Abstract
Interpretation of bone mineral density (BMD) results in premenopausal women is particularly challenging, since the relationship between BMD and fracture risk is not the same as for postmenopausal women. In most cases, Z scores rather than T scores should be used to define “low BMD” in premenopausal women. The finding of low BMD in a premenopausal woman should prompt thorough evaluation for secondary causes of bone loss. If a secondary cause is found, management should focus on treatment of this condition. In a few cases where the secondary cause cannot be eliminated, treatment with a bone active agent to prevent bone loss should be considered. In women with no fractures and no known secondary cause, low BMD is associated with microarchitectural defects similar to young women with fractures; however, no longitudinal data are available to allow use of BMD to predict fracture risk. BMD is likely to be stable in these women with isolated low BMD, and pharmacologic therapy is rarely necessary. Assessment of markers of bone turnover and follow-up bone density measurements can help to identify those with an ongoing process of bone loss that may indicate a higher risk for fracture, and possible need for pharmacologic intervention.
Keywords: Premenopausal Osteoporosis, Bisphosphonates, Teriparatide
Introduction
The finding of low bone mineral density (BMD) in a premenopausal woman presents specific diagnostic and management challenges that are quite different from postmenopausal women. In this review, we will discuss BMD interpretation in young women, epidemiology of fracture risk in premenopausal women, evaluation of premenopausal women with low BMD, and management issues that pertain to this particular population. This article serves as an update to prior reviews of this topic [1–3].
BMD testing in premenopausal women
The International Society for Clinical Densitometry (ISCD) recommends BMD testing in premenopausal women with a history of fragility fracture(s) and in those with a known secondary cause of osteoporosis or bone loss [4]. However, routine bone density screening in healthy premenopausal women is not recommended. This is because there are no prospective studies relating BMD by dual energy X-ray absorptiometry (DXA) to incident fractures in premenopausal women. Thus, unlike postmenopausal women, there are no data to support the use of BMD measurements to predict short-term fracture risk or to guide therapeutic decisions (see below) in healthy premenopausal women. Regardless of this, many healthy women are referred for BMD testing for various reasons. When results are lower than expected, these women may consult their physician for evaluation and advice regarding treatment options.
BMD and the diagnosis of “osteoporosis” in premenopausal women
In postmenopausal women, osteoporosis and osteopenia may be diagnosed based on BMD T scores measured by DXA, with or without the presence of a fragility fracture. Low BMD T scores predict future fractures in postmenopausal women.
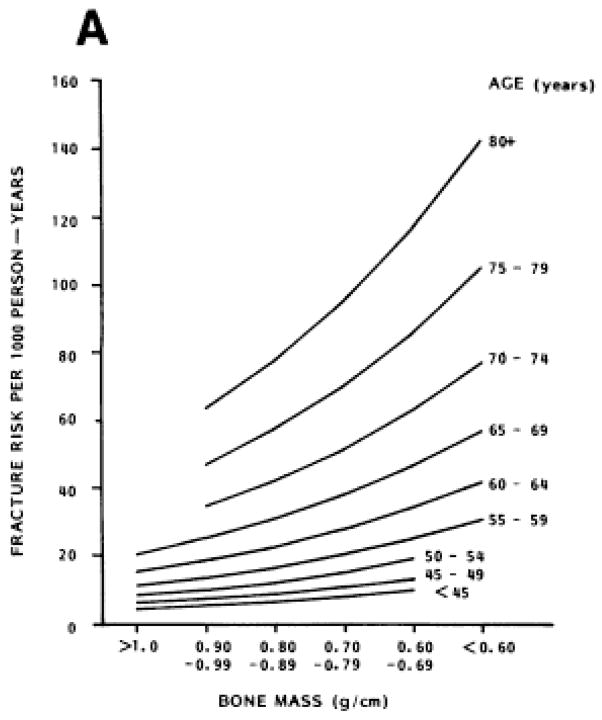
However, in a premenopausal woman, low BMD does not have the same clinical implications. In premenopausal women, the incidence and prevalence of fractures is orders of magnitude lower than in postmenopausal women [5, 6]. As seen in Figure 1, the relationship between BMD (using an older single-photon technique) and fracture risk is not the same in premenopausal and postmenopausal women and fracture incidence rates are low, even in those premenopausal women with low BMD measurements [7]. BMD by the currently-used DXA technique does have some relationship to fracture risk in the premenopausal years, but this relationship has been examined only in cross-sectional studies that have reported lower BMD by DXA in those with fractures. For example, premenopausal women with Colles fractures have been found to have significantly lower BMD at the non-fractured radius [8], lumbar spine, and femoral neck [9] than controls without fractures. Female military recruits with stress fractures were also found to have lower BMD than controls [10–12]. No prospective study has related BMD by DXA to incident fracture risk in premenopausal women.
Figure 1.
Reprinted with permission from [7]
Radial bone mass was measured using single photon absorptiometry in this 6.5 year prospective study of 521 Caucasian women.
For these reasons, the ISCD does not recommend using T scores to categorize BMD measurements in most premenopausal women. Even though T and Z scores are similar in young individuals, the ISCD recommends use of Z scores, which compare a young woman’s BMD to the mean of an age-, gender-, and ethnicity-matched reference population [4]. Young women with BMD Z scores below −2.0 should be categorized as having BMD that is “below expected range for age” and those with Z scores above −2.0 should be categorized as having BMD that is “within the expected range for age”[4]. The diagnostic category of “osteopenia” based upon BMD T scores should not be used in premenopausal women. The ISCD [4] and others[13–16], have recommended that young, otherwise healthy women should not be diagnosed with osteoporosis solely on the basis of low BMD by DXA, unless there is a history of fragility fracture or a secondary cause of osteoporosis.
There are a few circumstances for which some organizations recommend the use of T scores. The ISCD suggests that T score criteria be applied to perimenopausal women [4]. Additionally, the International Osteoporosis Foundation (IOF) recommends maintaining the T score −2.5 cutoff at the spine or hip for the diagnosis of osteoporosis in those young adults who have completed growth, and who suffer from a chronic disorder known to affect bone mass (an ongoing secondary cause) [17].
Isolated (idiopathic) low bone mineral density and bone structure in premenopausal women
Women with low BMD without a history of adult low trauma fracture and without a known cause of bone loss can be said to have idiopathic low BMD [18]. Based upon current ISCD and IOF recommendations, such women would not be considered to have “osteoporosis”.
