Abstract
We have developed nanoparticles based on Murine Leukemia Virus virus-like-particles (VLPs) that efficiently deliver therapeutic bioactive proteins in their native state into target cells. Nuclear transcription factors and toxic proteins were incorporated into the VLPs from stable producer cells without transducing viral-encoded genetic material. Delivery of nuclear transcription factors required incorporation of nuclear export signals (NESs) into the vector backbone for the efficient formation of VLPs. In the presence of an appropriate targeting Env glycoprotein, transcription factors delivered and activated nuclear transcription in the target cells. Additionally, we show delivery of the bacterial toxin, MazF, which is an ACA-specific mRNA interferase resulted in the induction of cell death. The stable producer cells are protected from the toxin through co-expression of the anti-toxin MazE and continuously released MazF incorporating VLPs. This highly adaptable platform can be harnessed to alter and regulate cellular processes by bioactive protein delivery.
Keywords: Protein transduction, Viral-like particles, Intracellular delivery, Transcriptional factors, Bacterial toxin
1. Introduction
The transfer of therapeutic proteins to patients has great potential for treatment of diseases including genetic disorders and cancer. Protein delivery into target cells for protein therapeutics faces several significant limitations including the targeting and stability of the proteins in vivo. The limiting ability of proteins to cross the cellular membrane and escape the endosomal–lysosomal pathways are major barriers for intracellular delivery of macromolecules [1,2]. Improving the safety and efficacy of pharmaceutical protein production and intracellular delivery remains a big challenge.
Retroviral-like particles (VLPs) present an approach to avoid sequestration or degradation of target proteins within the endosomal–lysosomal pathway. The process of retroviral entry into a target cell is tightly regulated by the interactions between the retroviral envelope glycoprotein and a specific host cell receptor. This provides the potential to modify the envelope glycoprotein protein for targeted delivery of VLPs to specific tissues and cell types. Approximately 2000 copies of the Gag can be delivered into a transduced cell from each VLP [3]. Transient retroviral protein transduction (PT) systems have successfully delivered foreign functional proteins including Flp recombinase, cytosine deaminase, uracil phosphorybosyltransferase, and caspase to cells [4,5].
In this study, we develop a protein delivery system based on Murine Leukemia Virus (MLV) VLPs capable of continuous largescale production and delivery of bioactive proteins. Unlike viral vectors, these particles do not package viral RNA. Cellular expression of the Gag protein generates replication-defective VLPs in the presence of targeting Env glycoproteins without the delivery of a viral genome [6]. Two classes of proteins were tested for incorporation into VLPs: nuclear transcription factors (TFs) and toxic proteins. In our system, the PT-VLPs are derived from a stable engineered mammalian producer cell line optimized for the release of high-titer VLPs, and tested for altered cellular behavior in the recipient cells.
2. Materials and methods
2.1. Plasmid construction
Construction of protein transduction vector (pPT) was base on pNCA-C (IN− D184N/K376A) proviral vector as backbone [7,8]. A new protease site (the nucleotide in bold, encoding RSSLY/PALTP indicates protease cleavage site) and three restriction sites: MluI, SwaI, and NotI (the nucleotides in italics) were incorporated into the C-terminus of matrix (MA) with overlapping PCR and the EcoRI/XhoI restriction sites (5’ CCGCCTCGGTCTTCACTGTACCCCGCACTGACCCCCACGCGTGGGCATTTAAATTGGGCGCGGCCGCTCCGATCC). The sequences encoding the foreign proteins were amplified by PCR and subcloned at the SwaI/NotI sites or Mlu/NotI sites to generate pPT-Protein constructs. The MazF or MazF(E24A) sequences were amplified from pACyC-MazF or pBAD33-MazF(E24A) (gifts from Dr. Masayori Inouye, Rutgers-RWJMS). The mouse TFs sequences were amplified from pMXs-TF purchased from Addgene. All primers are summarized in Table S1.
For the pL-GPTP (Gag-PT-Pol) vector construction, the 6-kb EcoRI-NdeI fragment from the pPT plasmid was subcloned into pSin-EF2-LIN28-Pur (Addgene 16580) to replace the Lin28 gene. The Gag N-terminal and IN C-terminal sequences were amplified from pPT-Oct4 plasmid using primers EcoRI 1010 fwd + XhoI 2118 rev and NdeI 5851 fwd + NsiI 6286 rev. The PCR products were subcloned in pL-GPTP by 0.5 kb EcoRI-MluI fragment exchange to replace the 5’ LTR sequence and 0.5 kb NdeI-NsiI fragment exchanged to complete the IN sequences. Finally, the pPT-protein plasmid was subcloned into pL-GPTP by MluI/PshAI fragment exchange to generate the individual pL-GPTP plasmids. For pL-GPT2NP (Gag-PT-2NES-Pol) plasmids, the NES nucleotide sequence (encoding (NINELALKFAGLDL) was inserted into the p12 N-terminus and NC C-terminus in pL-GPTP vectors by overlapping PCR with EcoRI/PshAI and PshAI/XcmI restriction sites.
The puromycin gene within pSin-EF2-LIN28-Pur vector was replaced by KpnI fragment exchange with a Zeocin resistance gene using overlapping PCR. The env EA6-3X sequence was amplified from pHIT-EA6-3X plasmid [9], fragment exchanged using EcoRI/NdeI with Lin28 in pSin-EF2-LIN28-Zeo, generating pL-Env. EA6-3X chimeric Env, encodes the ecotropic M-MLV receptor binding domain bearing the N261I/E311V/G552R mutations and the amphotropic TM [9].
For pL-MazE-GFP construction, the IRES-Puro sequence was removed from the pSin-EF1-GFP-IRES-Puro backbone [10] by KpnI digestion followed by self-ligation. The MazE or truncated MazE sequences (MazE42-GFP and MazE61E–GFP) [11] were amplified from pCold-MazE (a gift from Dr. Masayori Inouye, Rutgers-RWJMS) by PCR and subcloned into pSin-EF1α-GFP-ΔKpnI at the SpeI restriction sites.
Inducible GFP reporter constructs with specific TRE elements were generated by modifying pSin-EF1α-GFP-IRES-Puro. TATA-specific TRE sequences were amplified from DNA within Qiagen Reporter Arrays (CCA-106L-2) with AgeI/SpeI restriction sites, replacing the EF-1α promoter sequences, generating pL-TFTRE-GFP.
2.2. Cell culture
All of the cell lines were cultured as previously described [10]. The 293TCeB cells were maintained in DMEM containing 10 µg/mL Blasticidin S (Invivogen). The chimeric Gag VLPs producer cell lines in 293TCeB were maintained in DMEM containing 2.5 µg/mL puromycin, 400 µg/mL Zeocin (Invivogen), and 10 µg/mL Blasticidin S. HEK293T cell was purchased from American Type Culture Collection and the mouse embryonic fibroblast cell line (SNL) was ordered from Cell Biolabs, Inc. (CBA-316). HeLa MCAT and HEK293T MCAT cell line were created as previously described [12] and maintained in DMEM containing 10 µg/mL Blasticidin S.
2.3. Lentiviral production and generation of VLP producer cell lines
All lentiviral particles were produced as previously described [10]. Three days post-infection, puromycin and Zeocin selections were performed to obtain the stable VLP producer cell lines.
For generation of lentiviral particles that contained Gag-MazF-2NES-Pol sequences, HEK293T was first infected by pL-MazE-GFP lentiviral particles. HEK293T–MazE-GFP cells were used to transfect the pL-G-MazF-2NP, pCMV-ΔR8.2 Δvpr, and pHIT-G to generate Gag-MazF-2NES containing lentiviral particles.
2.4. Cell viability
The 3-(4,5-Dimethyl-2-thiazolyl)-2,5-diphenyl-2H–tetrazolium bromide (MTT) (Sigma, M5655) assay was used to measure cell viability. Initially, 500 cells were plated into each well of a 96-well tissue culture plate and treated with 0–30 µg/mL of CA containing VLPs or 0–500 nM of methotrexate (Sigma, A6770) for one week. 100 µL medium contain 0.5 mg/mL MTT was added to each well and incubated at 37° C. After 4–6 h incubation, the medium was discarded and the 100 µL acidic isopropanol containing 0.04 N HCl and 0.1% NP40 was added to dissolve the crystals for 10 min at room temperature. The optical density was read at OD570 nm immediately.
