Abstract
Aims/hypothesis
The aim of the study was to compare longitudinal changes in insulin sensitivity (SI) and beta cell function between women with and without a history of gestational diabetes mellitus (GDM).
Methods
The prospective follow-up cohort included 235 parous non-diabetic Mexican-American women, 93 with and 142 without a history of GDM. The participants underwent dual-energy x-ray absorptiometry, OGTTs and IVGTTs at baseline and at a median of 4.1 years follow-up. The baseline values and rates of change of metabolic measures were compared between groups.
Results
At baseline, women with prior GDM (mean age 36.3 years) had similar values of SI but higher percentages of body fat and trunk fat (p≤0.02), a lower acute insulin response and poorer beta cell compensation (disposition index [DI]) (p<0.0001) than women without GDM (mean age 37.9 years). During the follow-up, women with GDM had a faster decline in SI (p=0.02) and DI (p=0.02) than their counterparts without GDM, with no significant differences in changes of weight or fat (p>0.50). Adjustment for baseline age, adiposity, calorie intake, physical activity, age at first pregnancy, additional pregnancies and changes in adiposity during follow-up increased the between-group differences in the rates of change of SI and DI (p≤0.003).
Conclusions/interpretation
Mexican-American women with recent GDM had a faster deterioration in insulin sensitivity and beta cell compensation than their parous counterparts without GDM. The differences were not explained by differences in adiposity, suggesting more deleterious effects of existing fat and/or reduced beta cell robustness in women with GDM.
Keywords: Beta cell function, Gestational diabetes mellitus, Longitudinal change, Insulin sensitivity
Introduction
Women with a history of gestational diabetes mellitus (GDM) have an increased risk of developing type 2 diabetes, the risk being approximately fourfold to ten fold higher than in women without a history of GDM [1–3]. Cross-sectional studies indicate that women with GDM have a lower insulin sensitivity (SI) and lower beta cell compensation for that resistance than healthy women [4–7]. It is unclear whether those cross-sectional differences are constant over time between women with and without GDM, or reflect a more rapid deterioration in glucose regulation over time in women with GDM than women without it. We address that distinction in the present report, using data from a well characterised longitudinal cohort of Mexican-American women with and without a history of GDM.
Methods
Study participants
This study was conducted in the context of an observational study of obesity, insulin resistance and beta cell dysfunction in Mexican-Americans (the BetaGene study). Details of recruitment to the BetaGene study have been previously described elsewhere [8].
Briefly, the participants are Mexican-American individuals (both parents and three or more grandparents being Mexican or of Mexican descent) with a fasting glucose level <7.0 mmol/l who are either (1) women who have had GDM within the previous 5 years, (2) the siblings or cousins of women with a history of GDM, or (3) women with normal glucose levels during pregnancy in the previous 5 years. Women with and without previous GDM were identified from the patient populations at Los Angeles County/University of Southern California Medical Center, the Kaiser Permanente Southern California health plan membership and obstetric/gynaecological clinics at local Southern California hospitals. Women without previous GDM were frequency-matched to women with GDM by age, BMI and parity. A total of 1,250 individuals were recruited to the BetaGene study with completed OGTT and IVGTT testing [9]. Among them, 903 were women, of whom 735 were parous and had a known GDM status.
The BetaGene follow-up study (BetaGene-II) was designed to recall 400 participants from the original BetaGene study to repeat the metabolic testing for an assessment of changes over time. Subjects with a fasting glucose level ≥7.0 mmol/l were ineligible for follow-up testing. The BetaGene participants were initially recruited between January 2000 and June 2005, and follow-up visits were conducted between June 2006 and October 2012. A total of 390 participants were recruited into BetaGene-II with completed OGTT and IVGTT testing at follow-up; 288 of these were women, 235 of whom were parous and had a known GDM status at baseline. For this report, only parous women with a known prior GDM status at baseline who participated in the follow-up study were included. The BetaGene and BetaGene-II studies were approved by the institutional review boards of the participating institutions, and all participants provided written informed consent before participation.
Testing procedures and assays
Metabolic testing at each study point (baseline and follow-up) was conducted during two separate visits to the clinical trials unit at the University of Southern California. The first visit consisted of a physical examination, dietary and physical activity questionnaires, a dual-energy x-ray absorptiometry (DEXA) scan for body composition, and a 2 h, 75 g OGTT. An insulin-modified frequently sampled IVGTT was performed on the second visit to measure insulin resistance and beta cell function [10], as previously described [11–13]. The number of pregnancies, age at first pregnancy, parity and prior GDM status were ascertained at the initial visit. Identical testing procedures were used at follow-up visits.
