Abstract
BACKGROUND
Recombinant interleukin-2 (rIL-2) induces cellular cytotoxicity against leukemia blasts. Patients with acute myeloid leukemia (AML) in first complete remission (CR) may harbor minimal residual disease that is susceptible to rIL-2–activated effector cells.
METHODS
In the Cancer and Leukemia Group B (CALGB) 19808 study, patients with AML in first CR were randomly assigned after all planned chemotherapy to receive a 90-day course of subcutaneously administered rIL-2 or no further therapy. The primary objective was to compare disease-free survival (DFS) between the 2 treatment arms. A total of 534 patients achieved a CR, 214 of whom were randomized. Six courses of low-dose daily rIL-2 were given for the expansion of cytotoxic effector cells, each followed by 3-day high-dose boluses given to trigger cytotoxicity against minimal residual disease.
RESULTS
On the protocol-specified intention-to-treat analysis, the hazards ratio for DFS was 0.75 (95% confidence interval, 0.52–1.09; P =.13); the 5-year DFS rate was 42% in the observation arm and 53% in the rIL-2 treatment arm. The hazards ratio for overall survival (OS) was 0.88 (95% confidence interval, 0.54–1.23; P =.34); the 5-year OS rate was 58% for the observation arm and 63% for the rIL-2 treatment arm. Twenty-five of the 107 patients randomized to treatment with rIL-2 either refused or were unable to initiate therapy and 30 patients did not complete their assigned therapy. However, significant toxicities were not commonly observed. The trial design did not anticipate the difficulties patients would encounter with protocol compliance.
CONCLUSIONS
The efficacy of immunotherapy with rIL-2 administered after intensive postremission treatment was not assessed as planned because of unexpected refusals by patients and/or their physicians to comply with protocol-directed therapy. Neither DFS nor OS was found to be significantly improved.
Keywords: acute myeloid leukemia, recombinant interleukin-2, phase 3, immunotherapy, adjuvant therapy
INTRODUCTION
Recombinant interleukin-2 (rIL-2) induces cytotoxicity against tumor targets mediated by cytotoxic T cells and natural killer (NK) cells.1,2 Responses have been described in patients with recurrent and refractory acute myeloid leukemia (AML).3,4 Prior studies of rIL-2 treatment alone in patients with AML in first complete remission (CR) have not demonstrated improvements in outcomes.5
Beginning in 1994, the Cancer and Leukemia Group B (CALGB) initiated a series of trials in patients with AML in first CR6–8 using doses and schedules of rIL-2 that induce the expansion and cytotoxicity of NK cells with acceptable toxicity.9 We hypothesized that immunotherapy would be most effective when administered at the time of minimal residual disease. We now report outcomes from a randomization between rIL-2 and observation for patients in CR after the completion of all planned postremission therapy on the CALGB (Alliance) 19808 trial. Patients were randomized after receiving either high-dose cytarabine (HiDAC) consolidation or autologous hematopoietic stem cell transplantation (AHSCT), depending on cytogenetic risk. The feasibility and safety of treatment with rIL-2 after AHSCT has been previously shown.10,11 The goal was to assess the impact of rIL-2 immunotherapy on disease-free survival (DFS).
MATERIALS AND METHODS
Previously untreated patients aged <60 years with AML in first CR were randomized between 2 treatment arms: rIL-2 and observation. Patients with acute promyelocytic leukemia, therapy-related AML, or AML arising from a prior myeloproliferative disorder or those with a history of bone marrow-proven myelodysplastic syndrome of >3 months were excluded. Each participant signed an Institutional Review Board-approved protocol-specific informed consent in accordance with federal and institutional guidelines.
Induction and Postremission Therapy
The details of the CALGB 19808 study have been described previously.12 Briefly, induction chemotherapy consisted of daunorubicin, etoposide, and cytarabine, with or without valspodar (PSC-833), an inhibitor of p-glycoprotein. The first 302 patients were randomized to treatment with or without valspodar.
Postremission therapy depended on cytogenetic risk. Patients with core-binding factor AML (ie, those patients with t(8;21)(q22;q22), inv(16)(p13q22), or t(16;16)(p13;q22)) received 3 courses of HiDAC.13
Those patients with non–core-binding factor AML in CR received HiDAC with high-dose infusional etoposide followed by AHSCT as previously described.14,15 If unable to undergo transplantation, patients received an alternative consolidation sequence consisting of HiDAC with high-dose infusional etoposide followed by 2 courses of HiDAC.16
rIL-2 Treatment Plan
The doses and schedule of rIL-2 were developed in the prior phase 1/2 CALGB 9621 study.8 Patients were randomized between rIL-2 and observation after recovery from postremission chemotherapy or AHSCT. A maximum of 120 days was permitted from day 1 of the final course of consolidation chemotherapy or day 0 of AHSCT. The absolute neutrophil count had to be >0.75 × 109/L and the platelet count >10 × 109/L without transfusions. A bone marrow biopsy documenting a leukemia-free state with trilineage hematopoiesis was required. Consent for the immunotherapy randomization was obtained at the time of protocol registration, before induction therapy.