2.5. Immunofluorescence & confocal microscopy
In brief, cells seeded on poly-L-lysine coated glass coverslips were fixed and permeabilized with −20°C methanol, blocked by 5% BSA, and stained as previous described [13]. For consecutive antibody studies, the donkey anti-goat antibody was used prior to either the goat anti-mouse or rabbit antibodies. HEK293T & 293TCeB cells expressing the chimeric Gag protein were imaged on a Zeiss LSM510 META confocal microscope with a 63 × water immersion objective at the Robert Wood Johnson Medical School Confocal and Electronic Imaging Center.
2.6. Western blot and antibodies
VLPs of Gag-TF chimeras were harvested from producer cell supernatants and concentrated by centrifugation at 15,000 × g for 30 min. All antibodies used for the Western blots and immunofluorescence staining are listed in Table S2.
2.7. TF Activity assays
For TF activity assays, the specific pL-TFTRE-GFP lentiviral vector was produced as described [10] and introduced into SNL cell to establish the stable sensor cell line in the absence of drug selection. The bioactivity of PT was determined by measuring the percentages of GFP-positive cells by flow cytometry [10] at day 8 post Gag-TF-2NES VLPs treatment.
2.8. Quantitative PCR assays
cDNA templates from viral RNA or cellular mRNA were synthesized as previous described [8,10]. For genomic DNA purification, infected cells were collected at 10 days post-infection, genomic DNA was purified using DNeasy Blood & Tissue Kit (Qiagen) and used as template for PCR.
qPCR reactions were carried out using the Power SYBR Green PCR Master Mix (4367659, Applied Biosystems) on a Mastercycler ep realplex real-time PCR system (Eppendorf). qPCR reactions were performed in a total volume of 20 µl with 2 µl of template cDNA, plasmid DNA (for standard curve), or genomic DNA (~300 ng) with 250 nM of each primer. qPCR reactions were performed under the following conditions: 1 cycle at 95°C for 10 min, followed by 95°C for 30 s, 55°C for 30 s, and 72°C for 30 s for 40 cycles. The primers and probe used for quantification are shown on Table S1.
3. Results
3.1. Generation of non-infectious VLPs from stable producer cells
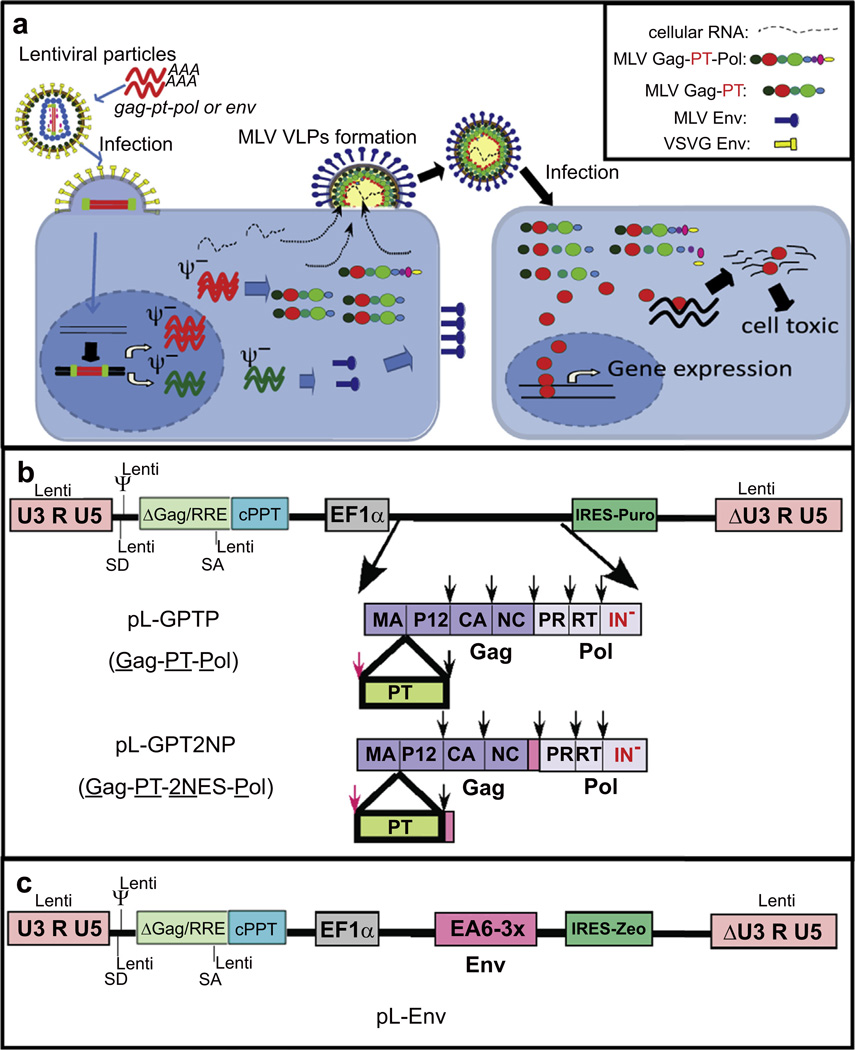
Gammaretroviruses are nanoparticles capable of specifically delivering sequestered proteins into target cells [4,5]. Fig. 1 outlines the approach to modify VLPs to assemble target proteins of interest, specifically nuclear transcription factors and toxic proteins. Viral particles (VPs) are assembled on the plasma membrane through interaction of four viral components, the precursor Gag and Gag-Pol, Env plus RNA (Fig. 1a). In this scheme, the proteins of interest are incorporated into the Gag precursor protein. VLP release is independent of an Env protein or specific viral RNAs [14–17] and particles are thus non-infectious but require an optimal ratio of Gag to Gag-Pol precursor proteins. Upon particle formation, the viral protease cleaves the precursor proteins, releasing the individual viral proteins and the proteins of interest, which are then delivered into the target cells and can traffic independently.
Fig. 1.
Generation of bioactive protein containing MLV VLPs from stable packaging cells. (a) A schematic representation of the VLP packaging cell, assembly of VLPs, and the intracellular delivery of bioactive proteins. Co-infection of HEK293T cells with VSV-G pseudotyped lentiviral particles packaging the EF1-α-gag-pt-pol or EF1-aenv cassette results in stable expression of PT and Env, followed by the formation and subsequent release of VLPs. Subsequent infection of the PT VLPs releases the proteolytically cleaved PT protein (red circle). PT proteins that are TF can enter the nucleus and drive gene expression. PT proteins that are toxins can drive apoptosis. (b) A schematic representation of lentiviral based vectors expressing MLV Gag-PT-Pol protein. PT was incorporated within the MLV Gag-Pol between MA and p12. An additional protease site (RSSLY/PALTP; red arrow) was inserted at the junction of MA and the PT protein, allowing for complete excision from the precursor protein by the viral PR. A second vector (pL-GPT2NP, encodes the NES inserted as an N-terminal fusion of p12 and C-terminal fusion of NC (NINELALKFAGLDL; pink box). IN− encoded the IN D184N/K376A mutation. (c) Schematic of lentiviral based vectors expressing Env protein EA6-3X [9]. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Fig. 1b outlines the vectors developed to establish high efficiency producer cells for continuous production of non-infectious protein transduction particles. The protein transduction system differs from VPs in that the VLPs are used for delivering protein rather than a gene. Therefore, incorporation of the viral RNA into the particles or inclusion of functions required for the establishment of an integrated provirus is not required, and are in fact, disadvantageous [18,19]. Proteins of interest were inserted within the gag gene between the MA and p12 proteins, bracketed by MLV PR cleavages sites (MLV Gag-PT-Pol) [4]. The MLV gag-pol bearing mutations in the integrase (IN) (D184N/K376A) was expressed from an internal elongation factor 1α (EF1α) promoter within the lentiviral vector (pL-GPTP). The MLV genome contained deletion in the sequences of 5’ and 3’ LTR, as well as the packaging site and MLV splice donor site. Thus, even if RNA from this construct is packaged it cannot be reverse-transcribed, and sites required for integration of the virus are absent. An env is expressed from an independent lentiviral vector (pL-Env) (Fig. 1c). Transduction of this PT sequence and env using a lentiviral packaging system into HEK293T cells and selection for puromycin and Zeocin resistance allows for the identification of producer cells [18–20]. For PT studies, a modified ecotropic MLV Env (EA6-3X) was utilized because of its properties of high titer with decreased syncytia formation in mouse cells or human cells expressing the MCAT receptor [9].
