Abstract
OBJECTIVE
To determine which neuroimaging, clinical and sociodemographic factors predict neurodevelopment at 18–22 months age among extremely preterm infants with intraparenchymal hemorrhage (IPH).
STUDY DESIGN
Cranial ultrasounds performed before 42 days of age and cranial ultrasounds/magnetic resonance images of the brain performed near discharge were reviewed for hemorrhage location and other abnormalities. Clinical and sociodemographic factors were extracted from existing databases. The primary outcome was presence of cerebral palsy (CP) and the secondary outcome was cognitive development (Bayley Scales of Infant Development).
RESULT
Of 1168 infants (<1000 g or <27 weeks), 141 infants had an IPH and 48 infants were seen in follow-up. All infants with extensive hemorrhages (involving three or more lobes) developed CP. In early imaging (before 42 days of age), ventriculomegaly, intraventricular hemorrhage (IVH) and extensive hemorrhage were predictors of CP. In imaging performed near discharge, ventriculomegaly, intraventricular echodensity and having a ventricular shunt were predictors of CP. Clinical, imaging and sociodemographic factors were not associated with low cognitive score.
CONCLUSION
In preterm infants surviving with IPH, extensive hemorrhage, ventriculomegaly, IVH and having a shunt increased the risk of developing CP.
Keywords: intraventricular hemorrhage, extremely low birth weight, cerebral palsy, prematurity
INTRODUCTION
Intraparenchymal hemorrhage (IPH) is one of the most serious complications of preterm birth because of its lifelong neurologic consequences. Hemorrhage in the brain parenchyma occurs most commonly in the periventricular white matter with an adjacent intraventricular hemorrhage (IVH). IPH is believed to be caused by venous infarction associated with the IVH.1,2 It is commonly referred to as periventricular hemorrhagic infarction (PVHI) or grade 4 IVH because of the presumed mechanism of hemorrhage.
IPH is seen in 10–15% of preterm infants who have a germinal matrix hemorrhage.1 The incidence of IPH increases with decreasing birth weight, with the greatest risk in infants weighing <750 g.1 Mortality in infants with IPH ranges from 30 to 60%.1 Those who survive are at increased risk for cognitive and/or motor delays and complications including blindness, deafness, seizures and cerebral palsy (CP). Studies have reported a 45–80% prevalence of CP in survivors of IPH.3–7 This wide range of outcomes has led to speculation about biological and environmental risk factors affecting the degree of recovery from severe IVH, but this complex interaction is not well understood.8
The predictive validity of imaging findings for developmental outcomes has not been well established. It seems likely that the location and size of the IPH would affect developmental outcomes, but these associations have not been consistently observed. Recent studies have shown an association between either extent (number of lobes involved)4,6 or laterality (unilateral vs bilateral)5 of hemorrhage, but not both, and neurodevelopmental outcomes (evaluated at >18 months). Other studies have shown an association between ventricular dilation or ventricular decompression and worse neurologic outcomes.7,9–12
The objective of this study was to determine whether neuroimaging findings and selected perinatal, neonatal clinical and sociodemographic factors were predictive of neurodevelopmental outcomes at 18–22 months age in infants surviving with an IPH. This study used detailed imaging categorization and prospectively collected clinical factors to provide prognostic information for clinicians that could be used to counsel families of these infants.
METHODS
Study design
This is a retrospective cohort study of very preterm infants with IPH. The birth cohort included all infants born January 1998 to December 2008 who were admitted to the neonatal intensive care unit at Children’s Memorial Hermann Hospital (outborn infants were included if admitted before 15 days of age) and met criteria to be seen at the University of Texas Medical School at Houston High-Risk Infant Clinic after discharge. Infants with a follow-up window before 1 January 2008 were selected based on a birth weight of less than 1000 g; those with a follow-up window after 1 January 2008 were selected based on a gestational age less than 27 weeks and were inborn only.
Infants in the birth cohort who had an IPH documented on a head ultrasound performed on or before 42 days of age were included in the IPH cohort. Infants with genetic conditions and congenital brain malformations were excluded. Infants from the IPH cohort who survived to 18 months postmenstrual age and were seen in follow-up were included in the follow-up cohort.
This study was approved by the Institutional Review Board of the University of Texas Health Science Center at Houston.
