Abstract
Rapid and somewhat surprising advances have recently been made towards understanding the molecular mechanisms causing heritable disorders of hypophosphatemia. The results of clinical, genetic, and translational studies have interwoven novel concepts underlying the endocrine control of phosphate metabolism, with far-reaching implications for treatment of both rare, Mendelian diseases as well as common disorders of blood phosphate excess such as chronic kidney disease (CKD). In particular, diseases caused by changes in the expression and proteolytic control of the phosphaturic hormone Fibroblast growth factor-23 (FGF23) have come to the forefront in terms of directing new models explaining mineral metabolism. These hypophosphatemic disorders, as well as others resulting from independent defects in phosphate transport or metabolism, will be reviewed herein, and implications for emerging therapeutic strategies based upon these new findings will be discussed.
Keywords: phosphate, hypophosphatemia, hyperphosphatemia, osteomalacia, FGF23, αKlotho, mineralization, rickets, furin, Fam20c, DMP1, ENPP1, GALNT3
Introduction
Hypophosphatemia can result in the striking presence of rickets in children that arises through a delay in endochondral ossification (1) and osteomalacia in children and adults, therefore proper maintenance of blood phosphate levels is crucial for bone health. Over the last two decades highly significant clinical, genetic, and translational studies of disorders of hypophosphatemia have provided key molecular insights into the systemic control of phosphate handling. Although the molecular mechanisms are unique to each disorder and reviewed herein, elevated Fibroblast growth factor-23 (FGF23), acting through its co-receptor αKlotho (αKL), is associated with multiple syndromes characterized by the hallmark biochemical phenotype of hypophosphatemia with low or inappropriately normal 1,25(OH)2 vitamin D (1,25D). These disorders include: autosomal dominant hypophosphatemic rickets (ADHR), X-linked hypophosphatemic rickets (XLH), autosomal recessive hypophosphatemic rickets (ARHR types 1–3), as well as the paraneoplastic disorder tumor-induced osteomalacia (TIO), which arises from over and unregulated production of FGF23 by a tumor. Hyperphosphatemic familial tumoral calcinosis (hfTC) is the biochemical reciprocal to ADHR and XLH, and is associated with loss of function mutations in FGF23 itself, genes controlling FGF23 stability, as well as αKL. This review will highlight the Mendelian disorders involving hypophosphatemic rickets, focusing upon recent aspects of novel FGF23 control of mineral metabolism, as well as emerging therapeutic approaches that could have impact on common diseases such as chronic kidney disease (CKD).
Multi-tissue Control of Systemic Phosphate Homeostasis
The majority of systemic phosphate homeostasis occurs through endocrine control of feedback loops involving phosphate absorption in the intestine, reabsorption in the kidney, and long-term storage in bone. Although its primary role is to control serum calcium levels, parathyroid hormone (PTH) has a major effect on serum phosphate concentrations. The type II sodium phosphate co-transporters NPT2a and NPT2c reside in the apical surface of the renal proximal tubule, and NPT2a is rapidly down-regulated following PTH delivery via the PTH/PTHrP receptor (2). PTH up-regulates the kidney 1-α-hydroxyase (Cyp27b1) mRNA thus increasing the production of active 1,25D. Circulating 1,25D stimulates calcium and phosphate absorption in the intestine and reduces PTH production. When mice were injected with 1,25D, serum FGF23 concentrations rose before detectable changes in serum phosphate, and these FGF23 increases have been faithfully mimicked in bone cell lines in vitro (3,4). FGF23 has the same effect as PTH on NPT2a and NPT2c expression in the renal proximal tubule (PT), but elicits opposite effects on 1,25D by down-regulating Cyp27b1 and increasing Cyp24a1 (5). When 1,25D is elevated in the blood as a product of Cyp27b1 activity, 1,25D increases FGF23 in bone, and circulating FGF23 completing the negative-feedback loop in kidney. Thus, the net effect of elevated circulating ‘intact’ FGF23 (iFGF23) is hypophosphatemia with inappropriately low or normal 1,25D. Evidence from short term (hours) studies also supports that FGF23 suppresses PTH by directly acting in the parathyroid gland as this tissue is known to express αKL (6,7), consistent with a feedback loop where PTH increases FGF23 in bone (8). Interestingly, parathyroid-specific conditional deletion of αKL points to an FGF23 effect on PTH secretion that is mediated through an NFAT pathway, as serum PTH and calcium-responsiveness were not different in the absence of parathyroid αKL (9). Certainly, these endocrine feedback loops are complex as highlighted by the fact that in the setting of late-stage CKD, both PTH and FGF23 are elevated, pointing to the concept that depending upon the physiological or pathophysiological context and the length of time, these hormone systems may interact in ways that are different than those seen is studies lasting a short duration.
FGF23 Activity Bridges Bone and Kidney Phosphate Handling
FGF23 requires the co-receptor αKlotho (αKL) to mediate its biological effects. αKL is produced as several isoforms: the ‘membrane’ bound αKL (‘mKL’) form is a 130 kD single-pass transmembrane protein characterized by a large extracellular domain that interacts with FGF23 as well as a short intracellular region not capable of independent signaling (10). The mKL protein can be cleaved near the transmembrane domain by membrane-bound secretases (11), giving rise to a circulating form of αKL, ‘cut-’ or ‘cleaved-KL’ (‘cKL’). mKL permits FGF23 signaling after the recruitment of canonical FGF receptors (FGFRs) (12), whereas the potential functional significance of cKL in terms of FGF23 is emerging (see below). Some findings support specific interactions between FGFR1c and αKL (13), but additional studies indicate that FGFR3c and FGFR4 can mediate FGF23-αKL signaling (17). Studies involving conditional-null mice support that FGFR1 may be more important for phosphate homeostasis, whereas FGFR3c and FGFR4 may play a role in vitamin D regulation (14). Consistent with FGFR signaling, renal FGF23 bioactivity appears to be primarily mediated through mitogen activated protein kinase (MAPK) cascades (15). The vast majority of αKL protein, as well as initial MAPK signaling following bolus FGF23 injections, primarily localizes to the renal distal tubule (DT), whereas the NPT2a and -2c transporters, Cyp27b1, and Cyp24a1 localize to the proximal tubule (PT) (16,17). Although immunofluorescence supports that a small proportion of αKL may localize to the PT, conditional deletion of αKL from the DT (using Ksp-Cre) resulted in hyperphosphatemia and elevated iFGF23, similar to global αKL deletion but less extreme (17). In addition to its effects in the PT, recent evidence suggests a direct role for FGF23 in regulating DT Ca2+ channels (18). Although to date there has been no stand-alone ‘phosphate sensor’ protein identified analogous to the Ca2+-sensing receptor (CASR), FGF23 increases are associated with increased phosphate intake in mice and humans (19,20). Recent evidence also supports that calcium stimulates FGF23 in vitro and in vivo (21), thus FGF23 is required to complete critical feedback loops for maintaining mineral ion homeostasis.
FGF23 serum assays provide a valuable physiological tool
Circulating FGF23 concentrations are measured using several commercial ELISAs, and as described below, these assays have proven important for understanding a novel, secondary level of control for plasma bioactive FGF23 protein (see ADHR and ARHR-3). Human- and rodent-specific ELISAs were developed that utilize two capture antibodies that recognize FGF23 antigens ‘C-terminal’ (cFGF23) to the FGF23 176RXXR179/S SPC cleavage site (from Immutopics, Int’l.; (22)), and thus recognizes both bioactive, intact FGF23 (iFGF23) and C-terminal proteolytic fragments that arise from intracellular cleavage of the mature hormone. An ELISA that uses conformation-specific monoclonal antibodies that essentially ‘span’ the 176RXXR179/S180 SPC site in terms of antigen recognition sites detects bioactive ‘intact’ FGF23 (iFGF23) across a wide variety of species (from Kainos, Inc.; (23)). The intact and C-terminal assays largely agree for FGF23 concentrations in patients with XLH and TIO, but as described herein, the ELISAs can be diagnostic for altered FGF23 regulation under specific disease circumstances.
Hypophosphatemic Disorders Reveal Novel Control Points in Mineral Metabolism
The heritable hypophosphatemias involving FGF23 are caused by alterations in genes that affect bone cell function or differentiation, with a common denominator of elevating iFGF23 by ‘over-riding’ the potent suppressive effects of low serum phosphate on FGF23 transcription (summarized in Figure 1 and see below). Furthermore, the control of serum iFGF23:cFGF23 ratios is emerging as being an important product of a complex synopsis of factors associated with calcium and phosphate balance in combination with factors that appear to lie outside traditional models of phosphate handling.
