Abstract
As a part of our continuing investigation of the manzamine alkaloids we studied the in vitro activity of the β-carboline containing manzamine alkaloids against Fusarium solani, Fusarium oxysporium, and Fusarium proliferatum by employing several bioassay techniques including one-dimensional direct bioautography, dilution, and plate susceptibility, and microtiter broth assays. In addition, we also studied the metabolism of the manzamine alkaloids by Fusarium spp. in order to facilitate the redesign of the compounds to prevent resistance of Fusarium spp. through metabolism. The present research reveals that the manzamine alkaloids are inactive against Fusarium spp. and the fungi transform manzamines via hydrolysis, reduction, and a retro Pictet-Spengler reaction. This is the first report to demonstrate an enzymatically retro Pictet-Spengler reaction. The results of this study reveal the utility of the rational design of metabolically stable antifungal agents from this class and the development of manzamine alkaloids as antimalarial drugs through the utilization of Fusarium’s metabolic products to reconstruct the molecule.
Keywords: Manzamine, Fusariumspp, Biotransformation, Metabolism, Resistance
Introduction
The manzamines are a group of alkaloids with complex heterocyclic ring system bearing a β-carboline moiety. The compounds are a product of sponges and unique host-microbial community. Since the first report by Higa et al. in 1986, more than 60 additional manzamine-type alkaloids have been isolated from sponges collected from different geographical regions. To date, there are 16 species belonging to eight families of sponges that have been confirmed to yield the β-carboline containing manzamine and manzamine-related alkaloids. These sponges have been collected from Okinawa, the Philippines, Indonesia, the Red Sea, Italy, South Africa, and Papua New Guinea (Hu et al. 2003). The manzamine alkaloids are reported to possess a diverse range of bioactivities (Ichiba et al. 1988; Kobayashi et al. 1994; Kondo et al. 1992; Mayer et al. 2000; Edrada et al. 1996; El Sayed et al. 1997; Rao et al. 2003, 2004; Yousaf et al. 2004). Manzamine A has been shown to inhibit GSK-3 and CDK-5 and is effective in decreasing tau phosphorylation after treatment in a human neuroblastoma cell line. The underlying mechanism for these activities appears to be kinase inhibition (Hamann et al. 2007). Manzamine A is a promising drug lead for the treatment of malaria. In vivo studies showed that a single intraperitoneal and oral administration of manzamine A or 8-hydroxymanzamine A prolonged the survival of Plasmodium berghei-infected mice more than 10 days (Ang et al. 2000).
The fungicidal activity of manzamine A, ent-8-hydroxymanzamine A, and ent-manzamine F against phyto-pathogenic fungi was reported for Stagonospora nodorum, Phytophtora infestans, and Pyricularia grisei (Peng et al. 2003). However, the compounds showed no activity against Puccinia recondita and Fusarium culmorum with the exception of manzamine F which inhibited 31 % of the growth of F. culmorum at a concentration of 1.7 μM. Fusarium spp. are common soil saprophytes and plant pathogens (Agrios 1988). The genus is also reported to be the cause of opportunistic infections in human immunocompromised patients (Guarro and Gene 1995). Fusarium spp. and its mycotoxins constitute major agricultural and health problems significantly impacting the economy in addition to human and animal health (Bryden et al. 2001). Opportunistic mycoses which are associated with the use of immunosuppressive drugs and AIDS combined with increasing numbers of fungi resistant to available antifungal drugs have driven the need for new anti-fungal drug leads (Kasanah and Hamann 2005). Fusarium spp. can cause a potentially severe opportunistic fungal infection that is primarily encountered in patients with leukemia, immunocompromised patients such as those with allogenic bone marrow or solid organ transplant recipients. There are five species of Fusarium that are most infective to humans: Fusarium solani, Fusarium oxysporium, Fusarium moniliforme, Fusarium verticilloides and Fusarium proliferatum. There are limited numbers of antifungal drugs effective against Fusarium spp. (Lionakis et al. 2003). Recent reports demonstrated that amphotericin B and voriconazole were active against strains of F. solani and F. oxysporium, while itraconazole showed minimal activity (Lewis et al. 2005). Another study showed in vitro synergy of caspofungin and amphotericin B against clinical isolates of Fusarium spp. (Arikan et al. 2002). The discovery of novel antifungal leads that are defined, safe, and have a specific mechanism of action is essential for both the pharmaceutical as well as agrochemical industries (Wedge and Nagle 2000).
We have studied the biotransformation of manzamine alkaloids extensively in order to generate analogs that are more active, less toxic, and that could be utilized in the construction of semisynthetic libraries. 12,34-Oxamanzamine F was first isolated from a sponge and was also shown to be a product of biotransformation of ent-8-hydroxymanzamine A by F. oxysporium ATCC 7601 and Nocardia ATCC 21145 (Yousaf et al. 2002). F. solani and Streptomyces seokies were reported to transform 8-hydroxymanzamine A to manzamine A. Further studies showed that F. solani could transform manzamine F to manzamine E and manzamine X to 6-deoxymanzamine X (Kasanah et al. 2003, 2004). To date, no new manzamine analogs have been reported from microbial transformation studies suggesting that the products isolated from the sponge are likely generated through biotransformation and biocatalysis of manzamine A.
In this paper, we report the metabolism of manzamine as one possible mechanism of inherent resistance of Fusarium spp. to this class of alkaloids. In addition, we discuss the impact of resistance and modification of structure of this lead class of compounds of antimalarial and antifungal agents. The manzamine alkaloids isolated from the sponge Acanthostrongylophora sp. were evaluated for in vitro anti-fungal activity against the phytopathogenic strains of F. solani, F. oxysporum, and F. proliferatum. We employed several bioassays including one-dimensional direct-bioautography, dilution, and plate susceptibility and micro-titer broth assays in order to establish in vitro activity of the manzamine alkaloids against Fusarium spp. and to carefully characterize the products.
