Abstract
Susceptibility to fracture is increased across the spectrum of chronic kidney disease (CKD). Moreover, fracture in patients with end-stage kidney disease (ESKD) results in significant excess mortality. The incidence and prevalence of CKD and ESKD are predicted to increase markedly over the coming decades in conjunction with the aging of the population. Given the high prevalence of both osteoporosis and CKD in older adults, it is of the utmost public health relevance to be able to assess fracture risk in this population. Dual-energy X-ray absorptiometry (DXA), which provides an areal measurement of bone mineral density (aBMD), is the clinical standard to predict fracture in individuals with postmenopausal or age-related osteoporosis. Unfortunately, DXA does not discriminate fracture status in patients with ESKD. This may be, in part, because excess parathyroid hormone (PTH) secretion may accompany declining kidney function. Chronic exposure to high PTH levels preferentially causes cortical bone loss, which may be partially offset by periosteal expansion. DXA can neither reliably detect changes in bone volume nor distinguish between trabecular and cortical bone. In addition, DXA measurements may be low, normal, or high in each of the major forms of renal osteodystrophy (ROD). Moreover, postmenopausal or age-related osteoporosis may also affect patients with CKD and ESKD. Currently, transiliac crest bone biopsy is the gold standard to diagnose ROD and osteoporosis in patients with significant kidney dysfunction. However, bone biopsy is an invasive procedure that requires time-consuming analyses. Therefore, there is great interest in developing non-invasive high-resolution imaging techniques that can improve fracture risk prediction for patients with CKD. In this paper, we review studies of fracture risk in the setting of ESKD and CKD, the pathophysiology of increased fracture risk in patients with kidney dysfunction, the utility of various imaging modalities in predicting fracture across the spectrum of CKD, and studies evaluating the use of bisphosphonates in patients with CKD.
Keywords: chronic kidney disease, renal osteodystrophy, fracture, bone imaging
The worldwide population is aging. In the United States alone, the number of persons aged 65 years and over is expected to rise from 32 to 69 million between 1990 and 2050, and the number over the age of 85 years will increase from 3 to 15 million.1 The global population is demonstrating similar trends as the number of persons aged 65 years and over is expected to rise from 323 to 1555 million between 1990 and 2050.1 These demographic trends have raised great concern about the burden of several common diseases associated with aging.
Osteoporosis, defined by the World Health Organization as a disorder of bone resulting in decreased bone strength, is an extremely common disorder of aging that currently affects 10–12 million people in the United States alone.2 Future projections, based on the aging of the United States population, indicate that the number of people with osteoporosis will increase exponentially during the first half of this century.1 For example, the estimated 7.8 million women and 2.3 million men affected with osteoporosis at the hip today is expected to increase to 10.5 and 3.3 million, respectively, by 2020.1
Fractures represent the main clinical manifestation of osteoporosis. Half of all women over the age of 50 years will suffer an osteoporotic fracture during their lifetime.3 Moreover, the increased prevalence of osteoporosis at the hip is expected to lead to a tripling of the number of hip fractures worldwide by 2050.4 The medical and economic burden of fragility fracture is substantial. Burge and colleagues5 have recently estimated that the incidence of osteoporotic fractures will increase by almost 50% from two million fractures in 2005 to three million fractures in 2025. The associated cost of those fractures is estimated at $25.3 billion.5 When the impact of hip fracture on the quality of life is considered in disability-adjusted life years, the global burden of disease has been estimated at 1.75 million years, with approximately one-quarter occurring in China and India, and 50% occurring in Western countries alone.6
Declining kidney function is another extremely common disorder of aging. Over the past decade, there has been a worldwide epidemic of chronic kidney disease (CKD).7,8 In a recent analysis of the Third National Health and Nutrition Examination Survey (NHANES III), Coresh et al.9 found that the prevalence of significant kidney impairment was remarkably high in the elderly US population. Of those aged 70 years and above, only 26% had normal kidney function, whereas 49% had mildly and 25% had moderately decreased kidney function. Notably, the short-term risk of osteoporotic fracture increases dramatically after the age of 70 years.10 However, despite the fact that osteoporotic fracture and mild-to-moderate CKD (Stages 1–4) are highly co-prevalent in the elderly,11 relatively few studies have examined the contribution of impaired kidney function to the risk of fragility fracture.
Given the rapid worldwide growth of an elderly population at risk for both osteoporosis and CKD, and the many potential mechanisms by which CKD could decrease bone strength12 and increase fracture risk, it is imperative that we develop effective diagnostic strategies to identify patients with CKD who are also at risk for fracture. It is also essential that we develop effective therapeutic strategies that decrease fracture risk in patients with CKD. This review will examine the scope of the problem of osteoporotic fractures in patients with end-stage kidney disease (ESKD) and CKD, the utility of currently available methods to identify patients at risk for fragility fracture, and what is known about the safety and efficacy of available therapies for osteoporosis in patients with CKD.
FRACTURE RISK IN END-STAGE KIDNEY DISEASE
The risk of fracture is greatly increased in patients with ESKD.13 Using the United States Renal Data System, Alem et al.14 demonstrated a four-fold increased risk of hip fracture in men and women on hemodialysis. Although the risk exceeded that of the normal population in all age groups of patients with ESKD, for those less than 65 years old the relative risk ranged between 10- and 100-fold higher, most likely due to the generally low incidence of hip fracture in normal men and women below the age of 65 years.14 Other studies of patients with ESKD have shown that yearly incidence rates for fracture by site are about 1% per year for hip fracture and about 2.6% for any fracture.14–16 This compares with the incidence rates of 0.07–0.22% for fracture at the hip in the general population.17–19 Risk factors for fracture in patients with ESKD include, both traditional risk factors for osteoporotic fracture (older age, female gender, low body weight, postmenopausal status, osteoporosis history, family history of osteoporosis, previous fracture, propensity to fall, and the use of psychoactive medications, such as benzodiazepines and selective serotonin reuptake inhibitors) and also risk factors that are specific to patients with ESKD,20 including the duration of kidney replacement therapy, exposure to glucocorticoids, history of a kidney transplant, and both low and high parathyroid hormone (PTH) levels.14,16,21,22 It is noteworthy that fracture rates in patients with ESKD are similar to fracture incidence rates of non-uremic individuals who are older by 10–20 years.16,22
FRACTURE RISK IN PRE-DIALYSIS CKD
A growing body of literature suggests that patients with CKD who do not yet require renal replacement therapy are also at an increased risk of fragility fracture (Table 1).23–27 In a cross-sectional analysis of the NHANES III, we showed that moderate-to-severe kidney disease was independently associated with more than a 2-fold increase in history of hip fracture. This association was stronger than several traditional risk factors for fracture including age, gender, race, body weight, and bone mineral density (BMD) measured at the hip by dual-energy X-ray absorptiometry (DXA).24 A similar relationship was noted in an analysis of the Cardiovascular Health Study. After multivariate adjustment for risk factors associated with osteoporotic fracture, there was a statistically significant 16% increased risk of incident hip fracture for women per standard deviation increase in cystatin C.27 Furthermore, in a case–control cohort study that selected women with either hip (n =149) or vertebral (n =150) fracture from the Study of Osteoporotic Fractures, the risk of trochanteric fracture was significantly increased by 5-fold and 3.5-fold with a glomerular filtration rate (GFR) less than 45 ml/min and between 45 and 59 ml/min, respectively.25 This study found no significant association between kidney function and the risk of vertebral fracture. In a cross-sectional study of elderly men and women treated for osteoporosis,23 a creatinine clearance of less than 65 ml/min was associated with a significant increase in the risk of hip, spine, and wrist fractures. In summary, these studies are consistent in demonstrating that fracture risk is increased in patients with moderate-to-severe CKD.
Table 1.
