Abstract
Background
To understand the molecular response of tumor cells to therapeutic ionizing radiation (IR), we previously reported that human breast cancer cells derived following chronic exposure to fractionated ionizing radiation (MCF+FIR) showed a transient radioresistance. MCF+FIR cells also demonstrated increased activity of NF-Î B, increased expression of the mitochondrial antioxidant enzyme (MnSOD), and increased expression of a cell cycle regulatory protein (Cyclin B1). The present studies were designed to determine the relationship of NF-Î B, MnSOD and Cyclin B1 expression in cellular adaptive responses to ionizing radiation.
Materials and Methods
The first intron of the cyclin B1 gene with a putative NF-Î B element was cloned into the pGL3 luciferase reporter (pGL3CB1EI1). PGL3CB1EI1 and control NF-Î B luciferase activities were determined in MCF-7 and MCF+FIR cells treated with a single dose of radiation, over expression of the dominant negative mutant IkB (mIkB) or over expression of the SOD2 gene.
Results
MCF+FIR cells derived from fractionated IR demonstrated increased transactivation of the pGL3CB1EI1 and NF-Î B controlled reporter activities, relative to the parental cell line. Transfection of dominant negative mutant IkB that inhibits NF-Î B nuclear translocation, inhibited pGL3CB1EI1 and NF-Î B activity, indicating the NF-Î B dependence of pGL3CB1EI1 mediated transcription. In addition, over expression of the human SOD2 gene (MnSOD) inhibited NF-Î B and pGL3CB1EI1 activity, indicating that superoxide or some species derived from superoxide may have participated in the up-regulation of reporter activity in response to chronic exposure to fractionated ionizing radiation. These results provide evidence suggesting that a signaling pathway involving NF-Î B and Cyclin B1 may contribute to adaptive radioresistance induced by chronic exposure to fractionated IR and support the conclusion that MnSOD appears to be a negative regulator of this pathway.
Keywords: Cyclin B1, ionizing radiation, NF-Î B, mitochondrial antioxidant enzyme, MnSOD
Mammalian cells treated with ionizing radiation (IR) induce a stress response associated with an enhanced tolerance to the subsequent cytotoxicity of radiation (1-3). This adaptive cell phenotypic alteration is originally observed in cells pre-exposed to a low or very low dose of IR (4-7). Tumor cells treated in vitro with relatively high doses of IR, e.g., fractionated ionizing radiation (FIR) used for clinical radiotherapy, also demonstrate a transient radioresistant phenotype (8-11). The molecular mechanisms responsible for IR induced radioresistance remain to be elucidated. Accumulating gene profiling data have demonstrated that exposure to IR is able to activate the transcription of many stress genes, and a small fraction of IR inducible proteins, i.e., transcription factors and elements signaling antiapoptosis and cell cycle control, are believed to be required for increasing cell survival after a lethal dose of IR (11-13). Using chronic exposure of FIR, we have described a gene expression profile altered in breast cancer MCF-7 cells after radiation (9-11). A group of stress responsive elements, i.e., transcription factor NF-Î B, mitochondrial antioxidant enzyme manganese-containing superoxide dismutase (MnSOD) and cell cycle protein Cyclin B1 are activated in the radiation-adapted radioresistance (11, 14).
Cyclin B1 has been shown to be an essential cell cycle component required for the process of transition going from G2- to M-phases (15-18). Cyclin B1 and the phosphorylated Cdc2 are able to form the complex with 14-3-3 proteins (19) that accelerates Cyclin B1/Cdc2 translocation into nucleus and cell cycle regulation. Cyclin B1 has been reported to increase when G2 delay is extended (20) and G2 delay is decreased when Cyclin B1 is over expressed by gene transfection (17). Clinically, Cyclin B1 is found to be associated with the radioresistance observed in patients with squamous cell carcinoma (21, 22) and regional recurrence of head and neck tumors treated by radiotherapy (23). cDNA microarray profiles and Western blotting have shown that Cyclin B1 is activated in human breast cancer MCF-7 cells exposed to fractionated ionizing radiation (9, 10) and in MCF-7 cells stably transfected with human SOD2 gene that encode MnSOD (11). Antisense blocking of MnSOD or Cyclin B1 expression has been shown to increase the radiosensitivity of radiation-derived MCF+FIR cells (9). However, the mechanisms of cyclin B1 activation in radiation-adapted radioresistance have not been identified.
NF-Î B regulates a great variety of genes involved in signaling different stress responses (24) and NF-Î B itself has been shown to be sensitive to IR-induced cell damage (25, 26). IR induces IKK, the protein kinase that phosphorylates IkB-α at Ser-32 and Ser-36, which accelerates activation and nuclear translocation of NF-Î B (25). After activation, a major function of NF-Î B appears to be signaling the antiapoptotic responses in cells exposed to IR (13, 26-28). Inhibition of NF-Î B activation is able to increase apoptosis and radiosensitivity (9, 10, 26, 29-31), and activation of NF-Î B is found causally related to the adaptive resistance to IR in human breast carcinoma MCF-7 cells (9, 11) and human keratinocytes transformed by papilloma virus (10). Blocking NF-Î B by over expressing the mutant form of IkB that reduces NF-Î B translocation to the nucleus decreases cell survival and Cyclin B1 expression (11). These results suggest that determining mechanisms of radiation-induced Cyclin B1 expression may be important to understanding adaptive responses to IR.