3.2. Incorporation of nuclear TFs within MLV based VLPs
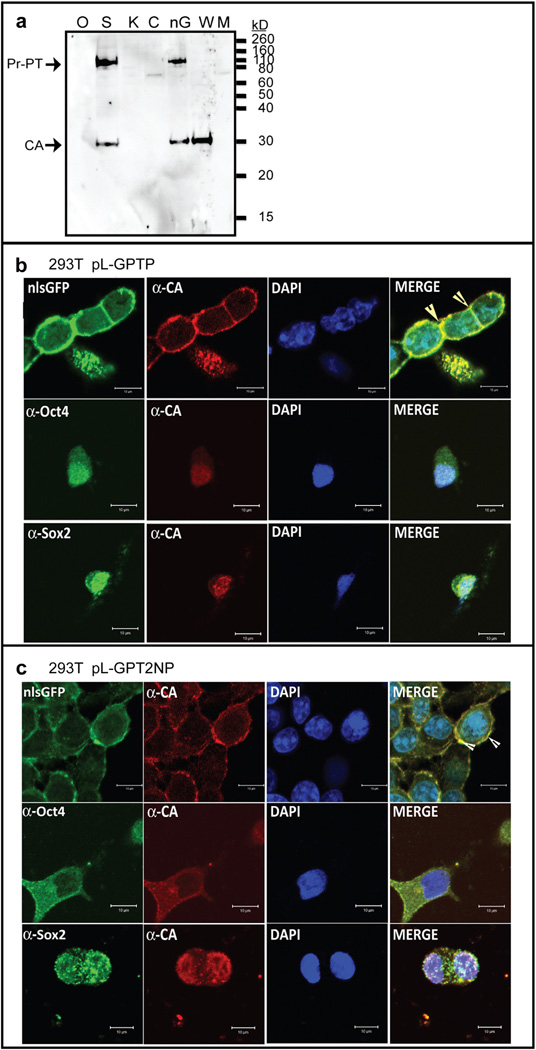
Initial Gag constructs (Fig. 1b) aimed at studying the delivery of nuclear transcription factors (Gag-TF), including mouse Oct4 (Gag-Oct4), Sox2 (Gag-Sox2), c-Myc (Gag-c-Myc), and Klf4 (Gag-Klf4). As a control, delivery of GFP bearing an NLS sequence (nlsGFP) was also monitored. VLP release was monitored by immunoblotting, detecting viral capsid (CA) protein in concentrated supernatants. Although the Gag-nlsGFP and Gag-Sox2 driven by EF1α promoter allowed for high production of VLPs from the HEK293T stable producer cell line, CA was not detectable in the concentrated supernatants from Gag-Oct4, Gag-Klf4, and Gag-cMyc packaging cell lines (Fig. 2a). Similar results were observed when the Gag-TF was directly expressed in an MLV based vector (Fig. S1a), with processed CA detected in nlsGFP > Sox2 > Oct4 > Klf4 and c-Myc (Fig. S1b) and the processed Oct4, Sox2, and GFP proteins detected in VLPs using their cognate antibodies (Fig. S1c).
Fig. 2.
Generation of VLPs containing transcriptional factors from HEK293T packaging cells. (a) Quantification of VLPs expression by Western blot analysis of CA. Concentrated VLPs were purified from pL-GPTP expressing cell culture (1 mL). CA was detected with a polyclonal anti-CA antibody (81S-263) and served as a loading control. Sizes for Pr-PT, and CA are indicated. O: Oct4, S: Sox2, K: Klf4, C: c-Myc, and nG: nlsGFP. M: culture supernatant from mock 293T cell. WT: wild type MLV virion. (b&c) Distribution of Gag-PT or Gag-PT-2NES in the packaging cell was analyzed by immunofluorescence. Representative images of HEK293T cells infected with lentivirus packaging pL-GPTP (panel b) or pL-GPT2NP (panel c) after selection in puromycin. Stable packaging cells were analyzed by GFP signal or immunofluorescence staining using anti-CA (Red), anti-Oct4 (Green), or anti-Sox2 (Green) antibody as described under “Materials and Methods.” Nuclei were stained with DAPI (Blue). Arrows indicate Gag chimeric protein accumulate and assemble on the inner surface of the plasma membrane. Cells were visualized with a Zeiss LSM510 META confocal microscope and bars represent 10 mm. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
The viral life-cycle is a careful balance between the late functions, where the precursor proteins need to be transported to the plasma membrane for assembly, and the early functions, where a subset of these viral proteins need to function in the nucleus upon infection. To further investigate the possibility that the properties or localization of the Gag-TF fusion proteins might be altered in the stable HEK293T production cell line, we examined producer cells by immunostaining and confocal microscopy. A variety of nuclear localization signals (NLSs) have been identified that function in the transport of nuclear proteins, however these are absent in the MLV Gag proteins [21]. Wildtype MLV precursor Gag and Gag-Pol therefore assemble at the plasma membrane. Surprisingly, the Gag-nlsGFP localized to the plasma membrane of HEK293T producer cells similar to WT MLV particles and released VLPs (Fig. 2a), despite encoding the classical SV40 t-antigen NLS (Fig. 2b).
In contrast, the precursor Gag-TF fusion proteins localized to the nucleus as a consequence of the NLS motifs within the TFs (Fig. 2b and Fig. S2a). Experiments monitored the co-localization of CA and the individual TF, to validate the localization of the precursor protein, along with DAPI staining of the DNA. Results varied with the properties of the individual TFs. Interestingly, both Gag-Sox2 and Gag-Oct4 accumulated in nucleus in the confocal image (Fig. 2b), although Gag-Sox2 showed the highest levels of VLPs released compared with the other Gag-TF fusion proteins in HEK293T packaging cell line (Fig. 2a). Previous studies indicated that Sox2, unlike other TF studied, localizes to both the nucleus and cytoplasm in pre-implantation embryos [13], suggesting that Sox2 can shuttle between these two subcellular compartments [22]. Since the imaging used to analyze chimeric Gag-TF localization is qualitative, it is possible that the small percent of Gag-Sox2 fusion protein that localized to the cytoplasm is sufficient to assemble VLP on the plasma membrane. These results indicate that nuclear import of chimeric MLV Gag-TF protein impairs plasma membrane localization and the subsequent release of VLPs.
