Abstract
The primate somatosensory system provides an excellent model system with which to investigate adult neural plasticity. We have previously shown that transection of the median and ulnar nerves is followed by an expansion in the representation of radial nerve skin, and that this plasticity proceeds in stages. Immediately following nerve injury, new receptive fields are “unmasked” in a fraction of the affected cortex. The remaining deprived cortex regains responsiveness to tactile stimulation over the following days to weeks. Given these progressive changes, it has been suggested that different mechanisms might account for the earlier and later phases of reorganization. In the present experiments, we have quantified receptor autoradiographic binding data for GABAA and GABAB, AMPA, and NMDA receptors in the primary somatosensory cortices of adult squirrel monkeys at four post-nerve injury survival durations: immediately (1–3 hr), 3-days, 1-month, and 2-months. We find dramatic reductions in GABAA binding in layer IV within hours following nerve injury, and this reduction is maintained across all survival durations. This finding is consistent with the idea that the earliest reorganizational changes are due to a relaxation in tonic inhibitory mechanisms permitting the expression of formerly subthreshold receptive fields. GABAB receptor binding is decreased in layer IV by 1 month after nerve injury, while binding for AMPA receptors is increased in layer IV by this time. These findings are consistent with our previous suggestion that the second stage of reorganization proceeds via mechanisms comparable to those revealed to account for NMDA-dependent long-term potentiation in the hippocampus.
Indexing terms: AMPA receptors, NMDA receptors, GABAA receptors, GABAB receptors, hippocampal LTP, homeostatic plasticity
Introduction
Over the last 25–30 years, the idea that the mature brain can alter its functional organization in response to changes in experience has gained broad acceptance. Throughout much of this period, the somatosensory system of primates has provided a useful model for workers in a number of laboratories, including our own (e.g., Merzenich et al., 1983a, b; Florence et al., Florence et al., 1998, 2000; Kaas et al., 1999; Pons et al., 1991; Xu and Wall, 1997, 1999; Garraghty et al., 1994, Churchill et al., 1998, 2001; Schroeder et al., 1997). One commonly employed procedure involves a partial deafferentation of the hand by transecting one or more of the nerves providing its innervation (Merzenich et al., 1983a, Garraghty and Kaas, 1991; Garraghty et al., 1994), followed by electrophysiological mapping of central somatotopic representations with the goal of determining the fate of the deprived cortex. The early, pioneering experiments of Merzenich, Kaas, and colleagues (1983) showed that transection of the median nerve, deafferenting the thumb side of the volar surface of the hand, was followed by a reorganization of the cortical map in which the deprived cortex regained responsiveness to tactile stimulation of adjacent skin surfaces on the hand with intact innervation. Moreover, this reorganization was shown to proceed in no fewer than two stages, an immediate, unmasking stage in which neurons in deprived cortex expressed new receptive fields directly after the nerve transection, and a more protracted second stage in which neurons throughout the remaining deprived cortex regained responsiveness to tactile stimulation over the ensuing days to weeks. We have shown that this latter stage of reorganization is prevented if N-methyl-D-Aspartate (NMDA) glutamatergic receptors are blocked (Garraghty & Muja, 1996). The unmasking phase of reorganization, on the other hand, transpires whether NMDA receptors are blocked, or not (Myers et al., 2000). The immediate plasticity is assumed to reflect an unmasking of suppressed inputs due to a relaxation in afferent-driven inhibition, and we have reported reductions in GABAA receptor binding in layer IV of deprived area 3b cortex within hours of nerve transection (Wellman et al., 2002), a finding that is consistent with a down-regulation of inhibition. In the present experiments, we have extended our observations of GABA receptors to longer post-transection survival times, and we have measured binding for the two major classes of glutamate receptors as well.
Materials and Methods
The median and ulnar nerves were transected in thirteen adult squirrel monkeys (Saimiri sciureus). Monkeys were anesthetized with a mixture of ketamine hydrochloride (30 mg/kg) and xylazine (4 mg/kg), with supplemental doses given as necessary to maintain stage III, plane 2 level of anesthesia. A longitudinal incision was made through the skin of the ventral forearm under aseptic conditions. Using a dissection microscope, the median and ulnar nerves were located by blunt dissection, separated from the surrounding tissue, and transected about midway between the elbow and wrist. The epineural sheath of the proximal stump was peeled back 0.5–1.0 cm, and the exposed nerve was avulsed. The empty epineural sheath was then reextended, folded back on itself and ligated.
Following post-transection survival durations of 2–5 hours (n=3), 3 days (n=3), 1 month (n=4) or 2 months (n=3), the nerve-transected animals and 6 unoperated controls were deeply anesthetized with either ketamine/xylazine or sodium pentobarbitol and decapitated. Brains were rapidly removed and somatosensory cortex was dissected, frozen in dry ice, and stored at −70°C until sectioning. For each animal, 16 coronal sections through somatosensory cortex were cut at 14 μm on a cryostat and thaw-mounted on chrome-alum gelatin-coated slides. GABAA receptors were labeled with [3H]muscimol using a procedure similar to that of Xia and Haddad (1992). Sections were rinsed 30 min at room temperature in 50 mM Tris/Citrate (pH 7.0). To assess total binding, sections were incubated 45 min at 4°C in 50 mM Tris/Citrate (pH 7.0) plus 50 nM [3H]muscimol (17.5 Ci/mmol; NEN, Boston, MA); nonspecific binding was assessed by incubation with the tritiated ligand plus 100 μM unlabeled GABA (RBI, Natick, MA). After five 2-sec rinses in ice-cold 50 mM Tris/Citrate (pH 7.0), sections were dipped once in ice-cold dH2O.
To label GABAB receptors, slide-mounted sections were incubated for 30 min. at room temperature in 50 mM Tris-HCl, pH 7.4, containing 2.5 mM CaCl2 and 50 nM [3H]GABA (93.2 Ci/mmol; NEN, Boston, MA). To block binding of GABA to GABAA receptors, 40 μM unlabeled isoguvacine (RBI, Natick, MA) was added to this buffer. Adjacent sections were incubated with the tritiated ligand, unlabeled isoguvacine, and 200 μM unlabeled GABA (RBI, Natick, MA) to assess nonspecific binding (Pratt & Bowery, 1993). Sections were rinsed 3 times (3 sec each) in 50 mM Tris-HCl, pH 7.4 and then dipped once in dH2O.
NMDA receptor binding was assessed using a procedure similar to that of Johnson et al. (1994). Sections were incubated at room temperature with 10 nM [3H]MK-801 to assess total binding; anatomically adjacent sections were incubated with the tritiated ligand plus 200 μM unlabeled ketamine to assess nonspecific binding. Following 2 rinses (10 min each) in ice-cold buffer, sections were dipped once in ice-cold acetone:glutaraldehyde solution (2.5% v:v).
To assess potential changes in AMPA receptor binding, sections were labeled with [3H]α-amino-3-hydroxy-5-methyl-4-isoxazolepropionate (AMPA), using a procedure similar to those of Olsen et al. (1987) and Tocco et al. (1992a, b). Slides were rinsed 30 min at 35°C in 100 mM Tris-Acetate (pH 7.2–7.4) containing 100 mM potassium thiocyanate and 100 μM EGTA before incubation 45 min at 4°C in this buffer plus 34 nM [3H]AMPA (53.0 Ci/mmol). Nonspecific binding was assessed by competing the tritiated ligand against 250 μM quisqualate. After incubation, all slides were rinsed twice (10 sec) in ice-cold buffer, followed by one 5-sec rinse in ice-cold buffer diluted 1:1 with dH2O. Slides were then dipped once in ice-cold dH2O, and dried in a stream of warm air.
All sections were then dried in a stream of warm air, fixed in paraformaldehyde vapors, then placed in autoradiographic cassettes (Hypercassettes; Amersham), opposed to film (3H Hyperfilm; Amersham) along with standardized autoradiographic microscales (Amersham), and stored at 4°C for either four (for [3H]muscimol, GABA, or MK-801 binding) or two weeks (for [3H]AMPA binding). The films were then developed (Kodak D-19), fixed (Kodak Fixer), and air dried. Slides were stained with cresylecht violet or thionin.
