Abstract
Context
The standard criteria used to evaluate tumor response, the Response Evaluation Criteria in Solid Tumors (RECIST), were developed to assess tumor shrinkage after cytotoxic chemotherapy and may be limited in assessing response to biologic agents, which have a cytostatic mechanism of action..
Objective
To validate novel tumor response criteria based on morphologic changes observed on computed tomography (CT) in patients with colorectal liver metastases (CLM) treated with bevacizumab-containing chemotherapy regimens.
Design, Setting, and Patients
234 CLM were analyzed in 50 patients who underwent hepatic resection after preoperative chemotherapy that included bevacizumab at a comprehensive US cancer center from 2004 to 2007; date of last follow-up was March 2008. All patients underwent routine contrast-enhanced CT at the start and end of preoperative therapy. Three blinded, independent radiologists evaluated images for morphologic response, based upon metastases changing from heterogeneous masses with ill-defined margins into homogeneous lesions with sharp borders. These criteria were validated with a separate cohort of 82 patients with unresectable CLM treated with bevacizumab-containing chemotherapy.
Main Outcome Measures
Response determined using morphologic criteria and RECIST was correlated with pathologic response in resected liver specimens and with patient survival.
Results
Interobserver agreement for scoring morphologic changes was good among three radiologists (κ=0.68–0.78; 95% confidence interval, 0.51–0.93). In resected tumor specimens with morphologic optimal, incomplete, and no response, the median percentages of residual tumor cells were 20% (interquartile range [IQR], 10%–30%), 50% (IQR, 30%–60%), and 70% (IQR, 60%–70%), respectively (P<.001). With RECIST partial response, stable disease, and progressive disease, the median percentages of residual tumor cells were 30% (IQR, 10%–60%), 50% (IQR, 20%–70%), and 70% (IQR, 65%–70%), respectively (P=.04). Among patients who underwent hepatic resection, median overall survival was not yet reached with optimal morphologic response and 35 months (95% CI, 20.2 to 29.8 months) with incomplete or no morphologic response (P=.03). In the validation cohort, patients with optimal morphologic response had median overall survival of 31 months (95% CI, 26.8 to 35.2 months) compared to 19 months (95% CI, 14.6 to 23.4 months) with incomplete or no morphologic response (P=.009). RECIST did not correlate with survival in neither the surgical nor validation cohort.
Conclusions
Among patients with CLM treated with bevacizumab-containing chemotherapy, CT-based morphologic criteria had a statistically significant association with pathologic response and overall survival.
INTRODUCTION
The addition of bevacizumab, a monoclonal antibody against vascular endothelial growth factor, to cytotoxic chemotherapy is associated with improved survival in patients with stage IV colorectal cancer and higher pathologic response rates in patients undergoing resection of colorectal liver metastases (CLM).1, 2 Response to bevacizumab, which exerts an antiangiogenic mechanism of action, may be inadequately assessed by traditional size-based radiologic criteria, the Response Evaluation Criteria in Solid Tumors (RECIST), which were designed for assessing tumor volume reduction following cytotoxic chemotherapy.3–5 In support of this, a recent phase III trial showed that the addition of bevacizumab to oxaliplatin-based chemotherapy for metastatic colorectal cancer improved progression-free survival without affecting RECIST-defined response rates.6
Recently, pathologic response to preoperative chemotherapy has been shown to correlate with improved survival and has been proposed as a new outcome endpoint after resection of CLM.7–9 To date, a noninvasive method of predicting pathologic response to chemotherapy in CLM, particularly biologic agents, is lacking. We observed that after bevacizumab-containing therapy, CLM tend not only to decrease in size but also to undergo unique morphological changes on computed tomography (CT). Metastases that have heterogeneous attenuation, variable degree of enhancement, and ill-defined borders before treatment transform into homogeneous, hypoattenuating lesions with well-defined borders. We hypothesized that these changes on CT reflect the pathologic response in patients treated with bevacizumab-containing chemotherapy before hepatic resection of CLM. To test this hypothesis, we correlated tumor response based on these morphologic criteria and tumor response based on RECIST with pathologic response. We then tested the morphologic criteria in patients with unresectable CLM treated with bevacizumab. The morphologic criteria and RECIST were correlated with survival in both resected and unresectable patients.
METHODS
Initial Patient Cohort
From a prospective hepatobiliary database at The University of Texas M. D. Anderson Cancer Center, we identified 234 CLM in 50 consecutive patients who received first-line chemotherapy with bevacizumab before undergoing hepatic resection between March 2004 and March 2007. All patients underwent contrast-enhanced CT scans of the abdomen at the start and end of preoperative therapy as part of their standard evaluation. Patients who had undergone prior liver resection were excluded. Postoperatively, patients were followed with history and physical examination, CT scans, and serum carcinoembryonic antigen levels at 3- to 6-month intervals for the first 2 to 3 years after resection and at more extended intervals thereafter. The median follow-up time was 18 months (range, 3–42 months). Date of last follow-up was March 2008. This study involved retrospective review of medical information and was conducted under the approval of the institutional review board, which waived the requirement for informed consent.