Dietary intake was assessed by the 126-item semi-quantitative Harvard Food Frequency Questionnaire [14]. Total calories and macronutrients were calculated by the Harvard Channing Laboratory. Only baseline dietary data were available for this report. Physical activity was assessed by the questionnaires developed by the Hawaii–Los Angeles Multiethnic Cohort Study [15, 16]. Individuals were categorised according to whether or not they met the United States Department of Health and Human Services (DHHS) recommendation of at least 75 min a week of vigorous or 150 min a week of moderate activity [17]. Our previous work showed that individuals who reported meeting the DHHS recommendation had better glucose and insulin profiles and enhanced beta cell function compared with subjects who did not meet the recommendation [9].
Plasma glucose was measured by glucose oxidase (YSI Model 2300; Yellow Springs Instruments, Yellow Springs, OH, USA), and insulin was measured by two-site immunoenzymometric assay (TOSOH Bioscience, San Francisco, CA, USA), which has <0.1% cross-reactivity with proinsulin and intermediate split products.
Data analysis
BMI was calculated as the weight in kilograms divided by the square of the height in metres. Insulin responses to glucose were calculated as the incremental AUC for insulin during the first 10 min of the IVGTT (the acute insulin response [AIR]) and the difference in plasma insulin level between the 30 min and fasting time points of the OGTT (30′ΔInsulin). The minimal model (MINMOD Millennium version 5.18) was used to derive SI and AIR from the IVGTT results [18]. The disposition index (DI), a measure of beta cell compensation for insulin resistance, was computed as the product of SI and AIR. The incremental AUC for the 2 h OGTT glucose curve (ΔGlucose AUC) was calculated using the trapezoid method.
Demographic, anthropometric and metabolic characteristics were compared between women with and without a prior history of GDM using generalised estimating equations (GEEs), assuming a family-level exchangeable correlation structure, to account for possible correlations between siblings or cousins within families. Results for fasting insulin level, post-challenge insulin concentration, insulin response, SI and DI were natural log-transformed to approximately normal distribution prior to analysis. For presentation purposes, medians and interquartile ranges (IQRs) were used for descriptive statistics, and geometric means were used for covariate adjusted results for which standard deviations were calculated using the delta method [19]. Rates of change in anthropometrics and metabolic measures between baseline and follow-up were calculated as ([follow-up – baseline] / follow-up time) to take account of individual differences in the length of follow-up. Rates of change were compared between the GDM and non-GDM groups using GEE without adjustment for covariates, as well as with adjustments for baseline age, age at first pregnancy, body fat percentage, caloric intake and level of physical activity. The adjusted analyses were repeated by replacing the baseline fat percentages with the baseline BMI and trunk fat as covariates. The impact of change in adiposity, additional pregnancy and physical activity during follow-up on the differences in rates of change of the metabolic factors between the two groups was evaluated by further adjustment for these follow-up variables. All statistical tests were two-sided. SAS version 9.2 (SAS Institute, Cary, NC, USA) was used for data analyses.
Results
Baseline characteristics
The sample was composed of 235 parous women from 100 independent families, 93 with and 142 without a history of GDM at baseline. Compared with women without a history of GDM, the women with prior GDM were approximately 2 years younger, were of the same parity and were around 3 years older in terms of age at first pregnancy (Table 1). The GDM group had an average of around a 1.5 kg/m2 higher BMI, a 1.2% greater percentage body fat and a 1.9 kg greater trunk fat compared with the non-GDM group (p≤0.03). The values for the fasting glucose, 2 h glucose and incremental glucose AUCs from the OGTTs were significantly higher in the GDM group (p≤0.003), as were the fasting and 2 h insulin levels (p≤0.001). The OGTT 30′ΔInsulin was similar between the two groups. At baseline, more women in the prior GDM group had impaired fasting glucose ([IFG], defined as a fasting glucose level ≥5.6 mmol/l; 23% vs 8%, p=0.002) and impaired glucose tolerance ([IGT], defined as a 2 h glucose level ≥7.8 mmol/l; 59.1% vs 30.3%, p<0.0001). SI was slightly but not significantly lower in the prior GDM group (p=0.11). AIR and DI were significantly lower in that group compared with the women without GDM (p<0.0001). Self-reported total calorie intake was slightly, but not significantly, higher in the GDM group (p=0.14). Fewer than 50% of women in each group met the DHHS physical activity recommendation (p=0.61 between groups). Baseline glucose, insulin, AIR and DI remained significantly elevated in the GDM group after adjustment for age, age at first pregnancy, percentage body fat, energy intake and physical activity.
Table 1.