Material and Methods
General Experimental Section
Liquid chromatography–mass spectrometry (LC-MS) was measured on a Bruker Daltonik GmbH, Germany, equipped with diode array detector (Agilent Technologies), Agilent 1100 binary pump and Autosampler (Agilent). The electrospray ionization positive mass spectra were acquired using a micrOTOF series mass spectrometer. Conditions, optimized using flow injection of standard and sample solutions, were as follows: electrospray ionization capillary voltage, 4.5 kV; end plate offset voltage, −500 V; nebulizer pressure, 2 bar; dry gas flow, 6 L/min; dry gas temperature 180 °C; source detector voltage 1,600 V; and TOF detector voltage 2,190 V. The electrospray ionization gas was nitrogen. All TOF measurements were performed at high resolution and the TOF analyzer was scanned at m/z 50–1,000 with a 1-s integration time. A flow rate of 0.4 mL/min was used for the analytical column C-8 (2), Phenomenex, Luna 5u, 150×4.6 mm. HPLC grade water and acetonitrile both containing 0.1 % formic acid were used as the mobile phase with a 15-min elution time.
Chromatographic Conditions
Thin layer chromatography (TLC) analysis for monitoring the metabolite was carried out on precoated silica gel UV254 plates (E. Merck, Darmstadt, Germany); mobile phase, hexanes/acetone (7:3) and chloroform/methanol (9:1). Detection of metabolites was performed under UV light at a wavelength of 254 nm. The crude extracts were purified using silica gel gravitational column and eluted with hexanes/acetone (from 100 to 70 % hexanes) and chloroform/methanol (from 100 to 70 % chloroform). Purification of metabolites was carried out by RP-HPLC, Phenomenex, Luna 5u, C-8, 100 Å, 250×10 mm column, a gradient of water/acetonitrile (0.1 % TFA) from 30 to 100 % acetonitrile over 60 min at a flow rate of 5 mL/min. The metabolites were detected at a wavelength of 254 nm.
Fusarium Isolates
F. solani (F-0007), F. oxysporum (F-0001), and F. proliferatum (F0029-1) were obtained from USDA-ARS Laboratory, Natural Products Utilization Research Unit, and National Center for Natural Products Research, the University of Mississippi. The fungi were isolated from an orchid species by Dr. D. E. Wedge and identified by Dr. W. H. Elmer of the Connecticut Agricultural Experiment Station. The cultures were stored on silica gel in the USDA-NPURU repository. The isolates were found to be pathogenic to orchid species.
Manzamine Alkaloids
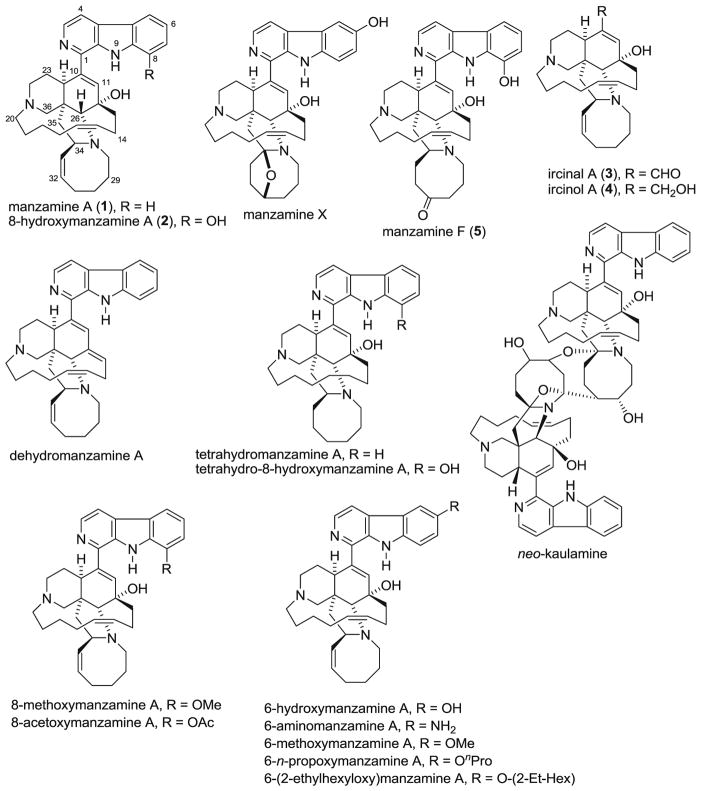
Manzamine alkaloids were isolated from a marine sponge of the genus Acanthostrongylophora sp. collected from Manado, Indonesia. The structures of manzamine and its analogs used in this study are shown in Fig. 1. The isolation and characterization of compounds used as standards was described previously in Rao et al. 2004.
Fig. 1.
Structures of manzamine alkaloids and its analogs used in this study
Conidia Preparation
Fusarium spp. cultures were initiated on potato dextrose agar (PDA, Difco, Detroit, MI) from spores. Conidia were subsequently harvested from 7-day-old culture by flooding plates with 10-mL sterile distilled water and dislodging using an L-shaped glass rod. Conidial suspensions were filtered through sterile Miracloth (Calbiochem-Novabiochem Corp., La Jolla, CA) to remove mycelia. The concentration of conidia was determined photometrically at 625 nm from a standard curve. The conidia suspension (3×105 conidia/mL) was used for all experiments.
One-Dimensional Direct Bioautography
Each compound was dissolved in chloroform at a concentration of 100 μg/mL (175–180 μM) and 1 mg/mL (1.75–1.8 mM). One hundred microliters of solution was spotted on a glass TLC plate (silica gel GF, 250 μm, 10×20 cm, Uniplates, Analtech, Inc., Newark, DE) and allowed to dry. The initial concentration of each compound tested was 100 μg/mL (175–180 μM). After drying, the plates were sprayed with conidial solution of Fusarium spp. under sterile conditions. The conidial solution was prepared with potato dextrose broth 12 g/500 mL, 0.1 % bacto agar, 0.1 % Tween-80 and contained 3×105 conidia/mL of each Fusarium spp. Plates were placed in a moisture chamber (Pioneer Plastic, Inc., Dixon, KY) and incubated for 3 days at 26 °C. After incubation plates were removed from the moisture chamber, dried at room temperature, and exhaustively sprayed with 0.25 % MTT (3-(4,5-dimethylthiazl-2-yl)-2,5-diphenyltetra-zolium bromide, Sigma, St. Louis, MO) prepared in phosphate buffer pH 7 (Sigma, St Louis, MO). The plates were placed back into a moisture chamber and incubated further at 26 °C for another day. The active compound was visualized showing clear zones of fungal growth inhibition on TLC plates with purple background. Amphotericin B, captan, benomyl, and azoxystrobin were used as positive controls to evaluate the activity of manzamine and its analogs. If the compound showed no antifungal activity, the concentration was increased to 1 mg/mL (1.75–1.8 mM) to investigate resistance of Fusarium spp. to the manzamine alkaloids.
