Abstract
T cells genetically modified to stably express immunoreceptors are being assessed for therapeutic potential in clinical trials. T cells expressing a chimeric antigen receptor (CAR) are endowed with a new specificity to target tumor-associated antigen (TAA) independent of major histocompatibility complex. Our approach to non-viral gene transfer in T cells uses ex vivo numeric expansion of CAR+ T cells on irradiated artificial antigen presenting cells (aAPC) bearing the targeted TAA. The requirement for aAPC to express a desired TAA limits the human application of CARs with multiple specificities when selective expansion through co-culture with feeder cells is sought. As an alternative to expressing individual TAAs on aAPC, we expressed one ligand that could activate CAR+ T cells for sustained proliferation independent of specificity. We expressed a CAR ligand (designated CARL) that binds the conserved IgG4 extracellular domain of CAR and demonstrated CARL+ aAPC propagate CAR+ T cells of multiple specificities. CARL avoids technical issues and costs associated with deploying clinical-grade aAPC for each TAA targeted by a given CAR. Employing CARL enables one aAPC to numerically expand all CAR+ T cells containing the IgG4 domain, and simplifies expansion, testing, and clinical translation of CAR+ T cells of any specificity.
Keywords: CAR (chimeric antigen receptor), aAPC (artificial antigen presenting cell), gene therapy, Sleeping Beauty, T cell, ex vivo propagation, manufacturing
INTRODUCTION
The adoptive transfer of antigen-specific T cells is a rapidly developing field of cancer immunotherapy with innovative approaches to their manufacture being tested and new antigens being targeted. T cells can be genetically-modified for immunotherapy to express chimeric antigen receptors (CARs) that recognize tumor-associated antigens (TAAs) independent of HLA expression. Recent results from early-phase clinical trials demonstrate that CAR+ T-cell (CART) therapies can lead to partial and complete remissions of malignant diseases, including in some recipients with advanced/relapsed B-cell tumors.1,2
Currently, many CART therapies are based upon ex vivo propagation from the donated T cells obtained from steady-state apheresis or venipuncture.3–8 Approaches for numeric expansion typically use either CAR-independent T-cell proliferation based upon cross-linking CD3 and CD28 with antibodies4–6,9 or CAR-dependent propagation using TAA expressed on artificial antigen presenting cells (aAPC).10–13 Other methods to selectively propagate T cells to constitutively express CAR include co-expression with transgenes for selection under cytocidal concentrations of drug and/or sorting, such as using magnetic beads that recognize introduced proteins co-expressed with CAR.10,14 After electro-transfer of DNA plasmids derived from Sleeping Beauty (SB) system, we employ CAR-mediated expansion to selectively propagate T cells that stably express the introduced single-chain immunoreceptor by repeated additions of γ-irradiated K562 cells genetically modified to co-express co-stimulatory molecules and the TAA targeted by the introduced CAR.3,11,15 However, this necessitates that each aAPC design must be manufactured to express the TAA targeted by a given CAR. Furthermore, some TAA that are biochemically or structurally complex, such as glycosphingolipids, are difficult to recapitulate on the surface of aAPC.16
Here, we describe an approach to achieve CAR-mediated expansion that avoids the requirement for cytotoxic drugs, magnetic selection, or TAA-specific proliferation. A monoclonal antibody (mAb, clone 2D3), previously reported by our laboratory,11 was shown to bind to the conserved exodomain (derived from modified human hinge and Fc region of IgG4)17 of a CAR. The antigen-specificity of this mAb was constructed as a single-chain variable fragment (scFv) and expressed on K562 cells to serve as an aAPC.3,18 This scFv on the cell surface of aAPC is able to ligate a panel of CARs with diverse specificities that contain the IgG4 extracellular scaffold, leading to selective expansion of genetically modified T cells that have redirected specificity for multiple TAAs. This scFv serves as a ligand for CAR (designated CARL) that can substitute for TAA and thus provides investigators with one source of aAPC that may be used to generate populations of CAR+ T cells with any specificity.
MATERIALS AND METHODS
Cells and culture conditions
K562 cells (European Collection of Cell Cultures through Sigma-Aldrich, St. Louis, MO; Cat. No. 89121407), noted for expression of desired endogenous adhesion molecules and the absence of most HLA class I and all class II molecules,18 were used to derive CD19+ and CARL+ K562 that served as aAPC. Immortalized tumor targets CD19neg, GD2+ EL-4 murine thymoma (Cat. No. TIB-40) and CD19+, GD2neg NALM-6 (pre-B cell leukemia, Cat. No. CRL-1567) were purchased from American Type Culture Collection (ATCC, Manassas, VA). Identity of cell lines was validated by the MDACC Cancer Center Support Grant Characterized Cell Line Core using short tandem repeat DNA fingerprinting. Peripheral blood was donated by consenting healthy volunteer adults at Gulf Coast Regional Blood Center (Houston, TX). Peripheral blood mononuclear cells (PBMC) were isolated using Ficoll-Paque Plus density centrifugation (GE Healthcare Biosciences, Piscataway Township, NJ; Cat. No. 17-1440-02) before freezing in a mixture of 10% DMSO (Sigma, Allentown, PA; Cat. No. D2650), 50% heat-inactivated fetal bovine serum (FBS-Thermo Scientific Hyclone, Bridgewater, NJ, Cat. No. SH30070.03), and 40% RPMI 1640 (Thermo Scientific Hyclone; Cat. No. SH30096.01). All cells were cultured in a 37° C humidified incubator with complete media (CM) prepared from RPMI 1640, 10% heat-inactivated FBS, and 2 mM GlutaMAX supplement (Life Technologies, Grand Island, NY; Cat. No. 35050061).