Comparison of baseline and follow-up characteristics between women with and without a history of GDM
| Variable | Baseline | Follow-up | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| GDM (n=93) | Non-GDM (n=142) | p valuea | GDM (n=93) | Non-GDM (n=142) | p valuea | |
| Age (years) | 36.3 (5.6) | 37.9 (8.4) | 0.06 | 40.8 (5.9) | 42.6 (8.5) | 0.09 |
| Age at first pregnancy (years) | 24.3 (5.8) | 21.2 (4.1) | <0.0001 | N/A | N/A | N/A |
| Parity | 2.8 (1.3) | 2.8 (1.3) | 0.81 | 3.1(1.3) | 2.9 (1.2) | 0.31 |
| BMI (kg/m2) | 30.7 (5.6) | 29.2 (5.6) | 0.03 | 31.1 (5.5) | 29.8 (5.6) | 0.08 |
| Weight (kg) | 75.2 (14.3) | 72.0 (14.2) | 0.06 | 77.0 (14.5) | 73.5 (14.0) | 0.08 |
| Body fat percentage (%) | 39.1 (4.8) | 37.9 (5.6) | 0.02 | 39.4 (4.7) | 38.4 (5.3) | 0.09 |
| Trunk fat (kg) | 15.7 (5.3) | 13.8 (5.2) | 0.003 | 16.2 (5.4) | 14.3 (5.0) | 0.01 |
| Fasting glucose (mmol/l) | 5.05 (0.73) | 4.82 (0.55) | 0.003 | 5.31 (0.59) | 4.93 (0.50) | <0.0001 |
| 2 h glucose (mmol/l) | 8.16 (1.94) | 7.10 (1.94) | <0.0001 | 9.14 (2.38) | 7.53 (2.22) | <0.0001 |
| ΔGlucose AUC (mmol/l × min)b,c | 393 (150) | 281 (211) | <0.0001 | 422 (197) | 297 (284) | <0.0001 |
| Fasting insulin (pmol/l)c | 55.6 (48.6) | 41.7 (45.1) | <0.0001 | 75.0 (55.6) | 48.6 (56.3) | 0.0002 |
| 2 h insulin (pmol/l)c | 535 (563) | 396 (340) | 0.001 | 773 (726) | 455 (554) | 0.0001 |
| 30′ΔInsulin (pmol/l)c | 337 (389) | 368 (396) | 0.63 | 379 (430) | 422 (447) | 0.07 |
| SI (min−1 per μU/ml × 10−4)c | 2.50 (2.06) | 3.00 (2.06) | 0.11 | 2.08 (1.38) | 2.53 (1.79) | 0.01 |
| AIR (pmol/l × min)c | 2007 (2257) | 3510 (3359) | <0.0001 | 2209 (2335) | 3455 (3103) | <0.0001 |
| DI (SI × AIR)c | 5722 (4639) | 9989 (8118) | <0.0001 | 4281 (3967) | 7800 (5906) | <0.0001 |
| Energy intake(kJ/day)c | 10138(4012) | 9414(3602) | 0.14 | N/A | N/A | N/A |
| Physical activity (DHHS recommendation) | ||||||
| No | 52 (56%) | 73 (53%) | 0.61 | 29 (32.2%) | 38 (29.2%) | 0.64 |
| Yes | 41 (44%) | 66 (47%) | 61 (67.8%) | 91 (70.8%) | ||
Data are mean (SD), or median (IQR) where indicated, or n (%)
Using the GEE
Incremental area under the OGTT curve, calculated by the trapezoid method
Log-transformed; data are median (IQR)
NA, not available
Changes during follow-up
Women in both the GDM and non-GDM groups were followed for a median of 4.1 years (IQR 3.3–5.5 and 3.6–5.7 years, respectively). At follow-up (Table 1), women in the GDM group remained slightly more obese, with significantly higher glucose, fasting and 2 h insulin levels, diminished acute insulin secretion and lower beta cell compensation than women in the non-GDM group. The GDM group progressed to have a significantly lower SI than the non-GDM group (p=0.01). Rates of IFG and IGT were increased to 35.5% vs 13.4% (p<0.0001) and 71.0% vs 39.4% (p<0.0001), respectively, for the GDM vs non-GDM group. During follow-up, a higher percentage of women in the GDM group experienced one or more additional pregnancies (21/93=23% vs 19/142=13%; p=0.07). Of the women who became pregnant during the follow-up interval, a higher percentage of women in the GDM group developed GDM in their subsequent pregnancies compared with those in the non-GDM group (12/21=57% vs 2/19=11%; p=0.01). At follow-up testing, 67.8% and 70.8% of women in the GDM and non-GDM groups, respectively, met the DHHS physical activity recommendation (p=0.64 between groups).