Agar Susceptibility Assay
This test was performed on a Petri dish containing 10-mL PDA (Difco). A manzamine solution and antifungal standard (1 mg/mL) were spread on agar and allowed to dry. The conidia suspension Fusarium spp. (100 μL) was spread equally over the plate. The negative control consisted of a conidia suspension, solvent (chloroform), and no fungicidal compound. The positive control consisted of conidia suspension and antifungal standards (benomyl, captan, azoxystrobin, amphotericin B). All plates were incubated at 28 °C for 5 days. Each experiment was replicated three times. The compound was considered active if it inhibited the growth of Fusarium spp. on plates at concentration tested.
Microdilution Broth Assay
Manzamine and its analogs were evaluated in a dose response format for antifungal activity using a 96-well microdilution broth assay at concentrations of 0.3, 3, and 30 μM against F. proliferatum, F. solani, and F. oxysporium according to procedure published by Wedge et al. in 1998. Microtiter plates (Nunc, Micro Well, Roskilde, Denmark) were covered with plastic lids and incubated in a growth chamber at 24±1 °C for a 12-h photoperiod under 60±5 μmol/m2 s. Fungal growth was monitored photometrically by measuring absorbance at 620 nm for 24, 48, and 72 h. Mean absorbance values and standard error were used to evaluate fungal growth.
Biotransformation Experiments
Cultures of Fusarium spp. were prepared as follows: 25 mL of potato dextrose broth in 125-mL flask was inoculated with 3×105 conidia/mL and fed manzamine A (1), 8-hydroxymanzamine A (2), ircinal A (3), and manzamine F (5) at a concentration of 100 μg/mL. The metabolites were monitored by TLC after 5 and 7 days. Cultures that contained metabolites were harvested after 7 days, while other cultures which did not contain any metabolites were allowed to grow for 2 weeks. The metabolites were analyzed as follows: the cultures were extracted with chloroform and ethyl acetate and evaporated under vacuum. The crude extracts were evaluated by TLC and subjected to separation using column chromatography to yield fractions. Further purification of fractions was done using SPE C-8 (Alltech) and evaluated by LC-MS to validate metabolite production.
Quantitative Analysis of Metabolites
Separate standard calibration curves were prepared over a concentration range of 0.2–20 μg/mL for 8-hydroxymanzamine A (2), ircinal A (3), and manzamine F (5). For each curve, six different concentrations (0.2, 0.5, 1.0, 5.0, 10, and 20 μg/mL) were used and the calculated peak area plotted against the concentration of each metabolite. The data showed a linear relationship with correlation coefficients (R2) of 0.994, 0.969, and 0.999 for alkaloids 2, 3, and 5, respectively. The quantitation of metabolites was accomplished by comparing the relative peak area of the extracted ion chromatogram of m/z 565±0.5, 411±0.5, and 581±0.5, in accordance to each metabolite present in the mixture for 2, 3, and 5, respectively.
Results
The in vitro bioactivity of manzamine alkaloids against Fusarium spp
Three bioassays were employed to examine the in vitro activity of manzamine alkaloids against Fusarium spp. In summary, the data collected from the microdilution broth assay exhibited that F. oxysporum, F. proliferatum, and F. solani were not susceptible to the manzamine alkaloids. The fungi were sensitive to the antifungal standards, and the response depends both upon the fungus and metabolites. F. oxysporum and F. solani are more susceptible to amphotericin B while F. proliferatum is more susceptible to captan (Figs. 2, 3, and 4).
Fig. 2.
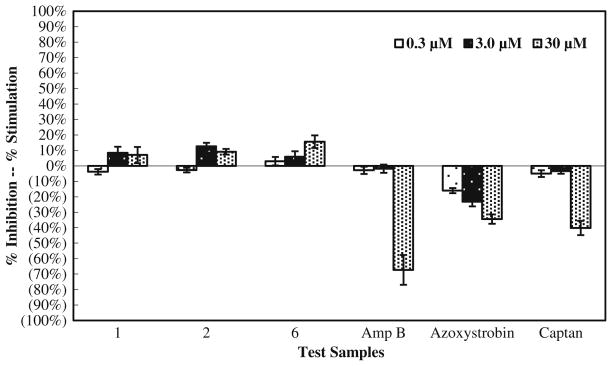
The growth of F. oxysporum in a dose-dependent response to manzamine and its analogs in a microdilution broth assay
Fig. 3.
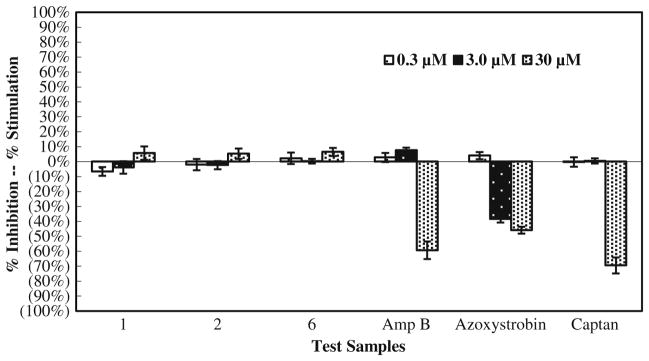
The growth of F. proliferatum in a dose-dependent response to manzamine and its analogs in a microdilution broth assay
Fig. 4.