DNA expression plasmids
Codon-optimized CD19RCD28mZ(CoOp)/pSBSO,11 which codes for CAR between transposition sites mediated by SB transposase,19 was used as the vector backbone for cloning of the following transgenes. The DNA plasmid 19G4CAR (also designated CD19RCD28,20 Figure S1.A) codes for a 2nd generation CD19-specific CAR containing a modified IgG4 exodomain, CD28 transmembrane, and CD28/CD3ζ endodomain. The synthesis of DNA plasmid GD2G4CAR (Figure S1.B), specific to sphingolipid GD2, utilized the same 19G4CAR backbone. The GD2-specific scFv derived from murine mAb (clone 14G2a)21 was commercially synthesized (Geneart, Life Technologies) as codon-optimized cDNA with NheI and XmnI restriction enzyme (RE) sites flanking the scFv. The 19G4CAR plasmid backbone and GD2-specific scFv cDNA were excised using these REs and ligated to replace CD19-specific scFv with GD2-specific scFv. A DNA plasmid (Figure S1.C) coding for a control CAR that contains no scFv region, designated G4CAR, but does contain an Igκ FLAG peptide sequence (METDTLLLWVLLLWVPGSTGDYKDEGTS), was derived from 19G4CAR using primer-directed PCR amplification from the beginning of the IgG4 domain hinge (primer 5’GGTACCTCTGGGGGGCAGGGCCTGCATG3’) to the terminus of the CD3ζ domain (primer 5’GGGCCCAGCGCTGAGAGCAAGTACGGCCCTCCC3’) and sequence verified. The G4CAR was ligated into the 19G4CAR backbone ApaI and KpnI RE sites. DNA plasmid coding for a CD19-specific CAR with no IgG4 (Figure S1.D), designated 19CAR, encodes from amino to carboxyl termini a GM-CSF (amino acid 1-22; NP_758452.1), CD19-specific scFv (245 amino acids), CD8α extracellular domain and hinge (amino acids 136-203; NP_001759) followed by the same CD28 transmembrane and CD28 and CD3ζ domains as other CARs.20 The full length of this transgene was synthesized by GeneArt, cut with ClaI and SpeI REs, and ligated into the 19G4CAR backbone replacing the 19G4CAR codon, which had been excised using EcoRV and SpeI. The scFv sequence of CARL was derived from the cDNA library of the 2D3 hybridoma.11 This was achieved by extracting RNA from 5x106 cells using the RNeasy Mini Kit (Qiagen, Gaithersburg, MD; Cat No. 74104) according to manufacturer’s protocol. A cDNA library was generated by reverse transcription using oligo-dT primers per the protocol provided in the Superscript III First Strand kit (Invitrogen; Cat No. 18080-051). PCR (using Amplitaq Gold) was performed on the cDNA using the degenerate primers for the FR1 region22 to amplify the mouse VH and VL regions. The VH and VL amplified products were ligated into the TOPO TA vector and sequenced. The CARL construct for surface expression on aAPC was composed of GM-CSF leader peptide (amino acid 1-22; NP_758452.1) fused to the 2D3-derived scFv, and tethered by CD8α (amino acid 136-182; NP_001759.3) to the transmembrane and intracellular portions of CD28 (amino acid 56-123; NP_001230006.1) followed by CD3ζ (amino acid. 48-163; NP_ 000725.1) intracellular domain. Design of all transgenes utilized Vector NTI Advance™ 11 software (Invitrogen). All transgenes were human codon optimized before synthesis at GeneArt. The CARL construct was excised and ligated into a SB expression plasmid, designated Zeo-2A-CARL (Figure S1.E), to co-express a zeocin resistance gene linked via a modified T2A peptide sequence- (amino acids ATGEGRGSLLTCGDVEEPGP). Truncated human CD19 was synthesized by GeneArt containing the extracellular and transmembrane portions of human CD19 (amino acid 1-313; NP_001171569.1). This gene was excised and ligated into SB DNA plasmid, designated CD19-2A-Neo (Figure S1.F), to co-express with neomycin phosphotransferase linked via a modified F2A peptide sequence (amino acids (G)4S(G)4SVKQTLNFDLLKLAGDVESNPGP).
Artificial antigen presenting cells (aAPC)
CARL+ and CD19+ aAPC were derived by the independent electroporation of parental K562 cells with Zeo-2A-CARL or CD19-2A-Neo and SB11 transposase DNA plasmids using the Amaxa 2D nucleofector under program T-16 with Kit V (Lonza, Allendale, NJ; Cat No. VCA-1003). After 3 days incubation, each transfection was placed under drug selection in a 6-well plate using either 0.5 mg/mL Zeocin or 1 mg/mL G418 for Zeocin resistance or Neomycin resistance, respectively (Invivogen, San Diego, CA; Cat. No. ant-zn-1 and ant-gn-1). This was achieved by dispersing 10,000 cells with drug in 3 mL semi-solid Methocult H4230 media (StemCell Technologies, Vancouver, BC, Canada; Cat No. 04230). After 10 days visually-perceptible individual (clonal) colonies were transferred to individual flasks and grown in CM. Each clone was tested for uniform expression of CARL or CD19 using flow cytometry. Clones of CARL+ (designated Zeo-2A-CARL MC5) and CD19+ (designated CD19-2A-Neo MC2) aAPC were grown to large numbers, γ-irradiated at 100 cGy, and cryopreserved. Before freezing, the aAPC were routinely tested for the presence of transgenes, absence of mycoplasma, and absence of endotoxin.
Propagation of CAR+ T cells (CART)
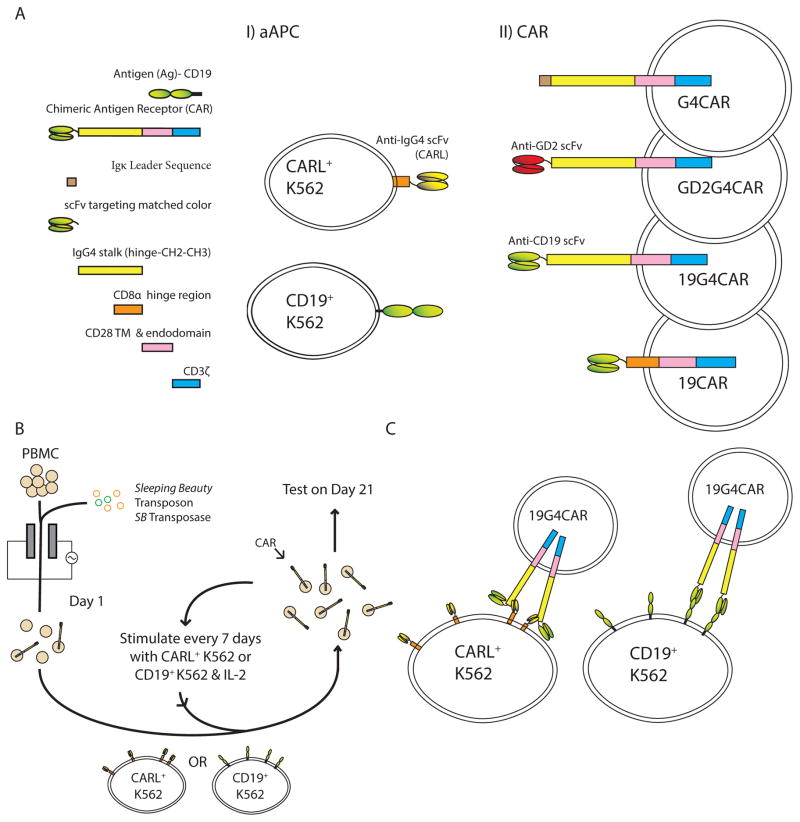
The designs of each CAR and antigen (CARL and CD19) as expressed on the respective T cell or aAPC are shown in Figure 1A. The propagation of CART is depicted in Figure 1B. Each CAR from Figure 1A.II was co-cultured with aAPC from Figure 1A.I. At the initiation of the experiment (defined as Day 0), thawed PBMC were washed twice, and maintained in CM for 3 to 4 hours before electroporation using the Amaxa 2D Nucleofector under program U-14 with human T cell Nucleofector kit (Lonza Biosciences; Cat No. VPA-1002). After resting overnight in CM, viable PBMC (counted by exclusion of 0.1% Trypan Blue) were resuspended in CM and mixed at a 1:2 ratio (mononuclear cell to γ-irradiated aAPC) using thawed aAPC that were washed twice and counted. The co-culture contained 106 total cells/ mL in CM and 50 IU/mL recombinant human IL-2 (Proleukin, Prometheus Labs, San Diego, CA). The live-cell counts were determined by Trypan Blue exclusion on Days 1, 7, 14, and 21 of co-culture. Flow cytometry for CD3, CD4, CD8, and human IgG (to assess CAR expression) occurred on Days 1, 7, 14, and 21, and for CD45RO, CD62L, and CD28 occurred on Days 14 and 21. Irradiated aAPC were re-added to co-cultures on Days 7 and 14 by re-stimulating mononuclear cells with γ-irradiated aAPC at 1:2 ratio. On Day 21 products of propagation were assessed for specific killing, and DNA and RNA were extracted. Each experiment was repeated at least 4 times using 5 donors.
