Abstract
The corticotropin-releasing hormone type I receptor (CRHR1) gene has been implicated in the liability for neuropsychiatric disorders, particularly under conditions of stress. Based on the hypothesized effects of CRHR1 variation on stress reactivity, measures of adulthood traumatic stress exposure were analyzed for their interaction with CRHR1 haplotypes and SNPs in predicting the risk for alcoholism. Phenotypic data on 2,533 non-related Caucasian individuals (1167 alcoholics and 1366 controls) were culled from the publically available Study of Addiction: Genetics and Environment (SAGE) genome-wide association study (GWAS). Genotypes were available for 19 tag SNPs. Logistic regression models examined the interaction between CRHR1 haplotypes / SNPs and adulthood traumatic stress exposure in predicting alcoholism risk. Two haplotype blocks spanned CRHR1. Haplotype analyses identified one haplotype in the proximal block 1 (p = 0.029) and two haplotypes in the distal block 2 (p = 0.026, 0.042) that showed nominally significant (corrected p < .025) genotype × traumatic stress interactive effects on the likelihood of developing alcoholism. The block 1 haplotype effect was driven by SNPs rs110402 (p = 0.019) and rs242924 (p = 0.019). In block 2, rs17689966 (p = 0.018) showed significant, and rs173365 (p = 0.026) showed nominally significant, gene × environment (G × E) effects on alcoholism status. This study extends the literature on the interplay between CRHR1 variation and alcoholism, in the context of exposure to traumatic stress. These findings are consistent with the hypothesized role of the extra hypothalamic CRF system dysregulation in the initiation and maintenance of alcoholism. Molecular and experimental studies are needed to more fully understand the mechanisms of risk and protection conferred by genetic variation at the identified loci.
Keywords: CRHR1, alcoholism, corticotropin-releasing hormone, genetics, stress
INTRODUCTION
Twin and adoption studies have shown that the heritability of alcoholism may be as high as 50-60% (Kendler et al., 1997, Prescott & Kendler, 1999). The neuropathophysiology of alcoholism and its underlying genetic architecture is complex and remains largely elusive. In recent years, progress in the identification of genetic risk has occurred through the use of intermediate phenotypes for alcohol use disorders (Ducci & Goldman, 2008, Hines et al., 2005), including the effects of alcohol on the neural pathways of stress. The corticotropin releasing factor (CRF) is critical to the stress response through its activation of the corticotropin-releasing hormone type I receptor (CRHR1), as CRF stimulates the synthesis and release of adrenocorticotropic hormone (ACTH) by the pituitary which in turn stimulates the synthesis and release of cortisol by the adrenal cortex. Hypothalamic-pituitary-adrenal (HPA) axis dysregulation can develop in response to childhood trauma, which permanently alters the stress response to acute stressors into adulthood (Nemeroff, 2004). Further, dysregulation of the extra hypothalamic CRF system is associated with the development and maintenance of alcoholism in both preclinical (Koob, 2008, Koob, 2010) and clinical (Sinha, 2001) models. Specifically, neurobiological models have emphasized the role of CRF in extrahypothalamic systems in the extended amygdala including dysregulated response to stressors, which is thought to contribute to the maintenance of alcoholism (Heilig & Koob, 2007, Koob, 2010). To that end, pharmacotherapies that can effectively target CRF system dysregulation are of great interest for the treatment of alcohol dependence (AD) (Ciccocioppo et al., 2009). For example, a selective CRHR1 antagonist was found to reduce alcohol self-administration in animals with a history of chronic alcohol exposure (Funk et al., 2007) and to block stress-induced reinstatement in alcohol preferring rats (Hansson et al., 2006).
In light of the literature supporting the biological and clinical plausibility of CRHR1 involvement in stress reactivity and addiction vulnerability, recent molecular genetic studies have examined the CRHR1 gene for its association with alcoholism, with and without accounting for environmental stress measures (Blomeyer et al., 2008, Chen et al., 2010, Kranzler et al., 2011b, Nelson et al., 2009 Treutlein et al., 2006). As expected, there is more extensive linkage disequilibrium (LD) across CRHRI in Caucasians than in individuals of African ancestry (Bradley et al., 2008, Roy et al., 2012). Additionally, the two populations can be distinguished by a 900 kb inversion polymorphism in those of European descent that is absent in those of African heritage (Stefansson et al., 2005). However in both ethnicities there is a distinct proximal haplotype block (henceforth called haplotype block 1) that spans intron 1 of the gene. Bradley et al (2008) identified a three single nucleotide polymorphism (SNP) haplotype (rs7209436, rs110402, rs242924), commonly known at the TAT haplotype, within this block that was protective against major depression in both African Americans and Caucasians who had experienced significant childhood trauma (Bradley et al., 2008). Kranzler et al. (2011) found that the TAT haplotype interacted with adverse childhood experiences to predict depression in African American women; however, no genotype or gene × environment (G × E) effects were found for alcoholism risk in this sample (Kranzler et al., 2011b). It appears that rs110402 is a tag SNP for this haplotype. Treutlein et al. (2006) examined the association between 14 CRHR1 haplotype tagging SNPs (tag SNPs) and alcohol use in two independent samples, one comprised of adolescents and one of adult drinkers (Treutlein et al., 2006). This study implicated two SNPs (rs242938 and rs1876831), located distal to haplotype block 1, with alcohol use phenotypes in both samples. A follow-up study of the adolescent sample reported an interaction between these two markers and measures of negative life events as predictors of the progression from heavy drinking to alcohol use disorders (Blomeyer et al., 2008). Nelson and colleagues (2009) examined the interaction between CRHR1 genotype and childhood sexual abuse as predictors of heavy drinking and AD in a large Caucasian sample (n = 1,128). Results revealed a significant G × E effect for alcohol consumption and alcoholism risk, such that the haplotype comprising the aforementioned SNPs, rs242938 and rs1876831, had a protective moderating effect (Nelson et al., 2009). However, the specific interaction of adult trauma exposure with CRHR1 polymorphisms as a risk factor for alcoholism has not yet been explored.
In light of the literature reviewed above, the aim of our study was to determine the main and interactive effects of CRHR1 haplotypes and SNPs and adulthood traumatic stress exposure on alcoholism risk in a large sample of cases and controls of European ancestry.