Genotoxic stress often requires the co-operation of several signaling pathways. This study was designed to answer the question of whether radiation-induced Cyclin B1 expression is regulated at the level of transcription by NF-Î B and MnSOD as well as determining the relevance of this pathway to IR-induced adaptive responses. An intronic NF-Î B element but not the NF-Î B elements in the 5' and 3' flanking regions has been identified for an active induction of the human SOD2 gene (32). Therefore, NF-Î B of the intronic region of IR inducible genes may play an important role in gene expression. The present study addresses the possibility that the first intron of cyclin B1 with a NF-Î B element is regulated by radiation via NF-Î B and mitochondrial antioxidant MnSOD that is also induced by radiation. Using a specific luciferase reporter controlled by the first intron of cyclin B1 isolated from human breast cancer MCF-7 cells and NF-Î B reporters, we demonstrate here that radiation-induced NF-Î B is responsible for the transactivation controlled by the first intron of cyclin B1 and mitochondria MnSOD appears to function as a negative control for NF-Î B-mediated gene regulation, suggesting a role for superoxide in this process.
Materials and Methods
Plasmid construction
Cyclin B1 1st intron was amplified from genomic DNA from MCF-7 cells by two-step PCR using the following primers: 5’-TGCGGGGTTTAAATCTGAGGCTAGG-3’ and 5’- TAGGCATTTTGGCCTGCAGTTG-3’ for 1st step, 5’-TGTTGGTTTCTGCTGGTTGTAGGTC-3’ and 5’-CTTTGCGCC TGCCATGTTGATCTT-3’ for 2nd step. PCR products were separated by agarose electrophoresis, and the DNA fragment was recovered from a gel to construct the Cyclin B1 first intron controlled luciferase reporter pGL3CB1EI1. The enzyme site attached DNA fragment by PCR was cloned into pGL3E luciferase assay vector (Clontech Laboratories Co., Palo Alto, CA, USA). The NF-Î B luciferase reporters were the same as described before (11).
Cell culture and gene transfection
MCF-7 human breast cancer cell line was obtained originally from the American Type Culture Collection (Manassas, VA, USA). MCF+FIR cells were established as described before (11). All the wild-type and transfected cell lines were maintained in Dulbecco's modified Eagle's medium supplemented with 10% (v/v) fetal-bovine serum. For gene transfection, cells were plated at a density of 2 × 105 cells in a 12-well plate for overnight culture, and the cells were transfected with 1 mg of experimental plasmids by LipofectAMINE 2000 reagent (Invitrogen, Carlsbad, CA, USA). After transfection, the cells were exposed to a single-dose IR and further incubated for 24 h. The transfected cells were washed with ice-cold PBS and lysed in a passive lysis buffer (Promega, Madison, WI, USA) for measuring luciferase activity by Luciferase Reporter Assay System (Promega) or in protein extraction buffer for Western blotting. The samples of each well were analyzed for protein concentration using the BCA protein assay (PIERCE, Rockford, IL, USA) for equal loading controls.
Ionizing radiation
The wild-type MCF-7 and gene-transfected cell lines were maintained in a humidified incubator (5% CO2) at 37°C. Ionizing radiation was performed at room temperature by using Cs-137 mark I irradiator (Dose rate 436 cGy/min; J.L. Shepherd & Associates).
Western blotting
MCF-7, MCF+FIR, and mutant IkB transfected cells were collected from T-25 flasks, washed with cold PBS, and lysed in protein extraction buffer. Protein concentrations were determined using the BCA protein assay kit (PIERCE) and equal amount of proteins were separated by SDS-PAGE and transferred onto PVDF membrane (Millipore, Rockford, IL, USA). Proteins were detected using antibody against each proteins and visualized by ECL Western blotting detection system (Amersham Biotech, Piscataway, NJ, USA).