DXA is a 2-dimensional technique that measures areal BMD and is affected by bone size. Thus, areal BMD measurements may be low in individuals with smaller bones [19]. This raises the important question of whether otherwise healthy young women with low areal BMD by DXA and no fractures truly have low volumetric BMD or reduced bone strength [2, 13, 20, 21]. Our three dimensional bone imaging and transiliac bone biopsy studies have addressed this question at multiple skeletal sites; we have shown that healthy, normally menstruating, premenopausal women with unexplained low BMD and no adult low trauma fractures have microarchitectural disruption (thinner cortices, thinner, more widely spaced and heterogeneously distributed trabeculae) and lower estimated bone strength that is comparable to a concurrently recruited cohort of premenopausal women with low trauma fractures. These commonalities remained even after correcting for the smaller bone size of those with low BMD but no fractures [18, 22, 23]. However, this study was limited by a small sample size and the possibility of ascertainment bias, since a high proportion of those in the low aBMD group had a family history of osteoporosis (84%), childhood fractures (26%) or high trauma adult fractures (16%) which may have led them to volunteer for the study. Although these results may not be generalizable to all premenopausal women with low aBMD by DXA, they suggest that very low BMD may represent a pre-symptomatic phase of osteoporosis in young women [18, 22, 23]. Similar results were reported in a group of premenopausal women with constitutional thinness (BMI < 16.5 kg/m2, normal menses and no known systemic disease), who were found to have low BMD, smaller bone size, and lower estimated breaking strength in comparison to normal women [24].
Even though the currently available data suggest that young women with idiopathic low BMD and no history of fracture are likely to have abnormal bone microarchitecture that is consistent with osteoporosis, this does not mean that low BMD measurements should be used to make therapeutic decisions in premenopausal women in the absence of a fracture history, as is common practice in postmenopausal women. This is because (1) there are no longitudinal data available to allow us to use BMD by DXA to predict short term risk of fracture in premenopausal women, (2) fracture risk increases greatly with age and for this reason is generally much lower in premenopausal women, and (3) the risks and benefits of osteoporosis medications may differ in premenopausal as compared postmenopausal women. Moreover, few studies have addressed risks and benefits of these medications in premenopausal women (see below).
Special Issues Related to Interpretation and Evaluation of Low BMD Measurements in Premenopausal Women
Peak bone mass is defined as the maximum BMD achieved by age 30, as measured by DXA. In healthy girls, the peak period of bone mass gain occurs between ages 11 and 14 [25]. Although approximately 95% of peak bone mass is acquired by the late teens, there are small gains from ages 20 to 29 [25–27]. Therefore, when interpreting low BMD measurements in premenopausal women under age 30, one must consider the possibility that they may not yet have reached their peak bone mass.
Everyone attains a peak bone density, but not everyone attains an optimal peak bone density. Genetic, nutritional and environmental factors clearly affect the peak of BMD attained: Women with genetically determined low peak bone mass may have no evidence of excessive bone loss. Low peak bone mass can also result from a past nutritional, environmental, or medication-related insult to bone – one that is no longer operative, and no longer leading to an active measurable process of bone loss at the time of evaluation in adulthood. Alternatively, some women may have a persistent cause of bone fragility and bone loss that could lead to low peak bone mass as well as ongoing bone loss in adulthood.
There are expected changes in bone mass associated with both pregnancy and lactation. Small decreases in bone density are expected during pregnancy and lactation is associated with rapid bone loss of 3–10% over 3–6 months [28, 29], with recovery of bone mass expected over 6–12 months, thereafter. Therefore, when interpreting a low BMD measurement in a premenopausal woman, the clinician must take into account the timing of recent pregnancy and lactation.
Low trauma fracture and the diagnosis of “osteoporosis” in premenopausal women
A low trauma fracture is defined as that which occurs with force equivalent to a fall from a standing height or less. This definition leaves some room for interpretation by the patient and the clinician, and the “low trauma” categorization of a fracture may not be clear in all cases. In each case of unusual fracture, the diagnosis of osteomalacia should be considered and other causes of pathologic fracture (eg malignancy, bone lesion) should be ruled out. Those premenopausal women with a history of one or more low-trauma fractures and no other pathology are defined as having osteoporosis, even if their BMD Z scores are not frankly low (i.e., <−2.0). These women have clinical evidence of decreased bone strength.
Several studies have shown that risk of a postmenopausal fracture is increased in women who have experienced a fracture before menopause[30–32]. In the Study of Osteoporotic Fractures (SOF), women with a history of premenopausal fracture were 35 percent more likely to fracture during the early postmenopausal years than women without a history of premenopausal fracture [31], even after controlling for a number of potential confounding variables. These findings suggest that certain life-long traits, perhaps including fall frequency, neuromuscular protective response to falls, bone mass, or various aspects of bone quality can affect the life-long risk of fracture [32].
Pathological processes (secondary causes) of low BMD and low-trauma fractures in premenopausal women
The majority of premenopausal women with low BMD measurements or low-trauma fractures have an underlying disorder or medication exposure that has interfered with bone mass accrual during adolescence and/or has caused excessive bone loss after peak bone mass has been reached. The same “secondary causes” may be associated with bone loss in older women. In a population study from Olmstead County, Minnesota, 90% of men and women aged 20–44 with established osteoporosis (i.e., fractures) were found to have a secondary cause [33]. In contrast, several other case series records of young women with osteoporosis evaluated in tertiary referral centers have found that only 44–56% have secondary causes. However, this discrepancy likely reflects referral bias of more obscure cases to specialists [34–36].
Potential secondary causes are listed in Table 1. The main goal of the clinician who encounters a premenopausal woman with low BMD measurements is to diagnose and treat any correctable contributing secondary cause. Often this can be accomplished by performing a detailed history and physical examination, but in some circumstances an exhaustive biochemical evaluation is necessary.
Table 1.
Secondary causes of osteoporosis in premenopausal women
| Any disease that affects skeletal development or bone mass acquisition during puberty and adolescence. |
Connective tissue diseases
|
Premenopausal amenorrhea/estrogen deficiency
|
Other endocrinopathies and abnormalities of calcium metabolism
|
Gastrointestinal/Nutritional
|
Inflammatory conditions
|
Other conditions:
|
Medications (not all have been studied in premenopausal populations)
|
Conditions with potential effects:
|
Evaluation
The cornerstone of the evaluation is a careful medical history. The physician should elicit information about family history, fractures, kidney stones, amenorrhea or evidence of premenopausal estrogen deficiency, timing of any recent pregnancies and lactation, dieting and exercise behavior, subtle gastrointestinal symptoms (that may suggest celiac disease or other causes of malabsorption), and medications, including over-the-counter supplements. Physical examination can identify signs of Cushing’s syndrome, thyroid hormone excess, or connective tissue disorders (e.g., blue sclerae in some forms of osteogenesis imperfecta).
The laboratory evaluation (see Table 2) should also be aimed at identifying secondary causes of osteoporosis. Biochemical evidence of hyperthyroidism, hyperparathyroidism, Cushing’s syndrome, early menopause, renal or liver disease, celiac disease and other forms of malabsorption, or idiopathic/primary hypercalciuria should be sought.
Table 2.
Laboratory Evaluation in Premenopausal Women with Unexplained Low BMD [3]
Initial Laboratory Evaluation
|
Additional Laboratory Evaluation
|
Bone turnover markers are of uncertain utility in premenopausal women. Elevated bone turnover markers might suggest ongoing bone loss, a diagnosable secondary cause, or higher short-term risk of fracture. However, bone turnover markers also increase after a fracture, and, when bone turnover markers are assessed in women during very early adulthood, they may be elevated as a result of active bone accrual in that individual, and may not reflect a process of bone loss.
