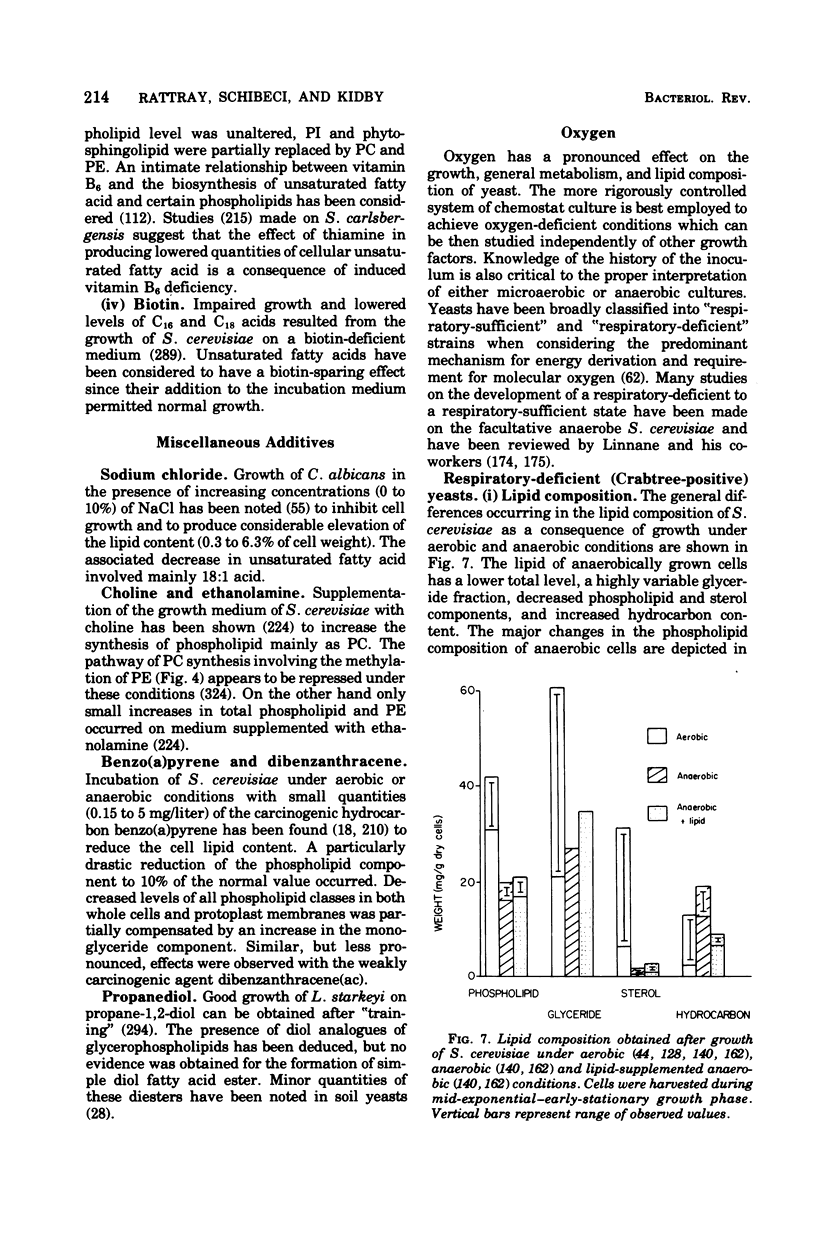
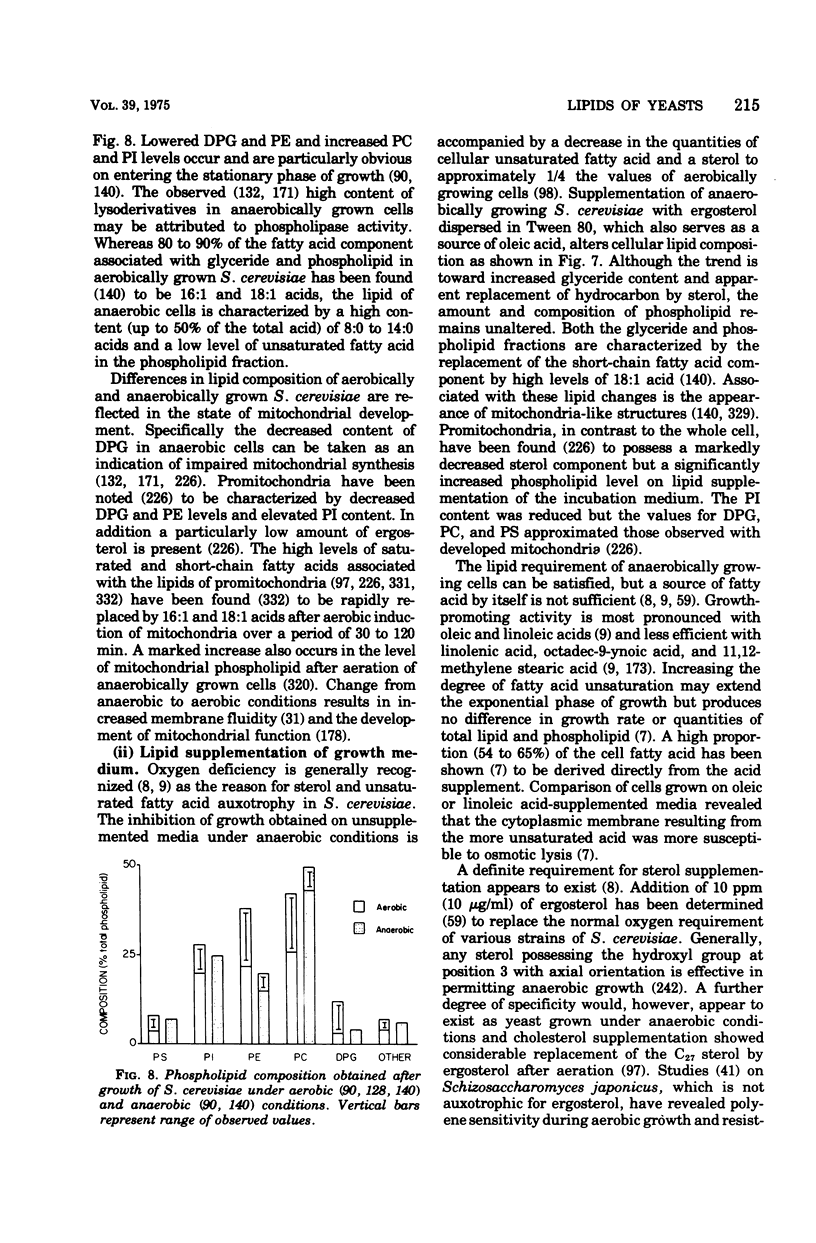
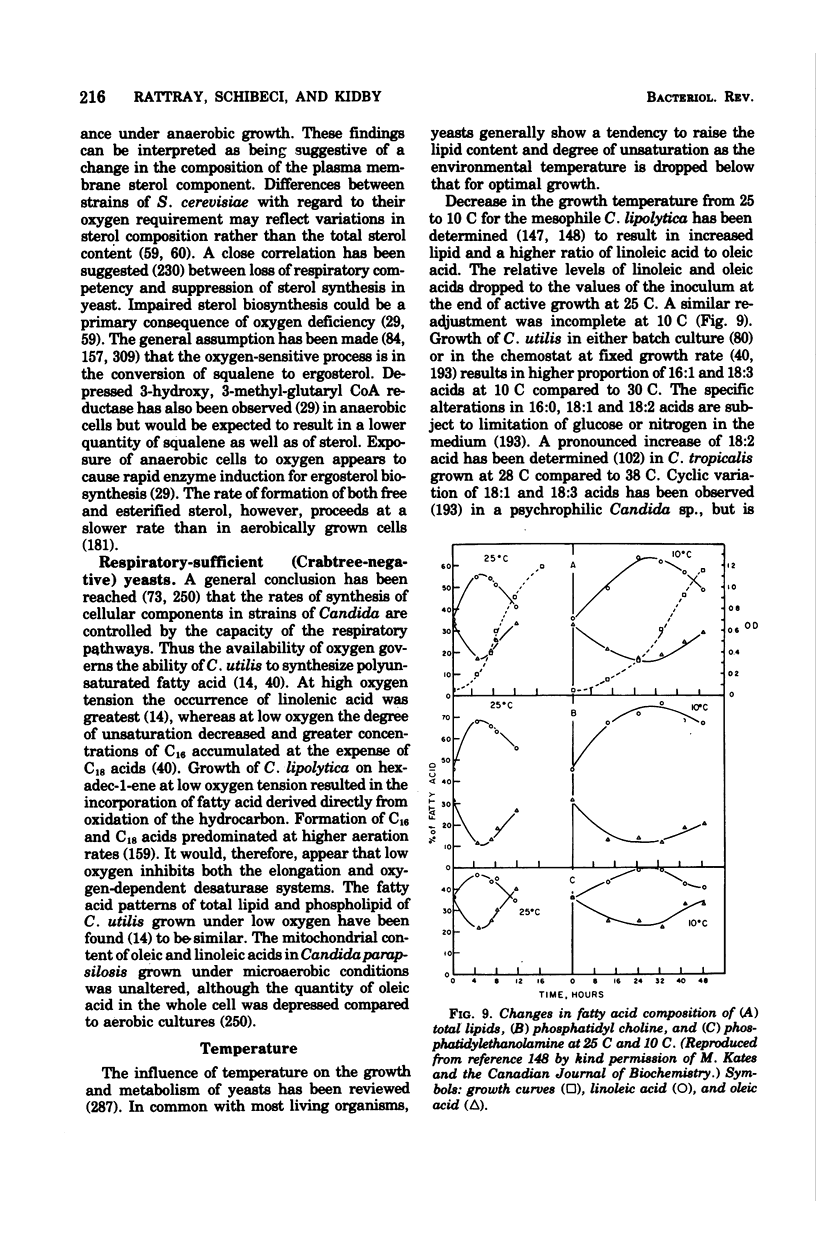
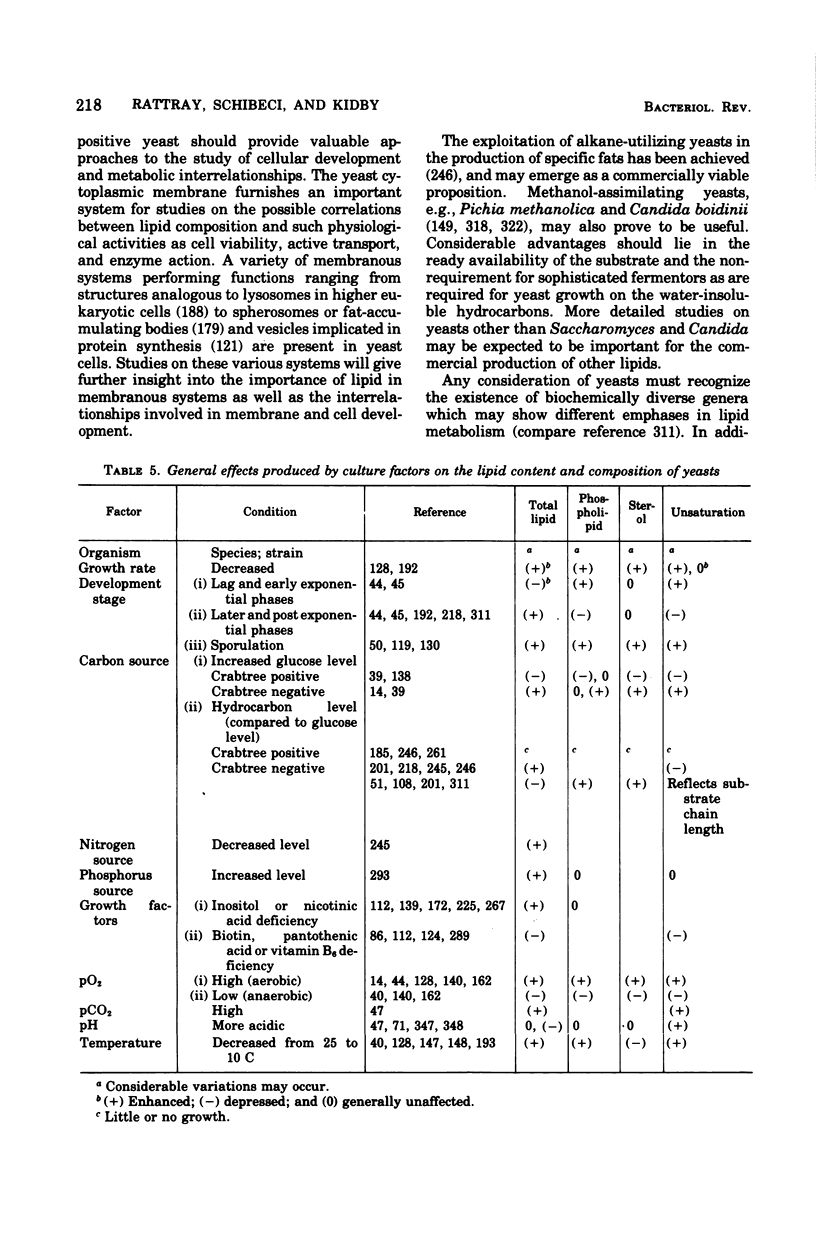
Full text
PDF


























Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANDREASEN A. A., STIER T. J. B. Anaerobic nutrition of Saccharomyces cerevisiae. I. Ergosterol requirement for growth in a defined medium. J Cell Physiol. 1953 Feb;41(1):23–36. doi: 10.1002/jcp.1030410103. [DOI] [PubMed] [Google Scholar]
- ANDREASEN A. A., STIER T. J. Anaerobic nutrition of Saccharomyces cerevisiae. II. Unsaturated fatty acid requirement for growth in a defined medium. J Cell Physiol. 1954 Jun;43(3):271–281. doi: 10.1002/jcp.1030430303. [DOI] [PubMed] [Google Scholar]
- Accoceberry B., Stahl A. Séparation des membranes internes et externes de mitochondries de la levure Saccharomyces cerevisiae. C R Acad Sci Hebd Seances Acad Sci D. 1972 Jun 5;274(23):3135–3138. [PubMed] [Google Scholar]
- Adams B. G., Parks L. W. Evidence for dual physiological formsof ergosterol in Saccharomyces cerevisiae. J Cell Physiol. 1967 Oct;70(2):161–168. doi: 10.1002/jcp.1040700205. [DOI] [PubMed] [Google Scholar]
- Adams B. G., Parks L. W. Isolation from yeast of a metabolically active water-soluble form of ergosterol. J Lipid Res. 1968 Jan;9(1):8–11. [PubMed] [Google Scholar]
- Ainsworth P. J., Tustanoff E. R., Ball A. J. Membrane phase (transitions) as a diagnostic tool for studying mitochondriogenesis. Biochem Biophys Res Commun. 1972 Jun 28;47(6):1299–1305. doi: 10.1016/0006-291x(72)90214-8. [DOI] [PubMed] [Google Scholar]
- Al-Doory Y., Larsh H. W. Quantitative Studies of Total Lipids of Pathogenic Fungi. Appl Microbiol. 1962 Nov;10(6):492–495. doi: 10.1128/am.10.6.492-495.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander R. B., Brand F. C., Alexander G. J. Effect of pentamethylenetetrazol on structure and enzyme activity of yeast. I. Changes in mitochondria and in sterol levels. Biochim Biophys Acta. 1965 Nov 15;111(1):318–325. doi: 10.1016/0304-4165(65)90499-x. [DOI] [PubMed] [Google Scholar]
- Alterthum F., Rose A. H. Osmotic lysis of sphaeroplasts from Saccharomyces cerevisiae grown anaerobically in media containing different unsaturated fatty acids. J Gen Microbiol. 1973 Aug;77(2):371–382. doi: 10.1099/00221287-77-2-371. [DOI] [PubMed] [Google Scholar]
- Angus W. W., Lester R. L. The regulated catabolism of endogenous and exogenous phosphatidylinositol by Saccharomyces cerevisiae leading to extracellular glycerophosphorylinositol and inositol. J Biol Chem. 1975 Jan 10;250(1):22–30. [PubMed] [Google Scholar]
- Angus W. W., Lester R. L. Turnover of inositol and phosphorus containing lipids in Saccharomyces cerevisiae; extracellular accumulation of glycerophosphorylinositol derived from phosphatidylinositol. Arch Biochem Biophys. 1972 Aug;151(2):483–495. doi: 10.1016/0003-9861(72)90525-5. [DOI] [PubMed] [Google Scholar]
- Athar M. A., Winner H. I. The development of resistance by candida species to polyene antibiotics in vitro. J Med Microbiol. 1971 Nov;4(4):505–517. doi: 10.1099/00222615-4-4-505. [DOI] [PubMed] [Google Scholar]
- BARTNICKI-GARCIA S., NICKERSON W. J. Isolation, composition, and structure of cell walls of filamentous and yeast-like forms of Mucor rouxii. Biochim Biophys Acta. 1962 Mar 26;58:102–119. doi: 10.1016/0006-3002(62)90822-3. [DOI] [PubMed] [Google Scholar]
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- BOULTON A. A. SOME OBSERVATIONS ON THE CHEMISTRY AND MORPHOLOGY OF THE MEMBRANES RELEASED FROM YEAST PROTOPLASTS BY OSMOTIC SHOCK. Exp Cell Res. 1965 Feb;37:343–359. doi: 10.1016/0014-4827(65)90183-7. [DOI] [PubMed] [Google Scholar]
- Babczinski P., Tanner W. Involvement of dolicholmonophosphate in the formation of specific mannosyl-linkages in yeast glycoproteins. Biochem Biophys Res Commun. 1973 Oct 1;54(3):1119–1124. doi: 10.1016/0006-291x(73)90808-5. [DOI] [PubMed] [Google Scholar]
- Babij T., Moss F. J., Ralph B. J. Effects of oxygen and glucose levels on lipid composition of yeast Candida utilis grown in continuous culture. Biotechnol Bioeng. 1969 Jul;11(4):593–603. doi: 10.1002/bit.260110407. [DOI] [PubMed] [Google Scholar]
- Bandlow W. Membrane separation and biogenesis of the outer membrane of yeast mitochondria. Biochim Biophys Acta. 1972 Sep 1;282(1):105–122. doi: 10.1016/0005-2736(72)90315-x. [DOI] [PubMed] [Google Scholar]
- Baraud J., Cassagne C., Genevois L., Joneau M. Présence d'hydrocarbures dans l'insaponifiable des lipides de levures. C R Acad Sci Hebd Seances Acad Sci D. 1967 Jul 3;265(1):83–85. [PubMed] [Google Scholar]
- Baraud J., Maurice A., Napias C. Composition et répartition des lipides au sein des cellules de Saccharomyces cerevisiae. Bull Soc Chim Biol (Paris) 1970;52(4):421–432. [PubMed] [Google Scholar]
- Baraud J., Maurice A., Napias C., Velours J. Actions de carbures cancérogènes sur les lipides des membranes protoplastiques et des mitochondries de Saccharomyces cerevisiae. Biochim Biophys Acta. 1973 Mar 8;296(3):481–492. [PubMed] [Google Scholar]
- Barber E. D., Lands W. E. Quantitative measurement of the effectiveness of unsaturated fatty acids required for the growth of Saccharomyces cerevisiae. J Bacteriol. 1973 Aug;115(2):543–551. doi: 10.1128/jb.115.2.543-551.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bard M. Biochemical and genetic aspects of nystatin resistance in saccharomyces cerevisiae. J Bacteriol. 1972 Sep;111(3):649–657. doi: 10.1128/jb.111.3.649-657.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bard M., Woods R. A., Haslam J. M. Porphyrine mutants of Saccharomyces cerevisiae: correlated lesions in sterol and fatty acid biosynthesis. Biochem Biophys Res Commun. 1974 Jan 23;56(2):324–330. doi: 10.1016/0006-291x(74)90845-6. [DOI] [PubMed] [Google Scholar]
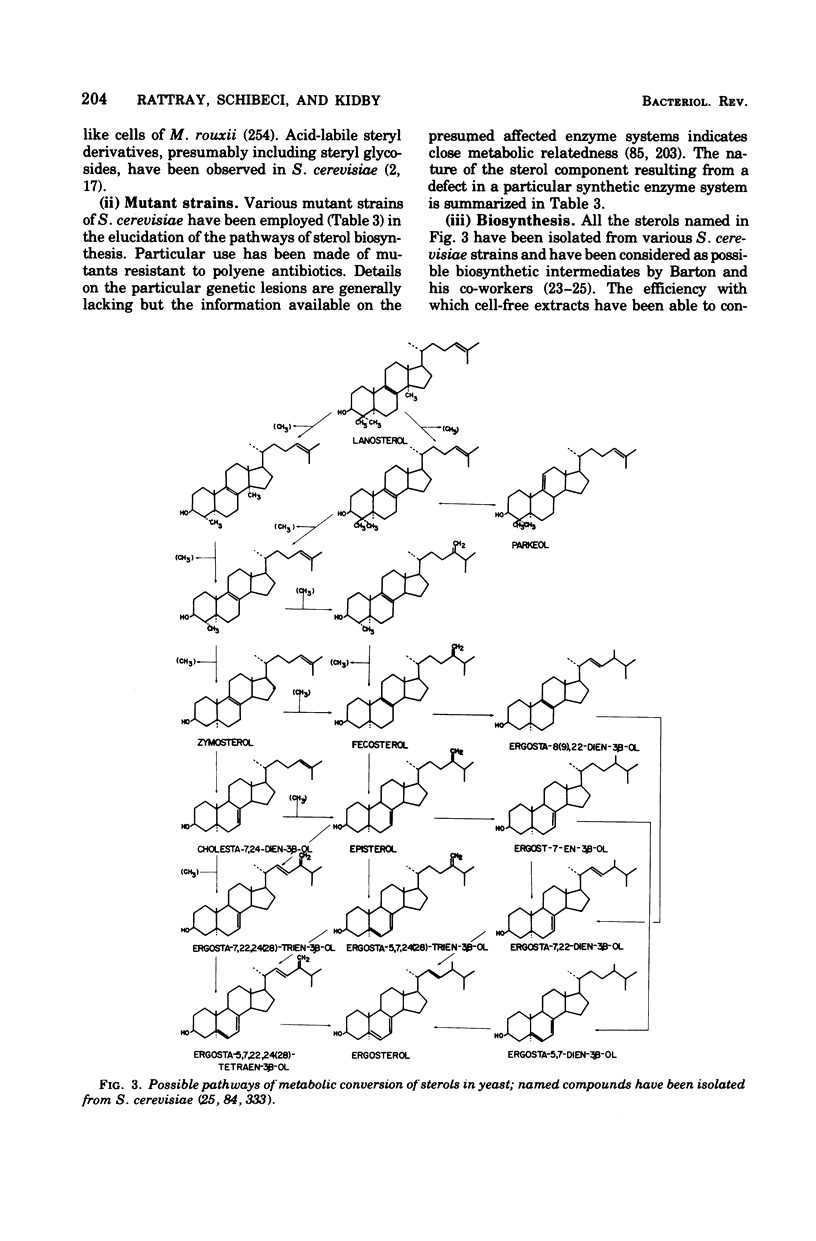
- Barton D. H., Kempe U. M., Widdowson D. A. Investigations on the biosynthesis of steroids and terpenoids. VI. The sterols of yeast. J Chem Soc Perkin 1. 1972;4:513–522. doi: 10.1039/p19720000513. [DOI] [PubMed] [Google Scholar]
- Bednarz-Prashad A. J., Mize C. E. Mitochondrial membranes of inositol-requiring Saccharomyces carlsbergensis: covalent binding of a radioactive marker to the outer membrane. Biochemistry. 1974 Sep 24;13(20):4237–4242. doi: 10.1021/bi00717a026. [DOI] [PubMed] [Google Scholar]
- Bell G. H. The metabolism of long-chain fatty acids and alcohols by Candida tropicalis and Saccharomyces cerevisiae. Antonie Van Leeuwenhoek. 1973;39(1):137–149. doi: 10.1007/BF02578849. [DOI] [PubMed] [Google Scholar]
- Bergelson L. D., Vaver V. A., Prokazova N. V., Ushakov A. N., Popkova G. A. Diol lipids. Biochim Biophys Acta. 1966 Jun 1;116(3):511–520. doi: 10.1016/0005-2760(66)90121-4. [DOI] [PubMed] [Google Scholar]
- Berndt J., Boll M., Löwel M., Gaumert R. Regulation of sterol biosynthesis in yeast: induction of 3-hydroxy-3-methylglutaryl-CoA reductase by glucose. Biochem Biophys Res Commun. 1973 Apr 16;51(4):843–848. doi: 10.1016/0006-291x(73)90003-x. [DOI] [PubMed] [Google Scholar]
- Bertoli E., Barbaresi G., Castelli A., Lenaz G. Effect of phospholipids from saccharomyces cerevisiae at different stages of development on restoration of succinooxidase activity in lipid-depleted mitochondria. J Bioenerg. 1971 Aug;2(3):135–140. doi: 10.1007/BF01648908. [DOI] [PubMed] [Google Scholar]
- Bianchi D. E. The lipid content of cell walls obtained from juvenile, yeast-like and filamentous cells of Candida albicans. Antonie Van Leeuwenhoek. 1967;33(3):324–332. doi: 10.1007/BF02045577. [DOI] [PubMed] [Google Scholar]
- Brennan P. J., Flynn M. P., Griffin P. F.S. Acylglucoses in Escherichia coli, Saccharomyces cerevisiae and Agaricus bisporus. FEBS Lett. 1970 Jul 3;8(6):322–324. doi: 10.1016/0014-5793(90)80004-3. [DOI] [PubMed] [Google Scholar]