Figure 1.
Study design to compare ability of chimeric antigen receptor (CAR) ligand (CARL) versus CD19 TAA on K562 cells for the selective propagation of CAR+ T cells (CART). A) Artificial antigen presenting cells (aAPC) demonstrated in I) were derived from parental K562 cells following transgene transfer, stable integration, and clonal selection. Each aAPC clone expresses either CARL, a scFv derived from 2D3 mAb that binds IgG4 exodomain of CAR, or truncated human CD19. II) CART used to evaluate specificity towards CARL or CD19 are shown. SB-derived DNA plasmids coding for a panel of CARs were individually electro-transferred into PBMC and recursively stimulated with CD19+ K562 or CARL+ K562 in the presence of soluble recombinant human IL-2. Each CAR follows a modular design. 19G4CAR contains the IgG4 scaffold and targets CD19 through the same scFv as 19CAR which lacks IgG4 scaffold and instead uses CD8α hinge and extracellular domain. GD2G4CAR contains the IgG4 scaffold and targets GD2. G4CAR contains the IgG4 scaffold, but has no scFv. All CARs employ of a 2nd generation design containing CD28 and CD3ζ signaling endodomains. B) On Day 0, synchronous electroporation of PBMC was undertaken with DNA plasmid coding for SB transposase (SB11- green) and SB DNA plasmids coding for CAR species (orange). To achieve outgrowth of T cells stably expressing CARs, the genetically modified cells were co-cultured, beginning on Day 1, upon γ-irradiated CD19+ or CARL+ K562 in the presence of 50 IU/mL IL-2. Cytokine was added with stimulation or during media change. Re-stimulation of CAR with aAPC occurred every 7 days until Day 21. C) Diagram of docking between CARL+ K562 cells and 19G4CAR+ T cells as compared with CD19+ K562 cells with 19G4CAR+ T cells.
Flow cytometry
We used a FACSCalibur (BD Biosciences, Billerica, MA) to acquire samples prepared in FACS staining solution as previously described.23 After washing once in FACS staining solution, cells were stained for 30 minutes at 4° C without blocking in FACS staining solution containing a 1:33 dilution of antibody. When anti-human Fc antibody was used, the anti-Fc stained sample was washed and re-stained for alternative surface markers before re-suspension in FACS buffer for flow cytometer analysis. Measurement of intracellular cytokine used the same protocol for cell surface staining followed by 20 min. fixation using BD cytofix/cytoperm kit fixative (BD Biosciences; Cat No. 554714), followed by washing twice in perm/wash buffer containing 20% FBS and staining with a 1:33 dilution of antibody in perm/wash buffer. Antibody incubation lasted 30 minutes at 4°C before samples were washed in perm/wash buffer and resuspended in FACS staining solution for acquisition. FlowJo v 10.0.5 (Tree Star Inc., Ashland, OR) was used for analysis of flow cytometry data. See Supplement Table S1 for antibodies used.
Chromium release assay (CRA)
CRA was performed as previously described.11 In brief, on Day 21 of T-cell co-culture on aAPC, the tumor targets (i) EL-4, (ii) NALM-6, and (iii) K562 were loaded with 51Cr for 3 hours, and, after washing, co-cultured with effector T cells for 6 hours at 37° C using a ratio of 5 T cells to 1 target cell.
Abundance and diversity of TCR repertoire
The direct TCR expression assay (DTEA), as previously reported,24 was used to measure the abundance of mRNA transcripts coding for 45 TCR α alleles, 46 TCR β alleles, 13 TCR γ alleles, and 5 TCR δ alleles from RNA obtained on Day 0 (T cells in PBMC before electroporation) and Day 21 (from T cells after electroporation/ propagation). Day 0 samples were negatively sorted for CD56 (Miltenyi Biotec, Cambridge, MA; Cat. No. 130-050-401) and then positively sorted for CD3 (Miltenyi Biotec; Cat. No. 130-050-101). The resulting CD3+CD56neg T cells (2 to 3 x 106 from each sample) were snap frozen as were 2 x 106 cells directly harvested at Day 21 of co-culture. RNA was extracted from thawed samples using the ALLprep DNA/RNA mini kit (Qiagen; Cat. No. 80204).
Statistics
Statistical analysis was performed using Prism v6.0 (Graph Pad Software Inc.). Student’s t-test (unpaired) was used to perform two sample comparisons. One- or two-way ANOVA F–test was used to perform group comparisons, and if found significant (p < 0.05); a t-test (unpaired) was undertaken to assess and report differences. Spearman’s nonparametric correlation was performed on housekeeping gene normalized DTEA transcript counts to assess the divergence of the TCR repertoire in T cells from an experimental group and autologous Day 0 PBMC. If the Spearman correlation coefficient was greater than or equal to 0.8 (ρ ≥ 0.8) within the 95% confidence interval of the correlation coefficient, then the two TCR repertoires were considered to be highly correlated.
