and behavioral alterations (Cromwell et al., 2007). Stock PCB was dissolved in absolute ethanol, mixed with 100 g of rat chow (Mowlan Teklad, Madison, WI, USA), and the ethanol was allowed to evaporate to obtain rat chow with PCB mixed in equal amounts (by weight) of PCB 47 and PCB 77. The formulation of 12.5ppm and 25ppm doses (i.e., 12.5mg PCB/kg diet and 25mg PCB/kg diet) was done by adding an appropriate weight of this concentrated mixture to plain diet to a final weight of 1000 g. Control animals were fed mash diet, which did not contain PCB. Animals were exposed until weaning (PND 20). Animals for the social recognition test and for the social investigation test came from different litters, but the maternal dietary regimes were identical.
2.2. Social recognition test
Pups were weaned from the mother at 20 days of age, habituated to the social recognition setup for 2min and placed in isolate housing for 18–24 h prior to experimentation at 21 days of age. The social recognition test was done using an arena composed of two rectangular chambers, a social box and a non-social box. The dimensions of each of these boxes were 25cm × 20cm × 13 cm. The chambers were connected to each other by means of a transparent Plexiglas tube. Each observer or restrained animal was placed in the social box and spatially restrained by a wire mesh grid so that it could not enter the tube or the non-social box Thus, the animals could only interact through the wire mesh when the test rat entered the social box. Initially, the test rat was placed in a transparent tube that connected the two boxes with barriers in place on each end to prevent the animal from entering the boxes prior to start of session. Animals from each group were placed facing either box in counterbalanced fashion. The effect of PCB on social recognition was estimated by comparing the amount of time spent and the number of entrances in the social-side of the apparatus, with similar measures for the non-social box side. Three consecutive trials of 5min each were completed. In the second trial the subject rat was exposed to the same restrained rat, and in the third trial a novel restrained rat was introduced. The inter-exposure interval time was 3min. Pairs of rats for the above tests received the same dietary treatment: control–control, PCB 12.5–PCB 12.5, or PCB 25–PCB 25.
2.3. Social investigation testing
The social port investigation paradigm was designed to examine variations in gregariousness between adult littermate animals. Each animal was tested three times in the social investigation paradigm: (1) first test session was after social housing with the littermate; (2) the second test session after 24 h of isolation housing and (3) the third test session was followed by 24 h of housing with littermate. Each session/day lasted 10 min. The social investigation apparatus consisted of two opaque Plexiglas cages (65cm × 24cm × 15 cm) with one exploration ports measuring 1.58cm in radius on each end of each box. Cages containing corn cob bedding were placed end-to-end on a secure table top such that the port designated the social port was facing the neighboring cage. The non-social port was on the opposing end of the cage and was facing open space. The ports were of sufficient diameter to allow the animals to place their noses into the ports, but were not large enough to allow escape. The space between social ports in adjacent cages was approximately 7 cm. Photo beams were located at the each port to count the number of times an animal nosepoked into either port. The photo beams transferred information to a DOS-based custom computer program (Headhov3) for analysis.
2.4. PVN area measurement
Animals were anesthetized with a lethal dose of sodium pentobarbital and decapitated. Brains were removed, weighed and placed in a 10% formalin solution for 20–24 h in 4 °C. After 4 days, they were placed in a solution of 30% sucrose in 10% formalin for 48 h. Brains were mounted on a freezing microtome and cut in a coronal plane with slice thickness of 40 μm. From each brain, 8 sections of PVN were taken between bregma −1.80 and −2.30. Sections were collected and mounted on a slide. Brain sections were stained with cresyl violet. The slices were examined microscopically using 40× and 100× magnifications. Using measurement software (Motic Image, Version 1.3, Motic China Group Co., Ltd.), the boundary of the PVN was traced in each of the slices and the procedure was repeated for animals from both control and PCB fed mothers. An average area (μm2) for the total PVN region (bilateral) was obtained for each animal.
2.5. Statistical analysis
All dependent measures were evaluated with an omnibus ANOVA followed by planned pairwise comparisons (independent and paired sample t-tests). One-way ANOVAs (3 levels: control, PCB 12.5 or PCB 25) were used to analyze among groups for food consumption and body weights of the dams and rat pups and adult animals and to compare the PVN area. For the social recognition and investigation a two-factor mixed design ANOVA was used with PCB treatment condition as the between factor (3 levels) and social test (novel vs. familiar tests or housing vs. isolation tests) as the repeated measure. Pairwise comparisons were completed using single step post hoc tests. For between group comparisons the Tukey test was used based on the studentized range distribution and for repeated measures comparisons the Bonferroni multiple comparison test was used.
3. Results
3.1. Food consumption and body weights
In order to determine any potential alteration in feeding regimen or general health status caused by PCB ingestion, we monitored food chow intake for all dams during gestation and maintained records of daily body weights. Not surprisingly given previous results, we found no significant differences among the three groups for amount of food consumed, weight gained during the last week of gestation, or final body weight the day before parturition (Table 1). Body weights of juvenile animals obtained during the period of the social recognition test were not significantly different among the groups (Table 2). The animals exposed to 12.5ppm weighed the least while the animals exposed to twice that dosage were nearly identical to the controls in body weight. Adult males used in the social recognition test displayed significantly different weights (Table 2). Tukey post hoc comparisons of these three groups showed that animals exposed to PCB 25ppm (see Table 2 for mean; 95% CI [381, 429]) were significantly heavier compared to either the controls (95% CI [313.3, 347.1], p < 0.001) or animals exposed to 12.5ppm PCB (95% CI [296.9, 344.8], p < 0.001).
Table 1.
Food consumption, weight gain and body weights of dams.
| Control dam | PCB 12.5 dam | PCB 25 dam | F (p value) | |
|---|---|---|---|---|
| Food consumed (g) | 24 ± 2.3 | 23.9 ± 3.2 | 24.8 ± 3.3 | 0.635 (0.547) |
| Weight gain (g) | 81.3 ± 9.7 | 80.0 ± 3.8 | 81.1 ± 3.4 | 0.013 (0.987) |
| Body weight (g) | 367.6 ± 6.2 | 346.3 ± 9.4 | 349 ± 8.4 | 1.9 (0.184) |
Food consumed provides a measure for how much chow was consumed per day on average for the 21-day gestation period. Weight gain is given as an average for the final 7 days prior to parturition and body weight was measured as an average the day before parturition.
Table 2.