Validation Patient Cohort
From a prospective gastrointestinal medical oncology database at The University of Texas M. D. Anderson Cancer Center, we identified 82 patients with unresectable CLM treated with bevacizumab combined with cytotoxic chemotherapy between March 2004 and April 2007. Patients were considered unresectable if two contiguous hepatic segments could not be preserved, vascular inflow and outflow or biliary drainage was inadequate, or the volume of the future liver remnant was ≤ 20% of the total estimated liver volume.10 All patients underwent contrast-enhanced CT scans of the abdomen before starting chemotherapy and at 2–3 month intervals thereafter. Median follow-up was 25 months (range, 6–57 months). Date of last follow-up was April 2009.
Imaging Analysis
CT scans were performed with 4- or 16-slice CT (LightSpeed, GE Healthcare, Piscataway, NJ) using a collimation of 5 mm and reconstruction at 2.5 mm. Images were acquired with one of two methods: a triphasic liver protocol following a noncontrast evaluation of the liver or a single-phase technique. For the triphasic liver protocol, images were obtained 30, 50, and 70 seconds after the start of intravenous injection of ioversol at a rate of 5 cc/sec. For the single-phase technique, images were obtained 60 to 70 seconds after the start of ioversol injection at a rate of 2 to 3 cc/sec. Because of the routine concomitant acquisition of chest CT and delayed images through the kidneys, single-phase scans also permitted partial evaluation of the liver during the early and delayed phases of enhancement.
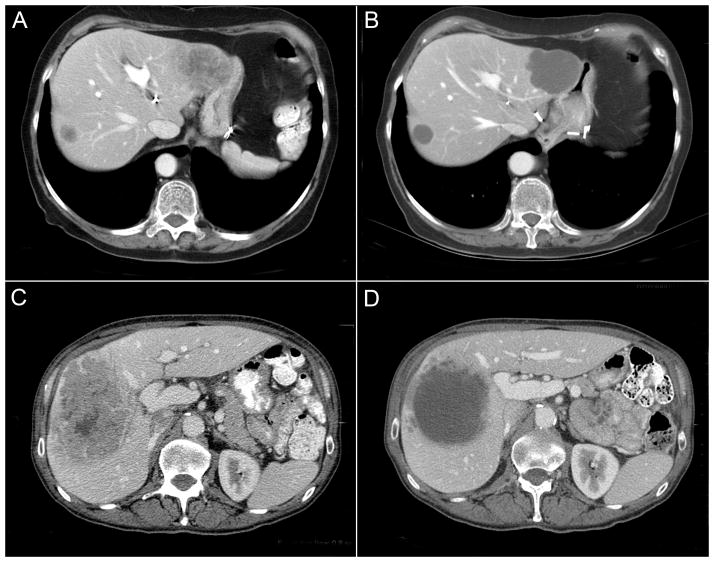
Response to treatment was assessed independently by three radiologists with 2, 15, and 20 years of experience in abdominal oncologic imaging. Radiologists were blinded to pathologic results, patient treatment, and outcomes. Discrepancies between radiologists were resolved by consensus review. Response was evaluated using new morphologic criteria, assigning each metastasis to one of three groups (Table 1). A group 3 metastasis was characterized by heterogeneous attenuation and a thick, poorly defined tumor-liver interface (Fig 1A and 1C). A group 1 metastasis was characterized by homogeneous attenuation with a thin, sharply defined tumor-liver interface (Fig 1B). A group 2 metastasis had morphology that could not be rated as 3 or 1 (Fig 1D). When present, a peripheral rim of hyperattenuating contrast enhancement was designated a group 3 characteristic, and resolution of this enhancement was classified group 1.
Table 1.
CT morphologic groups
| Morphology group | CT tumor characteristics | ||
|---|---|---|---|
| Overall attenuation | Tumor-liver interface | Peripheral rim of enhancement | |
| 3 | Heterogeneous | Ill-defined | May be present |
| 2 | Mixed | Variable | If initially present, partially resolved |
| 1 | Homogeneous and hypoattenuating | Sharp | If initially present, completely resolved |
Fig 1.
Pre- and posttreatment CT scans showing RECIST stable disease and morphologic optimal response (A, B), characterized by homogeneous attenuation and sharp tumor-liver interface; morphologic incomplete response (C, D), with homogeneous attenuation but ill-defined tumor-liver interface remaining after treatment.
Morphologic response criteria were defined as optimal if the metastasis changed from a group 3 or 2 to a 1, incomplete if the group changed from 3 to 2, and none if the group did not change or increased. In patients with multiple tumors, morphologic response criteria were assigned based on the response seen in the majority of tumors. Response by RECIST was defined as previously described: complete response, disappearance of all tumors; partial response, > 30% decrease in sum of the longest diameter of target tumor; progressive disease, > 20% increase in the sum of the longest diameter of target tumor; and stable disease, none of the complete response, partial response, or progressive disease criteria met.11 The appearance of new metastases was defined as progression by RECIST and morphology assessment.