- Brown C. M., Johnson B. Influence of the concentration of glucose and galactose on the physiology of Saccharomyces cerevisiae in continuous culture. J Gen Microbiol. 1970 Dec;64(3):279–287. doi: 10.1099/00221287-64-3-279. [DOI] [PubMed] [Google Scholar]
- Brown C. M., Rose A. H. Fatty-acid composition of Candida utilis as affected by growth temperature and dissolved-oxygen tension. J Bacteriol. 1969 Aug;99(2):371–378. doi: 10.1128/jb.99.2.371-378.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulder C. J. Anaerobic growth, ergosterol content and sensitivity to a polyene antibiotic, of the yeast Schizosaccharomyces japonicus. Antonie Van Leeuwenhoek. 1971;37(3):353–358. doi: 10.1007/BF02218505. [DOI] [PubMed] [Google Scholar]
- Bulder C. J., Reinink M. Unsaturated fatty acid composition of wild type and respiratory deficient yeasts after aerobic and anaerobic growth. Antonie Van Leeuwenhoek. 1974;40(3):445–455. doi: 10.1007/BF00399357. [DOI] [PubMed] [Google Scholar]
- CHALLINOR S. W., POWER D. M., TONGE R. J. EFFECTS OF INOSITOL-DEFICIENCY ON YEAST WITH PARTICULAR REFERENCE TO CHEMICAL COMPOSITION OF THE CELL AND OF THE CELL WALL. Nature. 1964 Jul 18;203:250–251. doi: 10.1038/203250a0. [DOI] [PubMed] [Google Scholar]
- Capek A., Simek A., Brůna L., Sváb A., Budesínský Z. Antimicrobial agents. XX. Ergosterol content of Candida albicans cells during adaptation to antimycotics. Folia Microbiol (Praha) 1974;19(1):79–80. doi: 10.1007/BF02874507. [DOI] [PubMed] [Google Scholar]
- Castelli A., Barbaresi G., Bertoli E. I., Orlando P. Fatty acids of subcellular fractions of Saccharomyces cerevisiae during the phases of growth. Ital J Biochem. 1969 Mar-Apr;18(2):78–90. [PubMed] [Google Scholar]
- Castelli A., Barbaresi G., Bertoli E. I. Studies on the lipids of S. cerevisiae during the growth phases. Ital J Biochem. 1969 Mar-Apr;18(2):91–99. [PubMed] [Google Scholar]
- Castelli A., Bertoli E., Buongiorno M. S., Curatola G., Lenaz G. Studies on the morphogenesis of yeast mitochondria. II. Composition of Saccharomyces cerevisiae phospholipids during morphogenesis and their effect on restoration of succinoxidase activity of lipid mitochondria. Ital J Biochem. 1972 Jan-Apr;21(1):8–22. [PubMed] [Google Scholar]
- Castelli A., Littarru G. P., Barbaresi G. Effect of pH and CO2 concentration changes on lipids and fatty acids of Saccharomyces cerevisiae. Arch Mikrobiol. 1969;66(1):34–39. doi: 10.1007/BF00414661. [DOI] [PubMed] [Google Scholar]
- Chassang A., Roger M., Vezinhet F., Galzy P. Variation of the lipid content of yeast cells during sporulation. Folia Microbiol (Praha) 1972;17(4):241–247. doi: 10.1007/BF02880197. [DOI] [PubMed] [Google Scholar]
- Clausen M. K., Christiansen K., Jensen P. K., Behnke O. Isolation of lipid particles from baker's yeast. FEBS Lett. 1974 Jul 15;43(2):176–179. doi: 10.1016/0014-5793(74)80994-4. [DOI] [PubMed] [Google Scholar]
- Cobon G. S., Crowfoot P. D., Linnane A. W. Biogenesis of mitchondria. Phospholipid synthesis in vitro by yeast mitochondrial and microsomal fractions. Biochem J. 1974 Nov;144(2):265–275. doi: 10.1042/bj1440265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobon G. S., Haslam J. M. The effect of altered membrane sterol composition on the temperature dependence of yeast mitochondrial ATPase. Biochem Biophys Res Commun. 1973 May 1;52(1):320–326. doi: 10.1016/0006-291x(73)90990-x. [DOI] [PubMed] [Google Scholar]
- Combs T. J., Guarneri J. J., Pisano M. A. The effect of sodium chloride on the lipid content and fatty acid composition of Candida albicans. Mycologia. 1968 Nov-Dec;60(6):1232–1239. [PubMed] [Google Scholar]
- Cosson J., Spiridakis A. ADP-dependent thermal reactivation of triton-inactivated ATPase from mitochondrially determined oligomycin-resistant mutants of Saccharomyces cerevisiae. Biochem Biophys Res Commun. 1974 Aug 5;59(3):1039–1046. doi: 10.1016/s0006-291x(74)80084-7. [DOI] [PubMed] [Google Scholar]
- Criddle R. S., Schatz G. Promitochondria of anaerobically grown yeast. I. Isolation and biochemical properties. Biochemistry. 1969 Jan;8(1):322–334. doi: 10.1021/bi00829a045. [DOI] [PubMed] [Google Scholar]
- DISALVO A. F., DENTON J. F. LIPID CONTENT OF FOUR STRAINS OF BLASTOMYCES DERMATITIDIS OF DIFFERENT MOUSE VIRULENCE. J Bacteriol. 1963 Apr;85:927–931. doi: 10.1128/jb.85.4.927-931.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DULANEY E. L., STAPLEY E. O., SIMPF K. Studies on ergosterol production by yeasts. Appl Microbiol. 1954 Nov;2(6):371–379. doi: 10.1128/am.2.6.371-379.1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DYKE K. G. THE CHEMICAL COMPOSITION OF THE CELL WALL OF THE YEAST, NADSONIA ELONGATA. Biochim Biophys Acta. 1964 Feb 10;82:374–384. doi: 10.1016/0304-4165(64)90309-5. [DOI] [PubMed] [Google Scholar]
- David M. H., Kirsop B. H. A correlation between oxygen requirements and the products of sterol synthesis in strains of Saccharomyces cerevisiae. J Gen Microbiol. 1973 Aug;77(2):529–531. doi: 10.1099/00221287-77-2-529. [DOI] [PubMed] [Google Scholar]
- Dawson P. S., Craig B. M. Lipids of Candida utilis: changes with growth. Can J Microbiol. 1966 Aug;12(4):775–785. doi: 10.1139/m66-105. [DOI] [PubMed] [Google Scholar]
- De Deken R. H. The Crabtree effect: a regulatory system in yeast. J Gen Microbiol. 1966 Aug;44(2):149–156. doi: 10.1099/00221287-44-2-149. [DOI] [PubMed] [Google Scholar]
- Deierkauf F. A., Booij H. L. Changes in the phosphatide pattern of yeast cells in relation to active carbohydrate transport. Biochim Biophys Acta. 1968 Mar 1;150(2):214–225. doi: 10.1016/0005-2736(68)90165-x. [DOI] [PubMed] [Google Scholar]
- Diatlovitskaia E. V., Greshnykh K. P., Bergel'son L. D. Fosfolipidy drozhzhei, vyrashchennykh na n-alkanakh. Biokhimiia. 1968 Jan-Feb;33(1):83–88. [PubMed] [Google Scholar]
- Domer J. E., Hamilton J. G. The readily extracted lipids of Histoplasma capsulatum and Blastomyces dermatitidis. Biochim Biophys Acta. 1971 May 4;231(3):465–478. doi: 10.1016/0005-2760(71)90114-7. [DOI] [PubMed] [Google Scholar]
- Dubé J., Setterfield G., Kiss G., Lusena C. V. Fate of the plasma membrane of Saccharomyces cerevisiae during cell rupture. Can J Microbiol. 1973 Feb;19(2):285–290. doi: 10.1139/m73-043. [DOI] [PubMed] [Google Scholar]
- Duvnjak Z., Azoulay E. Influence des acides gras sur le dévloppment de "Candida tropicalis sur alcanes. Ann Inst Pasteur (Paris) 1972 May;122(5):987–1007. [PubMed] [Google Scholar]
- Einsele A., Fiechter A., Knöpfel H. P. Respiratory activity of Candida tropicalis during growth on hexadecane and on glucose. Arch Mikrobiol. 1972;82(3):247–253. doi: 10.1007/BF00412196. [DOI] [PubMed] [Google Scholar]
- Eletr S., Williams M. A., Watkins T., Keith A. D. Perturbations of the dynamics of lipid alkyl chains in membrane systems: effect on the activity of membrane-bound enzymes. Biochim Biophys Acta. 1974 Mar 15;339(2):190–201. doi: 10.1016/0005-2736(74)90317-4. [DOI] [PubMed] [Google Scholar]
- FOLCH J., LEES M., SLOANE STANLEY G. H. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957 May;226(1):497–509. [PubMed] [Google Scholar]
- Farrell J., Rose A. H. Temperature effects on solute accumulation by Candida utilis. Arch Mikrobiol. 1971;79(2):122–139. doi: 10.1007/BF00424920. [DOI] [PubMed] [Google Scholar]
- Fryberg M., Oehlschlager A. C., Unrau A. M. Biosynthesis of ergosterol in yeast. Evidence for multiple pathways. J Am Chem Soc. 1973 Aug 22;95(17):5747–5757. doi: 10.1021/ja00798a051. [DOI] [PubMed] [Google Scholar]
- Fryberg M., Oehlschlager A. C., Unrau A. M. Biosynthetic routes to ergosterol in yeast. Biochem Biophys Res Commun. 1972 Aug 7;48(3):593–597. doi: 10.1016/0006-291x(72)90389-0. [DOI] [PubMed] [Google Scholar]
- Fryberg M., Oehlschlager A. C., Unrau A. M. Sterol biosynthesis in antibiotic-resistant yeast: nystatin. Arch Biochem Biophys. 1974 Jan;160(1):83–89. doi: 10.1016/s0003-9861(74)80011-1. [DOI] [PubMed] [Google Scholar]
