Abstract
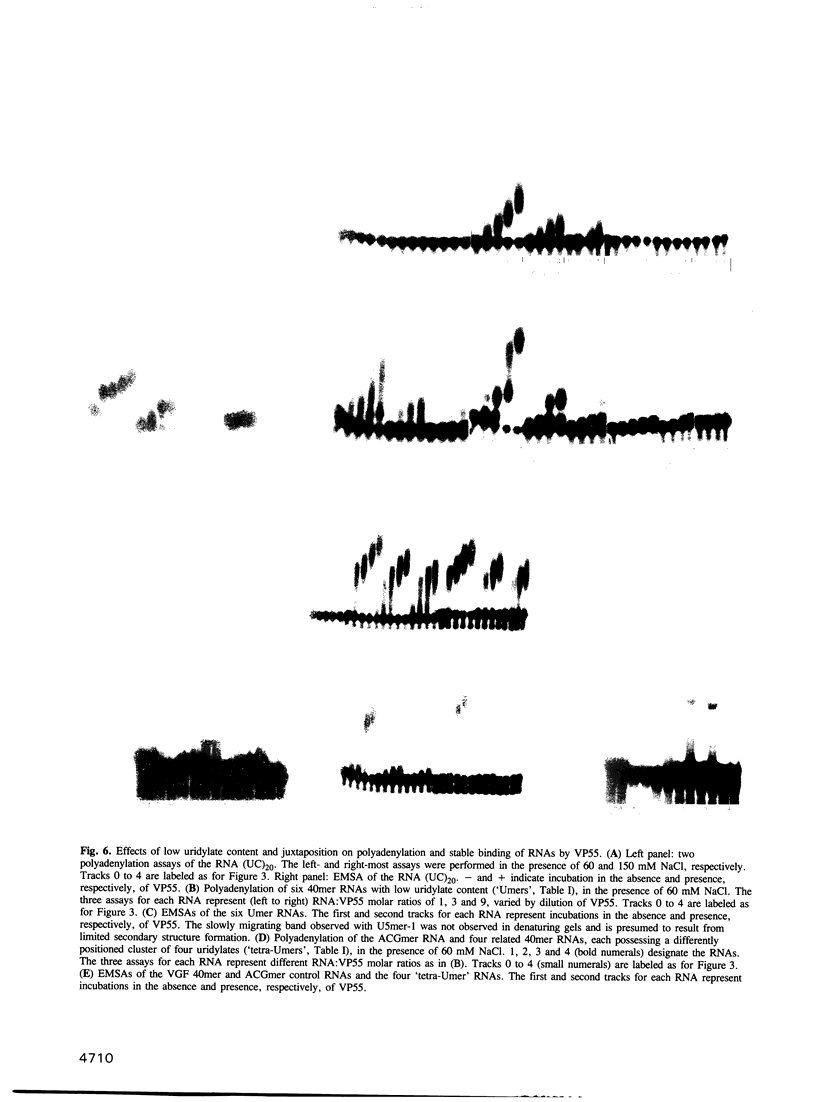
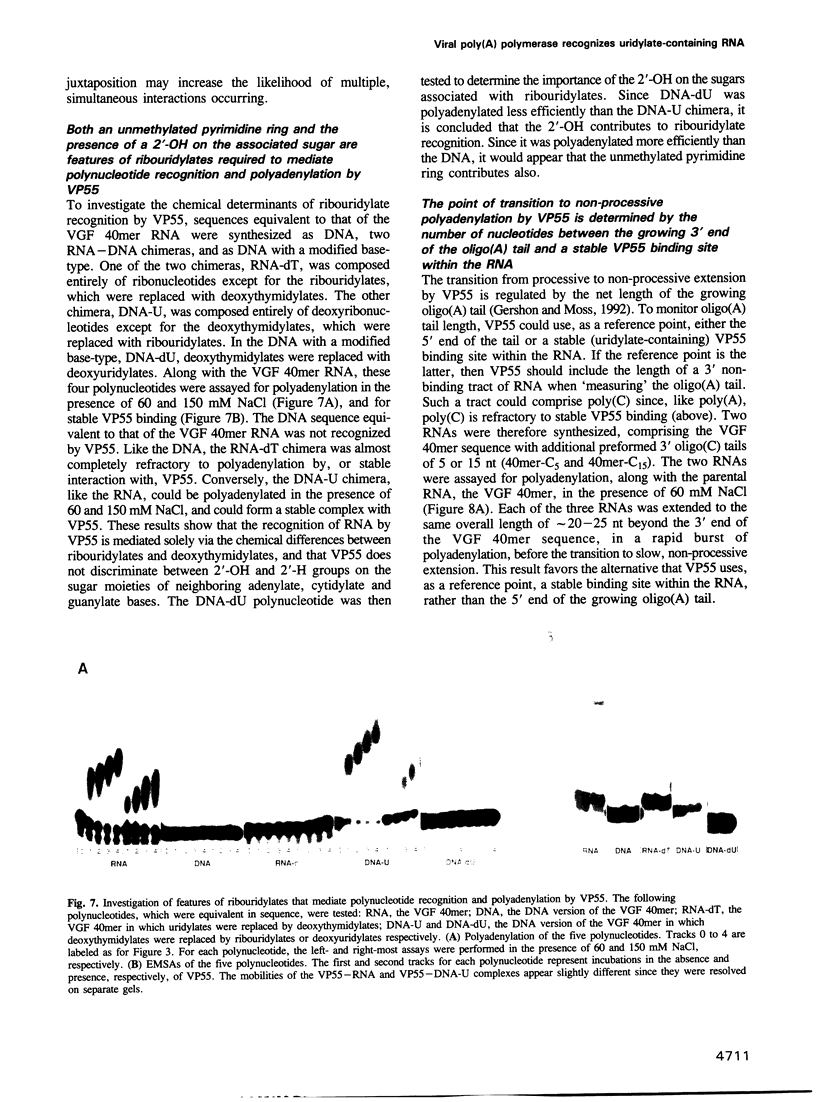
VP55, the catalytic subunit of vaccinia virus poly(A) polymerase, has the remarkable property of adding 30-35 adenylates to RNA 3' ends in a rapid processive burst before an abrupt transition to slow, non-processive adenylate addition. Here, we demonstrate that this property results from the affinity of the enzyme for uridylate residues within the 3' 31-40 nt of the RNA primer. At physiological salt concentrations, both polyadenylation and stable VP55 binding required the presence of multiple uridylates within a 31-40 nt length of RNA, though specific RNA sequences were not necessary. Even DNA in which the deoxythymidylate residues were replaced with ribouridylates, could be polyadenylated in a processive manner. Both the unmethylated pyrimidine ring and a 2'-OH on the associated sugar are features of ribouridylates that are important for priming. The abrupt termination of processive polyadenylation was attributed to translocation of VP55 along the nascent poly(A) tail, which lacks uridylates for stable binding. As evidence for translocation and interaction with newly synthesized RNA, other homopolymer tails were synthesized by VP55 in the presence of Mn2+, which relaxes its donor nucleotide specificity. Only during poly(U) tail synthesis did processive nucleotide addition fail to terminate.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bandziulis R. J., Swanson M. S., Dreyfuss G. RNA-binding proteins as developmental regulators. Genes Dev. 1989 Apr;3(4):431–437. doi: 10.1101/gad.3.4.431. [DOI] [PubMed] [Google Scholar]
- Bell L. R., Maine E. M., Schedl P., Cline T. W. Sex-lethal, a Drosophila sex determination switch gene, exhibits sex-specific RNA splicing and sequence similarity to RNA binding proteins. Cell. 1988 Dec 23;55(6):1037–1046. doi: 10.1016/0092-8674(88)90248-6. [DOI] [PubMed] [Google Scholar]
- Bienroth S., Keller W., Wahle E. Assembly of a processive messenger RNA polyadenylation complex. EMBO J. 1993 Feb;12(2):585–594. doi: 10.1002/j.1460-2075.1993.tb05690.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gershon P. D., Ahn B. Y., Garfield M., Moss B. Poly(A) polymerase and a dissociable polyadenylation stimulatory factor encoded by vaccinia virus. Cell. 1991 Sep 20;66(6):1269–1278. doi: 10.1016/0092-8674(91)90048-4. [DOI] [PubMed] [Google Scholar]