Results and Discussion
Activation of transcription factor NF-Î B, cell cycle regulating protein Cyclin B1, and the mitochondrial antioxidant enzyme MnSOD, as well as other factors appear to be required for signaling a prosurvival pathway in irradiated cells (9-11, 23, 33, 34). Our prior results demonstrate that Cyclin B1 is strongly inducible in breast cancer MCF-7 cells that showed adaptive radioresistance after chronic exposure to fractionated IR (9, 11). Furthermore, blocking Cyclin B1 by antisense transfection increases MCF-7 radiosensitivity (9, 11). Interestingly, Cyclin B1 has been found to be casually related to the radioresistance in head and neck cancers that show a persistent radioresistance following clinical radiotherapy (23). The present study provides evidence that radioresistant MCF+FIR cells derived following exposure to fractionated IR show increases in Cyclin B1 expression. Comparing to NF-Î B controlled reporters, luciferase reporters pGL3CB1EI1 controlled by the first intron of cyclin B1 that contains a potential NF-Î B element is found sensitive to radiation in wild-type MCF-7 and MCF+FIR cells. Transactivation of pGL3CB1EI1 and NF-Î B reporters are inhibited by overexpression of mutant IkB, suggesting NF-Î B dependence of cyclin B1 induction. Co-transfection of pGL3CB1EI1 with MnSOD expression vectors show a negative control on NF-Î B and pGL3CB1EI1-mediated transcription. Overall, these results suggest that a specific signaling network involving NF-Î B, Cyclin B1 and MnSOD contribute to radioresistance in breast cancer cells treated with fractionated ionizing radiation.
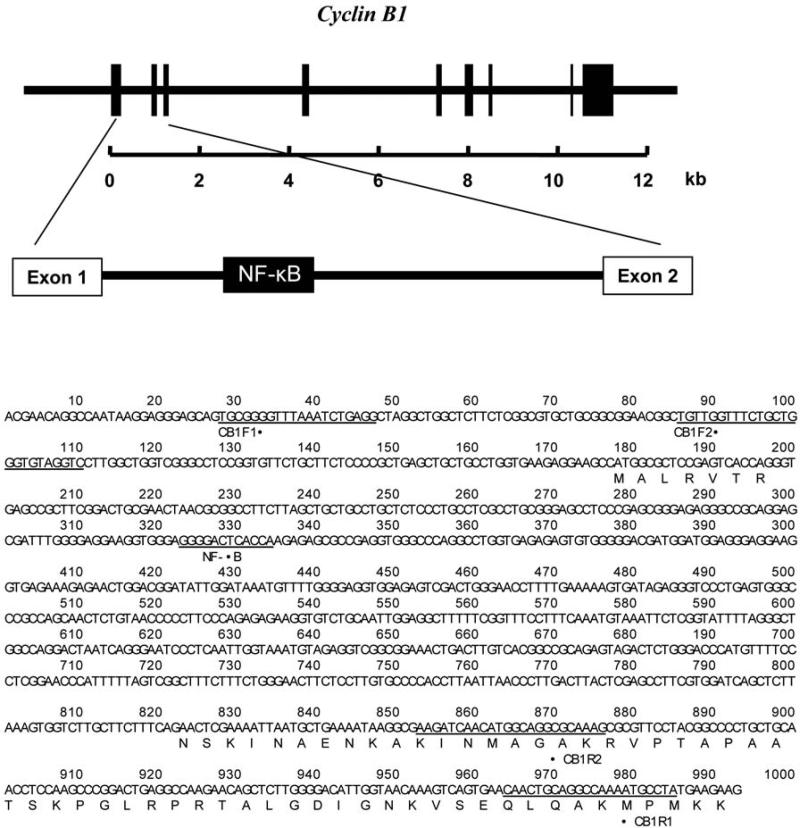
Construction of cyclin B1 1st intron reporters
The promoter and intron regions of human cyclin B1 gene (GenBank No. M25753) was analyzed and computational search for transcription factor-binding sites revealed that, except the NF-Î B binding sites located in the promoter region, a putative NF-Î B site was identified in the first intron as shown in Figure 1. Published work that intronic but not promoter NF-Î B elements are responsible for MnSOD induction encourage us to focus on the function of the first intron of cyclin B1 that may play a potential role in radiation-induced cyclin B1 expression. In order to elucidate the mechanism underlying NF-Î B-mediated regulation, we generated the luciferase reporter pCGLCB1EI1 with PCR fragment of the whole first intron that contains the NF-Î B site, as shown in Figure 1. Co-transfection experiments were performed in parental MCF-7 and radiation-adapted MCF+FIR cells to determine the pGLCB1EI1 luciferase response with the wild-type NF-Î B controlled reporters.
Figure 1.
A putative binding site of NF-Î B of cyclin B1 first intron. A putative NF-Î B binding site was located in the first intron of human cyclin B1 gene (GenBank #M25753). The marked regions (CB1F2 and CB1R2) were designed to produce fragment of cDNA from human breast cancer MCF-7 cells used for construction of cyclin B1-first-intron luciferase reporters (pGL3CB1EI1).