Studies of fracture risk associated with CKD
| Study | Definition of kidney function | Fracture site | Fracture risk (95% CI)a |
|---|---|---|---|
| Dukas et al. (2005)23 | <65 ml/min | Hip | OR 1.57 (1.18–2.09) |
| Wrist | OR 1.79 (1.39–2.31) | ||
| Vertebral | OR 1.31 (1.19–1.55) | ||
| Nickolas et al. (2006)24 | <59 ml/min | Hip | OR 2.32 (1.13–4.74) |
| Ensrud et al. (2007)25 | 45–59 ml/min | Hip | HR 1.24 (0.60–2.56) |
| <45 ml/min | HR 1.41 (0.59–3.36) | ||
| 45–59 ml/min | Trochanteric | HR 3.69 (1.21–11.24) | |
| <45 ml/min | HR 5.04 (1.38–18.45) | ||
| Jamal et al. (2007)26 | <45 ml/min | Any fracture | OR 1.3 (1.0–1.6)b |
| Vertebral | OR 2.5 (1.6–3.9)b | ||
| Fried et al. (2007)27 | <60 ml/min | Hip | HR 1.38 (0.99–1.94)b |
| Per s.d. increase in cystatin C | Hip | HR 1.16 (1.01–1.33)b |
CI, confidence interval; CKD, chronic kidney disease; HR, hazard ratio; OR, odds ratio.
After multivariate adjustment.
Women only.
RENAL OSTEODYSTROPHY: DEFINITION, CLASSIFICATION, AND DIAGNOSIS
In 2004, the National Kidney Foundation defined renal osteodystrophy (ROD) as a constellation of bone disorders, present or exacerbated by CKD, that lead to bone fragility and fractures, abnormal mineral metabolism, and extraskeletal manifestations.28 However, this definition did not gain international acceptance, in part, because of its lack of bone specificity. The Kidney Disease: Improving Global Outcomes (KDIGO) committee refined the definition of ROD, specifically limiting the term to the various types of bone pathology found in patients with CKD. Another term, CKD-mineral and bone disorders (CKD-MBD), was chosen to refer more broadly to the clinical, biochemical, and imaging abnormalities that are associated with ROD. CKD-MBD is defined as a systemic disorder of mineral and bone metabolism due to CKD and manifested by either one or a combination of (1) abnormalities of calcium, phosphorous, PTH, or vitamin D metabolism; (2) abnormalities of bone turnover, mineralization, volume, linear growth, or strength; and (3) vascular or other soft tissue calcification. KDIGO recommends that the initial evaluation of CKD-MBD proceed with biochemical testing (PTH, calcium, phosphorous, alkaline phosphatase, bicarbonate, and imaging for soft tissue calcification). Bone biopsy is recommended in cases where there are inconsistencies in biochemical parameters, unexplained bone pain, or unexplained bone fracture.
OSTEOPOROSIS: DEFINITION, PATHOGENESIS AND DIAGNOSIS
Osteoporosis is generally considered to account for the majority of fragility fractures. Osteoporosis is defined as a skeletal disorder characterized by compromised bone strength that leads to an increased risk of fracture.29 The amount of bone mineral present or the BMD is the major determinant of the strength of bone in the general population. Although BMD can be measured by a number of different radiographic and ultrasonographic techniques, the most widespread imaging modality in clinical use for measurement of BMD is DXA of the spine and proximal femur. However, the strength of bone is also influenced by the quality of the bone that is present. Bone quality is, in turn, determined by a number of material and structural characteristics of bone, including architecture and microarchitecture, bone remodeling activity or turnover, mineralization, collagen properties, and accumulation of microdamage.30,31 Histologically, osteoporosis is characterized by a reduced quantity of normally mineralized bone. In addition, the osteoporotic bone is structurally abnormal. Microstructural studies reveal thinning and increased porosity of the cortices and fewer, disconnected, widely spaced bony trabeculae. The microarchitectural changes usually result from an increase in the rate of bone remodeling and/or an imbalance between the bone resorbing activity of osteoclasts and the bone forming activity of osteoblasts. The most common scenario leading to osteoporosis is one in which bone resorption is increased and bone formation is also increased, but insufficiently to compensate. However, the histological changes of osteoporosis can also develop as a result of a decrease in bone formation whereas resorption proceeds at a normal pace. Measurement of biochemical markers that reflect osteoclast and osteoblast activities can be used to assess the rate of bone remodeling activity.32
In patients without kidney disease, the independent contribution of bone microarchitecture to skeletal strength is becoming increasingly recognized. Using a peripheral quantitative computed tomography (QCT) machine with a resolution (~80 μm) capable of imaging trabecular microstructure, Boutroy et al.33 investigated a group of post-menopausal women with osteopenia, and reported that those with a history of fracture had lower density of trabecular bone and more heterogeneous trabecular distribution at the radius than those without fracture, despite comparable BMD measurements.
PATHOGENESIS OF DECREASED BONE STRENGTH IN PATIENTS WITH KIDNEY DISEASE
Given the general definition of osteoporosis quoted above and our increasing understanding of the contribution of bone quality to bone strength, it is not surprising that fracture risk increases in patients with ESKD at a younger age than the typical individual with senile or postmenopausal osteoporosis.16,22 ESKD is characterized by several metabolic and hormonal abnormalities, including decreased renal synthesis of 1,25(OH)2D3, hyperphosphatemia, hypocalcemia, increased secretion of PTH, chronic metabolic acidosis, premature hypogonadism, and, more recently, recognized 25(OH) vitamin D deficiency; all may adversely affect the bone remodeling process in one or more of the following ways—increasing bone resorption, decreasing bone formation, or impairing mineralization of osteoid. These changes in bone remodeling have the potential to accelerate the deterioration of bone microstructure that accompanies normal aging—trabecular thinning and perforation, dropout of trabeculae, cortical thinning, and porosity—and therefore may prematurely decrease bone quality and strength and increase susceptibility to fragility fracture.34,35 In this regard, Amling et al.34 compared trabecular bone volume and connectivity, trabecular thickness, and number in spine autopsy specimens from 9 patients on hemodialysis and 26 normal controls. Although trabecular bone volume was similar between cases and controls, trabecular connectivity was reduced and more trabecular perforations were observed in patients with ESKD. Schober et al.35 compared transiliac bone biopsies from 40 men and women with ESKD and 142 healthy women. Endocortical resorption, cortical thinning, and a 45% reduction in mineralized cortical bone were common features, regardless of the histological classification of ROD. In addition, cancellous bone mineralization was significantly decreased by 36% in patients with osteitis fibrosa and nonsignificantly reduced by 9% in patients with osteomalacia. Thus, bone biopsy studies have demonstrated that ESKD is associated with microstructural alterations at both the cortical and trabecular levels.
The increased risk of fracture noted in patients with pre-dialysis CKD may be related to the fact that abnormalities in vitamin D metabolism, parathyroid function, and calcium–phosphate balance may result in full-blown ROD long before kidney function deteriorates to the level of ESKD. For example, in a large cross-sectional study of patients with early CKD, serum 1,25(OH)2D declined in a linear fashion as estimated GFRs (eGFR) decreased.36 Notably, 13% of patients with eGFR ≥80 already had low serum concentrations of 1,25(OH)2D and 12% patients had high serum concentrations of PTH.36 Moreover, approximately 40% of subjects with eGFR between 40 and 49 ml/min had elevated serum PTH levels. As elevated PTH levels are catabolic for cortical bone, these biochemical alterations could cause deterioration in cortical architecture, leading to reduced cortical density and increased cortical porosity37 much earlier in the course of CKD than previously thought. As cortical bone contributes substantially to bone mechanical competence,30,31 these architectural changes could account for the increased fracture susceptibility noted in studies of patients with CKD. In support of this notion, biopsy studies performed early in the course of CKD already demonstrate characteristics consistent with ROD, although routine biochemical and radiographic studies may still be normal.38,39 In a study of the efficacy of alfacalcidiol in the prevention and treatment of ROD, Hamdy et al.38 demonstrated that 75% of subjects with pre-dialysis CKD had abnormal bone histology (74% osteitis fibrosa, 19% mixed bone disease, 1% osteomalacia, 1% aluminum bone disease, and 5% adynamic bone disease). In this report, it was noteworthy that the subjects’ creatinine clearances ranged from 15 to 50 ml/min and that none had clinical, biochemical, or radiographic evidence of bone disease.