Assessment of Pathologic Response to Chemotherapy
Hematoxylin and eosin-stained specimens sectioned into 5-mm-thick slices were evaluated by two gastrointestinal pathologists who were blinded to treatment regimen, radiologic results, and patient outcomes. The extent of residual carcinoma was assessed semiquantitatively as a percentage relative to the total tumor surface area, as previously described.2, 8 Pathologic response was scored as minor if ≥ 50% residual tumor cells were present, major with 1% to 49% residual tumor cells, and complete if no residual tumor cells were detected.
Statistical Analysis
Continuous variables were compared using the Kruskal-Wallis or Mann-Whitney test; discrete variables, expressed as number and percentage, were compared using the chi-square test or Fisher exact test, when appropriate. A post hoc power analysis showed that based on the actual number of patients enrolled in the current study, there was greater than 95% statistical power to detect a difference in pathologic response rate of 32% between patients with optimal v incomplete or no morphologic response at a conventional P value of .05. Kappa statistics were used to determine interobserver agreement of the proposed morphologic criteria among three radiologists. Survival was determined from time of hepatic resection until the time of death or last follow-up. If more than 3 months had lapsed since the date of last follow-up, then survival was calculated according to whether patients were alive at the time the study was closed, as recorded in tumor registry data or medical records. Five patients with residual disease in the liver, lung, or an intact primary tumor at the time of hepatectomy were excluded from the survival analysis. Among unresectable patients, survival was calculated from the start of bevacizumab-containing chemotherapy. Survival curves were generated using the Kaplan-Meier method, and differences were evaluated with the log-rank test. Analyses were performed with SPSS software (version 12.0, SPSS Inc., Chicago, IL). All statistical tests were two- sided, and significance was set at P<.05.
RESULTS
Initial Surgical Cohort
Two hundred thirty-four lesions in 50 patients were evaluated; their demographic and clinicopathologic characteristics are presented in Table 2. Of the 130 CT scans reviewed, 53 were performed with triphasic liver protocol and 77 with single-phase technique. Among the 33 patients with multiple tumors, the morphologic responses of the metastases within the same patient were concordant in all but two patients.
Table 2.
Baseline characteristics
| No. (%) of patients
|
||
|---|---|---|
| Initial Surgical Cohort (n=50) | Unresectable Patients (n=82) | |
| Age | ||
| Median | 57 years | 57 years |
| Range | 34–84 years | 26–69 years |
|
| ||
| Sex | ||
| Female | 21 (42%) | 37 (45%) |
| Male | 29 (58%) | 45 (55%) |
|
| ||
| Primary tumor | ||
| Colon | 36 (72%) | 63 (77%) |
| Rectum | 14 (28%) | 19 (23%) |
|
| ||
| Sites of metastases | ||
| Liver only | 48 (95%) | 45 (55%) |
| Liver and extrahepatic | 2 (4%) | 37 (45%) |
|
| ||
| Liver metastases | ||
| Solitary | 17 (34%) | 4 (5%) |
| Multiple | 33 (66%) | 78 (95%) |
|
| ||
| Tumor sizea | ||
| Median | 2.3 cm | 4.9 cm |
| Range | 0.4–13 cm | 1.5–18 cm |
|
| ||
| Chemotherapy | ||
| FOLFIRI with bevacizumab | 7 (14%) | 47 (57%) |
| FOLFOX with bevacizumab | 43 (86%) | 35 (43%) |
|
| ||
| Hepatectomy | ||
| Minor | 24 (48%) | N/A |
| Major | 26 (52%) | |
|
| ||
| Radiofrequency ablation at time of hepatectomy | 8 (16%) | N/A |
|
| ||
| No. of chemotherapy cycles before hepatectomy | ||
| Median | 6 | N/A |
| Range | 3–12 | |
|
| ||
| Interval between last CT scan and surgery | ||
| Median | 18 days | N/A |
| Range | 2–56 days | |
|
| ||
| Overall survivalb | ||
| Median | Not reached | 25 months |
| 3-year | 51% | 24% |
Abbreviations: FOLFIRI, infusional 5-fluorouracil (5-FU) and leucovorin with irinotecan; FOLFOX, infusional 5-FU and leucovorin with oxaliplatin; CT, computed tomography; NA, not applicable.
Based on the largest diameter of the resected tumor specimen in surgical patients and largest diameter on pre-chemotherapy CT in unresectable patients.
From time of hepatectomy in surgical cohort and from start of bevacizumab-containing chemotherapy in unresectable cohort.
Morphologic response was significantly associated with the percentage of residual tumor cells: median values were 70% (interquartile range [IQR], 60%–70%), 50% (IQR, 30%–60%), and 20% (IQR, 10%–30%) residual tumor in patients with no response, incomplete response, and optimal response, respectively (P<.001, Fig 2A). RECIST was also significantly associated with the percentage of residual tumor cells: median values were 70% (IQR, 65%–70%), 50% (IQR, 20%–70%), and 30% (IQR, 10%–60%) in patients with progressive disease, stable disease, and partial response, respectively (P=.04, Fig 2B).