Density of receptor binding in somatosensory cortex was quantified using a computer-based image analysis system (MCID; Imaging Research Inc., St. Catharines, Ontario). Histological slides and corresponding autoradiograms were placed on a light box (Imaging Research, Inc.), digitized, and aligned. Regions of interest were then defined on the histological sections and samples taken from the corresponding areas of the autoradiograms. For each animal, average optical density was measured in layers II–III, IV, and V–VI (identified with standard morphological criteria of neuronal cell type and packing density) of the hand and hindlimb representations in area 3b of somatosensory cortex (Sur et al., 1982) in each section (e.g., see Fig. 1A). Samples from the hand representation were taken immediately lateral to the central sulcus and extending no more than 1.5 mm laterally, based on previous electrophysiological data (e.g., Garraghty & Kaas, 1991; Garraghty & Muja, 1996; Churchill et al., 1998; Myers et al., 2000), while samples from the hindlimb representation were taken immediately medial to the central sulcus and extending no more than 1.5 mm medially. Measures were standardized against autoradiographic microscales (Amersham) included on each film and expressed in fmol/mg wet tissue weight. Specific binding was calculated by subtracting nonspecific from total binding for each pair of sections, and average specific binding in hand and hindlimb representations was computed for each animal. Finally, to control for differences in overall amounts of binding across animals, the ratio of binding in the hand representation to binding in the hindlimb representation was computed for each animal. Ratios in somatosensory cortex contralateral to the nerve transections were compared to ratios averaged across the two hemispheres of the control monkeys, and from the intact hemisphere of nerve-injured animals.
Figure 1.
Counter-stained coronal sections (Figs. 1A and C), and corresponding digitized autoradiograms of GABAA (Fig. 1B) and GABAB (Fig. 1D) receptor binding in area 3b of an intact control monkey. Samples from the hand representation were taken immediately lateral to the central sulcus (C) and extending no more that 1.5 mm laterally (demarcated by the dark arrows in Figs. 1 A and C; see Sur et a., 19122). Samples from the hindlimb representation were taken medial to the central sulcus (demarcated by the white arrows in Figs. 1A and C.). Consistent with previous reports involving macaque monkeys (Rakic et al., 191212; Shaw et al., 1991), both GABAA (Fig. 1B) and GABAB (Fig. 1D) receptor binding were highest in superficial layers of area 3b. Also consistent with previously reported data (Shaw et al., 1991), our data show that GABAB receptor binding is substantially lower than GABAA receptor binding across all cortical layers. L indicates lateral sulcus. Scale bar = 2 mm.
For presentation purposes, digital light micrographs of histological sections were obtained using an Olympus SZ60 stereozoom microscope and a Kodak MDS290 digital camera. Contrast and brightness of digital images were adjusted in Adobe Photoshop CS. For statistical comparisons, we employed the Mann-Whitney U test (Daniel, 1978). Data from animals surviving nerve transections for 3 days or less were compared to controls. Generally, data from animals that survived the nerve transections for 1–2 months were compared to the corresponding data from the 3-day survival animals.
Results
GABA Receptors
Figure 1 presents counter-stained coronal sections (Figs. 1A and C), and corresponding digitized autoradiograms of GABAA (Fig. 1B) and GABAB (Fig. 1D) receptor binding in area 3b of an intact control monkey. Samples from the hand representation were taken immediately lateral to the central sulcus (C) and extending no more that 1.5 mm laterally (demarcated by the dark arrows in Figs. 1 A and C; see Sur et a., 1982). Samples from the hindlimb representation were taken medial to the central sulcus (demarcated by the white arrows in Figs. 1A and C.). Consistent with previous reports involving macaque monkeys (Rakic et al., 1988; Shaw et al., 1991), both GABAA (Fig. 1B) and GABAB (Fig. 1D) receptor binding were highest in superficial layers of area 3b. Also consistent with previously reported data (Shaw et al., 1991), our data show that GABAB receptor binding is substantially lower than GABAA receptor binding across all cortical layers.
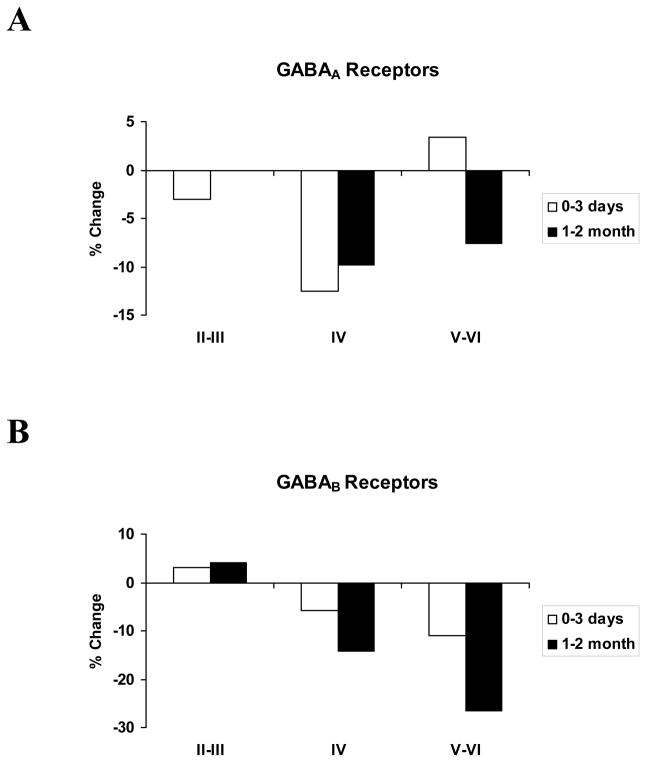
GABAA receptors
Figure 2A presents GABAA receptor binding data. Graphed values are percentage changes in GABAA binding in the cortex deprived of its normal, dominant activating inputs by nerve transection. For the superficial layers of cortex, there were no significant changes in GABAA binding at any of the post-injury time points. For layer IV, on the other hand, there was a 12.5% reduction in GABAA receptor binding early after nerve injury. This reduction is statistically significant (U= 31, p<.01; cf, Wellman et al., 2002). With longer survival durations, the average reduction was, at 9.8%, not significantly different from that found at 3 days (U=12, p>.10). For the lower layers of area 3b, there was no change in GABAA receptor binding over the first 3 days following nerve injury. A small, marginally significant decline in binding ensued over the following 1–2 months (U=4, .05<p<.10).
Figure 2.
A: We find that GABAA receptor binding levels in layer IV decline immediately after nerve injury, and remain reduced 1–2 months later. A smaller reduction in binding is present for layers V–VI only with the longer survival period. B: GABAB receptor binding levels show progressive declines in layer IV and in layers V–VI. These reductions could be due to a decrease in binding affinity, or they could be due to an internalization of the ligand binding sites of the receptors.
GABAB receptors
Figure 2B presents comparable data for GABAB receptors. As for the GABAA receptors, no changes were detected in GABAB binding in the upper cortical layers at any of the survival durations studied. GABAB binding in layer IV 0–3 days after nerve injury was 5.7 % lower in deprived cortex, relative to controls, but this small difference is most likely due to chance (U=51, p>.10). Following 1–2 months post-injury survival durations, binding levels in deprived cortex were 14.3% lower than controls, reflecting a small, but statistically significant decline in binding level relative to that seen at 3 days (U=1, p<.05). In the infragranular layers, GABAB binding was found to be 8.1% lower in deprived cortex 0–3 days after nerve injury, relative to controls, but this difference was also most likely due to chance (U=50, p>.10). Interestingly, in this one instance, we detected a systematic difference in binding levels in deprived cortex between animals sacrificed on the day of nerve transection and those that survived for 3 days. There was a 21.5% decline in GABAB binding by the third day, a significant decrease relative to the animals that were sacrificed within hours of nerve transection (U=1.5, p<.05). With longer survivals, GABAB receptor binding had declined by 26.5% relative to controls. This was not a significant additional decline in binding relative to that seen at 3 days, but it is significantly different from controls (U=11, p<.025), and from the binding levels found hours after nerve transection (U=2, p<.025).
Glutamate Receptors
Figure 3 presents counter-stained coronal sections (Figs. 3A and C) together with corresponding digitized autoradiograms of AMPA (Fig. 1B) and NMDA (Fig. 1D) receptor binding in area 3b of an intact control monkey. Our data show that the laminar distribution of binding to both NMDA and AMPA receptors is comparable to that previously documented in both primary sensory and motor cortices of Old World macaques, with the highest density of binding occurring in the superficial layers (Geyer et al., 1998; Young et al., 1990).
Figure 3.