Pup and adult rat weights at time of testing.
| Ss | Body weights (g) | F (p value) |
|---|---|---|
| Cont-pups | 23.0 ± 0.27 | |
| 12.5 pups | 19.7 ± 0.27 | 2.5 (0.118) |
| 25 pups | 22.7 ± 1.9 | |
| Cont-adults | 330.2 ± 9.6 | |
| 12.5 adults | 320.9 ± 7.8 | 18.8 (p < 0.001) |
| 25 adults | 405.5 ± 7.0* |
Bold = significantly different from cont-adults and 12.5 adults (p < 0.001).
3.2. Social recognition test
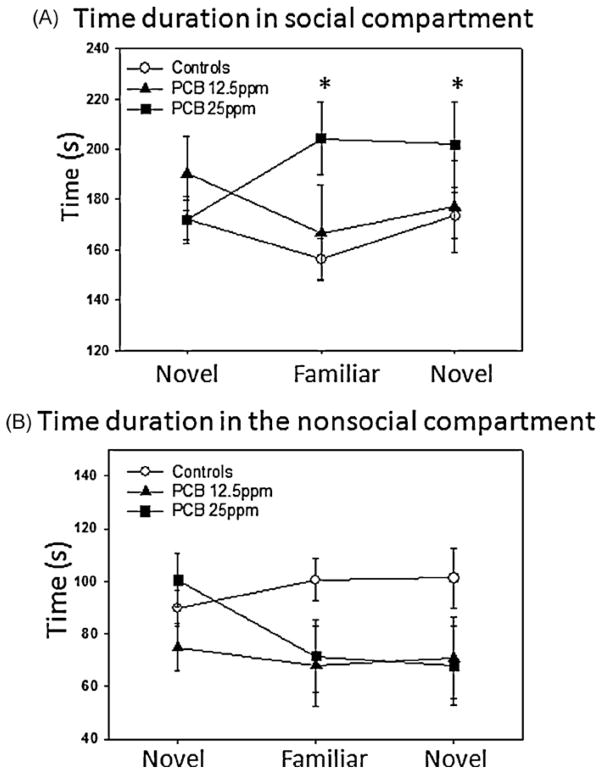
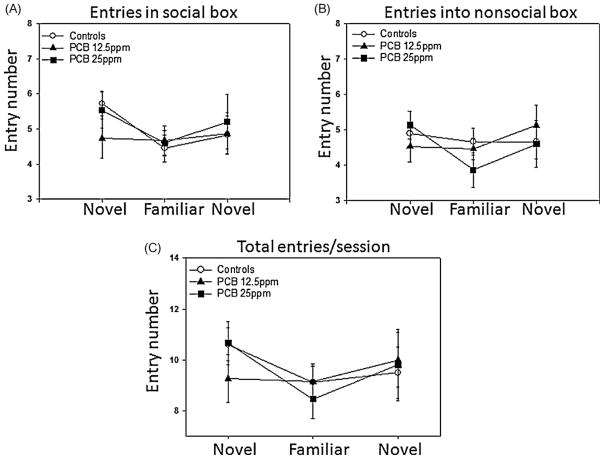
Social recognition tests were completed using 48 juvenile male animals (CONT, n = 18;PCB12.5 ppm, n = 15 andPCB25 ppm, n = 15). All tests were completed pairing animals from the same condition (subject and restrained animal were from same condition, not the same litter). First, we examined time spent in the social box and found a significant interaction effect between the condition and social experience factors (F(4,90) = 2.98, p < 0.05). This result supports a significant difference between groups regarding the way that they respond to repeated presentations of the conspecific. There were no significant main effects found. Pairwise comparisons using the Tukey test revealed a significantly greater duration of time in the social box by the PCB 25ppm group (M= 204.3, 95% CI [175.2, 233.3]) compared to the controls (M= 156, 95% CI [129.7, 182.7], p < 0.05) during the second test period when the conspecific is familiar. As controls and the lesser PCB dose group decreased or habituated to the same animal, the group receiving more PCB increased time in the social box (Fig. 1A). To examine this more closely, we completed the Bonferroni multiple comparison test to examine the degree of increase within the PCB 25ppm group over sessions. There was a marginal trend for increased time in the social box between the initial session and the second session for this group (initial session: M= 172, 95% CI [155, 188.9] vs. second session: M= 204.3, 95% CI [172, 235.5], p = 0.08). There were no significant differences among the groups for time spent in the non-social box (Fig. 1B). Interestingly, the controls expressed the typical increase in non-social box time for session 2 (familiar animal) while the PCB exposed groups decreased or did not change the amount of non-social time for this session. Entries into the different compartments can provide a measure of social preference in addition to general activity. No significant differences were obtained for either the more selective measures of social box (Fig. 2A) vs. non-social box entries (Fig. 2B) or for the general measure of total entries (Fig. 2C) during the sessions.
Fig. 1.
Time spent in investigation of a novel, familiar, and second novel conspecific. Each point and bar represents the mean ± SEM for either control (n = 18), PCB 12.5 (n = 15), and PCB 25 (n = 15) 21-day-old male rats. (A) Mean time spent in social investigation. (B)Meantime spent in non-social investigation. *p < 0.05 vs. first novel trial.
Fig. 2.
Number of entries into social compartment with a novel, familiar, and second novel conspecific and into a non-social compartment. Each point and bar represents the mean ± SEM for either control (n = 18), PCB 12.5 (n = 15), or PCB 25 (n = 15) 21-day-old male rats. (A) Number of entries into the social compartment. (B) Number of entries into the non-social compartment. (C) Total number of compartment entries. PCB did not alter total number of entries into either compartment or total number of entries per session.