MATERIALS AND METHODS
Participants
Data were culled from the Study of Addiction: Genetics and Environment (SAGE) genome-wide association study (GWAS). Complete data for this study were available for a total of 2,533 Caucasian participants in the SAGE (dbGaP study accession phs000092 v1.p1) data set. Alcohol dependent cases and non-alcoholic controls were selected from three large datasets: (1) Collaborative Study on the Genetics of Alcoholism (COGA);(2) Family Study of Cocaine Dependence (FSCD); and (3) Collaborative Genetic Study of Nicotine Dependence (COGEND). Of the 2,533 Caucasian participants identified, and for whom complete data were available, 1,167 were classified as alcohol dependent “cases” and 1,366 as “controls.” The sample characteristics are presented in Table 1. A detailed demographic description of the SAGE sample has been provided elsewhere (Bierut et al., 2010). All subjects provided written informed consent for genetic studies and agreed to have their DNA and clinical data available to investigators through the National Institutes of Health repositories. All data for this study were de-identified.
Table 1.
Sample characteristics across cases and controls
| Variable | Alcohol Dependent Cases | Controls |
|---|---|---|
|
| ||
| N | 1167 | 1366 |
| Sex (% male) | 61.3 | 29.9 |
| Age (SD) | 38.1 (9.9) | 38.7 (9.4) |
| Physical and / or Sexual Trauma (%) | 42.5 | 26.7 |
| Both Physical and Sexual Trauma (%) | 9.9 | 3.6 |
| Sexual Trauma: % female, % male | 42.3; 7.6 | 14.4; 3.7 |
| Physical Trauma: % female, % male | 39.3; 42.5 | 18.9; 27.2 |
Psychiatric Assessments
A common psychiatric assessment was performed across all three studies based on the Semi-Structured Assessment for the Genetics of Alcoholism (SSAGA) (Bucholz et al., 1994), allowing for pooling of phenotypic data. In all studies, cases were identified as having a lifetime history of AD according to the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV). Controls were individuals who had been exposed to alcohol (and possibly to other drugs) but had not developed alcohol or drug abuse and/or dependence in their lifetime. Controls were also screened to exclude individuals with major Axis I disorders, such as schizophrenia, mood disorders, and anxiety disorders.
Traumatic Stress Exposure in Adulthood
Exposure to traumatic stress was derived from the PTSD items of the SSAGA, the Semi Structured Assessment of Cocaine Dependence (SSACD), or the Semi Structured Assessment of Nicotine Dependence (SSAND). Traumatic stress exposure was assessed via participant self-report of ever having “experienced or witnessed something that is so horrible that it would be distressing or upsetting to almost anyone.” It was up to the judgment of both the participant and the interviewer to assess the magnitude of distress caused by the event. Further probing by the interviewer indicated whether the trauma exposure involved physical assault, rape, sexual assault or other. For hypothesis testing, we used the SAGE items capturing the following three types of trauma: (a) sexual, (b) physical, and (c) non-physical or sexual. Examples of traumatic events are: military combat; an assault, rape, or kidnapping; seeing someone seriously injured or killed; a flood, earthquake, large fire, or other disaster; an airplane crash or serious car accident; a shooting or bombing; or any situation where you feared there was a serious threat to your life or to the life of another person. Endorsements of traumatic events that did not involve direct physical or sexual trauma were then coded as “non-physical or sexual.” Because exposure to non-physical or non-sexual trauma was overly prevalent in this sample (64.4%), the present analyses focused on direct sexual and physical traumatic events only. A total of 50.5% of cases and 28.7% of controls endorsed exposure to either sexual or physical trauma; details are provided in Table 1. Variables on childhood physical abuse, sexual abuse, and neglect were available, although these data were deemed unreliable due to the large number of missing (unknown) values representing 71.14%, 71.97%, and 85.98% of the available data, respectively.
Genotypes
All DNA samples were genotyped on the Illumina Human 1M beadchip. After extensive data cleaning and quality control procedures, described in detail elsewhere (Bierut et al., 2010), a total of 948,658 SNPs were analyzed in the primary GWAS. For the purpose of this study we focused the analyses on the 21 SNPs covering the CRHR1 gene, which is approximately 51.55 kb in length and maps to 17q21.31. Two SNPs (rs4792882 and rs16940655) did not have sufficient genotypic variance (< 2% minor allele frequency; MAF) and were therefore excluded, leaving a total of 19 SNPs for analyses. SNPs gene location, chromosomal position, allele frequency, and Hardy-Weinberg Equilibrium (HWE) are presented in Table 2.
Table 2.
Effect of the CRHR1 SNP Genotype × Stress interaction on the likelihood of alcohol dependence diagnosis
|
CRHR1
SNP |
Chromosomal
Position * |
CRHR1
Location |
MAF |
HWE
p-value |
Base
variation |
p-value |
|---|---|---|---|---|---|---|
| rs4792886 | 41232603 | intron | 0.096 | 0.837 | G > A | 0.498 |
| rs110402 | 41235818 | intron | 0.461 | 0.087 | C > T | 0.019 |
| rs242924 | 41241147 | intron | 0.461 | 0.139 | C > A | 0.019 |
| rs242942 | 41247413 | intron | 0.110 | 0.730 | G > A | 0.408 |
| rs3785877 | 41247968 | intron | 0.041 | 0.074 | G > A | 0.269 |
| rs171440 | 41249267 | intron | 0.487 | 0.558 | C > T | 0.056 |
| rs242939 | 41251360 | intron | 0.070 | 0.796 | A > G | 0.912 |
| rs4566211 | 41251477 | intron | 0.218 | 0.496 | G > A | 0.150 |
| rs242936 | 41254990 | intron | 0.104 | 0.769 | C > T | 0.623 |
| rs17762954 | 41255567 | intron | 0.218 | 0.418 | C > T | 0.175 |
| rs173365 | 41256855 | intron | 0.429 | 0.811 | C > T | 0.026 |
| rs1396862 | 41258778 | intron | 0.218 | 0.496 | C > T | 0.150 |
| rs17763086 | 41261262 | intron | 0.218 | 0.511 | T > G | 0.141 |
| rs17763104 | 41261576 | intron | 0.119 | 0.534 | G > A | 0.908 |
| rs16940665 | 41263677 | coding | 0.218 | 0.458 | T > C | 0.143 |
| rs17689918 | 41265869 | intron | 0.218 | 0.537 | G > A | 0.147 |
| rs17689966 | 41266236 | intron | 0.428 | 0.738 | A > G | 0.018 |
| rs1876829 | 41267224 | intron | 0.222 | 0.608 | A > G | 0.095 |
| rs16940686 | 41268811 | 3UTR | 0.040 | 0.153 | G > T | 0.223 |
Note:
Genome Build 36.3.