Transiliac crest bone biopsies may be useful in certain clinical scenarios when it is necessary to examine bone remodeling, rule out osteomalacia, differentiate between different types of renal osteodystrophy, or complete an examination for rare secondary causes. Measurement of the bone formation rate from a bone biopsy sample performed after tetracycline labeling may be a more precise index of bone remodeling activity than the serum or urine bone turnover markers. In the authors’ experience, a bone biopsy may also occasionally identify unsuspected causes of bone fragility, such as Gaucher disease or mastocytosis.
Idiopathic Osteoporosis (IOP)
In some cases of low trauma fracture in premenopausal women, no known secondary cause can be found after extensive evaluation. These women are said to have idiopathic osteoporosis (IOP). Based on current guidelines, the term idiopathic osteoporosis applies only to those with a history of low trauma fractures, and not to those with low BMD and no history of fractures. That being several studies of idiopathic osteoporosis in women (and men) have included both those with fractures and those with low BMD alone. Based on such studies, IOP is predominantly reported in Caucasians, and family history of osteoporosis is common [33, 36–38]. Available evidence suggests that IOP is a heterogeneous disorder, in which abnormalities in skeletal microstructure and strength may be due to diverse pathogenic mechanisms. Analyses of hormones, biochemistries and bone remodeling on bone biopsy samples have distinguished groups with both high and low bone remodeling states. In our studies of premenopausal women with IOP, those with low bone turnover exhibited the most marked deficits in microarchitecture and had higher insulin-like growth factor -1 (IGF-1) levels, while those with high bone turnover manifested a biochemical pattern suggestive of primary/idiopathic hypercalciuria[18, 37]
Management Issues
General Measures
For all patients, it is appropriate to recommend a set of general measures that benefit bone health. Such measures include getting adequate amounts of weightbearing exercise, protein, calories, calcium and vitamin D, as well as lifestyle modifications such as smoking cessation and avoidance of excess alcohol [39–43]. Current guidelines from the Institute of Medicine[44] recommend a total of 1000 mg of calcium (from diet and supplements) and 600 IU of vitamin D for premenopausal women. These recommendations should be tailored to the individual based upon evaluation of calcium metabolism. Exercise recommendations must also be tailored to the individual patient, since excessive exercise in premenopausal women may lead to weight loss and/or hypothalamic amenorrhea, exacerbating low bone density.
In premenopausal women with isolated low BMD and no history of fractures, in whom no secondary cause can be identified after thorough evaluation, pharmacological therapy is rarely necessary. Low BMD in such young women may be due to genetically determined low peak bone mass, or may be due to past insults to the growing or adult skeleton (poor nutrition, medications, estrogen deficiency) that are no longer operative. Although these women may have bone microarchitectural abnormalities underlying their low BMD [18, 22], they usually have stable BMD [45], and a low short-term risk of fracture compared to postmenopausal women at the same BMD. Peris et al. have reported that BMD improved slightly on average in women with unexplained osteoporosis managed with only calcium (to achieve a total intake of 1500 mg/day), vitamin D (400–800 IU/daily) and exercise and followed over time [45]. Bone density should be remeasured after one or two years to identify women with declining BMD who may require ongoing evaluation for secondary causes and/or therapeutic intervention.
In premenopausal women with low BMD or low trauma fractures and a known secondary cause of osteoporosis, management should address the underlying cause whenever possible. Correction or treatment of several of these conditions, including estrogen deficiency, hypercalciuria [46], celiac disease [47–49], Crohn’s disease [50], endogenous and iatrogenic hypercortisolism and hyperparathyroidism [51], has been associated with measureable improvements in BMD in some populations, although many have not been specifically studied in premenopausal women.
In some women, it is not possible to address or alleviate the secondary cause directly. Premenopausal women with chronic inflammatory conditions, those requiring long-term glucocorticoids, and those being treated with medications leading to estrogen deficiency (eg for breast cancer or endometriosis) may require pharmacological therapy to prevent excessive bone loss or fractures. Options for treatment include antiresorptive drugs, such as estrogen, bisphosphonates or denosumab, or anabolic agents such as teriparatide. Selective estrogen receptor modulators (SERMS), such as raloxifene, should not be used to treat bone loss in menstruating women since they block estrogen action on bone and lead to further bone loss [52, 53].
Contraceptives
Combination oral contraceptives
Replacement of estrogen in premenopausal women who are estrogen deficient may have beneficial effects on bone mass [54–56], although oral reproductive hormone replacement has been shown to be ineffective in most studies examining bone mass in anorexia nervosa, a more complex condition [56–58] (see below). Studies of oral contraceptives in healthy premenopausal women without known pre-existing estrogen deficiency have examined varying estrogen doses in populations of different ages. The majority of these studies show no effect of oral contraceptives on bone mass [56, 59, 60]; however some have documented an adverse effect of low dose (< 30 μg ethinyl estradiol) oral contraceptives on bone mass in adolescents and young adult women [61–63]. The effects of oral contraceptives on fracture risk are unknown.
Depot medroxyprogesterone acetate
Depot medroxyprogesterone acetate (DMPA) is an injectable, progestin-only contraceptive that acts primarily via inhibition of gonadotropin secretion, thus leading to a hypoestrogenic state. BMD losses have been documented in several studies of both adolescents [64, 65] and adult women [66–68] using DMPA over several years. BMD recovery has been documented after medication cessation [69], but recovery may be more complete at the spine than at the hip [68, 70]. The FDA’s review of these data led to their requirement of a “black box warning” stating that, “Bone loss is greater with increasing duration of use and may not be completely reversible.” Whether fracture risk increases during or after DMPA use is unknown. In a study of female military recruits, DMPA use was one of several factors associated with an increased risk of stress fracture, but only in white women [71]; It is unclear if results in this high risk population can be applied to other premenopausal women. The American College of Obstetrics and Gynecology Committee Opinion states that women initiating DMPA receive counseling about the benefits and potential risks of this medication, but that short-term BMD losses are likely to be reversible, thus “Practitioners should not perform BMD monitoring solely in response to DMPA use” [72]. Some populations, such as women close to menopause who may not have ample time to experience post-DMPA BMD recovery, and women with active secondary causes of bone loss, may be at increased risk for lasting effects of the medication. BMD measurement may be indicated in such cases.
Bisphosphonates
In general, bisphosphonate therapy should be reserved for those premenopausal women with a history of fragility fractures, or with a known ongoing process of bone loss. The United States Food and Drug Administration has approved bisphosphonates only for those premenopausal women who are receiving glucocorticoids. Bisphosphonates carry a Category C rating for safety in pregnancy from the United States Food and Drug Administration (US FDA) because they accumulate in the skeleton, cross the placenta and accumulate in the fetal skeleton in a rat model, and have been reported to cause toxic effects in pregnant rats [73]. Human data on bisphosphonates in pregnancy are largely anecdotal. Although one report documented transient neonatal hypocalcemia and bilateral talipes equinovarus in 2 neonates [74], several other reports have documented no adverse maternal and fetal outcomes [75–77]. Effective contraception should be encouraged during bisphosphonate use, and it should be kept in mind that there is also the potential for adverse effects after stopping bisphosphonates, since they remain in the skeleton for years. Because of these risks, bisphosphonates should be used with great caution, and as a last resort, in women who may have future pregnancies.