Variables
Clinical variables included gestational age, birth weight, gender, multiple gestation, antenatal steroids, early- and late-onset sepsis, meningitis, length of mechanical ventilation, necrotizing enterocolitis, retinopathy of prematurity and ventricular shunt placement for post-hemorrhagic ventricular dilation. Early-onset sepsis was defined as positive blood culture within the first 72 h after birth. Late-onset sepsis was defined as a positive blood culture after 72 h of age. Necrotizing enterocolitis was defined as modified Bell’s staging criteria II or higher. Retinopathy of prematurity included all stages of retinopathy.
Sociodemographic variables included maternal age, maternal marital status, maternal ethnicity, maternal education, infant’s medical insurance and primary language used at home. If the social information was not recorded during the hospitalization, the information obtained immediately after discharge was used.
Maternal and infant clinical and sociodemographic information were obtained from two neonatology databases, including information collected for the NICHD (National Institute of Child Health and Human Development) Neonatal Research Network Generic Database, and electronic medical records.
Imaging classification
Ultrasound was performed using high-frequency (7–12 mHz) linear array tranducers (Philips HDI 500 and IU22 units, Philips Healthcare, Amsterdam, The Netherlands). Standard coronal and sagittal images were obtained from a transfontanelle approach with additional axial posterior fossa images through the posterolateral fontanelle. Magnetic resonance imaging (MRI) studies were performed on 1.5- and 3.0-T units (GE Healthcare, Buckinghamshire, UK; Philips). MRI imaging protocols varied slightly over the years of the study, but all studies included the following sequences: axial and sagittal T1 (spin-echo (SE) repetition time/echo time 470/12); axial, coronal T2 (fast SE repitition time/echo time 3000–5000/100); axial diffusion-weighted (SE-echo-planar imaging b-value =1200) and axial FLAIR. The slice thickness for most MR sequences was 3 mm with 0 gap, field of view 18–20. Imaging studies were evaluated by a single pediatric radiologist with 25 years of experience. The radiologist was masked to patient clinical outcomes.
Neuroimaging studies were classified into early imaging (performed on or before 42 days of age) and late imaging (performed after 42 days with most being near 38 weeks postmenstrual age). Early imaging included only head ultrasounds and late imaging included a head ultrasound and/or MRI of the brain. In our unit, preterm infants with a birth weight less than 1500 g routinely receive a cranial ultrasound at 7–14 days of age and earlier if clinically indicated. Additional studies were obtained as clinically indicated. Early imaging was defined as on or before 42 days of age to include all imaging that was done as routine screening for hemorrhage. Later imaging was performed for clinical indications or before hospital discharge. Until June 2003, a late cranial ultrasound was routinely performed at 90 days of age. Beginning in 2003, an MRI was routinely performed near 38 weeks postmenstrual age in infants with a birth weight less than 1000 g; a cranial ultrasound was routinely performed at 38 weeks postmenstrual age in infants with a birth weight of 1001–1500 g.
For imaging studies before 2003, data from imaging reports in the medical record were used because the images were not available for review. For imaging studies performed after 2003, the images were reviewed and categorized in a standard manner by a single pediatric radiologist at our institution.
For head ultrasound imaging, echodensity, ventriculomegaly, cysts and other abnormalities were documented. Ventricular size was measured at the atrial level and was classified as normal or venticulomegaly (small, moderate, large). Size of cysts and areas of abnormal echogenicity were also classified as small, moderate or large. The most severe finding on any image for each abnormality was used. For brain MRIs, abnormal signal, ventriculomegaly, cysts, white matter atrophy and diffuse excessive high signal intensity were documented. Size of ventricles, cysts and areas of abnormal signal intensity were classified as small, moderate or large.
Echodensity location and laterality were noted. Echodensity location was classified as: frontal lobe, parietal lobe, temporal lobe, occipital lobe, cerebellum, germinal matrix, choroid plexus, lateral ventricle, 3rd or 4th ventricle, caudate nucleus, basal ganglia and thalamus. Echodensity extent was classified by number of lobes involved on one side of the cerebrum. If an infant had bilateral hemorrhages, the echodensity extent of the most severe side was used.
Outcomes
Neurodevelopmental outcomes were obtained at 18–22 months corrected age during a clinic visit. The primary outcome of interest was presence of CP. Secondary outcomes included low cognitive score, vision impairment and hearing impairment. Neurologic examinations were performed by physicians and staff in the clinic who were trained and certified to achieve consistency.
CP was defined as having two of the following criteria: a delay in motor milestones, abnormalities observed in the neuromotor exam except for isolated toe walking or isolated hypotonia, and abnormalities in primitive reflexes. CP severity was categorized as mild, moderate or severe. Mild CP was defined as having impairment in motor function that slightly interferes with, but does not prevent, age-appropriate motor abilities. Moderate CP was defined as impairment in motor function that interferes with age-appropriate motor activities, with either no ambulation or ambulation only with assistive devices. Severe CP was defined as significant impairment in motor function that interferes with all age-appropriate motor activities, with no ambulation, no sitting or supported sitting. Types of CP were categorized as diplegia, hemiplegia, quadriplegia or other.