The 90 days of treatment with rIL-2 consisted of 6 courses of 10 to 16 days of low-dose rIL-2 (1 × 106 U/m2 subcutaneously [SC] daily) interspersed with five 3-day high-dose courses (12 × 106 U/m2−15 × 106 U/m2 SC daily) (Fig. 1). The last low-dose sequence extended to 16 days to provide a total of 90 days of therapy. Low-dose rIL-2 therapy was to be continued, with transfusion support as needed to maintain a platelet count >10 × 109/L. If the absolute neutrophil count was <0.5 × 109/L, therapy was held until recovery to >0.75 × 109/L, at which time rIL-2 could be restarted with a 20% dose reduction. If transfusion requirements increased, or if incapacitating fatigue developed or any nonhematologic toxicity of >grade 2 other than fever, a 3-day to 7- day therapy interruption was prescribed, followed by up to 3 dose reductions to 0.8 × 106 U/m2, 0.6 × 106 U/m2, or 0.4 × 106 U/m2.
Figure 1.
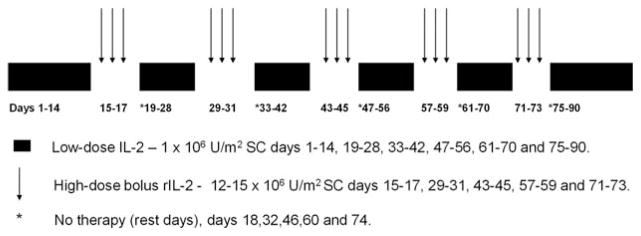
Treatment plan for recombinant interleukin-2 (rIL-2) maintenance therapy is shown. Low-dose rIL-2 was given subcutaneously (SC) for 10 to 16 days. Each low-dose sequence was followed by 3 days of high-dose rIL-2 administered SC. The course was completed with 16 days of low-dose rIL-2 therapy without further administration of high-dose rIL-2. Patients were given a rest day after each high-dose sequence.
Patients without a >grade 2 toxicity other than fever after the first 3-day high-dose sequence were to have their dose of rIL-2 increased from 12 × 106 U/m2 to 15 × 106 U/m2 daily starting with the second high-dose sequence. Patients experiencing >grade 2 nonhematologic toxicity after high-dose rIL-2 underwent dose interruptions of 3 to 7 days, after which the previously tolerated low dose of rIL-2 was given until the next high-dose cycle. Up to 3 reductions of 3 × 106 U/m2 each were allowed for patients who had been escalated to 15 × 106 U/m2 and 2 reductions were permitted for patients who were not escalated above 12 × 106 U/m2. Missed high-dose treatments were not made up.
Criteria for Response and Toxicity
National Cancer Institute Workshop criteria were used to define CR.17 The National Cancer Institute Common Toxicity Criteria were used to grade toxicity.
Quality Control, Quality Assurance, and Auditing
Institutional Review Board approval was required for every participating institution with written informed consent obtained from each patient. Patient registration, data collection, and all statistical analyses were performed by the Alliance for Clinical Trials in Oncology Statistics and Data Management Center. The medical records of 83 patients (39% of the 214 randomized patients) were audited; records from each participating institution were reviewed. In addition, this study was monitored by the CALGB (Alliance) Data and Safety Monitoring Committee following standard National Cancer Institute policies.
Statistical Analysis
Randomization on the current study used a randomized permuted block design with a block size of 8 and a 1:1 allocation to the 2 treatments (rIL-2 or observation) with stratification by the assigned induction regimen (cytarabine, daunorubicin, and etoposide [ADE] and cytarabine, daunorubicin, etoposide, and valspodar [ADEP]) and cytogenetic risk group (favorable or others). The randomization process was set up and maintained by the CALGB (Alliance) Statistics and Data Management Center.
The primary endpoint was DFS from the date of the immunotherapy randomization. The design assumed that 240 patients would achieve CR, complete all other treatment, and be randomized between rIL-2 and observation. These patients would be followed until a total of 192 failures were observed, providing 90% power to detect a hazards ratio (HR) <0.625 with respect to DFS. Analyses of DFS and overall survival (OS) used 2-sided log-rank tests18 at the .05 significance level as well as proportional hazards regression models.19 For the analysis of DFS, patients who were alive and in CR were censored at the time of last follow-up. OS was measured from the date of the immunotherapy randomization until the date of death; patients alive at the time of last follow-up were censored. Protocol-specified interim analyses were performed every 6 months after 100 patients were entered as previously described.20
The primary analyses compared the outcomes of patients randomized to the rIL-2 treatment arm with those who were randomized to the observation arm. Secondary analyses compared outcomes for patients who did not receive any rIL-2 with those who received at least 1 dose of rIL-2. In addition, a landmark analysis21 was conducted to compare outcomes by the amount of rIL-2 received by day 120 after randomization. In these analyses, survival duration was defined as the time from the landmark of 120 days to the event.
Survival estimates were calculated using the Kaplan-Meier method,22 and treatment groups were compared using a 2-sided log-rank test at a significance level of .05. Univariable Cox proportional hazards models were performed for both DFS and OS and the estimated HRs and corresponding 95% confidence intervals (95% CIs) were calculated to compare treatment groups within various subgroups defined by clinical parameters. Forest plots23 were generated to summarize these results and to evaluate any treatment covariate or subgroup effect.