3.3. Social investigation test
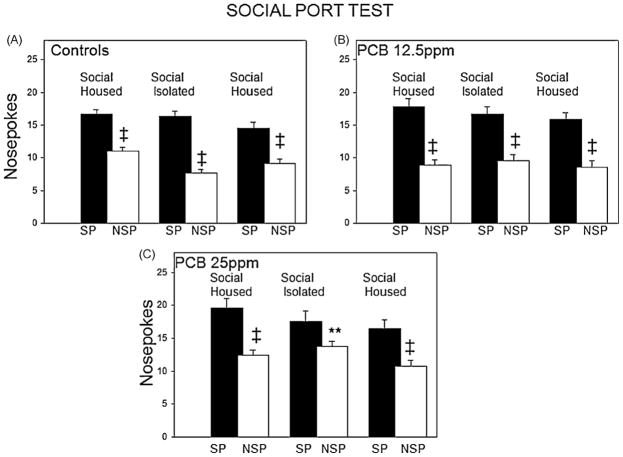
Sixty-two adult males were used in the social port test for investigative behavior (age range 90–121 days old; CONT, n = 30 from 6 litters; PCB 12.5, n = 14 from 4 litters; PCB 25, n = 18 from 4 litters). A significant main effect for condition (PCB exposure) was obtained (F(1,59) = 6.51, p < 0.01). A strong main effect for social housing experience was found as well (F(5,295) = 52.55, p < 0.001). Finally, a significant interaction between the condition and social experience factors was found (F(10,295) = 1.87, p < 0.05). To confirm the enhancement of social pokes after isolation, we completed the Bonferroni multiple test for within group comparisons. For the controls, a significantly greater number of nosepokes to the social port was found for each session and the greatest difference between the social and non-social port pokes was seen after isolation (Fig. 3A; p < 0.001 for each comparison). Similar but not has robust results were obtained for the PCB 12.5ppm exposure group (Fig. 3B; p < 0.01). Interestingly, the group exposed to the most PCB did not express a significant difference between social and nonsocial pokes following isolation (Fig. 3C; p = 1.00). We examined these effects more closely using the Tukey test for comparisons between the different groups. Significant differences were found between the greater dose of PCB (M= 13.7, 95% CI [12.2, 15.2]) and controls (M= 7.67, 95% CI [6.49, 8.84], p < 0.01) for the non-social nosepokes after social isolation. The PCB 25 exposed rats performed significantly more non-social directed pokes compared to controls after this condition (see Fig. 3). The PCB 12.5ppm subgroup was intermediate between the other subgroups and not significantly different in responsiveness compared to controls. The general trend was in the same direction as with the animals exposed to more PCB; all PCB exposed animals demonstrating more non-social port activity. However, comparisons between the two PCB dosage groups found that the PCB 25ppm group had significantly higher nonsocial port pokes after both social (PCB 12.5 ppm; M= 8.9, 95% CI [7.1, 10.6] vs. PCB 25 ppm; M= 12.4, 95% CI [10.9, 14], p < 0.05) and isolate housing experiences (PCB 12.5ppm (M= 9.57, 95% CI [7.8, 11.3], p < 0.01)).
Fig. 3.
Social port test results. Control animals demonstrated the preference for the social port side of the apparatus to make nosepokes and this preference increased following social isolation (A). PCB 12.5ppm group showed a preference for the social port which was similar across experiences (B). The animals exposed to the most PCB (PCB 25 ppm) also showed a preference for the social port but this preference was depressed relative to the other two groups after the 24 h of social isolation (C). ‡p < 0.05 for within group comparisons for the nosepokes and signifies the social port preference; **p < 0.05 for between group comparison (control vs. PCB 25 ppm).
3.4. PVN area measurement
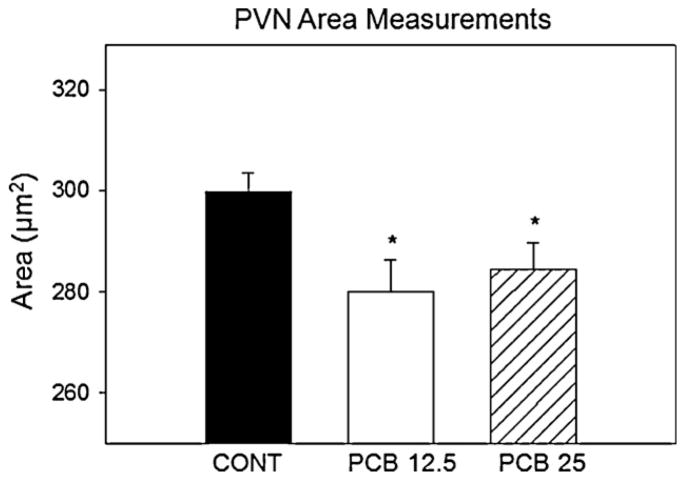
We measured the area of the periventricular nucleus of the hypothalamus using Nissl stained sections. The measures used were from the 48 animals tested in the social recognition test, sacrificed at 29 days of age. A significant difference for the average area was found among the three groups (F(2,47) = 4.60, p < 0.05). Pairwise comparisons were completed (Tukey test) and results showed that PCB exposed animals had a smaller area of PVN compared to controls (Fig. 4). The greatest reduction was the lesser PCB dose (M= 280.1, 95% CI [269.9, 290.4]) compared to controls (M= 300, 95% CI [290.5, 309.3], p < 0.05). The greater dose (PCB 25 ppm) animals showed a trend for a reduction in PVN area (M= 284.7, 95% CI [274.4, 294.9], p = 0.08) with no significant difference between the two PCB exposed groups (p = 0.807).
Fig. 4.
Normalized PVN area. Each point and bar represents the mean ± SEM for either control (n = 10), PCB 12.5 (n = 10), or PCB 25 (n = 10) young male rats. PCB exposure led to a significant decrease in PCN area as an average measured across anterior–posterior regions of the hypothalamus.
4. Discussion
4.1. PCB exposure and the importance of adaptive social behavior
Overall, the results from the two tests of social motivation support the idea that perinatal PCB exposure alters dynamic interactions of recognition and approach at different developmental time periods. The lack of typical habituation to the familiar conspecific at the juvenile stage signals potential deficits in the ability to alter approach behavior based upon short-term social memories separated by short intervals (Burman and Mendl, 1999, 2000). A potential reason that social recognition seen in the control group was not as strong as that shown in previous studies is that we used juvenile aged animals for both the test subject and the restrained subject in our study. Most previous work on social recognition using rats has used one juvenile and one adult animal and found more robust habituation–dishabituation (Thor and Holloway, 1982; Thor et al., 1982). The PCB-induced change in isolation-induced investigation in adult rats suggests that social behavior changes persist and remain modulated by experience. These results are not as straightforward as other behavioral work and at both developmental time periods, the behavioral changes are an interaction of specific sensitivities related to the developmental stage and effects on general processes related to stress, arousal and emotional behavior.