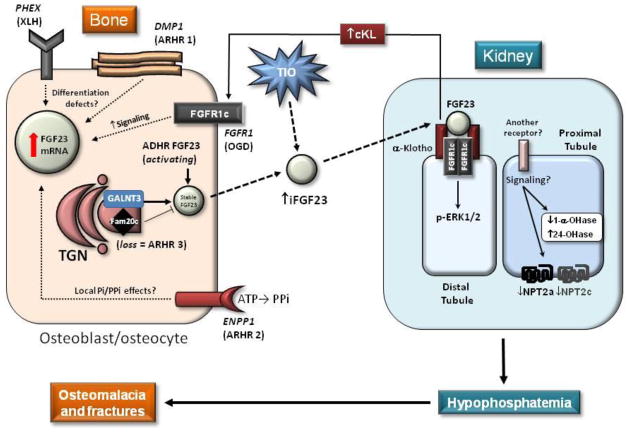
Figure 1. Schematic summary of genetic hypophosphatemias.
Loss of function mutations in PHEX (causes XLH) and DMP1 (ARHR-1) result in osteoblast to osteocyte differentiation defects and are associated with elevated FGF23 mRNA and circulating iFGF23 protein. FGFR1c activating mutations lead to inappropriate receptor signaling in OGD, and ENPP1 inactivating mutations (ARHR-2) may alter bone cell differentiation or the cell’s responsiveness to PPi and Pi leading to increased FGF23 through mechanisms that remain unclear. The FGF23 ADHR mutations within the RXXR/SAE domain reduce the ability of SPC proteases to cleave and inactivate iFGF23. Loss of FAM20c (ARHR-3 or Raine’s Syndrome) results in reduced DMP1 expression, and may have direct effects on the phosphorylation of FGF23 resulting in stabilization of iFGF23 through permitting GALNT3 activity. TIO tumors over produce FGF23 mRNA due to unknown molecular changes and secrete high levels of iFGF23. Circulating iFGF23 acts in kidney through mKL and FGFRs (potentially FGFR1c) to down regulate NPT2a and NPT2c and Cyp27b1, and up regulate Cyp24a1. Whether FGF23 acts in the kidney distal tubule first, then the proximal tubule through an alternate receptor system is unclear. A syndrome of iFGF23 excess also occurs through over-production of cKL which likely acts through osteoblast/osteocyte FGFRs to increase FGF23 mRNA. The net effect of increased iFGF23 is to reduce renal Pi reabsorption and 1,25D production, thus resulting in hypophosphatemia, causing osteomalacia and fractures.
Autosomal dominant hypophosphatemic rickets (ADHR; OMIM 193100)
Recent advances in the study of ADHR have led to novel insights underlying the molecular mechanisms controlling iFGF23 concentrations. ADHR is caused by gain of function mutations in FGF23 that occur within the RXXR/S motif that comprises a subtilisin-like proprotein convertase (SPC) proteolytic cleavage site (176RHTR179/S180), that separates the conserved FGF-like N-terminal domain from the variable C-terminal tail (24). The ADHR mutations substitute arginine (R) residues at positions 176 or 179 to glutamine (Q) or tryptophan (W) (24–26), and make FGF23 more stable (Figure 1). Indeed, following expression in vitro, ADHR mutant FGF23 is detected primarily as the full-length, intact 32 kDa form upon immunoblotting, whereas WT FGF23 is more labile thus less readily detected as the intact form, with more apparent 20 kDa (N-terminal) and 12 kDa (C-terminal) fragments. The trans-Golgi network (TGN) enzyme UDP-GalNAc transferase 3 (GALNT3) is known to O-glycosylate FGF23 on T178 within the 176RHTR179/S180 motif and prevent its proteolysis by SPC enzymes (27), indicating a dynamic role for this enzyme in controlling iFGF23 production. In support of this model, inactivating GALNT3 mutations in humans and gene ablation in the GALNT3-null mouse results in hfTC through increased SPC proteolysis of iFGF23, and thus the inability to reduce serum phosphate (28,29) (also see GALNT3 interactions with FAM20c in ARHR-3 section below). In the converse situation to ADHR, FGF23 loss of function mutations also result in hyperphosphatemia and hfTC due to destabilization of iFGF23 (30–32). Both GALNT3-hfTC and FGF23-hfTC mutations result in a signature ELISA profile of low serum iFGF23 with a correspondingly marked elevation of cFGF23 (30,33), created by a positive feedback loop in a physiological attempt to compensate for the prevailing elevated serum phosphate but in the context of an unstable FGF23 protein. ADHR is unique among the hereditary disorders of hypophosphatemia, as this disease is characterized by either early or delayed onset with variable expressivity (34). The origin of the ‘waxing and waning’ of the ADHR phenotype has recently been addressed through both clinical and translational studies linking FGF23 and iron handling (see below).
FGF23 and Iron Metabolism
Iron metabolism has recently been associated with altering FGF23 expression and being at least in part, responsible for the clinical ADHR phenotype. The late-onset ADHR disease courses can occur during physiological situations of iron deficiency/anemia, including puberty and menses onset as well as post-partum (34), and specific formulations of iron therapy, including iron maltose, elevated iFGF23 and resulted in hypophosphatemia in patients treated for anemia (35,36). Additionally, FGF23 was found to inversely correlate with serum ferritin in anemic patients tested with the cFGF23 ELISA (37). In ADHR, changes in iFGF23 parallel those of cFGF23 due to the fact that the intact and C-terminal ELISAs both detect iFGF23. Using the intact and C-terminal assays, Imel and colleagues found that serum iron was inversely correlated with iFGF23 and cFGF23 in patients with ADHR, but only cFGF23 in normal individuals (38). This relationship was maintained in ADHR patients longitudinally for 1,25D and serum phosphate (38).
To provide insight into the molecular mechanisms underlying these observational phenomena, a line of ADHR knock-in mice (carrying the R176Q ADHR point mutation) were placed on iron-deficient diet (39). Interestingly, the wild type (WT) and ADHR mice had marked increases in bone FGF23 mRNA when made anemic, which was associated with stabilization of Hypoxia-inducible factor (HIF)-1α transcription factor, a known intracellular responder to iron deprivation and hypoxia (40). Through these analyses, it was revealed that a significant portion of the low-iron ADHR mice had elevated iFGF23 and cFGF23 leading to a hypophosphatemic osteomalacia phenotype, whereas the WT mice had normal iFGF23 but elevated cFGF23 and normal phosphate metabolism. The ADHR iron deficient mice had reduced kidney NPT2a, and inappropriate levels of Cyp27b1 (suppressed) and Cyp24a1 (elevated), similar to FGF23 transgenic mice (39). Taken together, the human and mouse findings supported the concept that FGF23 mRNA was induced in bone by iron deficiency anemia, but normal humans and mice could proteolytically cleave the excess FGF23 to maintain proper circulating iFGF23. In contrast, FGF23 carrying an ADHR stabilizing mutation is not cleaved as efficiently as WT FGF23 by intracellular SPC proteases, reflected as elevated iFGF23. Further highlighting the importance of intracellular processing and the relationships between iron and FGF23, was a prospective study performed in anemic women that examined the delivery of ferric carboxymaltose versus iron dextran (41). Both compounds normalized serum iron, however the iron dextran reduced cFGF23 and iFGF23, whereas the iron maltose reduced cFGF23 but caused a transient rise in iFGF23 that was associated with hypophosphatemia (41). These observations would explain the elevated iFGF23 in some patients receiving iron therapy and indicate that specific iron pharmaceutical preparations alter the intracellular proteolytic processing of FGF23. The altered cleavage and secondary regulation of iFGF23 could potentially occur from imbalances in GALNT3 or SPC (or FAM20c, see below) expression or activity, or some combinations of all of these. Consistent with dynamic iFGF23 regulation, patients with polyostotic fibrous dysplasia (FD) have increased production and altered proteolytic cleavage of iFGF23, leading to renal phosphate wasting (42). Evidence points to the idea that sustained activation of Gsα increases GALNT3 activity and reduces furin activity, thus promoting an intracellular milieu favoring secretion of iFGF23 in these patients (43).
Iron deficiency is also common during late pregnancy, therefore to test for early onset ADHR in a translational study, breeding WT and ADHR mice were placed on low iron diets the last week of pregnancy (to mimic the last trimester) until weaning (44). Interestingly, both the WT and ADHR mice had a hypophosphatemic phenotype with increased iFGF23, and the ADHR mice were affected to a greater degree for all variables (iFGF23, serum Pi, alterations in 1,25D and its kidney metabolic enzymes) most likely due to the stabilizing R176Q FGF23 mutation. Further, studies in normal rats demonstrated that hypoxia treatment under a situation of normal serum iron and phosphate elevated cFGF23 but not iFGF23, similar to WT mice receiving low iron diet. The in vivo hypoxic effect on FGF23 was mimicked in UMR-106 cells, which increased FGF23 mRNA after culture in a reduced atmospheric O2 tension of 10% (normal = 21% O2), and HIF1α stabilization was temporally correlated with FGF23 mRNA increases (44). These studies point to a role for FGF23 in neonatal life, perhaps aiding in the coordination of iron and phosphate metabolism during early bone formation. In summary, recent studies of iron metabolism in normal individuals and in ADHR now points to novel ‘sensing’ for the optimization of iFGF23:cFGF23 ratios to control phosphate metabolism through a dynamic secondary level of protein regulation, emerging as interplay between FGF23 mRNA production, GALNT3 glycosylation, and SPC cleavage. Additionally, new data support a role for FGF23 phosphorylation in mediating the control of iFGF23 (see ARHR3 below).