Cognitive development was assessed using the Bayley Scales of Infant Development (BSID) Mental Developmental Index (MDI) or cognitive score. The assessments were performed by examiners who were masked to the infant’s hospital course. The BSID II was used before 2006 and the BSID III was used thereafter. Children who could not be tested because of severe developmental delay were given an MDI score of 49 using the BSID II and a cognitive score of 54 using the BSID III. Vision impairment was defined as uncorrectable vision impairment, likely blind. Hearing impairment was defined as hearing impairment needing a hearing aid or cochlear implant.
Statistical analysis
All outcomes were coded as binary variables. Because some infants had a MDI score from the BSID II, whereas others had a cognitive score from the BSID III; we calculated the percentile of each infant’s MDI score (BSID II) or cognitive score (BSID III) using the reference population of all infants in the birth cohort who were seen in follow-up during the same time period for each test. We then were able to use the percentile cognitive score for all infants in the follow-up cohort. Cognitive outcomes were coded into two groups: less than or equal to the 25th percentile and greater than 25th percentile based on the birth cohort.
For each of the outcomes of CP and cognitive outcome, two sets of analysis were performed. One used predictors available early in the hospitalization (first 42 days after birth) and another was performed using predictors available later in the hospitalization (near discharge).
Univariate analysis of the independent variables and outcomes were initially performed using χ2 or Fisher’s exact tests. Next, diagnostic test properties (sensitivity, specificity and likelihood ratios) were calculated for variables that were significant (P<0.05) from the univariate analysis. Variables that approached significance (P<0.1) were also included. All analyses were completed using Stata/IC 12.1 (Stata Corp, College Station, TX, USA).
Some categorical variables such as echodensity extent, maternal race, maternal education and child’s insurance were recoded as follows: the odds ratios or risk ratios were calculated for individual categories and then data were combined into two or three categories. Continuous variables were checked for linearity assumptions. Gestational age was recoded from a continuous variable into a two categories for the outcome of CP and then again for the outcome of cognitive development. Ventilator duration was also recoded into a binary variable.
The analysis for presence of CP was repeated, using the IPH cohort, for the combined outcome of CP or death to check whether the associations would change when death was analyzed as a competing outcome. Further analysis was performed for associations between low cognitive score, blindness or deafness and IPH location. For images with a study radiologist categorization available, we also examined the inter-rater agreement between ultrasound report findings and our radiologist findings. The kappa statistic was calculated for the following imaging variables: ventriculomegaly, cysts and echodensity extent.
RESULTS
Study population and baseline characteristics
From the years 1998–2008, 1168 infants were included in the birth cohort (Figure 1). One hundred and forty-two infants had no cranial imaging performed after birth with the majority having died in the first few weeks after birth. Eight hundred and eighty-five infants with cranial imaging did not have an IPH. One hundred and forty-one infants had an IPH and two were excluded from the study cohort because of central nervous system malformations. Eighty-five infants with IPHs died before follow-up with most having died before hospital discharge; six infants were lost to follow-up and forty-eight infants were seen in follow-up at 18–22 months corrected age.
Figure 1.
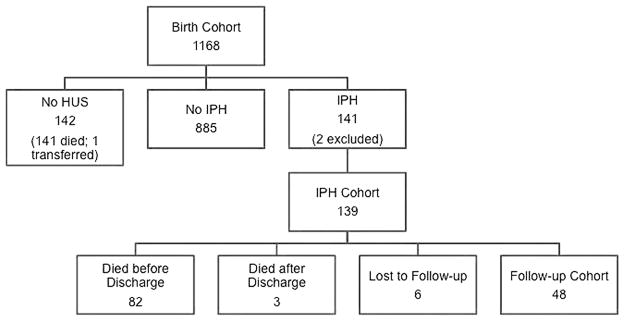
Study population. HUS, head ultrasound; IPH, intraparenchymal hemorrhage.
Infants with IPHs had a median gestational age of 24 weeks and median birth weight of 670 g (Table 1). As expected, infants who died without cranial imaging were younger and smaller than those with imaging performed. The clinical characteristics of the follow-up cohort are shown in Table 2.
Table 1.