Rates of change of the anthropometric and metabolic factors by group are presented in Table 2. Body weight, total body fat percentage and trunk fat increased similarly in the two groups. Fasting and 2 h OGTT glucose and fasting, 2 h insulin and 30′ΔInsulin values all increased over time in both groups, with higher rates of increase for the GDM group, although the differences between groups were not statistically significant. SI, AIR and DI all decreased over time in the two groups. The rates of decline in SI and DI were significantly greater in the GDM group (unadjusted p=0.02 for both). Two women in the GDM group and none of the women in the non-GDM group had developed diabetes at follow-up (p=0.23 by Fisher’s exact test).
Table 2.
Comparison of the rates of change of anthropometric and metabolic measurements between women with and without a history of GDM
| Rate of change during follow-upa | GDM (n=93) | Non-GDM (n=142) | Beta-coefficientb (95% CI) | ||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Mean (SD) | Mean (SD) | Unadjusted | Model I | Model II | Model III | Model IV | |
| Weight (kg/year) | 0.43 (1.63) | 0.37 (1.58) | 0.11 (−0.29, 0.51) | 0.03 (−0.37, 0.42) | 0.16 (−0.22, 0.55) | 0.18 (−0.23, 0.59) | 0.10 (−0.19, 0.38) |
| Body fat percentage (%/year) | 0.07 (0.70) | 0.17 (0.80) | −0.07 (−0.26, 0.12) | −0.07 (−0.26, 0.12) | 0.02 (−0.16, 0.20) | 0.01 (−0.18, 0.20) | – |
| Trunk fat (kg/year) | 0.12 (0.73) | 0.148 (0.70) | 0.00 (−0.02, 0.02) | 0.00 (−0.02, 0.02) | 0.01 (−0.01, 0.02) | 0.01 (−0.01, 0.02) | 0.00 (−0.01, 0.01) |
| Fasting glucose (mmol l−1 year−1) | 0.08 (0.22) | 0.02 (0.18) | 0.05 (−0.01, 0.10) | 0.04 (−0.02, 0.09) | 0.04 (−0.02, 0.10) | 0.04 (−0.02, 0.10) | 0.04 (−0.02, 0.10) |
| 2 h glucose (mmol l−1 year−1) | 0.28 (0.71) | 0.13 (0.47) | 0.15 (−0.01, 0.31) | 0.14 (−0.03, 0.31) | 0.14 (−0.03, 0.31) | 0.15 (−0.04, 0.33) | 0.15 (−0.04, 0.34) |
| ΔGlucose AUC (log[mmol/l × min]/year) | 0.029 (0.143) | 0.018 (0.165) | 0.01 (−0.03, 0.05) | 0.01 (−0.03, 0.05) | 0.01 (−0.03, 0.05) | 0.01 (−0.03, 0.06) | 0.02 (−0.03, 0.06) |
| Fasting insulin (log[pmol l−1 year−1]) | 0.058 (0.151) | 0.033 (0.161) | 0.02 (−0.02, 0.06) | 0.02 (−0.02, 0.06) | 0.02 (−0.02, 0.06) | 0.03 (−0.02, 0.07) | 0.02 (−0.02, 0.06) |
| 2 h insulin (log[pmol l−1 year−1]) | 0.097 (0.214) | 0.045 (0.159) | 0.04 (−0.01, 0.09) | 0.03 (−0.02, 0.09) | 0.03 (−0.02, 0.09) | 0.03 (−0.02, 0.09) | 0.04 (−0.02, 0.09) |
| 30′ΔInsulin (log[pmol l−1 year−1]) | 0.027 (0.156) | 0.024 (0.142) | 0.00 (−0.04, 0.04) | −0.01 (−0.05, 0.04) | 0.00 (−0.04, 0.04) | 0.01 (−0.03, 0.05) | 0.01 (−0.03, 0.05) |
| SI (log[min−1 (μU/ml)−1.× 10−4]/year) | −0.062 (0.118) | −0.025 (0.123) | −0.04* (−0.07, −0.01) | −0.04* (−0.07, −0.01) | −0.05** (−0.08, −0.02) | −0.05** (−0.08, −0.02) | −0.05** (−0.08, −0.02) |
| AIR (log[pmol/l × min]/year) | −0.018 (0.152) | −0.008 (0.109) | −0.01 (−0.05, 0.03) | −0.03 (−0.07, 0.02) | −0.02 (−0.06, 0.02) | −0.02 (−0.06, 0.02) | −0.02 (−0.07, 0.02) |
| DI (log unit/year) | −0.080 (0.159) | −0.033 (0.139) | −0.05* (−0.09, −0.01) | −0.06** (−0.11, −0.02) | −0.06** (−0.11, −0.02) | −0.07** (−0.12, −0.03) | −0.07** (−0.12, −0.03) |
Rate of change was calculated as: (follow-up value – baseline value) / follow-up years
Estimated from the GEE, representing differences in rate of change between the GDM and non-GDM groups for the variables in the first column
Model I: adjusted for age, and age at first pregnancy. Model II: adjusted for the covariates in Model I + baseline percentage body fat. Model III: adjusted for the covariates in Model II + baseline total calorie intake and physical activity. Model IV: adjusted for the covariates in Model III + rate of change in percentage body fat and any additional pregnancy during follow-up
p<0.05,
p<0.01
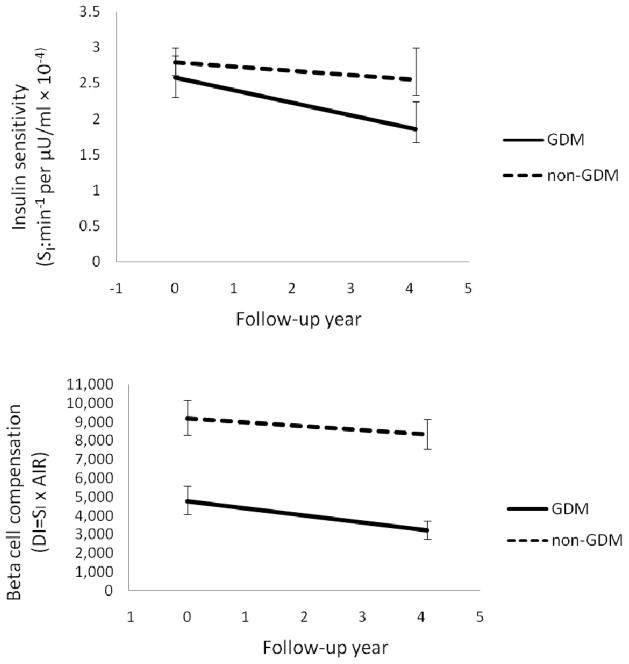