X-Linked hypophosphatemic rickets (XLH; OMIM 307800)
XLH is caused by loss of function mutations in the PHEX gene (phosphate-regulating gene with homologies to endopeptidases on the X chromosome) (45), a member of the M13 family of membrane-bound metalloproteases, which has highest expression in bone cells (osteoblasts, osteocytes) and teeth (odontoblasts) (46). Patients with XLH have elevated iFGF23 as well as the hallmark biochemical features of FGF23-related diseases. Inactivating PHEX mutations have previously been associated with a differentiation defect in osteocytes in the Hyp mouse model of XLH (47) which appears to be similar to that found in the Dmp1-null mouse (see ARHR-1), and Hyp bone FGF23 mRNA expression is elevated (Figure 1) (48,49). The increased FGF23 mRNA levels indicate that the increase in serum FGF23 in XLH is due to both over production by skeletal cells, as well as potentially a decrease in secondary iFGF23 proteolysis by SPC family members (50,51). The interactions between FGF23 and PHEX are indirect as FGF23 is not a PHEX substrate (52). There is evidence that osteopontin (OPN) is cleaved by PHEX, supporting that the OPN anti-mineralizing peptide would build up in XLH patients (and the Hyp mouse) (53), however the relevance of this finding to the pathophysiology of XLH is unclear and a complete list of physiological PHEX substrates remains unknown.
Serum phosphate concentrations tend to improve, but do not normalize during XLH therapy, which currently consists of a combination of oral phosphate and calcitriol replacement. However serum FGF23 concentrations tend to continue to rise in response to phosphate and vitamin D supplementation, providing support for altered ‘sensing’ of serum phosphate concentrations (54). To test the mechanisms underlying this phenomenon, mice with inactivating PHEX mutations (Hyp) were crossed with mice lacking GALNT3 (55). The PHEX/GALNT3 double mutant mice were hypophosphatemic with serum phosphate concentrations slightly, but significantly higher than Hyp with wild type GALNT3 alleles. Surprisingly, in the presence of only a slightly improved serum phosphate concentration, the double mutant mice markedly up-regulated FGF23 mRNA, well above the Hyp mouse with WT GALNT3 alleles (>20 fold in 12 week old Hyp mice) (55). In contrast to patients with ADHR, XLH patients and controls showed similar correlations between serum iron and iFGF23/cFGF23 (albeit XLH was on an increased scale) supporting that iron responsiveness is likely not a central factor in the pathogenesis of XLH (51). These results indicate that the PHEX mutation, in addition to potentially resulting in altered osteocyte cell differentiation, alters biological sensing for phosphate.
It is well established that male and female Hyp mice and XLH patients have similar relative serum phosphate and 1,25D concentrations (1). To further examine a phosphate ‘set point’ in XLH, a study compared female mice homozygous for PHEX inactivating mutations to females heterozygous for PHEX mutations as well as male hemizygotes (51). Both models had similar iFGF23, and the iFGF23 and serum phosphate were also similar between heterozygous females and hemizygous males (51). This lack of a gene dosage effect on circulating FGF23 and phosphate further suggests that inactivating PHEX mutations may create a lower set point for maintaining blood phosphate concentrations.
Autosomal recessive hypophosphatemic rickets (ARHR)
ARHR Type 1, (OMIM 241520)
Dentin Matrix Protein-1 (DMP1), a member of the Small Integrin-Binding LIgand, N-linked Glycoprotein (SIBLING) family, is highly expressed in osteocytes. Both Dmp1-null mice and patients with ARHR-1 have rickets and osteomalacia with isolated renal phosphate loss associated with elevated iFGF23. Mutational analyses revealed that ARHR families carry mutations in the DMP1 start codon, the C-terminus, as well as in exon splicing sites (56,57). Translational studies using the Dmp1-null mouse demonstrate that loss of DMP1 causes defective osteocyte maturation, leading to elevated FGF23 expression and pathological changes in bone mineralization (Figure 1) (56). Importantly, Dmp1-null mice appear to be biochemical phenocopies of the Hyp mouse, suggesting that PHEX may also have a role in osteocyte maturation in a parallel pathway to DMP1 that leads to over expression of FGF23 (Figure 1). Initial data support that a portion of the bone mineralization defect in the Dmp1-null mouse can be attributed to increased Wnt/β-catenin activity, along with suppression of DKK1, a Wnt/β-catenin signaling inhibitor. Further, over expression of DKK1 in the Dmp1-null mouse led to healing of the osteomalacia via enhanced bone formation rate and mineralization (58). Similarly, DKK1 production is normalized by over-expression of the 57-kDa C-terminal DMP1 fragment (59). Generation of a combined DMP1 and αKlotho deficient mouse implicated DMP1 as playing an anti-apoptotic role during hyperphosphatemia, which could be clinically relevant in protecting cells within a high phosphate environment (60).
ARHR Type 2, (OMIM 613312)
ARHR-2 is due to loss of function mutations in ectonucleotide pyrophosphatase/phosphodiesterase-1 (ENPP1), a gene that controls physiologic mineralization and pathologic chondrocalcinosis by producing inorganic pyrophosphate (PPi), an anti-mineralization metabolite. Indeed, patients with recessive ENPP1 mutations have iFGF23 modestly above the normal mean as well as hypophosphatemia (61,62). Evidence supports that ENPP1 may regulate osteoblastic differentiation in an manner independent of extracellular phosphate (Figure 1) (63). Therefore, the ENPP1 ARHR-2 mutations could potentially result in a cell differentiation defect and over expression of FGF23, similar to the biological situation for DMP1 (ARHR-1) and PHEX (XLH). ENPP1 hydrolyzes ATP to PPi and ENPP1 is responsive to extracellular Pi (64), therefore the ARHR-2 alterations may indicate that maintaining proper ratios of PPi to inorganic phosphate (Pi) in bone could play a role in iFGF23 production (Figure 1). Mutations in ENPP1 are also causative for idiopathic infantile arterial calcification (IIAC), as well as ossification of the posterior longitudinal ligament of the spine (OPLL), underscoring ENPP1’s role as an inhibitor of tissue calcification. Study of the Enpp1-null mouse revealed elevated FGF23, decreased serum phosphate and calcium, aortic and kidney calcification, as well as joint and spine ectopic cartilage formation (65). Altered trabecular architecture and bone geometry were also observed, with reduced trabecular number, trabecular bone volume, and trabecular and cortical thickness in the tibia and femur.
ARHR Type 3, (OMIM 259775 Raine’s syndrome; RNS)
ARHR-3 is a variant of Raine’s syndrome, caused by loss of function mutations in the Family with sequence similarity 20, member C (FAM20c) (also known as Golgi Casein Kinase (G-CK) or Dentin matrix protein-4 (DMP4)), and is usually lethal in infancy. However, not all Raine’s syndrome mutations may be lethal (66). It was determined using exome sequencing that compound heterozygous mutations in FAM20c were associated with increased plasma iFGF23 in a Raine’s patient (67), who also manifested tapering hypophosphatemia with age, severe tooth decay from infancy, ectopic brain calcifications, and osteosclerosis (67). Interestingly, Fam20c-null mice are characterized by elevated iFGF23, hypophosphatemia with inappropriately normal 1,25D concentrations, severe tooth defects and a ricketic phenotype (68). It was discovered that FAM20c is an ‘atypical’ kinase localized to the endoplasmic reticulum/TGN that phosphorylates proteins within an ‘S-x-E’ motif, including a number of secreted SIBLING family members such as osteopontin (OPN) and DMP1 (69,70). Thus, loss of FAM20c may reduce DMP1 phosphorylation and inhibit its expression or function, thereby producing a ‘DMP1-knockout’ phenocopy in the Fam20c-null mice (68). In accord with the noted similarities between the Dmp1-null and Fam20c-null mice, it was discovered that DMP1 levels were reduced in Fam20c knockdown cells (68). It remains unexplained why the dominant phenotype in patients with ARHR-3 is a severe, osteosclerotic disease whereas the Fam20c-null mice have a ricketic bone phenotype.