RESULTS
Between November 15, 2000 and March 31, 2006, 734 patients were registered. A CR was achieved by 549 of the 716 evaluable patients (77%). After the completion of all chemotherapy, 214 patients agreed to be randomized between rIL-2 therapy and observation. Table 1 describes the well-balanced demographic and clinical characteristics of the randomization arms.
TABLE 1.
Immunotherapy Randomization
| No. of registered patients | 734 |
| No. evaluable for response to induction therapy | 716 |
| No. with CR | 549 (77%) |
| No.(% of CR) | |
| Refused randomization | 82 (15%) |
| Refused postremission therapy | 15 (3%) |
| Developed disease recurrence before immunotherapy randomization | 73 (13%) |
| Death after CR, before or after consolidation therapy | 20 (4%) |
| Allogeneic HSCT in first CR | 63 (11%) |
| Unresolved toxicity | 45 (8%) |
| Failure to recovery blood counts by day 120 after AHSCT or last HiDAC | 21 (4%) |
| Other/unknown | 16 (3%) |
| Randomized to rIL-2 | 107 (19%) |
| Randomized to observation | 107 (19%) |
Abbreviations: AHSCT, autologous hematopoietic stem cell transplantation; CR complete remission; HiDAC, high-dose cytarabine; HSCT, hematopoietic stem cell transplantation; rIL-2, recombinant interleukin-2.
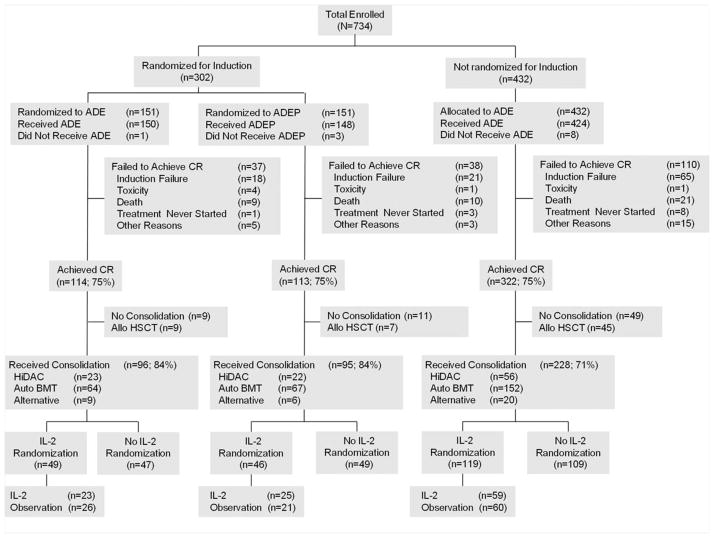
Of 549 patients who achieved a CR, 63 (11%) were removed from protocol therapy to receive allogeneic transplantation. Eighty-two patients (15%) refused randomization and 73 patients (13%) developed disease recurrence before randomization (Fig. 2) (Table 2). Among the 107 patients assigned to receive rIL-2, 31 patients (29%) did not begin treatment, almost all (25 patients) because of patient refusal. Another 30 patients (28%) initiated treatment but failed to complete their 90-day course. Reasons for discontinuing therapy included 19 patient refusals (18%) with grade 1 or grade 2 toxicity, and 5 patient refusals (5%) with grade 3 or grade 4 toxicity; 6 patients (6%) developed disease recurrence while receiving rIL-2. Seventy-six of the 107 patients assigned to receive rIL-2 therapy (71%) received at least 1 dose of rIL-2; 46 patients (43%) received at least 50% of the planned therapy and 25 patients (23%) received at least 90% of the planned therapy.
Figure 2.
A CONSORT (Consolidated Standards Of Reporting Trials) flow diagram demonstrating the progress of patients in complete remission (CR) up to the point of randomization between recombinant interleukin-2 (rIL-2) and observation is shown. “Received consolidation” includes only those patients who received protocol-specified therapy. Sixty-one of 549 patients in CR (11%) received allogeneic transplants. “Alternative” refers to the therapy given to patients without core-binding factor acute myeloid leukemia who did not receive autologous or allogeneic transplants. ADE indicates cytarabine, daunorubicin, and etoposide; ADEP, cytarabine, daunorubicin, etoposide, and valspodar (PSC-833); allo HSCT, allogeneic hematopoietic stem cell transplantation; HiDAC, high-dose cytarabine; BMT, bone marrow transplantation.
TABLE 2.
Grade 3 and 4 Toxicities Observed in at Least 5% of Patients Treated With rIL-2a
| Toxicity | Grade 3 No. | Grade 4 No. |
|---|---|---|
| Anemia | 5 (7%) | 0 |
| Neutropenia | 6 (8%) | 9 (12%) |
| Thrombocytopenia | 12 (16%) | 6 (8%) |
| Hypotension | 10 (13%) | 1 (1%) |
| Fatigue | 9 (12%) | 0 |
| Febrile neutropenia | 4 (5%) | 1 (1%) |
| Infection without neutropenia | 4 (5%) | 0 |
| Dyspnea | 4 (5%) | 0 |
Abbreviation: rIL-2, recombinant interleukin-2.
The National Cancer Institute Common Toxicity Criteria were used to grade toxicity.
Toxicity
Severe adverse events were uncommon. Grade 3 and 4 toxicities were observed in at least 5% of patients and are shown in Table 2. Thrombocytopenia, neutropenia, fatigue, and hypotension were the most common grade 3 and 4 events and were readily reversible.