The potential risks of long-term use of bisphosphonates, including osteonecrosis of the jaw [78] and atypical femoral fractures [79], are also of particular concern in a young population requiring long-term treatment. In young women, plans for duration of bisphosphonate use must be discussed as part of the process of initiation of this therapy, and the goal should be for the shortest possible duration of bisphosphonate use.
Denosumab
Denosumab is a RANK ligand inhibitor that is currently FDA approved for the treatment of osteoporosis in postmenopausal women. The efficacy and safety of this medication have not been defined in premenopausal women. Additionally, this medication caries an FDA designation of Category X for safety in pregnancy.
Teriparatide
There are few data on the effects of teriparatide or PTH(1-34) in premenopausal women, but this medication has been studied in women with medication-induced amenorrhea [80], women with IOP [81], women with pregnancy or lactation associated fractures [82] (see below), and those on glucocorticoids [83, 84](see below). In young women treated with the GnRH analog nafarelin for endometriosis, spine BMD declined by 4.9%, while those treated with PTH(1-34) 40 μg daily together with nafarelin had an increase of 2.1% (p<0.001) [80]. It is not clear whether these results would apply to premenopausal women with normal gonadal status. In an observational study of teriparatide 20 μg daily in 21 premenopausal women with IOP, BMD increased by 9.8% at the lumbar spine and 2.9% at the total hip (both p<0.05) after 18 months of treatment [81]. However, among this unique cohort, a small subset with very low baseline bone turnover had little or no increase in BMD on this medication [81]. Because the long-term effects of teriparatide in young women are not known, use of this medication should be reserved for those at highest risk for fracture or those who are experiencing recurrent fractures. In young women less than 25 years of age, documentation of fused epiphyses is recommended prior to consideration of teriparatide treatment, as this medication is contraindicated during growth.
Specific Clinical Situations
Glucocorticoid-Induced Osteoporosis
Glucocorticoids can lead to decreased reproductive hormone production and menstrual irregularities. Thus, it is reasonable to consider estrogen replacement as the initial management step in those with hypogonadism in the setting of glucocorticoid exposure.
Alendronate and risedronate have been approved by the US FDA for use in premenopausal women receiving glucocorticoids. However, relatively few premenopausal women participated in the relevant large registration trials for bisphosphonates in glucocorticoid-induced osteoporosis and none of the premenopausal women in those trials fractured [85–87]. A few studies have specifically evaluated premenopausal women with autoimmune and connective tissue diseases, and have demonstrated protective effects of intermittent cyclical etidronate and oral pamidronate [88, 89]. Guidelines from the American College of Rheumatology suggest that bisphosphonates be considered for prevention and treatment of glucocorticoid-induced osteoporosis in premenopausal women taking at least 7.5 mg of prednisone or equivalent per day for ≥ 3 months [90]. However, because of potential harm to the fetus in women who may become pregnant, they also urge great caution in the use of bisphosphonates in premenopausal women [90].
A recent study comparing teriparatide and alendronate for glucocorticoid-induced osteoporosis included some premenopausal women. Overall, teriparatide was associated with significantly greater increases in lumbar spine and total hip BMD and resulted in significantly fewer incident vertebral fractures than alendronate [84]. The BMD responses were similar in premenopausal women as in men and postmenopausal women, but no fractures occurred in either premenopausal group [83].
Pregnancy- and lactation- associated osteoporosis
In a report of 9 premenopausal women with a history of fracture(s) and a diagnosis of pregnancy- and lactation- associated osteoporosis, use of bisphosphonates over an average of 24 months was associated with substantial increases of 11–23% in lumbar spine bone density [76]. A case report has also documented substantial BMD increases in 3 women with pregnancy/lactation-associated vertebral fractures treated with teriparatide over 18 months postpartum[82]. Since bone density is expected to increase postpartum and after weaning in normal women, it is not clear to what extent medication use provided an incremental benefit for patients included in these studies.
Anorexia Nervosa
Body weight and nutritional recovery are thought to be the most important determinants of BMD in women with anorexia nervosa [57, 91, 92]. Estrogen preparations are generally ineffective in this group[58]. Both alendronate and risedronate have been shown to significantly increase BMD in young women with anorexia [91, 93].
Summary and Conclusions
The finding of low BMD (Z score ≤ −2.0) in a premenopausal woman has quite different clinical implications in comparison to a similar finding in a postmenopausal woman. This finding may be the result of genetically determined low peak BMD, past insults to skeleton or currently active causes of continuing bone loss. Because secondary causes are commonly found and many potential causes of low BMD are treatable, the finding of a Z score ≤ −2.0 at the spine, hip or forearm should lead to evaluation for potential secondary causes of osteoporosis and bone loss. For premenopausal women in whom a secondary cause is known or found, treatment of this underlying cause should be the focus of management to improve bone health. Pharmacologic intervention with a bone-active agent can be considered, on a case-by-case basis, for women with fragility fractures, documented persistent bone loss, or in cases of ongoing estrogen deficiency, inflammatory disease, glucocorticoid treatment, or other conditions that may result in a high short term risk for bone loss and fracture. Some data are available suggesting beneficial effects of both bisphosphonates and teriparatide in women in certain clinical settings. Although some bisphosphonates are approved for use in premenopausal women in the setting of ongoing glucocorticoid exposure, these medications should be used with caution in women who may have future pregnancies. For women with stable low BMD, no fractures, and no known secondary cause, the relationship between BMD and fracture risk is not clear. In such women, pharmacologic therapy is rarely necessary.
Acknowledgments
Please note that this is an updated article to: Cohen, A and Shane E. Treatment of Premenopausal Women with Low Bone Mineral Density. Current Osteoporosis Reports 2008, 6:39-46.
Footnotes
Conflict of Interest
A Cohen works for an institution that has received research grants from Novartis and Eli Lilly. E Shane works for an institution that has received research grants from Novartis.
Human and Animal Rights and Informed Consent
All studies by A. Cohen and E. Shane, et al. involving human subjects were performed after approval by the appropriate Institutional Review Boards. Written informed consent was obtained from all participants.