These types of general alterations following PCB exposure interacting with changes to specific social systems could disable vital social abilities leading to changes in behaviors used in diverse situations that include social learning (Sanchez-Andrade and Kendrick, 2009). For example, PCB exposure has been shown to alter reproductive behavior (Wang et al., 2002), general reproductive success (Donahue et al., 2002) partner preference (Cummings et al., 2008) and early social cue learning related to maternal care (Cromwell et al., 2007). Play behavior in animals is another very important social behavior (Ikemoto and Panksepp, 1992; Burghardt, 2005). A relationship between PCB exposure and play has been found in children (Vreugdenhil et al., 2002). Children with greater PCB exposure were more likely to have a gender-reversal in the type of play behavior expressed (Vreugdenhil et al., 2002). Exposure to other xenobiotics has been shown to alter social behavior including play (Bekkedal et al., 1999; Kelly and Tran, 1997; Korz and Gattermann, 1997). Additionally, neonates of women regularly consuming high levels of fish (>40 equivalent pounds of fish/lifetime) taken from Lake Ontario showed a significant decrement on performance on the National Behavioral Assessment Scale habituation cluster between an initial test (12–24 h) and a second test (25–48 h) after birth (Lonky et al., 1996). This result appears similar to deficits in habituation seen in young rats in the present study. Colbert et al. (2005) found perinatal exposure to low levels of vinclozoln changes social play and reproductive strategies in rats. Exposure to small amounts of lead or cadmium can alter social memory formation or play behavior in rat (Holloway and Thor, 1988, 1987). Without normal play behavior, cognitive and emotional deficits can arise and lead to greater behavioral impairments (van den Berg et al., 1999).
Explanations for how PCB may alter social behavior are diverse and depend on more investigation to identify possible causal factors. Previous work by our group has shown that perinatal PCB exposure does not seem to reduce non-social approach toward a familiar odor (Cromwell et al., 2007). In addition, we have been investigating motor competencies and have found that basic motor functions are generally intact in these juvenile and adult animals exposed to these congeners at this dosage as opposed to motor disruption in young rats exposed to the antithyroid substance thiouracil (Toth et al., 2009). Sensory deficits could be playing a role in the alterations observed. PCB has been shown to alter both the developmental trajectory of the auditory system (Goldey et al., 1995) and to alter certain properties of the olfactory system (Apfelbach et al., 1998). Vocalizations and olfactory cues are most likely crucial stimuli for the animal to receive and encode as social signals (Brudzynski, 2009; Tobin et al., 2010). One possible important environmental and social factor that could be involved is alteration of maternal care during early neonatal periods. Alterations in maternal care during early neonatal periods in rats have long terms consequences on behavioral and physiological functions (Cameron et al., 2005). Previous work has found that PCB exposure alters maternal care directed to the rat pups (Simmons et al., 2005). An elegant cross-fostering design showed that a subset of these changes persist in litters when the pups from a PCB treated mother are fostered to a control dam non-exposed to PCB (Cummings et al., 2005). This suggests that the behavior of the pups and motivation to the dam are altered in unique ways that might reflect toxicant exposure to the dam providing care. Signs that early care alterations may moderate PCB effects include the change in body weight during young adulthood. While PCB did not significantly alter pup body weight in the present study, there was a trend toward greater body weight in those exposed to the greater PCB concentration (25 ppm), as observed previously (Cromwell et al., 2007). Future investigation is necessary to unravel different explanations with regard to sensory and motor systems involved in these effects and the selective nature of the deficits for social-related interactions.
4.2. PCB, acute social isolation, and behavioral deficits
PCB alters a wide array of endocrine systems and neurochemicals in the brain and periphery (Corey et al., 1996). Surprisingly little research has been done examining how PCB exposure alters hormones such as oxytocin or vasopressin (Mlynarczuk and Kotwica, 2006; Coburn et al., 2007). Growing evidence supports an important role for these neuropeptides in social behavior in a broad range of species (Caldwell et al., 2008; Neumann, 2008; Popik and van Ree, 1991). We found that area of the hypothalamic PVN is reduced by the earlier PCB exposure in male rats with social recognition impairment. This result could be correlated with the effects of PCB on sexual maturation of reproductive behavior and steroid hormone systems related to the onset of this behavior (Ottinger et al., 2009; Steinberg et al., 2008; Lilienthal et al., 2006). Previous work has shown that exposure to a more complex PCB mixture (Aroclor 1254) than that used in the present study leads to morphological alterations of hippocampal formation (Pruitt et al., 1999). It is surprising that the greatest effects on behavior were observed at the highest dose exposure while the greatest effects on PVN size were observed for the lesser dose. One possibility is that PCB effects PVN size differently from the typical dose–response curve and instead works via the biological mechanism of hormesis (Phillips et al., 2008). This can lead to greater changes with exposure to lesser amounts of PCB rather than greater amounts and these changes may be part of an adaptive response to the toxicant effects. The connection between PVN changes and PCB suggests that processes related to oxytocin and vasopressin are candidates for future work. PVN region has been shown to be changed in several ways following PCB exposure. For example, indicators of hormone synthesis and release from PVN have been shown to be reduced after PCB administration (Coburn et al., 2007).
PCB exposure has been shown to alter several other hormone systems including sex steroid hormones (Goncharov et al., 2009; Murugesan et al., 2008), glucocorticoids (Meserve et al., 1992) and thyroid hormones (Donahue et al., 2004). Alterations to these systems could also impact social behavior and lead to persistent changes in investigation and approach and social interactions. One factor that could be influencing the present results could be an interaction between PCB exposure and the stress response following isolation (Zhao et al., 2009). Social isolation produces an elevation of the hormonal stress response via the hypothalamus–pituitary–adrenal (HPA) axis (Flak et al., 2009; Veenema, 2009), and Meserve et al. (1992) found gestational and lactational exposure to PCB to depress response of each segment of this axis in 15 day old rats. Regulation of emotional behavior and increased anxiety result from acute and chronic social isolation (Lapiz et al., 2001). Social memories are also altered by periods of isolation (Diergaarde et al., 2004). In the present study the animals were naïve to the isolation 24 h prior to the social recognition test. This isolation could have constituted a stressor that interacted with the previous PCB exposure to cause an increased arousal and deficit in familiarity-induced habituation. In a similar way, the social port test effect was seen only following isolation (24 h) and despite the isolation being relatively acute, it could combine with the effects of PCB-induced endocrine disruption to produce altered motivation or social learning. Orito et al. (2007) found that prenatal exposure to PCB (PCB126) produced an anxiogenic state in rats. The link between stress, PCB and behavior is likely complex yet very important to examine closely so that experimental work in toxicology is not confounded by factors such as housing experience (Mack et al., 2003).