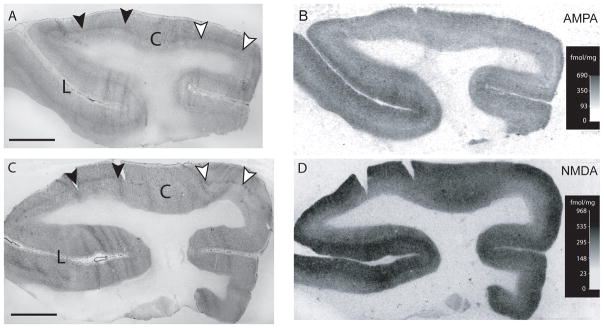
Counter-stained coronal sections (Figs. 3A and C), and corresponding digitized autoradiograms of AMPA (Fig. 3B) and NMDA (Fig. 1D) receptor binding in area 3b of an intact control monkey. The laminar distribution of binding to both NMDA and AMPA receptors was similar to that previously documented in both primary sensory and motor cortices of macaques, with the highest density of binding occurring in the superficial layers (Geyer et al., 19912; Young et al., 1990). C, central sulcus; L, lateral sulcus. Scale bar = 2mm.
AMPA receptors
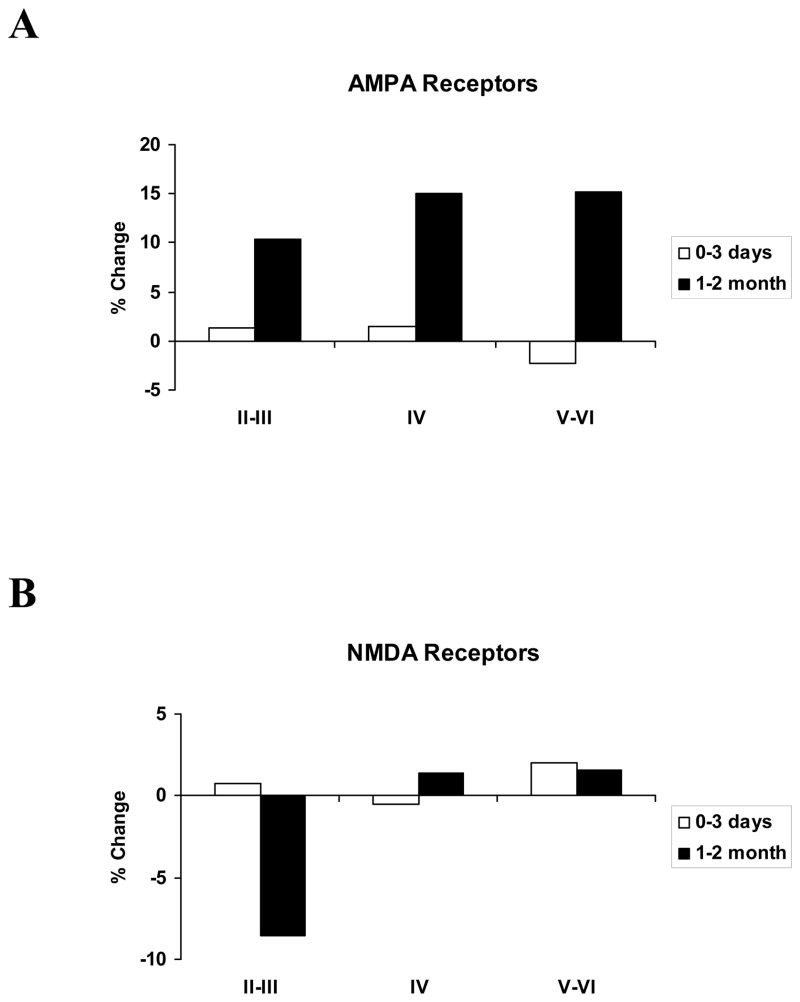
Figure 4A presents AMPA receptor binding data. For the superficial layers of cortex, AMPA receptor binding levels in deprived cortex 0–3 days after nerve injury were virtually identical to controls. In animals that survived nerve injury for 1–2 months, AMPA binding levels were 10.4% higher in deprived cortex than in controls, but this was not a statistically reliable change from the AMPA binding levels at 3 days after nerve injury (U=6, p>.10). In layer IV, as for the superficial cortical layers, there was essentially no change in AMPA binding levels over the first few days after nerve transection. At the longer survival durations, however, AMPA receptor binding in the deprived cortex was found to be increased by 15.0% relative to normal, and this was a significant increase over the binding levels measured 3 days after nerve transection (U=2, p<.025). For the infragranular layers, there was again no difference in AMPA receptor binding 0–3 days after nerve transection relative to controls. With the longer survivals, bindings levels were 15.1% higher than controls, and marginally different from the levels measured 3 days after nerve transection (U=4, .05<p<.10).
Figure 4.
A: Binding for AMPA receptors in deprived area 3b does not change early after nerve transection, but 1–2 months after nerve injury, binding is increased across all cortical layers. B: Binding for NMDA receptors in deprived area 3b is unchanged across all layers at all time points days after nerve transection.
NMDA receptors
Figure 4b presents NMDA receptor binding data. The only notable departure from control binding levels was for the superficial layers in animals surviving nerve transection 1–2 months. This 8.5% difference is not significantly different from that measured 3 days after nerve injury (U=5, p>.10).
Discussion
We report receptor autoradiographic binding data for GABAA, GABAB, AMPA, and NMDA receptors in cortex that has been deprived of it normal activating inputs via peripheral nerve transection in comparison to binding data from intact cortex. The main findings are: 1) there is a reduction in GABAA receptor binding in layer IV of cortex within hours of nerve transection, and this reduced binding in layer IV is maintained to at least 2-months after nerve injury. 2) There was a reduction in GABAB receptor binding in layer IV by 1 month after nerve transection. 3) In infragranular layers of cortex, GABAB binding had declined significantly by the third post-transection day, and remained reduced with longer survival durations. 4) AMPA receptor binding in layer IV was found to be significantly increased by 1 month after nerve transection. 5) The binding patterns for these particular receptors in squirrel monkey cortex are qualitatively consistent with comparable data reported previously by others for macaque cortex (e.g., Rakic et al., 1988; Young et al., 1990; Shaw et al., 1991; Geyer et al., 1998). Presumably these similarities reflect conserved features shared by a common ancestor of modern-day Old and New World primates.
GABA Receptors
GABAA receptors in layer IV
We have previously reported that GABAA receptor binding in layer IV is significantly reduced within hours of nerve transection (Wellman et al., 2002). We can now add that this early reduction in GABAA receptor binding in layer IV persists for at least 2 months. We can also add that GABAB receptor binding in layer IV is reduced by one month after nerve injury. As has been demonstrated by a number of investigators (e.g., Merzenich et al., 1983; Cusick et al., 1990; Calford and Tweedale, 1991), the topographic reorganization that follows peripheral nerve injury proceeds in two phases. The first, classically referred to as unmasking (Merzenich et al., 1983), is detectable immediately after nerve transection. Merzenich et al (1983) suggested that these “immediately unmasked inputs must be present, but almost completely suppressed in the normal case.” Evidence for this follows from several findings. First, Merzenich and colleagues (1983) reported that most of the unmasked receptive fields were on dorsal surfaces of digits (innervated by the radial nerve) whose glabrous surfaces had been deafferented by the median nerve transection, as if the representation of the dorsum skin had preferential access to cortical areas where corresponding glabrous surfaces were normally represented. We provided additional circumstantial support for this idea by combining radial nerve transection with transection of either the median or ulnar nerve (Garraghty et al., 1994). We found that complete or incomplete reorganization could be predicted by a model of dominant and latent representations of the median, ulnar, and radial nerves in cortex. Subsequently, suprathreshold electrical stimulation of peripheral nerves was used to show that the radial nerve normally activates neurons in “median nerve cortex,” whereas the ulnar nerve does not (Schroeder et al., 1995), and that these “latent” inputs progressively grow in amplitude and shorten in latency after the median nerve is transected (Schroeder et al., 1997). Another line of research began with the demonstration that the receptive field sizes of many neurons in cat somatosensory cortex expanded when the noncompetitive GABAA antagonist picrotoxin was applied to cortex (Batuev et al., 1982). Shortly thereafter Hicks and Dykes (1983) found the same was true for the competitive GABAA antagonist biculline. These findings were subsequently replicated in primate somatosensory cortex by Alloway and Burton (1991) using bicuculline. Thus, it appears most likely that a relaxation in tonic inhibitory mechanisms could well account for the immediate unmasking that follows peripheral nerve injury.