- Gershon P. D., Moss B. Stimulation of poly(A) tail elongation by the VP39 subunit of the vaccinia virus-encoded poly(A) polymerase. J Biol Chem. 1993 Jan 25;268(3):2203–2210. [PubMed] [Google Scholar]
- Gershon P. D., Moss B. Transition from rapid processive to slow nonprocessive polyadenylation by vaccinia virus poly(A) polymerase catalytic subunit is regulated by the net length of the poly(A) tail. Genes Dev. 1992 Aug;6(8):1575–1586. doi: 10.1101/gad.6.8.1575. [DOI] [PubMed] [Google Scholar]
- Gil A., Sharp P. A., Jamison S. F., Garcia-Blanco M. A. Characterization of cDNAs encoding the polypyrimidine tract-binding protein. Genes Dev. 1991 Jul;5(7):1224–1236. doi: 10.1101/gad.5.7.1224. [DOI] [PubMed] [Google Scholar]
- Horabin J. I., Schedl P. Regulated splicing of the Drosophila sex-lethal male exon involves a blockage mechanism. Mol Cell Biol. 1993 Mar;13(3):1408–1414. doi: 10.1128/mcb.13.3.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kates J., Beeson J. Ribonucleic acid synthesis in vaccinia virus. II. Synthesis of polyriboadenylic acid. J Mol Biol. 1970 May 28;50(1):19–33. doi: 10.1016/0022-2836(70)90101-4. [DOI] [PubMed] [Google Scholar]
- Moss B., Ahn B. Y., Amegadzie B., Gershon P. D., Keck J. G. Cytoplasmic transcription system encoded by vaccinia virus. J Biol Chem. 1991 Jan 25;266(3):1355–1358. [PubMed] [Google Scholar]
- Moss B., Rosenblum E. N., Gershowitz A. Characterization of a polyriboadenylate polymerase from vaccinia virions. J Biol Chem. 1975 Jun 25;250(12):4722–4729. [PubMed] [Google Scholar]
- Nevins J. R., Joklik W. K. Isolation and partial characterization of the poly(A) polymerases from HeLa cells infected with vaccinia virus. J Biol Chem. 1977 Oct 10;252(19):6939–6947. [PubMed] [Google Scholar]
- Nevins J. R., Joklik W. K. Poly (A) sequences of vaccinia virus messenger RNA: nature, mode of addition and function during translation in vitra and in vivo. Virology. 1975 Jan;63(1):1–14. doi: 10.1016/0042-6822(75)90365-7. [DOI] [PubMed] [Google Scholar]
- Patton J. G., Mayer S. A., Tempst P., Nadal-Ginard B. Characterization and molecular cloning of polypyrimidine tract-binding protein: a component of a complex necessary for pre-mRNA splicing. Genes Dev. 1991 Jul;5(7):1237–1251. doi: 10.1101/gad.5.7.1237. [DOI] [PubMed] [Google Scholar]
- Query C. C., Bentley R. C., Keene J. D. A common RNA recognition motif identified within a defined U1 RNA binding domain of the 70K U1 snRNP protein. Cell. 1989 Apr 7;57(1):89–101. doi: 10.1016/0092-8674(89)90175-x. [DOI] [PubMed] [Google Scholar]