- Furukawa Y., Kimura S. Effect of pantothenic acid deficiency on lipid metabolism in the yeast. J Vitaminol (Kyoto) 1971 Dec 10;17(4):219–224. doi: 10.5925/jnsv1954.17.219. [DOI] [PubMed] [Google Scholar]
- GOLDSTEIN J. L., SWAIN T., TJHIO K. H. Factors affecting the production of leucoanthocyanins in sycamore cambial cell cultures. Arch Biochem Biophys. 1962 Jul;98:176–178. doi: 10.1016/0003-9861(62)90165-0. [DOI] [PubMed] [Google Scholar]
- Gallo M., Bertrand J. C., Roche B., Azoulay E. Alkane oxidation in Candida tropicalis. Biochim Biophys Acta. 1973 Mar 8;296(3):624–638. doi: 10.1016/0005-2760(73)90123-9. [DOI] [PubMed] [Google Scholar]
- Gatt S., Barenholz Y. Enzymes of complex lipid metabolism. Annu Rev Biochem. 1973;42(0):61–90. doi: 10.1146/annurev.bi.42.070173.000425. [DOI] [PubMed] [Google Scholar]
- Getz G. S., Jakovcic S., Heywood J., Frank J., Rabinowitz M. A two-dimensional thin-layer chromatographic system for phospholipid separation. The analysis of yeast phospholipids. Biochim Biophys Acta. 1970 Dec 15;218(3):441–452. doi: 10.1016/0005-2760(70)90007-x. [DOI] [PubMed] [Google Scholar]
- Gill C. O., Ratledge C. Inhibition of glucose assimilation and transport by n-decane and other n-alkanes in Candida 107. J Gen Microbiol. 1973 Mar;75(1):11–22. doi: 10.1099/00221287-75-1-11. [DOI] [PubMed] [Google Scholar]
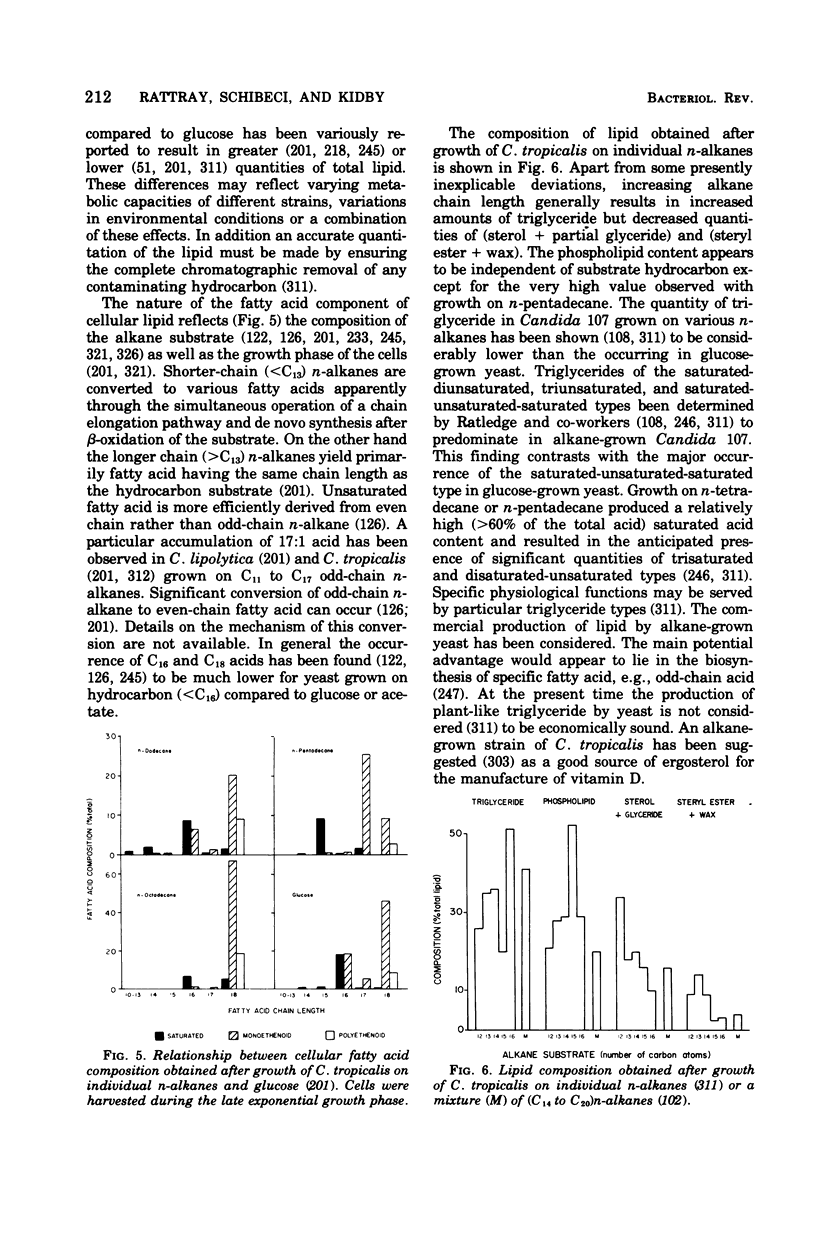
- Gill C. O., Ratledge C. Regulation of de novo fatty acid biosynthesis in the n-alkane-utilizing yeast, Candida 107. J Gen Microbiol. 1973 Oct;78(2):337–347. doi: 10.1099/00221287-78-2-337. [DOI] [PubMed] [Google Scholar]
- Gollub E. G., Trocha P., Liu P. K., Sprinson D. B. Yeast mutants requiring ergosterol as only lipid supplement. Biochem Biophys Res Commun. 1974 Jan 23;56(2):471–477. doi: 10.1016/0006-291x(74)90866-3. [DOI] [PubMed] [Google Scholar]
- Goma G., Pareilleux A., Durand G. Etude de l'ordre de la réaction de dégradation d'un n-alcane par Candida lipolytica. C R Acad Sci Hebd Seances Acad Sci D. 1971 Jul 5;273(1):109–112. [PubMed] [Google Scholar]
- Gordon P. A., Stewart P. R., Clark-Walker G. D. Fatty acid and sterol composition of Mucor genevensis in relation to dimorphism and anaerobic growth. J Bacteriol. 1971 Jul;107(1):114–120. doi: 10.1128/jb.107.1.114-120.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon P. A., Stewart P. R. Effect of lipid status on cytoplasmic and mitochondrial protein synthesis in anaerobic cultures of Saccharomyces cerevisiae. J Gen Microbiol. 1972 Sep;72(2):231–242. doi: 10.1099/00221287-72-2-231. [DOI] [PubMed] [Google Scholar]
- Gordon P. A., Syewart P. R. The effect of antibiotics on lipid synthesis during respiratory development in Saccharomyces cerevisiae. Microbios. 1971 Sep;4(14):115–132. [PubMed] [Google Scholar]
- Greene M. L., Kaneshiro T., Law J. H. Studies on the production of sphingolipid bases by the yeast, Hansenula ciferri. Biochim Biophys Acta. 1965 Jun 1;98(3):582–588. doi: 10.1016/0005-2760(65)90155-4. [DOI] [PubMed] [Google Scholar]
- Greenspan M. D., Germershausen J. I. Effect of halofenate and clofibrate on growth and lipid synthesis in Saccharomyces cerevisiae. J Bacteriol. 1973 Feb;113(2):847–855. doi: 10.1128/jb.113.2.847-855.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HARRISON J. S., TREVELYAN W. E. PHOSPHOLIPID BREAKDOWN IN BAKER'S YEAST DURING DRYING. Nature. 1963 Dec 21;200:1189–1190. doi: 10.1038/2001189a0. [DOI] [PubMed] [Google Scholar]
- HARTMAN L., HAWKE J. C., SHORLAND F. B., DI MENNA M. E. The fatty acid composition of Rhodotorula graminis fat. Arch Biochem Biophys. 1959 Apr;81(2):346–352. doi: 10.1016/0003-9861(59)90212-7. [DOI] [PubMed] [Google Scholar]
- Hamilton-Miller J. M. Chemistry and biology of the polyene macrolide antibiotics. Bacteriol Rev. 1973 Sep;37(3):166–196. [PMC free article] [PubMed] [Google Scholar]
- Hamilton-Miller J. M. Fungal sterols and the mode of action of the polyene antibiotics. Adv Appl Microbiol. 1974;17(0):109–134. doi: 10.1016/s0065-2164(08)70556-2. [DOI] [PubMed] [Google Scholar]
- Hamilton-Miller J. M. Polyene-resistance in yeasts: a consideration of physiological and biochemical mechanisms. Microbios. 1973 Nov-Dec;8(31):209–213. [PubMed] [Google Scholar]
- Hamilton-Miller J. M. Sterols from polyene-resistant mutants of Candida albicans. J Gen Microbiol. 1972 Nov;73(1):201–203. doi: 10.1099/00221287-73-1-201. [DOI] [PubMed] [Google Scholar]
- Hammond S. M., Lambert P. A., Kliger B. N. The mode of action of polyene antibiotics; induced potassium leakage in Candida albicans. J Gen Microbiol. 1974 Apr;81(2):325–330. doi: 10.1099/00221287-81-2-325. [DOI] [PubMed] [Google Scholar]
- Harries P. C., Ratledge C. Distribution of fatty acids in triglyceride from a yeast species grown on a fraction of n-alkanes predominant in tridecane. Chem Ind. 1969 May 3;18:582–583. [PubMed] [Google Scholar]
- Hartwell L. H. Saccharomyces cerevisiae cell cycle. Bacteriol Rev. 1974 Jun;38(2):164–198. doi: 10.1128/br.38.2.164-198.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haskell B. E., Snell E. E. Effect of vitamin B6 deficiency on the composition of yeast lipids. Arch Biochem Biophys. 1965 Dec;112(3):494–505. doi: 10.1016/0003-9861(65)90086-x. [DOI] [PubMed] [Google Scholar]
- Haslam J. M., Proudlock J. W., Linnane A. W. Biogenesis of mitochondria. 20. The effects of altered membrane lipid composition on mitochondrial oxidative phosphorylation in Saccharomyces cerevisiae. J Bioenerg. 1971 Dec;2(5):351–370. doi: 10.1007/BF01963830. [DOI] [PubMed] [Google Scholar]
- Haslam J. M., Spithill T. W., Linnane A. W., Chappell J. B. Biogenesis of mitochondria. The effects of altered membrane lipid composition on cation transport by mitochondria of Saccharomyces cerevisiae. Biochem J. 1973 Aug;134(4):949–957. doi: 10.1042/bj1340949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatanaka H., Ariga N., Nagai J., Katsuki H. Accumulation of a sterol intermediate during reaction in the presence of homocysteine with cell-free extract of yeast. Biochem Biophys Res Commun. 1974 Sep 23;60(2):787–793. doi: 10.1016/0006-291x(74)90309-x. [DOI] [PubMed] [Google Scholar]
- Henry S. A. Death resulting from fatty acid starvation in yeast. J Bacteriol. 1973 Dec;116(3):1293–1303. doi: 10.1128/jb.116.3.1293-1303.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry S. A., Fogel S. Saturated fatty acid mutants in yeast. Mol Gen Genet. 1971;113(1):1–19. doi: 10.1007/BF00335003. [DOI] [PubMed] [Google Scholar]
- Henry S. A., Halvorson H. O. Lipid synthesis during sporulation of Saccharomyces cerevisiae. J Bacteriol. 1973 Jun;114(3):1158–1163. doi: 10.1128/jb.114.3.1158-1163.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry S. A., Keith A. D. Membrane properties of saturated fatty acid mutants of yeast revealed by spin labels. Chem Phys Lipids. 1971 Dec;7(4):245–265. doi: 10.1016/0009-3084(71)90004-1. [DOI] [PubMed] [Google Scholar]
- Holley R. A., Kidby D. K. Role of vacuoles and vesicles in extracellular enzyme secretion from yeast. Can J Microbiol. 1973 Jan;19(1):113–117. doi: 10.1139/m73-017. [DOI] [PubMed] [Google Scholar]
- Hornei S., Köhler M., Weide H. Das Fettsäurespektrum eines Candida-Stammes nach Kultur auf n-Alkanen. Z Allg Mikrobiol. 1972;12(1):19–27. doi: 10.1002/jobm.3630120105. [DOI] [PubMed] [Google Scholar]
- Hoshi M., Kishimoto Y., Hignite C. 2,3-Erythro-dihydroxyhexacosanoic acid and homologs: isolation from yeast cerebrin phosphate and determination of their structures. J Lipid Res. 1973 Jul;14(4):406–414. [PubMed] [Google Scholar]
- Hug H., Fiechter A. Assimilation of aliphatic hydrocarbons by Candida tropicalis. II. Fatty acid profiles from cells grown on substrates of different chain length. Arch Mikrobiol. 1973;88(2):87–96. [PubMed] [Google Scholar]
- Hunter K., Rose A. H. Lipid composition of Saccharomyces cerevisiae as influenced by growth temperature. Biochim Biophys Acta. 1972 Apr 18;260(4):639–653. doi: 10.1016/0005-2760(72)90013-6. [DOI] [PubMed] [Google Scholar]