In addition to the effects of FAM20c on the SIBLING family members, it has recently come to light that FGF23 is a direct substrate for this kinase (71). In this regard, a key FAM20c phosphorylation event occurs on S180 (‘SAE’ motif) within the FGF23 SPC protease site 176RHT178R179/S180AE. In vitro results support that the phosporylated-S180 (pS180) prohibits the GALNT3-mediated glycosylation of T178, thus making FGF23 susceptible to SPC cleavage (Figure 2) (71). Interestingly, when a FAM20c cDNA carrying a Raine’s syndrome loss of function T268M mutation was co-expressed with FGF23, the mutant FAM20c only partially inhibited GALNT3 glycosylation of FGF23. This breakthrough supports that Raine syndrome FAM20c mutations that permit survival likely have partial function and reduced ability to phosphorylate FGF23 S180. This change in FGF23 is predicted to shift the cellular balance towards increased GALNT3 glycosylation on T178 and stabilizing FGF23, thus increasing iFGF23 secretion (Figure 2) (71). Additionally, of the SPC proteases PSCK1, -2, and -3 (furin) co-expressed with FGF23, only furin was capable of cleaving pS180-FGF23, supporting that this enzyme is a principle regulator of iFGF23, and that the regulation of furin activity or expression could potentially change the balance of iFGF23:cFGF23 under biological circumstances of altered Pi and in disease.
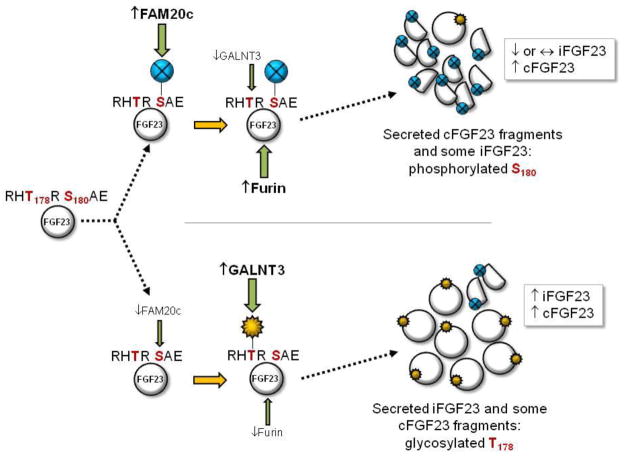
Figure 2. Model for dynamic control of iFGF23:cFGF23 ratios.
The study of diseases involving altered iFGF23:cFGF23 ratios, including ADHR and Raine’s syndrome, has introduced a model for dynamic FGF23 regulation. FGF23 protein (far left) carries an RHT178R-S180AE motif that comprises an ‘RXXR/S’ subtilisin-like proprotein convertase (SPC) site. (Upper) When FAM20c activity/expression is increased, S180 is phosphorylated and GALNT3 O-glycosylation of T178 may be inhibited making iFGF23 a furin substrate, thus levels of secreted iFGF23 can remain constant (or potentially decrease) with a corresponding increase in cFGF23 proteolytic fragments. (Lower) When FAM20c activity/expression is reduced, GALNT3 O-glycosylates FGF23 T178, which prevents furin cleavage resulting in increased secretion of iFGF23.
Renal Failure and Implications for FGF23 Processing
Elevated FGF23 in CKD is correlated with increased mortality, disease onset, progression of left ventricular hypertrophy (LVH), as well as loss of renal transplant allografts (72–74), and a direct effect of FGF23 to induce LVH has been demonstrated through in vivo studies in mice (75). Additionally, ex vivo studies point to direct effects of FGF23 on acutely increasing cardiac cell [Ca2+]i which may chronically lead to decreases in contractile function or stimulate cardiac hypertrophy (76). Understanding the associations between FGF23 and the CKD disease manifestations involving PTH, phosphate and calcium are likely to be important, considering initial studies demonstrated that administration of the calcimimetic. Cinacalcet reduced plasma FGF23 (25% reduction) versus placebo (31% increase in FGF23) in end stage renal disease patients (77). Further, it was reported that iFGF23 is the primary circulating form in patients with late-stage CKD, with disproportionally less production of C-terminal FGF23 fragments (78), supporting a defect in the proteolytic regulation of iFGF23 in this disease. Finally, in acute kidney disease iFGF23 is up-regulated before changes in serum phosphate, PTH or 1,25D (79). Taken together, these disorders point to imbalances in the proteolytic regulation of FGF23, potentially tipping the iFGF23:cFGF23 ratio towards positive iFGF23 production.
αKL over-expression
A loss of function mutation in αKlotho was shown to be responsible for hfTC, as a patient with a H193R missense mutation in the extracellular KL-1 FGF23 binding domain had hyperphosphatemia with ectopic calcifications in the heel and brain (80). Importantly, this patient had markedly elevated iFGF23 and cFGF23, consistent with kidney resistance to circulating FGF23 (80). In a case that was the biochemical converse, a patient harboring a balanced chromosomal translocation (t9:13) with the breakpoint adjacent to the αKlotho gene was found to be hypophosphatemic with inappropriately normal 1,25D and hyperparathyroidism (81). This chromosomal abnormality was associated with elevated circulating levels of cKL as well as iFGF23 (81) (Figure 1). To model this disorder, the cKL form of αKL was administered to mice using an adeno-associated virus (AAV-cKL). This treatment resulted in markedly increased iFGF23 and an identical biochemical phenotype to the translocation patient (82). Interestingly, extended AAV-cKL treatment resulted in a 150-fold increase in bone FGF23 mRNA, which would explain the increased serum iFGF23 concentrations despite low blood phosphate. Parallel studies in bone cell lines indicated that cKL-FGFR signaling was likely involved in the elevated expression of FGF23 (82) (Figure 1). In support of FGFR-mediated effects on FGF23 production, some patients with osteoglophonic dysplasia (OGD) due to activating FGFR1c mutations have elevated iFGF23 (83,84), and FGF2 increases bone FGF23 production (85). The physiological significance of cKL-mediated FGF23 regulation could be related to controlling circulating iFGF23 in a feedback loop between kidney and bone.
Emerging Treatments
Current therapy for FGF23 mediated hypophosphatemic disorders consists of oral administration of high dose calcitriol and phosphate. Although this therapy frequently results in healing of rickets and normalization of bone histology, growth tends to be suboptimal. Complications of therapy include diarrhea from phosphate, secondary and tertiary hyperparathyroidism, nephrocalcinosis, and potential renal insufficiency. The goal of therapy should not be to normalize serum phosphate as overly aggressive therapy can promote renal insufficiency. In light of the potential complications of standard therapy, investigators are considering therapeutic options aimed at reducing circulating bioactive FGF23 concentrations or FGF23 receptor blockade.
Recently, Carpenter, et al, reported results of a single dose Phase 1–2 trial using a humanized FGF23 neutralizing antibody to treat XLH patients (86). In brief, subcutaneous injection of the antibody resulted in a dose dependent increase in TMP/GFR, serum phosphate and calcitriol. Although hyperphosphatemia was not observed, calcitriol concentrations were elevated at several doses. Additionally, calcitriol levels peaked and returned to baseline earlier than serum inorganic phosphorus concentrations. The patients did not exhibit increased nephrocalcinosis or develop hypercalciuria, hypercalcemia, elevated serum parathyroid hormone (PTH) and creatinine, or produce antibodies to the therapeutic.
Conclusions
In conclusion, recent studies of heritable and acquired disorders of hypophosphatemia have led to critical insight into mineral metabolism. Notable findings included identification of novel pathways regulating FGF23 production, as well as previously unrecognized mechanisms for dictating circulating iFGF23 concentrations through dynamic glycosylation, phosphorylation, and proteolysis. Additionally, determining the extent and mechanisms for potential FGF23 effects in cardiac tissue during situations of chronically elevated FGF23 are ongoing. These discoveries are providing fertile ground for clinical and translational investigation to identify new therapeutic targets for both rare and common disorders of phosphate handling, including direct targeting of circulating FGF23, with potential for major impact on patient care and treatment.
Acknowledgments
This work was supported by grants R01-DK063934 and R01-DK95784 (KEW), R01-AR42228 (MJE), and support from T32-HL007910-15 (for JMH) from the National Institutes of Health; and a Showalter Scholar Award funded in part through the Ralph W. and Grace M. Showalter Research Trust Fund (KEW).
Footnotes
Conflict of interest statement:
KE White received royalties from Kyowa Hakko Kirin Co., Ltd. for licensing of the FGF23 gene and the anti-FGF23 monoclonal antibody trials.
JM Hum declares no conflicts of interest.
MJ Econs received royalties from Kyowa Hakko Kirin Co., Ltd. for licensing of the FGF23 gene and the anti-FGF23 monoclonal antibody trials and has also been a consultant to Kyowa Hakko Kirin for the anti-FGF23 monoclonal antibody trials.
Human and Animal Rights and Informed Consent
All studies by the authors involving animal and/or human subjects were performed after approval by the appropriate institutional review boards. When required, written informed consent was obtained from all participants.
Contributor Information
Kenneth E. White, Email: kenewhit@iu.edu.
Julia M. Hum, Email: jhum@iu.edu.
Michael J. Econs, Email: mecons@iu.edu.