Table 2 also presents the adjusted results. Adjustment for baseline age and age at first pregnancy (Model I) attenuated the intergroup difference for fasting and 2 h glucose, did not change the difference for SI (remained at p=0.02) and increased the difference for DI (to p=0.004). Further adjustment for baseline percentage body fat (Model II) and total calorie intake and physical activity at baseline (Model III) increased the intergroup differences for rate of change of SI and DI (to p=0.002 for both). Figure 1 depicts the means of SI and DI at baseline and at the median follow-up time for the GDM and non-GDM groups after adjustment for age at baseline, age at first pregnancy, baseline percentage body fat, dietary calorie intake and physical activity. SI was similar at baseline and deteriorated more rapidly in women with a history of GDM. DI was considerably lower at baseline and also deteriorated more rapidly in the GDM group. Further adjustment for changes in percentage body fat and/or additional pregnancies did not affect the results (Table 2, Model IV). Additional adjustment for changes in physical activity had no impact on the results (data not shown).
Fig. 1.
Changes in (a) SI and (b) beta cell compensation (DI from the IVGTTs; DI=SI×AIR) in women with (solid line) and without (dashed line) a history of GDM at baseline. Data are geometric means and 95% CI at baseline and at the median follow-up time for each group after adjustment for age, age at first pregnancy, baseline percentage body fat, total calorie intake and level of physical activity
To determine whether the difference in rate of change of DI between the GDM and non-GDM groups was due to a difference in the rate of change of insulin resistance, we added the rate of change of SI to Model IV as an additional covariate, with the rate of change of DI as the outcome. Adjustment for the rate of change of SI reduced the intergroup difference in the rate of DI change by 49%, and the remaining intergroup difference in the rate of DI change became marginally significant (p=0.07).
As a sensitivity analysis, we performed data analysis using unrelated individuals. Doing so excluded 49 women with GDM and 86 women without GDM. The resulting sample sizes were 44 women with GDM and 56 women without GDM. Results from the unrelated individuals were similar to the results reported when including all the women (data not shown). Excluding 33 women (21 in the GDM group and 12 in the non-GDM group) who had IFG at baseline gave similar conclusions.
Discussion
This study is unique in that it provided more than 4 years of follow-up with detailed physiological measurements to compare the natural history of glucose regulation outside pregnancy between parous Mexican-American women with and without a history of GDM. Four main findings were observed. First, women with a history of GDM showed a faster deterioration in SI over time than their non-GDM counterparts. Second, women with prior GDM had lower beta cell compensation at study entry and their beta cell compensation continued to deteriorate at a faster rate during follow-up than was seen for women without prior GDM. Third, the faster deterioration in SI and beta cell compensation in the prior GDM group occurred in the absence of apparent differences in body fat and was not explained by additional pregnancies or differences in physical activity during follow-up. Finally, the excess decline in beta cell compensation in women with GDM was not entirely due to increasing insulin resistance since adjustment for the change in SI only accounted for 49% of the between-group difference and the remaining difference was still marginally significant. Thus, our results indicate that Mexican-American women with a recent history of GDM show a more rapid decline in SI and beta cell compensation than their parous non-GDM counterparts, independent of baseline age, obesity, diet and physical activity as well as changes in obesity and additional pregnancies.