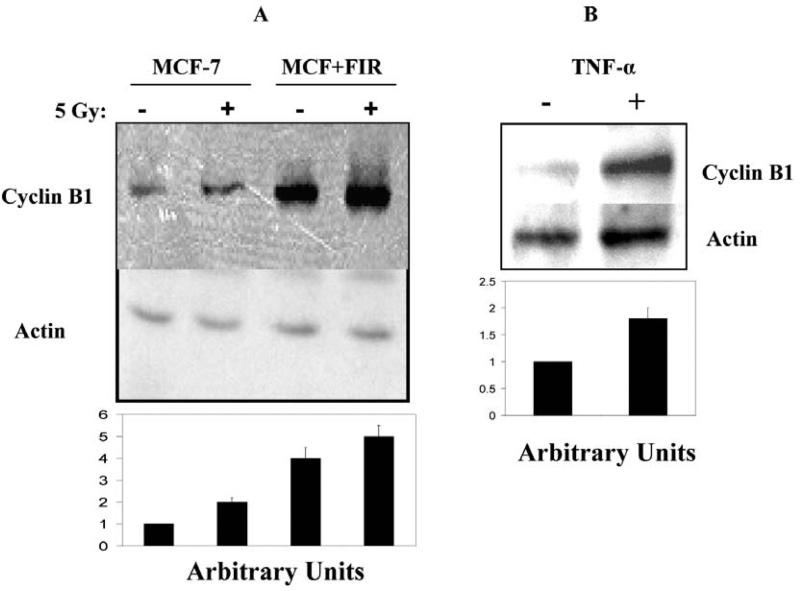
Induction of Cyclin B1 expression by radiation and TNF-α
Currently, specific genes causing radiation adaptive resistance have not been fully identified. Accumulating results, especially gene microarray profiles, suggest a group of IR-induced cell cycle elements may decide the fate of irradiated cells (35, 36). We have shown that Cyclin B1 expression was induced following multiple doses of IR in human breast cancer MCF-7 cells that demonstrated radioresistant phenotype (9). The function of Cyclin B1 in the signaling radioresistant phenotype is further evidenced by the facts that radiation-induced adaptive radioresistance is reduced by antisense blocking Cyclin B1 expression (9) and that Cyclin B1 is activated in radioresistant tumors (23). We hypothesized that IR-induced adaptive radioresistance is mediated by induction of Cyclin B1 that is due to activation of NF-Î B. Following this reasoning, we analyzed the induction of Cyclin B1 in wild-type MCF-7 cells treated with TNF-α that induces NF-Î B activation as a positive control. Figure 2 shows that a single dose of 5 Gy increases Cyclin B1 expression, and the basal Cyclin B1 protein level is elevated in the multiple dose-adapted radioresistant MCF+FIR cells that showed a further induction of Cyclin B1 after a single dose 5 Gy IR (Figure 2 A). In agreement with IR induction, Cyclin B1 is also induced by exposure to TNF-α (Figure 2B). Since TNF-α has been well documented to be an inducer of NF-Î B, these results suggest, but do not prove, that IR-induced Cyclin B1 expression is a consequence of NF-Î B induction.
Figure 2.
A. Cyclin B1 expression was induced by IR and TNF-α in wild-type MCF-7 and radiation-treated MCF-7 cells MCF+FIR. Wild-type MCF-7 and radiation-treated radioresistant MCF+FIR cell populations were exposed to a single dose 5 Gy IR and cyclin B1 expression levels were measured with Western blot 24 h after radiation. B. Cyclin B1 expression was induced in MCF-7 cells by TNF-α. Wild-type MCF-7 and radiation-treated radioresistant MCF+FIR cells were exposed to TNF-α (1 ng/ml for 24 h) and cyclin B1 expression levels were measured with Western blot 24 h after exposure to TNF-α.
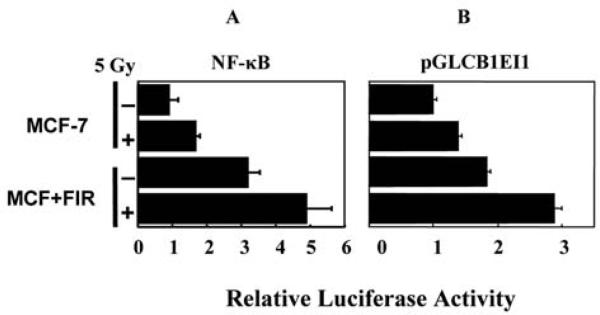
Transcription controlled by the first intron of Cyclin B1 was paralleled by NF-Î B-mediated transactivation by IR
To get insights of transcriptional activation of cyclin B1, Figure 3 further demonstrates that luciferase expression controlled by the first intron of Cyclin B1 (pCGLCB1EI1) or a wild-type NF-Î B is similarly induced by radiation in parental MCF-7 as well as the IR-adapted MCF+FIR cells (Figure 3A). In addition, MCF+FIR cells demonstrated an increased basal level of NF-Î B and pGKCB1EI1-mediated luciferase activity that was increased by 30% and 90%, respectively, for pGLCB1EI1 and NF-Î B-controlled luciferase activities compared to the level of IR-treated wild-type MCF-7 cells. Exposure to a single dose of 5 Gy IR further enhanced NF-Î B and pGLCB1EI1 activity in MCF+FIR cells (~35%; Figure 3). The present results provide the first evidence suggesting that IR-induced NF-Î B activation is responsible for Cyclin B1 expression and this signaling pathway may play an important role in long-term radiation induced radioresistance.