Outcomes
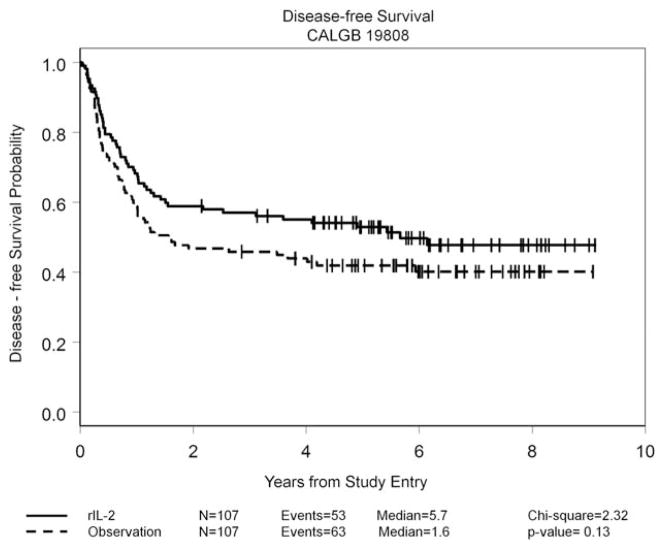
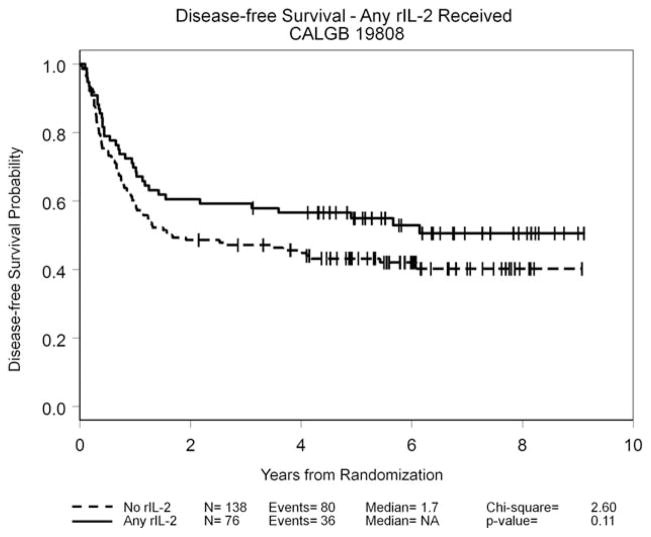
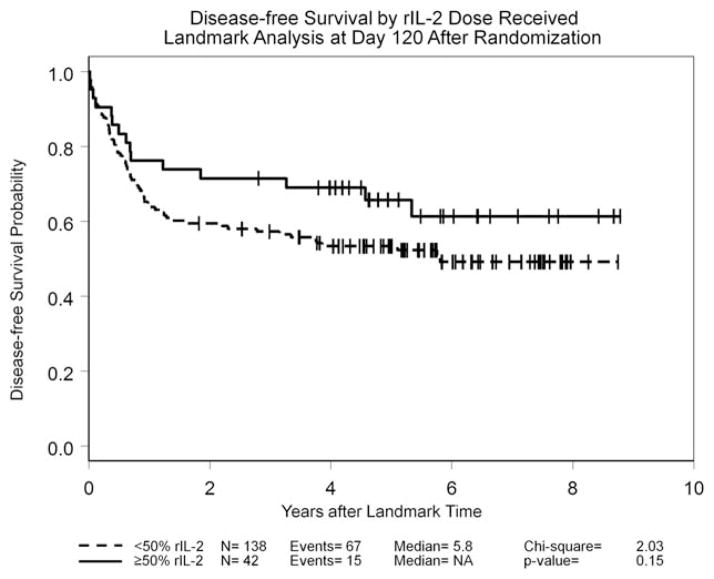
By the protocol-specified intention-to-treat analysis, the HR for DFS was 0.75 (95% CI, 0.52–1.09; P =.13); the 5-year DFS rate was 42% for the observation arm and 53% for the rIL-2 treatment arm. Details are given in Figure 3. The HR for OS was 0.88 (95% CI, 0.54–1.23; P =.34); the 5-year OS rate was 58% for the observation arm and 63% for the rIL-2 treatment arm. In an exploratory “as-treated” analysis in which patients who were randomized to receive rIL-2 therapy but who either did not receive or refused their assigned treatment were combined with the 107 patients in the observation arm, outcomes among the 76 patients who received at least 1 dose of rIL-2 were compared with the 138 patients who did not receive any immunotherapy. The 5-year DFS rate was 43% in the no immunotherapy group (median, 1.7 years) and 55% in the group treated with rIL-2 (median DFS not reached; P =.11) (Fig. 4), whereas the 5-year OS rates were 60% in the observation group and 61% in the group treated with rIL-2; neither group had reached the median OS at the time of last follow-up. An additional exploratory landmark analysis comparing outcomes by amount of rIL-2 received by day 120 after randomization (≥ 50% of the planned dose vs <50% or no rIL-2 received) demonstrated similar trends with respect to DFS (P =.15 in both instances) (Fig. 5) and no difference with respect to OS (P =.5). Univariable analyses for DFS and OS using 9 variables demonstrated that only the European LeukemiaNet genetic grouping24 significantly affected DFS and OS, whereas the baseline lactate dehydrogenase level was found to be significantly associated with OS (data not shown). A forest plot analysis showed HRs favoring rIL-2 with respect to DFS among all 16 variables studied (Fig. 6) and OS in 13 of the 16 variables studied (data not shown), but the 95% CI crossed unity in each instance.