PREDICTION OF FRACTURE RISK BY DXA IN ESKD AND CKD
In normal postmenopausal women and older men without significant kidney dysfunction, the measurement of the amount of mineralized bone mass of certain areas of the skeleton, particularly the spine and proximal femur, by DXA is an established clinical tool for discriminating among those with and without prevalent fractures and for identifying those who are at an increased risk of incident fracture.40 However, DXA provides a two-dimensional areal view of a three-dimensional structure and has very poor spatial resolution. In recent years, it has become apparent that the measurement of areal BMD (aBMD) by DXA has substantial limitations as a measure of bone strength, and therefore as a clinical technique.41 For example, studies in postmenopausal women show that half of all fractures occur in women with aBMD values above the World Health Organization’s diagnostic threshold for osteoporosis (T-score ≤ −2.5).42,43 In addition, age and history of adult fracture predict future fracture independent of BMD.44 The increase in spine BMD associated with different therapies for osteoporosis varies considerably (1–7%), underestimates the reduction in the risk of vertebral fracture that accompanies such therapies (35–60%), and explains only 4–30% of the reduction in fracture risk.45,46 Reductions in the risk of fracture associated with an institution of antiresorptive therapy also occur well before maximum gains in BMD are achieved.47 Finally, certain therapies (for example, sodium fluoride) are associated with large gains in BMD, yet do not reduce and may even be associated with increased risk of fracture.48 These studies suggest that characteristics other than BMD are related to fracture risk, characteristics not measured by DXA. In this regard, the WHO is currently defining six risk factors that are completely independent of BMD (age, weight, female gender, Caucasian race, prior fracture, and glucocorticoid use).
The limitations of measuring aBMD by DXA in men and women without significant kidney dysfunction are magnified in patients with ESKD and CKD. A number of studies have reported that the prevalence of low BMD measurements is higher than expected among patients with ESKD.49 However, it is essential for nephrologists to recognize that the meaning of low BMD measurements is unclear in patients with ESKD and CKD, as DXA measurements can be low, normal, or high in each of the three major forms of kidney bone disease—hyperparathyroidism, adynamic bone disease, and osteomalacia.50–52 In addition, postmenopausal and senile osteoporosis may coexist with all the forms of bone disease seen in patients with kidney dysfunction,53 who share multiple risk factors for low BMD with the general population, including old age, hypogonadism, and poor functional status. In this regard, a recent analysis of NHANES III concluded that low BMD in patients with CKD was attributable to traditionally accepted risk factors for osteoporosis, rather than to CKD.54 Also important, most studies suggest that in patients with ESKD and CKD, DXA measurements do not discriminate between those with and without prevalent fractures.52 Although a recent meta-analysis by Jamal et al.55 demonstrated a significant association between low BMD at the lumbar spine and radius (mid, distal, and one-third) and fracture status in patients on dialysis, the studies used in this meta-analysis did not permit adjustment for variables, such as body weight, duration of dialysis, previous fragility fracture, physical activity, and corticosteroid use, which are all robustly associated with BMD. Finally, the site at which BMD is measured in patients with CKD does not consistently predict fracture at that particular site,52 in contrast to patients without kidney dysfunction, in whom BMD measured at any site can predict fracture risk for that and other sites.56–59 Prospective studies that rigorously control for factors associated with BMD are necessary to identify non-invasive imaging technologies that predict fracture in the CKD population.
The lack of predictive value of DXA for fracture in the setting of kidney failure is likely because metabolic abnormalities that accompany kidney disease differentially affect the cortical and trabecular compartments of bone. In particular, chronic excess PTH secretion is catabolic for cortical bone, causing marked subperiosteal and intracortical erosion. In contrast, chronic PTH excess may also cause increased osteoblastic bone formation beneath the periosteum that may compensate for the loss of cortical bone to some extent, as well as increased trabecular thickness and increased trabecular number.37,60,61 The lumbar spine and proximal femur comprise substantial amounts of both cortical and trabecular bones. DXA provides a composite measurement of the cortical and trabecular compartment, and the resolution is not high enough to discriminate between cortical and trabecular bone. Therefore, DXA cannot detect the predominant cortical bone loss and periosteal expansion that accompanies hyperparathyroidism. In addition, vertebral bodies of patients with kidney disease typically develop the areas of endplate osteosclerosis (manifested radiographically as ‘rugger jersey spine’), which may falsely elevate DXA measurements and further complicate the interpretation of the scans. Theoretically, DXA measurements of the one-third radius site, which consists predominantly of cortical bone, may be more useful in assessing the degree of cortical versus trabecular bone loss. In the pre-dialysis population, the prevalence of low BMD is much higher at the one-third radius site (33%) than either the lumbar spine (19%) or the femoral neck (26%).50,62–64 Perhaps for these reasons, the International Society of Clinical Densitometry recommends that BMD of the one-third radius, rather than the hip or spine, be used to evaluate bone loss in disorders of hyperparathyroidism (http://www.iscd.org/Visitors/positions). However, despite the use of different anatomical sites for BMD assessment, DXA does not provide an accurate assessment of fracture risk in CKD.52,65,66 In Table 2, we summarize studies that have determined diagnostic test characteristics for DXA to discriminate fracture status in cohorts of patients with ESKD. Notably, DXA is a poor test to predict fracture status, no matter which site is imaged.
Table 2.
Studies of imaging modalities to assess fracture status in patients with CKD that report test diagnostic characteristics
| Study | Kidney function | Imaging modality | Site imaged | Fracture site | Area under the receiver operator curve | Sensitivity | Specificity |
|---|---|---|---|---|---|---|---|
| Jamal et al. (2006)66 | ESRD | DXA pQCT | Total hip | Any site | 56 | 26 | 78 |
| Radius (cortical density) | Any site | 89 | NA | NA | |||
| Radius (trabecular density) | Any site | 52 | NA | NA | |||
| Jamal et al. (2002)65 | ESRD | DXA | Femoral neck | Any site | 47.3 | 26 | 78 |
| Ultrasound | Calcaneous | Any site | 56.2 | 29 | 78 | ||
| Fontaine et al. (2000)85 | ESRD | DXA | Femoral neck | Any site | 75.5 | NA | NA |
| Radius (mid) | Any site | 83.7 | NA | NA | |||
| Radius (one-third) | Any site | 78.0 | NA | NA | |||
| Lumbar Spine | Any site | 72.8 | NA | NA | |||
| Yamaguchi et al. (1996)52 | ESRD | DXA | Lumbar spine | Vertebral | 72.9 | 70 | 70 |
| Non-spine | 69.3 | 63 | 63 | ||||
| Radius (one-third) | Vertebral | 75.2 | 69 | 69 | |||
| Radius (ultradistal) | Non-spine | 86.9 | 82 | 82 | |||
| Atsumi et al. (1999)21 | ESRD | DXA | Total body | Vertebral | 55 | NA | NA |
| Lumbar spine | Vertebral | 60 | NA | NA |
CKD, chronic kidney disease; DXA, dual-energy X-ray absorptiometry; ESRD, end-stage renal disease; NA, not applicable; pQCT, peripheral quantitative computed tomography.
TRANSILIAC BONE BIOPSY TO ASSESS BONE QUALITY AND STRENGTH IN PATIENTS WITH ESKD OR CKD
Currently, a transiliac crest bone biopsy, performed after tetracycline labeling of bone-forming sites, is the gold standard for assessing the material and structural characteristics that contribute to bone quality and hence to bone strength. Specific analyses performed on bone biopsy samples can assess the microarchitecture of cancellous and cortical bone, the amount and location of past and ongoing remodeling activity, and the accumulation of fatigue damage or microcracks. Quantitative backscattered electron imaging of bone biopsy sections provides additional information on the amount and distribution of bone mineral across the bony trabeculae and Fourier Transform Infrared Spectroscopy provides information on the ratio of collagen cross-links across trabeculae. Furthermore, micro-CT (resolution 20 μm) can be applied to intact biopsy specimens to provide three-dimensional images of trabecular microarchitecture, whereas finite element analysis of the micro-CT scans can provide estimates of mechanical strength. Transiliac bone biopsy is currently the best technique available for detecting microstructural alterations that occur in the setting of secondary hyperparathyroidism, particularly thinning and trabecularization of the cortex and increased bone volume of the cancellous compartment. However, an invasive procedure and time-consuming measurements are required. Furthermore, bone biopsies provide limited information on three-dimensional trabecular connectivity and orientation. Thus, there has been great interest in developing non-invasive techniques that can provide an accurate assessment of bone microarchitecture and strength without the necessity of a biopsy.
NON-INVASIVE BONE IMAGING TECHNOLOGIES IN ESKD AND CKD
Recently, several bone imaging modalities have been developed that show promise in providing more accurate estimates of bone quality and strength and that may prove useful in assessing bone strength in patients with ESKD and CKD.