References
Papers of particular interest, published recently, have been highlighted as:
•• Of major importance
• Of importance
- 1.Cohen A. Osteoporosis in Premenopausal Women. In: Marcus R, Feldman D, Dempster D, Luckey M, Cauley J, editors. Osteoporosis. 4. Elsevier; 2013. [Google Scholar]
- 2.Cohen A, Shane E. Premenopausal Osteoporosis. In: Rosen CJ, editor. Primer on the Metabolic Bone Diseases and Disorders of Mineral Metabolism. Washington, D.C: American Society for Bone and Mineral Research; 2008. pp. 289–293. [Google Scholar]
- 3.Cohen A, Shane E. Treatment of premenopausal women with low bone mineral density. Curr Osteoporos Rep. 2008;6:39–46. doi: 10.1007/s11914-008-0007-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lewiecki EM, Gordon CM, Baim S, Leonard MB, Bishop NJ, Bianchi ML, et al. International Society for Clinical Densitometry 2007 Adult and Pediatric Official Positions. Bone. 2008;43:1115–1121. doi: 10.1016/j.bone.2008.08.106. [DOI] [PubMed] [Google Scholar]
- 5.Thompson PW, Taylor J, Dawson A. The annual incidence and seasonal variation of fractures of the distal radius in men and women over 25 years in Dorset, UK. Injury. 2004;35:462–466. doi: 10.1016/S0020-1383(03)00117-7. [DOI] [PubMed] [Google Scholar]
- 6.Melton LJ, 3rd, Amadio PC, Crowson CS, O’Fallon WM. Long-term trends in the incidence of distal forearm fractures. Osteoporos Int. 1998;8:341–348. doi: 10.1007/s001980050073. [DOI] [PubMed] [Google Scholar]
- 7.Hui SL, Slemenda CW, Johnston CC., Jr Age and bone mass as predictors of fracture in a prospective study. J Clin Invest. 1988;81:1804–1809. doi: 10.1172/JCI113523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wigderowitz CA, Cunningham T, Rowley DI, Mole PA, Paterson CR. Peripheral bone mineral density in patients with distal radial fractures. J Bone Joint Surg Br. 2003;85:423–425. doi: 10.1302/0301-620x.85b3.13336. [DOI] [PubMed] [Google Scholar]
- 9•.Hung LK, Wu HT, Leung PC, Qin L. Low BMD is a risk factor for low-energy Colles’ fractures in women before and after menopause. Clin Orthop Relat Res. 2005:219–225. doi: 10.1097/01.blo.0000155345.04782.14. This study demonstrates a relationship between BMD and a history of low-energy fracture in premenopausal women. [DOI] [PubMed] [Google Scholar]
- 10.Lappe J, Davies K, Recker R, Heaney R. Quantitative ultrasound: use in screening for susceptibility to stress fractures in female army recruits. J Bone Miner Res. 2005;20:571–578. doi: 10.1359/JBMR.041208. [DOI] [PubMed] [Google Scholar]
- 11.Lauder TD, Dixit S, Pezzin LE, Williams MV, Campbell CS, Davis GD. The relation between stress fractures and bone mineral density: evidence from active-duty Army women. Arch Phys Med Rehabil. 2000;81:73–79. doi: 10.1016/s0003-9993(00)90225-9. [DOI] [PubMed] [Google Scholar]
- 12.Shaffer RA, Rauh MJ, Brodine SK, Trone DW, Macera CA. Predictors of stress fracture susceptibility in young female recruits. Am J Sports Med. 2006;34:108–115. doi: 10.1177/0363546505278703. [DOI] [PubMed] [Google Scholar]
- 13.Gourlay ML, Brown SA. Clinical considerations in premenopausal osteoporosis. Arch Intern Med. 2004;164:603–614. doi: 10.1001/archinte.164.6.603. [DOI] [PubMed] [Google Scholar]
- 14.Leib ES. Treatment of low bone mass in premenopausal women: when may it be appropriate? Curr Osteoporos Rep. 2005;3:13–18. doi: 10.1007/s11914-005-0022-x. [DOI] [PubMed] [Google Scholar]
- 15.Licata AA. “Does she or doesn’t she…have osteoporosis?” The use and abuse of bone densitometry. Endocr Pract. 2000;6:336–337. [PubMed] [Google Scholar]
- 16.Lindsay R. Bone mass measurement for premenopausal women. Osteoporos Int. 1994;4 (Suppl 1):39–41. doi: 10.1007/BF01623434. [DOI] [PubMed] [Google Scholar]
- 17.Ferrari S, Bianchi ML, Eisman JA, Foldes AJ, Adami S, Wahl DA, et al. Osteoporosis in young adults: pathophysiology, diagnosis, and management. Osteoporos Int. 2012 doi: 10.1007/s00198-012-2030-x. [DOI] [PubMed] [Google Scholar]
- 18.Cohen A, Dempster D, Recker R, Stein EM, JL, Zhou H, et al. Abnormal Bone Microarchitecture and Evidence of Osteoblast Dysfunction in Premenopausal Women with Idiopathic Osteoporosis. J Clin Endocrinol Metab. 2011;96:3095–3105. doi: 10.1210/jc.2011-1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Adams JS, Bishop NJ. Chapter 29: DXA in adults and children. In: Rosen CJ, editor. Primer on the Metabolic Bone Diseases and Disorders of Mineral Metabolism. Washington, D.C: American Society for Bone and Mineral Research; 2008. pp. 152–158. [Google Scholar]
- 20.Khan AA, Syed Z. Bone densitometry in premenopausal women: synthesis and review. J Clin Densitom. 2004;7:85–92. doi: 10.1385/jcd:7:1:85. [DOI] [PubMed] [Google Scholar]
- 21.Lewiecki EM. Low bone mineral density in premenopausal women. South Med J. 2004;97:544–550. doi: 10.1097/00007611-200406000-00006. [DOI] [PubMed] [Google Scholar]
- 22.Cohen A, Liu XS, Stein EM, McMahon DJ, Rogers HF, Lemaster J, et al. Bone microarchitecture and stiffness in premenopausal women with idiopathic osteoporosis. J Clin Endocrinol Metab. 2009;94:4351–4360. doi: 10.1210/jc.2009-0996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cohen A, Lang TF, McMahon DJ, Liu XS, Guo XE, Zhang C, et al. Central QCT Reveals Lower Volumetric BMD and Stiffness in Premenopausal Women with Idiopathic Osteoporosis, Regardless of Fracture History. J Clin Endocrinol Metab. 2012;97:4244–4252. doi: 10.1210/jc.2012-2099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Galusca B, Zouch M, Germain N, Bossu C, Frere D, Lang F, et al. Constitutional thinness: unusual human phenotype of low bone quality. J Clin Endocrinol Metab. 2008;93:110–117. doi: 10.1210/jc.2007-1591. [DOI] [PubMed] [Google Scholar]
- 25.Theintz G, Buchs B, Rizzoli R, Slosman D, Clavien H, Sizonenko PC, et al. Longitudinal monitoring of bone mass accumulation in healthy adolescents: evidence for a marked reduction after 16 years of age at the levels of lumbar spine and femoral neck in female subjects. J Clin Endocrinol Metab. 1992;75:1060–1065. doi: 10.1210/jcem.75.4.1400871. [DOI] [PubMed] [Google Scholar]