Significant traumatic stress × CRHR1 SNP interactions are bolded.
Statistical Analyses
Since there is no known functional CRHR1 polymorphism we used a haplotype-driven approach to capture potential CRHR1 variation. This approach is likely to detect the disease association of any allele, known or unknown, of moderate abundance and effect size for areas of the genome with conserved block structure, such as across the CRHR1 gene. Within each of the two haplotype blocks, we performed one analysis -- a logistical regression analysis -- and this identified the haplotypes that had an independent main effect as well as a G × E effect on outcome (see tables 4 and 6). In secondary analyses we then went further to determine whether any of the tightly linked SNPs provided the signal for the haplotype effects. Since at the outset we could not hypothesize which haplotype block might be associated with disease, for the purpose of Type I error correction a nominal p-value threshold of p = 0.025 was set (p = 0.05 divided by the number of haplotype blocks identified (i.e., 2).
Table 4.
Effect of CRHR1 haplotype block 1 haplotypes and trauma on the likelihood of alcohol dependence diagnosis
| Block 1 | B | OR | Std Err | z | P |
|---|---|---|---|---|---|
| Intercept | 1.28 | 3.60 | 0.11 | 11.15 | < 0.0001 |
| Sex | −1.32 | 0.27 | 0.07 | −20.18 | < 0.0001 |
| Trauma | 1.23 | 3.42 | 0.10 | 12.68 | < 0.0001 |
| H1 | 0.19 | 1.21 | 0.09 | 2.14 | 0.032 |
| H3 | 0.12 | 1.13 | 0.15 | 0.79 | 0.43 |
| Trauma*H1 | −0.30 | 0.74 | 0.14 | −2.19 | 0.029 |
| Trauma*H3 | −0.34 | 0.71 | 0.23 | −1.45 | 0.15 |
H2 (most frequent haplotype) is the reference haplotype (see Table 3a).
Statistically significant effects are bolded. OR = Odds ratio.
Table 6.
Effect of CRHR1 haplotype block 2 haplotypes and trauma on alcohol dependence
| Block 2 | B | Std Err | z | P |
|---|---|---|---|---|
| Intercept | 1.28 | 0.12 | 11.10 | < 0.0001 |
| Sex | −1.32 | 0.065 | −20.05 | < 0.0001 |
| Trauma | 1.24 | 0.099 | 12.62 | < 0.0001 |
| H1 | 0.22 | 0.11 | 1.95 | 0.051 |
| H2 | 0.11 | 0.14 | 0.79 | 0.43 |
| H3 | 0.14 | 0.17 | 0.79 | 0.43 |
| H5 | −0.0022 | 0.23 | −0.01 | 0.99 |
| H6 | 0.058 | 0.18 | 0.33 | 0.74 |
| H7 | 0.44 | 0.21 | 1.05 | 0.040 |
| Trauma*H1 | −0.37 | 0.17 | −2.22 | 0.026 |
| Trauma*H2 | −0.10 | 0.21 | −0.48 | 0.63 |
| Trauma*H3 | −0.22 | 0.27 | −0.79 | 0.43 |
| Trauma*H5 | −0.62 | 0.35 | −1.76 | 0.078 |
| Trauma*H6 | −0.19 | 0.27 | −0.71 | 0.48 |
| Trauma*H7 | −0.69 | 0.34 | −2.03 | 0.042 |
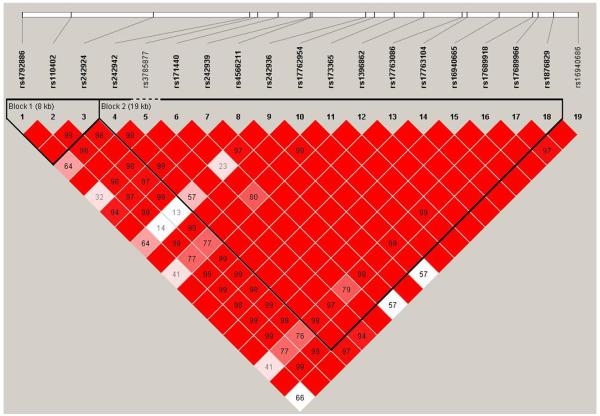
The second haplotype block is comprised of 15 SNPs: rs242942 to rs1876829 (Figure 1)
H4 (most frequent haplotype) is the reference haplotype.
Statistically significant effects are bolded.
Haplotype Analyses
Haplotype frequencies were estimated using a Bayesian approach implemented with PHASE (Stephens & Donnelly, 2003). Linkage disequilibrium (LD) relationships of the 19 SNPs were examined using Haploview software (Barett et al. 2005) the PHASE v2.1.1 Software (Stephens et al., 2001). Haplotype blocks were defined using validated a-priori methods (Gabriel et al., 2002). In order to test the effects of CRHR1 haplotypes on the likelihood of meeting criteria for alcoholism, as well as its interaction with trauma exposure, logistic regression models were run for each haplotype block using R Statistical Software. These models included sex (to correct for sex imbalance between cases and controls) and trauma exposure as covariates, and each haplotype within the respective block (1 or 2), as well as the interaction between haplotype and traumatic stress (G × E). For all haplotype analyses the most frequent haplotype was used as the reference group.
Secondary SNP Analyses
In order to determine which SNPs might be contributing to a haplotype effect, a series of logistic regression models were conducted testing whether AD status (cases versus controls) was predicted by CRHR1 SNP, traumatic stress exposure, and their interaction (G × E). Genotypes were coded log-additively (0, 1, 2 copies of the minor allele). All models included sex as a covariate to control for the sex imbalance between cases and controls.
RESULTS
Haplotype Analyses
Haplotype blocks and pairwise D’ values are shown in Figure 1. Two haplotype blocks were identified: block 1 includes three SNPs (8 kb): rs4792886, rs110402 and rs242924 while block 2 includes 15 SNPs (19 kb) and extends from rs242942 to rs1876829. The 3′UTR SNP rs16940686 was distal to block 2. Haplotypes within each block are presented in Table 3, along with their respective frequencies in cases and controls.
Figure 1. CRHR1 haplotype block structure in Caucasians.