Birth cohort characteristics
| Characteristic | Infants without cranial imaging (n =141) | Infants with cranial imaging
|
|
|---|---|---|---|
| Infants without IPH (n =885) | Infants with IPH (n =141) | ||
| Gestational age, wk, range (median) | 20–29 (23) | 21–34 (25) | 21–33 (24) |
| Birth weight, g, range, (median) | 310–1140 (560) | 410–1120 (755) | 445–982 (670) |
| Outborn (n, %) | 18 (13) | 172 (19) | 67 (48) |
| Female (n, %) | 69 (49) | 413 (47) | 88 (63) |
| Multiple gestation (n, %) | 36 (26) | 187 (21) | 38 (27) |
| Race (n, %) | |||
| Black | 58 (41) | 405 (46) | 61 (43) |
| White | 42 (30) | 234 (26) | 38 (27) |
| Hispanic | 34 (24) | 222 (25) | 34 (24) |
| Other | 3 (2) | 20 (2) | 8 (6) |
| Antenatal steroids (n, %) | 49 (35) | 643 (73) | 69 (49) |
Abbreviations: IPH, intraparenchymal hemorrhage; wk, week.
Table 2.
Clinical and demographic characteristics of infants seen in follow-up, n =48
| Characteristic | Value |
|---|---|
| Gestational age, mean (s.d.), wk | 25.1 (1.3) |
| Birth weight, mean (s.d.), g | 726 (127) |
| Outborn, n (%) | 19 (40) |
| Female, n (%) | 21 (44) |
| Multiple gestation, n (%) | 10 (21) |
| Antenatal steroids, n (%) | 24 (50) |
| Early or late-onset sepsis, n (%) | 18 (38) |
| Meningitis, n (%) | 3 (6) |
| NEC, n (%) | 3 (6) |
| ROP (all stages), n (%) | 46 (96) |
| Shunt, n (%) | 11 (23) |
| Duration of mechanical ventilation, 50%ile (25–75%ile), d | 55 (25–77) |
| Maternal age, mean (s.d.), yr | 25 (7) |
| Maternal race, n (%) | |
| Black/Black-Hispanic | 26 (54) |
| White non-Hispanic | 14 (29) |
| Hispanic/other | 8 (17) |
| Maternal marital status, married, n (%) | 20 (42) |
| Maternal/caretaker education, n (%) | |
| <12th Grade | 16 (33) |
| High school degree | 12 (25) |
| Any college | 20 (42) |
Abbreviations: d, day; NEC, necrotizing enterocolitis; ROP, retinopathy of prematurity; wk, week; yr, year
Imaging results
Among the infants seen in follow-up, the parietal lobe was the most common location of hemorrhage (Figure 2). In the early imaging, 42% of the infants had hemorrhages involving two or more lobes of the brain, 13% had hemorrhages involving three or more lobes, 21% had bilateral IPHs, 88% had an associated IVH, 75% had ventriculomegaly and 23% had cerebellar hemorrhages.
Figure 2.
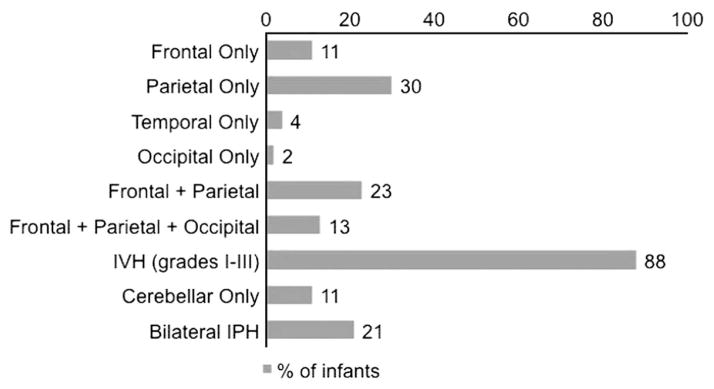
Echodensity location in infants seen in follow-up. IPH, intraparenchymal hemorrhage; IVH, intraventricular hemorrhage.
Late imaging included 39 infants with head imaging performed near 38 weeks postmenstrual age. There were 20 infants with head ultrasounds, 29 infants had brain MRIs and 10 infants had both an ultrasound and MRI. Among the infants with MRI studies, one infant had diffuse excessive high signal intensity and nine infants had white matter atrophy. By combining the most common findings seen in both types of studies (ultrasound and MRI), 16 (41%) had cysts with 2 infants having multiple bilateral periventricular cysts, 25 (64%) infants had ventriculomegaly and 21 (54%) infants had an intraventricular echodensity.