- 26.Bachrach LK, Hastie T, Wang MC, Narasimhan B, Marcus R. Bone mineral acquisition in healthy Asian, Hispanic, black, and Caucasian youth: a longitudinal study. J Clin Endocrinol Metab. 1999;84:4702–4712. doi: 10.1210/jcem.84.12.6182. [DOI] [PubMed] [Google Scholar]
- 27.Recker RR, Davies KM, Hinders SM, Heaney RP, Stegman MR, Kimmel DB. Bone gain in young adult women. Jama. 1992;268:2403–2408. [PubMed] [Google Scholar]
- 28.Sowers M, Corton G, Shapiro B, Jannausch ML, Crutchfield M, Smith ML, et al. Changes in bone density with lactation. Jama. 1993;269:3130–3135. [PubMed] [Google Scholar]
- 29.Karlsson MK, Ahlborg HG, Karlsson C. Maternity and bone mineral density. Acta Orthop. 2005;76:2–13. doi: 10.1080/00016470510030274. [DOI] [PubMed] [Google Scholar]
- 30.Honkanen R, Tuppurainen M, Kroger H, Alhava E, Puntila E. Associations of early premenopausal fractures with subsequent fractures vary by sites and mechanisms of fractures. Calcif Tissue Int. 1997;60:327–331. doi: 10.1007/s002239900237. [DOI] [PubMed] [Google Scholar]
- 31.Hosmer WD, Genant HK, Browner WS. Fractures before menopause: a red flag for physicians. Osteoporos Int. 2002;13:337–341. doi: 10.1007/s001980200035. [DOI] [PubMed] [Google Scholar]
- 32•.Wu F, Mason B, Horne A, Ames R, Clearwater J, Liu M, et al. Fractures between the ages of 20 and 50 years increase women’s risk of subsequent fractures. Arch Intern Med. 2002;162:33–36. doi: 10.1001/archinte.162.1.33. This study documents a 74% increased risk of fracture after age 50 in those women reporting fractures sustained between ages 20 and 50 years. [DOI] [PubMed] [Google Scholar]
- 33.Khosla S, Lufkin EG, Hodgson SF, Fitzpatrick LA, Melton LJ., 3rd Epidemiology and clinical features of osteoporosis in young individuals. Bone. 1994;15:551–555. doi: 10.1016/8756-3282(94)90280-1. [DOI] [PubMed] [Google Scholar]
- 34.Cohen A, Fleischer J, Freeby MJ, McMahon DJ, Irani D, Shane E. Clinical characteristics and medication use among premenopausal women with osteoporosis and low BMD: the experience of an osteoporosis referral center. J Womens Health (Larchmt) 2009;18:79–84. doi: 10.1089/jwh.2008.0887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Moreira Kulak CA, Schussheim DH, McMahon DJ, Kurland E, Silverberg SJ, Siris ES, et al. Osteoporosis and low bone mass in premenopausal and perimenopausal women. Endocr Pract. 2000;6:296–304. doi: 10.4158/EP.6.4.296. [DOI] [PubMed] [Google Scholar]
- 36.Peris P, Guanabens N, Martinez de Osaba MJ, Monegal A, Alvarez L, Pons F, et al. Clinical characteristics and etiologic factors of premenopausal osteoporosis in a group of Spanish women. Semin Arthritis Rheum. 2002;32:64–70. doi: 10.1053/sarh.2002.33725. [DOI] [PubMed] [Google Scholar]
- 37.Cohen A, Recker RR, Lappe J, Dempster DW, Cremers S, McMahon DJ, et al. Premenopausal women with idiopathic low-trauma fractures and/or low bone mineral density. Osteoporos Int. 2012;23:171–182. doi: 10.1007/s00198-011-1560-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kulak CAM, Schussheim DH, McMahon DJ, Kurland E, Silverberg SJ, Siris ES, et al. Osteoporosis and low bone mass in premenopausal and perimenopausal women. Endocr Pract. 2000;6:296–304. doi: 10.4158/EP.6.4.296. [DOI] [PubMed] [Google Scholar]
- 39.Wallace BA, Cumming RG. Systematic review of randomized trials of the effect of exercise on bone mass in pre- and postmenopausal women. Calcif Tissue Int. 2000;67:10–18. doi: 10.1007/s00223001089. [DOI] [PubMed] [Google Scholar]
- 40.Tudor-Locke C, McColl RS. Factors related to variation in premenopausal bone mineral status: a health promotion approach. Osteoporos Int. 2000;11:1–24. doi: 10.1007/s001980050001. [DOI] [PubMed] [Google Scholar]
- 41.Mein AL, Briffa NK, Dhaliwal SS, Price RI. Lifestyle influences on 9-year changes in BMD in young women. J Bone Miner Res. 2004;19:1092–1098. doi: 10.1359/JBMR.040310. [DOI] [PubMed] [Google Scholar]
- 42.Vainionpaa A, Korpelainen R, Leppaluoto J, Jamsa T. Effects of high-impact exercise on bone mineral density: a randomized controlled trial in premenopausal women. Osteoporos Int. 2005;16:191–197. doi: 10.1007/s00198-004-1659-5. [DOI] [PubMed] [Google Scholar]
- 43.Baran D, Sorensen A, Grimes J, Lew R, Karellas A, Johnson B, et al. Dietary modification with dairy products for preventing vertebral bone loss in premenopausal women: a three-year prospective study. J Clin Endocrinol Metab. 1990;70:264–270. doi: 10.1210/jcem-70-1-264. [DOI] [PubMed] [Google Scholar]
- 44.Ross AC, Manson JE, Abrams SA, Aloia JF, Brannon PM, Clinton SK, et al. The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: what clinicians need to know. J Clin Endocrinol Metab. 2011;96:53–58. doi: 10.1210/jc.2010-2704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45•.Peris P, Monegal A, Martinez MA, Moll C, Pons F, Guanabens N. Bone mineral density evolution in young premenopausal women with idiopathic osteoporosis. Clin Rheumatol. 2007;26:958–961. doi: 10.1007/s10067-006-0405-0. This retrospective study showed small increases in BMD after 2 to 3 years of follow-up in 16 premenopausal women with idiopathic osteoporosis (mean age 36 years) who were treated with only calcium and vitamin D. [DOI] [PubMed] [Google Scholar]
- 46.Adams JS, Song CF, Kantorovich V. Rapid recovery of bone mass in hypercalciuric, osteoporotic men treated with hydrochlorothiazide. Ann Intern Med. 1999;130:658–660. doi: 10.7326/0003-4819-130-8-199904200-00012. [DOI] [PubMed] [Google Scholar]
- 47.Ciacci C, Maurelli L, Klain M, Savino G, Salvatore M, Mazzacca G, et al. Effects of dietary treatment on bone mineral density in adults with celiac disease: factors predicting response. Am J Gastroenterol. 1997;92:992–996. [PubMed] [Google Scholar]
- 48.Mautalen C, Gonzalez D, Mazure R, Vazquez H, Lorenzetti MP, Maurino E, et al. Effect of treatment on bone mass, mineral metabolism, and body composition in untreated celiac disease patients. Am J Gastroenterol. 1997;92:313–318. [PubMed] [Google Scholar]