The figure was generated using Haploview software (Barrett et al. 2005). Haplotype block 1 extends from SNP rs4792886 to rs242924 (8 kb). Haplotype block 2 extends from SNP rs242942 to rs1876829 (19 kb). The numbers in the squares (0 – 100) refer to pairwise linkage disequilibrium (LD) measured as D’. Haplotype blocks were defined using a setting of average pairwise D’ of ≥ 80. The darker the square, the greater the LD.
Table 3.
Grouping of haplotypes within haplotype blocks*
| (3a) Haplotype block 1 | |||||
|---|---|---|---|---|---|
| rs4792886 | rs110402 | rs242924 | Haplotype Frequencies | ||
| Cases (%) | Control (%) | ||||
| G | C | C | H1 | 45 | 44 |
| G | T | A | H2 | 46 | 46 |
| A | C | C | H3 | 9 | 10 |
| (3b) Haplotype Block 2 | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| rs242942 | rs3785877 | rs171440 | rs242939 | rs4566211 | rs242936 | rs17762954 | rs173365 | rs1396862 | rs17763086 | rs17763104 | rs16940665 | rs17689918 | rs17689966 | rs1876829 |
Haplotype
Frequencies |
||
| Cases (%) |
Control (%) |
||||||||||||||||
| G | G | C | A | A | C | T | T | T | G | G | C | A | G | G | H1 | 23 | 21 |
| G | G | C | A | G | C | C | C | C | T | A | T | G | A | A | H2 | 12 | 12 |
| G | G | C | A | G | C | C | T | C | T | G | T | G | G | A | H3 | 6 | 6 |
| G | G | T | A | G | C | C | C | C | T | G | T | G | A | A | H4 | 45 | 45 |
| G | G | T | A | G | T | C | T | C | T | G | T | G | G | A | H5 | 3 | 4 |
| A | G | C | G | G | T | C | T | C | T | G | T | G | G | A | H6 | 7 | 7 |
| A | A | C | A | G | C | C | T | C | T | G | T | G | G | A | H7 | 4 | 4 |
Base pairs in shaded boxes indicate the minor allele for that SNP.
Haplotype block 1: Haplotype Analyses
From Table 3a it can be seen that there were two major haplotypes of nearly equal frequency (denoted H1 and H2) and a minor (i.e., low frequency) haplotype (H3). Cases and controls had similar haplotype frequencies. The results of the logistic regression analysis where H2 (the most frequent haplotype) is the reference haplotype are presented in Table 4. Within the overall model there was an effect of sex (p < .0001) and trauma (p < .0001) such that males and trauma-exposed individuals were more likely to be alcohol dependent.
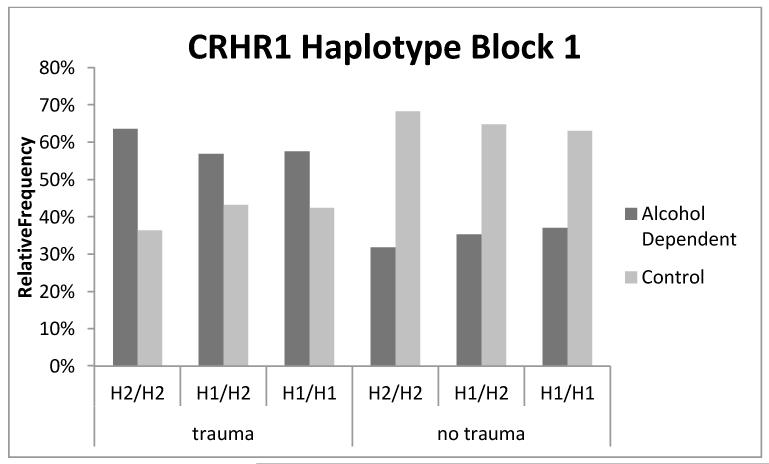
In addition, the H1 haplotype was associated with greater alcoholism risk (p = 0.032). The H1 × trauma interaction was also significant (p = 0.029) and the parameter estimate was negative (β = −0.30, SE = 0.14, p = .029). As shown in Figure 2, among individuals not exposed to trauma, the H1/H1 diplotype is a risk factor for AD relative to the H2/H2 diplotype; however, with trauma exposure carriers of the H1/H1 diplotype are more resilient to the development of AD relative to H2/H2 carriers.
Figure 2. Percentage of individuals with a given block1 haplotype and trauma exposure status who meet criteria for alcohol dependence.
Percentages were calculated from raw counts for each haplotype, trauma status, and alcohol dependence category. For example, of those individuals with the H1/H1 diplotype who were exposed to trauma, 63% were found to be alcohol dependent. A smaller percentage (34%) of those individuals who carried the H1/H1 diplotype and were not exposed to trauma were alcohol dependent.
Haplotype Block 1: Secondary SNP Analyses
As shown in Table 2, a significant (corrected p < .025) genotype × traumatic stress interaction on the likelihood of developing alcoholism was found for two of the block 1 SNPs: rs110402 and rs242924, These SNPs are in allelic identity (MAF = 0.461). The results of the full models for each SNP are shown in Table 5. As can be seen from Table 3A, these two SNPs appear to be driving the block 1 haplotype results. Thus the major alleles of rs110402 and rs242924, included in haplotype H1, are protective against risk of AD in individuals exposed to trauma.
Table 5.
The influence of selected CRHR1 SNPs and trauma on alcohol dependence
| SNPs | Gene Effect | Trauma |
G × E
Interaction |
Sex |
Whole
Model |
Whole
Model |
|||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| L-Rχ2 | P | L-Rχ2 | P | L-Rχ2 | P | L-Rχ2 | P | P value | Df | Var | |
| Block 1 | |||||||||||
|
| |||||||||||
| rs110402 | 4.12 | 0.045 | 21.54 | <.0001 | 5.50 | 0.019 | 190.5 | <0.001 | <0.0001 | 4 | 0.12 |
|
| |||||||||||
| rs242924 | 4.20 | 0.041 | 21.57 | <.0001 | 5.53 | 0.019 | 189.2 | <0.001 | <0.0001 | 4 | 0.12 |
|
| |||||||||||
| Block 2 | |||||||||||
|
| |||||||||||
| rs173365 | 2.48 | 0.115 | 70.89 | <.0001 | 4.96 | 0.026 | 188.0 | <0.001 | <0.0001 | 4 | 0.11 |
|
| |||||||||||
| rs17689966 | 2.34 | 0.126 | 73.36 | <.0001 | 5.63 | 0.018 | 188.8 | <0.001 | <0.0001 | 4 | 0.11 |
Results are for effect likelihood ratio (L-R) tests and are given for p < 0.05 (with exception of genotype main effects)
All other SNPs showed no significant main or interactive effects on alcohol dependence.