Conventional central and peripheral QCT
QCT can be applied to peripheral sites (radius and tibia) and central sites (lumbar spine and proximal femur). QCT provides a true volumetric measurement of BMD of the trabecular and cortical compartments, rather than the aBMD measurement provided by DXA. Moreover, the resolution of QCT is high enough to differentiate between the trabecular and cortical compartments of bone, at least in the proximal femur. Both central and peripheral QCT scans yield cortical and trabecular measurements that correlate well with comparable indices measured by micro-CT of bone biopsy specimens in patients without CKD.67 Central QCT has been used to evaluate BMD in patients with ESKD on dialysis and in kidney transplant recipients.68,69 Torres et al. compared 17 ESKD patients with 29 healthy controls, and found that spine BMD measured by central QCT was highly correlated with trabecular bone volume measured by quantitative histomorphometry of the iliac crest. However, not surprisingly, the type of ROD (high or low turnover) did not correlate with BMD, and normal, low, or high BMD was found in each type of ROD. Grotz et al. compared 33 female kidney transplant recipients with 74 women undergoing evaluation for osteoporosis with mid-vertebral high-resolution CT. Women in either group who had experienced a ≥15% reduction in vertebral height demonstrated altered trabecular microstructure, including lower BMD, lower trabecular area, number, thickness, and increased trabecular separation. Furthermore, compared with controls of similar BMD, kidney allograft recipients had significantly lower trabecular number and increased trabecular separation.69 Peripheral QCT has also been used to assess bone architecture in pre-dialysis patients, hemodialysis patients, and in kidney transplant recipients (Figure 1).70–73 Using peripheral QCT, Russo et al.72 demonstrated selective loss of cortical bone in patients on hemodialysis. In a study of kidney transplant recipients, cortical bone loss was found to result from resorption of the endosteal rather than the periosteal cortex.71 In a study of dialysis patients, Jamal et al.66 found that peripheral QCT of the distal radial cortical compartment was superior to DXA measurements of hip BMD to predict prevalent fracture (Table 2). In this same study, pQCT measurements of distal radial trabecular BMD did not classify fracture status. However, the resolution of conventional pQCT (350 μm) is not sufficient to measure trabecular microstructure, which may contribute substantially to bone strength and susceptibility to fracture.
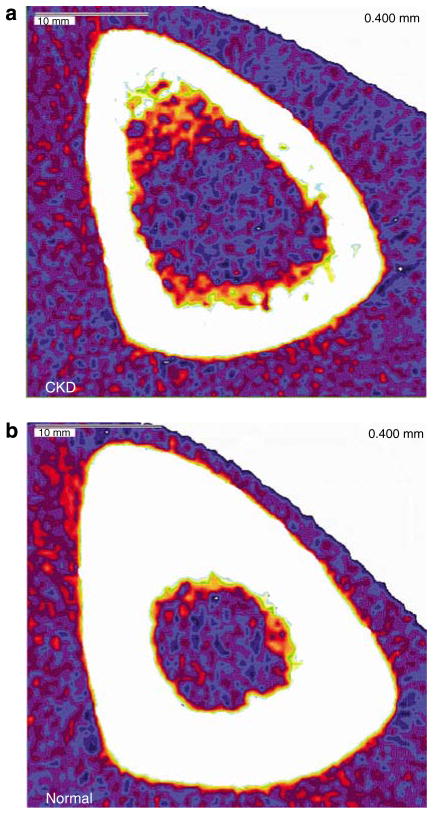
Figure 1. Example images from techniques to evaluate bone structure.
pQCT scans of the tibia mid-diaphysis in (a) 64-year-old male with CKD Stage 4, and (b) 62-year-old male with normal kidney function. In comparison with the cortex of the healthy control, the cortex of the patient with CKD is noted to have decreased cortical density and cortical thinning.
Micro-magnetic resonance imaging
Recent advances in micro-magnetic resonance imaging (micro-MRI) have made it possible to obtain high-resolution three-dimensional images of trabecular bone architecture in a volume of interest selected from a set of contiguous slices acquired in peripheral sites (distal radius, tibia, and calcaneus) (Figure 2). The images can then be analyzed in a manner analogous to a bone biopsy. Wehrli et al.74 used micro-MRI to evaluate 17 subjects with ESKD under the age of 50 years. They found that although there was substantial variability among the patients, cortical thickness and cross-sectional area were significantly lower than controls matched for age, gender, and body mass index. In addition, they were able to detect changes suggestive of trabecular disruption, including reduced trabecular number and an increased erosion index, which reflects disconnectivity; however, the differences did not quite achieve statistical significance. Thus, although larger studies are necessary, micro-MRI may have the potential to assess fracture risk non-invasively in patients with ESKD and CKD.
Figure 2. Example images from techniques to evaluate bone structure.
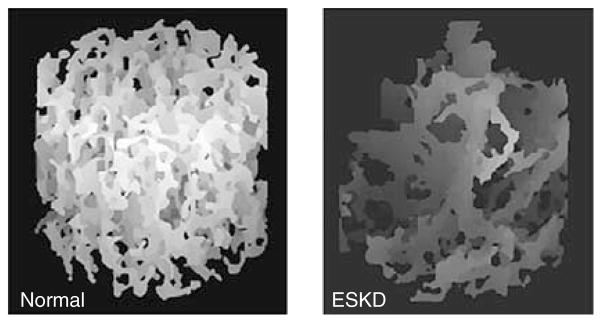
Tibia micro-MRI from a healthy control (left) and a patient with ESKD (right). Increased trabecular disconnectivity of the tibia from the patient with ESKD is noted by decreased trabecular density and loss of horizontal trabecular elements.
Ultra-high-resolution peripheral QCT
Recently, a newer technique, ultra-high-resolution peripheral QCT (HRpQCT), has been developed. Similar to conventional peripheral QCT methodology, the three-dimensional datasets provided by HRpQCT permit separate analysis of cancellous and cortical bone75,76 and can measure specific geometric parameters that correlate with bone strength, such as endosteal and periosteal circumferences, cortical area, and thickness.76,77 As the resolution of HRpQCT is <100 μm (versus 350 μm for standard peripheral QCT machines), HRpQCT technology yields scans that visualize the fine ultrastructural detail of trabecular microarchitecture, including trabecular thickness, number, and separation. HRpQCT is not only more sensitive than DXA for detecting small amounts of bone loss but can also localize that bone loss to the trabecular or cortical compartments. Thus, this technique may provide an improved method to assess microarchitectural features of bone that contribute to the increased fracture risk observed in patients with ESKD and CKD. Figure 3 shows representative HR-pQCT images from a healthy individual and both HR-pQCT and DXA images from a patient with ESKD. Cortical thinning and porosity and trabecular disconnectivity are notable on the HR-pQCT images. In addition, the DXA measurement of aBMD of the same patient demonstrates that DXA does not permit appreciation of the severity of the patient’s microstructural abnormalities.
Figure 3. Example images from techniques to evaluate bone structure.
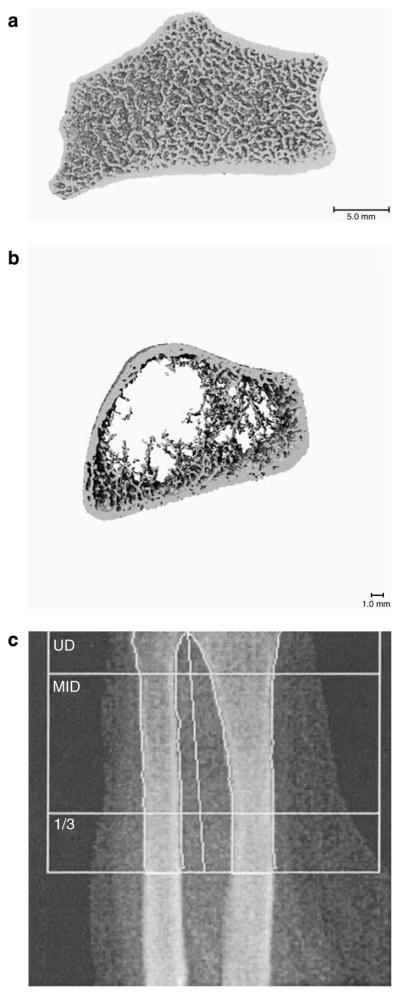
Representative HR-pQCT images from a healthy patient (a); a patient with ESKD (b); and a DXA image from the same patient with ESRD (c). HR-pQCT of the radius of the patient with ESKD demonstrates cortical thinning and extreme trabecular dropout. In comparison with DXA imaging of the same bone, HR-pQCT provides superior resolution with visualization of both trabecular and cortical bone compartments.