- Hutchison H. T., Cronan J. E., Jr The synthesis of cytidine diphosphate diglyceride by cell-free extracts of yeast. Biochim Biophys Acta. 1968 Dec 18;164(3):606–608. doi: 10.1016/0005-2760(68)90193-8. [DOI] [PubMed] [Google Scholar]
- Illingworth R. F., Rose A. H., Beckett A. Changes in the lipid composition and fine structure of Saccharomyces cerevisiae during ascus formation. J Bacteriol. 1973 Jan;113(1):373–386. doi: 10.1128/jb.113.1.373-386.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Indge K. J. The isolation and properties of the yeast cell vacuole. J Gen Microbiol. 1968 May;51(3):441–446. doi: 10.1099/00221287-51-3-441. [DOI] [PubMed] [Google Scholar]
- Jakovcic S., Getz G. S., Rabinowitz M., Jakob H., Swift H. Cardiolipin content of wild type and mutant yeasts in relation to mitochondrial function and development. J Cell Biol. 1971 Mar;48(3):490–502. doi: 10.1083/jcb.48.3.490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James R., Branton D., Wisnieski B., Keith A. Composition, structure and phase transition in yeast fatty acid auxotroph membranes: spin labels and freeze-fracture. J Supramol Struct. 1972;1(1):38–49. doi: 10.1002/jss.400010106. [DOI] [PubMed] [Google Scholar]
- Jayaraman J., Sastry P. S. Phospholipid metabolism in yeast cells during mitochondriogenesis. Indian J Biochem. 1971 Dec;8(4):278–282. [PubMed] [Google Scholar]
- Johnson B., Brown C. M. A possible relationship between the fatty acid composition of yeasts and the 'petite' mutation. Antonie Van Leeuwenhoek. 1972;38(2):137–144. doi: 10.1007/BF02328085. [DOI] [PubMed] [Google Scholar]
- Johnson B., Nelson S. J., Brown C. M. Influence of glucose concentration on the physiology and lipid composition of some yeasts. Antonie Van Leeuwenhoek. 1972;38(2):129–136. doi: 10.1007/BF02328084. [DOI] [PubMed] [Google Scholar]
- Johnston J. M., Paltauf F. Lipid metabolism in inositol-deficient yeast, Saccharomyces carlsbergensis. II. Incorporation of labeled precursors into lipids by whole cells and activities of some enzymes involved in lipid formation. Biochim Biophys Acta. 1970 Dec 15;218(3):431–440. [PubMed] [Google Scholar]
- Jollow D., Kellerman G. M., Linnane A. W. The biogenesis of mitochondria. 3. The lipid composition of aerobically and anaerobically grown Saccharomyces cerevisiae as related to the membrane systems of the cells. J Cell Biol. 1968 May;37(2):221–230. doi: 10.1083/jcb.37.2.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jwanny E. W., Chenouda M. S., Osman H. G. Utilization of hydrocarbons by microorganisms. Biochemical activities of alkanes-grown Candida lipolytica. Z Allg Mikrobiol. 1974;14(3):205–212. doi: 10.1002/jobm.3630140305. [DOI] [PubMed] [Google Scholar]
- KATES M., BAXTER R. M. Lipid composition of mesophilic and psychrophilic yeasts (Candida species) as influenced by environmental temperature. Can J Biochem Physiol. 1962 Sep;40:1213–1227. [PubMed] [Google Scholar]
- KESSLER G., NICKERSON W. J. Glucomannan-protein complexes from cell walls of yeasts. J Biol Chem. 1959 Sep;234:2281–2285. [PubMed] [Google Scholar]
- KLEIN H. P. Synthesis of lipids in resting cells of Saccharomyces cerevisiae. J Bacteriol. 1955 Jun;69(6):620–627. doi: 10.1128/jb.69.6.620-627.1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamiryo T., Numa S. Reduction of the acetyl coenzyme A carboxylase content of Saccharomyces cerevisiae by exogenous fatty acids. FEBS Lett. 1973 Dec 15;38(1):29–32. doi: 10.1016/0014-5793(73)80505-8. [DOI] [PubMed] [Google Scholar]
- Kanetsuna F., Carbonell L. M., Moreno R. E., Rodriguez J. Cell wall composition of the yeast and mycelial forms of Paracoccidioides brasiliensis. J Bacteriol. 1969 Mar;97(3):1036–1041. doi: 10.1128/jb.97.3.1036-1041.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karst F., Lacroute F. Isolation of pleiotropic yeast mutants requiring ergosterol for growth. Biochem Biophys Res Commun. 1973 Jun 8;52(3):741–747. doi: 10.1016/0006-291x(73)90999-6. [DOI] [PubMed] [Google Scholar]
- Karst F., Lacroute F. Yeast mutant requiring only a sterol as growth supplement. Biochem Biophys Res Commun. 1974 Jul 10;59(1):370–376. doi: 10.1016/s0006-291x(74)80216-0. [DOI] [PubMed] [Google Scholar]
- Kates M., Paradis M. Phospholipid desaturation in Candida lipolytica as a function of temperature and growth. Can J Biochem. 1973 Feb;51(2):184–197. doi: 10.1139/o73-024. [DOI] [PubMed] [Google Scholar]
- Kawaguchi A. Control of ergosterol biosynthesis in yeast. Existence of lipid inhibitors. J Biochem. 1970 Feb;67(2):219–227. doi: 10.1093/oxfordjournals.jbchem.a129245. [DOI] [PubMed] [Google Scholar]
- Kawaguchi A., Hatanaka H., Katsuki H. Control of ergosterol biosynthesis in yeast. Biochem Biophys Res Commun. 1968 Nov 8;33(3):463–468. doi: 10.1016/0006-291x(68)90596-2. [DOI] [PubMed] [Google Scholar]
- Keith A. D., Resnick M. R., Haley A. B. Fatty acid desaturase mutants of Saccharomyces cerevisiae. J Bacteriol. 1969 May;98(2):415–420. doi: 10.1128/jb.98.2.415-420.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kidby D. K., Davies R. Invertase and disulphide bridges in the yeast wall. J Gen Microbiol. 1970 Jun;61(3):327–333. doi: 10.1099/00221287-61-3-327. [DOI] [PubMed] [Google Scholar]
- Klug M. J., Markovetz A. J. Degradation of hydrocarbons by members of the genus Candida. II. Oxidation of n-alkanes and l-alkenes by Candida lipolytica. J Bacteriol. 1967 Jun;93(6):1847–1852. doi: 10.1128/jb.93.6.1847-1852.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klug M. J., Markovetz A. J. Fermentation of 1-hexadecene by Candida lipolytica. Biotechnol Bioeng. 1969 May;11(3):427–440. doi: 10.1002/bit.260110314. [DOI] [PubMed] [Google Scholar]
- Klug M. J., Markovetz A. J. Utilization of aliphatic hydrocarbons by micro-organisms. Adv Microb Physiol. 1971;5:1–43. doi: 10.1016/s0065-2911(08)60404-x. [DOI] [PubMed] [Google Scholar]
- Kovác L., Subík J., Russ G., Kollár K. On the relationship between respiratory activity and lipid composition of the yeast cell. Biochim Biophys Acta. 1967 Aug 8;144(1):94–101. doi: 10.1016/0005-2760(67)90080-x. [DOI] [PubMed] [Google Scholar]
- LAMPEN J. O., ARNOW P. M., BOROWSKA Z., LASKIN A. I. Location and role of sterol at nystatin-binding sites. J Bacteriol. 1962 Dec;84:1152–1160. doi: 10.1128/jb.84.6.1152-1160.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LIGHT R. J., LENNARZ W. J., BLOCH K. The metabolism of hydroxystearic acids in yeast. J Biol Chem. 1962 Jun;237:1793–1800. [PubMed] [Google Scholar]
- Lanyi J. K., Plachy W. Z., Kates M. Lipid interactions in membranes of extremely halophilic bacteria. II. Modification of the bilayer structure by squalene. Biochemistry. 1974 Nov 19;13(24):4914–4920. doi: 10.1021/bi00721a006. [DOI] [PubMed] [Google Scholar]
- Lebeault J. M., Azoulay E. Metabolism of alkane by yeast. Lipids. 1971 Jul;6(7):444–447. doi: 10.1007/BF02531226. [DOI] [PubMed] [Google Scholar]
- Lebeault J. M., Roche B., Duvnjak Z., Azoulay E. Isolation and study of the enzymes involved in the metabolism of hydrocarbons by Candida tropicalis. Arch Mikrobiol. 1970;72(2):140–153. doi: 10.1007/BF00409520. [DOI] [PubMed] [Google Scholar]
- Lebeault J. M., Roche B., Duvnjak Z., Azoulay E. Protoplasts obtained from Candida tropicalis grown on alkanes. J Bacteriol. 1969 Dec;100(3):1218–1221. doi: 10.1128/jb.100.3.1218-1221.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lester R. L., Steiner M. R. The occurrence of diphosphoinositide and triphosphoinositide in Saccharomyces cerevisiae. J Biol Chem. 1968 Sep 25;243(18):4889–4893. [PubMed] [Google Scholar]
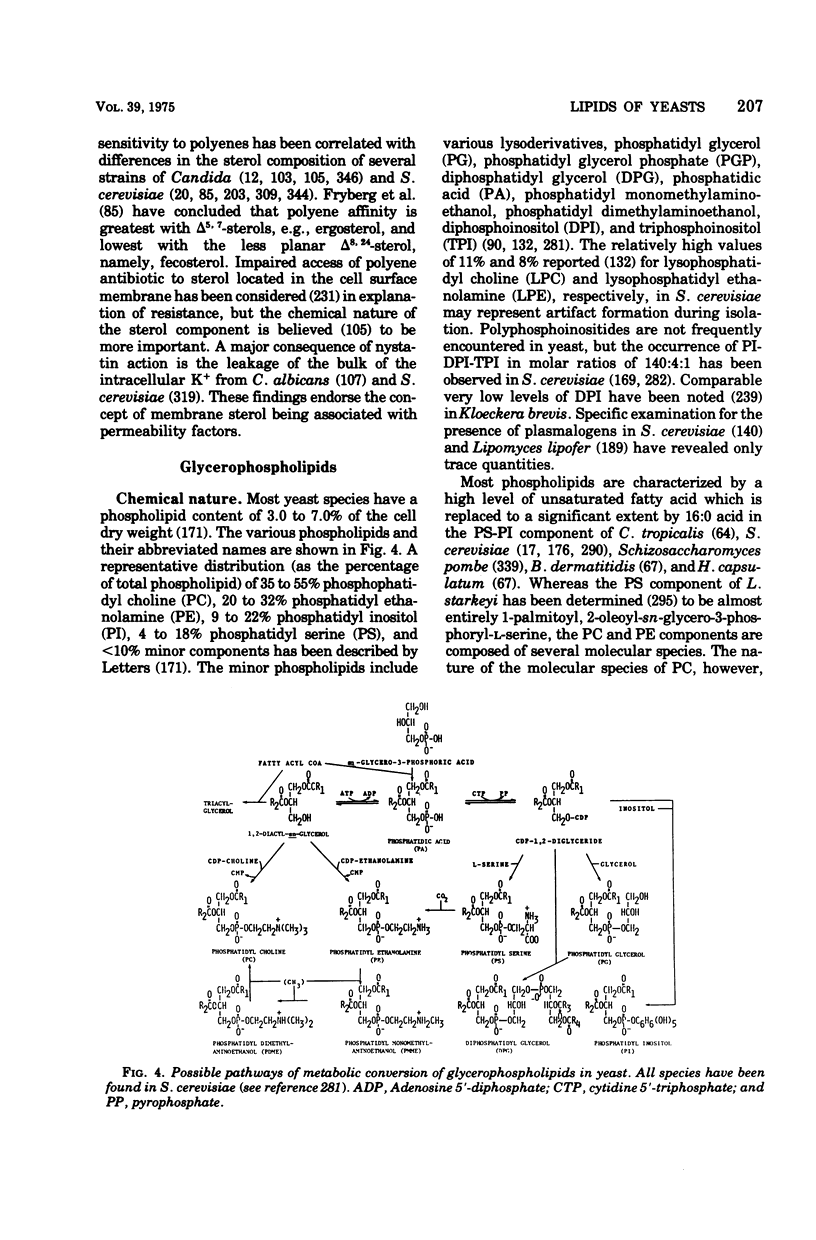
- Letters R. Phospholipids of yeast. II. Extraction, isolation and characterisation of yeast phospholipids. Biochim Biophys Acta. 1966 Jun 1;116(3):489–499. [PubMed] [Google Scholar]