- 49.McFarlane XA, Bhalla AK, Robertson DA. Effect of a gluten free diet on osteopenia in adults with newly diagnosed coeliac disease. Gut. 1996;39:180–184. doi: 10.1136/gut.39.2.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mauro M, Radovic V, Armstrong D. Improvement of lumbar bone mass after infliximab therapy in Crohn’s disease patients. Can J Gastroenterol. 2007;21:637–642. doi: 10.1155/2007/216162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lumachi F, Camozzi V, Ermani M, FDEL, Luisetto G. Bone mineral density improvement after successful parathyroidectomy in pre- and postmenopausal women with primary hyperparathyroidism: a prospective study. Ann N Y Acad Sci. 2007;1117:357–361. doi: 10.1196/annals.1402.012. [DOI] [PubMed] [Google Scholar]
- 52.Powles TJ, Hickish T, Kanis JA, Tidy A, Ashley S. Effect of tamoxifen on bone mineral density measured by dual-energy x-ray absorptiometry in healthy premenopausal and postmenopausal women. J Clin Oncol. 1996;14:78–84. doi: 10.1200/JCO.1996.14.1.78. [DOI] [PubMed] [Google Scholar]
- 53.Vehmanen L, Elomaa I, Blomqvist C, Saarto T. Tamoxifen treatment after adjuvant chemotherapy has opposite effects on bone mineral density in premenopausal patients depending on menstrual status. J Clin Oncol. 2006;24:675–680. doi: 10.1200/JCO.2005.02.3515. [DOI] [PubMed] [Google Scholar]
- 54.Sagsveen M, Farmer JE, Prentice A, Breeze A. Gonadotrophin-releasing hormone analogues for endometriosis: bone mineral density. Cochrane Database Syst Rev. 2003:CD001297. doi: 10.1002/14651858.CD001297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cundy T, Ames R, Horne A, Clearwater J, Roberts H, Gamble G, et al. A randomized controlled trial of estrogen replacement therapy in long-term users of depot medroxyprogesterone acetate. J Clin Endocrinol Metab. 2003;88:78–81. doi: 10.1210/jc.2002-020874. [DOI] [PubMed] [Google Scholar]
- 56.Liu SL, Lebrun CM. Effect of oral contraceptives and hormone replacement therapy on bone mineral density in premenopausal and perimenopausal women: a systematic review. Br J Sports Med. 2006;40:11–24. doi: 10.1136/bjsm.2005.020065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Miller KK, Lee EE, Lawson EA, Misra M, Minihan J, Grinspoon SK, et al. Determinants of skeletal loss and recovery in anorexia nervosa. J Clin Endocrinol Metab. 2006;91:2931–2937. doi: 10.1210/jc.2005-2818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sim LA, McGovern L, Elamin MB, Swiglo BA, Erwin PJ, Montori VM. Effect on bone health of estrogen preparations in premenopausal women with anorexia nervosa: a systematic review and meta-analyses. Int J Eat Disord. 2010;43:218–225. doi: 10.1002/eat.20687. [DOI] [PubMed] [Google Scholar]
- 59.Lopez LM, Grimes DA, Schulz KF, Curtis KM. Steroidal contraceptives: effect on bone fractures in women. Cochrane Database Syst Rev. 2011:CD006033. doi: 10.1002/14651858.CD006033.pub2. [DOI] [PubMed] [Google Scholar]
- 60.Wei S, Winzenberg T, Laslett LL, Venn A, Jones G. Oral contraceptive use and bone. Curr Osteoporos Rep. 2011;9:6–11. doi: 10.1007/s11914-010-0037-9. [DOI] [PubMed] [Google Scholar]
- 61.Scholes D, Ichikawa L, LaCroix AZ, Spangler L, Beasley JM, Reed S, et al. Oral contraceptive use and bone density in adolescent and young adult women. Contraception. 2010;81:35–40. doi: 10.1016/j.contraception.2009.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cromer BA. Bone mineral density in adolescent and young adult women on injectable or oral contraception. Curr Opin Obstet Gynecol. 2003;15:353–357. doi: 10.1097/00001703-200310000-00002. [DOI] [PubMed] [Google Scholar]
- 63.Polatti F, Perotti F, Filippa N, Gallina D, Nappi RE. Bone mass and long-term monophasic oral contraceptive treatment in young women. Contraception. 1995;51:221–224. doi: 10.1016/0010-7824(95)00036-a. [DOI] [PubMed] [Google Scholar]
- 64.Cromer BA, Stager M, Bonny A, Lazebnik R, Rome E, Ziegler J, et al. Depot medroxyprogesterone acetate, oral contraceptives and bone mineral density in a cohort of adolescent girls. J Adolesc Health. 2004;35:434–441. doi: 10.1016/j.jadohealth.2004.07.005. [DOI] [PubMed] [Google Scholar]
- 65.Scholes D, LaCroix AZ, Ichikawa LE, Barlow WE, Ott SM. Change in bone mineral density among adolescent women using and discontinuing depot medroxyprogesterone acetate contraception. Arch Pediatr Adolesc Med. 2005;159:139–144. doi: 10.1001/archpedi.159.2.139. [DOI] [PubMed] [Google Scholar]
- 66.Berenson AB, Breitkopf CR, Grady JJ, Rickert VI, Thomas A. Effects of hormonal contraception on bone mineral density after 24 months of use. Obstet Gynecol. 2004;103:899–906. doi: 10.1097/01.AOG.0000117082.49490.d5. [DOI] [PubMed] [Google Scholar]
- 67.Clark MK, Sowers MR, Nichols S, Levy B. Bone mineral density changes over two years in first-time users of depot medroxyprogesterone acetate. Fertil Steril. 2004;82:1580–1586. doi: 10.1016/j.fertnstert.2004.04.064. [DOI] [PubMed] [Google Scholar]
- 68.Scholes D, LaCroix AZ, Ichikawa LE, Barlow WE, Ott SM. Injectable hormone contraception and bone density: results from a prospective study. Epidemiology. 2002;13:581–587. doi: 10.1097/00001648-200209000-00015. [DOI] [PubMed] [Google Scholar]
- 69.Kaunitz AM, Arias R, McClung M. Bone density recovery after depot medroxyprogesterone acetate injectable contraception use. Contraception. 2008;77:67–76. doi: 10.1016/j.contraception.2007.10.005. [DOI] [PubMed] [Google Scholar]
- 70.Clark MK, Sowers M, Levy B, Nichols S. Bone mineral density loss and recovery during 48 months in first-time users of depot medroxyprogesterone acetate. Fertil Steril. 2006;86:1466–1474. doi: 10.1016/j.fertnstert.2006.05.024. [DOI] [PubMed] [Google Scholar]
- 71.Lappe JM, Stegman MR, Recker RR. The impact of lifestyle factors on stress fractures in female Army recruits. Osteoporos Int. 2001;12:35–42. doi: 10.1007/s001980170155. [DOI] [PubMed] [Google Scholar]
- 72.ACOG Committee Opinion No. 415. Depot medroxyprogesterone acetate and bone effects. Obstet Gynecol. 2008;112:727–730. doi: 10.1097/AOG.0b013e318188d1ec. [DOI] [PubMed] [Google Scholar]
- 73.Minsker DH, Manson JM, Peter CP. Effects of the bisphosphonate, alendronate, on parturition in the rat. Toxicol Appl Pharmacol. 1993;121:217–223. doi: 10.1006/taap.1993.1148. [DOI] [PubMed] [Google Scholar]