The growth of F. solani in a dose-dependent response to manzamine and its analogs in a microdilution broth assay
Metabolisms of Manzamine Alkaloids by Fusarium spp
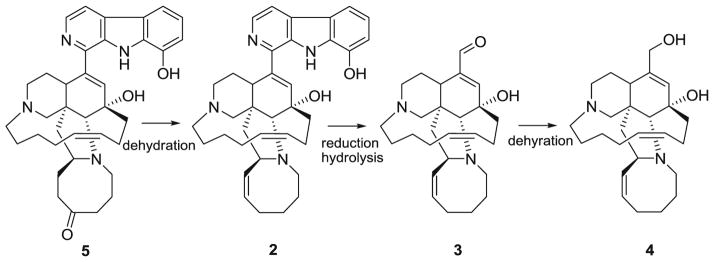
The metabolism study of the manzamine alkaloids by Fusarium spp. was designed to help improve our understanding of how Fusarium spp. are inherently resistant to these types of compound and to provide an opportunity to redesign this class of kinase inhibitors for optimized activity and reduced toxicity. The metabolism studies were conducted for manzamines 1, 2, and 5. Herein, we discuss biotransformation of 5 to 4 in detail. Other biotransformation products are tabulated in Table 1.
Table 1.
Metabolites of manzamine alkaloids by Fusarium spp.
| Fusarium spp. | Starting compounds | Metabolites |
|---|---|---|
| F. solani (F-0007) | Manzamine A (1) | 8-Hydroxymanzamine A (2) |
| Manzamine F (5) | 8-Hydroxymanzamine A (2) Ircinal A (3), ircinol A (4). |
|
| Ircinal a (3) | Ircinol a (4) | |
| 8-Hydroxymanzamine a (2) | Ircinal a (3) | |
| F. oxysporium (F-0001) | Manzamine a (1) | 8-Hydroxymanzamine A (2) |
| Manzamine F (5) | Ircinol A (4) 8-Hydroxymanzamine A (2) |
|
| Ircinal A (3) | No metabolite | |
| 8-Hydroxymanzamine A (2) | No metabolite | |
| F. proliferatum (F-0029-1) | Manzamine A (1) | No metabolite |
| Manzamine F (5) | No metabolite | |
| Ircinal A (3) | No metabolite | |
| 8-Hydroxymanzamine A (2) | No metabolite |
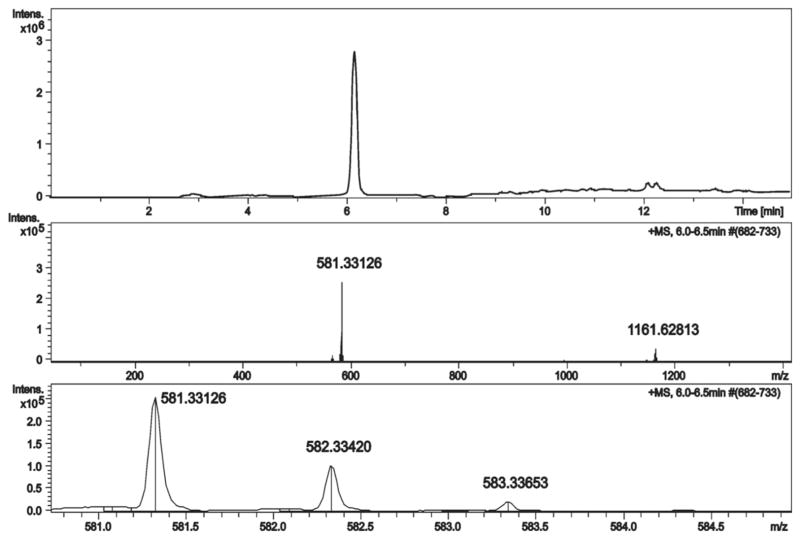
Data from LC-MS spectra (Fig. 5) revealed a single signal at retention time 6.2 min that corresponds to the authentic standard of 6 at m/z 581.3313. No traces of signal were observed that correspond to other standard manzamine alkaloids at the LC-MS detection limit (10 pg).
Fig. 5.
LC-MS spectra of starting material (manzamine F 5)
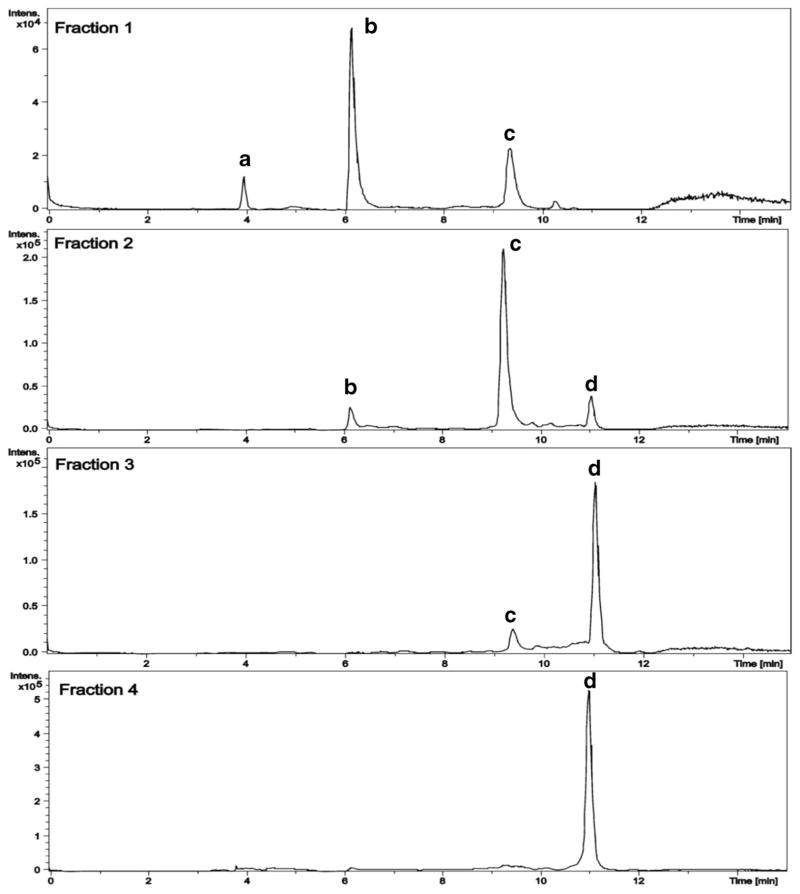
Purification of metabolites by HPLC yielded four major fractions which were analyzed further by LC-MS and compared with authentic manzamine standards. Chromatograms of four fractions after HPLC are shown in Fig. 6.