Figure 3.
NF-Î B and pGL3CB1EI1 controlled luciferase reporters were highly activated in MCF+FIR cells. Wild-type MCF-7 and radiation-treated radioresistant MCF+FIR cells were transfected with NF-Î B or pGL3CB1EI1 reporters for 6 h and cultured for 18 h before irradiation with a single dose of 5 Gy. Luciferase activities were measured 24 h after radiation and normalized to the value of basal MCF-7 cells without radiation (mean±S.D., n =3).
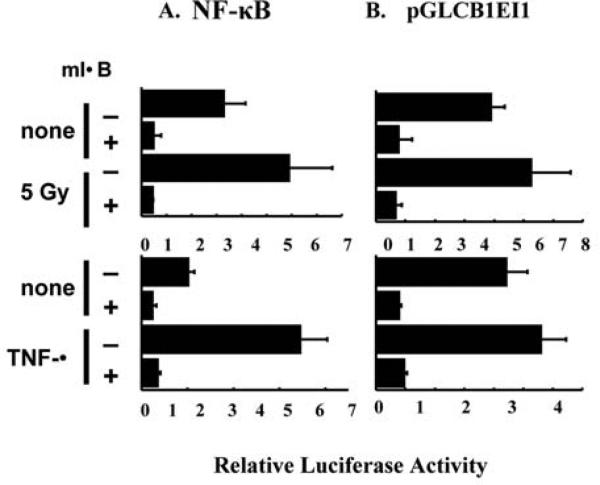
Blocking NF-Î B inhibited pGLCB1EI1 transcription
Next, we determined if over expression of the dominant negative mutant IkB (mIkB) that inhibits NF-Î B nuclear translocation inhibits pGLCB1EI1-controlled luciferase transcription. Experiments in Figure 4 indicate that over expression of mIkB that was co-transfected with luciferase NF-Î B or pGL3ECCNB1, inhibited the basal and IR-induced NF-Î B as well as pCGLCB1EI1-controlled luciferase activity (Figure 4A and 4B). mIkB-mediated inhibition of luciferase activity by pGLCB1EI1 was found to be in consistent with that of NF-Î B inhibition by mIkB. As a control, over expression of mIkB also inhibited TNF-α induced NF-Î B and pGLCB1EI1 luciferase activity. These results clearly support the hypothesis that NF-Î B is involved in radiation-induced increases in Cyclin B1 expression.
Figure 4.
Over expression of dominant negative mutant IkB inhibited basal and IR-induced NF-Î B and pCGLCB1EI1 luciferase activities. Luciferase reporters of NF-Î B or pGL3CB1EI1 were co-transfected respectively with 0.5 mg of dominant negative mutant IkB expression vector in MCF-7 cells, and luciferase activities were measured 24 h after 5 Gy IR or exposure to 1 ng/ml TNF-α (mean±S.D., n=4).
Over expression of human SOD2 genes inhibited NF-Î B and pGLCB1EI1-controlled transcription
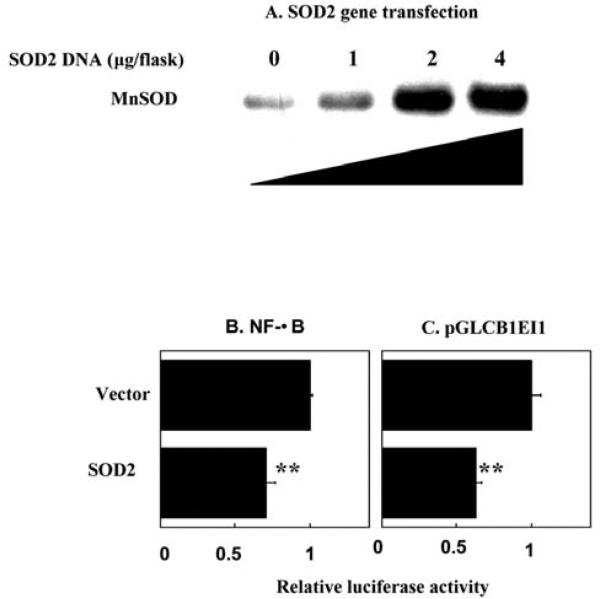
Another critical feature of adaptive radioresistance in tumor cells is the finding that mitochondrial antioxidant MnSOD, that is low or absent in many human cancer cells (37), appears to be sensitive to radiation and involved in signaling prosurvival (11). MnSOD has been shown to regulate several redox sensitive transcription factors including NF-Î B (38) and Cyclin B1 is found increased in MCF-7 cells transfected with SOD2 gene (MCF+SOD) (11). Therefore, the question of whether MnSOD up- or down-regulates cyclin B1 is to be answered. To clarify the relationship between MnSOD expression and NF-Î B -mediated cyclin B1 induction, we analyzed the luciferase reporters controlled by pCGLCB1EI1 or NF-Î B under the condition of over expression of human SOD2 gene. As shown in Figure 5A, by transient transfection of SOD2 plasmids, MnSOD protein levels showed a dose-dependently increase with the amount of SOD2 DNA transfected (Figure 5A). However, a significant inhibition was induced in cells cotransfected with NF-Î B (Figure 5B) or pGLCB1EI1 (Figure 5C) luciferase reporters with MnSOD expression vectors compared to the control of empty vectors. Together, these results suggest that although NF-Î B, Cyclin B1 and MnSOD are induced by IR, overexpression of MnSOD functions as a negative regulator NF-Î B-Cyclin B1 pathway. Since MnSOD is only active after translocation to mitochondria, these results suggest that alterations in the NF-Î B-Cyclin B1 pathway may be mediated by mitochondrial superoxide in cells exposed to fractionated ionizing radiation.