The fact remains, however, that the bulk of cortex deprived of its normal activating inputs after nerve injury is not responsive to tactile stimulation immediately. Thus, a release from tonic inhibition, while perhaps necessary, is not apparently sufficient for the second phase of reorganization. Certainly, if the reduced binding for GABAA receptors shortly after nerve injury reflects a relaxation in inhibitory circuits that permits the expression of the unmasked receptive fields, one would imagine that their maintained expression at longer survival intervals might similarly be due to a release from inhibition. The second stage of reorganization, on the other hand, would seem to require other mechanisms. We have shown previously that functional NMDA receptors are necessary for the second phase of reorganization (Garraghty and Muja, 1996), but not for the immediate unmasking (Myers et al., 2000). Others (Juliano et al., 1991; Webster et al., 1991; Avendaño et al., 1995) have demonstrated a role for acetylcholine in long-term cortical plasticity. From the perspective of the involvement of NMDA receptors, normal cholinergic function along with reductions in intracortical inhibition could well collaborate to permit the NMDA receptor-dependent plasticity to proceed. Thus, if the maintained decrease in GABAA receptor binding in layer IV across the survival durations tested in the present experiments does reflect reductions in intracortical inhibition, cortical neurons might well be slightly depolarized, enabling NMDA receptors.
GABAB receptors in layer IV
The additional decline in GABAB receptor binding in layer IV might also play a role in the cortical plasticity, as GABAB blockers have also been shown to produce increases in receptive field sizes of neurons in intact raccoon somatosensory cortex (Chowdhury and Rasmusson, 2002a), and in raccoon somatosensory cortex undergoing reorganization after digit amputation (Chowdhury and Rasmusson, 2002b). The potential role of the reduction in GABAB receptor binding in somatotopic reorganization after nerve injury remains less clear, however, as this receptor subtype is present both pre- and postsynaptically.
Activation of presynaptic GABAB receptors decreases neurotransmitter release by impeding Ca2+ conductance (see Cooper et al., 2003). GABAB receptors on the terminals of thalamocortical axons have been shown to modulate activity in mouse somatosensory cortex (Porter and Nieves, 2004). If thalamocortical axons in primates also have GABAB receptors, a reduction in this pool of GABAB receptors would presumably result in an increase in glutamate release. Such an increase would be expected to affect terminations on both excitatory and inhibitory neurons in cortex (Porter and Nieves, 2004), however, so it is not immediately clear exactly how topographic plasticity would be promoted. Moreover, one would more readily imagine that if there were changes in thalamocortical glutamate release in deprived cortex, it would be a decrease and not an increase. Indeed, in visual cortex, silencing activity in one eye of adult monkeys has been shown to result in decreases in immunostaining for GAD (the synthesizing enzyme for GABA, glutamic acid decarboxylase), GABA (Hendry and Jones, 1988), and GABAA receptors (Hendry et al., 1990, 1994) in deprived cortex. Such reductions would certainly not be expected to follow an increase in thalamocortical glutamate release. Similarly, we have shown comparable reductions in immunostaining for GABA in primate somatosensory cortex after nerve injury (Garraghty et al., 1991), and report reductions in GABAA receptor in autoradiographic labeling of GABAA receptors in the present results and ones reported previously (Wellman et al., 2002). Welker and colleagues (1989) have also reported reductions in GAD staining in adult mouse cortex following whisker ablations. Thus, it seems relatively improbable that the reductions in GABAB receptor binding in layer IV reported here involve presynaptic receptors on thalamocortical terminals.
GABAB receptors could also be located presynaptically on local circuit neurons in layer IV. If so, one would expect increased transmitter release at their terminations. If intracortical presynaptic GABAB receptors are reflected in the decreased binding we report here, the effect would seemingly not include autoreceptors on GABAergic local circuit neurons, as this would presumably result in an increase in GABA release, an outcome that is incompatible with the overall reduction in GABA in the cortex reported previously (Garraghty et al., 1991). Moreover, while GABAB autoreceptors have been shown to contribute to NMDA-dependent LTP (e.g., Davies et al., 1991), this contribution involves their activation, resulting in reduced GABA release, and consequently, a reduction in postsynaptic GABAA receptor-mediated inhibition, enabling NMDA receptors. This scenario is certainly not consistent with a reduction in binding for GABAB autoreceptors, and would require an increase, and not a decrease (Garraghty et al., 1991) in GABA.
Alternatively, the reduced layer IV binding reported here could involve GABAB receptors on the terminals of intracortical glutamatergic neurons. One could posit that excitatory layer IV intracortical connections (e.g., Cowan and Stricker, 2004) might convey inputs from neurons outside of the deprived region of cortex, thereby permitting the transmission of new receptive fields. Indeed, others (e.g, Darien-Smith and Gilbert, 1995; Florence et al., 1998) have suggested a prominent role for lateral intracortical connections in plasticity in adult primate cortex. We cannot rule out the possibility that lateral connections contribute to somatosensory cortical plasticity after peripheral nerve injury. We can note, however, that the latency of the reorganized cortical response to radial nerve stimulation after median nerve transection is not detectably different from the median nerve’s activation latency in intact cortex (Schroeder et al., 1997), suggesting that the new receptive fields are not being delivered indirectly. We can also note that the intracortical changes reported by Darian-Smith and Gilbert (1994) were predominantly supragranular, whereas the changes in GABAB receptor binding detected in the present experiments were confined to granular and infragranular layers.
Finally, the affected pool of GABAB receptors could be located postsynaptically. GABAB receptors are metabotropic, and their postsynaptic inhibitory effects are due to second messenger-mediated phosphorylation of K+ channels. The resulting inhibitory postsynaptic potential is, thus, delayed relative to the faster chloride-mediated effects of the ionotropic GABAA receptors (see Cooper et al., 2003). This temporal difference has led to the suggestion that the delayed inhibition arising from GABAB receptor activation would be more effective in blocking excitation mediated by NMDA receptors than that due to AMPA receptor activation (Mott et al., 1999). More recently, Otmakhova and Lisman (2004) have reported that antagonists of GABAB receptors block the afterhyperpolarization recorded in hippocampal CA1 neurons when the perforant path is stimulated. They suggest that the GABAB receptor-mediated afterhyperpolarization acts as a “brake” on the NMDA response in the CA1 neurons. They also note that the perforant path inputs terminate on distal dendrites in stratum lacunosum-moleculare, and Miyashita and Kubo (1997) have shown that this layer has much higher levels of immunoreactivity for the GABAB-receptor associated protein GIRK1 than the more proximal stratum radiatum. Subsequently, Kulik et al. (2003) showed that GABAB receptors in stratum lacunosum-moleculare are predominantly postsynaptic, and located in spines very close to excitatory synapses. This spatial arrangement suggests a special role for GABAB inhibition at the distally-located excitatory perforant path terminations in stratum lacunosum-moleculare. We find a reduction in GABAB receptor binding in layer IV within a month after nerve injury. This time-frame overlaps with the second phase of reorganization that we have shown to be NMDA receptor-dependent (Garraghty & Muja, 1996). Interestingly, we have also shown that progressive changes in the dendritic arbors of neurons in reorganized somatosensory cortex are confined to more distal portions of the arbors (Churchill et al., 2004), and we hypothesize that the latent inputs that gain expression during reorganization terminate distally. In any event, the changes in GABAB binding in layer IV of somatosensory cortex after nerve injury correlates temporally with the second, NMDA receptor-dependent phase of reorganization. We suggest that the reduced binding for GABAB receptors in layer IV reported here more likely reflects changes in postsynaptic rather than presynaptic receptors.
GABA receptors in infragranular layers
We also find some changes in GABA receptors in infragranular layers of cortex. A marginally significant reduction in GABAA receptor binding emerges with 1–2 month post-transection survival durations. A more substantial effect is found for GABAB receptors where binding levels are significantly reduced by 3 days after nerve injury, with this reduction persisting over the longer survival durations. Indeed, the reduced binding levels for GABAB receptors in the infragranular layers was the largest change we detected at any level of cortex for any of the receptors we examined. Both pre- and postsynaptic GABAB receptors almost certainly exist in the infragranular layers, and all of the issues discussed briefly above regarding layer IV are potentially relevant. The fundamental difference is that infragranular neurons are presumably expressing receptive fields that are conveyed to them via vertically oriented functional cortical columns. As such, one could imagine that there would be no need for modulations in synaptic connections. We can only speculate that changes in the deeper layers of cortex might play some role in descending modulation of somatosensory nuclei in brainstem and thalamus. Both the cuneate nucleus and the ventroposterior lateral nucleus undergo reorganization after peripheral nerve injury in adult monkeys (e.g., Garraghty & Kaas, 1991; Xu and Wall, 1997; Churchill et al., 2001), and both have been shown to be sensitive to corticofugal influences (e.g., Ergenzinger et al., 1998; Wang and Wall, 2005).