PREVENTION OF FRACTURE IN PATIENTS WITH ESKD AND CKD
As awareness has increased that patients with ESKD and CKD are at such high risk of fracture, so has the concern about how best to prevent these debilitating events. Unfortunately, we are a long way from knowing how to accomplish this goal, especially in patients with ESKD.
Bisphosphonates, which inhibit osteoclast-mediated bone resorption, are the mainstay for fracture risk reduction in men and women with normal kidney function.78 However, one cannot assume that a fracture in patient with ESKD or severe CKD has osteoporosis secondary to the same pathophysiologic etiologies as postmenopausal, senile, or glucocorticoid-induced osteoporosis. Fractures develop in kidney disease patients with hyperparathyroidism, osteomalacia, and adynamic bone disease. Without a bone biopsy, it can be very difficult to determine precisely what type of bone disease a fracturing patient with ESKD has. Moreover, there are no prospective studies that address the safety or efficacy of bisphosphonates in patients with ESKD. As bisphosphonates are cleared by renal mechanisms, there is also concern that they may accumulate in the skeleton to an even greater extent than they do in patients with normal kidney function. In general, most experts do not recommend treating patients with ESKD with bisphosphonates. Similarly, the only current anabolic therapy for osteoporosis, PTH 1–34 or teriparatide, is also not approved for use in this population. Cunningham et al.,79 in a pooled analysis of safety data from four randomized clinical trials of calcimimetics in 1184 patients with ESKD and severe hyperparathyroidism, found that cinacalcet was associated with a significant 54% reduction in the risk of fracture. Although active metabolites of vitamin D have not been demonstrated to reduce fracture incidence in patients with ESKD, they have been shown to reduce serum PTH levels80–82 and improve BMD in patients with CKD,38,82 and also to improve bone strength in animal studies.83 Thus, there is the hope that by addressing some of the mechanisms thought to lead to increased bone fragility (hyperparathyroidism, mineralization defects), judicious use of these agents will prevent or arrest declining bone strength and lead to reduced fracture risk in dialysis patients.
Slightly more information is available on the safety and efficacy of bisphosphonates in patients with milder degrees of kidney dysfunction. The major registration clinical trials investigating the efficacy of bisphosphonates to prevent osteoporotic fractures generally excluded potential subjects with significant kidney dysfunction. However, because potential subjects were screened on the basis of elevated serum creatinine concentrations rather than on the basis of creatinine clearance or GFR, sizable populations of patients with moderate-to-severe kidney disease were actually enrolled in these studies. Recently, two studies have been published in which Phase III clinical trials of risedronate and alendronate have been reanalyzed with a view toward evaluating their effects in the subjects with Stage 3 and 4 CKD, as assessed by estimating creatinine clearance with the Cockcroft–Gault formula (Table 3). Both studies addressed the efficacy of these bisphosphonates in increasing BMD and preventing fracture and their potential for nephrotoxicity in the subset of patients with CKD.26,84 Miller et al.84 performed a retrospective analysis of the risedronate Phase III clinical trial database that included pooled data from nine clinical trials of postmenopausal women to evaluate the influence of baseline kidney function on the safety and efficacy of risedronate (5 mg/day). Approximately half of the subjects had moderate (45%) or severe (7%) renal dysfunction, and were equally distributed between the placebo and treatment groups. Mean follow-up was 2 years and maximum duration of risedronate treatment was 3 years. There were no differences between groups in the rate of reported adverse renal events. Lumbar spine and trochanteric BMD increased significantly in all groups of kidney impairment. Femoral neck BMD increased in the group with moderate kidney dysfunction but not in the severe group. Importantly, fracture incidence was reduced for all groups of kidney impairment. Pre- and post-treatment bone biopsy samples were available for subjects with mild (n =43) and moderate (n =14) CKD. Both mineralization surface and activation frequency were decreased in treated subjects. Jamal et al.26 recently published a retrospective analysis of the Fracture Intervention Trial (FIT) evaluating the use of alendronate in osteoporotic women with impaired kidney function as estimated by the Cockcroft–Gault formula. In this study population, 10% had severe CKD (GFR <45 ml/min) and 37% had moderate CKD (eGFR 45–59 ml/min). They found no difference in outcomes among subjects with an eGFR of 45–59 ml/min and those with normal kidney function, defined as an eGFR ≥60 ml/min. Thus, these groups were pooled and compared with the women with severe CKD. Women with severe CKD were at an increased risk of prevalent vertebral fractures and history of non-spine fractures after the age of 45 years, compared with women without CKD. Alendronate was associated with an increase in BMD at the total hip, femoral neck, and spine for subjects with and without severe CKD. Women with reduced eGFR were at increased risk of sustaining incident spine and non-spine fractures during the study. However, compared with placebo-treated women, alendronate reduced the risk of non-vertebral and vertebral fractures to a similar degree in subjects with and without kidney impairment. Bone biopsies were not performed in FIT. There was a small but significant increase in serum creatinine over the course of the 3-year study, which did not differ between the groups with severe CKD and those with eGFR ≥45 ml/min. Similarly, the rate of other adverse events did not differ according to the degree of renal impairment.
Table 3.
Studies of bisphosphonates in patients with CKD
| Study | Bisphosphonate | Range of kidney function (calculate by Cockcroft–Gault formula) | Mean duration of follow-up (years) | Bone histomorphometry | Comparison of adverse event rates in kidney dysfunction to the normal kidney function group | Clinical benefit |
|---|---|---|---|---|---|---|
| Jamal et al. (2007)26 | Alendronate | > 20 ml/min | 3 | No | Equivalent | Alendronate was equivalently effective at increasing femoral neck and spine BMD in patients with and without kidney dysfunction. Spine and non-spine fractures were also reduced to a similar degree |
| Miller et al. (2005)84 | Risedronate | > 13 ml/min | 2 | Yes | Equivalent | Risedronate increased BMD at all sites (spine and hip) and decreased fracture incidence for all kidney failure groups (mild, moderate, and severe) |
BMD, bone mineral density; CKD, chronic kidney disease.
The results of these recent retrospective analyses of the major oral bisphosphonate registration trials suggest that 2–3 years of therapy is efficacious in preventing fractures in patients with moderate-to-severe CKD and provide some reassurance that oral bisphosphonate use does not appear to accelerate age-related declines in kidney function. However, we do not advocate indiscriminate use of oral bisphosphonates in patients with severe CKD to prevent fractures. In such patients, it is unclear whether a low BMD measurement on a DXA scan or even fragility fractures are manifestations of osteoporosis or one of the forms of ROD that should be managed with phosphorous restriction and supplementation with parent vitamin D and active vitamin D metabolites. A careful clinical and biochemical evaluation that includes measurement of serum calcium, phosphate, total, and bone alkaline phosphatase, PTH, 25-hydroxyvitamin D, and 1,25-dihydroxyvitamin D is essential before committing such patients to oral bisphosphonate therapy and a bone biopsy should be strongly considered. Any decision to embark upon oral bisphosphonate therapy in such patients should be individualized and carefully considered. Although we can expect increasing use of intravenous bisphosphonates, such as zoledronic acid and ibandronate, for the management of postmenopausal osteoporosis, the renal safety of intravenous bisphosphonates is not well defined in patients with eGFR <30 ml/min or in patients with diabetes or hypertension who are at high risk for CKD.
FUTURE PERSPECTIVES
Fracture is an important complication of CKD that is associated with excess morbidity and mortality. Given the rapid growth of an elderly population at risk for both osteoporosis and CKD, and the many potential mechanisms by which CKD could decrease bone strength and increase fracture risk, it is imperative that we develop effective diagnostic strategies to identify patients with CKD who are also at risk for fracture. More studies incorporating transiliac crest bone biopsy are urgently needed. Similarly, studies are needed that assess the utility of novel imaging technologies, such as micro-MRI and HR-pQCT, to provide a more accurate assessments of bone strength and risk of fracture.