3.3. Optimization of release of chimeric MLV VLPs
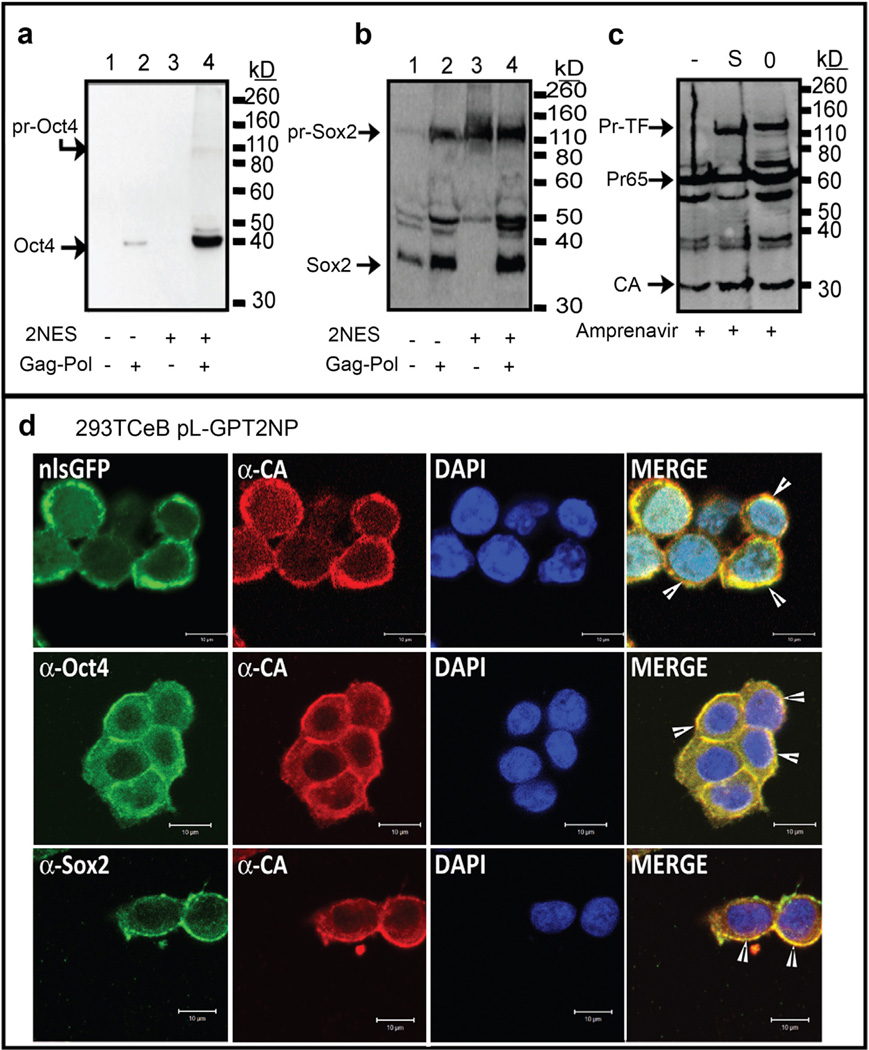
The retroviral Gag protein plays a dominant role in the assembly of new VPs at the cell membrane and is the core component of the VLPs. In MLV infected cells, it has been proposed that approximately 18% of the Gag precursor is present in the nucleus [23], but the mechanism of nuclear import and export for MLV Gag is still unclear. To address the relocalization of the precursor Gag-TF proteins to the nucleus (Fig. 2a), we tested whether a MLV Gag-TF chimera could be restored to localize to the cytoplasm and generate VLPs through the incorporation of optimized cyclic-AMP-dependent-kinase-inhibitor (PKI)-type NESs [24,25]. NES sequences were inserted within the p12 and NC genes at positions tolerant of insertions [4] (Fig. 1b). The localization of chimeric Gag-TF-2NES proteins was analyzed by immunostaining and confocal microscopy. Remarkably, insertion of the two NES sequences resulted in the predominant localization of the four Gag-TF proteins to the cytoplasm (Fig. 2c and Fig. S2b). Although the presence of functional NESs was able to export the Gag-TF protein from the nucleus to the cytoplasm (Fig. 2c and Fig. S2b), restoration of cytoplasm localization with the NES failed to rescue release of VLPs. Fig. 3ab examines the presence of Oct4 and Sox2 within VLPs using antibodies specific to the respective TF. For Gag-Oct4, no Oct4 protein was incorporated into VLPs in the absence or presence of the 2 NESs within Gag (Fig 3a, lanes 1 vs 3). For Gag-Sox2, insertion of the 2 NESs inhibited proteolytic processing of the precursor protein, with the detection of a majority of the Gag proteins in the virions as the precursor protein (Fig. 3b, lanes 1 vs. 3). Thus, insertion of the NES within Gag resulted in the export of MLV Gag-TF to the cytoplasm but did not result in the assembly and release of VLPs from the plasma membrane.
Fig. 3.
Co-expression of WT Gag-Pol enhances VLPs release from the Gag-PT-2NES-Pol packaging cell. (a–c) Western blot of VLPs using anti-Oct4 (a), anti-Sox2 (b), or anti-CA (c) antibodies. Presence of NES, WT Gag-Pol, and Amprenavir is as indicated. Position of migration of the WT Pr65Gag and those precursor encoding the transduced proteins are indicated with arrows. (c) EA6-3X pseudotyped VLPs were harvested from Amprenavir (10 µM) treated 293TCeB Gag-Oct4-2NES-Pol (O) and Gag-Sox2-2NES-Pol (S) packaging cells. Mock VLPs was collected from 293TCeB cells. Western blot using a polyclonal anti-CA antibody to reveal the stoichiometry of Gag-TF to WT Gag in VLPs is shown. Position of migration of the Gag-TF-2NES precursor (Pr-TF), Gag precursor (Pr65), and CA are indicated. (d) Immunofluorescence of 293TCeB packaging cells infected with pL-GPT2NP pseudotyped lentivirus. Green represents the GFP signal or anti-Oct4, anti-Sox2 monoclonal antibody-stained signal. Red and blue represent the anti-CA antibody signal and the DAPI stained nucleus, respectively. Scale bars represent 10 µm. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
To facilitate chimeric MLV VLPs release from the plasma membrane, we next generated Gag-TF or Gag-TF-2NES producing cells in 293TCeB cells [20], which stably express MLV gag and pol genes from the CeB plasmid in the absence of a packaging signal (Ψ−). The presence of WT Gag-Pol increased both the yield and the level of processing of the Gag-TF within the VLPs (Fig. 3ab). For Gag-Sox2-2NES, similar to Gag-Oct4-2NES, fully processed TF is now readily detected within the VLPs (Fig. 3ab, lane 4). To determine the ratio of the WT Gag to the Gag-TF, cells were treated were the protease inhibitor Amprenavir and the viral proteins released were examined (Fig. 3c and Fig. S2d). Comparison of the WT Gag to those encoding the TF indicated roughly equal levels of Gag-TF to WT Gag (Fig. 3c and Fig. S2d).
Confocal microscopy of the 293TCeB cells expressing the Gag-TF-2NES constructs confirms the co-localization of the protein transduction proteins at the plasma membrane (Fig. 3d and Fig. S2c). For both Gag-Oct4 and Gag-Sox2 2NES constructs, the proteins now accumulated on the cell boundaries (Fig. 3d), rather than the general cytoplasmic localization observed in Fig. 2. These results support a model that incorporation of NES sequences into MLV Gag-TF-2NES chimeras facilitates cytoplasmic retention of the Gag-TF polyprotein. Co-expression of wild type Gag-Pol facilitates chimeric Gag to assemble at the plasma membrane and results in the efficient release of VLPs from the producer cell (Fig. 3ab).
