Abstract
Tetrabromobisphenol A (TBBPA) is a ubiquitous flame retardant. A high-throughput immunoassay would allow for monitoring of human and environmental exposures as a part of risk assessment. Naturally occurring antibodies in camelids that are devoid of light chain, show great promise as an efficient tool in monitoring environmental contaminants, but they have been rarely used for small molecules. An alpaca was immunized with a TBBPA hapten coupled to thyroglobulin and a variable domain of heavy chain antibody (VHH) T3–15 highly selective for TBBPA was isolated from a phage displayed VHH library using heterologous coating antigens. Compared to the VHHs isolated using homologous antigens, VHH T3–15 had about a 10-fold improvement in sensitivity in an immunoassay. This assay, under the optimized conditions of 10% methanol in the assay buffer (pH 7.4), had an IC50 for TBBPA of 0.40 ng mL–1 and negligible cross reactivity (<0.1%) with other tested analogues. After heating the VHH at 90 °C for 90 min about 20% of the affinity for coating antigen T3-BSA remained. The recoveries of TBBPA from spiked soil and fetal bovine serum samples ranged from 90.3% to 110.7% by ELISA and agreed well with a liquid chromatography–tandem mass spectrometry method. We conclude the many advantages of VHH make them attractive for the development of immunoassays to small molecules.
Introduction
Tetrabromobisphenol A (2,2-bis(3,5-dibromo-4-hydroxyphenyl)propane; TBBPA) is the largest brominated flame retardant (BFR) in terms of production volume globally.1 It is widely used in improving fire safety as an additive flame retardant or a reactive retardant with incorporation into plastics and other materials as its diglycidyl ether. TBBPA can be released to the environment during its production, usage, and disposal. Although TBBPA has lower toxicity than many other BFRs such as polybrominated diphenyl ethers (PBDEs), it can cause hepatic and kidney lesions in pregnant mice and their offspring when pregnant dams are exposed to TBBPA in the diet2 and endocrine disruption due to the structural resemblance of TBBPA to thyroid hormones.3 By mimicking β-estradiol, TBBPA can bind to the human estrogen sulfotransferase (SULT1E1), a key hormone metabolizing enzyme,4 and it was also reported to induce transcription of E2-activated genes in mosquitofish in vivo.5 TBBPA has been detected in abiotic samples, such as soil (<0.3 ng g–1 dry weight (dw) in farming land and 3.4–32.2 ng g–1 dw in industrial soils),6 sediment (3.8–230 ng g–1 dw in Dongjiang river in China7 and 34–270 ng g–1 dw in a stream of the industry8), sewage sludge (up to 1329 ng g–1 dw in Ebro River basin9 and up to 472 ng g–1 dw from Catalonia in Spain10), and indoor dust (490–520 ng g–1 in Japan),11 as well as in biotic samples, such as human breast milk (up to 12.46 ng g–1 lipid weight (lw) in Beijing12 and 30–550 pg g–1 lw in Boston13) and serum (up to 3.4 pmol g–1 lw in computer technicians).14
TBBPA is usually determined by chromatographic methods, such as HPLC–MS/MS (the limit of detection (LOD) reported as 0.5 ng g–1),15 UPLC–MS/MS (LOD of 60 pg g–1)12 and GC–MS (LOD of 90 pg g–1).6 Even though highly sensitive and selective, these instrumental methods are very time-consuming, costly, and require complex operations such as derivatization for GC–MS analysis. Immunoassays based on polyclonal antibody (PAb) and monoclonal antibody (mAb) against TBBPA have proven to be sensitive, selective, and capable of high throughput in the monitoring of this environmental pollutant in water, soils, and sediments.16,17 In an earlier work, the haptens of TBBPA were synthesized, and the PAb-based immunoassay was developed, with a half-maximum signal inhibition concentration (IC50) of 0.87 ng mL–1.16 The biotin–streptavidin-amplified PAb-based and the mAb-based immunoassays for TBBPA showed an IC50 of 0.58 ng mL–117 and 3.87 ng mL–1,18 respectively.
Since antibodies devoid of light chain were discovered in the serum of the camel (Camelus dromedarius),19 camelid antibodies have been gaining more attention in many biotechnological applications.20 Conventional IgGs (mAb and PAb) are about 150 kDa, while the antigen-binding fragment, a variable domain of heavy chain antibody (VHH), is about 15 kDa,21 making it suitable for construction of a high throughput selection system, such as phage, yeast, or ribosome.22 It has been demonstrated that VHHs have high thermal stability, high solubility, and are easily and cheaply expressed in high yield.23 Although both VHH and heavy chains of conventional antibodies have four framework regions (FR1, FR2, FR3 and FR4) and three complementarity determining regions (CDR1, CDR2 and CDR3), binding by the VHH paratope, in absence of the variable light chain, is compensated for by the extension of the CDR1 loop, highly hydrophilic amino acid of FR2 and the longer convex-shaped CDR3.24 Due to these advantages, VHH has been used as a therapeutic tool in various disease areas.25−27 More recently, VHHs were exploited in detecting environmental contaminants such as azoxystrobin28 and triclocarban,29 as well as the breakdown product of some pyrethroid insecticides, 3-phenoxybenzoic acid (3-PBA).30 Thus, they are showing promise for small-molecule analysis in general and in environmental analysis in particular.