It is also essential that we develop effective therapeutic strategies that decrease fracture risk in patients with CKD. Prospective treatment studies that specifically target this population are urgently required. Such studies must address fracture risk reduction, the potential for adverse effects of bisphosphonates, and other agents on renal function and should incorporate micro-MRI and HR-pQCT, which may prove to be useful outcome measures for clinical trials of interventions to reduce fractures. Also crucial are prospective studies of the effects of bisphosphonates on bone histomorphometry in patients with CKD, which specifically address the concern that long-term use of these agents may oversuppress bone remodeling and precipitate adynamic bone disease. Similarly, more data are needed on the effects of calcimimetics and active metabolites of vitamin D on fracture risk reduction in patients with CKD. There remains a great deal of work to do to deal with the burgeoning incidence of fracture in older individuals with CKD.
References
- 1.Melton LJ., III Epidemiology worldwide. Endocrinol Metab Clin North Am. 2003;32:1–13. v. doi: 10.1016/s0889-8529(02)00061-0. [DOI] [PubMed] [Google Scholar]
- 2.Looker AC, Orwoll ES, Johnston CC, Jr, et al. Prevalence of low femoral bone density in older U.S. adults from III NHANES. J Bone Miner Res. 1997;12:1761–1768. doi: 10.1359/jbmr.1997.12.11.1761. [DOI] [PubMed] [Google Scholar]
- 3.Cummings SR, Black DM, Nevitt MC, et al. Bone density at various sites for prediction of hip fractures. The Study of Osteoporotic Fractures Research Group. Lancet. 1993;341:72–75. doi: 10.1016/0140-6736(93)92555-8. [DOI] [PubMed] [Google Scholar]
- 4.Cummings SR, Melton LJ. Epidemiology and outcomes of osteoporotic fractures. Lancet. 2002;359:1761–1767. doi: 10.1016/S0140-6736(02)08657-9. [DOI] [PubMed] [Google Scholar]
- 5.Burge R, Dawson-Hughes B, Solomon DH, et al. Incidence and economic burden of osteoporosis-related fractures in the United States, 2005–2025. J Bone Miner Res. 2007;22:465–475. doi: 10.1359/jbmr.061113. [DOI] [PubMed] [Google Scholar]
- 6.Johnell O, Kanis JA. An estimate of the worldwide prevalence, mortality and disability associated with hip fracture. Osteoporos Int. 2004;15:897–902. doi: 10.1007/s00198-004-1627-0. [DOI] [PubMed] [Google Scholar]
- 7.Levey AS, Atkins R, Coresh J, et al. Chronic kidney disease as a global public health problem: approaches and initiatives—a position statement from Kidney Disease Improving Global Outcomes. Kidney Int. 2007;72:247–259. doi: 10.1038/sj.ki.5002343. [DOI] [PubMed] [Google Scholar]
- 8.Gilbertson DT, Liu J, Xue JL, et al. Projecting the number of patients with end-stage renal disease in the United States to the year 2015. J Am Soc Nephrol. 2005;16:3736–3741. doi: 10.1681/ASN.2005010112. [DOI] [PubMed] [Google Scholar]
- 9.Coresh J, Astor BC, Greene T, et al. Prevalence of chronic kidney disease and decreased kidney function in the adult US population: Third National Health and Nutrition Examination Survey. Am J Kidney Dis. 2003;41:1–12. doi: 10.1053/ajkd.2003.50007. [DOI] [PubMed] [Google Scholar]
- 10.Harvey N, Earl S, Cooper C. Epidemiology of osteoporotic fractures. In: Favus M, editor. Primer on the Metabolic Bone Diseases and Disorders of Calcium Metabolism. 6. American Society of Bone and Mineral Research; Washington, DC: 2006. pp. 244–248. [Google Scholar]
- 11.Klawansky S, Komaroff E, Cavanaugh PF, Jr, et al. Relationship between age, renal function and bone mineral density in the US population. Osteoporos Int. 2003;14:570–576. doi: 10.1007/s00198-003-1435-y. [DOI] [PubMed] [Google Scholar]
- 12.Moe S, Drueke T, Cunningham J, et al. Definition, evaluation, and classification of renal osteodystrophy: a position statement from Kidney Disease: Improving Global Outcomes (KDIGO) Kidney Int. 2006;69:1945–1953. doi: 10.1038/sj.ki.5000414. [DOI] [PubMed] [Google Scholar]
- 13.Gal-Moscovici A, Sprague SM. Osteoporosis and chronic kidney disease. Semin Dial. 2007;20:423–430. doi: 10.1111/j.1525-139X.2007.00319.x. [DOI] [PubMed] [Google Scholar]
- 14.Alem AM, Sherrard DJ, Gillen DL, et al. Increased risk of hip fracture among patients with end-stage renal disease. Kidney Int. 2000;58:396–399. doi: 10.1046/j.1523-1755.2000.00178.x. [DOI] [PubMed] [Google Scholar]
- 15.Ball AM, Gillen DL, Sherrard D, et al. Risk of hip fracture among dialysis and renal transplant recipients. JAMA. 2002;288:3014–3018. doi: 10.1001/jama.288.23.3014. [DOI] [PubMed] [Google Scholar]
- 16.Jadoul M, Albert JM, Akiba T, et al. Incidence and risk factors for hip or other bone fractures among hemodialysis patients in the Dialysis Outcomes and Practice Patterns Study. Kidney Int. 2006;70:1358–1366. doi: 10.1038/sj.ki.5001754. [DOI] [PubMed] [Google Scholar]
- 17.Fielden J, Purdie G, Horne G, et al. Hip fracture incidence in New Zealand, revisited. N Z Med J. 2001;114:154–156. [PubMed] [Google Scholar]
- 18.Kanis JA, Johnell O, De Laet C, et al. International variations in hip fracture probabilities: implications for risk assessment. J Bone Miner Res. 2002;17:1237–1244. doi: 10.1359/jbmr.2002.17.7.1237. [DOI] [PubMed] [Google Scholar]
- 19.Reginster JY, Gillet P, Gosset C. Secular increase in the incidence of hip fractures in Belgium between 1984 and 1996: need for a concerted public health strategy. Bull World Health Organ. 2001;79:942–946. [PMC free article] [PubMed] [Google Scholar]
- 20.Stehman-Breen CO, Sherrard DJ, Alem AM, et al. Risk factors for hip fracture among patients with end-stage renal disease. Kidney Int. 2000;58:2200–2205. doi: 10.1111/j.1523-1755.2000.00394.x. [DOI] [PubMed] [Google Scholar]
- 21.Atsumi K, Kushida K, Yamazaki K, et al. Risk factors for vertebral fractures in renal osteodystrophy. Am J Kidney Dis. 1999;33:287–293. doi: 10.1016/s0272-6386(99)70302-1. [DOI] [PubMed] [Google Scholar]
- 22.Coco M, Rush H. Increased incidence of hip fractures in dialysis patients with low serum parathyroid hormone. Am J Kidney Dis. 2000;36:1115–1121. doi: 10.1053/ajkd.2000.19812. [DOI] [PubMed] [Google Scholar]
- 23.Dukas L, Schacht E, Stahelin HB. In elderly men and women treated for osteoporosis a low creatinine clearance of <65 ml/min is a risk factor for falls and fractures. Osteoporos Int. 2005;16:1683–1690. doi: 10.1007/s00198-005-1903-7. [DOI] [PubMed] [Google Scholar]
- 24.Nickolas TL, McMahon DJ, Shane E. Relationship between moderate to severe kidney disease and hip fracture in the United States. J Am Soc Nephrol. 2006;17:3223–3232. doi: 10.1681/ASN.2005111194. [DOI] [PubMed] [Google Scholar]
- 25.Ensrud KE, Lui LY, Taylor BC, et al. Renal function and risk of hip and vertebral fractures in older women. Arch Intern Med. 2007;167:133–139. doi: 10.1001/archinte.167.2.133. [DOI] [PubMed] [Google Scholar]