Inter-rater agreement analyses using the kappa statistic were performed for early imaging findings. We compared the imaging reports to our study radiologist’s classification for all infants with IPH (n =81). The kappa statistic values for the imaging variables were fair to good and are shown in Table 3. Because of this agreement, we chose to include all the infants in the follow-up cohort in the final analyses.
Table 3.
Inter-rater agreement of early imaging variables
| Imaging variable | Kappa statistic |
|---|---|
| Ventriculomegaly | 0.65 |
| Cysts | 0.52 |
| Intraventricular echodensity | 0.59 |
| Echodensity in 2 or more lobes | 0.48 |
| Echodensity in 3 or more lobes | 0.54 |
CP outcomes
Fifty-four percent of the infants with IPHs developed CP. All infants with extensive hemorrhages (involving three or more lobes of the brain) (n =13) developed CP. Of the 11 infants with a cerebellar hemorrhage, only 2 developed CP and these infants also had hemorrhages in other lobes of the brain. There were five infants with isolated cerebellar hemorrhages and none of them developed CP.
Among the 26 infants with CP, 1 infant had diplegia, 6 had hemiplegia, 9 had quadriplegia and 10 had other types of CP. Ten (21%) infants had mild CP, nine (19%) had moderate CP and seven (14%) had severe CP. Among the infants with extensive hemorrhage (three or more lobes), 50% had severe CP.
In the univariate analyses of early clinical and sociodemographic variables, none were significant in prediction of CP. In the analysis of early imaging variables, we found that ventriculomegaly, IVH and extensive hemorrhage (involving three or more lobes) were predictors (P<0.05) of CP (Table 4). Location of hemorrhage was not associated with CP. The analysis of early imaging variables was repeated using the combined outcome of CP or death for all infants with IPH and similar results were found. We also did a secondary analysis that included only the 21 infants with images available for review by the study radiologist. Although the precision of the estimates was decreased as expected with the smaller sample, the relative risks were similar to the relative risks obtained for the full follow-up cohort.
Table 4.
Univariate analyses of early imaging, clinical, sociodemographic variables and outcome of CP
| Variable | P | OR (95% CI) | RR (95% CI) | Prevalence of CP (%)a |
|---|---|---|---|---|
| Ventriculomegaly | 0.01 | — | 1.69 (1.14–2.52) | 67 |
| Intraventricular echodensity | 0.05 | — | 1.32 (1.01–1.73) | 61 |
| Cysts | 0.07 | — | 5.92 (0.79–44.51) | 88 |
| Echodensity in 2 or more lobes | 0.07 | — | 1.97 (0.91–4.26) | 70 |
| Echodensity in 3 or more lobes | 0.03 | — | Unable to be calculate | 100 |
| Bilateral IPH | 0.77 | — | 0.85 (0.28–2.55) | — |
| Gestational age ≥25 weeks | 0.88 | — | 0.97 (0.62–1.50) | — |
| Birth weight | 0.59 | 1.00 (0.99–1.01)b | — | — |
| Male gender | 0.34 | — | 0.79 (0.48–1.29) | — |
| Maternal education <12th grade | 0.84 | — | 1.09 (0.48–2.44) | — |
| Maternal race (compared with Black) | ||||
| White | 0.48 | — | 0.81 (0.44–1.49) | — |
| Hispanic/other | 0.23 | 0.61 (0.24–1.57) | ||
| Child’s medical insurance—public/medicaid | 0.74 | — | 0.95 (0.68–1.31) | — |
| Primary language used at home—Spanish/other | 0.86 | — | 0.85 (0.13–5.52) | — |
Abbreviations: CI, confidence interval; CP, cerebral palsy; IPH, intraparenchymal hemorrhage; OR, odds ratio; RR, relative risk.
Prevalence of CP was only calculated for significant variables.
For each increase of 1 g in birth weight.
In the univariate analyses of late clinical and imaging variables, having a ventricular shunt, ventriculomegaly and intraventricular echodensity were predictors (P<0.05) of CP (Table 5). For the significant variables from the univariate analyses, the diagnostic test properties for the prediction of CP are shown in Tables 6 and 7. The prevalence of CP among survivors with IPH was 54% and the prevalence of CP with each significant variable is shown in Tables 4 and 5.
Table 5.