References
Papers of particular interest, published recently, have been highlighted as:
•• Of major importance
• Of importance
- 1.Tenenhouse HS, Econs MJ. Mendelian Hypophosphatemias. In: Valle D, editor. The Metabolic and Molecular Bases of Inherited Disease. The McGraw-Hill Companies; New York: 2001. pp. 1–9. [Google Scholar]
- 2.Bacic D, Lehir M, Biber J, Kaissling B, Murer H, Wagner CA. The renal Na+/phosphate cotransporter NaPi-IIa is internalized via the receptor-mediated endocytic route in response to parathyroid hormone. Kidney Int. 2006;69(3):495–503. doi: 10.1038/sj.ki.5000148. [DOI] [PubMed] [Google Scholar]
- 3.Farrow EG, Davis SI, Ward LM, Summers LJ, Bubbear JS, Keen R, Stamp TC, Baker LR, Bonewald LF, White KE. Molecular analysis of DMP1 mutants causing autosomal recessive hypophosphatemic rickets. Bone. 2009;44(2):287–94. doi: 10.1016/j.bone.2008.10.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu S, Tang W, Zhou J, Stubbs JR, Luo Q, Pi M, Quarles LD. Fibroblast growth factor 23 is a counter-regulatory phosphaturic hormone for vitamin D. J Am Soc Nephrol. 2006;17(5):1305–15. doi: 10.1681/ASN.2005111185. [DOI] [PubMed] [Google Scholar]
- 5.Shimada T, Mizutani S, Muto T, Yoneya T, Hino R, Takeda S, Takeuchi Y, Fujita T, Fukumoto S, Yamashita T. Cloning and characterization of FGF23 as a causative factor of tumor-induced osteomalacia. Proc Natl Acad Sci U S A. 2001;98(11):6500–5. doi: 10.1073/pnas.101545198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Krajisnik T, Bjorklund P, Marsell R, Ljunggren O, Akerstrom G, Jonsson KB, Westin G, Larsson TE. Fibroblast growth factor-23 regulates parathyroid hormone and 1alpha-hydroxylase expression in cultured bovine parathyroid cells. J Endocrinol. 2007;195(1):125–31. doi: 10.1677/JOE-07-0267. [DOI] [PubMed] [Google Scholar]
- 7.Ben-Dov IZ, Galitzer H, Lavi-Moshayoff V, Goetz R, Kuro-o M, Mohammadi M, Sirkis R, Naveh-Many T, Silver J. The parathyroid is a target organ for FGF23 in rats. J Clin Invest. 2007;117(12):4003–8. doi: 10.1172/JCI32409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rhee YEF, Lee R, Bivi N, Lezcano V, Plotkin L, White K, Bellido T. FGF23 Gene Expression Is Upregulated by PTH Receptor Activation In Osteocytes In Vitro and In Vivo: A Parathyroid-Bone Link Influencing the Endocrine Function of Osteocytes. J Bone Miner Res. 2009;24(Suppl 1) [Google Scholar]
- 9.Olauson H, Lindberg K, Amin R, Sato T, Jia T, Goetz R, Mohammadi M, Andersson G, Lanske B, Larsson TE. Parathyroid-specific deletion of Klotho unravels a novel calcineurin-dependent FGF23 signaling pathway that regulates PTH secretion. PLoS Genet. 2013;9(12):e1003975. doi: 10.1371/journal.pgen.1003975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Matsumura Y, Aizawa H, Shiraki-Iida T, Nagai R, Kuro-o M, Nabeshima Y. Identification of the human klotho gene and its two transcripts encoding membrane and secreted klotho protein. Biochem Biophys Res Commun. 1998;242(3):626–30. doi: 10.1006/bbrc.1997.8019. [DOI] [PubMed] [Google Scholar]
- 11.Bloch L, Sineshchekova O, Reichenbach D, Reiss K, Saftig P, Kuro-o M, Kaether C. Klotho is a substrate for alpha-, beta- and gamma-secretase. FEBS Lett. 2009;583(19):3221–4. doi: 10.1016/j.febslet.2009.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goetz R, Nakada Y, Hu MC, Kurosu H, Wang L, Nakatani T, Shi M, Eliseenkova AV, Razzaque MS, Moe OW, Kuro OM, Mohammadi M. Isolated C-terminal tail of FGF23 alleviates hypophosphatemia by inhibiting FGF23-FGFR-Klotho complex formation. Proc Natl Acad Sci U S A. 2009 doi: 10.1073/pnas.0902006107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Urakawa I, Yamazaki Y, Shimada T, Iijima K, Hasegawa H, Okawa K, Fujita T, Fukumoto S, Yamashita T. Klotho converts canonical FGF receptor into a specific receptor for FGF23. Nature. 2006;444(7120):770–4. doi: 10.1038/nature05315. [DOI] [PubMed] [Google Scholar]
- 14.Li H, Martin A, David V, Quarles LD. Compound deletion of Fgfr3 and Fgfr4 partially rescues the Hyp mouse phenotype. Am J Physiol Endocrinol Metab. 2011;300(3):E508–17. doi: 10.1152/ajpendo.00499.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kurosu H, Ogawa Y, Miyoshi M, Yamamoto M, Nandi A, Rosenblatt KP, Baum MG, Schiavi S, Hu MC, Moe OW, Kuro-o M. Regulation of fibroblast growth factor-23 signaling by klotho. J Biol Chem. 2006;281(10):6120–3. doi: 10.1074/jbc.C500457200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Farrow EG, Davis SI, Summers LJ, White KE. Initial FGF23-mediated signaling occurs in the distal convoluted tubule. J Am Soc Nephrol. 2009;20(5):955–60. doi: 10.1681/ASN.2008070783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17•.Olauson H, Lindberg K, Amin R, Jia T, Wernerson A, Andersson G, Larsson TE. Targeted deletion of Klotho in kidney distal tubule disrupts mineral metabolism. J Am Soc Nephrol. 2012;23(10):1641–51. doi: 10.1681/ASN.2012010048. A novel Klotho-derived renal-bone feedback loop was described after generating a mouse model with partial deletion of Klotho in the distal tubular segments. While the Ksp-Cre KL(−/−) mice had a normal phenotype, lacking the vascular and tubular calcifications found in Klotho(−/−) mice, they did exhibit hyperphosphatemia with elevated FGF23, increased expression of Npt2a, and increased vitamin D signaling highlighting the importance of the renal-bone Klotho feedback loop. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Andrukhova O, Smorodchenko A, Egerbacher M, Streicher C, Zeitz U, Goetz R, Shalhoub V, Mohammadi M, Pohl EE, Lanske B, Erben RG. FGF23 promotes renal calcium reabsorption through the TRPV5 channel. EMBO J. 2013;33(3):229–46. doi: 10.1002/embj.201284188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Antoniucci DM, Yamashita T, Portale AA. Dietary phosphorus regulates serum fibroblast growth factor-23 concentrations in healthy men. J Clin Endocrinol Metab. 2006;91(8):3144–9. doi: 10.1210/jc.2006-0021. [DOI] [PubMed] [Google Scholar]
- 20.Burnett SM, Gunawardene SC, Bringhurst FR, Juppner H, Lee H, Finkelstein JS. Regulation of C-terminal and intact FGF-23 by dietary phosphate in men and women. J Bone Miner Res. 2006;21(8):1187–96. doi: 10.1359/jbmr.060507. [DOI] [PubMed] [Google Scholar]
- 21.David V, Dai B, Martin A, Huang J, Han X, Quarles LD. Calcium regulates FGF-23 expression in bone. Endocrinology. 2013;154(12):4469–82. doi: 10.1210/en.2013-1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jonsson KB, Zahradnik R, Larsson T, White KE, Sugimoto T, Imanishi Y, Yamamoto T, Hampson G, Koshiyama H, Ljunggren O, Oba K, Yang IM, Miyauchi A, Econs MJ, Lavigne J, Juppner H. Fibroblast growth factor 23 in oncogenic osteomalacia and X-linked hypophosphatemia. N Engl J Med. 2003;348(17):1656–63. doi: 10.1056/NEJMoa020881. [DOI] [PubMed] [Google Scholar]
- 23.Yamazaki Y, Okazaki R, Shibata M, Hasegawa Y, Satoh K, Tajima T, Takeuchi Y, Fujita T, Nakahara K, Yamashita T, Fukumoto S. Increased circulatory level of biologically active full-length FGF-23 in patients with hypophosphatemic rickets/osteomalacia. J Clin Endocrinol Metab. 2002;87(11):4957–60. doi: 10.1210/jc.2002-021105. [DOI] [PubMed] [Google Scholar]
- 24.ADHR-Consortium. Autosomal dominant hypophosphataemic rickets is associated with mutations in FGF23. Nat Genet. 2000;26(3):345–8. doi: 10.1038/81664. [DOI] [PubMed] [Google Scholar]
- 25.White KE, Carn G, Lorenz-Depiereux B, Benet-Pages A, Strom TM, Econs MJ. Autosomal-dominant hypophosphatemic rickets (ADHR) mutations stabilize FGF-23. Kidney Int. 2001;60(6):2079–86. doi: 10.1046/j.1523-1755.2001.00064.x. [DOI] [PubMed] [Google Scholar]