Glutamate Receptors
AMPA receptors
We find no changes in the levels of AMPA receptor binding early after nerve injury. With longer survival durations, however, there is a significant increase in AMPA receptor binding in layer IV and a marginally significant increase in infragranular layers. As mentioned above regarding the decline in binding for GABAB receptors in layer IV, this increase in AMPA receptor binding coincides temporally with the second, NMDA receptor-dependent phase of reorganization (Garraghty and Muja, 1996).
In 1973, Bliss and Lomø reported in their now-classic study that repeated, intense stimulation of the perforant pathway led to a sustained increase in synaptic efficacy at its connections with cells in the dentate gyrus (see also, Bliss and Gardner-Medwin, 1973). Other investigators extended this finding to other synapses in the hippocampus proper (e.g., Schwartzkroin and Wester, 1975; Alger and Teyler, 1976), including those of Schaffer collaterals with cells in CA1. Following these early demonstrations, attention turned to identifying the cellular and molecular mechanisms responsible for the plasticity. At the neuronal receptor level, Collingridge and colleagues (1983) demonstrated that the blockade of NMDA receptors with APV prevented the induction of the long-term potentiation (LTP). Once LTP had been induced, however, blockade of NMDA receptors had no effect on the potentiated response. Thus, NMDA receptors were shown to be necessary for the induction of LTP but not for its maintenance. Similarly, NMDA receptors are necessary for the second phase of nerve injury-induced reorganization in adult primate cortex (Garraghty and Muja, 1996), but not for its maintenance (Myers et al., 2000; see also, Garraghty et al., 1998). Because the maintenance of LTP was shown not to be NMDA receptor-dependent, attention turned to other mechanisms. A body of evidence developed implicating changes in postsynaptic AMPA receptors. One of the possible changes in this class of receptors that could support an increase in synaptic efficacy was an increase in the number of AMPA receptors, a possibility that has been confirmed (e.g., Tocco et al., 1992b; Maren et al., 1993). Indeed, these observations served to open the floodgates in the study of AMPA receptor trafficking (for recent reviews, see Song and Huganir, 2002; Malinow and Malenka, 2002; Ju et al., 2004; Collingridge et al., 2004). Increased binding for AMPA receptors, thus, is yet another parallel between nerve injury-induced reorganization in primate somatosensory cortex and NMDA-dependent LTP in the hippocampus.
NMDA receptors
We find no net changes in NMDA receptor binding in primary somatosensory cortex at any time point tested in the present study. Interestingly, there has been a report of increased NMDA receptor binding in the CA1 region of adult rat hippocampus after induction of LTP (Grosshans et al., 2002), so we could conceivably have found changes in that subclass of glutamate receptors as well. In rat hippocampus, the finding could well relate to developmental changes in AMPA receptor levels (Petralia et al., 1999; see below).
How do Nerve Injury-Induced Cortical Plasticity and Hippocampal LTP Differ?
We have shown three parallels between adult primate cortical somatosensory plasticity and NMDA-dependent LTP in the hippocampus. Both require normally functioning NMDA receptors for induction. Neither requires normal NMDA function for their maintenance. Both result in an increase in AMPA receptor binding. Important differences between these forms of plasticity exist, however. As we have noted previously, hippocampal LTP is induced with a specific pattern of repetitive stimulation. There is absolutely no reason to believe that peripheral nerve transection would be followed by any change in the pattern of activity in the remaining nerve(s). Rather, it would seem more likely that relatively low levels of activation are able to utilize NMDA receptor-dependent processes to effect somatosensory cortical plasticity. Second, hippocampal LTP can be induced within seconds, whereas the second, NMDA receptor-dependent phase of somatosensory cortical plasticity proceeds over days to weeks (e.g., Merzenich et al., 1983). These differing time-courses clearly suggest the existence of important mechanistic differences, perhaps ones that would be of relevance to the distinction in the memory literature between consolidation and storage. Relatedly, these two forms of plasticity differ in their durations. Hippocampal LTP is not permanent, and presumably it would be quite maladaptive were that not the case. If hippocampal LTP is involved in learning and memory, its role would apparently be in the consolidation of memories that are to be stored elsewhere. The changes that occur in somatosensory cortex after nerve injury are still evident over 2 years later (Churchill et al., 1998), and there is no reason to suspect that they would not persist permanently. The temporal difference in the durations of these two models of plasticity clearly implies the existence of molecular differences that relate to the transient nature of hippocampal LTP and the apparently permanent changes found in somatosensory cortex. Indeed, it is this difference that suggests that the molecular underpinnings of these two forms of plasticity are different in potentially very important ways.
How Might Cortical Somatosensory Plasticity Proceed?
As discussed earlier, the topographic reorganization of the cortex that follows peripheral nerve transection in adult monkeys proceeds in no less than two stages. The present results show that the first, unmasking phase is strongly correlated with a decrease in autoradiographic binding for GABAA receptors in layer IV of cortex. Others (e.g., Diamond et al., 1994) have reported that plastic changes are first observed in superficial layers of the cortex, and then later in layer IV. We have found no changes in binding for any of the GABA or glutamate receptor subtypes in superficial layers of the cortex at any time point after nerve injury. We suggest that the reduced binding for GABAA receptors in layer IV reported here reflects a reduction in afferent-driven inhibition, permitting the expression of masked inputs, in much the same way that application of the GABAA antagonist bicuculline results in the expansion of receptive fields of many cortical neurons (e.g., Alloway and Burton, 1991).
The second stage of reorganization has been shown to be NMDA receptor-dependent (Garraghty and Muja, 1996), and the present results demonstrate correlated changes in binding levels for AMPA and GABAB receptors. Each of these receptor types have been implicated in NMDA-dependent LTP in the hippocampus, and despite the differences between somatosensory and hippocampal plasticity, the similarities are striking. Much of the hippocampal work has involved the use of relatively immature subjects in which the numbers of “silent synapses” are large relative to adult animals (e.g., Petralia et al., 1999). Silent synapses are ones that have NMDA receptors but not AMPA receptors. Early in development, many thalamocortical synapses have been shown to be silent, and can be converted to functional synapses with LTP (e.g., Crair and Malenka, 1995; Isaac et al., 1997). These findings have prompted the suggestion that LTP is intimately involved in the topographic refinement of thalamocortical connections. At least in the hippocampus, LTP is made possible at silent synapses by the presence of GABAB autoreceptors on GABAergic neurons. At low rates of stimulation, GABA release activates postsynaptic GABAA receptors, reducing the opportunity for NMDA receptor-mediated responses. At high stimulation frequencies, on the other hand, GABA reduces its own release via it action at the presynaptic GABAB receptors (e.g., Davies et al., 1991). This enables an NMDA receptor-mediated postsynaptic response, and the induction of LTP, with the consequent increase in AMPA receptor-mediated postsynaptic responses (e.g., Liao et al., 1995). This scenario can result in the induction of LTP not only at silent synapses, but also in preparations with pharmacologically blocked AMPA receptors (e.g., Kauer et al., 1988; Muller et al., 1988). In intact monkey cortex, a small, delayed response to electrical stimulation of the radial nerve can be recorded in median nerve cortex (Schroeder et al., 1995). Perhaps this response is mediated by NMDA receptors at “silent synapses” on distal portions of the arbors of cortical neurons. In this view, the cortical neurons may be rendered more excitable by a reduction in postsynaptic GABA receptors, thereby enabling NMDA receptors. One potentially interesting implication of this view is that adult somatosensory cortex retains its remarkable facility to undergo plastic reorganizational changes at least in part because it retains features that are used to shape normal developmental outcomes in other brain structures.