Univariate analyses of late variables and outcome of CP
| Variable | P | RR (95% CI) | Prevalence of CPa (%) |
|---|---|---|---|
| Ventricular shunt | 0.02 | 8.46 (1.17–61.02) | 80 |
| Ventriculomegaly | 0.01 | 1.99 (1.09–3.63) | 72 |
| Intraventricular echodensity | 0.04 | 1.93 (0.96–3.91) | 71 |
| Cysts | 0.20 | 1.70 (0.73–3.96) | — |
Abbreviations: CI, confidence interval; CP, cerebral palsy; RR, relative risk.
Prevalence of CP was only calculated for significant variables.
Table 6.
Early prediction of cerebral palsy
| Ventriculomegaly | Extensive hemorrhage | Intraventricular echodensity | Cysts | Echodensity extent into 2 or more lobes | |
|---|---|---|---|---|---|
| Sensitivity % (95% CI) | 92 (75–99) | 23 (9–44) | 96 (80–100) | 27 (12–48) | 54 (33–73) |
| Specificity % (95% CI) | 46 (24–68) | 100 (85–100) | 27 (11–50) | 96 (77–100) | 73 (50–89) |
| LR + (95% CI) | 1.7 (1.1–2.5) | — | 1.3 (1.0–1.7) | 5.9 (0.79–44.5) | 2.0 (0.92–4.3) |
| LR− (95% CI) | 0.17 (0.041–0.69) | 0.77 (0.62–0.95) | 0.14 (0.018–1.1) | 0.61 (0.51–0.71) | 0.64 (0.39–1.0) |
Abbreviations: CI, confidence interval; LR +, likelihood ratio positive; LR−, likelihood ratio negative.
Table 7.
Late prediction of cerebral palsy
| Ventricular shunt | Ventriculomegaly | Intraventricular echodensity | |
|---|---|---|---|
| Sensitivity % (95% CI) | 38 (20–59) | 82 (60–95) | 68 (45–86) |
| Specificity % (95% CI) | 96 (77–100) | 59 (33–82) | 64 (38–86) |
| LR + (95% CI) | 8.5 (1.2–61) | 2.0 (1.1–3.6) | 1.9 (1.0–3.9) |
| LR− (95% CI) | 0.64 (0.47–0.88) | 0.31 (0.12–0.82) | 0.49 (0.24–1.0) |
Abbreviations: CI, confidence interval; LR +, likelihood ratio positive, LR−, likelihood ratio negative.
Cognitive outcomes
Forty-one of forty-eight infants had cognitive testing using the BSID. Thirty-three infants were tested using the BSID II and eight infants had the BSID III. Thirteen infants were untestable and were assigned a MDI score of 49 (BSID II) or a cognitive score of 54 (BSID III). The mean MDI score was 65 with a s.d. 18 and the mean BSID III cognitive score was 78 with a s.d. 17. By comparing the score percentiles for each infant to the percentiles for the entire birth cohort, 54% of infants with IPH had cognitive scores at or below the 25th percentile for the birth cohort patients seen in follow-up.
None of the early clinical, sociodemographic and imaging predictors were significantly associated with cognitive scores in the bottom quartile in the analysis. Maternal education was the strongest predictor (P =0.098). In the analysis of late predictors, none of the clinical, sociodemographic and imaging variables were associated with low cognitive outcome.
Other outcomes
In the IPH cohort, 19 infants had non-correctable vision impairment and 4 infants had severe hearing impairment. There was no association between vision impairment and occipital lobe hemorrhages (P =0.70).
DISCUSSION
In our study, 54% of infants surviving with IPHs developed CP, which is consistent with previous studies.2,6 Our mortality rate of 61% was somewhat higher than the mortality reported in other recent studies; this may be explained by a lower mean gestational age in our study than in the other studies.2,6,7 A recent study by Goldstein et al. showed an association between lower gestational age and death in infants with grade III and IV IVH, although lower gestational age was not associated with neurodevelopmental impairment among survivors.12
Extent of hemorrhage (number of lobes involved on the worst side) was significantly associated with development of CP. Infants with more than two lobes involved on the worst side had a higher risk of CP and all of our infants with hemorrhages extending into three or more lobes developed CP. This is consistent with the findings of the other two studies that examined this association.4,6 Similar to other studies, we did not find an association between extent of hemorrhage and cognitive outcome,4,13 possibly because all of these studies were limited by relatively small sample sizes.
In our study, laterality (unilateral vs bilateral) of hemorrhage was not associated with neurodevelopmental outcomes. This is consistent with findings seen in the two previous studies of similar size.4,6 In contrast, in a larger multicenter study of 69 infants with PVHI, Maitre et al. found that infants with bilateral PVHI had significantly worse motor and cognitive outcomes than infants with unilateral PVHI.5 In another study of 20 infants with grade 4 IVH, Merhar et al. found that infants with bilateral hemorrhage had lower Bayley scores than infants with unilateral hemorrhage.14 It is possible that some of the smaller studies did not have adequate power to identify this association.