Figure 3.
Disease-free survival among the 214 patients in complete remission randomized between treatment with recombinant interleukin-2 (rIL-2) and observation is shown by intent-to-treat analysis. CALGB indicates Cancer and Leukemia Group B.
Figure 4.
Exploratory analysis of disease-free survival according to whether any recombinant interleukin-2 (rIL-2) treatment was received after the completion of postremission chemotherapy is shown. CALGB indicates Cancer and Leukemia Group B; NA, median not reached.
Figure 5.
Disease-free survival is shown using a landmark analysis comparing outcomes by amount of recombinant interleukin-2 (rIL-2) received by day 120 after randomization (≥ 50% of the planned dose vs <50% or no rIL-2 received). NA indicates median not reached.
Figure 6.
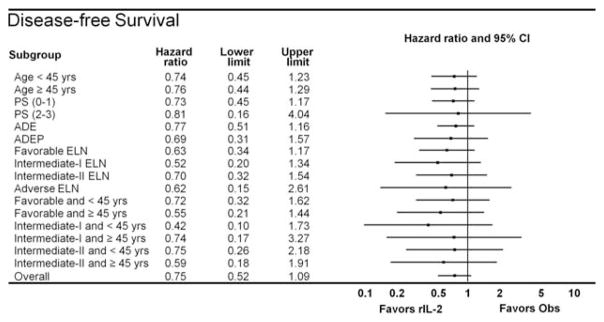
Forest plot analysis of disease-free survival is shown. Points to the left of the ordinate favor treatment with recombinant interleukin-2 (rIL-2) whereas those to the right favor no therapy (observation [obs]). 95% CI indicates 95% confidence interval; PS, performance status; ADE, cytarabine, daunorubicin, and etoposide; ADEP, cytarabine, daunorubicin, etoposide, and valspodar (PSC-833); ELN, European LeukemiaNet genetic grouping.
DISCUSSION
Multiple studies of rIL-2 in patients with AML in first CR have failed to demonstrate benefit. This has been summarized in a meta-analysis of 6 phase 3 randomized trials, including preliminary data from the current report.5 Five of the 6 cited trials were published between 2007 and 2010, well after the start of this study. One phase 3 trial that combined rIL-2 with histamine dihydrochloride in patients with AML in CR favored the rIL-2 arm.25
The CALGB has completed 2 phase 3 trials in older and younger patients with AML in first CR that evaluated the effect of the SC administration of rIL-2 on DFS. CALGB 9720, which was performed in patients aged ≥60 years, demonstrated no benefit for treatment with rIL-2.7 Our results in CALGB 19808 among patients aged <60 years did not reach statistical significance with respect to DFS and OS.
This randomized immunotherapy maintenance trial encountered constraints with respect to patient acceptance. Despite a lack of excessive toxicity, many patients declined to receive their assigned immunotherapy either after randomization or after experiencing modest toxicity. In our previous study of patients aged ≥60 years (CALGB 9720), among 320 patients in CR, 163 (51%) were randomized between rIL-2 and observation,7 a percentage that is similar to that in the current study, taking into account that 11% of patients in CALGB 19808 received allografts at the time of first CR. Consequently, 44% of nonallografted patients in CR were randomized. After randomization, 22 of the 81 older patients (27%) refused to begin or continue rIL-2 therapy, compared with 49 of the 107 younger patients (46%). In a subsequent study, CALGB 10503, patients aged <60 years with AML received similar induction and consolidation therapy as in the current study. Of 349 patients in first CR, 34% received a planned phase 2 decitabine maintenance sequence after completing all postremission therapy.26 In both trials, the investigational therapy was given after a prolonged course of intensive chemotherapy including AHSCT or multiple cycles of HiDAC. We speculate that “treatment fatigue” may be a reason contributing to the refusal of younger patients (15% in the current study and 9% in CALGB 10503) to receive additional outpatient treatment after intensive postremission therapy.26 Withdrawal from therapy in the absence of quantifiable toxicity may be an important obstacle to drug development. Consideration should be given to establishing feasibility measurements alongside toxicity scales as new therapies are evaluated.
NK cells are a central component of the innate immune response. CD56brightCD16dim/negative NK cells expand at the time of interaction between low-dose rIL-2 and its high-affinity receptor. Short courses of rIL-2 at higher doses can activate cytotoxicity against NK cell-resistant tumor targets.20 These effects have been described in vitro using concentrations of rIL-2 readily achieved with SC dosing,27 as well as in patients with immunodeficiencies and malignancies.9,28 Significant expansion of NK cells with cytolytic activity against autologous blasts was observed in a trial of continuous intravenous infusion low-dose rIL-2 after HiDAC consolidation in younger patients with AML in first CR.29
The NK cell population that is expanded by low-dose rIL-2 expresses fewer inhibitory killer immunoglobulin receptors than CD56dim NK cells, whose cytotoxicity is inhibited by the major histocompatibility complex class I ligands present on target cells.30 Conversely, rIL-2 also activates regulatory T cells (Tregs), which can dampen the autologous antitumor immune response.31
The failure to reach statistical significance for DFS, the primary endpoint, is consistent with the results of other studies of IL-2 as reported in the recently published meta-analysis.5 Unfortunately, in the current study, the number of patients randomized was far fewer than anticipated, with a resultant decrease in power. The observed number of events was only 60% of the planned number. Thus, the power of this trial to detect the originally specified HR was only 0.71, rather than the planned 0.90. Because of this, the results reported herein are inconclusive with respect to the original design objective. The observed HR with the originally planned 192 events would have reached a significance level of .05 (95% CI, 0.57–0.99). There can be no way of knowing whether the observed HR would have been maintained over the additional events.