There still remains the question of what would drive the reductions in postsynaptic GABA receptors, permitting the second phase of reorganization to proceed. One potential mechanism that is gaining an increasing amount of attention is the concept of homeostatic plasticity (e.g., see Miller, 1996; Turrigiano and Nelson, 2000, 2004 for reviews). In this view, neurons defend their activity levels by making adjustments in transmitter receptors and/or ion channels as their levels rise above or fall below their homeostatic range. In monkey somatosensory cortex, the activity levels of many neurons may well fall below their homeostatic range when their normal activating inputs are drastically reduced by peripheral nerve transection. The immediate unmasking of new receptive fields by some of the deprived neurons permits them to remain within their range, whereas the regions of cortex throughout which plasticity proceeds over the ensuing days to weeks is populated by neurons with reduced activity. Because the GABAA binding levels are already reduced, perhaps the lowered levels of activity in these unresponsive regions of cortex is the stimulus for reducing the numbers or binding affinities of postsynaptic GABAB receptors, leading ultimately to the NMDA receptor-dependent emergence of responsiveness during the second stage of reorganization. The presumed slower time-course of homeostatic plasticity is consistent with the delayed second phase of cortical reorganization, as is the demonstration that, under some circumstances, the process is associated with an increase in postsynaptic AMPA receptors (Wierenga et al., 2005).
With even longer post-transection survival durations (11–28 months), the reorganized cortical map becomes more topographically refined than is the case with shorter survival intervals (Garraghty and Kaas, 1991; Churchill et al., 1998). This refinement is accomplished by a reduction in the receptive field sizes of neurons in the reorganized portion of the map. One imagines that these changes reflect a restoration of more nearly normal patterns of intracortical inhibition, a process that could manifest itself with recoveries in the binding levels of GABA receptors.
Acknowledgments
We thank Elizabeth Garman for technical assistance.
Grant sponsor: National Institutes of Health/National Institute of Neurological Disorders and Stroke; Grant number: NS37348
Literature Cited
- Alger BE, Teyler TJ. Long-term and short-term plasticity in the Ca1, CA3, and dentate regions of the rat hippocampal slice. Brain Res. 1976;110:463–480. doi: 10.1016/0006-8993(76)90858-1. [DOI] [PubMed] [Google Scholar]
- Alloway K, Burton H. Differential effects of GABA and bicuculline on rapidly and slowly adapting neurons in primary somatosensory cortex of primates. Exp Brain Res. 1991;85:598–610. doi: 10.1007/BF00231744. [DOI] [PubMed] [Google Scholar]
- Avendaño C, Umbriaco D, Dykes RW, Descarres L. Decrease and long-term recovery of choline acetyltransferase immunoreactivity in adult cat somatosensory cortex after peripheral nerve transections. J Comp Neurol. 1995;354:321–332. doi: 10.1002/cne.903540302. [DOI] [PubMed] [Google Scholar]
- Bashir ZI, Berretta N, Bortolotto ZA, Clark K, Davies CH, Frenguelli BG, Harvey J, Potier B, Collingridge GL. NMDA receptors and long-term potentiation in the hippocampus. In: Collingridge GL, Watkins JC, editors. The NMDA Receptor. 2. New York: Oxford University Press; 1994. pp. 294–312. [Google Scholar]
- Batuev AS, alexandrov AA, Scheynikov NA. Picrotoxin action on the receptive fields of the cat sensorimotor cortex neurons. J Neurosci Res. 1982;7:49–55. doi: 10.1002/jnr.490070106. [DOI] [PubMed] [Google Scholar]
- Bliss TV, Gardner-Medwin AR. Long-lasting potentiation of synaptic transmission in the dentate area of the unanaesthetized rabbit following stimulation of the perforant path. J Physiol (Lond) 1973;232:357–374. doi: 10.1113/jphysiol.1973.sp010274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bliss TV, Lomø T. Long-lasting potentiation of synaptic transmission in the dentate area of the anaesthetized rabbit following stimulation of the perforant path. J Physiol. 1973;232:331–356. doi: 10.1113/jphysiol.1973.sp010273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown TH, Kairiss EW, Keenan CL. Hebbian synapses: biophysical mechanisms and algorithms. Annu Rev Neurosci. 1990;13:475–511. doi: 10.1146/annurev.ne.13.030190.002355. [DOI] [PubMed] [Google Scholar]
- Buonomano DV, Merzenich MM. Cortical plasticity: from synapses to maps. Annu Rev Neurosci. 1998;21:149–186. doi: 10.1146/annurev.neuro.21.1.149. [DOI] [PubMed] [Google Scholar]
- Calford MB, Tweedale R. Interhemispheric transfer of plasticity in cerebral cortex. Science. 1990;249:805–807. doi: 10.1126/science.2389146. [DOI] [PubMed] [Google Scholar]
- Calford MB, Tweedale R. Immediate expansion of receptive fields of neurons in area 3b of macaque monkeys after digit denervation. Somatosens Motor Res. 1991;8:249–260. doi: 10.3109/08990229109144748. [DOI] [PubMed] [Google Scholar]
- Chowdhury SA, Rasmusson DD. Comparison of receptive field expansion produced by GABAB and GABAA receptor antagonists in raccoon primary somatosensory cortex. Exp Brain Res. 2002a;144:114–121. doi: 10.1007/s00221-002-1035-7. [DOI] [PubMed] [Google Scholar]
- Chowdhury SA, Rasmusson DD. Effect of GABAB receptor blockade on receptive fields of raccoon somatosensory cortical neurons during reorganization. Exp Brain Res. 2002b;145:150–157. doi: 10.1007/s00221-002-1130-9. [DOI] [PubMed] [Google Scholar]
- Churchill JD, Arnold LL, Garraghty PE. Somatotopic reorganization in the brainstem and thalamus following peripheral nerve injury in adult primates. Brain Res. 2001;910:142–152. doi: 10.1016/s0006-8993(01)02703-2. [DOI] [PubMed] [Google Scholar]
- Churchill JD, Muja N, Myers WA, Besheer J, Garraghty PE. Somatotopic consolidation: a third phase of reorganization after peripheral nerve injury in adult squirrel monkeys. Exp Brain Res. 1998;118:189–196. doi: 10.1007/s002210050271. [DOI] [PubMed] [Google Scholar]
- Churchill JD, Tharp JA, Wellman CL, Sengelaub DR, Garraghty PE. Morphological correlates of injury-induced reorganization in primate somatosensory cortex. BMC Neuroscience. 2004;5:43. doi: 10.1186/1471-2202-5-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collingridge GL, Bliss TVP. NMDA receptors – their role in long-term potentiation. Trends Neurosci. 1987;10:288–293. [Google Scholar]
- Collingridge GL, Isaac JT, Wang YT. Receptor trafficking and synaptic plasticity. Nature Rev Neurosci. 2004;5:952–962. doi: 10.1038/nrn1556. [DOI] [PubMed] [Google Scholar]
- Collingridge GL, Kehl SJ, McLennan H. Excitatory amino acids in synaptic transmission in the Schaffer-collateral commissural pathway of the rat hippocampus. J Physiol (Lond) 1983;334:33–46. doi: 10.1113/jphysiol.1983.sp014478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper JR, Bloom FE, Roth RH. The Biochemical Basis of Neuropharmacology. 8. New York: Oxford University Press; 2003. [Google Scholar]
- Cowan AI, Stricker C. Functional connectivity in layer IV local excitatory circuits of rat somatosensory cortex. J Neurophysiol. 2004;92:2137–2150. doi: 10.1152/jn.01262.2003. [DOI] [PubMed] [Google Scholar]
- Crair MC, Malenka RC. A critical period for long-term potentiation at thalamocortical synapses. Nature. 1995;375:325–328. doi: 10.1038/375325a0. [DOI] [PubMed] [Google Scholar]