3.4. Kinetics of protein delivery and transcriptional activity of transduced TFs
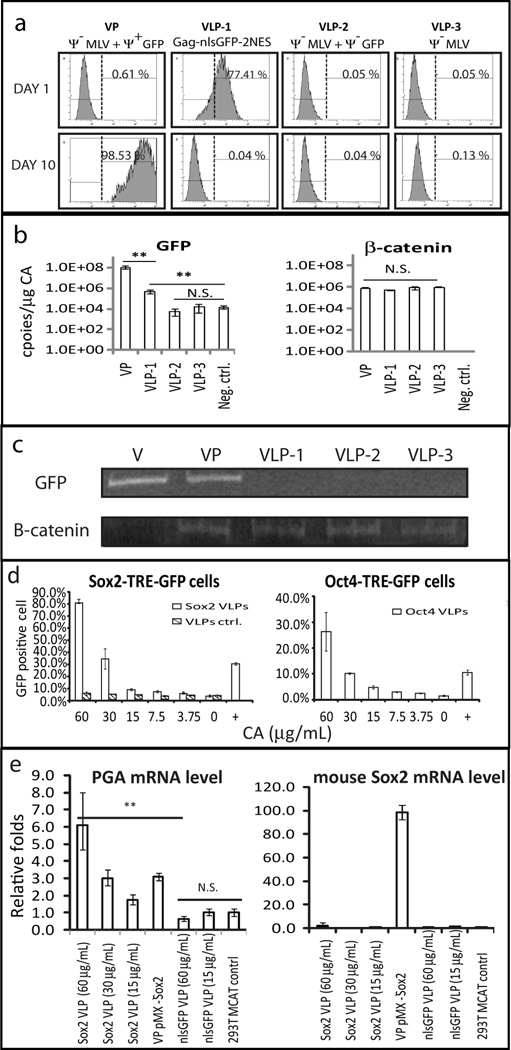
Experiments were performed to establish the transfer of the PT proteins into the recipient cells. VLPs were pseudotyped with the EA6-3X chimeric Env (N261I/E311V/G552R) (Fig. 1c) [9]. The EA6-3X chimeric Env gene was expressed in the 293TCeB Gag-PT-2NES-Pol producer cell from the lentiviral vector pL-Env (Fig. 1c). SNL cells were treated with Gag-nlsGFP-2NES VLPs and analyzed by flow cytometry to detect GFP protein levels in the protein-transduced cell. Parallel studies quantified the nucleic acids in the particles by quantitative PCR (qPCR) assays. The amount of capsid protein in the VPs or VLPs was determined by ELISA [26] and used to normalize the amount of particles in all samples. Fig. 4a shows that Gag-nlsGFP-2NES VLPs from the producer cell efficiently mediated GFP delivery into mouse cells and accumulated to steadystate levels within 24 h after protein transduction, followed by a steady decline until 100 h with return to baseline autofluorescence. On day 10 post-PT, the GFP expression was only detectable in the cells infected with integrating retroviral particles (Fig. 4a Ψ−MLV + Ψ+GFP) but not in the VLPs treated cells. GFP expressed in the producer cells independent of the VLP did not get incorporated into VLPs and was not transferred into the recipient cells (Fig. 4a, Ψ−MLV + Ψ−GFP).
Fig. 4.
Delivery of bioactive proteins to target cells is mediated by non-infectious VLPs. EA6-3X Env pseudotyped VLPs collected from 293TCeB Gag-nlsGFP-2NES-Pol (VLP-1; Gag-nlsGFP-2NES), 293TCeB GFP (VLP-2; Ψ− MLV + Ψ−GFP), and 293TCeB (VLP-3;Ψ− MLV) cells were used to infect SNL cells. Infectious VP collected from 293TCeB pMXs-GFP:EA6-3X (VP; Ψ−MLV + Ψ+GFP) served as a positive control. (a) Flow cytometry of GFP+ cells on Day 1 and Day 10 post-transduction VPs or VLPs (b) qPCR of VLPs or VP packaging nucleic acids. Copy number of individual RNA species in VP or VLPs was divided by the levels of CA(µg)in the preparations. Negative control: primer alone. (c) PCR analysis of genomic DNA to measure integrated GFP provirus. Genomic DNA was harvested from 10 days post transduction VP or VLP cell and used as template for PCR reactions. Endogenous β-catenin was used as an internal control. Vector (V): 1 ng pMXs-GFP plasmid served as positive control. PCR products were analyzed on a 10% PAG in TBE. (d) Activation of GFP expression following the treatment of TF containing VLPs in individual reporter cell line containing a stably integrated expression cassette (TFTRE-GFP). Cells were treated with the indicated levels of TF-VLPs every two days, harvested and the GFP+ cells was measured by flow cytometry analysis on day 8. Infectious pMXs-TF VPs served as a positive control (+). Wild type Gag-Pol serves as a negative control (VLPs ctrl). (e) qPCR of mouse sox2 gene and endogenous pepsinogen A gene (PGA) expression level in HEK293T MCAT cells treated with Sox2-VLPs & nlsGFP-VLPs. The cellular mRNA were harvested from 293T MCAT cells 8 days post-PT. The pepsinogen C gene (PGC) was used as an internal control. Results are mean with standard error, N = 3. Statistical significance was determined by one-way ANOVA (**: p < 0.01, n.s., not significant).
We next examined the RNA content of the chimeric VLPs. RNAs from the GFP gene and the cellular control gene β-catenin were measured by qPCR. Constructs bearing a packaging sequence were highly abundant within the VPs with over 108 copies/µg CA. Both the ψ-MLV viral RNA and cellular mRNA were non-selectively packaged into VLPs, (Fig. 4b) at levels >100 fold lower than mRNA bearing packaging signals. Incorporation of ψ-GFP mRNA that included MLV gag-pol (gag-gfp-2NES-pol) was higher than that from a construct expressing only GFP mRNA (ψ-gfp) (VLP-1 vs VLP-2). This is consistent with previous reports [27] that MLV Gag protein preferentially engaged ψ-MLV genome RNA rather than cellular mRNA in ψ-VLPs. This low level of incorporating ψ-RNA into VLPs did not result in transgene delivery into the host DNA. PCR analysis of the genomic DNA did not indicate the presence of integrated GFP DNA sequences (Fig. 4c).
The transcriptional activation of the PT delivered TFs were studied using reporter constructs under the control of promoters specific for each transcription factor. Promoters consisted of tandem repeats of the specific TF transcriptional response element (TF-TRE) upstream of a minimal promoter driving a reporter GFP gene. Reporter constructs were stably integrated into SNL cells by the pL-TF-TRE-GFP lentivirus. Cells were monitored over a period of one week after exposure to concentrated ecotropic pseudotyped VLPs. The cells were exposed to Gag-PT-2NES VLPs every two days and the GFP expression levels were determined by flow cytometry 8 days after protein transduction. In the Sox2-responsive cell, protein transduction with pseudotyped VLPs containing Gag-Sox2-2NES resulted in the activation of Sox2-TRE-minimal promoter-driven GFP reporter (Fig. 4d, left). At the highest level of PT, 80% of the target cells were GFP positive on day 8. Control treatment with wild type Gag assembled pseudotyped VLPs did not give rise to GFP reporter expression for all concentrations tested (Fig. 4d, left). The data indicate that the Sox2 protein delivered within the PT VLPs activated nuclear RNA Pol II transcription in the recipient cell. Similarly, protein transduction of Oct4, Klf4, and c-Myc activated their cognate TRE, driving transcription of the GFP reporter gene (Fig. 4d, right and Fig. S2e).
In addition, the Sox2 regulation of expression of endogenous genes was examined. It has been reported that ectopic expression of Sox2 will up-regulate expression of pepsinogen A mRNA (PGA) but not that of pepsinogen C mRNA (PGC) in HEK293 cells [28]. Protein-transduction of Sox2 (expressed from Gag-Sox2-2NES) into HEK293 MCAT cells expressing the ecotropic MLV receptor MCAT1, resulted in a significant (6-fold) up regulation of the ratio of PGA/ PGC mRNA as judged by qPCR, compared to control protein transduction of GFP (Gag-GFP-2NES) (Fig. 4e). PGA mRNA was similarly activated by ectopic over-expression of Sox2 (VP-pMX-Sox2). Interestingly, the level of transcriptional activation of all four TF proteins transduced by 60 µg/mL VLPs was significantly higher than cells infected with their respective integrating retroviral pMXs. Mouse Sox2 mRNA was only detected using the integrating pMX-Sox2 vector (Fig. 4e, right).
Overall, the chimeric VLPs did result in the transient delivery protein into the target cells. Serial exposure to chimeric VLPs allows for continued expression of the transduced protein in the recipient cells and can provide highly efficient transcriptional activation for TF delivery. Expression was not a result of background transfer of RNA. The expression from protein-transduction VLPs was comparable with the integrating MLV-derived vector and can be used for cell engineering.