Fig 2.
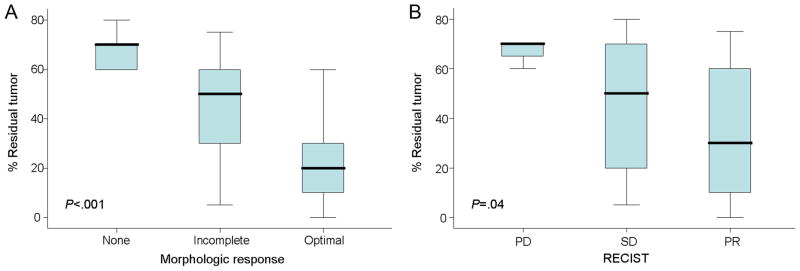
Correlation between morphologic response (A) or RECIST (B) and percentage of residual tumor cells. Lines inside boxes represent the median; endpoints of whiskers represent minimum and maximum values. Lower and upper edges of boxes represent 25th and 75th percentiles.
When pathologic response was stratified as minor, major, or complete, using previously determined cutoff values for the percentage of residual tumor cells,8 two patients had a complete response, 27 had a major response, and 21 had a minor response. Metastases with major pathologic response were characterized by replacement of tumor cells by fibrosis. Necrosis was observed in less than 5% of tumors. Complete or major pathologic response corresponded to morphologic optimal response in 22/29 (76%) patients, while minor pathologic response was associated with morphologic partial or no response in 17/21 (81%) patients. When correlated with RECIST, complete or major pathologic response corresponded to RECIST partial response in 23/29 (79%) patients, while minor pathologic response was associated with RECIST stable or progressive disease in 10/21 (48%) patients. None of the patients had RECIST complete response. Therefore, incomplete or no response by morphology was more specific for predicting minor pathologic response than RECIST stable or progressive disease (17/21, morphology v 10/21, RECIST, P=0.02). Morphologic optimal response and RECIST partial response had similar sensitivities for predicting complete or major pathologic response (22/29, morphology v 23/29, RECIST, P=0.75).
Outcome among Resected Patients
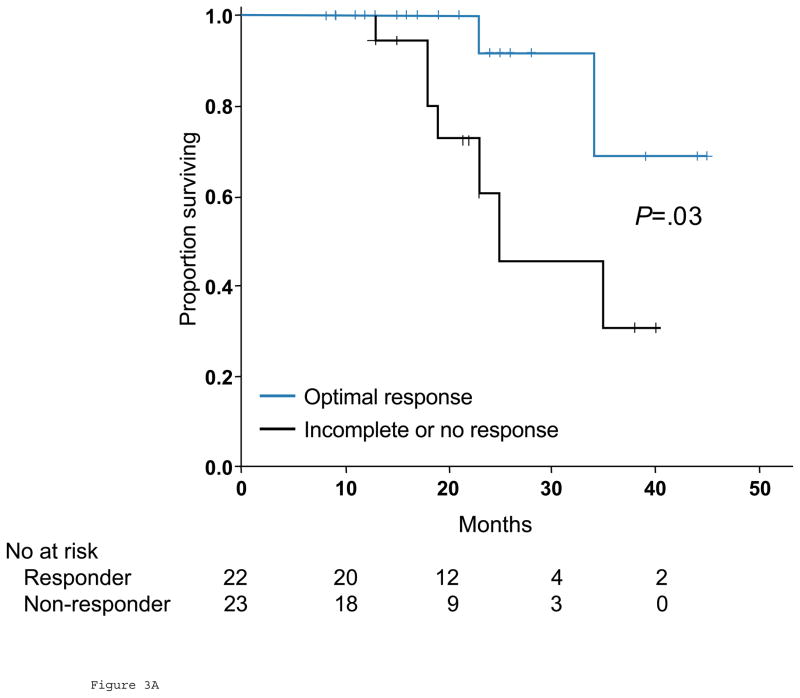
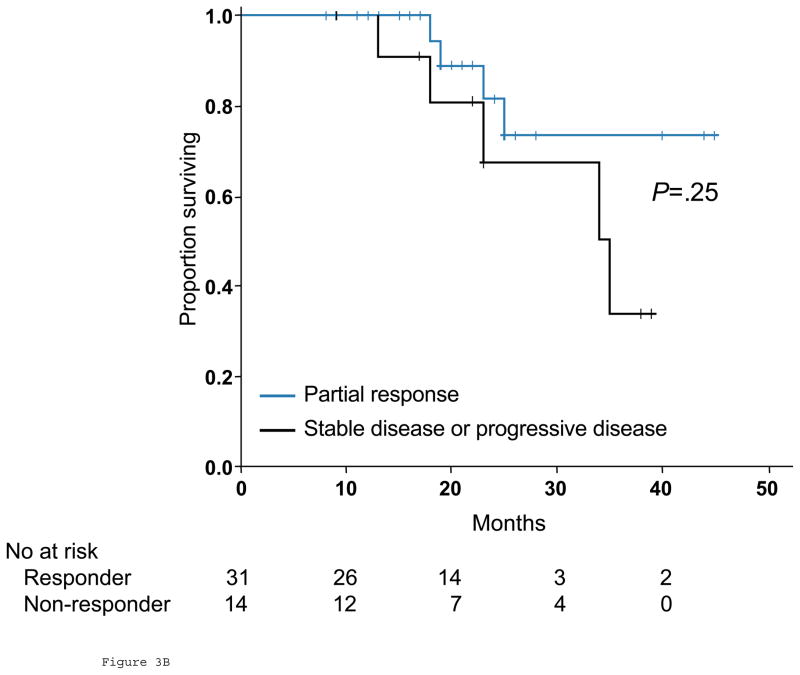
Thirty patients (60%) had disease recurrence during the study period, and 9 (18%) died of disease. Five patients (10%) with residual disease in the liver, lung, or an intact primary tumor at the time of hepatectomy were excluded from the survival analysis. Patients with optimal response by morphology were classified as responders, while the remaining patients were classified as nonresponders. When morphologic criteria were used in tumor response evaluation, median overall survival was not yet reached for responders and 35 months (95% CI, 20.2 to 29.8 months) for nonresponders (P=.03, Fig 3A). When RECIST were used, median