- Lewin L. M. Effects of meso-inositol deficiency on some important biological and chemical characteristics of yeast. J Gen Microbiol. 1965 Nov;41(2):215–224. doi: 10.1099/00221287-41-2-215. [DOI] [PubMed] [Google Scholar]
- Linnane A. W., Haslam J. M., Lukins H. B., Nagley P. The biogenesis of mitochondria in microorganisms. Annu Rev Microbiol. 1972;26:163–198. doi: 10.1146/annurev.mi.26.100172.001115. [DOI] [PubMed] [Google Scholar]
- Longley R. P., Rose A. H., Knights B. A. Composition of the protoplast membrane from Saccharomyces cerevisiae. Biochem J. 1968 Jul;108(3):401–412. doi: 10.1042/bj1080401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lonsane B. K., Singh H. D., Baruah J. N., Iyengar M. S. Ascospore formation in yeasts during active growth on hydrocarbons. Arch Mikrobiol. 1972;87(1):41–46. doi: 10.1007/BF00424777. [DOI] [PubMed] [Google Scholar]
- Lowdon M. J., Gordon P. A., Stewart P. R. Regulation of the synthesis of mitochondrial enzymes and cytochromes. Distinction between catabolite repression and anaerobiosis in Saccharomyces cerevisiae. Arch Mikrobiol. 1972;85(4):355–361. doi: 10.1007/BF00549273. [DOI] [PubMed] [Google Scholar]
- Ludvík J., Munk V., Dostálek M. Ultrastructural changes in the yeast Candida lipolytica caused by penetration of hydrocarbons into the cell. Experientia. 1968 Oct 15;24(10):1066–1068. doi: 10.1007/BF02138754. [DOI] [PubMed] [Google Scholar]
- MAHLER H. R., NEISS G., SLONIMSKI P. P., MACKLER B. BIOCHEMICAL CORRELATES OF RESPIRATORY DEFICIENCY. 3. THE LEVEL OF SOME UNSAPONIFIABLE LIPIDS IN DIFFERENT STRAINS OF BAKER'S YEAST. Biochemistry. 1964 Jul;3:893–895. doi: 10.1021/bi00895a005. [DOI] [PubMed] [Google Scholar]
- MARKOVETZ A. J., KALLIO R. E. ASSIMILATION OF ALKANES AND ALKENES BY YEASTS. J Bacteriol. 1964 Apr;87:968–969. doi: 10.1128/jb.87.4.968-969.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madyastha P. B., Parks L. W. The effect of cultural conditons on the ergosterol ester components of yeast. Biochim Biophys Acta. 1969 Jun 10;176(4):858–862. doi: 10.1016/0005-2760(69)90267-7. [DOI] [PubMed] [Google Scholar]
- Marchant R., Smith D. G. Membranous structures in yeasts. Biol Rev Camb Philos Soc. 1968 Nov;43(4):459–480. doi: 10.1111/j.1469-185x.1968.tb00967.x. [DOI] [PubMed] [Google Scholar]
- Marzuki S., Hall R. M., Linnane A. W. Induction of respiratory incompetent mutants by unsaturated fatty acid depletion in Saccharomyces cerevisiae. Biochem Biophys Res Commun. 1974 Mar 25;57(2):372–378. doi: 10.1016/0006-291x(74)90940-1. [DOI] [PubMed] [Google Scholar]
- Matile P., Wiemken A. The vacuole as the lysosome of the yeast cell. Arch Mikrobiol. 1967 Feb 20;56(2):148–155. doi: 10.1007/BF00408765. [DOI] [PubMed] [Google Scholar]
- McElroy F. A., Stewart H. B. The lipids of Lipomyces lipofer. Can J Biochem. 1967 Feb;45(2):171–178. doi: 10.1139/o67-020. [DOI] [PubMed] [Google Scholar]
- McMurrough I., Rose A. H. Effect of growth rate and substrate limitation on the composition and structure of the cell wall of Saccharomyces cerevisiae. Biochem J. 1967 Oct;105(1):189–203. doi: 10.1042/bj1050189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMurrough I., Rose A. H. Effects of temperature variation on the fatty acid composition of Candida utilis. J Bacteriol. 1971 Sep;107(3):753–758. doi: 10.1128/jb.107.3.753-758.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMurrough I., Rose A. H. Effects of temperature variation on the fatty acid composition of a psychrophilic Candida species. J Bacteriol. 1973 Apr;114(1):451–452. doi: 10.1128/jb.114.1.451-452.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendoza C. G., Villanueva J. R. Preparation and composition of the protoplast membrane of Candida utilis. Biochim Biophys Acta. 1967 May 2;135(2):189–195. doi: 10.1016/0005-2736(67)90113-7. [DOI] [PubMed] [Google Scholar]
- Merdinger E., Cwiakala C. E. Fatty acids of the lipids fromPullularia pullulans. Lipids. 1967 May;2(3):276–277. doi: 10.1007/BF02532568. [DOI] [PubMed] [Google Scholar]
- Meyer K. H., Schweizer E. Saturated fatty acid mutant of Saccharomyces cerevisiae with an intact fatty acid synthetase. J Bacteriol. 1974 Feb;117(2):345–350. doi: 10.1128/jb.117.2.345-350.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mian F. A., Fencl Z., Prokop A., Mohagheghi A., Fazeli A. Effect of growth rate on the glucose metabolism of yeast grown in continuous culture. Radiorespirometric studies. Folia Microbiol (Praha) 1974;19(3):191–198. doi: 10.1007/BF02895017. [DOI] [PubMed] [Google Scholar]
- Miller W. L., Gaylor J. L. Investigation of the component reactions of oxidative sterol demethylation. Oxidation of a 4 alpha-methyl sterol to a 4 alpha-carboxylic acid during cholesterol biosynthesis. J Biol Chem. 1970 Oct 25;245(20):5369–5374. [PubMed] [Google Scholar]
- Minnikin D. E., Abley P., McQuillin F. J. Location of double bonds in long chain esters by methoxymercuration-demercuration followed by mass spectroscopy. Lipids. 1974 Mar;9(3):135–140. doi: 10.1007/BF02532684. [DOI] [PubMed] [Google Scholar]
- Miura T., Sato R. Cellular senescence in yeast caused by carbon-source starvation. II. Induction and subcellular location of lipid peroxidation activity. J Biochem. 1974 Sep;76(3):593–601. doi: 10.1093/oxfordjournals.jbchem.a130603. [DOI] [PubMed] [Google Scholar]
- Molzahn S. W., Woods R. A. Polyene resistance and the isolation of sterol mutants in Saccharomyces cerevisiae. J Gen Microbiol. 1972 Sep;72(2):339–348. doi: 10.1099/00221287-72-2-339. [DOI] [PubMed] [Google Scholar]
- Moss F. J., Rickard P. A., Beech G. A., Bush F. E. The response by microorganisms to steady state growth in controlled concentrations of oxygen and glucose. I. Candida utilis. Biotechnol Bioeng. 1969 Jul;11(4):561–580. doi: 10.1002/bit.260110404. [DOI] [PubMed] [Google Scholar]
- Munk V., Dostálek M., Volfová O. Cultivation of yeast on gas oil. Biotechnol Bioeng. 1969 May;11(3):383–391. doi: 10.1002/bit.260110310. [DOI] [PubMed] [Google Scholar]
- Myher J. J., Marai L., Kuksis A. Identification of fatty acids by GC-MS using polar siloxane liquid phases. Anal Biochem. 1974 Nov;62(1):188–203. doi: 10.1016/0003-2697(74)90380-7. [DOI] [PubMed] [Google Scholar]
- Nakamura M., Onishi S. I. Spin-labeled yeast cells. Biochem Biophys Res Commun. 1972 Jan 31;46(2):926–932. doi: 10.1016/s0006-291x(72)80230-4. [DOI] [PubMed] [Google Scholar]
- Napias C., Maurice A., Baraud J. Influence des carbures cancérogènes sur la structure lipidique des membranes protoplastique et mitochondriale de Saccharomyces cerevisiae. C R Acad Sci Hebd Seances Acad Sci D. 1972 Mar 20;274(12):1849–1852. [PubMed] [Google Scholar]
- Necas O., Svoboda A. Effect of proteases, phospholipases and polysaccharide-splitting enzymes on plasma membrane particles and on the synthesis of the fibrillar cell wall component in yeast protoplasts. Folia Microbiol (Praha) 1974;19(2):81–87. doi: 10.1007/BF02872839. [DOI] [PubMed] [Google Scholar]
- Nes W. R. Role of sterols in membranes. Lipids. 1974 Aug;9(8):596–612. doi: 10.1007/BF02532509. [DOI] [PubMed] [Google Scholar]
- Nielsen H. S. Variation in Lipid Content of Strains of Histoplasma capsulatum Exhibiting Different Virulence Properties for Mice. J Bacteriol. 1966 Jan;91(1):273–277. doi: 10.1128/jb.91.1.273-277.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishi K., Ichikawa H., Tomochika K., Okabe A., Kanemasa Y. Lipid composition of Candida albicans and effect of growth temperature on it. Acta Med Okayama. 1973 Aug;27(3):73–81. [PubMed] [Google Scholar]
- Nishikawa Y., Nakamura I., Kamihara T., Fukui S. Effects of thiamine and pyridoxine on the composition of fatty acids in Saccharomyces carlsbergensis 4228. Biochem Biophys Res Commun. 1974 Jul 24;59(2):777–780. doi: 10.1016/s0006-291x(74)80047-1. [DOI] [PubMed] [Google Scholar]