- 74.Munns CF, Rauch F, Ward L, Glorieux FH. Maternal and fetal outcome after long-term pamidronate treatment before conception: a report of two cases. J Bone Miner Res. 2004;19:1742–1745. doi: 10.1359/JBMR.040711. [DOI] [PubMed] [Google Scholar]
- 75.Levy S, Fayez I, Taguchi N, Han JY, Aiello J, Matsui D, et al. Pregnancy outcome following in utero exposure to bisphosphonates. Bone. 2009;44:428–430. doi: 10.1016/j.bone.2008.11.001. [DOI] [PubMed] [Google Scholar]
- 76.O’Sullivan SM, Grey AB, Singh R, Reid IR. Bisphosphonates in pregnancy and lactation-associated osteoporosis. Osteoporos Int. 2006;17:1008–1012. doi: 10.1007/s00198-006-0112-3. [DOI] [PubMed] [Google Scholar]
- 77.Ornoy A, Wajnberg R, Diav-Citrin O. The outcome of pregnancy following pre-pregnancy or early pregnancy alendronate treatment. Reprod Toxicol. 2006;22:578–579. doi: 10.1016/j.reprotox.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 78.Khosla S, Burr D, Cauley J, Dempster DW, Ebeling PR, Felsenberg D, et al. Bisphosphonate-associated osteonecrosis of the jaw: report of a task force of the American Society for Bone and Mineral Research. J Bone Miner Res. 2007;22:1479–1491. doi: 10.1359/jbmr.0707onj. [DOI] [PubMed] [Google Scholar]
- 79.Shane E, Burr D, Ebeling PR, Abrahamsen B, Adler RA, Brown TD, et al. Atypical subtrochanteric and diaphyseal femoral fractures: report of a task force of the American Society for Bone and Mineral Research. J Bone Miner Res. 2010;25:2267–2294. doi: 10.1002/jbmr.253. [DOI] [PubMed] [Google Scholar]
- 80.Finkelstein JS, Klibanski A, Arnold AL, Toth TL, Hornstein MD, Neer RM. Prevention of estrogen deficiency-related bone loss with human parathyroid hormone-(1-34): a randomized controlled trial. Jama. 1998;280:1067–1073. doi: 10.1001/jama.280.12.1067. [DOI] [PubMed] [Google Scholar]
- 81•.Cohen A, Stein EM, Recker RR, Lappe JM, Dempster DW, Zhou H, et al. Teriparatide for idiopathic osteoporosis in premenopausal women: a pilot study. J Clin Endocrinol Metab. 2013;98:1971–1981. doi: 10.1210/jc.2013-1172. This paper presents BMD, biochemical, and paired bone biopsy results from a study of 21 premenopausal women with idiopathic osteoporosis treated with teriparatide for 18–24 months. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Choe EY, Song JE, Park KH, Seok H, Lee EJ, Lim SK, et al. Effect of teriparatide on pregnancy and lactation-associated osteoporosis with multiple vertebral fractures. J Bone Miner Metab. 2012;30:596–601. doi: 10.1007/s00774-011-0334-0. [DOI] [PubMed] [Google Scholar]
- 83••.Langdahl BL, Marin F, Shane E, Dobnig H, Zanchetta JR, Maricic M, et al. Teriparatide versus alendronate for treating glucocorticoid-induced osteoporosis: an analysis by gender and menopausal status. Osteoporos Int. 2009;20:2095–2104. doi: 10.1007/s00198-009-0917-y. In patients with glucocorticoid-induced osteoporosis, the effects of teriparatide versus alendronate were compared by gender and menopausal status. BMD response was similar in men, postmenopausal women and premenopausal women. [DOI] [PubMed] [Google Scholar]
- 84.Saag KG, Shane E, Boonen S, Marin F, Donley DW, Taylor KA, et al. Teriparatide or alendronate in glucocorticoid-induced osteoporosis. N Engl J Med. 2007;357:2028–2039. doi: 10.1056/NEJMoa071408. [DOI] [PubMed] [Google Scholar]
- 85.Adachi JD, Bensen WG, Brown J, Hanley D, Hodsman A, Josse R, et al. Intermittent etidronate therapy to prevent corticosteroid-induced osteoporosis. N Engl J Med. 1997;337:382–387. doi: 10.1056/NEJM199708073370603. [DOI] [PubMed] [Google Scholar]
- 86.Saag KG, Emkey R, Schnitzer TJ, Brown JP, Hawkins F, Goemaere S, et al. Alendronate for the prevention and treatment of glucocorticoid-induced osteoporosis. Glucocorticoid-Induced Osteoporosis Intervention Study Group. N Engl J Med. 1998;339:292–299. doi: 10.1056/NEJM199807303390502. [DOI] [PubMed] [Google Scholar]
- 87.Wallach S, Cohen S, Reid DM, Hughes RA, Hosking DJ, Laan RF, et al. Effects of risedronate treatment on bone density and vertebral fracture in patients on corticosteroid therapy. Calcif Tissue Int. 2000;67:277–285. doi: 10.1007/s002230001146. [DOI] [PubMed] [Google Scholar]
- 88.Nzeusseu Toukap A, Depresseux G, Devogelaer JP, Houssiau FA. Oral pamidronate prevents high-dose glucocorticoid-induced lumbar spine bone loss in premenopausal connective tissue disease (mainly lupus) patients. Lupus. 2005;14:517–520. doi: 10.1191/0961203305lu2149oa. [DOI] [PubMed] [Google Scholar]
- 89.Nakayamada S, Okada Y, Saito K, Tanaka Y. Etidronate prevents high dose glucocorticoid induced bone loss in premenopausal individuals with systemic autoimmune diseases. J Rheumatol. 2004;31:163–166. [PubMed] [Google Scholar]
- 90.Grossman JM, Gordon R, Ranganath VK, Deal C, Caplan L, Chen W, et al. American College of Rheumatology 2010 recommendations for the prevention and treatment of glucocorticoid-induced osteoporosis. Arthritis Care Res (Hoboken) 62:1515–1526. doi: 10.1002/acr.20295. [DOI] [PubMed] [Google Scholar]
- 91•.Golden NH, Iglesias EA, Jacobson MS, Carey D, Meyer W, Schebendach J, et al. Alendronate for the treatment of osteopenia in anorexia nervosa: a randomized, double-blind, placebo-controlled trial. J Clin Endocrinol Metab. 2005;90:3179–3185. doi: 10.1210/jc.2004-1659. In this 1-year trial in 32 adolescent girls with anorexia nervosa, some BMD increase was associated with alendronate (10 mg/day) versus placebo; however, body weight was the most important determinant of BMD. [DOI] [PubMed] [Google Scholar]
- 92.Golden NH, Lanzkowsky L, Schebendach J, Palestro CJ, Jacobson MS, Shenker IR. The effect of estrogen-progestin treatment on bone mineral density in anorexia nervosa. J Pediatr Adolesc Gynecol. 2002;15:135–143. doi: 10.1016/s1083-3188(02)00145-6. [DOI] [PubMed] [Google Scholar]
- 93.Miller KK, Grieco KA, Mulder J, Grinspoon S, Mickley D, Yehezkel R, et al. Effects of risedronate on bone density in anorexia nervosa. J Clin Endocrinol Metab. 2004;89:3903–3906. doi: 10.1210/jc.2003-031885. [DOI] [PubMed] [Google Scholar]