RESULTS
Numeric expansion of CAR+ T cells upon K562 cells expressing CARL or CD19
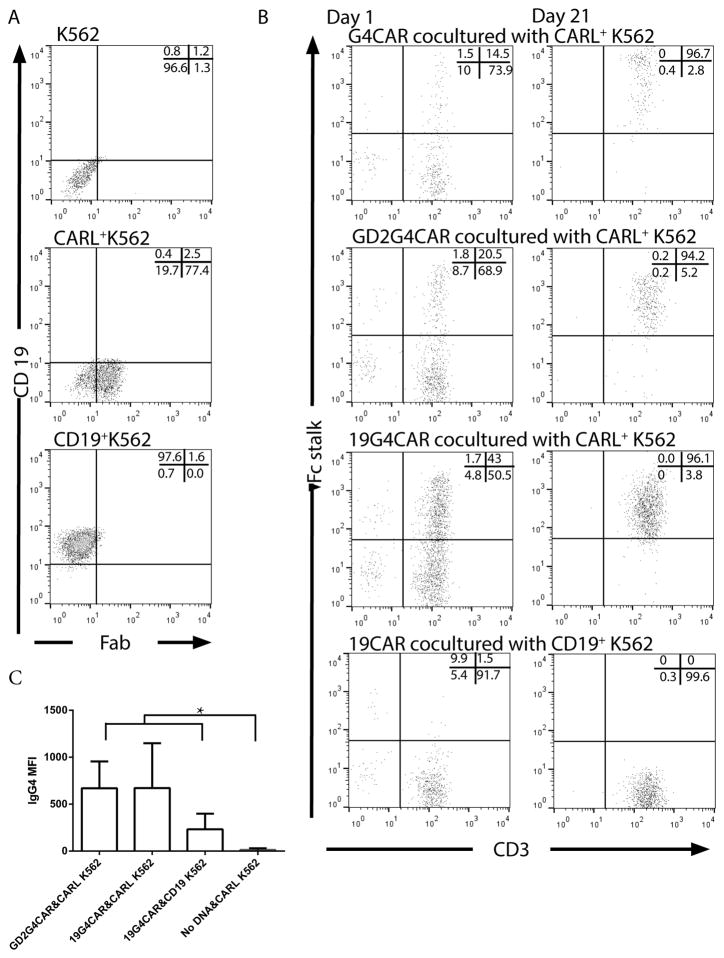
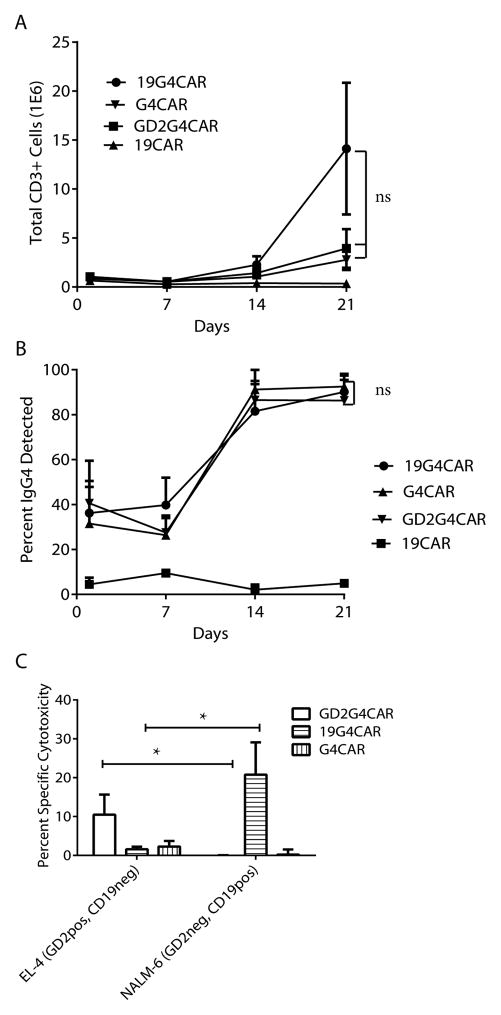
Mouse mAb clone 2D3 was obtained by repeated footpad injections of NSO cells expressing 19G4CAR into BALB/c mice and blocking studies defined the specificity of the mAb to the human IgG4 exodomain of 19G4CAR.11 We hypothesized that this mAb may be used to cross-link CAR and activate genetically modified T cells for sustained proliferation. Therefore, the scFv of 2D3 (designated CARL) was expressed on the cell surface to compare with human truncated CD19 TAA on K562 cells. The CARL and CD19 transgenes were cloned into DNA plasmids for co-expression with drug-selection genes between SB transposable elements. The SB transposon DNA plasmids for 2D3-derived scFv or CD19 were electro-transferred with SB11 transposase DNA plasmid into K562 cells in separate experiments. Genetically modified cells were propagated under drug selection from a single cell for homogeneous expression of CARL (as detected by antibody against mouse Fab) or CD19 (Figure 2A). A comparison of the γ-irradiated K562-derived aAPC to selectively propagate CART was undertaken following electroporation (defined as Day 0) of the panel of CAR constructs (Figure 1A.II) into PBMC using SB system. On Day 1, initial expression of CAR in T cells was evaluated by flow cytometry using antibody specific for human Fc (Figure 2B). The expression of CARs and number of total viable T cells were measured weekly for 21 days of co-culture with CD19+ K562 or CARL+ K562 with the following immunoreceptors on T cells; (i) 19G4CAR with specificity for CD19 and containing the IgG4 exodomain, (ii) 19CAR with specificity for CD19 and absence of IgG4 exodomain, (iii) G4CAR without scFv, but containing an IgG4 exodomain, and (iv) GD2G4CAR with specificity for GD2 and containing the IgG4 exodomain. All CAR species contained the same transmembrane and intracellular domains (CD28/CD3ζ) as the 2nd generation 19G4CAR.11 The co-cultures of CART with the two types of aAPC were found to have significantly different amounts of T cells by Day 21 depending on the choice of aAPC (p < 0.05) using two-way ANOVA followed by un-paired t-tests. The 19CAR+ T cells proliferated upon co-culture with CD19+ K562 and G4CAR+ T cells proliferated upon co-culture with CARL+ K562 in an exponential fashion, whereas 19CAR on CARL+ K562 and G4CAR on CD19+ K562 did not numerically expand (Figure 3A top panel). These data indicate that, as expected, the CD19 TAA on aAPC selectively supports the outgrowth of CD19-specific CART. Furthermore, they demonstrate that CARL can activate T cells to proliferate that contain a CAR species with an IgG4 exodomain. Next, the ability of CD19+ K562 and CARL+ K562 were assessed for ability to sustain the proliferation of T cells expressing 19G4CAR to evaluate how two modes of crosslinking (Figure 1C) can activate T cells. There were no significant differences in the accumulated number of viable T cells on Day 21 of co-culture based on the type of aAPC used (Figure 3A bottom panel), the expression of CAR as a percent of the population (Figure 3B bottom panel), or the mean fluorescence intensity (MFI). A trend (p = 0.09) towards a difference in MFI of CAR expression resulting from aAPC employed to expand 19G4CAR was noted (Figure 2C). There were no significant differences (Figure 3C & D) between the two aAPC types for propagating 19G4CAR+ T cells co-expressing cell-surface proteins associated with memory phenotype (p = 0.82),25 or other co-receptors (p = 0.26), as well as the specific lysis by 19G4CAR+ T cells (p = 0.16). Therefore, CD19-specific CAR+ T cells can be propagated in similar quantity and quality by K562-derived aAPC expressing CAR or CD19.