- Noble A. C., Duitschaever C. L. Fatty acid composition of lipids from Saccharomyces fragilis. Lipids. 1973 Nov;8(11):655–657. doi: 10.1007/BF02533153. [DOI] [PubMed] [Google Scholar]
- Nurminen T., Suomalainen H. Occurrence of long-chain fatty acids and glycolipids in the cell envelope fractions of baker's yeast. Biochem J. 1971 Dec;125(4):963–969. doi: 10.1042/bj1250963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyns E. J., Chiang N., Wiaux A. L. Comparative lipid content of Candida lipolytica grown on glucose and on n-hexadecane. Antonie Van Leeuwenhoek. 1968;34(2):197–204. doi: 10.1007/BF02046431. [DOI] [PubMed] [Google Scholar]
- Oldfield E., Chapman D. Dynamics of lipids in membranes: Heterogeneity and the role of cholesterol. FEBS Lett. 1972 Jul 1;23(3):285–297. doi: 10.1016/0014-5793(72)80300-4. [DOI] [PubMed] [Google Scholar]
- Ono T., Kesado T., Awaya J., Omura S. Target of inhibition by the anti-lipogenic antibiotic cerulenin of sterol synthesis in yeast. Biochem Biophys Res Commun. 1974 Apr 23;57(4):1119–1124. doi: 10.1016/0006-291x(74)90812-2. [DOI] [PubMed] [Google Scholar]
- Orme T. W., McIntyre J., Lynen F., Kühn L., Schweizer E. Fatty-acid elongation in a mutant of Saccharomyces cerevisiae deficient in fatty-acid synthetase. Eur J Biochem. 1972 Jan 21;24(3):407–415. doi: 10.1111/j.1432-1033.1972.tb19700.x. [DOI] [PubMed] [Google Scholar]
- Osumi M., Miwa N., Teranishi Y., Tanaka A., Fukui S. Ultrastructure of Candida yeasts grown on n-alkanes. Appearance of microbodies and its relationship to high catalase activity. Arch Microbiol. 1974;99(3):181–201. doi: 10.1007/BF00696234. [DOI] [PubMed] [Google Scholar]
- PARKS L. W., STARR P. R. A relationship between ergosterol and respiratory competency in yeast. J Cell Comp Physiol. 1963 Feb;61:61–65. doi: 10.1002/jcp.1030610107. [DOI] [PubMed] [Google Scholar]
- Palamarczyk G., Chojnacki T. Biosynthesis of lipid-linked sugars in Saccharomyces cerevisiae. FEBS Lett. 1973 Aug 15;34(2):201–203. doi: 10.1016/0014-5793(73)80793-8. [DOI] [PubMed] [Google Scholar]
- Paltauf F., Johnston J. M. Lipid metabolism in inositol-deficient yeast, Saccharomyces carlsbergensis. I. Influence of different carbon sources on the lipid composition of deficient cells. Biochim Biophys Acta. 1970 Dec 15;218(3):424–430. [PubMed] [Google Scholar]
- Paltauf F., Schatz G. Promitochondria of anaerobicallly grown yeast. II. Lipid composition. Biochemistry. 1969 Jan;8(1):335–339. doi: 10.1021/bi00829a046. [DOI] [PubMed] [Google Scholar]
- Papahadjopoulos D., Cowden M., Kimelberg H. Role of cholesterol in membranes. Effects on phospholipid-protein interactions, membrane permeability and enzymatic activity. Biochim Biophys Acta. 1973 Nov 30;330(1):8–26. doi: 10.1016/0005-2736(73)90280-0. [DOI] [PubMed] [Google Scholar]
- Parks L. W., Anding C., Ourisson G. Sterol transmethylation during aerobic adaptation of yeast. Eur J Biochem. 1974 Apr 16;43(3):451–458. doi: 10.1111/j.1432-1033.1974.tb03431.x. [DOI] [PubMed] [Google Scholar]
- Parks L. W., Bond F. T., Thompson E. D., Starr P. R. 8(9),22 -Ergostadiene-3 -ol, an ergosterol precursor accumulated in wild-type and mutants of yeast. J Lipid Res. 1972 May;13(3):311–316. [PubMed] [Google Scholar]
- Patel P. V., Johnston J. R. Kinetics of action of nystatin on yeast. J Appl Bacteriol. 1971 Jun;34(2):449–458. doi: 10.1111/j.1365-2672.1971.tb02305.x. [DOI] [PubMed] [Google Scholar]
- Pelechová J., Krumphanzl V., Uher J., Dyr J. Assimilation of hydrocarbons. I. Proportion of fatty acids in the cell fat. Folia Microbiol (Praha) 1971;16(2):103–109. doi: 10.1007/BF02887479. [DOI] [PubMed] [Google Scholar]
- Penman C. S., Duffus J. H. Ergosterol is the only sterol in Kluyveromyces fragilis. Antonie Van Leeuwenhoek. 1974;40(4):529–531. doi: 10.1007/BF00403816. [DOI] [PubMed] [Google Scholar]
- Pilát P., Prokop A., Volfová O., Panos J., Ragab A. M. The substrate specificity of two yeast strains utilizing hydrocarbons. Folia Microbiol (Praha) 1974;19(2):118–124. doi: 10.1007/BF02872844. [DOI] [PubMed] [Google Scholar]
- Power D. M., Challinor S. W. The effects of inositol-deficiency on the chemical composition of the yeast cell wall. J Gen Microbiol. 1969 Feb;55(2):169–176. doi: 10.1099/00221287-55-2-169. [DOI] [PubMed] [Google Scholar]
- Prostenik M., Kljaić K., Weinert M. Occurrence of (+)-erythro-2,3-dihydroxyhexacosanoic acid in cerebrin from the yeast Saccharomyces cerevisiae. Lipids. 1973 May;8(5):325–326. doi: 10.1007/BF02531914. [DOI] [PubMed] [Google Scholar]
- Prottey C., Seidman M. M., Ballou C. E. Preparation of 3H-phosphatidylmyoinositol with Kloeckera brevis. Lipids. 1970 May;5(5):463–468. doi: 10.1007/BF02531309. [DOI] [PubMed] [Google Scholar]
- Proudlock J. W., Haslam J. M., Linnane A. W. Biogenesis of mitochondria. 19. The effects of unsaturated fatty acid depletion on the lipid composition and energy metabolism of a fatty acid desaturase mutant of Saccharomyces cerevisiae. J Bioenerg. 1971 Dec;2(5):327–349. doi: 10.1007/BF01963829. [DOI] [PubMed] [Google Scholar]
- Proudlock J. W., Haslam J. M., Linnane A. W. Specific effect of unsaturated fatty acid depletion on mitochondrial oxidative phosphorylation in Saccharomyces cerevisiae. Biochem Biophys Res Commun. 1969 Nov 20;37(5):847–852. doi: 10.1016/0006-291x(69)90969-3. [DOI] [PubMed] [Google Scholar]
- Proudlock J. W., Wheeldon L. W., Jollow D. J., Linnane A. W. Role of sterols in Saccharomyces cerevisiae. Biochim Biophys Acta. 1968 Mar 4;152(2):434–437. doi: 10.1016/0005-2760(68)90060-x. [DOI] [PubMed] [Google Scholar]
- Pugh E. L., Kates M. Desaturation of phosphatidylcholine and phosphatidylethanolamine by a microsomal enzyme system from Candida lipolytica. Biochim Biophys Acta. 1973 Sep 25;316(3):305–316. doi: 10.1016/0005-2760(73)90071-4. [DOI] [PubMed] [Google Scholar]
- ROZIJN T. H., TONINO G. J. STUDIES ON THE YEAST NUCLEUS. I. THE ISOLATION OF NUCLEI. Biochim Biophys Acta. 1964 Sep 11;91:105–112. doi: 10.1016/0926-6550(64)90174-4. [DOI] [PubMed] [Google Scholar]
- Ratcliffe S. J., Hossack J. A., Wheeler G. E., Rose A. H. Modifications to the phospholipid composition of Saccharomyces cerevisiae induced by exogenous ethanolamine. J Gen Microbiol. 1973 Jun;76(2):445–449. doi: 10.1099/00221287-76-2-445. [DOI] [PubMed] [Google Scholar]
- Ratledge C. Microbial conversions of n-alkanes to fatty acids: A new attempt to obtain economical microbial fats and fatty acids. Chem Ind. 1970 Jun 27;26:843–854. [PubMed] [Google Scholar]
- Ratledge C., Saxton R. K. Quantitative extraction of lipid and fatty acids from a Candida species. Anal Biochem. 1968 Nov;26(2):288–294. doi: 10.1016/0003-2697(68)90339-4. [DOI] [PubMed] [Google Scholar]
- Rogers P. J., Stewart P. R. Mitochondrial protein synthesis in aerobic and micro-aerobic continuous cultures of Candida parapsilosis. Arch Microbiol. 1974;99(1):47–59. doi: 10.1007/BF00696221. [DOI] [PubMed] [Google Scholar]
- Rouser G., Kritchevsky G., Yamamoto A., Baxter C. F. Lipids in the nervous system of different species as a function of age: brain, spinal cord, peripheral nerve, purified whole cell preparations, and subcellular particulates: regulatory mechanisms and membrane structure. Adv Lipid Res. 1972;10:261–360. doi: 10.1016/b978-0-12-024910-7.50013-0. [DOI] [PubMed] [Google Scholar]
- Rylkin S. S., Berezov T. B., Gurina L. V., Belova L. A., Shul'ga A. V. O sostave kletochnoi stenki drozhzhei Candida tropicalis pri roste na gliukoze i n-alkanakh. Mikrobiologiia. 1974;43(3):551–552. [PubMed] [Google Scholar]
- SCHATZ G. SUBCELLULAR PARTICLES CARRYING MITOCHONDRIAL ENZYMES IN ANAEROBICALLY-GROWN CELLS OF SACCHAROMYCES CEREVISIAE. Biochim Biophys Acta. 1965 Feb 22;96:342–345. [PubMed] [Google Scholar]
- SENTHESHANMUGATHAN S., NICKERSON W. J. Composition of cells and cell walls of triangular and ellipsoidal forms of Trigonopsis variabilis. J Gen Microbiol. 1962 Mar;27:451–464. doi: 10.1099/00221287-27-3-451. [DOI] [PubMed] [Google Scholar]