overall survival was not yet reached in patients who achieved partial response and 34 months (95% CI, 20.0 to 48.0 months) with stable or progressive disease (P=.25, Fig 3B). On univariate analysis of traditional predictors of survival and potential radiologic predictors of outcome, only morphologic criteria demonstrated a significant correlation with overall survival (Table 3).
Fig 3.
Overall survival in responders and nonresponders by morphologic criteria (A) and RECIST (B) in surgical cohort.
Table 3.
Univariate Analysis of Predictors of Overall Survival among 45 Surgical Patients
| Variable | No. of Patients | Actuarial 2-Year Survival Rate | P |
|---|---|---|---|
| Gender | .71 | ||
| Male | 26 | 79% | |
| Female | 19 | 74% | |
|
| |||
| Age, years | .83 | ||
| ≤ 60 | 17 | 71% | |
| > 60 | 28 | 89% | |
|
| |||
| Primary site | .24 | ||
| Colon | 33 | 78% | |
| Rectum | 12 | 76% | |
|
| |||
| Primary tumor | .90 | ||
| Node-negative | 16 | 83% | |
| Node-positive | 29 | 74% | |
|
| |||
| Prehepatectomy serum CEA | NA | ||
| ≤ 200 ng/ml | 45 | 77% | |
| > 200 ng/ml | 0 | ||
|
| |||
| Resection margin | .11 | ||
| Negative | 43 | 79% | |
| Positive | 2 | 50% | |
|
| |||
| Disease-free interval | .55 | ||
| ≥ 12 months | 15 | 69% | |
| < 12 months | 30 | 81% | |
|
| |||
| No. of tumors | .79 | ||
| 1 | 16 | 75% | |
| > 1 | 29 | 78% | |
|
| |||
| Tumor size, cma | .11 | ||
| ≤ 5 | 40 | 79% | |
| > 5 | 5 | 50% | |
|
| |||
| Morphologic response | .03 | ||
| Optimal | 22 | 90% | |
| Incomplete or none | 23 | 42% | |
|
| |||
| RECIST | .25 | ||
| Partial response | 31 | 80% | |
| Stable or progressive disease | 14 | 64% | |
Abbreviations: CEA, carcinoembryonic antigen; NA, not applicable.
Based on the largest diameter of the resected tumor specimen.
Validation in Unresectable Patients
To validate the CT response criteria in assessing clinically significant tumor response of liver metastases, a separate cohort of 82 patients with unresectable CLM treated with bevacizumab-containing chemotherapy was analyzed. Their clinicopathologic features are presented in Table 2. Among the 78 patients with multiple liver metastases, the morphologic responses of the metastases within the same patient were concordant in all but 10 patients; in these patients, morphology score was assigned based on the dominant pattern observed.