Construction and use of phage display peptide libraries is a general and useful method to select the high-affinity antibody.31 A vast repertoire of antibody fragments can be displayed on the phage surface. Thus, a key step to an effective selection strategy is to enrich and isolate the specific VHH ligands from a large number of nonspecific phage clones. Biopanning with homologous coating antigens on solid plates is a common method of selecting ligands by competition for the small-molecule target.28−30 Heterology in immunizing antigen and coating antigen has been extensively employed to improve the sensitivity32 and selectivity33 of immunoassays for small molecule analytes, by minimizing nonspecific binding by the antibody. The heterology was also introduced for hybridoma screening for the target of interest in the production of mAb,34−36 e.g. mAbs with a broad-selectivity for parathion, methyl parathion, fenitrothion, and isocarbophos have been produced.36 On the basis of the advantages, we hypothesized that screening with heterologous coating antigens would be useful in the selective isolation of single-domain antibodies.
In this study, a VHH selective for TBBPA was isolated from a phage-display library with a novel heterologous selection system and was used to develop an enzyme-linked immunosorbent assay (ELISA) for monitoring environmental and human exposure to TBBPA. To our knowledge, this is the first systematic study on the influence of heterologous antigen selection on the VHH-based immunoassay sensitivity and specificity.
Experimental Section
Safety
Prior to disposal, all the containers (e.g., centrifuge tubes and flasks) for phage incubation were immersed in 10% bleach solution overnight, followed by autoclaving. All the disposable items (pipet tips and tubes) in contact with phages were autoclaved before being discarded. TBBPA and its analogues were discarded as hazardous waste according to campus policies.
Materials and Methods
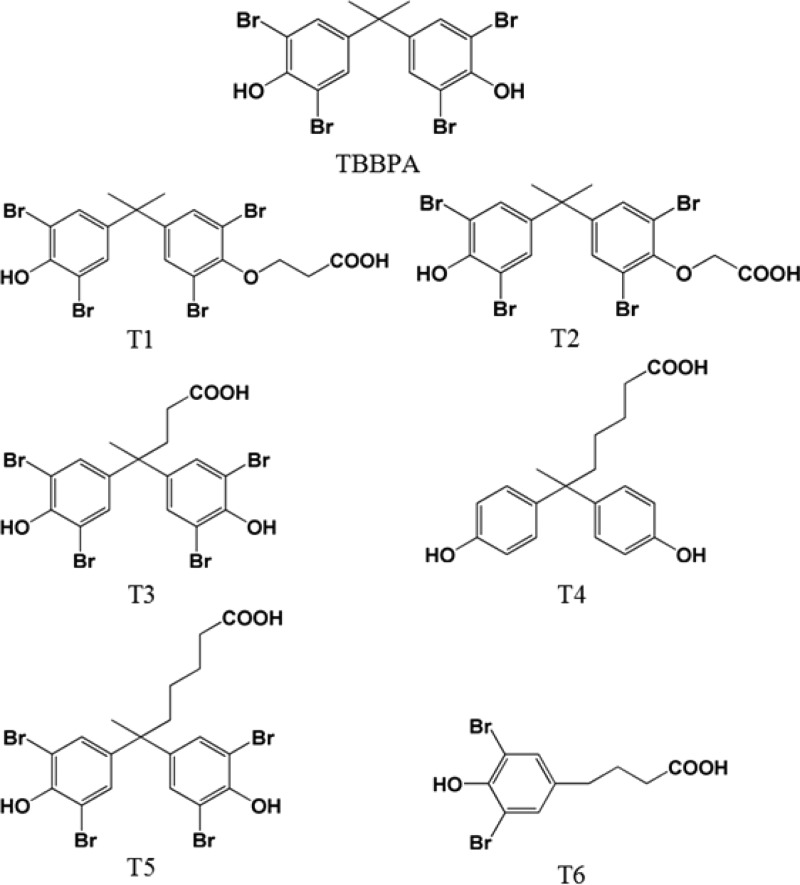
The synthesis of haptens T1–T6 (Figure 1) and the conjugation of haptens to carrier proteins were described in the previous report.16 TBBPA standard was purchased from TCI Co. Ltd. (Tokyo, Japan). TBBPA derivatives and other BFR analogues were purchased from AccuStandard (New Haven, CT). The anti-HA tag antibody (horseradish peroxidase (HRP) conjugate) (ab1265) and goat antirabbit IgG (HRP conjugate) (ab97051) were purchased from Abcam (Cambridge, MA). HisPur Ni-NTA resin, B-PER, Halt protease inhibitor cocktail, and Nunc MaxiSorp flat-bottom 96-well plates were purchased from Thermo Fisher Scientific Inc. (Rockford, IL, U.S.A.). Incomplete Freund’s adjuvant, thyroglobulin, bovine serum albumin (BSA), 3,3′,5,5′-tetramethylbenzidine (TMB), polyethylene glycol 8000 (PEG 8000), isopropyl-β-d-thiogalactopyranoside (IPTG) were purchased from Sigma-Aldrich Chemical Co. (St. Louis, MO, U.S.A.). Mouse anti-M13 phage mAb-HRP was from GE Healthcare (Piscataway, NJ, U.S.A.). Rabbit anti-alpaca PAb was produced in the laboratory. The phagemid vector pComb3X was a gift from Dr. Carlos F. Barbas (The Scripps Research Institute, La Jolla, CA, U.S.A.). Electrocompetent E. coli ER2738 cells were acquired from Lucigen Corporation (Middleton, WI). M13KO7 helper phage and SfiI were purchased from New England Biolabs (Ipswich, MA).