We conclude that immunotherapy for patients with AML in first CR using this dose and schedule of rIL-2 was not well accepted by patients after prolonged and intensive consolidation therapies, including AHSCT. However, the observed results provide important information regarding the use of rIL-2 in this setting. Patient refusal compromised the original trial design despite an acceptable level of toxicity associated with rIL-2 therapy. Significant improvements in outcomes were not observed. We believe that the unanticipated failure to meet the accrual goals to this final part of the overall study with the consequent loss of statistical power impacted on the outcomes of this trial. The goal of modulating the innate immune response against minimal residual disease in patients with AML remains valid in our view. Trial designs have to anticipate the potential problem of therapy-related fatigue by focusing on a patient’s antecedent intensity and length of therapy; the dislocations and inconveniences related to late investigational interventions; and, not least, the need to maintain physician commitment and enthusiasm throughout the process.
Supplementary Material
Acknowledgments
We wish to thank Daniel J. Sargent, PhD, and Sherry Breaux, MPH, for their critical reading of the article and helpful suggestions. We also thank Deedra Nicolet, MS, and Dean Margeson, MAS, for help with the data analysis. Above all, we are grateful to the patients, physicians, nurses, and data managers who participated in this trial.
The following institutions participated in the current study: Christiana Care Health Services Inc Community Clinical Oncology Program, Wilmington, DE (Stephen Grubbs, MD, supported by CA45418); Dana-Farber Cancer Institute, Boston, MA (Harold J. Burstein, MD, PhD, supported by CA32291); Dartmouth Medical School-Norris Cotton Cancer Center, Lebanon, NH (Konstantin Dragnev, MD, supported by CA04326); Duke University Medical Center, Durham, NC (Jeffrey Crawford, MD, supported by CA47577); Georgetown University Medical Center, Washington, DC (Minetta C. Liu, MD, supported by CA77597); Green Mountain Oncology Group Community Clinical Oncology Program, Bennington, VT (Herbert L. Maurer, MD, supported by CA35091); Illinois Oncology Research Association, Peoria, IL (John W. Kugler, MD, supported by CA35113); Monter Cancer Center of North Shore-Long Island Jewish Health Systems, Lake Success, NY (Daniel Budman, MD, supported by CA35279); Massachusetts General Hospital, Boston, MA (Jeffrey W. Clark, MD, supported by CA32291); Mount Sinai School of Medicine, New York, NY (Lewis R. Silverman, MD, supported by CA04457); Nevada Cancer Research Foundation Community Clinical Oncology Program, Las Vegas, NV (John A. Ellerton, MD, supported by CA35421); Rhode Island Hospital, Providence, RI (William Sikov, MD, supported by CA08025); Roswell Park Cancer Institute, Buffalo, NY (Ellis Levine, MD, supported by CA59518); Southeast Cancer Control Consortium Inc Community Clinical Oncology Program, Goldsboro, NC (James N. Atkins, MD, supported by CA45808); State University of New York Upstate Medical University, Syracuse, NY (Stephen L. Graziano, MD, supported by CA21060); The Ohio State University Medical Center, Columbus, OH (Clara D. Bloomfield, MD, supported by CA77658); University of California at San Diego, San Diego, CA (Barbara A. Parker, MD, supported by CA11789); University of California at San Francisco, San Francisco, CA (Charles J. Ryan, MD, supported by CA60138); University of Chicago, Chicago, IL (Hedy L. Kindler, MD, supported by CA41287); University of Illinois Minority-Based Community Clinical Oncology Program, Chicago, IL (David J. Peace, MD, supported by CA74811);University of Iowa, Iowa City, IA (Daniel A. Vaena, MD, supported by CA47642); University of Minnesota, Minneapolis, MN (Bruce A. Peterson, MD, supported by CA16450); University of Massachusetts Medical School, Worcester, MA (William V. Walsh, MD, supported by CA37135); University of Missouri/Ellis Fischel Cancer Center, Columbia, MO (Michael C. Perry, MD, supported by CA12046); University of Nebraska Medical Center, Omaha, NE (Apar Ganti, MD, supported by CA77298); University of Oklahoma, Oklahoma City, OK (Shubham Pant, MD, supported by CA37447); University of North Carolina at Chapel Hill, Chapel Hill, NC (Thomas C. Shea, MD, supported by CA47559); University of Vermont, Burlington, VT (Steven M. Grunberg, MD, supported by CA77406); Wake Forest University School of Medicine, Winston-Salem, NC (David D. Hurd, MD, supported by CA03927); Walter Reed Army Medical Center, Washington, DC (Brendan M. Weiss, MD, supported by CA26806); Washington University School of Medicine, St. Louis, MO (Nancy Bartlett, MD, supported by CA77440); Weill Medical College of Cornell University, New York, NY (John Leonard, MD, supported by CA07968); and Western Pennsylvania Cancer Institute, Pittsburgh, PA (John Lister, MD).