Var = variance explained by total model. Df = degrees of freedom.
Haplotype Block 2: Haplotype Analyses
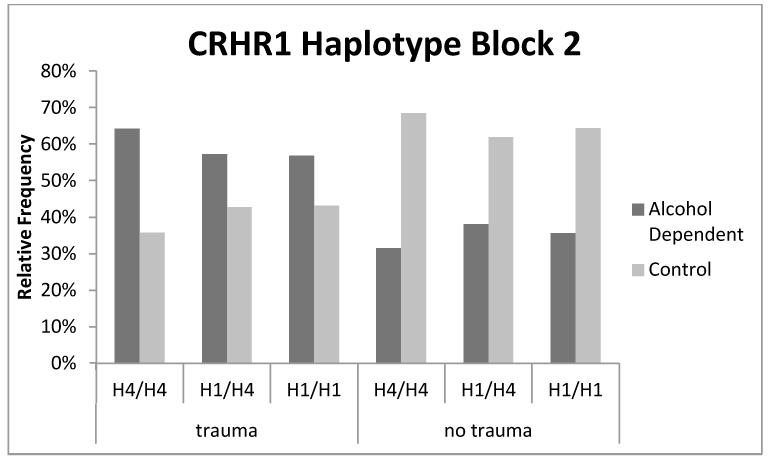
A similar pattern of results emerged for haplotype block 2 (see Table 6). There was a simple effect of sex and trauma (ps < .0001), such that males and trauma-exposed individuals were more likely to meet criteria for alcoholism. Further, there were simple effects for two haplotypes (H1, trend level, and H7), which were associated with increased risk of AD. In addition, there were significant haplotype × trauma interactions for both H1 and H7 (p = .026 and .042, respectively) such that the effects of traumatic stress exposure on alcoholism risk were mitigated by the H1 haplotype. As shown in Figure 3, in individuals not exposed to trauma, the H1/H1 diplotype is a risk factor for AD relative to the H4/H4 diplotype; however with trauma exposure, carriers of the H1/H1 diplotype are more resilient to the development of AD relative to H4/H4 carriers.
Figure 3. Percentage of individuals with a given block 2 haplotype and trauma exposure status who suffer from alcohol dependence.
Percentages were calculated from raw counts for each haplotype, trauma status, and alcohol dependence category. We observe that 56% of individuals who carried the H1/H1 diplotype and were exposed to trauma were found to be alcohol dependent. This percentage is slightly smaller than the percentage (62%) of individuals who carried the H4/H4 reference diplotype and were exposed to trauma who were found to be alcohol dependent. In the absence of trauma, 33% of individuals carrying the H1/H1 diplotype were found to be alcohol dependent.
Haplotype Block 2: Secondary SNP Analyses
As shown in Table 2, a significant (corrected p < .025) genotype × traumatic stress interaction on the likelihood of developing alcoholism was found for the block two SNPs rs173365 and rs17689966 that are in allelic identity (MAF = 0.428-0.429). Results of the full models for each of the two SNPs are presented in Table 5. As can be seen in Table 3b, base pairs for SNPs rs173365 and rs17689966 differ from the reference haplotype in both H1 and H7 haplotypes. However, these loci do not differ from H3, H5, and H6 suggesting that these SNPs alone do not account for the observed haplotype effects. Therefore, epistatic effects and/or additional markers not captured in these analyses likely contribute to the haplotype results observed for block 2. In models examining three-way interactions between genetic factors, trauma and sex, which included all lower order interaction terms, there were no significant three-way CRHR1 SNPs/haplotypes × Traumatic Stress × Sex interactions (ps > .10).
DISCUSSION
To further elucidate the putative role of stress dysregulation in the CRF system in the initiation and maintenance of addiction (Koob & Kreek, 2007), this study examined CRHR1 G × E interactions as predictors of alcoholism status in a large and well-defined sample of Caucasians. In contrast to many of the CRHR1 G × E studies in depression, our stressor was trauma experienced in adulthood rather than childhood. This is in common with several of the CRHR1 G × E studies on alcoholism and alcohol consumption phenotypes that have had positive outcomes (e.g., Blomeyer et al., 2008, Schmid et al., 2010).. As shown in Table 4, the haplotype block 1 H1 haplotype had both a main effect and an interactive effect with trauma on risk for AD. Specifically, in individuals not exposed to trauma, the H1/H1 diplotype was found to be a risk factor for AD relative to the H2/H2 diplotype; however, with trauma exposure carriers of the H1/H1 diplotype were found to be more resilient to the development of AD relative to H2/H2 carriers. A similar pattern of results emerged for the H1 haplotype in block 2, indicating that the relative risk of alcoholism was moderated by exposure to traumatic stress.
While the available data did not allow us to test the entire TAT haplotype reported by Bradley et al. (2008) and Kranzler et al. (2011) as a moderator of the effect of adverse childhood experiences on lifetime risk of major depression, our findings are consistent in suggesting a G × E effect for this common region of the CRHR1 gene. Our study has shown that the rs110402 and rs242924 SNPs were driving the block 1 haplotype analyses and that the major alleles of these two SNPs are protective against risk of AD in trauma exposed individuals but conversely increase risk for AD in individuals who have not experienced adulthood trauma. The direction of these findings is opposite to earlier studies showing that the TAT haplotype, including the minor alleles of rs110402 and rs242924, was protective against major depression in African Americans and Caucasians exposed to childhood trauma (Bradley et al., 2008, Kranzler et al., 2011a). Moreover, Tyrka et al. (2009) showed that in individuals exposed to childhood trauma, carriers of the major homozygotes of rs110402 and rs242924 showed elevated cortisol response to the dexamethasone suppression test (Tyrka et al., 2009).
One possible reason for the different outcomes between our study and the earlier studies is that we used stressors in adulthood and not childhood. For example, Binder et al. (2008) in an analysis of the HPA axis FKBP5 gene showed a G × E effect on posttraumatic stress disorder for childhood trauma but not for adult stressors (Binder et al., 2008). It has been shown that significant childhood trauma has a deleterious and permanent effect on the development and functioning of the extra hypothalamic CRF system (Enoch, 2010). Stressors in adulthood may work by different mechanisms. We did not have adequate data on exposure to childhood trauma in the SAGE dataset. Nevertheless, since early life stress is a predictor of adult psychopathology including alcoholism (Enoch, 2010), it is likely that a significant proportion of the alcoholics had been exposed to childhood trauma. Moreover, several studies have shown that exposure to childhood trauma predicts increased risk for subsequent trauma in adulthood including physical assault and rape (Enoch, 2010). Sample differences may account for the null findings in the Kranzler et al. (2011) study for interactive effects of the TAT haplotype and childhood trauma on lifetime risk of alcoholism.