Fig. 6.
LC-MS chromatograms of each fraction after purification by HPLC. aircinol A (4); bmanzamine F (5); c8-hydroxymanzamine A (2); dircinal A (3) (refer to Fig. 7 for mass spectra analysis)
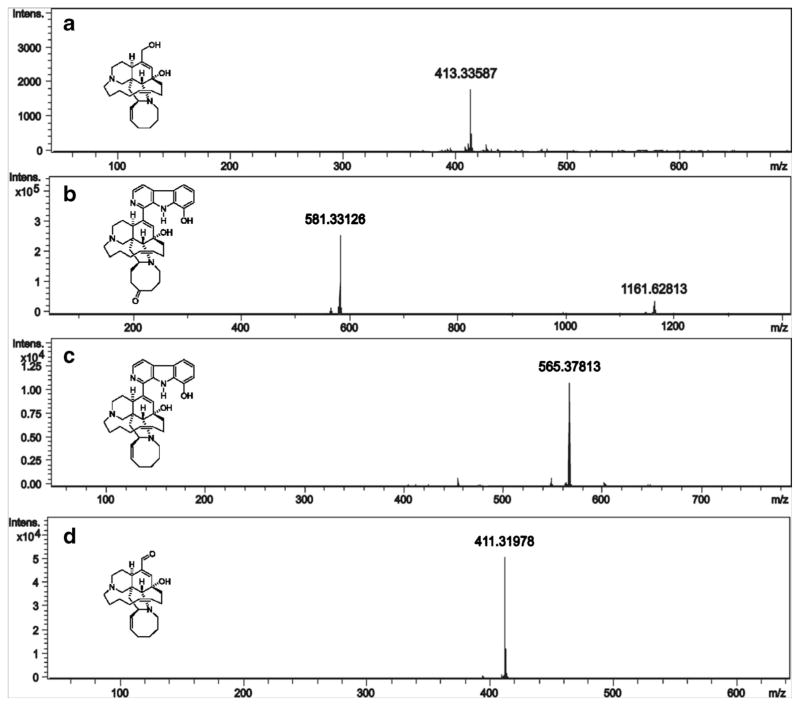
The chromatograms show that fractions 1, 2, and 3 contain a mixture of metabolites while fraction 4 possesses a single compound. Mass spectra analysis of each signal (Fig. 7) revealed that the signal with a retention time of 3.9 min was 4 (m/z 413. 3359), while the signal eluting at 6.2 min represented 5 (m/z 581.3313). The signal at the retention time of 9.3 min corresponded to 2 (m/z 565.3781) while 3 (m/z 411.3198) was detected as a peak with retention time at 11.0 min.
Fig. 7.
Mass spectral analysis of each metabolite. a ircinol A (4); b manzamine F (5); c 8-hydroxymanzamine A (2); d ircinal A (3)
LC-MS analysis revealed that 2, 4, and starting material 5, were obtained from fraction 1 while fraction 2 contained 2as a major metabolite along with 5 and 3. Fraction 3 contained 2 and 3, while fraction 4 showed 3 as a major metabolite. In this study, we showed that metabolite 5 was converted to 2, 3, and 4. The yield of 2 recovered from the culture was 0.98 %±0.02. Ircinal A 3 was determined as a major metabolite with a yield of 4.49 %±0.96. The starting material 5 was recovered from the culture in a yield of 1.27 %±0.035. The transformation of 5 by F. solani may involve several pathways. Optimization of the biotransformation conditions improves the yield of desired metabolites from certain bio-transformation pathways. This is the first report of a retro Pictet-Spengler reaction catalyzed by fungi. The capability of Fusarium spp. to transform antifungal compounds is also reported in a number of papers (Turbek et al. 1992; Yue et al. 1998; Zhang and Smith 1983).
Discussion
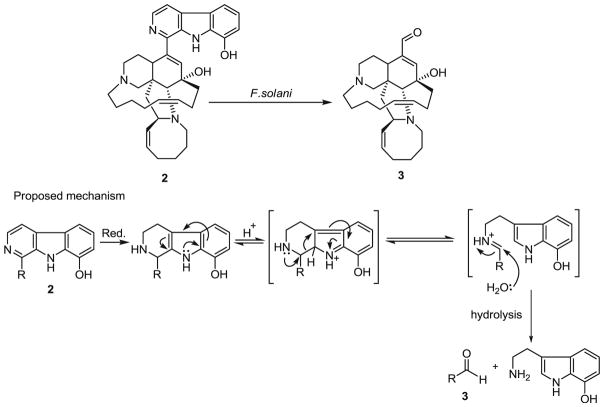
The cumulative data generated from these bioassays demonstrated that F. oxysporum, F. solani, and F. proliferatum are resistant to the manzamine alkaloids. Antifungal resistance is a broad concept that can be described as failure of a fungus to respond to the antifungal agent. The in vitro antifungal resistance has been classified as primary (intrinsic) and secondary (acquired). Primary resistance is present prior to exposure to antifungals while secondary resistance develops after exposure to antifungal agents due to genotypic alterations (Kontoyiannis and Lewis 2002). The resistance of Fusarium spp. to the manzamine alkaloids is clearly an example of intrinsic resistance due to the fact that Fusarium spp. has never been exposed to the natural products. Intrinsic resistance was reported for zygomycetes such as Fusarium spp., Acremonium spp., Scedosporium spp., and dematiaceous fungi (White et al. 1998; Perea and Patterson 2002). Based on metabolites detected from the culture, we propose that 5 was transformed by F. solani to 4 through the formation of 2 and 3. The proposed route of biotransformation is shown in Fig. 8.