3.5. Application of VLPs for delivery of a cytotoxic enzyme
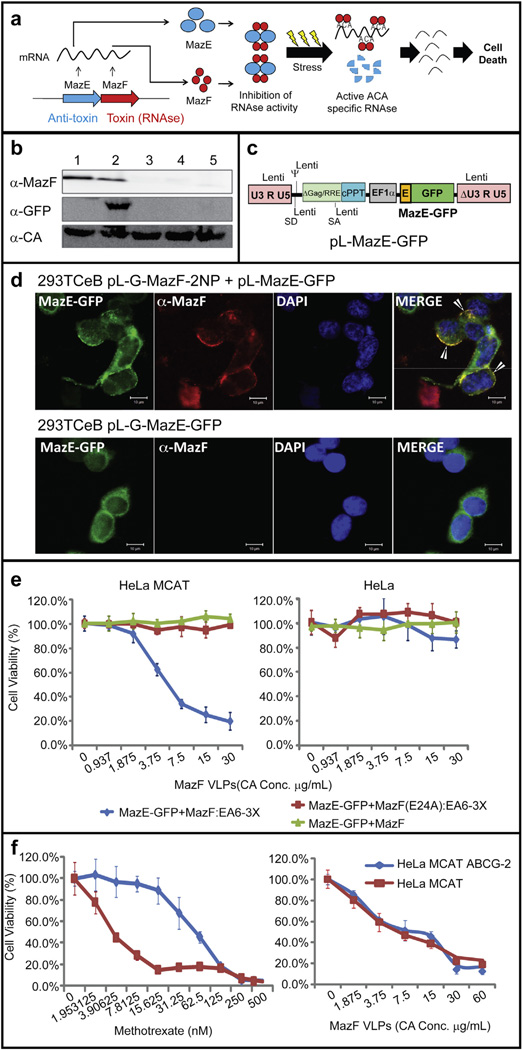
A second key area of interest for protein therapeutics is the delivery of exogenous toxic proteins into mammalian cells. A key challenge to these systems is to protect the producer cell from the effects of the toxin. The use of the bacterial MazEF “toxin-antitoxin” system provides a unique approach to this dilemma. MazF is a bacterial ACA-specific mRNA interferase regulated by the direct binding of the labile anti-toxin MazE (Fig. 5a) [29]. MazF cleaves RNA molecules containing ACA-sequences, thus inducing degradation of cellular mRNA, blocking de novo protein synthesis, and inducing apoptotic cell death in mammalian cells [30,31]. Expression of the initial pL-G-MazF-2NP construct resulted in the death of the producer 293TCeB cells after puromycin selection. Constructs bearing an inactivating mutation within the MazF endor-ibonuclease (E24A) resulted in the release of VLPs from 293TCeB cells (Fig. 5b lane 1), indicating that expression of the active MazF was incompatible with the viability of the packaging cell. To inhibit MazF function in E. coli, MazE binds to MazF to form a stable heterohexamer (MazF2-MazE2-MazF2) (Fig. 5a) [32]. Expression of MazE in the packaging cell lines (as a MazE-GFP fusion protein, Fig. 5c) results in the protection of these cells, allowing for release of VLPs packaging the Gag-MazF-2NES (Fig. 5b, lane 2). Interestingly, we found that MazE-GFP protein will not only inhibit the endoribonuclease activity of MazF in the packaging cell but was also co-assembled into VLPs, which is mediated by MazF–MazE interactions (Fig. 5b&d). The packaging cells were also protected by truncated forms of MazE (MazE42 and MazE61 [11] but not as well as wild type MazE and only cells expressing low levels of Gag-MazF-2NES were able to survive after puromycin selection (Fig. 5b, lanes 3,4). MazE-GFP expression alone did not assemble into VLP in the absence of MazF (Fig. 5b, lane 5).
Fig. 5.
Biological activity of VLPs containing bacterial MazEF toxin-antitoxin cassette. (a) Schematic of the sequence-specific endoribonuclease toxin–antitoxin systems from the E. coli. (b) Western blot of ecotropic pseudotyped VLPs from 293TCeB cells. Lane 1, MazF (E24A); lane 2, MazF + MazE-GFP; lane 3, MazF + MazE42-GFP; lane 4, MazF + MazE61E-GFP; lane 5, MazE-GFP [11]. (c) Schematic of the pL-MazE-GFP lentiviral vector. (d) Immunofluorescence co-localization of Gag-MazF-2NESΨ(anti-MazF; Red) with MazE-GFP (Green) assembly on the inner surface of the plasma membrane (arrows) in 293TCeB packaging cells. Scale bars represent 10 µm. (e) Cell viability assay after MazF delivered via VLPs. Target HeLa cells in the presence and absence of the ecotropic MLV receptor MCAT1 (HeLa MCAT) were incubated for 7 days with VLPs consisting of MazF + MazE-GFP:EA6-3X, MazF(E24A) + MazE-GFP:EA6-3X, and MazF + MazE-GFP (bald VLPs, lacking Env). (f) ABCG-2 over-expression cell (HeLa MCAT ABCG-2) viability assay after MazF chimeric VLPs or methotrexate treatment. Results are mean with standard error, N=3. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
To examine the bioactivity of MazF in the recipient cells after protein transduction, the Gag-MazF-2NES VLPs pseudotyped with the EA6-3X Env were concentrated and exposed to the HeLa MCAT cell line. As shown in Fig. 5e, VLPs that contained both Gag-MazF-2NES and MazE-GFP in the same EA6-3X Env pseudotyped VLPs caused an 80% reduction in cell viability. However, no cell death was observed with VLPs containing only Gag-MazF(E24A) or particles lacking an Env for receptor-mediated internalization. Particle delivery into cells was dependent on expression of the MCAT viral receptor (Fig. 5e). This shows that the MazE anti-toxin protein inhibits the MazF endoribonuclease, maintaining its viability in the packaging cell line, and remains a complex in the VLPs. After VLPs enter into infected cells, the lability of the anti-toxin releases the MazF toxin to act within the target cells.
The sensitivity to MazF in chemotherapy-resistant cells was examined. Overexpression of the ATP-binding cassette (ABC) transporters, such as ABCG2, result in multiple drug resistance due to elimination of the drug from the cells and is critical in the clinical pharmacology of anticancer drugs [33–35]. As shown in previous reports [36], over-expression of ABCG2 decreased sensitivity to methotrexate in HeLa MCAT cells (Fig. 5f, left). However, the methotrexate-resistance cells remain sensitive to MazF toxin (Fig. 5f, right). These results demonstrate that VLP-mediated delivery of bacterial toxins to a target cell causes cell cytotoxicity and may serve as a therapeutic approach towards chemotherapy-resistant transformed cells.
4. Discussion
Protein therapeutics in general have limited ability to cross the cell membranes due to their surface charge distribution and large size. High doses of protein drugs over long periods of time may cause adverse immune responses. Incorporation of proteins within MLV-based VLPs can increase the efficiency of intracellular protein delivery and can help to avoid these limitations due to the fact that MLV-based retrovirus vectors are poorly immunogenic [37]. Compared with generation of retroviral-like particles by transient transfection in previous reports [4,5], the establishment of stable VLP producer cell lines allows for large-scale protein production and provides a platform for high titer delivery of protein therapeutic drugs.
In the absence of a viral genome, the retroviral Gag alone is necessary and sufficient for the formation and release of VLPs from the plasma membrane, and cellular RNAs can substitute for viral RNA for assembly. Nuclear trafficking of Gag has been reported in several retroviral genera, including MLV, human immunodeficiency virus type 1 (HIV-1), Rous sarcoma virus (RSV), and prototypic foamy virus (PFV) [23,38–40]. In RSV, Gag contains two NLSs and one NES sequence, which can bind to the nuclear import receptors (importin-11 and importin-alpha) and nuclear export factor (CRM-1) for the nuclear trafficking, respectively. Unlike RSV Gag, there is no evidence that MLV Gag contains a functional NLS or NES. Data in this study demonstrates that MLV Gag is directed to the nucleus by the insertion of foreign NLS-containing proteins and leads to the nuclear accumulation of the Gag chimera and thus decreasing the level of VLPs released from the plasma membrane of the infected cell. With the addition of the NES sequence within the p12 and NC genes and the co-expression of wild type Gag or Gag-Pol protein, the Gag chimera can cycle out of the nucleus using the CRM-1 dependent pathway defined for PKI-type NESs [24,25] and are capable of generating VLPs. Taken together, the modification of MLV Gag precursor protein with an NLS is counterbalanced by the NES sequence yielding little overall change to the MLV fitness.