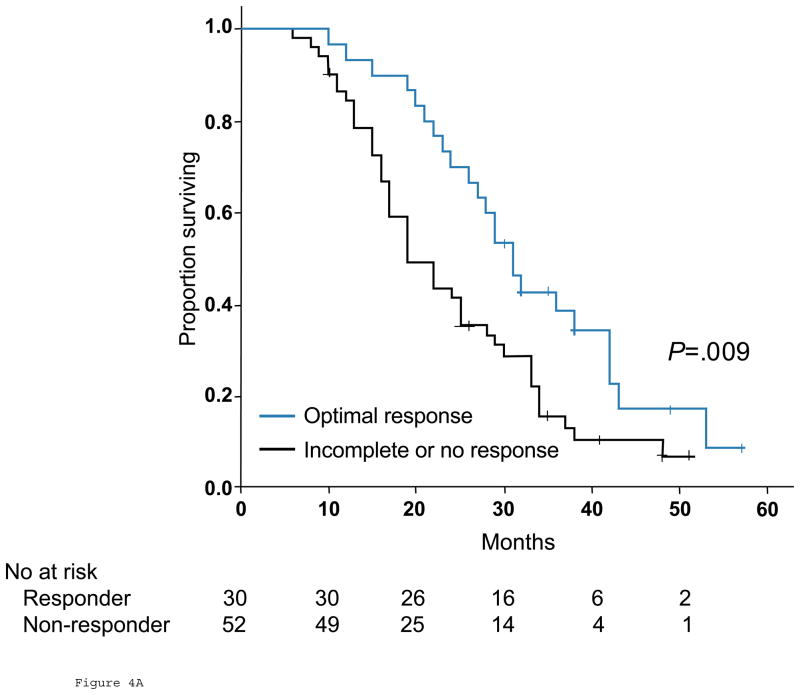
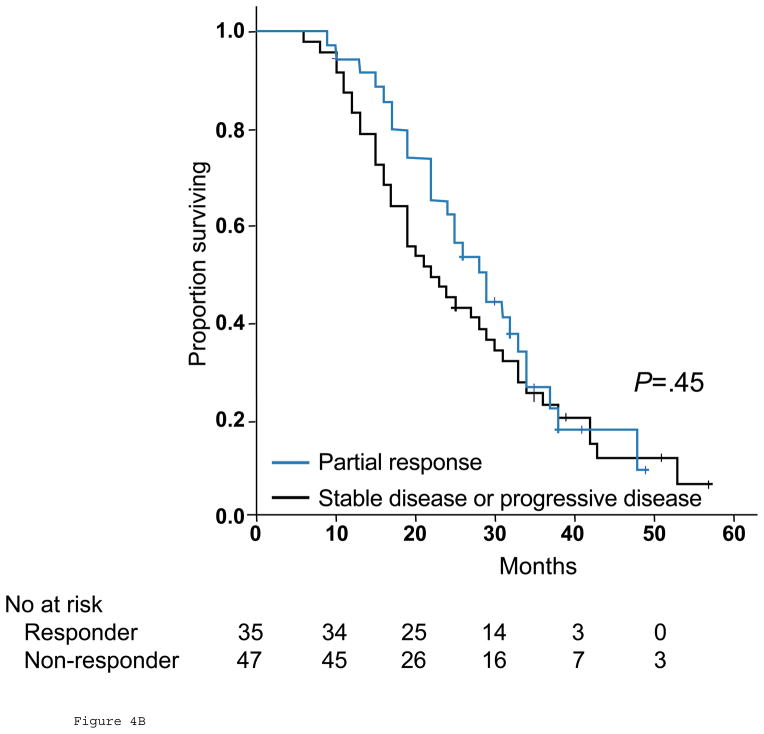
Among the 82 patients with stage IV colorectal cancer treated with chemotherapy only, those with optimal response by morphologic criteria had significantly better overall survival than patients with incomplete or no response, with median overall survival of 31 months (95% CI, 26.8 to 35.2 months), and 19 months (95% CI, 14.6 to 23.4 months), respectively (P=.009, Fig 4A). In contrast, response by RECIST was not associated with an improvement in survival; median overall survival was 28 months (95% CI, 22.5 to 33.5 months) in patients with partial response and 22 months (95% CI, 15.3 to 28.7 months) in those with stable or progressive disease (P=.45, Fig. 4B). The following variables did not significantly affect overall survival: gender, age, size or multiplicity of hepatic metastases, rectal primary, and the presence of extrahepatic metastases.
Fig 4.
Overall survival in responders and nonresponders by morphologic criteria (A) and RECIST (B) among 82 unresectable patients.
Interobserver Agreement for Morphologic Criteria
The interobserver agreement between the three radiologists for scoring morphologic changes was good: κ=0.78 (95% confidence interval [CI]: 0.63–.93) between readers 1 and 2, κ = 0.72 (95% CI: 0.56–0.88) between readers 1 and 3, and κ 0.68 (95% CI: 0.51–0.85) between readers 2 and 3. Among the radiologists, there were discrepancies in scoring morphologic criteria in 13 of the 50 surgical patients, which were resolved by consensus review.
DISCUSSION
We present novel qualitative morphologic CT criteria for predicting response to bevacizumab-containing chemotherapy in patients with CLM. These criteria were reproducible, as shown by the good interobserver agreement in scoring morphologic changes among three independent radiologists with varied experience in abdominal oncologic imaging. Morphologic criteria correlated strongly with the percentage of residual tumor cells and also with pathologic response stratified as complete, major, or minor, using 50% residual tumor cells as the cutoff value between major and minor pathologic response. Optimal morphologic response to preoperative therapy translated into a survival benefit after hepatic resection. In a separate validation cohort of patients with unresectable CLM, response by morphologic criteria was also associated with improved overall survival. RECIST was also sensitive for predicting complete or major pathologic response but with a significantly lower specificity for predicting minor pathologic response. RECIST was neither associated with the stratified pathologic response nor survival.