- 26.Shimada T, Muto T, Urakawa I, Yoneya T, Yamazaki Y, Okawa K, Takeuchi Y, Fujita T, Fukumoto S, Yamashita T. Mutant FGF-23 responsible for autosomal dominant hypophosphatemic rickets is resistant to proteolytic cleavage and causes hypophosphatemia in vivo. Endocrinology. 2002;143(8):3179–82. doi: 10.1210/endo.143.8.8795. [DOI] [PubMed] [Google Scholar]
- 27.Kato K, Jeanneau C, Tarp MA, Benet-Pages A, Lorenz-Depiereux B, Bennett EP, Mandel U, Strom TM, Clausen H. Polypeptide GalNAc-transferase T3 and familial tumoral calcinosis. Secretion of fibroblast growth factor 23 requires O-glycosylation. J Biol Chem. 2006;281(27):18370–7. doi: 10.1074/jbc.M602469200. [DOI] [PubMed] [Google Scholar]
- 28.Topaz O, Shurman DL, Bergman R, Indelman M, Ratajczak P, Mizrachi M, Khamaysi Z, Behar D, Petronius D, Friedman V, Zelikovic I, Raimer S, Metzker A, Richard G, Sprecher E. Mutations in GALNT3, encoding a protein involved in O-linked glycosylation, cause familial tumoral calcinosis. Nat Genet. 2004;36(6):579–81. doi: 10.1038/ng1358. [DOI] [PubMed] [Google Scholar]
- 29.Ichikawa S, Sorenson AH, Austin AM, Mackenzie DS, Fritz TA, Moh A, Hui SL, Econs MJ. Ablation of the Galnt3 gene leads to low-circulating intact fibroblast growth factor 23 (Fgf23) concentrations and hyperphosphatemia despite increased Fgf23 expression. Endocrinology. 2009;150(6):2543–50. doi: 10.1210/en.2008-0877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Benet-Pages A, Orlik P, Strom TM, Lorenz-Depiereux B. An FGF23 missense mutation causes familial tumoral calcinosis with hyperphosphatemia. Hum Mol Genet. 2005;14(3):385–90. doi: 10.1093/hmg/ddi034. [DOI] [PubMed] [Google Scholar]
- 31.Larsson T, Yu X, Davis SI, Draman MS, Mooney SD, Cullen MJ, White KE. A novel recessive mutation in fibroblast growth factor-23 causes familial tumoral calcinosis. J Clin Endocrinol Metab. 2005;90(4):2424–7. doi: 10.1210/jc.2004-2238. [DOI] [PubMed] [Google Scholar]
- 32.Bergwitz C, Banerjee S, Abu-Zahra H, Kaji H, Miyauchi A, Sugimoto T, Juppner H. Defective O-glycosylation due to a novel homozygous S129P mutation is associated with lack of fibroblast growth factor 23 secretion and tumoral calcinosis. J Clin Endocrinol Metab. 2009;94(11):4267–74. doi: 10.1210/jc.2009-0961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Garringer HJ, Fisher C, Larsson TE, Davis SI, Koller DL, Cullen MJ, Draman MS, Conlon N, Jain A, Fedarko NS, Dasgupta B, White KE. The role of mutant UDP-N-acetyl-alpha-D-galactosamine-polypeptide N-acetylgalactosaminyltransferase 3 in regulating serum intact fibroblast growth factor 23 and matrix extracellular phosphoglycoprotein in heritable tumoral calcinosis. J Clin Endocrinol Metab. 2006;91(10):4037–42. doi: 10.1210/jc.2006-0305. [DOI] [PubMed] [Google Scholar]
- 34.Econs MJ, McEnery PT. Autosomal dominant hypophosphatemic rickets/osteomalacia: clinical characterization of a novel renal phosphate-wasting disorder. J Clin Endocrinol Metab. 1997;82(2):674–81. doi: 10.1210/jcem.82.2.3765. [DOI] [PubMed] [Google Scholar]
- 35•.Prats M, Font R, Garcia C, Cabre C, Jariod M, Vea AM. Effect of ferric carboxymaltose on serum phosphate and C-terminal FGF23 levels in non-dialysis chronic kidney disease patients: post-hoc analysis of a prospective study. BMC Nephrol. 2013;14(1):167. doi: 10.1186/1471-2369-14-167. A post-hoc analysis of a prospective study of non-dialysis CKD patients with iron-deficiency anemia were administered a single injection of ferric carboxymaltose. The treatment reduced serum phosphate in patients for three months. Serum FGF23 levels were significantly reduced, and serum calcium, PTH and 1,25-dihyroxyvitamin D remained unchanged. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schouten BJ, Hunt PJ, Livesey JH, Frampton CM, Soule SG. FGF23 elevation and hypophosphatemia after intravenous iron polymaltose: a prospective study. J Clin Endocrinol Metab. 2009;94(7):2332–7. doi: 10.1210/jc.2008-2396. [DOI] [PubMed] [Google Scholar]
- 37.Durham BH, Joseph F, Bailey LM, Fraser WD. The association of circulating ferritin with serum concentrations of fibroblast growth factor-23 measured by three commercial assays. Ann Clin Biochem. 2007;44(Pt 5):463–6. doi: 10.1258/000456307781646102. [DOI] [PubMed] [Google Scholar]
- 38••.Imel EA, Peacock M, Gray AK, Padgett LR, Hui SL, Econs MJ. Iron modifies plasma FGF23 differently in autosomal dominant hypophosphatemic rickets and healthy humans. J Clin Endocrinol Metab. 2011 doi: 10.1210/jc.2011-1239. A link between iron deficiency and ADHR disease activity was made in this study that showed patients with ADHR had elevated iFGF23 and cFGF23 levels, which correlated inversely with serum iron concentrations Only cFGF23 correlated inversely with serum iron in control patients, whereas reduced serum phosphate and 1, 25D correlated with lower serum iron in ADHR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39••.Farrow EG, Yu X, Summers LJ, Davis SI, Fleet JC, Allen MR, Robling AG, Stayrook KR, Jideonwo V, Magers MJ, Garringer HJ, Vidal R, Chan RJ, Goodwin CB, Hui SL, Peacock M, White KE. Iron deficiency drives an autosomal dominant hypophosphatemic rickets (ADHR) phenotype in fibroblast growth factor-23 (Fgf23) knock-in mice. Proc Natl Acad Sci U S A. 2011;108(46):E1146–55. doi: 10.1073/pnas.1110905108. This paper identified the molecular mechanisms linking iron deficiency and FGF23, as well as uncovered FGF23 production and cleavage as two distinct levels of FGF23 regulation within bone. A low iron diet markedly increased bone transcription of FGF23 in normal mice, whereas knock-in mice carrying the R176Q-FGF23 ADHR mutation were resistant to proteolysis, resulting in elevated iFGF23 and a hypophosphatemic osteomalacia phenotype. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Peyssonnaux C, Zinkernagel AS, Schuepbach RA, Rankin E, Vaulont S, Haase VH, Nizet V, Johnson RS. Regulation of iron homeostasis by the hypoxia-inducible transcription factors (HIFs) J Clin Invest. 2007;117(7):1926–32. doi: 10.1172/JCI31370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41•.Wolf M, Koch TA, Bregman DB. Effects of iron deficiency anemia and its treatment on fibroblast growth factor 23 and phosphate homeostasis in women. J Bone Miner Res. 2013;28(8):1793–803. doi: 10.1002/jbmr.1923. In this randomized controlled trial of iron-deficient women it was demonstrated that iron deficiency reversibly stimulates FGF23 production as observed by different intravenous iron formulations. Iron dextran lowered cFGF23 levels with less of an effect on iFGF23, but ferric carboxymaltose raised iFGF23 and caused hypophosphatemia. [DOI] [PubMed] [Google Scholar]
- 42.Riminucci M, Collins MT, Fedarko NS, Cherman N, Corsi A, White KE, Waguespack S, Gupta A, Hannon T, Econs MJ, Bianco P, Gehron Robey P. FGF-23 in fibrous dysplasia of bone and its relationship to renal phosphate wasting. J Clin Invest. 2003;112(5):683–92. doi: 10.1172/JCI18399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bhattacharyya N, Wiench M, Dumitrescu C, Connolly BM, Bugge TH, Patel HV, Gafni RI, Cherman N, Cho M, Hager GL, Collins MT. Mechanism of FGF23 processing in fibrous dysplasia. J Bone Miner Res. 2012;27(5):1132–41. doi: 10.1002/jbmr.1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Clinkenbeard EL, Farrow EG, Summers LJ, Cass TA, Roberts JL, Bayt CA, Lahm T, Albrecht M, Allen MR, Peacock M, White KE. Neonatal Iron Deficiency Causes Abnormal Phosphate Metabolism by Elevating FGF23 in Normal and ADHR Mice. J Bone Miner Res. 2014;29(2):361–9. doi: 10.1002/jbmr.2049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.HYP-Consortium; A gene (PEX) with homologies to endopeptidases is mutated in patients with X-linked hypophosphatemic rickets. The HYP Consortium. Nat Genet. 1995;11(2):130–6. doi: 10.1038/ng1095-130. [DOI] [PubMed] [Google Scholar]