Our findings are consistent with the concept that GDM commonly represents the detection of a chronic condition characterised by insulin resistance and failing beta cell compensation for that resistance [7]. The women with prior GDM in our cohort were younger and did not show significantly greater insulin resistance at baseline because of our matching effect on BMI at baseline recruitment, but had much lower insulin secretion and beta cell compensation at baseline compared with the women without GDM. Thereafter, SI fell more rapidly without much increase in insulin secretion, resulting in a more rapid deterioration in beta cell compensation in the women with prior GDM. Both observations were consistent with a progressive beta cell defect that was first detected in pregnancy.
This pattern complements results from previous short-term studies [6, 20, 21] that have shown beta cell deterioration in women with GDM during and shortly after pregnancy. Although it remains possible that glucose intolerance is limited to pregnancy in some women, the weight of evidence points to a chronic disease state that is detected in pregnancy and eventually leads to impaired glucose levels and type 2 diabetes. The fact that there were no significant differences in glucose profiles between groups and that very few women in the GDM group developed diabetes in the 4 years of follow-up may be due to the fact that a substantial loss of beta cell compensation is required to raise glucose levels to the diabetic range [7, 22].
Factors leading to the faster increase in insulin resistance in the women with GDM remain to be discovered. The authors previously found, in a group of 60 women with prior GDM, that weight gain was significantly associated with a deterioration of beta cell function [23]. In this study, we also observed that weight gain was associated with falling SI (p=0.002) and beta cell compensation (p=0.01) (data not shown). However, we did not find that absolute fat, change in weight or fat, additional pregnancies during follow-up explained the excess deterioration of SI and beta cell compensation seen in the women with GDM. We did not observe a differential impact of weight gain on the deterioration of SI and beta cell compensation between women with and without GDM (interaction tests p>0.79 for both). This finding suggests biological differences in fat between the two groups. We do not have imaging data that would allow us to assess visceral or organ fat in this cohort, and we do not have measures of adipokines. A number of genetic variants have been shown to be associated with GDM [24], and it is possible that differences in genetic susceptibility may play a role in the faster decline in SI and beta cell function. These potential mechanisms for our observations remain to be explored.
We acknowledge some limitations to our study. First, our study sample included the siblings and cousins of the women with a history of GDM. We used the GEE approach to statistically adjust for the non-independence of the study participants; however, it is possible that the actual differences in rate of deterioration between women with and without GDM from a random sample of Mexican-American women might be different. Second, women with a fasting glucose level ≥ 7.0 mmol/l at baseline or follow-up were not included in the main study since SI could not be validly assessed using IVGTTs for individuals with overt diabetes. Therefore, our results represented the natural history of change prior to the development of overt diabetes. Third, we do not have follow-up dietary data for this report; therefore, the effect of any differences in dietary change could not be assessed. Finally, only Mexican-American women were included in this study and differences in metabolic deterioration between women with and without GDM in other racial/ethnic groups might be different; it has previously been shown that the risk of developing diabetes associated with GDM varies by race/ethnicity [2].
The strength of this study is our unique sample, including relatively large and long-term longitudinal follow-up with detailed OGTT- and IVGTT-based measures of SI and beta cell compensation at baseline and follow-up. Unlike previous longitudinal studies, which were mostly short term and centred around pregnancy [6, 20, 21], our cohort included women with and without a history of GDM with an average of more than 4 years of follow-up outside pregnancy. We also have DEXA-assessed adiposity levels and physical activity questionnaires at baseline and follow-up, so we were able to assess whether the excess decline in insulin sensitivity and beta cell compensation over time in women prior to GDM was caused by differences in changes in these two factors as well as additional pregnancies.
In summary, with over 4 years of follow-up with detailed physiological testing in Mexican-American women, we found that women with a history of GDM had poor metabolic profiles at baseline and a faster deterioration in SI and beta cell compensation than women without such a history. The differences were not explained by age, body fat content, diet, physical activity or additional pregnancies. Our findings provide important evidence supporting the concept that GDM is one manifestation of a progressive beta cell disease that is detected during pregnancy but continues unabated thereafter in the absence of specific interventions [25]. Moreover, our results suggest that the degree of adiposity [23] is not the only determinant of falling SI and beta cell function, suggesting that a variation in fat biology and/or genetic susceptibility may contribute to differences in deterioration between women with and without GDM. Our results highlight the need to improve SI and beta cell function in women with prior GDM. Indeed, interventions targeting a reduction in insulin resistance using pharmacological agents [26–28] or lifestyle style changes [28] can delay or prevent type 2 diabetes in women with a history of GDM.