Optimal morphologic response was defined as a change in metastases from lesions with heterogeneous attenuation and thick, irregular borders into bland, homogeneously hypodense masses with a sharp interface between the tumor and adjacent normal liver parenchyma, which in some cases could mimic a cyst. This homogeneous attenuation of metastases responding to treatment likely reflects the replacement of treated tumor by fibroconnective tissue rather than tumor necrosis, which was present in less than 5% of patients in this study. Rubbia-Brandt et al. also observed that histological tumor response is characterized by fibrous replacement of tumor rather than tumor necrosis in CLM.7 In patients with multiple liver metastases, the morphologic responses of the metastases within the same patient were uniform in 99 of 111 (89%) patients. This result confirms a previous study demonstrating that in patients with multiple CLM, the histological tumor responses within the same patient were similar.7
The impact of pathologic response to preoperative therapy on survival in patients with solid tumors is well-established.12, 13 In patients with CLM, histologic tumor regression, graded by the extent of fibrosis and presence of residual tumor cells, has been shown to correlate with survival.7 In this study, we scored pathologic response semiquantitatively as the percentage of residual tumor cells relative to the total tumor surface area. Pathologic response was scored as minor if ≥ 50% residual tumor cells were present, major with 1% to 49% residual tumor cells, and complete with 0%. Using this 50% cutoff to define major versus minor pathologic response, we recently showed that in patients with CLM, major pathologic response to preoperative chemotherapy independently predicted improved patient survival.8 In the present study, overall survival was correlated with morphologic response but not RECIST-based prognostic factors, such as tumor size and number. Although the sample size in the surgical cohort is small, these results highlight the importance of response rather than baseline clinical factors in determining patient outcome after liver resection.8, 14
The limitations of this study include its retrospective nature and potentially the predominant use of a single-phase CT technique. Although triphasic liver protocol CT is not required to apply the morphologic criteria and is probably not needed routinely in non-surgical patients with CLM, it might improve sensitivity by allowing evaluation of early and delayed phases of tumor enhancement. Recent studies on evaluating response to antiangiogenic agents with CT or MRI have focused on tumor perfusion.15–18 Although tumoral enhancement was a component of our morphology response criteria, the degree of enhancement could not be consistently assessed because of variations in scanning techniques. While CLM are considered hypovascular tumors, enhancement does occur and is characterized by an ill-defined rim of peripheral enhancement which is maximal during the arterial phase and fades away during the portal phase.19, 20 We reviewed many CTs that were single phase studies, lacking an arterial phase. Nevertheless, in cases where an early hyperattenuating rim of enhancement was present before treatment, it disappeared in all patients with an optimal morphologic response. In addition, the applicability of these criteria in assessing response to other biologic agents approved for CLM requires investigation.
In conclusion, we present novel qualitative radiologic criteria that predict the pathologic response to preoperative bevacizumab-containing chemotherapy in patients undergoing resection of CLM. Morphologic response correlated with pathologic response stratified as complete, major, or minor, as well as overall survival, whereas RECIST did not. This correlation between survival and morphologic response, but not RECIST, was confirmed in a non-surgical cohort. Thus, our results indicate that morphologic response may be a useful, noninvasive surrogate marker of pathologic response and improved survival in patients with CLM receiving a bevacizumab-containing regimen. It provides complementary information to traditional size-based criteria in assessing CT response to bevacizumab in CLM
Footnotes
Author Contributions: Dr. Vauthey had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Role of the Sponsor: None of the funders had any role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript.