Although hemorrhage is optimally detected early in the course, ventricular dilation may be seen early or late and it can be transient or progressive. Historically, the justification for near-discharge imaging has been to identify progressive white matter damage or loss. When looking at all imaging variables in our study, ventriculomegaly in either early or late imaging had a strong association with CP. This finding has been very consistent across multiple previous studies. Ment et al. found that ventriculomegaly at term gestation predicted CP and lower intelligent quotient at 4.5 years age among former very low birth weight infants.11 Roze et al. found that post-hemorrhagic ventricular dilation was a risk factor for lower intelligence and fine manipulative abilities at 4–12 years of age among survivors of PVHI.13 In a study by Brouwer et al. of 76 infants surviving with IPH, intervention for post-hemorrhagic ventricular dilation was associated with lower developmental quotients (Griffiths’ Mental Developmental Scale).7 Goldstein et al. also reported that shunt placement was associated with neurodevelopmental impairment among surviving infants with Grade IV hemorrhage.12
One of our objectives was to evaluate whether sociodemographic factors would explain the variability in outcomes among infants with IPH. A previous study found that social factors such as higher maternal education and two-parent families were associated with higher cognition among former 600–1250 g preterm infants.15 Similar findings were reported in two other studies.8,11 We did not see a statistically significant relationship between sociodemographic variables and cognitive outcome, although lower maternal education was the variable most strongly associated with cognitive outcome in the bottom quartile. Although some studies have shown an association between abnormal neonatal imaging and cognitive outcomes, the association has been more consistent for motor than cognitive outcomes, whereas sociodemographic factors have been more strongly associated with cognitive than motor outcomes. The association between sociodemographic factors and neurodevelopment is likely to increase over time after discharge.
The strengths of this study are prospective clinical data collection, detailed categorization of imaging findings, masked neurodevelopmental assessments and a high follow-up rate (89%). We were limited by the retrospective nature of this study in that some patients did not have late imaging and others did not have cognitive testing scores recorded. Another limitation is that our unit’s imaging protocols changed during the study time period. We were unable to have a uniform reading and coding for all head imaging, as older images were not available for review. Even though we had a combination of ultrasounds and MRIs for our late imaging studies, we used variables that could be extracted from either type of study. Like all other studies of IPH outcomes, our study was limited by small sample size. Larger studies, preferably with school-age outcomes, would be needed for more precise outcome prediction.
Based on our findings, clinicians could calculate the probability of CP using the imaging findings for an individual patient. For our patients, the prevalence or pre-test probability of CP among survivors with IPH was 54%. For similar risk patients, this gives a pre-test odds of 0.54/0.46 =1.17. For a patient without ventriculomegaly seen in early imaging, with a negative likelihood ratio of 0.2, the post-test odds would be 0.2 ×1.17 =0.2. This gives a post-test probability of developing CP 0.2/(0.2 +1) =17%. The 95% CI for the post-test probability would be 4–45%. Similar calculations could be performed for other findings or for different pre-test probabilities.
CONCLUSIONS
Among infants surviving with an IPH, ventriculomegaly, intraventricular echodensity or extensive hemorrhage seen in early imaging increased the probability of developing CP at 18–22 months age. Having a ventricular shunt or having ventriculomegaly or intraventricular echodensity seen in late imaging also increased the probability of developing CP. These findings may help clinicians when counseling families in the neonatal intensive care unit.
Acknowledgments
NICHD Neonatal Research Network (U10 HD21373): Jon E Tyson, MD MPH; Nora I Alaniz, BS; Beverly Foley Harris, RN BSN; Charles Green, PhD; Margarita Jiminez, MD MPH; Anna E Lis, RN BSN; Sarah Martin, RN BSN; Georgia E McDavid, RN; Brenda H Morris, MD; Margaret L Poundstone, RN BSN; Stacy Reddoch, BA; Saba Siddiki, MD; Patti L Pierce Tate, RCP; Laura L Whitely, MD; Sharon L Wright, MT (ASCP).
Footnotes
CONFLICT OF INTEREST
The authors declare no conflict of interest.