Figure 2.
Characterization of aAPC and CAR+ T cells. A) CD19 and CARL as SB transposons were integrated into parental K562 cells using SB11 transposase and clonally expanded for homogeneous expression of CD19 or CARL. Dot plots depict the expression of CD19 and CARL on parental K562 and derived clones. The stable expression of CARL is shown using antibody that detects mouse Fab. B) The expression level of CAR species as determined by flow cytometry is shown on Days 1 and 21 of co-culture with aAPC. Expression of chimeric IgG4 revealed CAR expression in all constructs except 19CAR which was determined using an antibody against human Fc. The percentage of cells in each flow plot quadrant is provided as an inset. C) The effect of aAPC design on abundance of CAR expression was assessed on Day 21 by measuring mean fluorescent intensity (MFI) of IgG4 signal by flow cytometry. The experiments are designated [CAR & aAPC] with unmodified mock electroporated T cells (No DNA plasmid) used as a control. Each experimental group contained 4 or 5 separate donor-derived PBMC. Statistical comparison was undertaken by One-way ANOVA followed by unpaired t-tests between each experiment (* = p < 0.05).
Figure 3.
Comparison of CAR+ T cells propagated on CD19+ or CARL+ aAPC. A) Total inferred T-cell number and B) CAR (IgG4) expression for each CART was measured every 7 days for 5 donors. Top panel: 19CAR+ or G4CAR+ T cells were numerically expanded on either CD19+ or CARL+ aAPC. Bottom panel: 19G4CAR+ T cells were propagated on either CD19+ or CARL+ aAPC. C) After 21 days of co-culture on CD19+ or CARL+ aAPC, 19G4CAR+ T cells from 5 donors were assessed for expression of markers associated with memory (top panel) or T cell co-receptors (bottom panel). D) Specific killing by electroporated/propagated T cells expressing 19CAR, G4CAR, and 19G4CAR, by CRA at a ratio of 5 effectors to 1 target. The tumor targets were EL-4 (murine thymoma- GD2+, CD19neg), NALM-6 (human B cell ALL- GD2neg, CD19+), and K562 (a human CML- GD2neg, CD19neg). Up to 5 donors were tested in 4 independent experiments. ns- No significance, * = p<0.05, *** = p<0.001
CARL+ K562 can numerically expand CAR+ T cells independent of specificity
The 2D3-derived scFv on aAPC was evaluated for ability to propagate not just CD19-specific T cells, but CAR+ T cells of alternative specificities. The CD19 and GD226 TAAs are not present on parental K562 cells to propagate T cells expressing GD2G4CAR, 19G4CAR, and G4CAR. Nonetheless, T cells bearing these three CARs numerically expanded on CARL+ K562. The number of total viable T cells on Day 21 of co-culture with CARL+ K562 cells did not significantly differ between 19G4CAR, G4CAR, and GD2G4CAR (p = 0.16, Figure 4A). Similarly, the percentage of each CAR expressed on T cells at Day 21 did not significantly differ among the three populations of genetically modified T cells (p = 0.68, Figure 4B). Finally, the electroporated and propagated T cells exhibited specific lysis of CD19 and GD2 TAAs recognized by CD19-specific and GD2-specific CARs. EL-4 cells, previously reported to express GD2,21 were specifically killed by GD2G4CAR+ T cells and not with T cells expressing G4CAR or 19G4CAR. As anticipated, CD19+ NALM-6 cells were targeted by T cells expressing 19G4CAR (Figure 4C). In summary, genetically modified T cells can be selectively propagated by CARL+ K562 cells resulting in T cells that retain specificity for TAA and stable expression of CAR. .
Figure 4.
Numeric expansion of CAR+ T cells using CARL+ aAPC. A) Total inferred T-cell number and B) CAR (IgG4) expression for each CART was measured every 7 days from 4 to 5 donors for 21 days of co-culture on aAPC. The differences between Day 21 total T-cell number and percent CAR expression was assessed using One-way ANOVA. C) The specific killing by panel of T cells expressing GD2G4CAR, 19G4CAR, and G4CAR, were tested using CRA at a ratio of 5 effectors to 1 target. The targets were EL-4 (GD2+, CD19neg), NALM-6 (GD2neg, CD19+), and parental K562 (GD2neg, CD19neg). Two-way ANOVA followed by unpaired t-tests was performed for 4 to 5 donors tested in 4 independent tests on Day 21 of co-culture on aAPC. ns- No significance, * = p<0.05.
The choice of aAPC does not skew the TCR repertoire for numerically expanded CART
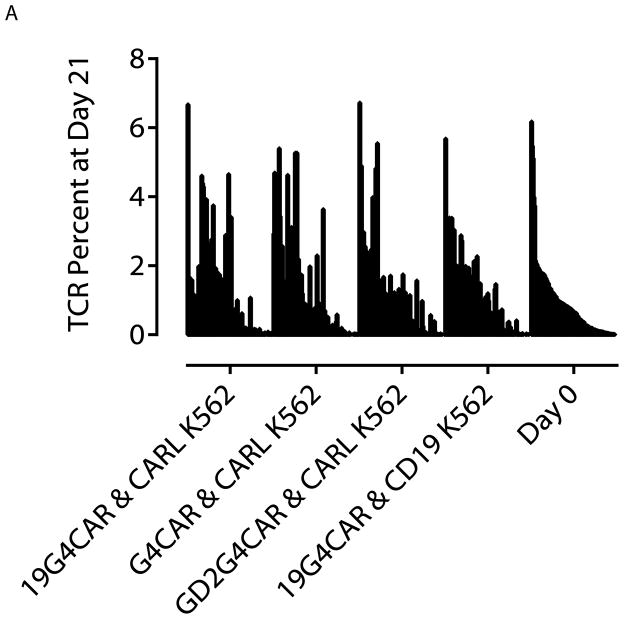
Each T cell in peripheral blood bears a distinct pair of αβ or γδ TCRs which can be analyzed using the direct TCR expression assay (DTEA) to determine the abundance of TCR chains. This assay was employed to determine whether CARL+ or CD19+ K562 influenced the distribution of TCR alleles after 21 days co-culture on aAPC. TCR variants were assayed on the nCounter Analysis System using a set of 111 TCR α, β, γ, and δ transcripts.23,24 By measuring the distribution of TCR alleles we could determine if the aAPC design preferentially supported the numeric expansion of some, but not all genetically modified T cells. The starting TCR distribution of T cells on Day 0 was ranked from the most to least frequent TCR usage and the rank order compared for T cells harvested on Day 21 of co-culture with aAPC (Figure 5A). This revealed no apparent monoclonal or oligoclonal outgrowth of electroporated T cells propagated on CARL+ or CD19+ K562 cells. The ranks of TCR frequencies on Day 0 and Day 21 from each experiment were compared using Spearman’s rank correlation test and found to significantly correlate (p < 0.0001; Table 1). The statistical comparison of TCR abundance and type from Day 0 and 21 indicated that all correlation coefficients (ρ) had values greater than 0.8 within the 95% confidence interval of ρ which is consistent with a strong correlation, indicating no change in TCR frequency. The measurement of TCR abundance demonstrates that CARL or TAA on aAPC do not skew the outgrowth of sub-populations of propagated T cells, but rather that both 2D3-derived scFv and CD19 on K562 cells can sustain the outgrowth of CAR+ T cells that maintain a polyclonal repertoire.