- Rohrmann G., Yuen L., Moss B. Transcription of vaccinia virus early genes by enzymes isolated from vaccinia virions terminates downstream of a regulatory sequence. Cell. 1986 Sep 26;46(7):1029–1035. doi: 10.1016/0092-8674(86)90702-6. [DOI] [PubMed] [Google Scholar]
- Schnierle B. S., Gershon P. D., Moss B. Cap-specific mRNA (nucleoside-O2'-)-methyltransferase and poly(A) polymerase stimulatory activities of vaccinia virus are mediated by a single protein. Proc Natl Acad Sci U S A. 1992 Apr 1;89(7):2897–2901. doi: 10.1073/pnas.89.7.2897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson M. S., Dreyfuss G. Classification and purification of proteins of heterogeneous nuclear ribonucleoprotein particles by RNA-binding specificities. Mol Cell Biol. 1988 May;8(5):2237–2241. doi: 10.1128/mcb.8.5.2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takagaki Y., MacDonald C. C., Shenk T., Manley J. L. The human 64-kDa polyadenylylation factor contains a ribonucleoprotein-type RNA binding domain and unusual auxiliary motifs. Proc Natl Acad Sci U S A. 1992 Feb 15;89(4):1403–1407. doi: 10.1073/pnas.89.4.1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei C. M., Moss B. Methylated nucleotides block 5'-terminus of vaccinia virus messenger RNA. Proc Natl Acad Sci U S A. 1975 Jan;72(1):318–322. doi: 10.1073/pnas.72.1.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilusz J., Shenk T. A uridylate tract mediates efficient heterogeneous nuclear ribonucleoprotein C protein-RNA cross-linking and functionally substitutes for the downstream element of the polyadenylation signal. Mol Cell Biol. 1990 Dec;10(12):6397–6407. doi: 10.1128/mcb.10.12.6397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuen L., Moss B. Multiple 3' ends of mRNA encoding vaccinia virus growth factor occur within a series of repeated sequences downstream of T clusters. J Virol. 1986 Oct;60(1):320–323. doi: 10.1128/jvi.60.1.320-323.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuen L., Moss B. Oligonucleotide sequence signaling transcriptional termination of vaccinia virus early genes. Proc Natl Acad Sci U S A. 1987 Sep;84(18):6417–6421. doi: 10.1073/pnas.84.18.6417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamore P. D., Patton J. G., Green M. R. Cloning and domain structure of the mammalian splicing factor U2AF. Nature. 1992 Feb 13;355(6361):609–614. doi: 10.1038/355609a0. [DOI] [PubMed] [Google Scholar]