4.3. Implications for human social disorders
PCBs as well as other toxicants have been proposed to play a role in human disease and emotional dysregulation. A recent study by Plusquellec et al. (2010) found that prenatal exposure to PCB (PCB 153) positively correlated with ratings of unhappiness and anxiety in Inuit preschoolers from Arctic, Quebec. Researchers have madea case for a relationship between toxicant exposure and social violence in humans (Hwang, 2007) suggesting that as toxicant entry into the environment is better regulated the level of violence has decreased substantially (Carpenter and Nevin, 2010). DeSoto (2009) noted that toxicant exposure is potentially a factor in the rise of social developmental disorders, such as autism and others (Kenet et al., 2007) have found PCB exposure to cause shifts in auditory processing in ways that could lead to social deficits. The changes in social behavior observed in the present study are similar to changes observed in some of the animal models of autism (Crawley, 2007; Presti-Torres et al., 2007; Harmon et al., 2008, 2009). Overall, examining how toxicant exposure alters crucial natural behaviors using controlled laboratory techniques is very important in the developing field of ‘ecotoxicology’ (Cohn and MacPhail, 1996). This fusion of natural observation and experimentation could reveal important insights into behavioral dynamics of development and provide an understanding of the ways toxicants are harmful and ways to possibly prevent this harm.
Footnotes
Conflict of interest statement
Nothing to declare.
References
- Apfelbach R, Engelhart A, Behnisch P, Hagenmaier H. The olfactory system as a portal of entry for airborne polychlorinated biphenyls (PCBs) to the brain? Arch Toxicol. 1998;72:314–317. doi: 10.1007/s002040050508. [DOI] [PubMed] [Google Scholar]
- Bekkedal MY, Rossi J, 3rd, Panksepp J. Fetal and neonatal exposure to trimethylolpropane phosphate alters rat social behavior and emotional responsivity. Neurotoxicol Teratol. 1999;21:435–443. doi: 10.1016/s0892-0362(99)00002-1. [DOI] [PubMed] [Google Scholar]
- Beyer A, Biziuk M. Environmental fate and global distribution of polychlorinated biphenyls. Rev Environ Contam Toxicol. 2009;201:137–158. doi: 10.1007/978-1-4419-0032-6_5. [DOI] [PubMed] [Google Scholar]
- Brudzynski SM. Communication of adult rats by ultrasonic vocalization: biological, sociobiological, and neuroscience approaches. ILAR J. 2009;50:43–50. doi: 10.1093/ilar.50.1.43. [DOI] [PubMed] [Google Scholar]
- Burghardt GM. The Genesis of Animal Play: Testing the Limits. MIT Press; Cambridge, MA: 2005. [Google Scholar]
- Burman OH, Mendl M. The effects of environmental context on laboratory rat social recognition. Anim Behav. 1999;58:629–634. doi: 10.1006/anbe.1999.1170. [DOI] [PubMed] [Google Scholar]
- Burman OH, Mendl M. Short-term social memory in the laboratory rat: its susceptibility to disturbance. Appl Anim Behav Sci. 2000;67:241–254. doi: 10.1016/s0168-1591(99)00120-3. [DOI] [PubMed] [Google Scholar]
- Caldwell HK, Lee HJ, Macbeth AH, Young WS., 3rd Vasopressin: behavioral roles of an “original” neuropeptide. Prog Neurobiol. 2008;84:1–24. doi: 10.1016/j.pneurobio.2007.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron NM, Champagne FA, Parent C, Fish EW, Ozaki-Kuroda K, Meaney MJ. The programming of individual differences in defensive responses and reproductive strategies in the rat through variations in maternal care. Neurosci Biobehav Rev. 2005;29:843–865. doi: 10.1016/j.neubiorev.2005.03.022. [DOI] [PubMed] [Google Scholar]
- Carpenter DO. Polychlorinated biphenyls (PCBs): routes of exposure and effects on human health. Rev Environ Health. 2006;21:1–23. doi: 10.1515/reveh.2006.21.1.1. [DOI] [PubMed] [Google Scholar]
- Carpenter DO, Nevin R. Environmental causes of violence. Physiol Behav. 2010;99:260–268. doi: 10.1016/j.physbeh.2009.09.001. [DOI] [PubMed] [Google Scholar]
- Chung YW, Nunez AA, Clemens LG. Effects of neonatal polychlorinated biphenyl exposure on female sexual behavior. Physiol Behav. 2001;74:363–370. doi: 10.1016/s0031-9384(01)00579-0. [DOI] [PubMed] [Google Scholar]
- Coburn CG, Curras-Collazo MC, Kodavanti PR. Polybrominated diphenyl ethers and ortho-substituted polychlorinated biphenyls as neuroendocrine disruptors of vasopressin release: effects during physiological activation in vitro and structure–activity relationships. Toxicol Sci. 2007;98:178–186. doi: 10.1093/toxsci/kfm086. [DOI] [PubMed] [Google Scholar]
- Cohn J, MacPhail RC. Ethological and experimental approaches to behavior analysis: implications for ecotoxicology. Environ Health Perspect. 1996;104 (Suppl 2):299–305. doi: 10.1289/ehp.96104s2299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colbert NK, Pelletier NC, Cote JM, Concannon JB, Jurdak NA, Minott SB, Markowski VP. Perinatal exposure to low levels of the environmental antiandrogen vinclozolin alters sex-differentiated social play and sexual behaviors in the rat. Environ Health Perspect. 2005;113:700–707. doi: 10.1289/ehp.7509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colciago A, Casati L, Mornati O, Vergoni AV, Santagostino A, Celotti F, Negri-Cesi P. Chronic treatment with polychlorinated biphenyls (PCB) during pregnancy and lactation in the rat. Part 2: Effects on reproductive parameters, on sex behavior, on memory retention and on hypothalamic expression of aromatase and 5alpha-reductases in the offspring. Toxicol Appl Pharmacol. 2009;239:46–54. doi: 10.1016/j.taap.2009.04.023. [DOI] [PubMed] [Google Scholar]
- Corey DA, Juarez de Ku LM, Bingman VP, Meserve LA. Effects of exposure to polychlorinated biphenyl (PCB) from conception on growth, and development of endocrine, neurochemical, and cognitive measures in 60 day old rats. Growth Dev Aging. 1996;60:131–143. [PubMed] [Google Scholar]