- Cusick CG, Wall JT, Whiting JH, Jr, Wiley RG. Temporal progression of cortical reorganization following nerve injury. Brain Res. 1990;537:355–358. doi: 10.1016/0006-8993(90)90385-o. [DOI] [PubMed] [Google Scholar]
- Daniel WW. Applied Nonparametric Statistics. Boston: Houghton Mifflin Co; 1978. [Google Scholar]
- Darian-Smith C, Gilbert CD. Axonal sprouting accompanies functional reorganization in adult cat striate cortex. Nature. 1994;368:737–740. doi: 10.1038/368737a0. [DOI] [PubMed] [Google Scholar]
- Darian-Smith C, Gilbert CD. Topographic reorganization in the striate cortex of the adult cat and monkey is cortically mediated. J Neurosci. 1995;15:1631–1647. doi: 10.1523/JNEUROSCI.15-03-01631.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies CH, Starkey SJ, Pozza MF, Collingridge GL. GABAB autoreceptors regulate the induction of LTP. Nature. 1991;349:609–611. doi: 10.1038/349609a0. [DOI] [PubMed] [Google Scholar]
- Diamond ME, Huang W, Ebner FF. Laminar comparison of somatosensory cortical plasticity. Science. 1994;265:1885–1888. doi: 10.1126/science.8091215. [DOI] [PubMed] [Google Scholar]
- Ergenzinger ER, Glasier MM, Hahm JO, Pons TP. Cortically induced thalamic plasticity in the primate somatosensory system. Nature Neurosci. 1998;1:226–229. doi: 10.1038/673. [DOI] [PubMed] [Google Scholar]
- Florence SL, Garraghty PE, Wall JT, Kaas JH. Sensory afferent projections and area 3b somatotopy following median nerve cut and repair in macaque monkeys. Cerebral Cortex. 1994;4:391– 407. doi: 10.1093/cercor/4.4.391. [DOI] [PubMed] [Google Scholar]
- Florence SL, Hackett TA, Strata F. Thalamic and cortical contributions to neural plasticity after limb amputation. J Neurophysiol. 2000;83:3154–2159. doi: 10.1152/jn.2000.83.5.3154. [DOI] [PubMed] [Google Scholar]
- Florence SL, Taub HB, Kaas JH. Large-scale sprouting of cortical connections after peripheral injury in adult macaque monkeys. Science. 1998;282:1117–1121. doi: 10.1126/science.282.5391.1117. [DOI] [PubMed] [Google Scholar]
- Garraghty PE, Churchill JD, Banks MK. Adult neural plasticity: similarities between two paradigms. Curr Dir Psych Sci. 1998;7:87–91. [Google Scholar]
- Garraghty PE, Kaas JH. Large-scale functional reorganization in adult monkey cortex after peripheral nerve injury. Proc Natl Acad Sci USA. 1991;88:6976–6980. doi: 10.1073/pnas.88.16.6976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garraghty PE, LaChica EA, Kaas JH. Injury-induced reorganization of somatosensory cortex is accompanied by reductions in GABA staining. Somatosens Motor Res. 1991;8:347–354. doi: 10.3109/08990229109144757. [DOI] [PubMed] [Google Scholar]
- Garraghty PE, Hanes DP, Florence SL, Kaas JH. Pattern of peripheral deafferentation predicts reorganizational limits in adult primate somatosensory cortex. Somatosens Motor Res. 1994;11:109–117. doi: 10.3109/08990229409028864. [DOI] [PubMed] [Google Scholar]
- Garraghty PE, Muja N. NMDA receptors and plasticity in adult primate somatosensory cortex. J Comp Neurol. 1996;367:319–326. doi: 10.1002/(SICI)1096-9861(19960401)367:2<319::AID-CNE12>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Geyer S, Matelli M, Luppino G, Schleicher A, Jansen Y, Palomero-Gallagher N, Zilles K. Receptor autoradiographic mapping of the mesial motor and premotor cortex of the macaque monkey. J Comp Neurol. 1998;397:231–250. doi: 10.1002/(sici)1096-9861(19980727)397:2<231::aid-cne6>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Grosshans DR, Clayton DA, Coultrap SJ, Browning MD. LTP leads to rapid surface expression of NMDA but not AMPA receptors in adult rat CA1. Nature Neurosci. 2002;5:27–33. doi: 10.1038/nn779. [DOI] [PubMed] [Google Scholar]
- Hendry SHC, Fuchs J, deBlas AL, Jones EG. Distribution and plasticity of immunocytochemically localized GABAA receptors in adult monkey visual cortex. J Neurosci. 1990;10:2438–2450. doi: 10.1523/JNEUROSCI.10-07-02438.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendry SHC, Hunstman M-M, Viñuela A, Möhler H, de Blas AL, Jones EG. GABAA receptor subunit immunoreactivity in primate visual cortex: distribution in macaques and humans and regulation by visual input in adulthood. J Neurosci. 1994;14:2383–2401. doi: 10.1523/JNEUROSCI.14-04-02383.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendry SHC, Jones EG. Activity-dependent regulation of GABA expression in the visual cortex of adult monkeys. Neuron. 1988;1:701–712. doi: 10.1016/0896-6273(88)90169-9. [DOI] [PubMed] [Google Scholar]
- Hicks TP, Dykes RW. Receptive field size for certain neurons in primary somatosensory cortex is determined by GABA-mediated intracortical inhibition. Brain Res. 1983;274:160–164. doi: 10.1016/0006-8993(83)90533-4. [DOI] [PubMed] [Google Scholar]
- Isaac JT, Crair MC, Nicoll RA, Malenka RC. Silent synapses during development of thalamocortical inputs. Neuron. 1997;18:269–280. doi: 10.1016/s0896-6273(00)80267-6. [DOI] [PubMed] [Google Scholar]
- Johnson AE, Liminga U, Liden A, Lindefors N, Gunne LM, Wiesel FA. Chronic treatment with a classical neuroleptic alters excitatory amino acid and GABAergic neurotransmission in specific regions of the rat brain. Neuroscience. 1994;63:1003–1020. doi: 10.1016/0306-4522(94)90568-1. [DOI] [PubMed] [Google Scholar]
- Ju W, Morishita W, Tsui J, Gaietta G, Deerinck TJ, Adams SR, Garner CC, Tsien RY, Ellisman MH, Malenka RC. Activity-dependent regulation of dendritic synthesis and trafficking of AMPA receptors. Nature Neurosci. 2004;7:244–253. doi: 10.1038/nn1189. [DOI] [PubMed] [Google Scholar]
- Juliano SL, Ma W, Eslin D. Cholinergic depletion prevents expansion of topographic maps in somatosensory cortex. Proc Natl Acad Sci USA. 1991;88:780–784. doi: 10.1073/pnas.88.3.780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaas JH, Florence SL, Jain N. Subcortical contributions to massive cortical reorganizations. Neuron. 1999;22:657–660. doi: 10.1016/s0896-6273(00)80725-4. [DOI] [PubMed] [Google Scholar]
- Kauer JA, Malenka RC, Nicoll RA. A persistent postsynaptic modification mediate long-term potentiation in the hippocampus. Neuron. 1988;1:911–917. doi: 10.1016/0896-6273(88)90148-1. [DOI] [PubMed] [Google Scholar]
- Kulik A, Vida I, Luján R, Haas CA, López-Bendito G, Shigemoto R, Frotscher M. Subcellular localization of metabotropic GABAB receptor subunits GABAB1a/b and GABAB2 in the rat hippocampus. J Neurosci. 2003;23:11026–11035. doi: 10.1523/JNEUROSCI.23-35-11026.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao D, Hessler NA, Malinow R. Activation of postsynaptically silent synapses during pairing-induced LTP in CA1 region of hippocampal slice. Nature. 1995;375:400–404. doi: 10.1038/375400a0. [DOI] [PubMed] [Google Scholar]
- Malinow R, Malenka RC. AMPA receptor trafficking and synaptic plasticity. Annu Rev Neurosci. 2002;25:103–126. doi: 10.1146/annurev.neuro.25.112701.142758. [DOI] [PubMed] [Google Scholar]
- Maren S, Tocco G, Baudry M, Thompson RF. Postsynaptic factors in the expression of long-term potentiation (LTP): increased glutamate receptor binding following LTP induction in vivo. Proc Natl Acad Sci USA. 1993;90:9654–9658. doi: 10.1073/pnas.90.20.9654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez JL, Jr, Derrick BE. Long-term potentiation and learning. Annu Rev Psych. 1996;47:173–203. doi: 10.1146/annurev.psych.47.1.173. [DOI] [PubMed] [Google Scholar]
- Merzenich MM, Kaas JH, Wall JT, Sur M, Nelson RJ, Felleman DJ. Progression of change following median nerve section in the cortical representation of the hand in areas 3b and 1 in adult owl and squirrel monkeys. Neuroscience. 1983;10:639–665. doi: 10.1016/0306-4522(83)90208-7. [DOI] [PubMed] [Google Scholar]