In the present study, the sample was comprised exclusively of Caucasians and we found consistent support for the main effect of sex and trauma exposure on the risk for alcoholism. These variables were significant covariates in all models and without these statistical controls the G × E effects were obscured. Rates of exposure to traumatic stress were also quite high in this sample with 50.51% of cases and 28.65% of controls endorsing exposure to either sexual or physical trauma. An even higher percentage of the sample endorsed exposure to non-physical or non-sexual traumas (64.37%) suggesting that the degree of traumatic exposure may impact the ability to detect meaningful G × E effects. Additional studies that can more effectively delineate the nature and intensity of traumatic stress (or childhood trauma) are required to effectively capture genetic effects on clinical outcomes. Failure to properly operationalize the stress or trauma experienced may lead to mixed findings in the G × E literature of psychiatric disorders (Caspi et al., 2010).
The results of our study are consistent with the findings by Treutlein and colleagues implicating two CRHR1 SNPs (rs242938 and rs1876831), distal to haplotype block 1, with increased alcohol use (Treutlein et al., 2006), particularly in the context of negative life events (Blomeyer et al., 2008). Although these studies rely on tag SNPs to capture variance in the CRHR1 gene, they emphasize the need to elucidate the molecular mechanisms underlying CRHR1 expression and function, as they may influence reactivity to stress and possibly alcoholism risk. To that end, recent studies have highlighted the role of the CRHR1 gene in alcohol use in animals (Molander et al., 2012) and in clinical samples (Ribbe et al., 2011). A notable preclinical study compared global CRHR1 knockout mice and conditional brain-specific CRHR1 knockout across a range of alcohol exposure conditions, including an alcohol deprivation paradigm that serves as a relapse analog (Molander et al., 2012). Results suggested that stress-induced augmentation of alcohol intake and escalation of alcohol intake was lower in the brain-specific knockout mice as compared to control animals. These findings indicate that the contribution of CRHR1 to basal alcohol intake and relapse-like drinking may be limited to situations with a high stress load, underscoring G × E effects for this locus. Further, a clinical study found that a CRHR1 SNP (rs110402; same as the one identified in this study) interacted with a Corticotropin releasing factor binding protein (CRHBP) SNP (rs3811939) to predict higher risk of comorbid alcoholism in a sample of patients with primary schizophrenia. Of interest, CRHR1 and CRHBP messenger RNA (mRNA) levels were quantified as a biological estimate of ligand efficiency of the CRF system and analyses revealed that these two markers were associated with blood mRNA levels across both alcohol dependent patients and non-dependent controls (Ribbe et al., 2011). Together, these recent studies emphasize the biological plausibility and provide initial mechanistic evidence that the CRF system and the CRHR1 gene in particular, is involved with alcoholism risk through interactions with the stress-pathway.
This study should be interpreted in light of its strengths and limitations. Study strengths include the well-ascertained and large sample of Caucasian individuals from a publically available database. Study limitations include the reliance on tag SNPs from the Illumina Human 1M beadchip to capture genetic variation in the CRHR1 gene. Moreover, the lack of detailed information on the timing, type, and intensity of the traumatic stress exposure and lack of data on childhood trauma precludes fine grained analyses of the specific stress conditions that may interact with CRHR1 genotype to determine alcoholism susceptibility. Specifically, studies have shown that alcohol use itself increases the risk of exposure to traumatic events (e.g., Harris et al., 2012) such that the relationship between alcohol use and trauma is likely bi-directional. As such, studies that can properly capture the temporal nature of the traumatic exposure in relation to the onset of alcohol problems are needed to more fully elucidate these complex relationship and its genetic determinants.
A large proportion of the sample had experienced traumatic events that did not involve direct physical or sexual trauma. By including these individuals in the ‘no trauma’ group it is conceivable that claims regarding haplotype / diplotype risk factors in ‘no trauma’ individuals might be inaccurate. Also, the sample is imbalanced with respect to sex, since there are many more men represented in the alcoholic cases than in the controls. Further, alcoholism is a complex phenotype such that dysregulation of the extra hypothalamic CRF system precipitated by traumatic exposure or otherwise, may be a more salient risk factor for some individuals than others. As such, more discrete clinical phenotypes, such as stress-induced craving (e.g., Ray, 2011), may be useful to elucidating the role of stress on alcoholism etiology and maintenance. Likewise, clinical predictors beyond adult traumatic exposure would be useful and may include constructs such as childhood trauma, chronic stress exposure, and recent stress exposure, all of which have been associated with worse psychiatric functioning (Huang et al., 2012, Mulia et al., 2008). In brief, refined phenotypes at the level of the outcome (dependent variable) and predictors (independent variables) are needed to more fully delineate stress-based pathways of risk and resilience for alcoholism.
It should be noted that the transcription of CRHR1 is complex: alternative splicing results in multiple transcript variants, one of which represents a read-through transcript with the neighboring gene MGC57346. A look at the UCSC gene browser (http://genome.ucsc.edu/) shows that haplotype block 1 extends for a great distance proximally and includes MGC57346. Although this gene is of unknown function, our block 1 haplotype association could theoretically result from MGC57346 rather than CRHR1. Finally, the haplotype results did not quite reach the corrected p <0.025 although the SNP results, underlying the haplotype results, were significant.
On balance, this study provides evidence of G × E effects implicating several CRHR1 SNPs and haplotypes with alcoholism risk in the context of traumatic stress exposure in adulthood. These findings are consistent with the hypothesized role of the extra hypothalamic CRF system dysregulation in alcoholism and suggest that further molecular genetic studies as well as translational investigations of the CRHR1 gene are warranted.
Contributor Information
Lara A. Ray, University of California Los Angeles, Department of Psychology, Brain Research Institute, Department of Psychiatry and Biobehavioral Sciences.
Mary Sehl, University of California Los Angeles, Department of Biomathematics & Department of Oncology.
Spencer Bujarski, University of California Los Angeles, Department of Psychology.
Kent Hutchison, University of Colorado, Boulder, Department of Psychology.
Sara Blaine, University of Colorado, Boulder, Department of Psychology.