- 26.Jamal SA, Bauer DC, Ensrud KE, et al. Alendronate treatment in women with normal to severely impaired renal function: an analysis of the fracture intervention trial. J Bone Miner Res. 2007;22:503–508. doi: 10.1359/jbmr.070112. [DOI] [PubMed] [Google Scholar]
- 27.Fried LF, Biggs ML, Shlipak MG, et al. Association of kidney function with incident hip fracture in older adults. J Am Soc Nephrol. 2007;18:282–286. doi: 10.1681/ASN.2006050546. [DOI] [PubMed] [Google Scholar]
- 28.Moe SM, Drueke TB. A bridge to improving healthcare outcomes and quality of life. Am J Kidney Dis. 2004;43:552–557. doi: 10.1053/j.ajkd.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 29.NIH Consensus Development Panel on Osteoporosis Prevention, Diagnosis, and Therapy. Osteoporosis prevention, diagnosis, and therapy. JAMA. 2001;285:785–795. [Google Scholar]
- 30.Seeman E. Pathogenesis of bone fragility in women and men. Lancet. 2002;359:1841–1850. doi: 10.1016/S0140-6736(02)08706-8. [DOI] [PubMed] [Google Scholar]
- 31.Seeman E, Delmas PD. Bone quality—the material and structural basis of bone strength and fragility. N Engl J Med. 2006;354:2250–2261. doi: 10.1056/NEJMra053077. [DOI] [PubMed] [Google Scholar]
- 32.Seibel MJ. Biochemical markers of bone remodeling. Endocrinol Metab Clin North Am. 2003;32:83–113. vi–vii. doi: 10.1016/s0889-8529(02)00077-4. [DOI] [PubMed] [Google Scholar]
- 33.Boutroy S, Bouxsein ML, Munoz F, et al. In vivo assessment of trabecular bone microarchitecture by high-resolution peripheral quantitative computed tomography. J Clin Endocrinol Metab. 2005;90:6508–6515. doi: 10.1210/jc.2005-1258. [DOI] [PubMed] [Google Scholar]
- 34.Amling M, Grote HJ, Vogel M, et al. Three-dimensional analysis of the spine in autopsy cases with renal osteodystrophy. Kidney Int. 1994;46:733–743. doi: 10.1038/ki.1994.328. [DOI] [PubMed] [Google Scholar]
- 35.Schober HC, Han ZH, Foldes AJ, et al. Mineralized bone loss at different sites in dialysis patients: implications for prevention. J Am Soc Nephrol. 1998;9:1225–1233. doi: 10.1681/ASN.V971225. [DOI] [PubMed] [Google Scholar]
- 36.Levin A, Bakris GL, Molitch M, et al. Prevalence of abnormal serum vitamin D, PTH, calcium, and phosphorus in patients with chronic kidney disease: results of the study to evaluate early kidney disease. Kidney Int. 2007;71:31–38. doi: 10.1038/sj.ki.5002009. [DOI] [PubMed] [Google Scholar]
- 37.Parfitt AM. A structural approach to renal bone disease. J Bone Miner Res. 1998;13:1213–1220. doi: 10.1359/jbmr.1998.13.8.1213. [DOI] [PubMed] [Google Scholar]
- 38.Hamdy NA, Kanis JA, Beneton MN, et al. Effect of alfacalcidiol on natural course of renal bone disease in mild to moderate renal failure. BMJ. 1995;310:358–363. doi: 10.1136/bmj.310.6976.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Malluche HH, Ritz E, Lange HP, et al. Bone histology in incipient and advanced renal failure. Kidney Int. 1976;9:355–362. doi: 10.1038/ki.1976.42. [DOI] [PubMed] [Google Scholar]
- 40.Blake GM, Fogelman I. The role of DXA bone density scans in the diagnosis and treatment of osteoporosis. Postgrad Med J. 2007;83:509–517. doi: 10.1136/pgmj.2007.057505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Seeman E. Clinical review 137: sexual dimorphism in skeletal size, density, and strength. J Clin Endocrinol Metab. 2001;86:4576–4584. doi: 10.1210/jcem.86.10.7960. [DOI] [PubMed] [Google Scholar]
- 42.Schuit SC, van der KM, Weel AE, et al. Fracture incidence and association with bone mineral density in elderly men and women: the Rotterdam Study. Bone. 2004;34:195–202. doi: 10.1016/j.bone.2003.10.001. [DOI] [PubMed] [Google Scholar]
- 43.Stone KL, Seeley DG, Lui LY, et al. BMD at multiple sites and risk of fracture of multiple types: long-term results from the Study of Osteoporotic Fractures. J Bone Miner Res. 2003;18:1947–1954. doi: 10.1359/jbmr.2003.18.11.1947. [DOI] [PubMed] [Google Scholar]
- 44.Johnell O, Kanis JA, Oden A, et al. Predictive value of BMD for hip and other fractures. J Bone Miner Res. 2005;20:1185–1194. doi: 10.1359/JBMR.050304. [DOI] [PubMed] [Google Scholar]
- 45.Delmas PD. How does antiresorptive therapy decrease the risk of fracture in women with osteoporosis? Bone. 2000;27:1–3. doi: 10.1016/s8756-3282(00)00301-x. [DOI] [PubMed] [Google Scholar]
- 46.Delmas PD, Seeman E. Changes in bone mineral density explain little of the reduction in vertebral or nonvertebral fracture risk with anti-resorptive therapy. Bone. 2004;34:599–604. doi: 10.1016/j.bone.2003.12.022. [DOI] [PubMed] [Google Scholar]
- 47.Delmas PD, Li Z, Cooper C. Relationship between changes in bone mineral density and fracture risk reduction with antiresorptive drugs: some issues with meta-analyses. J Bone Miner Res. 2004;19:330–337. doi: 10.1359/JBMR.0301228. [DOI] [PubMed] [Google Scholar]
- 48.Kleerekoper M. Prevention of postmenopausal bone loss and treatment of osteoporosis. Semin Reprod Med. 2005;23:141–148. doi: 10.1055/s-2005-869481. [DOI] [PubMed] [Google Scholar]
- 49.Lindberg JS, Moe SM. Osteoporosis in end-state renal disease. Semin Nephrol. 1999;19:115–122. [PubMed] [Google Scholar]
- 50.Rix M, Andreassen H, Eskildsen P, et al. Bone mineral density and biochemical markers of bone turnover in patients with predialysis chronic renal failure. Kidney Int. 1999;56:1084–1093. doi: 10.1046/j.1523-1755.1999.00617.x. [DOI] [PubMed] [Google Scholar]
- 51.Urena P, Bernard-Poenaru O, Ostertag A, et al. Bone mineral density, biochemical markers and skeletal fractures in haemodialysis patients. Nephrol Dial Transplant. 2003;18:2325–2331. doi: 10.1093/ndt/gfg403. [DOI] [PubMed] [Google Scholar]
- 52.Yamaguchi T, Kanno E, Tsubota J, et al. Retrospective study on the usefulness of radius and lumbar bone density in the separation of hemodialysis patients with fractures from those without fractures. Bone. 1996;19:549–555. doi: 10.1016/s8756-3282(96)00246-3. [DOI] [PubMed] [Google Scholar]
- 53.Slatopolsky E, Delmez J. Renal osteodystrophy. In: Coe FL, Favus MJ, editors. Disorders of Bone and Mineral Metabolism. Raven Press; New York: 1992. pp. 905–934. [Google Scholar]
- 54.Hsu CY, Cummings SR, McCulloch CE, et al. Bone mineral density is not diminished by mild to moderate chronic renal insufficiency. Kidney Int. 2002;61:1814–1820. doi: 10.1046/j.1523-1755.2002.00306.x. [DOI] [PubMed] [Google Scholar]
- 55.Jamal SA, Hayden JA, Beyene J. Low bone mineral density and fractures in long-term hemodialysis patients: a meta-analysis. Am J Kidney Dis. 2007;49:674–681. doi: 10.1053/j.ajkd.2007.02.264. [DOI] [PubMed] [Google Scholar]
- 56.Black DM, Cummings SR, Genant HK, et al. Axial and appendicular bone density predict fractures in older women. J Bone Miner Res. 1992;7:633–638. doi: 10.1002/jbmr.5650070607. [DOI] [PubMed] [Google Scholar]
- 57.Hui SL, Slemenda CW, Johnston CC., Jr Baseline measurement of bone mass predicts fracture in white women. Ann Intern Med. 1989;111:355–361. doi: 10.7326/0003-4819-111-5-355. [DOI] [PubMed] [Google Scholar]