References
- 1.Hurwitz H, Fehrenbacher L, Novotny W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004 Jun 3;350(23):2335–2342. doi: 10.1056/NEJMoa032691. [DOI] [PubMed] [Google Scholar]
- 2.Ribero D, Wang H, Donadon M, et al. Bevacizumab improves pathologic response and protects against hepatic injury in patients treated with oxaliplatin-based chemotherapy for colorectal liver metastases. Cancer. 2007 Dec 15;110(12):2761–2767. doi: 10.1002/cncr.23099. [DOI] [PubMed] [Google Scholar]
- 3.Sathornsumetee S, Cao Y, Marcello JE, et al. Tumor angiogenic and hypoxic profiles predict radiographic response and survival in malignant astrocytoma patients treated with bevacizumab and irinotecan. J Clin Oncol. 2008 Jan 10;26(2):271–278. doi: 10.1200/JCO.2007.13.3652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grothey A, Hedrick EE, Mass RD, et al. Response-independent survival benefit in metastatic colorectal cancer: a comparative analysis of N9741 and AVF2107. J Clin Oncol. 2008 Jan 10;26(2):183–189. doi: 10.1200/JCO.2007.13.8099. [DOI] [PubMed] [Google Scholar]
- 5.Choi H, Charnsangavej C, Faria SC, et al. Correlation of computed tomography and positron emission tomography in patients with metastatic gastrointestinal stromal tumor treated at a single institution with imatinib mesylate: proposal of new computed tomography response criteria. J Clin Oncol. 2007 May 1;25(13):1753–1759. doi: 10.1200/JCO.2006.07.3049. [DOI] [PubMed] [Google Scholar]
- 6.Saltz LB, Clarke S, Diaz-Rubio E, et al. Bevacizumab in combination with oxaliplatin-based chemotherapy as first-line therapy in metastatic colorectal cancer: a randomized phase III study. J Clin Oncol. 2008 Apr 20;26(12):2013–2019. doi: 10.1200/JCO.2007.14.9930. [DOI] [PubMed] [Google Scholar]
- 7.Rubbia-Brandt L, Giostra E, Brezault C, et al. Importance of histological tumor response assessment in predicting the outcome in patients with colorectal liver metastases treated with neo-adjuvant chemotherapy followed by liver surgery. Ann Oncol. 2007 Feb;18(2):299–304. doi: 10.1093/annonc/mdl386. [DOI] [PubMed] [Google Scholar]
- 8.Blazer DG, 3rd, Kishi Y, Maru DM, et al. Pathologic response to preoperative chemotherapy: a new outcome end point after resection of hepatic colorectal metastases. J Clin Oncol. 2008 Nov 20;26(33):5344–5351. doi: 10.1200/JCO.2008.17.5299. [DOI] [PubMed] [Google Scholar]
- 9.Adam R, Wicherts DA, de Haas RJ, et al. Complete pathologic response after preoperative chemotherapy for colorectal liver metastases: myth or reality? J Clin Oncol. 2008 Apr 1;26(10):1635–1641. doi: 10.1200/JCO.2007.13.7471. [DOI] [PubMed] [Google Scholar]
- 10.Charnsangavej C, Clary B, Fong Y, Grothey A, Pawlik TM, Choti MA. Selection of patients for resection of hepatic colorectal metastases: expert consensus statement. Ann Surg Oncol. 2006 Oct;13(10):1261–1268. doi: 10.1245/s10434-006-9023-y. [DOI] [PubMed] [Google Scholar]
- 11.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000 Feb 2;92(3):205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 12.Swisher SG, Hofstetter W, Wu TT, et al. Proposed revision of the esophageal cancer staging system to accommodate pathologic response (pP) following preoperative chemoradiation (CRT) Ann Surg. 2005 May;241(5):810–817. doi: 10.1097/01.sla.0000161983.82345.85. discussion 817–820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ajani JA, Mansfield PF, Crane CH, et al. Paclitaxel-based chemoradiotherapy in localized gastric carcinoma: degree of pathologic response and not clinical parameters dictated patient outcome. J Clin Oncol. 2005 Feb 20;23(6):1237–1244. doi: 10.1200/JCO.2005.01.305. [DOI] [PubMed] [Google Scholar]
- 14.Adam R, Pascal G, Castaing D, et al. Tumor progression while on chemotherapy: a contraindication to liver resection for multiple colorectal metastases? Ann Surg. 2004 Dec;240(6):1052–1061. doi: 10.1097/01.sla.0000145964.08365.01. discussion 1061–1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Provenzale JM. Imaging of angiogenesis: clinical techniques and novel imaging methods. AJR Am J Roentgenol. 2007 Jan;188(1):11–23. doi: 10.2214/AJR.06.0280. [DOI] [PubMed] [Google Scholar]
- 16.Sabir A, Schor-Bardach R, Wilcox CJ, et al. Perfusion MDCT enables early detection of therapeutic response to antiangiogenic therapy. AJR Am J Roentgenol. 2008 Jul;191(1):133–139. doi: 10.2214/AJR.07.2848. [DOI] [PubMed] [Google Scholar]
- 17.Kan Z, Phongkitkarun S, Kobayashi S, et al. Functional CT for quantifying tumor perfusion in antiangiogenic therapy in a rat model. Radiology. 2005 Oct;237(1):151–158. doi: 10.1148/radiol.2363041293. [DOI] [PubMed] [Google Scholar]
- 18.Miles KA, Leggett DA, Kelley BB, Hayball MP, Sinnatamby R, Bunce I. In vivo assessment of neovascularization of liver metastases using perfusion CT. Br J Radiol. 1998 Mar;71(843):276–281. doi: 10.1259/bjr.71.843.9616236. [DOI] [PubMed] [Google Scholar]
- 19.Outwater E, Tomaszewski JE, Daly JM, Kressel HY. Hepatic colorectal metastases: correlation of MR imaging and pathologic appearance. Radiology. 1991 Aug;180(2):327–332. doi: 10.1148/radiology.180.2.2068294. [DOI] [PubMed] [Google Scholar]
- 20.Muramatsu Y, Takayasu K, Moriyama N, et al. Peripheral low-density area of hepatic tumors: CT-pathologic correlation. Radiology. 1986 Jul;160(1):49–52. doi: 10.1148/radiology.160.1.3012632. [DOI] [PubMed] [Google Scholar]