Mary-Anne Enoch, Laboratory of Neurogenetics, National Institute on Alcohol Abuse and Alcoholism.
REFERENCES
- Barrett JC, Fry B, Maller J, Daly MJ. Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics. doi: 10.1093/bioinformatics/bth457. in press. [DOI] [PubMed] [Google Scholar]
- Bierut LJ, Agrawal A, Bucholz KK, Doheny KF, Laurie C, Pugh E, Fisher S, Fox L, Howells W, Bertelsen S, Hinrichs AL, Almasy L, Breslau N, Culverhouse RC, Dick DM, Edenberg HJ, Foroud T, Grucza RA, Hatsukami D, Hesselbrock V, Johnson EO, Kramer J, Krueger RF, Kuperman S, Lynskey M, Mann K, Neuman RJ, Nothen MM, Nurnberger JI, Jr., Porjesz B, Ridinger M, Saccone NL, Saccone SF, Schuckit MA, Tischfield JA, Wang JC, Rietschel M, Goate AM, Rice JP. A genome-wide association study of alcohol dependence. Proc Natl Acad Sci U S A. 2010;107:5082–5087. doi: 10.1073/pnas.0911109107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder EB, Bradley RG, Liu W, Epstein MP, Deveau TC, Mercer KB, Tang Y, Gillespie CF, Heim CM, Nemeroff CB, Schwartz AC, Cubells JF, Ressler KJ. Association of FKBP5 polymorphisms and childhood abuse with risk of posttraumatic stress disorder symptoms in adults. JAMA. 2008;299:1291–1305. doi: 10.1001/jama.299.11.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blomeyer D, Treutlein J, Esser G, Schmidt MH, Schumann G, Laucht M. Interaction between CRHR1 gene and stressful life events predicts adolescent heavy alcohol use. Biol Psychiatry. 2008;63:146–151. doi: 10.1016/j.biopsych.2007.04.026. [DOI] [PubMed] [Google Scholar]
- Bradley RG, Binder EB, Epstein MP, Tang Y, Nair HP, Liu W, Gillespie CF, Berg T, Evces M, Newport DJ, Stowe ZN, Heim CM, Nemeroff CB, Schwartz A, Cubells JF, Ressler KJ. Influence of child abuse on adult depression: moderation by the corticotropin-releasing hormone receptor gene. Arch Gen Psychiatry. 2008;65:190–200. doi: 10.1001/archgenpsychiatry.2007.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bucholz KK, Cadoret R, Cloninger CR, Dinwiddie SH, Hesselbrock VM, Nurnberger JI, Jr., Reich T, Schmidt I, Schuckit MA. A new, semi-structured psychiatric interview for use in genetic linkage studies: a report on the reliability of the SSAGA. J Stud Alcohol. 1994;55:149–158. doi: 10.15288/jsa.1994.55.149. [DOI] [PubMed] [Google Scholar]
- Caspi A, Hariri AR, Holmes A, Uher R, Moffitt TE. Genetic sensitivity to the environment: the case of the serotonin transporter gene and its implications for studying complex diseases and traits. Am J Psychiatry. 2010;167:509–527. doi: 10.1176/appi.ajp.2010.09101452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen AC, Manz N, Tang Y, Rangaswamy M, Almasy L, Kuperman S, Nurnberger J, Jr., O’Connor SJ, Edenberg HJ, Schuckit MA, Tischfield J, Foroud T, Bierut LJ, Rohrbaugh J, Rice JP, Goate A, Hesselbrock V, Porjesz B. Single-nucleotide polymorphisms in corticotropin releasing hormone receptor 1 gene (CRHR1) are associated with quantitative trait of event-related potential and alcohol dependence. Alcohol Clin Exp Res. 2010;34:988–996. doi: 10.1111/j.1530-0277.2010.01173.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciccocioppo R, Gehlert DR, Ryabinin A, Kaur S, Cippitelli A, Thorsell A, Le AD, Hipskind PA, Hamdouchi C, Lu J, Hembre EJ, Cramer J, Song M, McKinzie D, Morin M, Economidou D, Stopponi S, Cannella N, Braconi S, Kallupi M, de Guglielmo G, Massi M, George DT, Gilman J, Hersh J, Tauscher JT, Hunt SP, Hommer D, Heilig M. Stress-related neuropeptides and alcoholism: CRH, NPY, and beyond. Alcohol. 2009;43:491–498. doi: 10.1016/j.alcohol.2009.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ducci F, Goldman D. Genetic approaches to addiction: genes and alcohol. Addiction. 2008;103:1414–1428. doi: 10.1111/j.1360-0443.2008.02203.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enoch MA. The role of early life stress as a predictor for alcohol and drug dependence. Psychopharmacology (Berl) 2010;214:17–31. doi: 10.1007/s00213-010-1916-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funk CK, Zorrilla EP, Lee MJ, Rice KC, Koob GF. Corticotropin-releasing factor 1 antagonists selectively reduce ethanol self-administration in ethanol-dependent rats. Biol Psychiatry. 2007;61:78–86. doi: 10.1016/j.biopsych.2006.03.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabriel SB, Schaffner SF, Nguyen H, Moore JM, Roy J, Blumenstiel B, Higgins J, DeFelice M, Lochner A, Faggart M, Liu-Cordero SN, Rotimi C, Adeyemo A, Cooper R, Ward R, Lander ES, Daly MJ, Altshuler D. The structure of haplotype blocks in the human genome. Science. 2002;296:2225–2229. doi: 10.1126/science.1069424. [DOI] [PubMed] [Google Scholar]
- Hansson AC, Cippitelli A, Sommer WH, Fedeli A, Bjork K, Soverchia L, Terasmaa A, Massi M, Heilig M, Ciccocioppo R. Variation at the rat Crhr1 locus and sensitivity to relapse into alcohol seeking induced by environmental stress. Proc Natl Acad Sci U S A. 2006;103:15236–15241. doi: 10.1073/pnas.0604419103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris AH, Lembke A, Henderson P, Gupta S, Moos R, Bradley KA. Risk of future trauma based on alcohol screening scores: a two-year prospective cohort study among US veterans. Addict Sci Clin Pract. 2012;7:6. doi: 10.1186/1940-0640-7-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heilig M, Koob GF. A key role for corticotropin-releasing factor in alcohol dependence. Trends Neurosci. 2007;30:399–406. doi: 10.1016/j.tins.2007.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hines LM, Ray L, Hutchison K, Tabakoff B. Alcoholism: the dissection for endophenotypes. Dialogues Clin Neurosci. 2005;7:153–163. doi: 10.31887/DCNS.2005.7.2/lhines. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang MC, Schwandt ML, Ramchandani VA, George DT, Heilig M. Impact of multiple types of childhood trauma exposure on risk of psychiatric comorbidity among alcoholic inpatients. Alcohol Clin Exp Res. 2012;36:1099–1107. doi: 10.1111/j.1530-0277.2011.01695.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendler KS, Prescott CA, Neale MC, Pedersen NL. Temperance board registration for alcohol abuse in a national sample of Swedish male twins, born 1902 to 1949. Arch Gen Psychiatry. 1997;54:178–184. doi: 10.1001/archpsyc.1997.01830140090015. [DOI] [PubMed] [Google Scholar]