Fig. 8.
Proposed route of biotransformation of manzamine F (5) by F. solani
We propose here a possible mechanism of microbial transformation from metabolite 5 to 2 through a series of reasonable transformations. The first step is the reduction of the azacyclooctanone ring to a secondary alcohol moiety which is carried out in the presence of NADPH. The second step is the dehydration of the alcohol to afford a cyclooctene species which in turn participates in a [1,3]-sigmatropic rearrangement to yield 2.
In addition, the microbial metabolism of 2 to 3 (Fig. 9) is proposed to be either a reduction followed by a retro Pictet-Spengler (mechanism A) or hydrolysis-reductive cleavage (mechanism B) pathway. Mechanism A consists of enzymatic reduction of the heteroaromatic ring to the tetrahydro moiety which in turn undergoes a retro Pictet-Spengler reaction to yield 3and hydroxytryptamine. A retro Pictet-Spengler reaction could be involved either in an intermediate with direct bond-cleavage route (path a) or pass through a bond-rearrangement to a spiro skeleton which is converted to the iminium moiety (path b). Mechanism B involves the hydrolysis of the imine group on the β-carboline ring, followed by stabilization of the hemiaminal species to form the corresponding amino and ketone moieties. Reductive cleavage of the ketone with NADPH would result in the formation of the target compound 3 and 3-(2-amino-vinyl)-1H-indol-7-ol as a by-product. During the study of the capability of Fusarium spp. to modify manzamine alkaloids, we observed the metabolism products presented in Table 1.
Fig. 9.
Proposed mechanism of biotransformation of 2 to 3
The metabolism studies of the manzamine alkaloids revealed some highly promising results. Several mechanisms were involved in the transformation of the manzamine alkaloids including oxidation, reduction, hydrolysis, dehydration, and the retro Pictet-Spengler reaction. F. solani metabolized 5 to 2 followed by the formation of 3 and further reduction to 4. Reduction of 3 to 4 was validated when F. solani was fed metabolite 3. No metabolite was detected when F. oxysporum was fed metabolites 2 and 3; however, the fungus transformed 5to 2as well as 1 to 2. F. proliferatum showed the capability to transform the manzamine alkaloids to non-related manzamine metabolites. Reduction is the most dominant reaction catalyzed by Fusarium spp. in this study whereas a retro Pictet-Spengler reaction is the most important and useful mechanism for optimizing this class of drug leads. The biotransformation products are significantly different from those previously reported due to the variety of media used in the biotransformation studies. However, all the products can be found in the sponge (Kasanah et al. 2003, 2004). Fungal pathogens have strategies to counter the effects of antifungal agents in many ways including the biosynthesis of degradative enzymes, overexpression of target sites, altering the target sites, and activating efflux systems (White et al. 1998; Loeffler and Stevens 2003). Biotransformation and degradation of antifungal compounds derived from plants by phytopathogenic fungi including Fusarium spp. are common mechanisms of resistance (Morrissey and Osbourn 1999, Van Etten et al. 1989) and explain how these prolific phytopathogenic fungi may be resistant to many antifungal agents active against other fungi.
The Implication for Antifungal Drug Discovery
The morbidity and mortality associated with resistance and opportunistic fungal infections continue to increase. Seated mycosis caused by resistant filamentous fungi such as Fusarium spp., Scedosporium prolificans, and Aspergillus terreus requires new classes of antifungal agents with new specific targets, better activity and safety than existing antifungal drugs (Canuto and Rodero 2002). Numerous marine natural products have been reported to possess antifungal activity (Li et al. 1998; Molinski 2004). There are limited reports regarding the activity of these compounds against Fusarium spp. We learned from the results presented here that the β-carboline moiety is not a viable pharmacophore against Fusarium spp. due to the fungus’ ability to metabolize this functional group. There are many marine natural products which were discovered as antifungal leads but are cytotoxic as well as many cytotoxic leads which are good antifungal agents. Cytotoxic compounds will indeed exhibit antifungal activity in vitro. Manzamine A was discovered as a cytotoxic compound with a β-carboline ring system which is essential for activity. β-Carboline alkaloids are widely distributed in nature including plants, marine organisms, insects, as well as mammals. This pharmacophore is interesting due to the diverse biological activity such as inhibiting CDK, topoisomerase I, and monoamine oxidase, and interacting with benzodiazepine and 5-hydroxyserotonin receptors and intercalating DNA (Cao et al. 2007). Our results revealed that all manzamine alkaloids with or without modification to the β-carboline ring are inactive against Fusarium spp.
The Significance of a Proposed Retro Pictet-Spengler Reaction in the Optimization of Antimalarial and Antifungal Activity
The Pictet-Spengler reaction is an important reaction for the generation of the tetrahydro-β-carboline and tetrahydroisoquinoline ring systems (Nielsen et al. 2003). This type of reaction is widely involved in the biosynthesis of alkaloids derived from tryptophan including the manzamine alkaloids. The reverse reaction known as the retro Pictet-Spengler reaction is rarely reported and involves the reverse catalytic ring opening and hydrolysis of an iminium group (Zhang et al. 1989). Microbial transformations offer a high degree of stereospecificity and eliminate the need to protect and deprotect functional groups (Abraham and Spassov 2002; Rathbone and Bruce 2002). A biocatalytic approach to performing a retro Pictet-Spengler reaction would be highly valuable in the production of 3which is an important starting material for the development of manzamine-related analogs as antimalarial and antifungal agents. The production of 3, either directly from bacterial biosynthesis or transformation, can have a big impact on how this class of compounds will be developed in the future. The biotransformation of 5to 3 is particularly significant due to the reasonable yield and the absence of biological activity associated with 5.