References
- 1.Volpe JJ. Neurology of the Newborn. 4. W.B. Saunders Company; 2001. [Google Scholar]
- 2.Bassan H, Feldman HA, Limperopoulos C, Benson CB, Ringer SA, Veracruz E, et al. Periventricular hemorrhagic infarction: risk factors and neonatal outcome. Pediatr Neurol. 2006;35(2):85–92. doi: 10.1016/j.pediatrneurol.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 3.Papile LA, Munsick-Bruno G, Schaefer A. Relationship of cerebral intraventricular hemorrhage and early childhood neurologic handicaps. J Pediatr. 1983;103(2):273–277. doi: 10.1016/s0022-3476(83)80366-7. [DOI] [PubMed] [Google Scholar]
- 4.Bassan H, Limperopoulos C, Visconti K, Mayer DL, Feldman HA, Avery L, et al. Neurodevelopmental outcome in survivors of periventricular hemorrhagic infarction. Pediatrics. 2007;120(4):785–792. doi: 10.1542/peds.2007-0211. [DOI] [PubMed] [Google Scholar]
- 5.Maitre NL, Marshall DD, Price WA, Slaughter JC, O’Shea TM, Maxfield C, et al. Neurodevelopmental outcome of infants with unilateral or bilateral periventricular hemorrhagic infarction. Pediatrics. 2009;124(6):e1153–e1160. doi: 10.1542/peds.2009-0953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Roze E, Kerstjens JM, Maathuis CG, ter Horst HJ, Bos AF. Risk factors for adverse outcome in preterm infants with periventricular hemorrhagic infarction. Pediatrics. 2008;122(1):e46–e52. doi: 10.1542/peds.2007-3305. [DOI] [PubMed] [Google Scholar]
- 7.Brouwer A, Groenendaal F, van Haastert IL, Rademaker K, Hanlo P, de Vries L. Neurodevelopmental outcome of preterm infants with severe intraventricular hemorrhage and therapy for post-hemorrhagic ventricular dilatation. J Pediatr. 2008;152(5):648–654. doi: 10.1016/j.jpeds.2007.10.005. [DOI] [PubMed] [Google Scholar]
- 8.Vohr B, Garcia Coll C, Flanagan P, Oh W. Effects of intraventricular hemorrhage and socioeconomic status on perceptual, cognitive, and neurologic status of low birth weight infants at 5 years of age. J Pediatr. 1992;121(2):280–285. doi: 10.1016/s0022-3476(05)81204-1. [DOI] [PubMed] [Google Scholar]
- 9.Kuban KC, Allred EN, O’Shea TM, Paneth N, Pagano M, Dammann O, et al. Cranial ultrasound lesions in the NICU predict cerebral palsy at age 2 years in children born at extremely low gestational age. J Child Neurol. 2009;24(1):63–72. doi: 10.1177/0883073808321048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.O’Shea TM, Kuban KC, Allred EN, Paneth N, Pagano M, Dammann O, et al. Neonatal cranial ultrasound lesions and developmental delays at 2 years of age among extremely low gestational age children. Pediatrics. 2008;122(3):e662–e669. doi: 10.1542/peds.2008-0594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ment LR, Vohr B, Allan W, Westerveld M, Katz KH, Schneider KC, et al. The etiology and outcome of cerebral ventriculomegaly at term in very low birth weight preterm infants. Pediatrics. 1999;104(2 Pt 1):243–248. doi: 10.1542/peds.104.2.243. [DOI] [PubMed] [Google Scholar]
- 12.Goldstein RF, Cotten CM, Shankaran S, Gantz MG, Poole WK Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network. Influence of gestational age on death and neurodevelopmental outcome in premature infants with severe intracranial hemorrhage. J Perinatol. 2013;33(1):25–32. doi: 10.1038/jp.2012.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Roze E, Van Braeckel KN, van der Veere CN, Maathuis CG, Martijn A, Bos AF. Functional outcome at school age of preterm infants with periventricular hemorrhagic infarction. Pediatrics. 2009;123(6):1493–1500. doi: 10.1542/peds.2008-1919. [DOI] [PubMed] [Google Scholar]
- 14.Merhar SL, Tabangin ME, Meinzen-Derr J, Schibler KR. Grade and laterality of intraventricular haemorrhage to predict 18–22 month neurodevelopmental outcomes in extremely low birthweight infants. Acta Paediatr. 2012;101(4):414–418. doi: 10.1111/j.1651-2227.2011.02584.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Luu TM, Ment LR, Schneider KC, Katz KH, Allan WC, Vohr BR. Lasting effects of preterm birth and neonatal brain hemorrhage at 12 years of age. Pediatrics. 2009;123(3):1037–1044. doi: 10.1542/peds.2008-1162. [DOI] [PMC free article] [PubMed] [Google Scholar]