Figure 5.
Comparison of TCR repertoire changes induced by CAR-mediated expansion on aAPC. A) TCR repertoire was measured for 111 TCR α, β, γ, and δ alleles using DTEA. 24 TCR abundance was organized from the most to the least frequently occurring transcripts based on sorted CD3+CD56neg cells from Day 0. The set is visually represented next to TCR repertoire expressed by T cells at Day 21 of co-culture on CARL+ K562 cells and CD19+ K562 cells. Analysis was performed on 2 donors and a representative plot of one donor is shown.
Table 1.
Comparison of TCR abundance harvested from T cells before versus after propagation on aAPC*
| Day 0 | Day 21 | |||||
|---|---|---|---|---|---|---|
| 19G4CAR & CARL+ K562 | G4CAR & CARL+ K562 | GD2G4CAR & CARL+ K562 | 19G4CAR & CD19+ K562 | |||
| Day 0 | 0.748 (0.65–0.82) | 0.857 (0.80–0.90) | 0.867 (0.81–0.91) | 0.912 (0.87–0.94) | ||
| Day 21 | 19G4CAR & CARL+ K562 | 0.752 (0.65–0.83) | 0.805 (0.72–0.86) | 0.706 (0.59–0.79) | 0.734 (0.63–0.81) | |
| G4CAR & CARL+ K562 | 0.899 (0.85–0.93) | 0.71 (0.61–0.79) | 0.816 (0.74–0.87) | 0.839 (0.77–0.89) | ||
| GD2G4CAR & CARL+ K562 | 0.825 (0.75–0.88) | 0.72 (0.61–0.80) | 0.801 (0.72–0.86) | 0.881 (0.83–0.92) | ||
| 19G4CAR & CD19+ K562 | 0.916 (0.87–0.94) | 0.69 (0.57–0.78) | 0.887 (0.84–0.92) | 0.808 (0.73–0.87) | ||
Analysis DTEA data from two donors was normalized using housekeeping genes and assessed using Spearman correlation coefficient to compare distributions of TCR usage for two donors. The upper right of the table contains the correlation between experimental groups for one donor. The lower left of the table contains the second donor subjected to the same analysis. Each cell in the table contains the Spearman correlation coefficient (ρ) and within the brackets the 95% confidence interval. (A strong correlation is considered to be ρ ≥ 0.8.)
DISCUSSION
This study demonstrates that a ligand directed against a conserved extracellular domain on CARs can function to numerically expand CART while preserving redirected specificity of genetically modified T cells for TAA. This differs from other methods to select or sort for CART such as magnetic sorting,14 selection with cytotoxic drug,10 or TAA-mediated numeric expansion. A recent report demonstrated antigen-independent CAR-mediated T-cell activation using antibody binding to an extracellular Myc-tag of ErbB2-specific T cells.27 Our study differs as CARL recognizes a determinant native to an extracellular scaffold to induce proliferation of CART. This provides an apparent advantage, as the use of epitope tags may alter antigen recognition or increase immunogenicity. Our data demonstrate that a mAb-derived scFv sequence directed against conserved extracellular CAR domains can be used for cross-linking, activation, and propagation of CAR species on genetically modified T cells.4–6 Thus, CARL-mediated numeric expansion of CART will be useful to laboratories seeking to augment the selective outgrowth of CART within a tissue culture environment after gene transfer.
A benefit of our approach is that one CARL design could functionally substitutes for multiple TAAs. Specifically, the CARL in this report enables K562 cells to function as aAPC to propagate T cells expressing a panel of CARs to specifically lyse tumor cells expressing multiple TAAs. Alternatively, our technology allows for CARs to be designed which impart no specificity. This was demonstrated here as a proof-of-concept with G4CAR activating T cells to proliferate during co-culture with CARL+ K562 cells without ligating endogenous CD3. Implicit in this finding is that any T cell bearing an introduced CAR, or other immunoreceptor containing the CARL-binding domain, may be propagated upon cross-linking by CARL.
Recent studies have demonstrated that reducing the length of an IgG4 exodomain improved cytokine secretion, cytotoxicity, and proliferation of ROR1-specific CART28 and removal of IgG1 scaffold from a CAR appeared to improve killing of CD22+ targets.29 These improvements in potency support modifying the scFv distance from the T-cell membrane to enable a candidate CAR design to provide a fully-competent T-cell activation signal. The identification of the peptide recognized by 2D3-derived scFv is ongoing, and may enable us to alter the length of the extracellular domain to tune CAR+ T cells for optimal activation by TAA while preserving the ability of CARL to propagate genetically modified T cells.
One measure of redirected specificity is the ability of CAR to mediate T-cell killing of TAA+ targets. The cytotoxicity of CARL-propagated CART appears to be moderately reduced based on prior publications.19,30 This may be accounted by the design of the CARL+ and CD19+ aAPC, which were not engineered to express co-stimulatory molecules such as CD86, CD137L and membrane-bound IL-15, as are present on aAPC (designated clone 4) we previously used to generate CD19-specific 19G4CAR+ T cells.3 Furthermore, we used aAPC clone 4 in the presence of soluble recombinant IL-2 and IL-21 whereas CARL+ K562 cells were co-cultured with genetically modified T cells with only IL-2. Future studies will help elucidate the effect of costimulation on CART performance.