FUNDING SUPPORT
The research for Cancer and Leukemia Group B (CALGB) 19808 (Alliance) was supported in part by grants from the National Cancer Institute (CA31946) to the Alliance for Clinical Trials in Oncology (Monica M. Bertagnolli, MD, Chair) and to the Alliance Statistical Center (Daniel J. Sargent, PhD; CA33601).
Footnotes
The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute.
CONFLICT OF INTEREST DISCLOSURES
Dr. Powell has received consulting fees from Teva Pharmaceuticals for work outside of the current study. Dr. Allen has received speaking honoraria from Celgene Corporation, owns stock in Bristol-Myers Squibb, and has acted as a member of the Advisory Boards of Spectrum and Alloso for work outside of the current study. Drs. Kolitz and Allen were supported by grant CA35279. Dr. George, Ms. Maharry, Dr. Hars, and Ms. Hoke were supported by grant CA33601. Dr. Benson, Ms. Maharry, Dr. Marcucci, Ms. Bakan, Dr. Bloomfield, and Dr. Caligiuri were supported by grants CA77658, CA101140, and CA140158 and the Leukemia Clinical Research Foundation. Dr. Vij was supported by grant CA77440. Dr. Powell was supported by grant CA03927. Dr. DeAngelo was supported by grant CA32291. Dr. Shea was supported by grant CA47559. Drs. Stock and Larson were supported by grant CA41287.
References
- 1.Becknell B, Caligiuri MA. Interleukin-2, interleukin-15, and their roles in human natural killer cells. Adv Immunol. 2005;86:209–239. doi: 10.1016/S0065-2776(04)86006-1. [DOI] [PubMed] [Google Scholar]
- 2.Waldmann TA. The biology of interleukin-2 and interleukin-15: implications for cancer therapy and vaccine design. Nat Rev Immunol. 2006;6:595–601. doi: 10.1038/nri1901. [DOI] [PubMed] [Google Scholar]
- 3.Meloni G, Foa R, Vignetti M, et al. Interleukin-2 may induce prolonged remissions in advanced acute myelogenous leukemia. Blood. 1994;84:2158–2163. [PubMed] [Google Scholar]
- 4.Maraninchi D, Blaise D, Viens P, et al. High-dose recombinant interleukin-2 and acute myeloid leukemias in relapse. Blood. 1991;78:2182–2187. [PubMed] [Google Scholar]
- 5.Buyse M, Squifflet P, Lange BJ, et al. Individual patient data meta-analysis of randomized trials evaluating IL-2 monotherapy as remission maintenance therapy in acute myeloid leukemia. Blood. 2011;117:7007–7013. doi: 10.1182/blood-2011-02-337725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Farag SS, Archer KJ, Mrozek K, et al. Isolated trisomy of chromosomes 8, 11, 13 and 21 is an adverse prognostic factor in adults with de novo acute myeloid leukemia: results from Cancer and Leukemia Group B 8461. Int J Oncol. 2002;21:1041–1051. [PubMed] [Google Scholar]
- 7.Baer MR, George SL, Caligiuri MA, et al. Low-dose interleukin-2 immunotherapy does not improve outcome of patients age 60 years and older with acute myeloid leukemia in first complete remission: Cancer and Leukemia Group B Study 9720. J Clin Oncol. 2008;26:4934–4939. doi: 10.1200/JCO.2008.17.0472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kolitz J, George S, Dodge R, et al. Cancer and Leukemia Group B. Dose escalation studies of cytarabine, daunorubicin, and etoposide with and without multidrug resistance modulation with PSC-833 in untreated adults with acute myeloid leukemia younger than 60 years: final induction results of Cancer and Leukemia Group B Study 9621. J Clin Oncol. 2004;22:4290–4301. doi: 10.1200/JCO.2004.11.106. [DOI] [PubMed] [Google Scholar]
- 9.Meropol NJ, Barresi GM, Fehniger TA, Hitt J, Franklin M, Caligiuri MA. Evaluation of natural killer cell expansion and activation in vivo with daily subcutaneous low-dose interleukin-2 plus periodic intermediate-dose pulsing. Cancer Immunol Immunother. 1998;46:318–326. doi: 10.1007/s002620050493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cortes JE, Kantarjian HM, O’Brien S, et al. A pilot study of interleukin-2 for adult patients with acute myelogenous leukemia in first complete remission. Cancer. 1999;85:1506–1513. [PubMed] [Google Scholar]
- 11.Blaise D, Attal M, Reiffers J, et al. Randomized study of recombinant interleukin-2 after autologous bone marrow transplantation for acute leukemia in first complete remission. Eur Cytokine Netw. 2000;11:91–98. [PubMed] [Google Scholar]