Acknowledgments
We thank the participants who took part in the BetaGene study and in particular those who returned to participate in the BetaGene-II study. We also acknowledge the efforts of our recruiting and technical staff, the support of the University of Southern California General Clinical Research Center (M01-RR-00043), the SC CTSI (UL1-RR-031986) and their respective staff, and Kaiser Permanente Southern California Direct Community Benefit funds.
Funding
This work was supported by National Institutes of Health grants DK-061628 and UL1-TR000130; by Clinical Research Grant 7-09-CT-09 from the American Diabetes Association Research Award.
Abbreviations
- 30′ΔInsulin
Difference in plasma insulin level between the 30 min and fasting time points of the OGTT
- ΔGlucose AUC
Incremental AUC for the 2 h OGTT glucose curve
- AIR
Acute insulin response
- DEXA
Dual-energy x-ray absorptiometry
- DHHS
Department of Health and Human Services
- DI
Disposition index
- GDM
Gestational diabetes mellitus
- GEE
Generalised estimating equation
- IFG
Impaired fasting glucose
- IGT
Impaired glucose tolerance
- IQR
Interquartile range
- SI
Insulin sensitivity
Footnotes
Duality of interest
The authors declare that there is no duality of interest associated with this manuscript.
Contribution statement
AHX, RMW and TAB contributed to study conception and design; AHX, MT, ET, JML, RMW and TAB contributed to data acquisition; AHX, MT and MHB contributed to analysis and interpretation of data; AHX drafted the manuscript; MT, MHB, ET, JML, RMW and TAB reviewed and revised the manuscript critically for important intellectual content; all approved the version to be published.
References
- 1.Bellamy L, Casas JP, Hingorani AD, Williams D. Type 2 diabetes mellitus after gestational diabetes: a systematic review and meta-analysis. Lancet. 2009;373:1773–1779. doi: 10.1016/S0140-6736(09)60731-5. [DOI] [PubMed] [Google Scholar]
- 2.Xiang AH, Li BH, Black MH, et al. Racial and ethnic disparities in diabetes risk after gestational diabetes mellitus. Diabetologia. 2011;54:3016–3021. doi: 10.1007/s00125-011-2330-2. [DOI] [PubMed] [Google Scholar]
- 3.Feig DS, Zinman B, Wang X, Hux JE. Risk of development of diabetes mellitus after diagnosis of gestational diabetes. CMAJ. 2008;179:229–234. doi: 10.1503/cmaj.080012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Catalano PM, Tyzbir ED, Wolfe RR, et al. Carbohydrate metabolism during pregnancy in control subjects and women with gestational diabetes. Am J Physiol. 1993;264:E60–E67. doi: 10.1152/ajpendo.1993.264.1.E60. [DOI] [PubMed] [Google Scholar]
- 5.Xiang AH, Peters RK, Trigo E, Kjos SL, Lee WP, Buchanan TA. Multiple metabolic defects during late pregnancy in women at high risk for type 2 diabetes. Diabetes. 1999;48:848–854. doi: 10.2337/diabetes.48.4.848. [DOI] [PubMed] [Google Scholar]
- 6.Homko C, Sivan E, Chen X, Reece EA, Boden G. Insulin secretion during and after pregnancy in patients with gestational diabetes mellitus. J Clin Endocrinol Metab. 2001;86:568–573. doi: 10.1210/jcem.86.2.7137. [DOI] [PubMed] [Google Scholar]
- 7.Buchanan TA, Xiang AH. Gestational diabetes mellitus. J Clin Invest. 2005;115:485–491. doi: 10.1172/JCI24531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Watanabe RM, Allayee H, Xiang AH, et al. Transcription factor 7-like 2 (TCF7L2) is associated with gestational diabetes mellitus and interacts with adiposity to alter insulin secretion in Mexican Americans. Diabetes. 2007;56:1481–1485. doi: 10.2337/db06-1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen Z, Black MH, Watanabe RM, et al. Self-reported physical activity is associated with beta-cell function in Mexican American adults. Diabetes Care. 2013;36:638–644. doi: 10.2337/dc12-1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bergman RN. Toward physiological understanding of glucose tolerance. Minimal model approach. Diabetes. 1989;38:1512–1527. doi: 10.2337/diab.38.12.1512. [DOI] [PubMed] [Google Scholar]