We have shown that there is a loss of activity that may be attributed in part due to metabolism through a unique mechanism although other contributing factors or mechanisms cannot be dismissed. We report here the metabolism of 6 to 2, 3, and 4 by F. solani through a putative retro Pictet-Spengler mechanism. This transformation is highly significant due to the fact that 3 is an essential starting material in the derivatization of manzamine analogs and 5 is inactive and isolated in high yield from the sponge. Although the classification of this transformation as a retro Pictet-Spengler reaction needs further validation with stable isotopes, no other reasonable explanation can currently be provided. In addition, the results suggest that replacing the β-carboline moiety of these natural products with the heterocyclic moiety may yield antifungal kinase inhibitors that are metabolically stable.
Contributor Information
Noer Kasanah, Email: noer.kasanah@gmail.com, Department of Pharmacognosy, School of Pharmacy, The University of Mississippi, Oxford, MS, USA. Department of Fisheries, Faculty of Agriculture, Gadjah Mada University, Yogyakarta, Indonesia.
Lorelei Lucas Farr, Department of Pharmacognosy, School of Pharmacy, The University of Mississippi, Oxford, MS, USA.
Abbas Gholipour, Department of Pharmacognosy, School of Pharmacy, The University of Mississippi, Oxford, MS, USA.
David E. Wedge, Natural Product Utility-USDA-ARS-NPURU, The University of Mississippi, Oxford, MS, USA
Mark T. Hamann, Department of Pharmacognosy, School of Pharmacy, The University of Mississippi, Oxford, MS, USA
References
- Abraham WR, Spassov G. Biotransformation of alkaloids: a challenge. Heterocycles. 2002;56:711–741. [Google Scholar]
- Agrios GN. Plant pathology. 3. Academic; New York: 1988. pp. 408–409. [Google Scholar]
- Ang KK, Holmes MJ, Higa T, Hamann MT, Kara UA. In vivo antimalarial activity of beta-carboline alkaloid manzamine A. Antimicrob Agent Chemother. 2000;44:1645–1649. doi: 10.1128/aac.44.6.1645-1649.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arikan S, Lozano-Chiu M, Paetznik V, Rex JH. In vitro synergy of caspofungin and amphotericin B against Aspergillus and Fusarium spp. Antimicrob Agents Chemother. 2002;46:245–247. doi: 10.1128/AAC.46.1.245-247.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryden LW, Logrieco A, Abbas KH, Porter JK, Vesonder RF, Richard JL, Cole RJ. In: Other significant fusarium mycotoxin. Fusarium Summerell BA, Leslie FJ, Backhouse D, Bryden WL, Burgess LW, editors. American Phytopathological Society; 2001. p. 360. [Google Scholar]
- Canuto MM, Rodero FG. Antifungal drug resistance to azoles and polyenes. Lancet Infect Dis. 2002;2:550–563. doi: 10.1016/s1473-3099(02)00371-7. [DOI] [PubMed] [Google Scholar]
- Cao R, Peng W, Wang Z, Xu A. β-Carboline alkaloids: biochemical and pharmacological functions. Curr Med Chem. 2007;14:479–500. doi: 10.2174/092986707779940998. [DOI] [PubMed] [Google Scholar]
- Edrada RA, Prosksch V, Wray V, Witte L, Müeller WEG, Van Soest RWM. Four new bioactive manzamine-type alkaloids from the Philippine marine sponge Xestospongia ashmorica. J Nat Prod. 1996;59:1056–1060. doi: 10.1021/np9604083. [DOI] [PubMed] [Google Scholar]
- El Sayed KA, Dunbar CD, Perry TL, Wilkins SP, Hamann MT, Greenplate JT, Wideman MA. Marine natural products as prototypes of insecticidal agents. J Agric Food Chem. 1997;45:2735–2739. [Google Scholar]
- Guarro J, Gene J. Opportunistic fusarial infections in humans. Eur J Clin Microbiol Infect Dis. 1995;14:741–754. doi: 10.1007/BF01690988. [DOI] [PubMed] [Google Scholar]
- Hamann MT, Alonso D, Martin-Aparacio E, Fuertes A, Perez-Puerto JM, Castro A, Morales S, Navarro ML, del Monte-Millán M, Medina M, Pennaka H, Balaiah A, Peng J, Cook J, Wahyuono S, Martinez A. GSK-3 Inhibitory activity and SAR studies of the manzamine alkaloids. Potential for Alzheimer’s disease. J Nat Prod. 2007;70:1397–1405. doi: 10.1021/np060092r. [DOI] [PubMed] [Google Scholar]
- Hu JF, Hamann MT, Hill RT, Kelly M. The manzamine alkaloids. In: Cordell GA, editor. The alkaloids. Vol. 60. Elsevier; New York: 2003. pp. 207–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichiba T, Sakai R, Kohmoto S, Saucy G, Higa T. New manzamine alkaloids from a sponge of the genus Xestospongia. Tetrahedron Lett. 1988;29:3083–3086. [Google Scholar]
- Kasanah N, Hamann MT. Evaluation of SPK-843. Curr Opin Investig Drugs. 2005;6:845–853. [PMC free article] [PubMed] [Google Scholar]
- Kasanah N, Rao KV, Yousaf M, Wedge D, Hamann MT. Biocatalytic conversion of 8-hydroxymanzamine A to manzamine A. Tetrahedron Lett. 2003;44:1291–1293. [Google Scholar]
- Kasanah N, Rao KV, Yousaf M, Wedge DE, Hill RT, Hamann MT. Biotransformation studies of the manzamine alkaloid. Mar Biotech. 2004;6:S268–S272. [Google Scholar]
- Kobayashi J, Tsuda M, Kawasaki N, Sasaki T, Mikami Y. 6-hydroxymanzamine A and 3,4-dihydromanzamine A, new alkaloids from the Okinawan marine sponge Amphimedon sp. J Nat Prod. 1994;57:1737–1741. doi: 10.1021/np50114a021. [DOI] [PubMed] [Google Scholar]