It is possible that the aAPC used to activate CAR may selectively propagate a sub-population of genetically modified T cells over the co-culture period. However, we observed that both CARL+ and CD19+ K562 cells numerically expanded T cells bearing a similar percentage expression and density of CAR, a comparable immunophenotype. In addition, there were no significant differences in TCR repertoire expression and abundance before versus after propagation on aAPC indicating that the starting population of T cells matched the population present at the end of the co-culture period. These findings justify investigating whether CARL+ aAPC might be used to generate CART for human application. Furthermore, it is our expectation that expression of CARL on a single source of clinical-grade aAPC can be used to generate panels of CAR+ T cells, overcoming the current need to produce panels of aAPC with each expressing a given TAA for a given specificity of CART.
In summary, we report the development of an aAPC based on a CAR-specific mAb for the CAR-mediated propagation of CAR+ T cells with multiple specificities.
Supplementary Material
Figure S1. Vector maps for expression of transgenes. Each DNA plasmid expresses a transgene of interest under promoter human Elongation Factor 1 alpha (phEF-1α), using the beta hemoglobin poly-adenylation signal (BGH) to terminate transcription. The indirect repeats / direct repeats (IR/DR) allow for transgene transposition into the genome using SB11. All plasmids were propagated in bacteria using the origin of replication ColE1 and Kanamycin resistance (KanR) under the promoter pKan. A) 19G4CAR demonstrates the original plasmid design used in these studies and shows NheI and XmnI restriction enzyme (RE) sites used to generate B) GD2G4CAR from PCR-directed truncation of CD19-specific scFv on 19G4CAR which led to the generation of C) G4CAR and final ligation using ApaI and KpnI REs. D) 19CAR was designed without an IgG4 exodomain, instead expressing the CD8α hinge and exodomain. E) Zeo-2A-CARL, expressing CARL, and F) CD19-2A-Neo, expressing truncated human CD19 (tCD19), were designed to express CARL or CD19 on aAPC under drug selection conditions.
Table S1. Fluorochrome-conjugated antibodies used for flow cytometry.
Acknowledgments
The authors thank the Monoclonal Antibody Facility at MD Anderson Cancer Center for producing the 2D3 hybridoma. We also recognize Dr. Kirsten Switzer for her experimental help, Dr. Drew Deniger for suggestions regarding the manuscript, Dr. Brian Rabinovich for help with molecular biology, and Dr. George McNamara for assistance in editing. We also thank the Altman-Goldstein Discovery Fellowship for their generous contribution.
Support: Cancer Center Core Grant (CA16672); RO1 (CA124782,CA120956, CA141303; CA141303); R33 (CA116127); P01 (CA148600); Burroughs Wellcome Fund; Cancer Prevention and Research Institute of Texas; CLL Global Research Foundation; DARPA (Defense Sciences Office); Department of Defense; Estate of Noelan L. Bibler; Gillson Longenbaugh Foundation; Harry T. Mangurian, Jr., Fund for Leukemia Immunotherapy; Institute of Personalized Cancer Therapy; Leukemia and Lymphoma Society; Lymphoma Research Foundation; MDACC’s Sister Institution Network Fund; Miller Foundation; Mr. Herb Simons; Mr. and Mrs. Joe H. Scales; Mr. Thomas Scott; National Foundation for Cancer Research; Pediatric Cancer Research Foundation; William Lawrence and Blanche Hughes Children's Foundation; Altman-Goldstein Discovery Fellowship.
Financial Disclosure Statement: Dr. Cooper founded and owns InCellerate Inc. He has patents with Sangamo BioSciences with artificial nucleases. He consults with Targazyme, Inc. (American Stem cells, Inc.), GE Healthcare, and Ferring Pharmaceuticals. D. Rushworth and Drs. Jena and Cooper are named on a patent pending for the use of 2D3.
References
- 1.Kalos M, Levine BL, Porter DL, et al. T cells with chimeric antigen receptors have potent antitumor effects and can establish memory in patients with advanced leukemia. Sci Trans Med. 2011 Aug 10;3(95):95ra73. doi: 10.1126/scitranslmed.3002842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kochenderfer JN, Dudley ME, Feldman SA, et al. B-cell depletion and remissions of malignancy along with cytokine-associated toxicity in a clinical trial of anti-CD19 chimeric-antigen-receptor-transduced T cells. Blood. 2012 Mar 22;119(12):2709–2720. doi: 10.1182/blood-2011-10-384388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huls MH, Figliola MJ, Dawson MJ, et al. Clinical application of Sleeping Beauty and artificial antigen presenting cells to genetically modify T cells from peripheral and umbilical cord blood. JoVE. 2013;(72):e50070. doi: 10.3791/50070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang X, Naranjo A, Brown CE, et al. Phenotypic and functional attributes of lentivirus-modified CD19-specific human CD8+ central memory T cells manufactured at clinical scale. J Immunother. 2012 Nov-Dec;35(9):689–701. doi: 10.1097/CJI.0b013e318270dec7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hollyman D, Stefanski J, Przybylowski M, et al. Manufacturing validation of biologically functional T cells targeted to CD19 antigen for autologous adoptive cell therapy. J Immunother. 2009 Feb-Mar;32(2):169–180. doi: 10.1097/CJI.0b013e318194a6e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kochenderfer JN, Feldman SA, Zhao Y, et al. Construction and preclinical evaluation of an anti-CD19 chimeric antigen receptor. J Immunother. 2009 Sep;32(7):689–702. doi: 10.1097/CJI.0b013e3181ac6138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chinnasamy N, Wargo JA, Yu Z, et al. A TCR targeting the HLA-A*0201-restricted epitope of MAGE-A3 recognizes multiple epitopes of the MAGE-A antigen superfamily in several types of cancer. J Immuno. 2011 Jan 15;186(2):685–696. doi: 10.4049/jimmunol.1001775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Parkhurst MR, Yang JC, Langan RC, et al. T cells targeting carcinoembryonic antigen can mediate regression of metastatic colorectal cancer but induce severe transient colitis. Mol Ther. 2011 Mar;19(3):620–626. doi: 10.1038/mt.2010.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kung P, Goldstein G, Reinherz EL, Schlossman SF. Monoclonal antibodies defining distinctive human T cell surface antigens. Science. 1979 Oct 19;206(4416):347–349. doi: 10.1126/science.314668. [DOI] [PubMed] [Google Scholar]
- 10.Jensen MC, Clarke P, Tan G, et al. Human T lymphocyte genetic modification with naked DNA. Mol Ther. 2000 Jan;1(1):49–55. doi: 10.1006/mthe.1999.0012. [DOI] [PubMed] [Google Scholar]