- 46.Beck L, Soumounou Y, Martel J, Krishnamurthy G, Gauthier C, Goodyer CG, Tenenhouse HS. Pex/PEX tissue distribution and evidence for a deletion in the 3′ region of the Pex gene in X-linked hypophosphatemic mice. J Clin Invest. 1997;99(6):1200–9. doi: 10.1172/JCI119276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Miao D, Bai X, Panda D, McKee M, Karaplis A, Goltzman D. Osteomalacia in hyp mice is associated with abnormal phex expression and with altered bone matrix protein expression and deposition. Endocrinology. 2001;142(2):926–39. doi: 10.1210/endo.142.2.7976. [DOI] [PubMed] [Google Scholar]
- 48.Liu S, Zhou J, Tang W, Jiang X, Rowe DW, Quarles LD. Pathogenic role of Fgf23 in Hyp mice. Am J Physiol Endocrinol Metab. 2006;291(1):E38–49. doi: 10.1152/ajpendo.00008.2006. [DOI] [PubMed] [Google Scholar]
- 49.Perwad F, Azam N, Zhang MY, Yamashita T, Tenenhouse HS, Portale AA. Dietary and serum phosphorus regulate fibroblast growth factor 23 expression and 1,25-dihydroxyvitamin D metabolism in mice. Endocrinology. 2005;146(12):5358–64. doi: 10.1210/en.2005-0777. [DOI] [PubMed] [Google Scholar]
- 50.Yuan B, Feng JQ, Bowman S, Liu Y, Blank RD, Lindberg I, Drezner MK. Hexa-D-Arginine treatment increases 7B2*PC2 activity in hyp-mouse osteoblasts and rescues the HYP phenotype. J Bone Miner Res. 2012 doi: 10.1002/jbmr.1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ichikawa S, Gray AK, Bikorimana E, Econs MJ. Dosage effect of a Phex mutation in a murine model of X-linked hypophosphatemia. Calcif Tissue Int. 2013;93(2):155–62. doi: 10.1007/s00223-013-9736-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Benet-Pages A, Lorenz-Depiereux B, Zischka H, White KE, Econs MJ, Strom TM. FGF23 is processed by proprotein convertases but not by PHEX. Bone. 2004;35(2):455–62. doi: 10.1016/j.bone.2004.04.002. [DOI] [PubMed] [Google Scholar]
- 53.Barros NM, Hoac B, Neves RL, Addison WN, Assis DM, Murshed M, Carmona AK, McKee MD. Proteolytic processing of osteopontin by PHEX and accumulation of osteopontin fragments in Hyp mouse bone, the murine model of X-linked hypophosphatemia. J Bone Miner Res. 2013;28(3):688–99. doi: 10.1002/jbmr.1766. [DOI] [PubMed] [Google Scholar]
- 54.Imel EA, DiMeglio LA, Hui SL, Carpenter TO, Econs MJ. Treatment of X-linked hypophosphatemia with calcitriol and phosphate increases circulating fibroblast growth factor 23 concentrations. J Clin Endocrinol Metab. 2010;95(4):1846–50. doi: 10.1210/jc.2009-1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55•.Ichikawa S, Austin AM, Gray AK, Econs MJ. A Phex mutation in a murine model of X-linked hypophosphatemia alters phosphate responsiveness of bone cells. J Bone Miner Res. 2012;27(2):453–60. doi: 10.1002/jbmr.544. The responsiveness of bone cells to extracellular phosphate was compared across three murine models: XLH (Phex-null (K496X)), hyperphosphatemic tumoral calcinosis (Galnt3−/−) and a Galnt3/Phex double-mutant model. Whereas Phex mutant mice displayed increased FGF23 expression, reduced cleavage of FGF23 and elevated intact FGF23, Galnt3 knockout mice had increased cleavage of FGF23, which accompanied low intact FGF23 concentrations and hyperphosphatemia. The double-mutant model displayed an intermediate phenotype, suggesting that Phex mutations alter bone cells responsiveness to extracellular phosphate and produce a lower set point for ‘normal’ phosphate levels. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Feng JQ, Ward LM, Liu S, Lu Y, Xie Y, Yuan B, Yu X, Rauch F, Davis SI, Zhang S, Rios H, Drezner MK, Quarles LD, Bonewald LF, White KE. Loss of DMP1 causes rickets and osteomalacia and identifies a role for osteocytes in mineral metabolism. Nat Genet. 2006;38(11):1310–5. doi: 10.1038/ng1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lorenz-Depiereux B, Bastepe M, Benet-Pages A, Amyere M, Wagenstaller J, Muller-Barth U, Badenhoop K, Kaiser SM, Rittmaster RS, Shlossberg AH, Olivares JL, Loris C, Ramos FJ, Glorieux F, Vikkula M, Juppner H, Strom TM. DMP1 mutations in autosomal recessive hypophosphatemia implicate a bone matrix protein in the regulation of phosphate homeostasis. Nat Genet. 2006;38(11):1248–50. doi: 10.1038/ng1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lin S, Jiang Y, Zong Z, Liu M, Liu Y, Yuan B, Drezner MK, Ke HZ, Feng J. A key pathological role for the Wnt-b-catenin signaling pathway in hypophosphatemic rickets/osteomalacia. J Bone Miner Res. 2012:Oral1018. [Google Scholar]
- 59.Lu Y, Yuan B, Qin C, Cao Z, Xie Y, Dallas SL, McKee MD, Drezner MK, Bonewald LF, Feng JQ. The biological function of DMP-1 in osteocyte maturation is mediated by its 57-kDa C-terminal fragment. J Bone Miner Res. 2011;26(2):331–40. doi: 10.1002/jbmr.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rangiani A, Cao Z, Sun Y, Lu Y, Gao T, Yuan B, Rodgers A, Qin C, Kuro OM, Feng JQ. Protective roles of DMP1 in high phosphate homeostasis. PLoS One. 2012;7(8):e42329. doi: 10.1371/journal.pone.0042329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lorenz-Depiereux B, Schnabel D, Tiosano D, Hausler G, Strom TM. Loss-of-function ENPP1 mutations cause both generalized arterial calcification of infancy and autosomal-recessive hypophosphatemic rickets. Am J Hum Genet. 2010;86(2):267–72. doi: 10.1016/j.ajhg.2010.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Levy-Litan V, Hershkovitz E, Avizov L, Leventhal N, Bercovich D, Chalifa-Caspi V, Manor E, Buriakovsky S, Hadad Y, Goding J, Parvari R. Autosomal-recessive hypophosphatemic rickets is associated with an inactivation mutation in the ENPP1 gene. Am J Hum Genet. 2010;86(2):273–8. doi: 10.1016/j.ajhg.2010.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nam HK, Liu J, Li Y, Kragor A, Hatch NE. Ectonucleotide pyrophosphatase/phosphodiesterase-1 (Enpp1) regulates osteoblast differentiation. J Biol Chem. 2011 doi: 10.1074/jbc.M111.221689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rendenbach C, Yorgan TA, Heckt T, Otto B, Baldauf C, Jeschke A, Streichert T, David JP, Amling M, Schinke T. Effects of Extracellular Phosphate on Gene Expression in Murine Osteoblasts. Calcif Tissue Int. 2013 doi: 10.1007/s00223-013-9831-6. [DOI] [PubMed] [Google Scholar]
- 65.Mackenzie NC, Zhu D, Milne EM, van’t Hof R, Martin A, Darryl Quarles L, Millan JL, Farquharson C, MacRae VE. Altered bone development and an increase in FGF-23 expression in Enpp1(−/−) mice. PLoS One. 2012;7(2):e32177. doi: 10.1371/journal.pone.0032177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66•.Ababneh FK, AlSwaid A, Youssef T, Al Azzawi M, Crosby A, AlBalwi MA. Hereditary deletion of the entire FAM20C gene in a patient with Raine syndrome. Am J Med Genet A. 2013;161A(12):3155–60. doi: 10.1002/ajmg.a.36160. An exome sequencing study of siblings with hypophosphatemia and dental mineralization abnormalities found compound heterozygous mutations in FAM20C, linking FGF23 and FAM20c in humans. Examination of the respective Norwegian background population found no other undiagnosed FAM20C mutations. [DOI] [PubMed] [Google Scholar]
- 67.Rafaelsen SH, Raeder H, Fagerheim AK, Knappskog P, Carpenter TO, Johansson S, Bjerknes R. Exome sequencing reveals FAM20c mutations associated with fibroblast growth factor 23-related hypophosphatemia, dental anomalies, and ectopic calcification. J Bone Miner Res. 2013;28(6):1378–85. doi: 10.1002/jbmr.1850. [DOI] [PubMed] [Google Scholar]