- STANACEV N. Z., KATES M. Constitution of cerebrin from the yeast Torulopsis utilis. Can J Biochem Physiol. 1963 May;41:1330–1334. [PubMed] [Google Scholar]
- Safe S., Caldwell J. The effect of growth environment on the chloroform-methanol and alkali-extractable cell wall and cytoplasm lipid levels of Mucor rouxii. Can J Microbiol. 1975 Jan;21(1):79–84. doi: 10.1139/m75-011. [DOI] [PubMed] [Google Scholar]
- Safe S., Duncan J. Effect of oxygen levels on the fatty acids and lipids of Mucor rouxii. Lipids. 1974 Apr;9(4):285–289. doi: 10.1007/BF02532207. [DOI] [PubMed] [Google Scholar]
- Safe S. Lipid and alkali extractable fatty acids from Mucor rouxii: effect of thermal changes in growth environment and age of cells. Lipids. 1974 Dec;9(12):952–956. doi: 10.1007/BF02533817. [DOI] [PubMed] [Google Scholar]
- Safe S. The effect of environment on the free and hydrosoluble sterols of Mucor rouxii. Biochim Biophys Acta. 1973 Dec 20;326(3):471–475. doi: 10.1016/0005-2760(73)90147-1. [DOI] [PubMed] [Google Scholar]
- Sarachek A., Higgins N. P. Effects of ergosterol, palmitic acid and related simple lipids on the recovery of Candida albicans from ultraviolet irradiation. Arch Mikrobiol. 1972;82(1):38–54. doi: 10.1007/BF00424928. [DOI] [PubMed] [Google Scholar]
- Satyanarayana T., Klein H. P. Studies on acetyl-coenzyme A synthetase of yeast: inhibition by long-chain acyl-coenzyme A esters. J Bacteriol. 1973 Aug;115(2):600–606. doi: 10.1128/jb.115.2.600-606.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheda R., Bos P. Hydrocarbons as substrates of Yeasts. Nature. 1966 Aug 6;211(5049):660–660. doi: 10.1038/211660a0. [DOI] [PubMed] [Google Scholar]
- Schibeci A., Rattray J. B., Kidby D. K. Electron microscope autoradiography of labelled yeast plasma membrane. Biochim Biophys Acta. 1973 Nov 16;323(4):532–538. doi: 10.1016/0005-2736(73)90161-2. [DOI] [PubMed] [Google Scholar]
- Schibeci A., Rattray J. B., Kidby D. K. Isolation and identification of yeast plasma membrane. Biochim Biophys Acta. 1973 Jun 7;311(1):15–25. doi: 10.1016/0005-2736(73)90250-2. [DOI] [PubMed] [Google Scholar]
- Schweizer E., Bolling H. A Saccharomyces cerevisiae mutant defective in saturated fatty acid biosynthesis. Proc Natl Acad Sci U S A. 1970 Oct;67(2):660–666. doi: 10.1073/pnas.67.2.660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sentandreu R., Lampen J. O. Biosynthesis of mannan in Saccharomyces cerevisiae, isolation of a lipid intermediate and its identification as a mannosyl-1-phosphoryl polyrenol. FEBS Lett. 1972 Nov 1;27(2):331–334. doi: 10.1016/0014-5793(72)80652-5. [DOI] [PubMed] [Google Scholar]
- Shafai T., Lewin L. M. Effects of myo-inositol deficiency upon the lipid composition of the yeast, Saccharomyces carlsbergensis. Biochim Biophys Acta. 1968 Jul 1;152(4):787–790. doi: 10.1016/0005-2760(68)90127-6. [DOI] [PubMed] [Google Scholar]
- Shaw R. The polyunsaturated fatty acids of microorganisms. Adv Lipid Res. 1966;4:107–174. doi: 10.1016/b978-1-4831-9940-5.50011-9. [DOI] [PubMed] [Google Scholar]
- Skipton M. D., Watson K., Houghton R. L., Griffiths D. E. A comparative study of cells and mitochondria of Saccharomyces cerevisiae and of a hydrocarbon-utilizing yeast, Candida lipolytica. J Gen Microbiol. 1974 Sep;84(1):94–110. doi: 10.1099/00221287-84-1-94. [DOI] [PubMed] [Google Scholar]
- Smith S. W., Lester R. L. Inositol phosphorylceramide, a novel substance and the chief member of a major group of yeast sphingolipids containing a single inositol phosphate. J Biol Chem. 1974 Jun 10;249(11):3395–3405. [PubMed] [Google Scholar]
- Somlo M., Krupa M. ATPase from mitochondrially determined oligomycin-resistant mutants of S. cerevisiae: effect of triton X-100 and phospholipase A on oligomycin-sensitivity of ATPase from mitochondria and promitochondria. Biochem Biophys Res Commun. 1974 Aug 5;59(3):1165–1171. doi: 10.1016/s0006-291x(74)80101-4. [DOI] [PubMed] [Google Scholar]
- Southwell-Keely P. T., Lynen F. The mechanism of production of 11-octadecenoic acid (vaccenic acid) by yeast. Biochim Biophys Acta. 1974 Jan 23;337(1):22–28. doi: 10.1016/0005-2760(74)90036-8. [DOI] [PubMed] [Google Scholar]
- Souzu H. Proceedings: The phospholipid degradation and cellular death caused by freeze-thawing or freeze-drying of yeast. Cryobiology. 1973 Nov;10(5):427–431. doi: 10.1016/0011-2240(73)90070-9. [DOI] [PubMed] [Google Scholar]
- Stanacev N. Z., Davidson J. B., Stuhne-Sekalec L., Domazet Z. Biochemistry of polyglycerophosphatides. The mechanism of cardiolipin biosynthesis in isolated mitochondria. Can J Biochem. 1973 Mar;51(3):286–304. doi: 10.1139/o73-035. [DOI] [PubMed] [Google Scholar]
- Starkey R. L. Lipid Production by a Soil Yeast. J Bacteriol. 1946 Jan;51(1):33–50. [PMC free article] [PubMed] [Google Scholar]
- Starr P. R., Parks L. W. Transmethylation of sterols in aerobically adapting Saccharomyces cerevisiae. J Bacteriol. 1972 Jan;109(1):236–242. doi: 10.1128/jb.109.1.236-242.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steele S. D., Miller J. J. Ultrastructural changes in germinating ascospores of Saccharomyces cerevisiae. Can J Microbiol. 1974 Jul;20(7):929–933. doi: 10.1139/m74-142. [DOI] [PubMed] [Google Scholar]
- Steiner M. R., Lester R. L. In vitro studies of phospholipid biosynthesis in Saccharomyces cerevisiae. Biochim Biophys Acta. 1972 Feb 21;260(2):222–243. doi: 10.1016/0005-2760(72)90035-5. [DOI] [PubMed] [Google Scholar]
- Steiner S., Lester R. L. Metabolism of diphosphoinositide and triphosphoinositide in Saccharomyces cerevisiae. Biochim Biophys Acta. 1972 Jan 27;260(1):82–87. doi: 10.1016/0005-2760(72)90076-8. [DOI] [PubMed] [Google Scholar]
- Steiner S., Lester R. L. Studies on the diversity of inositol-containing yeast phospholipids: incorporation of 2-deoxyglucose into lipid. J Bacteriol. 1972 Jan;109(1):81–88. doi: 10.1128/jb.109.1.81-88.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner S., Smith S., Waechter C. J., Lester R. L. Isolation and partial characterization of a major inositol-containing lipid in baker's yeast, mannosyl-diinositol, diphosphoryl-ceramide. Proc Natl Acad Sci U S A. 1969 Nov;64(3):1042–1048. doi: 10.1073/pnas.64.3.1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stodola F. H., Deinema M. H., Spencer J. F. Extracellular lipids of yeasts. Bacteriol Rev. 1967 Sep;31(3):194–213. doi: 10.1128/br.31.3.194-213.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumper M. Control of fatty-acid biosynthesis by long-chain acyl CoAs and by lipid membranes. Eur J Biochem. 1974 Nov 15;49(2):469–475. doi: 10.1111/j.1432-1033.1974.tb03851.x. [DOI] [PubMed] [Google Scholar]
- Swanljung P., Swanljung H., Partis M. D. Correlation between oligomycin-sensitivity and ergosterol in a purified mitochondrial adenosine triphosphatase from Saccharomyces cerevisiae. Biochem J. 1972 Jun;128(2):479–480. doi: 10.1042/bj1280479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talamo B., Chang N., Bloch K. Desaturation of oleyl phospholipid to linoleyl phospholipid in Torulopsis utilis. J Biol Chem. 1973 Apr 25;248(8):2738–2742. [PubMed] [Google Scholar]
- Talwalkar R. T., Lester R. L. Synthesis of diphosphoinositide by a soluble fraction of Saccharomyces cerevisiae. Biochim Biophys Acta. 1974 Sep 19;360(3):306–311. doi: 10.1016/0005-2760(74)90060-5. [DOI] [PubMed] [Google Scholar]
- Talwalkar R. T., Lester R. L. The response of diphosphoinositide and triphosphoinostitide to perturbations of the adenylate energy charge in cells of Saccharomyces cerevisiae. Biochim Biophys Acta. 1973 Jun 21;306(3):412–421. doi: 10.1016/0005-2760(73)90180-x. [DOI] [PubMed] [Google Scholar]
- Thompson E. D., Knights B. A., Parks L. W. Identification and properties of a sterol-binding polysaccharide isolated from Saccharomyces cerevisiae. Biochim Biophys Acta. 1973 Mar 30;304(1):132–141. doi: 10.1016/0304-4165(73)90122-0. [DOI] [PubMed] [Google Scholar]