- Kondo K, Shigemori H, Kikuchi Y, Ishibashi M, Sasaki T, Kobayashi J. Ircinals A and B from Okinawan marine sponge Ircinia sp: plausible biogenetic precursor of manzamine alkaloids. J Org Chem. 1992;57:2480–2483. [Google Scholar]
- Kontoyiannis DM, Lewis RE. Antifungal drug resistance of pathogenic fungi. Lancet. 2002;359:1135–1144. doi: 10.1016/S0140-6736(02)08162-X. [DOI] [PubMed] [Google Scholar]
- Lewis RE, Wiederhold NP, Klepser ME. In vitro pharmacodynamics of amphotericin B, itraconazole and voriconazole against Aspergillus, Fusarium and Scedosporium spp. Antimicrob Agents Chemother. 2005;49:945–951. doi: 10.1128/AAC.49.3.945-951.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H-Y, Matsunaga S, Fusetani N. Antifungal metabolites from sponges. Curr Org Chem. 1998;2:649–682. [Google Scholar]
- Lionakis MS, Lewis RE, Samonis G, Kontoyianis DP. Pentamidine is active against Fusarium species. Antimicrob Agents Chemother. 2003;47:3252–32. doi: 10.1128/AAC.47.10.3252-3259.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loeffler J, Stevens DA. Antifungal drug resistance. Clin Infect Dis. 2003;36(Suppl):S31–S41. doi: 10.1086/344658. [DOI] [PubMed] [Google Scholar]
- Mayer AMS, Gunasakera SP, Pomponi SA, Sennett SH. Antiinflammatory uses of manzamines. WO 0056304A220000928 PCT Int Appl, CODEN: PIXXD2. 2000
- Molinski TF. Antifungal compounds from marine organisms. Curr Med Chem Anti-infective Agents. 2004;3:197–220. [Google Scholar]
- Morrissey JP, Osbourn AE. Fungal resistance to plant antibiotics as a mechanism of pathogenesis. Microbiol Mol Biol Rev. 1999;63:708–724. doi: 10.1128/mmbr.63.3.708-724.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen TE, Dines F, Morten M. The Pictet-Spengler reaction in solid phase combinatorial chemistry. Curr Opin Drug Disc Dev. 2003;6:801–814. [PubMed] [Google Scholar]
- Peng J, Shen X, El Sayed KA, Dunbar DC, Perry TL, Wilkins SP, Hamann MT, Bobzin S, Huesing J, Camp R, Prinsen M, Krupa D, Wideman MA. Marine natural products as prototype agro-chemical agents. J Agric Food Chem. 2003;51:2246–2252. doi: 10.1021/jf0207880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perea S, Patterson TF. Antifungal resistance in pathogenic fungi. Clin Infect Dis. 2002;35:1073–1080. doi: 10.1086/344058. [DOI] [PubMed] [Google Scholar]
- Rao KV, Santarsiero BD, Mesecar AD, Schinazi RF, Tekwani BL, Hamann MT. New manzamine alkaloids with activity against infectious and tropical parasitic diseases from Indonesian sponges. J Nat Prod. 2003;66:823–827. doi: 10.1021/np020592u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao KV, Kasanah N, Wahyuono S, Tekwani BL, Schinazi RF, Hamann MT. Three new manzamine alkaloids from common Indonesian sponges and their activity against infectious and tropical parasitic diseases. J Nat Prod. 2004;67:1314–1318. doi: 10.1021/np0400095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rathbone DA, Bruce NC. Microbial transformation of alkaloids. Curr Opin Microbiol. 2002;5:274–281. doi: 10.1016/s1369-5274(02)00317-x. [DOI] [PubMed] [Google Scholar]
- Turbek CS, Li DX, Choi GH, Schardl CL, Smith DA. An extra-cellular enzyme from Fusarium solani f sp. phaseoli which catalyze hydration of the isoflavonoid phytoalexin, phaseollidin. FEMS Microbiol Lett. 1992;94:187–190. doi: 10.1016/0378-1097(92)90606-o. [DOI] [PubMed] [Google Scholar]
- VanEtten HD, Matthews DE, Matthews PS. Phytoalexin detoxification: importance for pathogenicity and practical implications. Ann Rev Phytopathol. 1989;27:143–164. doi: 10.1146/annurev.py.27.090189.001043. [DOI] [PubMed] [Google Scholar]
- Wedge DE, Nagle DG. A New 2D TLC bioautography method for the discovery of novel antifungal agents to control plant pathogens. J Nat Prod. 2000;63:1050–1054. doi: 10.1021/np990628r. [DOI] [PubMed] [Google Scholar]
- White TC, Marr KA, Bowden RA. Clinical, cellular and molecular factors that contribute to antifungal drug resistance. Clin Microbiol Rev. 1998;11:382–402. doi: 10.1128/cmr.11.2.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yousaf M, El Sayed KA, Rao KV, Lim CW, Hu JF, Kelly M, Franzblau F, Peraud O, Hill RT, Hamann MT. 12,34 Oxamanzamine, novel biocatalytic and natural products from manzamine Indo-Pacific producing sponges. Tetrahedron. 2002;59:7397–7402. [Google Scholar]
- Yousaf M, Hammond NL, Peng JP, Wahyuono S, McIntosh KA, Charman WN, Mayer AMS, Hamann MT. New manzamine alkaloids from an Indo-Pacific sponge: pharmacokinetics, oral availability and the significant activity of several manzamine against HIV-1, AIDS opportunistic infections and inflammatory diseases. J Med Chem. 2004;47:3512–3517. doi: 10.1021/jm030475b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue Q, Bacon CW, Richardson MD. Biotransformation of 2-benzoxazolinone and 6-methoxybenzoxazolinone by Fusarium moniliforme. Phytochem. 1998;48:451–454. [Google Scholar]
- Zhang Y, Smith DA. Concurrent metabolism of phytoalexins phaseollin, kievitone and phaseollinisoflavan by Fusarium solani f.sp. phaseoli. Physiol Plant Pathol. 1983;23:89–100. [Google Scholar]
- Zhang LH, Gupta AK, Cook JM. Reinvestigation of mechanism of the acid catalyzed epimerization of reserpine to isoreserpine. J Org Chem. 1989;54:4708–4712. [Google Scholar]