Figure 5.
Over expression of human SOD2 genes inhibited luciferase activity of pCGLCB1EI1 and NF-Î B. MnSOD proteins were dose-dependently increased with SOD2 gene transfection (A). Gene expression vectors containing human SOD2 (11) gene were transiently transfected into MCF-7 cells and MnSOD protein levels were estimated using Western blotting. Luciferase reporters controlled by NF-Î B (B) or pGL3CB1EI1 (C) were co-transfected with (1-4 mg/flask) human MnSOD gene expression vector (SOD2) and luciferase activities were measured 24 h after exposure to 5 Gy IR. Luciferase activity was normalized to protein concentration (mean±S. D., n=4; **p<0.01).
Conclusion
NF-Î B is sensitive to redox alterations (39, 40) and both Cyclin B1 and the mitochondrial antioxidant enzyme MnSOD have been shown to be induced by radiation via NF-Î B regulation (11). Our present data demonstrate that Cyclin B1 expression is increased via NF-Î B activation in MCF-7 cells treated with fractionated ionizing radiation. Over expression of MnSOD demonstrated a negative control on NF-Î B and Cyclin B1 expression. Overall, these results suggest that regulation of the NF-Î B-Cyclin B1 pathway in cells exposed to fractionated IR may be involved with adaptive radioresistance and mediated, at least in part, by alterations in mitochondrial superoxide production.
Acknowledgements
This work was supported by the United States Department of Energy under contracts DE-FG02-03ER63634 and NIH grant CA101990 to JJL and DE-FG02-02ER63447 to DRS. TW is supported by the Beckman Postdoctoral Fellowships from Beckman Foundation. The authors would like to thank Drs. Larry W. Oberley and Daret K. St. Clair for insightful discussion and the gift of the SOD2 expression vectors.
References
- 1.Hill BT, Shellard SA, Hosking LK, Fichtinger-Schepman AM, Bedford P. Enhanced DNA repair and tolerance of DNA damage associated with resistance to cis-diammine-dichloroplatinum (II) after in vitro exposure of a human teratoma cell line to fractionated X-irradiation. Int J Radiat Oncol Biol Phys. 1990;19:75–83. doi: 10.1016/0360-3016(90)90137-9. [DOI] [PubMed] [Google Scholar]
- 2.Joiner MC, Lambin P, Malaise EP, Robson T, Arrand JE, Skov KA, Marples B. Hypersensitivity to very-low single radiation doses: its relationship to the adaptive response and induced radioresistance. Mutat Res. 1996;358:171–183. doi: 10.1016/s0027-5107(96)00118-2. [DOI] [PubMed] [Google Scholar]
- 3.Eichholtz-Wirth H, Marx K. Clonal variability of radiation-induced cisplatin resistant HeLa cells. Anticancer Res. 1998;18:2989–2991. [PubMed] [Google Scholar]
- 4.Azzam EI, de Toledo SM, Raaphorst GP, Mitchel RE. Low-dose ionizing radiation decreases the frequency of neoplastic transformation to a level below the spontaneous rate in C3H 10T1/2 cells. Radiat Res. 1996;146:369–373. [PubMed] [Google Scholar]
- 5.Boothman DA, Odegaard E, Yang CR, Hosley K, Mendonca MS. Molecular analyses of adaptive survival responses (ASRs): role of ASRs in radiotherapy. Hum Exp Toxicol. 1998;17:448–453. doi: 10.1177/096032719801700809. [DOI] [PubMed] [Google Scholar]
- 6.Feinendegen LE, Bond VP, Sondhaus CA, Altman KI. Cellular signal adaptation with damage control at low doses versus the predominance of DNA damage at high doses. C R Acad Sci III. 1999;322:245–251. doi: 10.1016/s0764-4469(99)80051-1. [DOI] [PubMed] [Google Scholar]
- 7.Feinendegen LE. Reactive oxygen species in cell responses to toxic agents. Hum Exp Toxicol. 2002;21:85–90. doi: 10.1191/0960327102ht216oa. [DOI] [PubMed] [Google Scholar]
- 8.Russell J, Wheldon TE, Stanton P. A radioresistant variant derived from a human neuroblastoma cell line is less prone to radiation-induced apoptosis. Cancer Res. 1995;55:4915–4921. [PubMed] [Google Scholar]