- Thompson E. D., Parks L. W. Effect of altered sterol composition on growth characteristics of Saccharomyces cerevisiae. J Bacteriol. 1974 Nov;120(2):779–784. doi: 10.1128/jb.120.2.779-784.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson E. D., Parks L. W. Lipids associated with cytochrome oxidase derived from yeast mitochondria. Biochim Biophys Acta. 1972 Apr 18;260(4):601–607. doi: 10.1016/0005-2760(72)90009-4. [DOI] [PubMed] [Google Scholar]
- Thompson E. D., Parks L. W. The effect of altered sterol composition on cytochrome oxidase and S-adenosylmethionine: delta 24 sterol methyltransferase enzymes of yeast mitochondria. Biochem Biophys Res Commun. 1974 Apr 23;57(4):1207–1213. doi: 10.1016/0006-291x(74)90825-0. [DOI] [PubMed] [Google Scholar]
- Thompson E. D., Starr P. R., Parks L. W. Sterol accumulation in a mutant of Saccharomyces cerevisiae defective in ergosterol production. Biochem Biophys Res Commun. 1971 Jun 18;43(6):1304–1309. doi: 10.1016/s0006-291x(71)80014-1. [DOI] [PubMed] [Google Scholar]
- Thorpe R. F., Ratledge C. Fatty acids of triglycerides and phospholipids from a thermotolerant strain of Candida tropicalis grown on n-alkanes at 30 and 40 degrees C. J Gen Microbiol. 1973 Sep;78(1):203–206. doi: 10.1099/00221287-78-1-203. [DOI] [PubMed] [Google Scholar]
- Topham R. W., Gaylor J. L. Further characterization of the 5 -hydroxysterol dehydrase of yeast. Biochem Biophys Res Commun. 1972 Apr 14;47(1):180–186. doi: 10.1016/s0006-291x(72)80026-3. [DOI] [PubMed] [Google Scholar]
- Trocha P. J., Jasne S. J., Sprinson D. B. Novel sterols in ergosterol deficient yeast mutants. Biochem Biophys Res Commun. 1974 Jul 24;59(2):666–671. doi: 10.1016/s0006-291x(74)80031-8. [DOI] [PubMed] [Google Scholar]
- Työrinoja K., Nurminen T., Suomalainen H. The cell-envelope glycolipids of baker's yeast. Biochem J. 1974 Jul;141(1):133–139. doi: 10.1042/bj1410133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Den Bosch H., Van Der Elzen H. M., Van Deenen L. L. On the phospholipases of yeast. Lipids. 1967 May;2(3):279–280. doi: 10.1007/BF02532570. [DOI] [PubMed] [Google Scholar]
- Venables P., Russell A. D. Some effects of nystatin on Saccharomyces cerevisiae. Microbios. 1972 Dec;6(24):239–246. [PubMed] [Google Scholar]
- Vignais P. M., Nachbaur J., Huet J., Vignais P. V. Studies on the phospholipids of yeast mitochondria. Biochem J. 1970 Feb;116(4):42P–43P. doi: 10.1042/bj1160042pb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volfová O., Pecka K. Cultivation of Candida lipolytica 4-1 on hydrocarbons. IV. Fatty acids formed during batch cultivation on model gas oils. Folia Microbiol (Praha) 1973;18(4):286–299. doi: 10.1007/BF02868045. [DOI] [PubMed] [Google Scholar]
- Volfová O., Pilát P. Studies on methanol-oxidizing yeasts. I. Isolation and growth studies. Folia Microbiol (Praha) 1974;19(4):249–256. doi: 10.1007/BF02873216. [DOI] [PubMed] [Google Scholar]
- Waechter C. J., Lester R. L. Differential regulation of the N-methyl transferases responsible for phosphatidylcholine synthesis in Saccharomyces cerevisiae. Arch Biochem Biophys. 1973 Sep;158(1):401–410. doi: 10.1016/0003-9861(73)90637-1. [DOI] [PubMed] [Google Scholar]
- Waechter C. J., Lester R. L. Regulation of phosphatidylcholine biosynthesis in Saccharomyces cerevisiae. J Bacteriol. 1971 Mar;105(3):837–843. doi: 10.1128/jb.105.3.837-843.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner F., Kleemann T., Zahn W. Microbial transformation of hydrocarbons. II. Growth constants and cell composition of microbial cells derived from n-alkanes. Biotechnol Bioeng. 1969 May;11(3):393–408. doi: 10.1002/bit.260110311. [DOI] [PubMed] [Google Scholar]
- Wallace P. G., Huang M., Linnane A. W. The biogenesis of mitochondria. II. The influence of medium composition on the cytology of anaerobically grown Saccharomyces cerevisiae. J Cell Biol. 1968 May;37(2):207–220. doi: 10.1083/jcb.37.2.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D. I., Ochoa A. Measurements on the interfacial areas of hydrocarbon in yeast fermentations and relationships to specific growth rates. Biotechnol Bioeng. 1972 May;14(3):345–360. doi: 10.1002/bit.260140307. [DOI] [PubMed] [Google Scholar]
- Weete J. D., Laseter J. L. Distribution of sterols in the fungi. I. Fungal spores. Lipids. 1974 Aug;9(8):575–581. doi: 10.1007/BF02532507. [DOI] [PubMed] [Google Scholar]
- Weinert M., Kljaić K., Prostenik M. Studies in the sphingolipids series. XXXV. Separation and chemical characterization of the cerebrins from the yeast Saccharomyces cerevisiae. Chem Phys Lipids. 1973 Sep;11(2):83–88. doi: 10.1016/0009-3084(73)90025-x. [DOI] [PubMed] [Google Scholar]
- Weiss B., Stiller R. L. A simple preparative procedure for the rapid isolation of phytosphingosine from the yeast Hansenula ciferrii. Lipids. 1970 Sep;5(9):782–785. doi: 10.1007/BF02531393. [DOI] [PubMed] [Google Scholar]
- Welch J. W., Burlingame A. L. Very long-chain fatty acids in yeast. J Bacteriol. 1973 Jul;115(1):464–466. doi: 10.1128/jb.115.1.464-466.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler G. E., Michell R. H., Rose A. H. Phosphatidylinositol kinase activity in Saccharomyces cerevisiae. Biochem J. 1972 Apr;127(3):64P–64P. doi: 10.1042/bj1270064pa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White G. L., Hawthorne J. N. Phosphatidic acid and phosphatidylinositol metabolism in Schizosaccharomyces pombe. Biochem J. 1970 Apr;117(2):203–213. doi: 10.1042/bj1170203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams M. A., Taylor D. W., Tinoco J., Ojakian M. A., Keith A. D. Utilization of C20-polyunsaturated fatty acids by a yeast fatty acid desaturase mutant. Biochem Biophys Res Commun. 1973 Oct 15;54(4):1560–1566. doi: 10.1016/0006-291x(73)91164-9. [DOI] [PubMed] [Google Scholar]
- Wisnieski B. J., Keith A. D., Resnick M. R. Double-bond requirement in a fatty acid desaturase mutant of Saccharomyces cerevisiae. J Bacteriol. 1970 Jan;101(1):160–165. doi: 10.1128/jb.101.1.160-165.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisnieski B. J., Kiyomoto R. K. Fatty acid desaturase mutants of yeast: growth requirements and electron spin resonance spin-label distribution. J Bacteriol. 1972 Jan;109(1):186–195. doi: 10.1128/jb.109.1.186-195.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witter B., Debuch H., Steiner M. Die Lipide von Endomycopsis vernalis bei verschiedener Stickstoff-Ernährung. Arch Microbiol. 1974;101(4):321–335. doi: 10.1007/BF00455948. [DOI] [PubMed] [Google Scholar]
- Woods R. A., Bard M., Gardner I. E., Molzahn S. W. Studies on the accumulation of ergosterol and 24(28)-dehydroergosterol in 3 strains of Saccharomyces cerevisiae. Microbios. 1974 Jun-Jul;10A SUPPL(41):73–80. [PubMed] [Google Scholar]
- Woods R. A., Bard M., Jackson I. E., Drutz D. J. Resistance to polyene antibiotics and correlated sterol changes in two isolates of Candida tropicalis from a patient with an amphotericin B-resistant funguria. J Infect Dis. 1974 Jan;129(1):53–58. doi: 10.1093/infdis/129.1.53. [DOI] [PubMed] [Google Scholar]
- Woods R. A. Nystatin-resistant mutants of yeast: alterations in sterol content. J Bacteriol. 1971 Oct;108(1):69–73. doi: 10.1128/jb.108.1.69-73.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- el-Refai A. H., el-Kady I. A. Sterol biosynthesis in Saccharomyces fermentati. Z Allg Mikrobiol. 1968;8(5):361–366. [PubMed] [Google Scholar]
- el-Refai A. H., el-Kady I. A. Sterol production of yeast strains. Z Allg Mikrobiol. 1968;8(5):355–360. doi: 10.1002/jobm.3630080503. [DOI] [PubMed] [Google Scholar]
- van Dijken J. P., Harder W. Optimal conditions for the enrichment and isolation of methanol-assimilating yeasts. J Gen Microbiol. 1974 Oct;84(2):409–411. doi: 10.1099/00221287-84-2-409. [DOI] [PubMed] [Google Scholar]