- 11.Singh H, Manuri PR, Olivares S, et al. Redirecting specificity of T-cell populations for CD19 using the Sleeping Beauty system. Can Res. 2008 Apr 15;68(8):2961–2971. doi: 10.1158/0008-5472.CAN-07-5600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Puls R, Minchin R. Gene transfer and expression of a non-viral polycation-based vector in CD4+ cells. Gene Ther. 1999 Oct;6(10):1774–1778. doi: 10.1038/sj.gt.3301022. [DOI] [PubMed] [Google Scholar]
- 13.Nakazawa Y, Huye LE, Dotti G, et al. Optimization of the PiggyBac transposon system for the sustained genetic modification of human T lymphocytes. J Immunother. 2009 Oct;32(8):826–836. doi: 10.1097/CJI.0b013e3181ad762b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang X, Chang WC, Wong CW, et al. A transgene-encoded cell surface polypeptide for selection, in vivo tracking, and ablation of engineered cells. Blood. 2011 Aug 4;118(5):1255–1263. doi: 10.1182/blood-2011-02-337360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Numbenjapon T, Serrano LM, Chang WC, Forman SJ, Jensen MC, Cooper LJ. Antigen-independent and antigen-dependent methods to numerically expand CD19-specific CD8+ T cells. Exp Hema. 2007 Jul;35(7):1083–1090. doi: 10.1016/j.exphem.2007.04.007. [DOI] [PubMed] [Google Scholar]
- 16.d'Azzo A, Tessitore A, Sano R. Gangliosides as apoptotic signals in ER stress response. Cell Death Diff. 2006 Mar;13(3):404–414. doi: 10.1038/sj.cdd.4401834. [DOI] [PubMed] [Google Scholar]
- 17.Cooper LJ, Topp MS, Serrano LM, et al. T-cell clones can be rendered specific for CD19: toward the selective augmentation of the graft-versus-B-lineage leukemia effect. Blood. 2003 Feb 15;101(4):1637–1644. doi: 10.1182/blood-2002-07-1989. [DOI] [PubMed] [Google Scholar]
- 18.Suhoski MM, Golovina TN, Aqui NA, et al. Engineering artificial antigen-presenting cells to express a diverse array of co-stimulatory molecules. Mol Ther. 2007 May;15(5):981–988. doi: 10.1038/mt.sj.6300134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Davies JK, Singh H, Huls H, et al. Combining CD19 redirection and alloanergization to generate tumor-specific human T cells for allogeneic cell therapy of B-cell malignancies. Can Res. 2010 May 15;70(10):3915–3924. doi: 10.1158/0008-5472.CAN-09-3845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kowolik CM, Topp MS, Gonzalez S, et al. CD28 costimulation provided through a CD19-specific chimeric antigen receptor enhances in vivo persistence and antitumor efficacy of adoptively transferred T cells. Can Res. 2006 Nov 15;66(22):10995–11004. doi: 10.1158/0008-5472.CAN-06-0160. [DOI] [PubMed] [Google Scholar]
- 21.Alvarez-Rueda N, Desselle A, Cochonneau D, et al. A monoclonal antibody to O-acetyl-GD2 ganglioside and not to GD2 shows potent anti-tumor activity without peripheral nervous system cross-reactivity. PloS One. 2011;6(9):e25220. doi: 10.1371/journal.pone.0025220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang Z, Raifu M, Howard M, et al. Universal PCR amplification of mouse immunoglobulin gene variable regions: the design of degenerate primers and an assessment of the effect of DNA polymerase 3' to 5' exonuclease activity. J Immuno Methods. 2000 Jan 13;233(1–2):167–177. doi: 10.1016/s0022-1759(99)00184-2. [DOI] [PubMed] [Google Scholar]
- 23.Deniger DC, Switzer K, Mi T, et al. Bispecific T-cells expressing polyclonal repertoire of endogenous gammadelta T-cell receptors and introduced CD19-specific chimeric antigen receptor. Mol Ther. 2013 Mar;21(3):638–647. doi: 10.1038/mt.2012.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang M, Maiti S, Bernatchez C, et al. A new approach to simultaneously quantify both TCR alpha- and beta-chain diversity after adoptive immunotherapy. Clin Can Res. 2012 Sep 1;18(17):4733–4742. doi: 10.1158/1078-0432.CCR-11-3234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Klebanoff CA, Gattinoni L, Restifo NP. CD8+ T-cell memory in tumor immunology and immunotherapy. Immuno Rev. 2006 Jun;211:214–224. doi: 10.1111/j.0105-2896.2006.00391.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Helfand SC, Hank JA, Gan J, Sondel PM. Lysis of human tumor cell lines by canine complement plus monoclonal antiganglioside antibodies or natural canine xenoantibodies. Cellular Immuno. 1996 Jan 10;167(1):99–107. doi: 10.1006/cimm.1996.0012. [DOI] [PubMed] [Google Scholar]
- 27.Duong CP, Westwood JA, Yong CS, et al. Engineering T cell function using chimeric antigen receptors identified using a DNA library approach. PloS One. 2013;8(5):e63037. doi: 10.1371/journal.pone.0063037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hudecek M, Lupo-Stanghellini MT, Kosasih PL, et al. Receptor Affinity and Extracellular Domain Modifications Affect Tumor Recognition by ROR1-Specific Chimeric Antigen Receptor T Cells. Clin Can Res. 2013 Jun 15;19(12):3153–3164. doi: 10.1158/1078-0432.CCR-13-0330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Haso W, Lee DW, Shah NN, et al. Anti-CD22-chimeric antigen receptors targeting B-cell precursor acute lymphoblastic leukemia. Blood. 2013 Feb 14;121(7):1165–1174. doi: 10.1182/blood-2012-06-438002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yvon E, Del Vecchio M, Savoldo B, et al. Immunotherapy of metastatic melanoma using genetically engineered GD2-specific T cells. Clin Can Res. 2009 Sep 15;15(18):5852–5860. doi: 10.1158/1078-0432.CCR-08-3163. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Vector maps for expression of transgenes. Each DNA plasmid expresses a transgene of interest under promoter human Elongation Factor 1 alpha (phEF-1α), using the beta hemoglobin poly-adenylation signal (BGH) to terminate transcription. The indirect repeats / direct repeats (IR/DR) allow for transgene transposition into the genome using SB11. All plasmids were propagated in bacteria using the origin of replication ColE1 and Kanamycin resistance (KanR) under the promoter pKan. A) 19G4CAR demonstrates the original plasmid design used in these studies and shows NheI and XmnI restriction enzyme (RE) sites used to generate B) GD2G4CAR from PCR-directed truncation of CD19-specific scFv on 19G4CAR which led to the generation of C) G4CAR and final ligation using ApaI and KpnI REs. D) 19CAR was designed without an IgG4 exodomain, instead expressing the CD8α hinge and exodomain. E) Zeo-2A-CARL, expressing CARL, and F) CD19-2A-Neo, expressing truncated human CD19 (tCD19), were designed to express CARL or CD19 on aAPC under drug selection conditions.
Table S1. Fluorochrome-conjugated antibodies used for flow cytometry.