- Crawley JN. Mouse behavioral assays relevant to the symptoms of autism. Brain Pathol. 2007;17:448–459. doi: 10.1111/j.1750-3639.2007.00096.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cromwell HC, Johnson A, McKnight L, Horinek M, Asbrock C, Burt S, Jolous-Jamshidi B, Meserve LA. Effects of polychlorinated biphenyls on maternal odor conditioning in rat pups. Physiol Behav. 2007;91:658–666. doi: 10.1016/j.physbeh.2007.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings JA, Nunez AA, Clemens LG. A cross-fostering analysis of the effects of PCB 77 on the maternal behavior of rats. Physiol Behav. 2005;85:83–91. doi: 10.1016/j.physbeh.2005.04.001. [DOI] [PubMed] [Google Scholar]
- Cummings JA, Clemens LG, Nunez AA. Exposure to PCB 77 affects partner preference but not sexual behavior in the female rat. Physiol Behav. 2008;95:471–475. doi: 10.1016/j.physbeh.2008.07.016. [DOI] [PubMed] [Google Scholar]
- DeSoto MC. Ockham’s Razor and autism: the case for developmental neurotoxins contributing to a disease of neurodevelopment. Neurotoxicology. 2009;30:331–337. doi: 10.1016/j.neuro.2009.03.003. [DOI] [PubMed] [Google Scholar]
- Diergaarde L, Gerrits MA, Stuy A, Spruijt BM, van Ree JM. Neonatal amygdala lesions and juvenile isolation in the rat: differential effects on locomotor and social behavior later in life. Behav Neurosci. 2004;118:298–305. doi: 10.1037/0735-7044.118.2.298. [DOI] [PubMed] [Google Scholar]
- Domingo JL, Bocio A. Levels of PCDD/PCDFs and PCBs in edible marine species and human intake: a literature review. Environ Int. 2007;33:397–405. doi: 10.1016/j.envint.2006.12.004. [DOI] [PubMed] [Google Scholar]
- Donahue DA, Bowen CL, Provost TL, Merserve LA. Effects of PCB on reproductive success in Sprague–Dawley rats exposed to Aroclor 1254 for one year. Ohio J Sci. 2002;102:102–105. [Google Scholar]
- Donahue DA, Dougherty EJ, Meserve LA. Influence of a combination of two tetrachlorobiphenyl congeners (PCB 47; PCB 77) on thyroid status, choline acetyltransferase (ChAT) activity, and short- and long-term memory in 30-day-old Sprague–Dawley rats. Toxicology. 2004;203:99–107. doi: 10.1016/j.tox.2004.06.011. [DOI] [PubMed] [Google Scholar]
- Faroon O, Jones D, de Rosa C. Effects of polychlorinated biphenyls on the nervous system. Toxicol Ind Health. 2001;16:305–333. doi: 10.1177/074823370001600708. [DOI] [PubMed] [Google Scholar]
- Flak JN, Ostrander MM, Tasker JG, Herman JP. Chronic stress-induced neurotransmitter plasticity in the PVN. J Comp Neurol. 2009;517:156–165. doi: 10.1002/cne.22142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldey ES, Kehn LS, Lau C, Rehnberg GL, Crofton KM. Developmental exposure to polychlorinated biphenyls (Aroclor 1254) reduces circulating thyroid hormone concentrations and causes hearing deficits in rats. Toxicol Appl Pharmacol. 1995;135:77–88. doi: 10.1006/taap.1995.1210. [DOI] [PubMed] [Google Scholar]
- Goncharov A, Rej R, Negoita S, Schymura M, Santiago-Rivera A, Morse G, Carpenter DO. Lower serum testosterone associated with elevated polychlorinated biphenyl concentrations in Native American men. Environ Health Perspect. 2009;117:1454–1460. doi: 10.1289/ehp.0800134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gore AC. Environmental toxicant effects on neuroendocrine function. Endocrine. 2001;14:235–246. doi: 10.1385/ENDO:14:2:235. [DOI] [PubMed] [Google Scholar]
- Harmon KM, Cromwell HC, Burgdorf J, Moskal JR, Brudzynski SM, Kroes RA, Panksepp J. Rats selectively bred for low levels of 50 kHz ultrasonic vocalizations exhibit alterations in early social motivation. Dev Psychobiol. 2008;50:322–331. doi: 10.1002/dev.20294. [DOI] [PubMed] [Google Scholar]
- Harmon KM, Greenwald ML, McFarland A, Beckwith T, Cromwell HC. The effects of prenatal stress on motivation in the rat pup. Stress. 2009;12:250–258. doi: 10.1080/10253890802367265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holloway WR, Jr, Thor DH. Low level lead exposure during lactation increases rough and tumble play fighting of juvenile rats. Neurotoxicol Teratol. 1987;9:51–57. doi: 10.1016/0892-0362(87)90070-5. [DOI] [PubMed] [Google Scholar]
- Holloway WR, Jr, Thor DH. Social memory deficits in adult male rats exposed to cadmium in infancy. Neurotoxicol Teratol. 1988;10:193–197. doi: 10.1016/0892-0362(88)90017-7. [DOI] [PubMed] [Google Scholar]
- Hwang L. Environmental stressors and violence: lead and polychlorinated biphenyls. Rev Environ Health. 2007;22:313–328. doi: 10.1515/reveh.2007.22.4.313. [DOI] [PubMed] [Google Scholar]
- Ikemoto S, Panksepp J. The effects of early social isolation on the motivation for social play in juvenile rats. Dev Psychobiol. 1992;25:261–274. doi: 10.1002/dev.420250404. [DOI] [PubMed] [Google Scholar]
- Jonsson HT, Jr, Keil JE, Gaddy RG, Loadholt CB, Hennigar GR, Walker EM., Jr Prolonged ingestion of commercial DDT and PCB; effects on progesterone levels and reproduction in the mature female rat. Arch Environ Contam Toxicol. 1975;3:479–490. doi: 10.1007/BF02220818. [DOI] [PubMed] [Google Scholar]
- Kelly SJ, Tran TD. Alcohol exposure during development alters social recognition and social communication in rats. Neurotoxicol Teratol. 1997;19:383–389. doi: 10.1016/s0892-0362(97)00064-0. [DOI] [PubMed] [Google Scholar]
- Kenet T, Froemke RC, Schreiner CE, Pessah IN, Merzenich MM. Perinatal exposure to a noncoplanar polychlorinated biphenyl alters tonotopy, receptive fields, and plasticity in rat primary auditory cortex. Proc Natl Acad Sci USA. 2007;104:7646–7651. doi: 10.1073/pnas.0701944104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan MA, Lichtensteiger CA, Faroon O, Mumtaz M, Schaeffer DJ, Hansen LG. The hypothalamo-pituitary-thyroid (HPT) axis: a target of nonpersistent ortho-substituted PCB congeners. Toxicol Sci. 2002;65:52–61. doi: 10.1093/toxsci/65.1.52. [DOI] [PubMed] [Google Scholar]