- 12.Kolitz JE, George SL, Marcucci G, et al. Cancer and Leukemia Group B. P-glycoprotein inhibition using valspodar (PSC-833) does not improve outcomes for patients younger than age 60 years with newly diagnosed acute myeloid leukemia: Cancer and Leukemia Group B study 19808. Blood. 2010;116:1413–1421. doi: 10.1182/blood-2009-07-229492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moore JO, George SL, Dodge RK, et al. Sequential multiagent chemotherapy is not superior to high-dose cytarabine alone as post-remission intensification therapy for acute myeloid leukemia in adults under 60 years of age: Cancer and Leukemia Group B Study 9222. Blood. 2005;105:3420–3427. doi: 10.1182/blood-2004-08-2977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Linker CA, Ries CA, Damon LE, et al. Autologous stem cell transplantation for acute myeloid leukemia in first remission. Biol Blood Marrow Transplant. 2000;6:50–57. doi: 10.1016/s1083-8791(00)70052-8. [DOI] [PubMed] [Google Scholar]
- 15.Linker CA, Owzar K, Powell B, et al. Cancer and Leukemia Group B. Auto-SCT for AML in second remission: CALGB study 9620. Bone Marrow Transplant. 2009;44:353–359. doi: 10.1038/bmt.2009.36. [DOI] [PubMed] [Google Scholar]
- 16.Kolitz JE, George SL, Barrier R, et al. A novel post-remission consolidation regimen for patients with acute myeloid leukemia (AML) <60 years old with normal or unfavorable cytogenetics: results from CALGB 9621. Blood (Abstract) 2003;102:175a. [Google Scholar]
- 17.Cheson BD, Cassileth PA, Head DR, et al. Report of the National Cancer Institute-sponsored workshop on definitions of diagnosis and response in acute myeloid leukemia. J Clin Oncol. 1990;8:813–819. doi: 10.1200/JCO.1990.8.5.813. [DOI] [PubMed] [Google Scholar]
- 18.Bland JM, Altman DG. The logrank test. BMJ. 2004;328:1073. doi: 10.1136/bmj.328.7447.1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Breslow NE. Analysis of survival data under the proportional hazards model. Int Stat Rev. 1975;43:45–57. [Google Scholar]
- 20.Freidlin B, Korn EL, George SL. Data monitoring committees and interim monitoring guidelines. Control Clin Trials. 1999;20:395–407. doi: 10.1016/s0197-2456(99)00017-3. [DOI] [PubMed] [Google Scholar]
- 21.Anderson JR, Cain KC, Gelber RD. Analysis of survival by tumor response. J Clin Oncol. 1983;1:710–719. doi: 10.1200/JCO.1983.1.11.710. [DOI] [PubMed] [Google Scholar]
- 22.Kaplan EL, Meier P. Nonparametric estimation for incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 23.Lewis S, Clarke M. Forest plots: trying to see the wood and the trees. BMJ. 2001;322:1479–1480. doi: 10.1136/bmj.322.7300.1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dohner H, Estey EH, Amadori S, et al. European LeukemiaNet. Diagnosis and management of acute myeloid leukemia in adults: recommendations from an international expert panel, on behalf of the European LeukemiaNet. Blood. 2010;115:453–474. doi: 10.1182/blood-2009-07-235358. [DOI] [PubMed] [Google Scholar]
- 25.Brune M, Castaigne S, Catalano J, et al. Improved leukemia-free survival after postconsolidation immunotherapy with histamine dihydrochloride and interleukin-2 in acute myeloid leukemia: results of a randomized phase 3 trial. Blood. 2006;108:88–96. doi: 10.1182/blood-2005-10-4073. [DOI] [PubMed] [Google Scholar]
- 26.Blum W, Donohue K, Marcucci G, et al. Challenges for Conducting Clinical Trials Evaluating Maintenance Chemotherapy In Acute Myeloid Leukemia (AML): a Cancer and Leukemia Group B (CALGB) Study [abstract] ASH Annual Meeting Abstracts. 2010;116:2176. [Google Scholar]
- 27.Caligiuri MA, Murray C, Robertson MJ, et al. Selective modulation of human natural killer cells in vivo after prolonged infusion of low dose recombinant interleukin-2. J Clin Invest. 1993;91:123–132. doi: 10.1172/JCI116161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Khatri VP, Baiocchi RA, Bernstein ZP, Caligiuri MA. Immunotherapy with low-dose interleukin-2: rationale for prevention of immune-deficiency-associated cancer. Cancer J Sci Am. 1997;3(suppl 1):S129–S136. [PubMed] [Google Scholar]
- 29.Stone RM, DeAngelo DJ, Janosova A, et al. Low dose interleukin-2 following intensification therapy with high dose cytarabine for acute myelogenous leukemia in first complete remission. Am J Hematol. 2008;83:771–777. doi: 10.1002/ajh.21253. [DOI] [PubMed] [Google Scholar]
- 30.Cooper MA, Fehniger TA, Caligiuri MA. The biology of human natural killer-cell subsets. Trends Immunol. 2001;22:633–640. doi: 10.1016/s1471-4906(01)02060-9. [DOI] [PubMed] [Google Scholar]
- 31.Hallett WH, Ames E, Alvarez M, et al. Combination therapy using IL-2 and anti-CD25 results in augmented natural killer cell-mediated antitumor responses. Biol Blood Marrow Transplant. 2008;14:1088–1099. doi: 10.1016/j.bbmt.2008.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.