- Koob GF. Alcoholism, corticotropin-releasing factor, and molecular genetic allostasis. Biol Psychiatry. 2008;63:137–138. doi: 10.1016/j.biopsych.2007.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF. The role of CRF and CRF-related peptides in the dark side of addiction. Brain Res. 2010;1314:3–14. doi: 10.1016/j.brainres.2009.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Kreek MJ. Stress, dysregulation of drug reward pathways, and the transition to drug dependence. Am J Psychiatry. 2007;164:1149–1159. doi: 10.1176/appi.ajp.2007.05030503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kranzler HR, Feinn R, Nelson EC, Covault J, Anton RF, Farrer L, Gelernter J. A CRHR1 haplotype moderates the effect of adverse childhood experiences on lifetime risk of major depressive episode in African-American women. Am J Med Genet B Neuropsychiatr Genet. 2011a;156B:960–968. doi: 10.1002/ajmg.b.31243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kranzler HR, Feinn R, Nelson EC, Covault J, Anton RF, Farrer L, Gelernter J. A CRHR1 haplotype moderates the effect of adverse childhood experiences on lifetime risk of major depressive episode in African-American women. Am J Med Genet B Neuropsychiatr Genet. 2011b;156:960–968. doi: 10.1002/ajmg.b.31243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molander A, Vengeliene V, Heilig M, Wurst W, Deussing JM, Spanagel R. Brain-specific inactivation of the Crhr1 gene inhibits post-dependent and stress-induced alcohol intake, but does not affect relapse-like drinking. Neuropsychopharmacology. 2012;37:1047–1056. doi: 10.1038/npp.2011.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulia N, Schmidt L, Bond J, Jacobs L, Korcha R. Stress, social support and problem drinking among women in poverty. Addiction. 2008;103:1283–1293. doi: 10.1111/j.1360-0443.2008.02234.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson EC, Agrawal A, Pergadia ML, Wang JC, Whitfield JB, Saccone FS, Kern J, Grant JD, Schrage AJ, Rice JP, Montgomery GW, Heath AC, Goate AM, Martin NG, Madden PA. H2 haplotype at chromosome 17q21.31 protects against childhood sexual abuse-associated risk for alcohol consumption and dependence. Addict Biol. 2009;15:1–11. doi: 10.1111/j.1369-1600.2009.00181.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prescott CA, Kendler KS. Genetic and environmental contributions to alcohol abuse and dependence in a population-based sample of male twins. Am J Psychiatry. 1999;156:34–40. doi: 10.1176/ajp.156.1.34. [DOI] [PubMed] [Google Scholar]
- Ray LA. Stress-induced and cue-induced craving for alcohol in heavy drinkers: Preliminary evidence of genetic moderation by the OPRM1 and CRH-BP genes. Alcohol Clin Exp Res. 2011;35:166–174. doi: 10.1111/j.1530-0277.2010.01333.x. [DOI] [PubMed] [Google Scholar]
- Ribbe K, Ackermann V, Schwitulla J, Begemann M, Papiol S, Grube S, Sperling S, Friedrichs H, Jahn O, Sillaber I, Gefeller O, Krampe H, Ehrenreich H. Prediction of the risk of comorbid alcoholism in schizophrenia by interaction of common genetic variants in the corticotropin-releasing factor system. Arch Gen Psychiatry. 2011;68:1247–1256. doi: 10.1001/archgenpsychiatry.2011.100. [DOI] [PubMed] [Google Scholar]
- Roy A, Hodgkinson CA, Deluca V, Goldman D, Enoch MA. Two HPA axis genes, CRHBP and FKBP5, interact with childhood trauma to increase the risk for suicidal behavior. J Psychiatr Res. 2012;46:72–79. doi: 10.1016/j.jpsychires.2011.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid B, Blomeyer D, Treutlein J, Zimmermann US, Buchmann AF, Schmidt MH, Esser G, Rietschel M, Banaschewski T, Schumann G, Laucht M. Interacting effects of CRHR1 gene and stressful life events on drinking initiation and progression among 19-year-olds. Int J Neuropsychopharmacol. 2010;13:703–714. doi: 10.1017/S1461145709990290. [DOI] [PubMed] [Google Scholar]
- Sinha R. How does stress increase risk of drug abuse and relapse? Psychopharmacology (Berl) 2001;158:343–359. doi: 10.1007/s002130100917. [DOI] [PubMed] [Google Scholar]
- Stephens M, Donnelly P. A comparison of bayesian methods for haplotype reconstruction from population genotype data. Am J Hum Genet. 2003;73:1162–1169. doi: 10.1086/379378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens M, Smith NJ, Donnelly P. A new statistical method for haplotype reconstruction from population data. Am J Hum Genet. 2001;68:978–989. doi: 10.1086/319501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treutlein J, Kissling C, Frank J, Wiemann S, Dong L, Depner M, Saam C, Lascorz J, Soyka M, Preuss UW, Rujescu D, Skowronek MH, Rietschel M, Spanagel R, Heinz A, Laucht M, Mann K, Schumann G. Genetic association of the human corticotropin releasing hormone receptor 1 (CRHR1) with binge drinking and alcohol intake patterns in two independent samples. Mol Psychiatry. 2006;11:594–602. doi: 10.1038/sj.mp.4001813. [DOI] [PubMed] [Google Scholar]
- Tyrka AR, Price LH, Gelernter J, Schepker C, Anderson GM, Carpenter LL. Interaction of childhood maltreatment with the corticotropin-releasing hormone receptor gene: effects on hypothalamic-pituitary-adrenal axis reactivity. Biol Psychiatry. 2009;66:681–685. doi: 10.1016/j.biopsych.2009.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]