- 9.Li Z, Xia L, Lee ML, Khaletskiy A, Wang J, Wong JYC, Li JJ. Effector genes altered in MCF-7 human breast cancer cells after exposure to fractionated ionizing radiation. Radiat Res. 2001;155:543–553. doi: 10.1667/0033-7587(2001)155[0543:egaimh]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 10.Chen X, Shen B, Xia L, Khaletzkiy A, Chu D, Wong JY, Li JJ. Activation of nuclear factor kappaB in radioresistance of TP53-inactive human keratinocytes. Cancer Res. 2002;62:1213–1221. [PubMed] [Google Scholar]
- 11.Guo G, Yan-Sanders Y, Lyn-Cook BD, Wang T, Tamae D, Ogi J, Khaletskiy A, Li Z, Weydert C, Longmate JA, Huang TT, Spitz DR, Oberley LW, Li JJ. Manganese superoxide dismutase-mediated gene expression in radiation-induced adaptive responses. Mol Cell Biol. 2003;23:2362–2378. doi: 10.1128/MCB.23.7.2362-2378.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schmidt-Ullrich RK, Contessa JN, Dent P, Mikkelsen RB, Valerie K, Reardon DB, Bowers G, Lin PS. Molecular mechanisms of radiation-induced accelerated repopulation. Radiat Oncol Investig. 1999;7:321–330. doi: 10.1002/(SICI)1520-6823(1999)7:6<321::AID-ROI2>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 13.McBride WH, Pajonk F, Chiang CS, Sun JR. NF-kappa B, cytokines, proteasomes, and low-dose radiation exposure. Mil Med. 2002;167:66–67. [PubMed] [Google Scholar]
- 14.Li Z, Khaletskiy A, Wang J, Wong JY, Oberley LW, Li JJ. Genes regulated in human breast cancer cells overexpressing manganese-containing superoxide dismutase. Free Radic Biol Med. 2001;30:260–267. doi: 10.1016/s0891-5849(00)00468-8. [DOI] [PubMed] [Google Scholar]
- 15.Muschel RJ, Zhang HB, Iliakis G, McKenna WG. Cyclin B expression in HeLa cells during the G2 block induced by ionizing radiation. Cancer Res. 1991;51:5113–5137. [PubMed] [Google Scholar]
- 16.Metting NF, Little JB. Transient failure to dephosphorylate the cdc2-cyclin B1 complex accompanies radiation-induced G2-phase arrest in HeLa cells. Radiat Res. 1995;143:286–292. [PubMed] [Google Scholar]
- 17.Kao GD, McKenna WG, Maity A, Blank K, Muschel RJ. Cyclin B1 availability is a rate-limiting component of the radiation- induced G2 delay in HeLa cells. Cancer Res. 1997;57:753–758. [PubMed] [Google Scholar]
- 18.Azzam EI, de Toledo SM, Gooding T, Little JB. Intercellular communication is involved in the bystander regulation of gene expression in human cells exposed to very low fluences of alpha particles. Radiat Res. 1998;150:497–504. [PubMed] [Google Scholar]
- 19.Taylor WR, Stark GR. Regulation of the G2/M transition by p53. Oncogene. 2001;20:1803–1815. doi: 10.1038/sj.onc.1204252. [DOI] [PubMed] [Google Scholar]
- 20.Maity A, Hwang A, Janss A, Phillips P, McKenna WG, Muschel RJ. Delayed cyclin B1 expression during the G2 arrest following DNA damage. Oncogene. 1996;13:1647–1657. [PubMed] [Google Scholar]
- 21.Soria JC, Jang SJ, Khuri FR, Hassan K, Liu D, Hong WK, Mao L. Overexpression of cyclin B1 in early-stage non-small cell lung cancer and its clinical implication. Cancer Res. 2000;60:4000–4004. [PubMed] [Google Scholar]
- 22.Hassan KA, El-Naggar AK, Soria JC, Liu D, Hong WK, Mao L. Clinical significance of cyclin B1 protein expression in squamous cell carcinoma of the tongue. Clin Cancer Res. 2001;7:2458–2462. [PubMed] [Google Scholar]
- 23.Hassan KA, Ang KK, El-Naggar AK, Story MD, Lee JI, Liu D, Hong WK, Mao L. Cyclin B1 overexpression and resistance to radiotherapy in head and neck squamous cell carcinoma. Cancer Res. 2002;62:6414–6417. [PubMed] [Google Scholar]
- 24.Baeuerle PA, Baltimore D. NF-kappa B: ten years after. Cell. 1996;87:13–20. doi: 10.1016/s0092-8674(00)81318-5. [DOI] [PubMed] [Google Scholar]