- Korz V, Gattermann R. Behavioral alterations in male golden hamsters exposed to chlorodibromomethane. Pharmacol Biochem Behav. 1997;58:643–647. doi: 10.1016/s0091-3057(97)00076-2. [DOI] [PubMed] [Google Scholar]
- La Rocca C, Mantovani A. From environment to food: the case of PCB. Ann Ist Super Sanita. 2006;42:410–416. [PubMed] [Google Scholar]
- Lapiz MD, Fulford A, Muchimapura S, Mason R, Parker T, Marsden CA. Influence of postweaning social isolation in the rat on brain development, conditioned behaviour and neurotransmission. Ross Fiziol Zh Im I M Sechenova. 2001;87:730–751. [PubMed] [Google Scholar]
- Lee HJ, Caldwell HK, Macbeth AH, Tolu SG, Young WS., 3rd A conditional knockout mouse line of the oxytocin receptor. Endocrinology. 2008;149:3256–3263. doi: 10.1210/en.2007-1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lilienthal H, Hack A, Roth-Härer A, Grande SW, Talsness CE. Effects of developmental exposure to 2,2′,4,4′,5-pentabromodiphenyl ether (PBDE-99) on sex steroids, sexual development, and sexually dimorphic behavior in rats. Environ Health Perspect. 2006;114:194–201. doi: 10.1289/ehp.8391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lonky E, Reihman J, Darvill T, Mather J, Sr, Daly H. Neonatal behavioral assessment scale performance in humans influenced by maternal consumption of environmentally contaminated Lake Ontario fish. J Great Lakes Res. 1996;22:198–212. [Google Scholar]
- Mack PA, Bell RM, Tubo BL, Ashline JA, Smiler KL. Validation study of social housing of canines in toxicology studies. Contemp Top Lab Anim Sci. 2003;42:29–30. [PubMed] [Google Scholar]
- Meserve LA, Murray BA, Landis JA. Influence of maternal ingestion of Aroclor 1254 (PCB) or FireMaster BP-6 (PBB) on unstimulated and stimulated corticosterone levels in young rats. Bull Environ Contam Toxicol. 1992;48:715–720. doi: 10.1007/BF00195992. [DOI] [PubMed] [Google Scholar]
- Mlynarczuk J, Kotwica J. Influence of polychlorinated biphenyls on LH-stimulated secretion of progestereone and oxytocin from bovine luteal cells. Pol J Vet Sci. 2006;9:101–108. [PubMed] [Google Scholar]
- Murugesan P, Muthusamy T, Balasubramanian K, Arunakaran J. Polychlorinated biphenyl (Aroclor 1254) inhibits testosterone biosynthesis and antioxidant enzymes in cultured rat Leydig cells. Reprod Toxicol. 2008;25:447–454. doi: 10.1016/j.reprotox.2008.04.003. [DOI] [PubMed] [Google Scholar]
- Neumann ID. Brain oxytocin: a key regulator of emotional and social behaviours in both females and males. J Neuroendocrinol. 2008;20:8588–8665. doi: 10.1111/j.1365-2826.2008.01726.x. [DOI] [PubMed] [Google Scholar]
- Orito K, Gotanda N, Murakami M, Ikeda T, Egashira N, Mishima K, Fujiwara M. Prenatal exposure to 3,3′,4,4′,5-pentachlorobiphenyl (PCB126) promotes anxiogenic behavior in rats. Tohoku J Exp Med. 2007;212:151–157. doi: 10.1620/tjem.212.151. [DOI] [PubMed] [Google Scholar]
- Ottinger MA, Lavoie ET, Abdelnabi M, Quinn MJ, Jr, Marcell A, Dean K. An overview of dioxin-like compounds. PCB, and pesticide exposures associated with sexual differentiation of neuroendocrine systems, fluctuating asymmetry, and behavioral effects in birds. J Environ Sci Health C: Environ Carcinog Ecotoxicol Rev. 2009;27:286–300. doi: 10.1080/10590500903310229. [DOI] [PubMed] [Google Scholar]
- Patisaul HB, Adewale HB. Long-term effects of environmental endocrine disruptors on reproductive physiology and behavior. Front Behav Neurosci. 2009;3:10. doi: 10.3389/neuro.08.010.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips KP, Foster WG, Leiss W, Sahni V, Karyakina N, Turner MC, Kacew S, Krewski D. Assessing and managing risks arising from exposure to endocrine-active chemicals. J Toxicol Environ Health B: Crit Rev. 2008;11:351–372. doi: 10.1080/10937400701876657. [DOI] [PubMed] [Google Scholar]
- Plusquellec P, Muckle G, Dewailly E, Ayotte P, Begin G, Desrosiers C, Despres C, Saint-Amour D, Poitras K. The relation of environmental contaminants exposure to behavioral indicators in Inuit preschoolers in Arctic Quebec. Neurotoxicology. 2010;31:17–25. doi: 10.1016/j.neuro.2009.10.008. [DOI] [PubMed] [Google Scholar]
- Popik P, van Ree JM. Oxytocin but not vasopressin facilitates social recognition following injection into the medial preoptic area of the rat brain. Eur Neuropsychopharmacol. 1991;1:555–560. doi: 10.1016/0924-977x(91)90010-r. [DOI] [PubMed] [Google Scholar]
- Presti-Torres J, de Lima MN, Scalco FS, Caldana F, Garcia VA, Guimaraes MR, Schwartsmann G, Roesler R, Schroder N. Impairments of social behavior and memory after neonatal gastrin-releasing peptide receptor blockade in rats: implications for an animal model of neurodevelopmental disorders. Neuropharmacology. 2007;52:724–732. doi: 10.1016/j.neuropharm.2006.09.020. [DOI] [PubMed] [Google Scholar]
- Provost TL, Juarez de Ku LM, Zender C, Meserve LA. Dose- and age-dependent alterations in choline acetyltransferase (ChAT) activity, learning and memory, and thyroid hormones in 15- and 30-day old rats exposed to 1.25 or 12.5 PPM polychlorinated biphenyl (PCB) beginning at conception. Prog Neuropsychopharmacol Biol Psychiatry. 1999;23:915–928. doi: 10.1016/s0278-5846(99)00035-4. [DOI] [PubMed] [Google Scholar]
- Pruitt DL, Meserve LA, Bingman VP. Reduced growth of intra- and infrapyramidal mossy fibers is produced by continuous exposure to polychlorinated biphenyl. Toxicology. 1999;138:11–17. doi: 10.1016/s0300-483x(99)00073-6. [DOI] [PubMed] [Google Scholar]
- Roegge CS, Schantz SL. Motor function following developmental exposure to PCBS and/or MEHG. Neurotoxicol Teratol. 2006;28:260–277. doi: 10.1016/j.ntt.2005.12.009. [DOI] [PubMed] [Google Scholar]