- 58.Johnston CC, Jr, Slemenda CW, Melton LJ., III Clinical use of bone densitometry. New Engl J Med. 1991;324:1105–1109. doi: 10.1056/NEJM199104183241606. [DOI] [PubMed] [Google Scholar]
- 59.Ross PD, Davis JW, Epstein RS, et al. Pre-existing fractures and bone mass predict vertebral fracture incidence in women. Ann Intern Med. 1991;114:919–923. doi: 10.7326/0003-4819-114-11-919. [DOI] [PubMed] [Google Scholar]
- 60.Parfitt AM. Hormonal influences on bone remodeling and bone loss: application to the management of primary hyperparathyroidism. Ann Intern Med. 1996;125:413–415. doi: 10.7326/0003-4819-125-5-199609010-00009. [DOI] [PubMed] [Google Scholar]
- 61.Parisien M, Silverberg SJ, Shane E, et al. The histomorphometry of bone in primary hyperparathyroidism: preservation of cancellous bone structure. J Clin Endocrinol Metab. 1990;70:930–938. doi: 10.1210/jcem-70-4-930. [DOI] [PubMed] [Google Scholar]
- 62.Atkinson PJ, Hancock DA, Acharya VN, et al. Changes in skeletal mineral in patients on prolonged maintenance dialysis. BMJ. 1973;4:519–522. doi: 10.1136/bmj.4.5891.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lindergard B, Johnell O, Nilsson BE, et al. Studies of bone morphology, bone densitometry and laboratory data in patients on maintenance hemodialysis treatment. Nephron. 1985;39:122–129. doi: 10.1159/000183355. [DOI] [PubMed] [Google Scholar]
- 64.Przedlacki J, Manelius J, Huttunen K. Bone mineral density evaluated by dual-energy X-ray absorptiometry after one-year treatment with calcitriol started in the predialysis phase of chronic renal failure. Nephron. 1995;69:433–437. doi: 10.1159/000188515. [DOI] [PubMed] [Google Scholar]
- 65.Jamal SA, Chase C, Goh YI, et al. Bone density and heel ultrasound testing do not identify patients with dialysis-dependent renal failure who have had fractures. Am J Kidney Dis. 2002;39:843–849. doi: 10.1053/ajkd.2002.32006. [DOI] [PubMed] [Google Scholar]
- 66.Jamal SA, Gilbert J, Gordon C, et al. Cortical pQCT measures are associated with fractures in dialysis patients. J Bone Miner Res. 2006;21:543–548. doi: 10.1359/jbmr.060105. [DOI] [PubMed] [Google Scholar]
- 67.Laib A, Ruegsegger P. Calibration of trabecular bone structure measurements of in vivo three-dimensional peripheral quantitative computed tomography with 28-microm-resolution microcomputed tomography. Bone. 1999;24:35–39. doi: 10.1016/s8756-3282(98)00159-8. [DOI] [PubMed] [Google Scholar]
- 68.Torres A, Lorenzo V, Gonzalez-Posada JM. Comparison of histomorphometry and computerized tomography of the spine in quantitating trabecular bone in renal osteodystrophy. Nephron. 1986;44:282–287. doi: 10.1159/000184007. [DOI] [PubMed] [Google Scholar]
- 69.Grotz WH, Mundinger FA, Muller CB, et al. Trabecular bone architecture in female renal allograft recipients—assessed by computed tomography. Nephrol Dial Transplant. 1997;12:564–569. doi: 10.1093/ndt/12.3.564. [DOI] [PubMed] [Google Scholar]
- 70.Hasegawa K, Hasegawa Y, Nagano A. Estimation of bone mineral density and architectural parameters of the distal radius in hemodialysis patients using peripheral quantitative computed tomography. J Biomech. 2004;37:751–756. doi: 10.1016/S0021-9290(03)00174-X. [DOI] [PubMed] [Google Scholar]
- 71.Negri AL, Lombas C, Cuevas C, et al. Evaluation of cortical bone by peripheral quantitative computed tomography in renal transplant recipients. Transplant Proc. 2005;37:1020–1022. doi: 10.1016/j.transproceed.2004.12.056. [DOI] [PubMed] [Google Scholar]
- 72.Russo CR, Taccetti G, Caneva P, et al. Volumetric bone density and geometry assessed by peripheral quantitative computed tomography in uremic patients on maintenance hemodialysis. Osteoporos Int. 1998;8:443–448. doi: 10.1007/s001980050089. [DOI] [PubMed] [Google Scholar]
- 73.Tsuchida T, Ishimura E, Miki T, et al. The clinical significance of serum osteocalcin and N-terminal propeptide of type I collagen in predialysis patients with chronic renal failure. Osteoporos Int. 2005;16:172–179. doi: 10.1007/s00198-004-1655-9. [DOI] [PubMed] [Google Scholar]
- 74.Wehrli FW, Leonard MB, Saha PK, et al. Quantitative high-resolution magnetic resonance imaging reveals structural implications of renal osteodystrophy on trabecular and cortical bone. J Magn Reson Imaging. 2004;20:83–89. doi: 10.1002/jmri.20085. [DOI] [PubMed] [Google Scholar]
- 75.Genant HK, Lang TF, Engelke K, et al. Advances in the noninvasive assessment of bone density, quality, and structure. Calcif Tissue Int. 1996;59 (Suppl 1):S10–S15. doi: 10.1007/s002239900169. [DOI] [PubMed] [Google Scholar]
- 76.Guglielmi G, Schneider P, Lang T, et al. Quantitative computed tomography at the axial and peripheral skeleton. Eur Radiol. 1997;7 (Suppl 2):S32–S42. [PubMed] [Google Scholar]
- 77.Augat P, Gordon CL, Lang TF, et al. Accuracy of cortical and trabecular bone measurements with peripheral quantitative computed tomography (pQCT) Phys Med Biol. 1998;43:2873–2883. doi: 10.1088/0031-9155/43/10/015. [DOI] [PubMed] [Google Scholar]
- 78.Altkorn D, Vokes T. Treatment of postmenopausal osteoporosis. JAMA. 2001;285:1415–1418. doi: 10.1001/jama.285.11.1415. [DOI] [PubMed] [Google Scholar]
- 79.Cunningham J, Danese M, Olson K, et al. Effects of the calcimimetic cinacalcet HCl on cardiovascular disease, fracture, and health-related quality of life in secondary hyperparathyroidism. Kidney Int. 2005;68:1793–1800. doi: 10.1111/j.1523-1755.2005.00596.x. [DOI] [PubMed] [Google Scholar]
- 80.Andress DL. Vitamin D treatment in chronic kidney disease. Semin Dial. 2005;18:315–321. doi: 10.1111/j.1525-139X.2005.18408.x. [DOI] [PubMed] [Google Scholar]
- 81.Coburn JW, Maung HM, Elangovan L, et al. Doxercalciferol safely suppresses PTH levels in patients with secondary hyperparathyroidism associated with chronic kidney disease stages 3 and 4. Am J Kidney Dis. 2004;43:877–890. doi: 10.1053/j.ajkd.2004.01.012. [DOI] [PubMed] [Google Scholar]
- 82.Rix M, Eskildsen P, Olgaard K. Effect of 18 months of treatment with alfacalcidol on bone in patients with mild to moderate chronic renal failure. Nephrol Dial Transplant. 2004;19:870–876. doi: 10.1093/ndt/gfg595. [DOI] [PubMed] [Google Scholar]
- 83.Jokihaara J, Porsti I, Pajamaki I, et al. Paricalcitol [19-nor-1,25-(OH)2D2] in the treatment of experimental renal bone disease. J Bone Miner Res. 2006;21:745–751. doi: 10.1359/jbmr.060114. [DOI] [PubMed] [Google Scholar]
- 84.Miller PD, Roux C, Boonen S, et al. Safety and efficacy of risedronate in patients with age-related reduced renal function as estimated by the Cockcroft and Gault method: a pooled analysis of nine clinical trials. J Bone Miner Res. 2005;20:2105–2115. doi: 10.1359/JBMR.050817. [DOI] [PubMed] [Google Scholar]
- 85.Fontaine MA, Albert A, Dubois B, et al. Fracture and bone mineral density in hemodialysis patients. Clin Nephrol. 2000;54:218–226. [PubMed] [Google Scholar]