Combining VLP particles with entry targeting approaches can address several of the bottleneck issues of protein and antibody-based delivery systems. VLPs pseudotyped with retargeted Envs can provide astounding specificity for targeting protein delivery. A variety of approaches have shown that retargeting of retroviral particle entry can be achievedvia alteration of the viral–host interactions, including single-chain antibodies [7]. Our laboratory has developed a screen to identify retroviral Env capable of productively infecting target cells through randomizing an 11 amino acid region of the receptor binding domain of feline leukemia virus (FeLV) [41–45] and established the effectiveness of intratumor delivery [46]. Additionally, successful retargeting for Sindbis Env pseudotyped onto lentiviral particles has also been achieved with the insertion of the Protein A ZZ domain and sandwiching targeting antibodies to the modified particles. Previous reports have shown that modified Sindbis Env pseudotyped retroviral particles can bind to specific IgG antibodies, retargeting to cancer cells, undifferentiated human embryonic stem cells, or induced pluripotent stem cells [10,47]. Delivery of therapeutic proteins using VLP-antibody conjugation could attenuate undesired attributes to cell surface or extracellular targets and decrease the nonspecific uptake by the normal cell. Furthermore, targeted pharmaceutical delivery of proteins by VLPs would incorporate more than a thousand protein drug molecules per targeted particle. The introduction of active protein into the cytosol or nucleus of living cells would provide the opportunity to regulate signaling pathways with intracellular processes, signaling cell proliferation, differentiation or apoptosis. Features of this antibody-VLP conjugation system are being tested in our laboratory with the prospect of next generation bioactive protein-delivery systems with enhanced efficacy and safety.
5. Conclusion
A wide-range of systems can benefit from the development of efficient mechanisms to target the delivery of therapeutic proteins into cells, including the restoration of normal cellular functions and/ or the ability to destroy targeted cells. In this study, we have developed MLV based VLPs producer cells that incorporate foreign proteins within the Gag precursor. These protein transduction agents maintain the critical viral functions needed for the production and delivery of biologically active proteins to the target cells, including viral assembly, release, proteolytic processing, entry through cell-specific receptors, and intracellular disassembly steps. We demonstrated the effectiveness of this approach by transferring the nuclear TFs and MazF toxin protein to the targeted cells. We have shown that VLPs containing MazF toxin protein are active and can induce cell death. In addition, we have shown that TFs are active within the nucleus of the target cells, activating tandem repeats of the TFs transcriptional response elements as well as the endogenous PGA expression.
Supplementary Material
Acknowledgements
This work was supported by NIH grant RO1CA49932 to MJR.
Footnotes
The authors declare no competing financial interests.
Appendix A. Supplementary data
Supplementary data related to this article can be found at doi:10.1016/j.biomaterials.2014.06.006.
References
- 1.Al-Taei S, Penning NA, Simpson JC, Futaki S, Takeuchi T, Nakase I, et al. Intracellular traffic and fate of protein transduction domains HIV-1 TAT peptide and octaarginine. Implications for their utilization as drug delivery vectors. Bioconjug Chem. 2006;17:90–100. doi: 10.1021/bc050274h. [DOI] [PubMed] [Google Scholar]
- 2.Belting M, Sandgren S, Wittrup A. Nuclear delivery of macromolecules: barriers and carriers. Adv Drug Deliv Rev. 2005;57:505–527. doi: 10.1016/j.addr.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 3.Nermut MV, Mulloy B. Consideration of the three-dimensional structure of core shells (capsids) in spherical retroviruses. Micron. 2007;38:462–470. doi: 10.1016/j.micron.2006.11.007. [DOI] [PubMed] [Google Scholar]
- 4.Voelkel C, Galla M, Maetzig T, Warlich E, Kuehle J, Zychlinski D, et al. Protein transduction from retroviral Gag precursors. Proc Natl Acad Sci U S A. 2010;107:7805–7810. doi: 10.1073/pnas.0914517107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kaczmarczyk SJ, Sitaraman K, Young HA, Hughes SH, Chatterjee DK. Protein delivery using engineered virus-like particles. Proc Natl Acad Sci U S A. 2011;108:16998–17003. doi: 10.1073/pnas.1101874108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sandrin V, Muriaux D, Darlix JL, Cosset FL. Intracellular trafficking of Gag and Env proteins and their interactions modulate pseudotyping of retroviruses. J Virol. 2004;78:7153–7164. doi: 10.1128/JVI.78.13.7153-7164.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Peng K, Donovan K, Schneider U, Cattaneo R, Lust J, Russell S. Oncolytic measles viruses displaying a single-chain antibody against CD38, a myeloma cell marker. Blood. 2003;101:2557–2562. doi: 10.1182/blood-2002-07-2195. [DOI] [PubMed] [Google Scholar]
- 8.Schneider WM, Wu DT, Amin V, Aiyer S, Roth MJ. MuLV IN mutants responsive to HDAC inhibitors enhance transcription from unintegrated retroviral DNA. Virology. 2012;426:188–196. doi: 10.1016/j.virol.2012.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.O’Reilly L, Roth MJ. G541R within the 4070A TM protein regulates fusion in murine leukemia viruses. J Virol. 2003;77:12011–12021. doi: 10.1128/JVI.77.22.12011-12021.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wu DT, Seita Y, Zhang X, Lu CW, Roth MJ. Antibody-directed lentiviral gene transduction for live-cell monitoring and selection of human iPS and hES cells. PLoS One. 2012;7:e34778. doi: 10.1371/journal.pone.0034778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Park JH, Yamaguchi Y, Inouye M. Intramolecular regulation of the sequence-specific mRNA interferase activity of MazF fused to a MazE fragment with a linker cleavable by specific proteases. Appl Environ Microbiol. 2012;78:3794–3799. doi: 10.1128/AEM.00364-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 13.Avilion AA, Nicolis SK, Pevny LH, Perez L, Vivian N, Lovell-Badge R. Multipotent cell lineages in early mouse development depend on SOX2 function. Genes Dev. 2003;17:126–140. doi: 10.1101/gad.224503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Delchambre M, Gheysen D, Thines D, Thiriart C, Jacobs E, Verdin E, et al. The GAG precursor of simian immunodeficiency virus assembles into virus-like particles. EMBO J. 1989;8:2653–2660. doi: 10.1002/j.1460-2075.1989.tb08405.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gheysen D, Jacobs E, de Foresta F, Thiriart C, Francotte M, Thines D, et al. Assembly and release of HIV-1 precursor Pr55gag virus-like particles from recombinant baculovirus-infected insect cells. Cell. 1989;59:103–112. doi: 10.1016/0092-8674(89)90873-8. [DOI] [PubMed] [Google Scholar]