- Sanchez-Andrade G, Kendrick KM. The main olfactory system and social learning in mammals. Behav Brain Res. 2009;200:323–335. doi: 10.1016/j.bbr.2008.12.021. [DOI] [PubMed] [Google Scholar]
- Schecter A, Colacino J, Haffner D, Patel K, Opel M, Papke O, Birnbaum L. Perfluorinated compounds, polychlorinated biphenyls, and organochlorine pesticide contamination in composite food samples from Dallas, Texas, USA. Environ Health Perspect. 2010;118:796–802. doi: 10.1289/ehp.0901347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seegal RF, Brosch KO, Okoniewski RJ. Coplanar PCB congeners increase uterine weight and frontal cortical dopamine in the developing rat: implications for developmental neurotoxicity. Toxicol Sci. 2005;86:125–131. doi: 10.1093/toxsci/kfi174. [DOI] [PubMed] [Google Scholar]
- Sekiguchi R, Wolterink G, van Ree JM. Analysis of the influence of vasopressin neuropeptides on social recognition of rats. Eur Neuropsychopharmacol. 1991;1:123–126. doi: 10.1016/0924-977x(91)90713-5. [DOI] [PubMed] [Google Scholar]
- Shields PG. Understanding population and individual risk assessment: the case of polychlorinated biphenyls. Cancer Epidemiol Biomarkers Prev. 2006;15:830–839. doi: 10.1158/1055-9965.EPI-06-0222. [DOI] [PubMed] [Google Scholar]
- Simmons SL, Cummings JA, Clemens LG, Nunez AA. Exposure to PCB 77 affects the maternal behavior of rats. Physiol Behav. 2005;84:81–86. doi: 10.1016/j.physbeh.2004.10.022. [DOI] [PubMed] [Google Scholar]
- Spiteri T, Agmo A. Ovarian hormones modulate social recognition in female rats. Physiol Behav. 2009;98:247–250. doi: 10.1016/j.physbeh.2009.05.001. [DOI] [PubMed] [Google Scholar]
- Steinberg RM, Juenger TE, Gore AC. The effects of prenatal PCBs on adult female paced mating reproductive behaviors in rats. Horm Behav. 2007;51:364–372. doi: 10.1016/j.yhbeh.2006.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg RM, Walker DM, Juenger TE, Woller MJ, Gore AC. Effects of perinatal polychlorinated biphenyls on adult female rat reproduction: development, reproductive physiology, and second generational effects. Biol Reprod. 2008;78:1091–1101. doi: 10.1095/biolreprod.107.067249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart PW, Lonky E, Reihman J, Pagano J, Gump BB, Darvill T. The relationship between prenatal PCB exposure and intelligence (IQ) in 9-year-old children. Environ Health Perspect. 2008;116:1416–1422. doi: 10.1289/ehp.11058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thor DH, Holloway WR. Persistence of social investigatory behavior in the male rat: evidence for long-term memory of initial copulatory experience. Anim Learn Behav. 1981;9:561–565. [Google Scholar]
- Thor DH, Holloway WR. Social memory of the male laboratory rat. J Comp Psychol. 1982;96:1000–1006. [Google Scholar]
- Thor DH, Holloway WR., Jr Testosterone dependent effects of methylphenidate on social investigatory behavior of the rat. Physiol Behav. 1986;37:869–873. [PubMed] [Google Scholar]
- Thor DH, Wainwright KL, Holloway WR. Persistence of attention to a novel conspecific: some developmental variables in laboratory rats. Dev Psychobiol. 1982;15:1–8. doi: 10.1002/dev.420150102. [DOI] [PubMed] [Google Scholar]
- Tilson HA, Kodavanti PR. The neurotoxicity of polychlorinated biphenyls. Neurotoxicology. 1998;19:517–525. [PubMed] [Google Scholar]
- Tobin VA, Hashimoto H, Wacker DW, Takayanagi Y, Langnaese K, Caquineau C, Noack J, Landgraf R, Onaka T, Leng G, Meddle SL, Engelmann M, Ludwig M. An intrinsic vasopressin system in the olfactory bulb is involved in social recognition. Nature. 2010;464:413–417. doi: 10.1038/nature08826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toth CL, Hiler KA, Smith WL, Pena S, Cromwell HC, Meserve LA. Comparison between PCB and thiouracil exposure: a behavioral study. Proc. 91st Ann. Soc. Endocrinol. Mtg; 2009. pp. 2–69. [Google Scholar]
- van den Berg CL, Hol T, Van Ree JM, Spruijt BM, Everts H, Koolhaas JM. Play is indispensable for an adequate development of coping with social challenges in the rat. Dev Psychobiol. 1999;34:129–138. [PubMed] [Google Scholar]
- Veenema AH. Early life stress, the development of aggression and neuroendocrine and neurobiological correlates: what can we learn from animal models? Front Neuroendocrinol. 2009;30:497–518. doi: 10.1016/j.yfrne.2009.03.003. [DOI] [PubMed] [Google Scholar]
- Vreugdenhil HJ, Slijper FM, Mulder PG, Weisglas-Kuperus N. Effects of perinatal exposure to PCBs and dioxins on play behavior in Dutch children at school age. Environ Health Perspect. 2002;110:A593–A598. doi: 10.1289/ehp.021100593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Yu G, Cascio C, Liu Y, Gingrich B, Insel TR. Dopamine D2 receptor-mediated regulation of partner preferences in female prairie voles (Microtus ochrogaster): a mechanism for pair bonding? Behav Neurosci. 1999;113:602–611. doi: 10.1037//0735-7044.113.3.602. [DOI] [PubMed] [Google Scholar]
- Wang XQ, Fang J, Nunez AA, Clemens LG. Developmental exposure to polychlorinated biphenyls affects sexual behavior of rats. Physiol Behav. 2002;75:689–696. doi: 10.1016/s0031-9384(02)00673-x. [DOI] [PubMed] [Google Scholar]
- Widholm JJ, Villareal S, Seegal RF, Schantz SL. Spatial alternation deficits following developmental exposure to Aroclor 1254 and/or methylmercury in rats. Toxicol Sci. 2004;82:577–589. doi: 10.1093/toxsci/kfh290. [DOI] [PubMed] [Google Scholar]
- Zhao X, Sun L, Jia H, Meng Q, Wu S, Li N, He S. Isolation rearing induces social and emotional function abnormalities and alters glutamate and neurodevelopment-related gene expression in rats. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33:1173–1177. doi: 10.1016/j.pnpbp.2009.06.016. [DOI] [PubMed] [Google Scholar]