- 25.Li N, Karin M. Ionizing radiation and short wavelength UV activate NF-kappaB through two distinct mechanisms. Proc Natl Acad Sci USA. 1998;95:13012–13017. doi: 10.1073/pnas.95.22.13012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bradbury CM, Markovina S, Wei SJ, Rene LM, Zoberi I, Horikoshi N, Gius D. Indomethacin-induced radiosensitization and inhibition of ionizing radiation-induced NF-kappaB activation in HeLa cells occur via a mechanism involving p38 MAP kinase. Cancer Res. 2001;61:7689–7696. [PubMed] [Google Scholar]
- 27.Barkett M, Gilmore TD. Control of apoptosis by Rel/NF-kappaB transcription factors. Oncogene. 1999;18:6910–6924. doi: 10.1038/sj.onc.1203238. [DOI] [PubMed] [Google Scholar]
- 28.Ravi R, Bedi GC, Engstrom LW, Zeng Q, Mookerjee B, Gelinas C, Fuchs EJ, Bedi A. Regulation of death receptor expression and TRAIL/Apo2L-induced apoptosis by NF-kappaB. Nat Cell Biol. 2001;3:409–416. doi: 10.1038/35070096. [DOI] [PubMed] [Google Scholar]
- 29.Shao R, Karunagaran D, Zhou BP, Li K, Lo SS, Deng J, Chiao P, Hung MC. Inhibition of nuclear factor-kappaB activity is involved in E1A-mediated sensitization of radiation-induced apoptosis. J Biol Chem. 1997;272:32739–32742. doi: 10.1074/jbc.272.52.32739. [DOI] [PubMed] [Google Scholar]
- 30.Lee SJ, Dimtchev A, Lavin MF, Dritschilo A, Jung M. A novel ionizing radiation-induced signaling pathway that activates the transcription factor NF-kappaB. Oncogene. 1998;17:1821–1826. doi: 10.1038/sj.onc.1202088. [DOI] [PubMed] [Google Scholar]
- 31.Kato T, Duffey DC, Ondrey FG, Dong G, Chen Z, Cook JA, Mitchell JB, Van Waes C. Cisplatin and radiation sensitivity in human head and neck squamous carcinomas are independently modulated by glutathione and transcription factor NF-kappaB. Head Neck. 2000;22:748–759. doi: 10.1002/1097-0347(200012)22:8<748::aid-hed2>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 32.Xu Y, Kiningham KK, Devalaraja MN, Yeh CC, Majima H, Kasarskis EJ, St Clair DK. An intronic NF-kappaB element is essential for induction of the human manganese superoxide dismutase gene by tumor necrosis factor-alpha and interleukin-1beta. DNA Cell Biol. 1999;18:709–722. doi: 10.1089/104454999314999. [DOI] [PubMed] [Google Scholar]
- 33.St Clair DK, Wan XS, Oberley TD, Muse KE, St Clair WH. Suppression of radiation-induced neoplastic transformation by overexpression of mitochondrial superoxide dismutase. Mol Carcinogenesis. 1992;6:238–242. doi: 10.1002/mc.2940060404. [DOI] [PubMed] [Google Scholar]
- 34.Epperly MW, Gretton JE, Sikora CA, Jefferson M, Bernarding M, Nie S, Greenberger JS. Mitochondrial localization of superoxide dismutase is required for decreasing radiation-induced cellular damage. Radiat Res. 2003;160:568–578. doi: 10.1667/rr3081. [DOI] [PubMed] [Google Scholar]
- 35.El-Deiry WS. Regulation of p53 downstream genes. Semin Cancer Biol. 1998;8:345–357. doi: 10.1006/scbi.1998.0097. [DOI] [PubMed] [Google Scholar]
- 36.Kasid U. Raf-1 protein kinase, signal transduction, and targeted intervention of radiation response. Exp Biol Med (Maywood) 2001;226:624–625. doi: 10.1177/153537020222600706. [DOI] [PubMed] [Google Scholar]
- 37.Oberley LW, McCormick ML, Sierra-Rivera E, St Clair DK. Manganese superoxide dismutase in normal and transformed human embryonic lung fibroblasts. Free Radical Biol Med. 1989;6:379–384. doi: 10.1016/0891-5849(89)90083-x. [DOI] [PubMed] [Google Scholar]
- 38.Li JJ, Oberley LW, Fan M, Colburn NH. Inhibition of AP-1 and NF-kappaB by manganese-containing superoxide dismutase in human breast cancer cells. FASEB J. 1998;12:1713–1723. doi: 10.1096/fasebj.12.15.1713. [DOI] [PubMed] [Google Scholar]
- 39.Kamata H, Hirata H. Redox regulation of cellular signalling. Cell Signal. 1999;11:1–14. doi: 10.1016/s0898-6568(98)00037-0. [DOI] [PubMed] [Google Scholar]
- 40.Harris AL. Hypoxia--a key regulatory factor in tumour growth. Nat Rev Cancer. 2002;2:38–47. doi: 10.1038/nrc704. [DOI] [PubMed] [Google Scholar]