- 11.Black MH, Fingerlin TE, Allayee H, et al. Evidence of interaction between PPARG2 and HNF4A contributing to variation in insulin sensitivity in Mexican Americans. Diabetes. 2008;57:1048–1056. doi: 10.2337/db07-0848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li X, Allayee H, Xiang AH, et al. Variation in IGF2BP2 interacts with adiposity to alter insulin sensitivity in Mexican Americans. Obesity (Silver Spring) 2009;17:729–736. doi: 10.1038/oby.2008.593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shu YH, Hartiala J, Xiang AH, et al. Evidence for sex-specific associations between variation in acid phosphatase locus 1 (ACP1) and insulin sensitivity in Mexican-Americans. J Clin Endocrinol Metab. 2009;94:4094–4102. doi: 10.1210/jc.2008-2751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Willett WC, Sampson L, Stampfer MJ, et al. Reproducibility and validity of a semiquantitative food frequency questionnaire. Am J Epidemiol. 1985;122:51–65. doi: 10.1093/oxfordjournals.aje.a114086. [DOI] [PubMed] [Google Scholar]
- 15.Kolonel LN, Henderson BE, Hankin JH, et al. A multiethnic cohort in Hawaii and Los Angeles: baseline characteristics. Am J Epidemiol. 2000;151:346–357. doi: 10.1093/oxfordjournals.aje.a010213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nothlings U, Wilkens LR, Murphy SP, Hankin JH, Henderson BE, Kolonel LN. Body mass index and physical activity as risk factors for pancreatic cancer: the Multiethnic Cohort Study. Cancer Causes Control. 2007;18:165–175. doi: 10.1007/s10552-006-0100-0. [DOI] [PubMed] [Google Scholar]
- 17.United States Department of Health and Human Services. [accessed 1 May 2011];2008 Physical Activity Guidelines for Americans. 2008 Available from www.health.gov/PAGuidelines/pdf/paguide.pdf.
- 18.Boston RC, Stefanovski D, Moate PJ, Sumner AE, Watanabe RM, Bergman RN. MINMOD Millennium: a computer program to calculate glucose effectiveness and insulin sensitivity from the frequently sampled intravenous glucose tolerance test. Diabetes Technol Ther. 2003;5:1003–1015. doi: 10.1089/152091503322641060. [DOI] [PubMed] [Google Scholar]
- 19.Rosner B. Fundamentals of biostatistics. Duxbury Press; Pacific Grove, CA: 2000. [Google Scholar]
- 20.Catalano PM, Huston L, Amini SB, Kalhan SC. Longitudinal changes in glucose metabolism during pregnancy in obese women with normal glucose tolerance and gestational diabetes mellitus. Am J Obstet Gynecol. 1999;180:903–916. doi: 10.1016/s0002-9378(99)70662-9. [DOI] [PubMed] [Google Scholar]
- 21.Retnakaran R, Qi Y, Sermer M, Connelly PW, Hanley AJ, Zinman B. Beta-cell function declines within the first year postpartum in women with recent glucose intolerance in pregnancy. Diabetes Care. 2010;33:1798–1804. doi: 10.2337/dc10-0351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xiang AH, Wang C, Peters RK, Trigo E, Kjos SL, Buchanan TA. Coordinate changes in plasma glucose and pancreatic beta-cell function in Latino women at high risk for type 2 diabetes. Diabetes. 2006;55:1074–1079. doi: 10.2337/diabetes.55.04.06.db05-1109. [DOI] [PubMed] [Google Scholar]
- 23.Xiang AH, Kawakubo M, Trigo E, Kjos SL, Buchanan TA. Declining beta-cell compensation for insulin resistance in Hispanic women with recent gestational diabetes mellitus: association with changes in weight, adiponectin, and C-reactive protein. Diabetes Care. 2010;33:396–401. doi: 10.2337/dc09-1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kwak SH, Kim SH, Cho YM, et al. A genome-wide association study of gestational diabetes mellitus in Korean women. Diabetes. 2012;61:531–541. doi: 10.2337/db11-1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Buchanan TA, Xiang AH, Page KA. Gestational diabetes mellitus: risks and management during and after pregnancy. Nat Rev Endocrinol. 2012;8:639–649. doi: 10.1038/nrendo.2012.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Buchanan TA, Xiang AH, Peters RK, et al. Preservation of pancreatic beta-cell function and prevention of type 2 diabetes by pharmacological treatment of insulin resistance in high-risk hispanic women. Diabetes. 2002;51:2796–2803. doi: 10.2337/diabetes.51.9.2796. [DOI] [PubMed] [Google Scholar]
- 27.Xiang AH, Peters RK, Kjos SL, et al. Effect of pioglitazone on pancreatic beta-cell function and diabetes risk in Hispanic women with prior gestational diabetes. Diabetes. 2006;55:517–522. doi: 10.2337/diabetes.55.02.06.db05-1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ratner RE, Christophi CA, Metzger BE, et al. Prevention of diabetes in women with a history of gestational diabetes: effects of metformin and lifestyle interventions. J Clin Endocrinol Metab. 2008;93:4774–4779. doi: 10.1210/jc.2008-0772. [DOI] [PMC free article] [PubMed] [Google Scholar]