- Miller KD. Synaptic economics: competition and cooperation in synaptic plasticity. Neuron. 1996;17:371–374. doi: 10.1016/s0896-6273(00)80169-5. [DOI] [PubMed] [Google Scholar]
- Miyashita T, Kubo Y. Localization and developmental changes of the expression of two inward rectifying K+-channel proteins in the rat brain. Brain Res. 1997;750:251–263. doi: 10.1016/s0006-8993(96)01365-0. [DOI] [PubMed] [Google Scholar]
- Morris RGM, Davis M. The role of NMDA receptors in learning and memory. In: Collingridge GL, Watkins JC, editors. The NMDA Receptor. 2. New York: Oxford University Press; 1994. pp. 340–375. [Google Scholar]
- Mott DD, Li Q, Okazaki MM, Turner DA, Lewis DV. GABAB-receptor-mediated currents in interneurons of the dentate-hilus border. J Neurophysiol. 1999;82:1438–1450. doi: 10.1152/jn.1999.82.3.1438. [DOI] [PubMed] [Google Scholar]
- Muller D, Joly M, Lynch G. Contributions of quisqualate and NMDA receptors to the induction and expression of LTP. Science. 1988;242:1694–1697. doi: 10.1126/science.2904701. [DOI] [PubMed] [Google Scholar]
- Myers WA, Churchill JD, Muja N, Garraghty PE. Role of NMDA receptors in adult primate cortical somatosensory plasticity. J Comp Neurol. 2000;418:373–382. [PubMed] [Google Scholar]
- Olsen RW, Szamraj O, Houser CR. [3H]AMPA binding to glutamate receptor subpopulations in rat brain. Brain Res. 1987;402:243–254. doi: 10.1016/0006-8993(87)90030-8. [DOI] [PubMed] [Google Scholar]
- Otmakhova NA, Lisman JE. Contribution of Ih and GABAB to synaptically induced afterhyperpolarizations in CA1: a brake on the NMDA response. J Neurophysiol. 2004;92:2027–2039. doi: 10.1152/jn.00427.2004. [DOI] [PubMed] [Google Scholar]
- Petralia RS, Esteban JA, Wang Y-X, Partridge JG, Zhao H-M, Wenthold RJ. Selective acquisition of AMPA receptors over postnatal development suggests a molecular basis for silent synapses. Nature Neurosci. 1999;2:31–36. doi: 10.1038/4532. [DOI] [PubMed] [Google Scholar]
- Pons TP, Garraghty PE, Ommaya AK, Kaas JH, Taub E, Mishkin M. Massive reorganization of the primary somatosensory cortex after peripheral sensory deafferentation. Science. 1991;252:1857–1860. doi: 10.1126/science.1843843. [DOI] [PubMed] [Google Scholar]
- Porter JT, Nievee D. Presynaptic GABAB receptors modulate thalamic excitation of inhibitory and excitatory neurons in the mouse barrel cortex. J Neurophysiol. 2004;92:2762–2770. doi: 10.1152/jn.00196.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratt GD, Bowery NG. Repeated administration of desipramine and a GABAB receptor antagonist, CGP 36742, discretely upregulates GABAB receptor binding sites in rat prefrontal cortex. Br J Pharmacol. 1993;110:724–735. doi: 10.1111/j.1476-5381.1993.tb13872.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakic P, Goldman-Rakic PS, Gallager D. Quantitative autoradiography of major neurotransmitter receptors in the monkey striate and extrastriate cortex. J Neurosci. 1988;8:3670–3690. doi: 10.1523/JNEUROSCI.08-10-03670.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder CE, Seto S, Arezzo JC, Garraghty PE. Electrophysiologic evidence for overlapping dominant and latent inputs to somatosensory cortex in squirrel monkeys. J Neurophysiol. 1995;74:722–732. doi: 10.1152/jn.1995.74.2.722. [DOI] [PubMed] [Google Scholar]
- Schroeder CE, Seto S, Garraghty PE. Emergence of radial nerve dominance in “median nerve cortex” after median nerve transection in an adult squirrel monkey. J Neurophysiol. 1997;77:522–526. doi: 10.1152/jn.1997.77.1.522. [DOI] [PubMed] [Google Scholar]
- Schwartzkroin PA, Wester K. Long-lasting facilitation of a synaptic potential following tetanization in the in vitro hippocampal slice. Brain Res. 1975;89:107–119. doi: 10.1016/0006-8993(75)90138-9. [DOI] [PubMed] [Google Scholar]
- Shaw C, Cameron L, March D, Cynader M, Zielinski B, Hendrickson A. Pre- and postnatal development of GABA receptors in Macaca monkey visual cortex. J Neurosci. 1991;11:3943–3959. doi: 10.1523/JNEUROSCI.11-12-03943.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song I, Huganir RL. Regulation of AMPA receptors during synaptic plasticity. Trends Neurosci. 2002;25:578–588. doi: 10.1016/s0166-2236(02)02270-1. [DOI] [PubMed] [Google Scholar]
- Sur M, Nelson RJ, Kaas JH. Representations of the body surface in cortical areas 3b and 1 of squirrel monkeys: comparisons with other primates. J Comp Neurol. 1982;211:177–192. doi: 10.1002/cne.902110207. [DOI] [PubMed] [Google Scholar]
- Tocco G, Annala AJ, Baudry M, Thompson RF. Learning of a hippocampal-dependent conditioning task changes the binding properties of AMPA receptors in rabbit hippocampus. Behav Neural Biol. 1992;58:222–231. doi: 10.1016/0163-1047(92)90510-b. [DOI] [PubMed] [Google Scholar]
- Tocco G, Maren S, Shors TJ, Baudry M, Thompson RF. Long-term potentiation is associated with increased [3H]AMPA binding in rat hippocampus. Brain Res. 1992;573:228–234. doi: 10.1016/0006-8993(92)90767-4. [DOI] [PubMed] [Google Scholar]
- Turrigiano GG, Nelson SB. Hebb and homeostasis in neuronal plasticity. Curr Opinion Neurobiol. 2000;10:358–364. doi: 10.1016/s0959-4388(00)00091-x. [DOI] [PubMed] [Google Scholar]
- Turrigiano GG, Nelson SB. Homeostatic plasticity in the developing nervous system. Nature Rev Neurosci. 2004;5:97–107. doi: 10.1038/nrn1327. [DOI] [PubMed] [Google Scholar]
- Wang X, Wall JT. Cortical influences on sizes and rapid plasticity of tactile receptive fields in the dorsal column nuclei. J Comp Neurol. 2005;489:241–248. doi: 10.1002/cne.20642. [DOI] [PubMed] [Google Scholar]
- Webster HH, Hanisch U-K, Dykes RW, Biesold D. Basal forebrain lesions with or without reserpine injection inhibit cortical reorganization in rat hindpaw primary somatosensory cortex following sciatic nerve section. Somatosens Motor Res. 1991;8:327–346. doi: 10.3109/08990229109144756. [DOI] [PubMed] [Google Scholar]
- Welker EE, Soriano E, Van der Loos H. Plasticity in the barrel cortex of the adult mouse: effects of peripheral deprivation on Gad-immunoreactivity. Exp Brain Res. 1989;74:441–452. doi: 10.1007/BF00247346. [DOI] [PubMed] [Google Scholar]
- Wellman CL, Arnold LL, Garman EE, Garraghty PE. Acute reductions in GABAA receptor binding in layer IV of adult primate somatosensory cortex after peripheral nerve injury. Brain Res. 2002;954:68–72. doi: 10.1016/s0006-8993(02)03343-7. [DOI] [PubMed] [Google Scholar]
- Wierenga CJ, Ibata K, Turrigiano GG. Postsynaptic expression of homeostatic plasticity at neocortical synapses. J Neurosci. 2005;25:2895–2905. doi: 10.1523/JNEUROSCI.5217-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia Y, Haddad GG. Ontogeny and distribution of GABAA receptors in rat brainstem and rostral brain regions. Neurosci. 1992;49:973–989. doi: 10.1016/0306-4522(92)90373-a. [DOI] [PubMed] [Google Scholar]
- Xu J, Wall JT. Rapid changes in brainstem of adult primates after peripheral nerve injury. Brain Res. 1997;774:211–215. doi: 10.1016/s0006-8993(97)81706-4. [DOI] [PubMed] [Google Scholar]
- Xu J, Wall JT. Evidence for brainstem and supra-brainstem contributions to rapid cortical plasticity in adult monkeys. J Neurosci. 1999;19:7578–7590. doi: 10.1523/JNEUROSCI.19-17-07578.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young AB, Dauth GW, Hollingsworth Z, Penney JB, Kaatz K, Gilman S. Quisqualate- and NMDA-sensitive [3H]glutamate binding in primate brain. J Neurosci Res. 1990;27:512–521. doi: 10.1002/jnr.490270412. [DOI] [PubMed] [Google Scholar]