- 68•.Wang X, Wang S, Li C, Gao T, Liu Y, Rangiani A, Sun Y, Hao J, George A, Lu Y, Groppe J, Yuan B, Feng JQ, Qin C. Inactivation of a novel FGF23 regulator, FAM20C, leads to hypophosphatemic rickets in mice. PLoS Genet. 2012;8(5):e1002708. doi: 10.1371/journal.pgen.1002708. This paper examined the FAM20C global KO and mineralized tissue conditional KO, and showed these mice develop hypophosphatemic rickets caused by elevations in FGF23 in serum and bone. In vitro experiments support FAM20C’s role as a promoter of osteogenesis and regulator of DMP1 expression or function. This study implicated FAM20C as a key regulator of FGF23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69••.Tagliabracci VS, Engel JL, Wen J, Wiley SE, Worby CA, Kinch LN, Xiao J, Grishin NV, Dixon JE. Secreted kinase phosphorylates extracellular proteins that regulate biomineralization. Science. 2012;336(6085):1150–3. doi: 10.1126/science.1217817. This significant paper demonstrated that Fam20C belongs to a family of novel atypical protein kinases that localize within the Golgi apparatus and phosphorylate secreted proteins important for biomineralization carrying S-x-E motifs. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ishikawa HO, Xu A, Ogura E, Manning G, Irvine KD. The Raine syndrome protein FAM20C is a Golgi kinase that phosphorylates bio-mineralization proteins. PLoS One. 2012;7(8):e42988. doi: 10.1371/journal.pone.0042988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71••.Tagliabracci VS, Engel JL, Wiley SE, Xiao J, Gonzales DJ, Appaiah HN, Koller A, Nizet V, White KE, Dixon JE. Dynamic regulation of FGF23 by Fam20C phosphorylation, GalNAc-T3 glycosylation, and furin proteolysis. Proc Natl Acad Sci U S A. 2014 doi: 10.1073/pnas.1402218111. In press. This paper highlighted Fam20C as a novel regulator of FGF23, providing a new mechanism of FGF23 processing. FGF23 was found to be a direct substrate for phosphorylation by Fam20C, reducing O-glycosylation by GALNT3 which promotes FGF23 proteolysis by furin. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Fliser D, Kollerits B, Neyer U, Ankerst DP, Lhotta K, Lingenhel A, Ritz E, Kronenberg F, Kuen E, Konig P, Kraatz G, Mann JF, Muller GA, Kohler H, Riegler P. Fibroblast growth factor 23 (FGF23) predicts progression of chronic kidney disease: the Mild to Moderate Kidney Disease (MMKD) Study. J Am Soc Nephrol. 2007;18(9):2600–8. doi: 10.1681/ASN.2006080936. [DOI] [PubMed] [Google Scholar]
- 73.Mirza MA, Larsson A, Melhus H, Lind L, Larsson TE. Serum intact FGF23 associate with left ventricular mass, hypertrophy and geometry in an elderly population. Atherosclerosis. 2009;207(2):546–51. doi: 10.1016/j.atherosclerosis.2009.05.013. [DOI] [PubMed] [Google Scholar]
- 74.Gutierrez OM, Mannstadt M, Isakova T, Rauh-Hain JA, Tamez H, Shah A, Smith K, Lee H, Thadhani R, Juppner H, Wolf M. Fibroblast growth factor 23 and mortality among patients undergoing hemodialysis. N Engl J Med. 2008;359(6):584–92. doi: 10.1056/NEJMoa0706130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Faul C, Amaral AP, Oskouei B, Hu MC, Sloan A, Isakova T, Gutierrez OM, Aguillon-Prada R, Lincoln J, Hare JM, Mundel P, Morales A, Scialla J, Fischer M, Soliman EZ, Chen J, Go AS, Rosas SE, Nessel L, Townsend RR, Feldman HI, St John Sutton M, Ojo A, Gadegbeku C, Di Marco GS, Reuter S, Kentrup D, Tiemann K, Brand M, Hill JA, Moe OW, Kuro OM, Kusek JW, Keane MG, Wolf M. FGF23 induces left ventricular hypertrophy. J Clin Invest. 2011;121(11):4393–408. doi: 10.1172/JCI46122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Touchberry CD, Green TM, Tchikrizov V, Mannix JE, Mao TF, Carney BW, Girgis M, Vincent RJ, Wetmore LA, Dawn B, Bonewald LF, Stubbs JR, Wacker MJ. FGF23 is a novel regulator of intracellular calcium and cardiac contractility in addition to cardiac hypertrophy. Am J Physiol Endocrinol Metab. 2013;304(8):E863–73. doi: 10.1152/ajpendo.00596.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Moe SM Investigators E. Cinacalcet decreases FGF23 levels in prevelent dialysis patients compared to placebo. J Am Soc Nephrol. 2014:TH-ORO23. [Google Scholar]
- 78.Shimada T, Urakawa I, Isakova T, Yamazaki Y, Epstein M, Wesseling-Perry K, Wolf M, Salusky IB, Juppner H. Circulating fibroblast growth factor 23 in patients with end-stage renal disease treated by peritoneal dialysis is intact and biologically active. J Clin Endocrinol Metab. 2010;95(2):578–85. doi: 10.1210/jc.2009-1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79•.Christov M, Waikar SS, Pereira RC, Havasi A, Leaf DE, Goltzman D, Pajevic PD, Wolf M, Juppner H. Plasma FGF23 levels increase rapidly after acute kidney injury. Kidney Int. 2013;84(4):776–85. doi: 10.1038/ki.2013.150. In response to acute kidney injury, both human and rodents showed increased circulating levels of FGF23 independent of other known stimuli of FGF23 production. The findings suggest that the rapid increases in iFGF23 and cFGF23 may be due to an additional level of FGF23 regulation, including decreased iFGF23 cleavage. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ichikawa S, Imel EA, Kreiter ML, Yu X, Mackenzie DS, Sorenson AH, Goetz R, Mohammadi M, White KE, Econs MJ. A homozygous missense mutation in human KLOTHO causes severe tumoral calcinosis. J Clin Invest. 2007;117(9):2684–91. doi: 10.1172/JCI31330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Brownstein CA, Adler F, Nelson-Williams C, Iijima J, Li P, Imura A, Nabeshima Y, Reyes-Mugica M, Carpenter TO, Lifton RP. A translocation causing increased alpha-klotho level results in hypophosphatemic rickets and hyperparathyroidism. Proc Natl Acad Sci U S A. 2008;105(9):3455–60. doi: 10.1073/pnas.0712361105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82•.Smith RC, O’Bryan LM, Farrow EG, Summers LJ, Clinkenbeard EL, Roberts JL, Cass TA, Saha J, Broderick C, Ma YL, Zeng QQ, Kharitonenkov A, Wilson JM, Guo Q, Sun H, Allen MR, Burr DB, Breyer MD, White KE. Circulating alphaKlotho influences phosphate handling by controlling FGF23 production. J Clin Invest. 2012;122(12):4710–5. doi: 10.1172/JCI64986. This paper highlighted that cKL, a cleavage product of the FGF23 co-receptor αKlotho, stimulates bone FGF23 production resulting in hypophosphatemia, hypocalcemia, and osteomalacia. This phenotype mirrors a patient with a KL translocation characterized by a metabolic bone syndrome due to increased FGF23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Farrow EG, Davis SI, Mooney SD, Beighton P, Mascarenhas L, Gutierrez YR, Pitukcheewanont P, White KE. Extended mutational analyses of FGFR1 in osteoglophonic dysplasia. Am J Med Genet A. 2006;140(5):537–9. doi: 10.1002/ajmg.a.31106. [DOI] [PubMed] [Google Scholar]
- 84.White KE, Cabral JM, Davis SI, Fishburn T, Evans WE, Ichikawa S, Fields J, Yu X, Shaw NJ, McLellan NJ, McKeown C, Fitzpatrick D, Yu K, Ornitz DM, Econs MJ. Mutations that cause osteoglophonic dysplasia define novel roles for FGFR1 in bone elongation. Am J Hum Genet. 2005;76(2):361–7. doi: 10.1086/427956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Xiao L, Esliger A, Hurley MM. Nuclear fibroblast growth factor 2 (FGF2) isoforms inhibit bone marrow stromal cell mineralization through FGF23/FGFR/MAPK in vitro. J Bone Miner Res. 2013;28(1):35–45. doi: 10.1002/jbmr.1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86•.Carpenter TO, Imel EA, Ruppe MD, Weber TJ, Klausner MA, Wooddell MM, Kawakami T, Ito T, Zhang X, Humphrey J, Insogna KL, Peacock M. Randomized trial of the anti-FGF23 antibody KRN23 in X-linked hypophosphatemia. J Clin Invest. 2014;124(4):1587–97. doi: 10.1172/JCI72829. This randomized Phase1-2 trial tested an anti-FGF23 monoclonal neutralizing antibody for treatment of XLH and patients showed increased TMP/GFR, serum phosphate, and calcitriol. [DOI] [PMC free article] [PubMed] [Google Scholar]