- 16.Smith AJ, Cho MI, Hammarskjold ML, Rekosh D. Human immunodeficiency virus type 1 Pr55gag and Pr160gag-pol expressed from a simian virus 40 late replacement vector are efficiently processed and assembled into virus like particles. J Virol. 1990;64:2743–2750. doi: 10.1128/jvi.64.6.2743-2750.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wills JW, Craven RC, Achacoso JA. Creation and expression of myristylated forms of Rous sarcoma virus gag protein in mammalian cells. J Virol. 1989;63:4331–4343. doi: 10.1128/jvi.63.10.4331-4343.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hacein-Bey-Abina S, Garrigue A, Wang GP, Soulier J, Lim A, Morillon E, et al. Insertional oncogenesis in 4 patients after retrovirus-mediated gene therapy of SCID-X1. J Clin Invest. 2008;118:3132–3142. doi: 10.1172/JCI35700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hacein-Bey-Abina S, Von Kalle C, Schmidt M, McCormack MP, Wulffraat N, Leboulch P, et al. LMO2-associated clonal T cell proliferation in two patients after gene therapy for SCID-X1. Science. 2003;302:415–419. doi: 10.1126/science.1088547. [DOI] [PubMed] [Google Scholar]
- 20.Cosset FL, Takeuchi Y, Battini JL, Weiss RA, Collins MK. High-titer packaging cells producing recombinant retroviruses resistant to human serum. J Virol. 1995;69:7430–7436. doi: 10.1128/jvi.69.12.7430-7436.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Boulikas T. Putative nuclear localization signals (NLS) in protein transcription factors. J Cell Biochem. 1994;55:32–58. doi: 10.1002/jcb.240550106. [DOI] [PubMed] [Google Scholar]
- 22.Li J, Pan G, Cui K, Liu Y, Xu S, Pei D. A dominant-negative form of mouse SOX2 induces trophectoderm differentiation and progressive polyploidy in mouse embryonic stem cells. J Biol Chem. 2007;282:19481–19492. doi: 10.1074/jbc.M702056200. [DOI] [PubMed] [Google Scholar]
- 23.Nash MA, Meyer MK, Decker GL, Arlinghaus RB. A subset of Pr65gag is nucleus associated in murine leukemia virus-infected cells. J Virol. 1993;67:1350–1356. doi: 10.1128/jvi.67.3.1350-1356.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guttler T, Madl T, Neumann P, Deichsel D, Corsini L, Monecke T, et al. NES consensus redefined by structures of PKI-type and Rev-type nuclear export signals bound to CRM1. Nat Struct Mol Biol. 2010;17:1367–1376. doi: 10.1038/nsmb.1931. [DOI] [PubMed] [Google Scholar]
- 25.Gomez Corredor A, Archambault D. The bovine immunodeficiency virus Rev protein: identification of a novel nuclear import pathway and nuclear export signal among retroviral Rev/Rev-like proteins. J Virol. 2012;86:4892–4905. doi: 10.1128/JVI.05132-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wu D-T, Aiyer S, Villanueva RA, Roth MJ. Development of an enzyme-linked immunosorbent assay based on the murine leukemia virus p30 capsid protein. J Virol Methods. 2013;193:332–336. doi: 10.1016/j.jviromet.2013.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rulli SJ, Jr., Hibbert CS, Mirro J, Pederson T, Biswal S, Rein A. Selective and nonselective packaging of cellular RNAs in retrovirus particles. J Virol. 2007;81:6623–6631. doi: 10.1128/JVI.02833-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tani Y, Akiyama Y, Fukamachi H, Yanagihara K, Yuasa Y. Transcription factor SOX2 up-regulates stomach-specific pepsinogen A gene expression. J Cancer Res Clin Oncol. 2007;133:263–269. doi: 10.1007/s00432-006-0165-x. [DOI] [PubMed] [Google Scholar]
- 29.Yamaguchi Y, Inouye M. Chapter 12 mRNA interferases, sequence-specific endoribonucleases from the toxin–antitoxin systems. In: Ciaran C, editor. Prog Mol Biol Transl Sci. 2009. pp. 467–500. [DOI] [PubMed] [Google Scholar]
- 30.Shimazu T, Degenhardt K, Nur EKA, Zhang J, Yoshida T, Zhang Y, et al. NBK/BIK antagonizes MCL-1 and BCL-XL and activates BAK-mediated apoptosis in response to protein synthesis inhibition. Genes Dev. 2007;21:929–941. doi: 10.1101/gad.1522007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chono H, Matsumoto K, Tsuda H, Saito N, Lee K, Kim S, et al. Acquisition of HIV-1 resistance in T lymphocytes using an ACA-specific E. coli mRNA interferase. Hum Gene Ther. 2011;22:35–43. doi: 10.1089/hum.2010.001. [DOI] [PubMed] [Google Scholar]
- 32.Kamada K, Hanaoka F, Burley SK. Crystal structure of the MazE/MazF complex: molecular bases of antidote-toxin recognition. Mol Cell. 2003;11:875–884. doi: 10.1016/s1097-2765(03)00097-2. [DOI] [PubMed] [Google Scholar]
- 33.Doyle LA, Yang W, Abruzzo LV, Krogmann T, Gao Y, Rishi AK, et al. A multidrug resistance transporter from human MCF-7 breast cancer cells. Proc Natl Acad Sci U S A. 1998;95:15665–15670. doi: 10.1073/pnas.95.26.15665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Maliepaard M, van Gastelen MA, de Jong LA, Pluim D, van Waardenburg RC, Ruevekamp-Helmers MC, et al. Overexpression of the BCRP/MXR/ABCP gene in a topotecan-selected ovarian tumor cell line. Cancer Res. 1999;59:4559–4563. [PubMed] [Google Scholar]
- 35.Miyake K, Mickley L, Litman T, Zhan Z, Robey R, Cristensen B, et al. Molecular cloning of cDNAs which are highly overexpressed in mitoxantrone-resistant cells: demonstration of homology to ABC transport genes. Cancer Res. 1999;59:8–13. [PubMed] [Google Scholar]
- 36.Volk EL, Farley KM, Wu Y, Li F, Robey RW, Schneider E. Overexpression of wild-type breast cancer resistance protein mediates methotrexate resistance. Cancer Res. 2002;62:5035–5040. [PubMed] [Google Scholar]
- 37.Fong TC, Sauter SL, Ibanez CE, Sheridan PL, Jolly DJ. The use and development of retroviral vectors to deliver cytokine genes for cancer therapy. Crit Rev Ther Drug Carrier Syst. 2000;17:1–60. [PubMed] [Google Scholar]
- 38.Dupont S, Sharova N, DeHoratius C, Virbasius CM, Zhu X, Bukrinskaya AG, et al. A novel nuclear export activity in HIV-1 matrix protein required for viral replication. Nature. 1999;402:681–685. doi: 10.1038/45272. [DOI] [PubMed] [Google Scholar]
- 39.Scheifele LZ, Garbitt RA, Rhoads JD, Parent LJ. Nuclear entry and CRM1-dependent nuclear export of the Rous sarcoma virus Gag polyprotein. Proc Natl Acad Sci U S A. 2002;99:3944–3949. doi: 10.1073/pnas.062652199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schliephake AW, Rethwilm A. Nuclear localization of foamy virus Gag precursor protein. J Virol. 1994;68:4946–4954. doi: 10.1128/jvi.68.8.4946-4954.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bupp K, Roth MJ. Altering retroviral tropism using a random-display envelope library. Mol Ther. 2002;5:329–335. doi: 10.1006/mthe.2002.0546. [DOI] [PubMed] [Google Scholar]
- 42.Bupp K, Roth MJ. Targeting a retroviral vector in the absence of a known cell-targeting ligand. Hum Gene Ther. 2003;14:1557–1564. doi: 10.1089/104303403322495061. [DOI] [PubMed] [Google Scholar]
- 43.Mazari PM, Linder-Basso D, Sarangi A, Chang Y, Roth MJ. Single-round selection yields a unique retroviral envelope utilizing GPR172A as its host receptor. Proc Natl Acad Sci U S A. 2009;106:5848–5853. doi: 10.1073/pnas.0809741106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mazari PM, Argaw T, Valdivieso L, Zhang X, Marcucci KT, Salomon DR, et al. Comparison of the convergent receptor utilization of a retargeted feline leukemia virus envelope with a naturally-occurring porcine endogenous retrovirus A. Virology. 2012;427:118–126. doi: 10.1016/j.virol.2012.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sarangi A, Bupp K, Roth MJ. Identification of a retroviral receptor used by an envelope protein derived by peptide library screening. Proc Natl Acad Sci U S A. 2007;104:11032–11037. doi: 10.1073/pnas.0704182104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang X, Sarangi A, Wu DT, Kanduri J, Roth MJ. Gene delivery in a mouse xenograft of a retargeted retrovirus to a solid 143B osteosarcoma. Virol J. 2013;10:194. doi: 10.1186/1743-422X-10-194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Morizono K, Xie Y, Ringpis GE, Johnson M, Nassanian H, Lee B, et al. Lentiviral vector retargeting to P-glycoprotein on metastatic melanoma through intravenous injection. Nat Med. 2005;11:346–352. doi: 10.1038/nm1192. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.