Figure 1.
Structures of TBBPA and its haptens. Conjugate of T5 with thyroglobulin was used as the immunization antigen.
Selection of anti-TBBPA Phage-VHHs from VHH library
A four-year old castrated male alpaca was immunized subcutaneously with T5-thyroglobulin six times biweekly. The VHH phage display library was constructed with the blood lymphocytes collected after the sixth injection using the method described previously.30 Briefly, the total mRNA was transcribed to complementary DNA, and VHH fragments were amplified by polymerase chain reaction (PCR). VHH IgG variable domains were ligated into the plasmid pComb3X using restriction sites SfiI. Then the ligated material was electroporated into electrocompetent cells E. coli ER 2738.
One well of a microtiter plate was coated overnight with 100 μL of T5-BSA (10 μg mL–1) at 4 °C, and an additional four wells with 100 μL of 3% BSA in coating buffer. The plate was blocked with 3% skim milk in PBS (0.01 mol L–1 phosphate, 0.137 mol L–1 NaCl, 3 mmol L–1 KCl, pH 7.4) for 1 h at ambient temperature. A 100-μL aliquot of phage-display VHH library was added into the first well with 5% methanol (MeOH) and incubated for 2 h with gentle shaking at ambient temperature. After washing 10 times with PBST (0.05% Tween-20 in PBS), this well was eluted with 100 μL of TBBPA (1000 ng mL–1) in PBS containing 5% MeOH for 1 h at ambient temperature with shaking. The eluent was transferred in equal aliquots to the next four BSA-coated wells to remove VHH phage that binds nonspecifically. Then the eluent was collected for the determination of phage titer and phage amplification. The phage eluent was amplified with addition of the M13KO7 helper phage (1 × 1012 cfu mL–1) for the next round of panning, which was described by Barbas et al.37 The entire panning process was repeated three times, except the concentrations of coating antigen and TBBPA to elute the VHH phage were decreased gradually. The concentrations of T5-BSA for the second, third, and fourth panning were 5, 2.5, and 1 μg mL–1, respectively. Meanwhile, the concentrations of TBBPA were decreased to 200, 40, and 10 ng mL–1, respectively. After four rounds of panning, several phage clones were tested for their binding ability with TBBPA by a competitive phage ELISA,30 and the optimal one was selected for the remaining studies.
Similarly, the heterologous coating antigens T1-BSA and T3-BSA were separately coated to isolate VHHs from the VHH library.
Expression and Purification of VHH
The cloned plasmids pComb3X containing the anti-TBBPA VHHs were extracted from ER2738 and heat shock transformed to Top 10F′ cells. A 1-mL aliquot of overnight culture was diluted in 100 mL of Super Broth with 50 μg mL–1 carbenicillin and 2 mL of 1 M MgCl2. After shaking for 8 h, the culture was induced with 1 mM IPTG and incubated in a shaker at 37 °C overnight. The culture was centrifuged, and the cell pellet was lysed with B-PER lysis buffer (4 mL g–1 pellet) containing protease inhibitors at ambient temperature for 10 min. The cell lysate supernatants were collected by centrifugation at 13000 × g for 10 min, followed by purifying on a 1-mL Ni-NTA resin column. The column was equilibrated and washed with 40 mM imidazole (dissolved in 10 mM PBS, pH 7.4). The VHH was eluted with 150 mM imidazole, and the purified VHH was stored at −20 °C after dialysing with three buffer changes with PBS at 4 °C.
VHH Competitive ELISA
The optimal concentrations of coating antigens and VHH dilutions were selected by a checkerboard titration. A 100-μL solution of T3-BSA (2 μg mL–1) was coated on a 96-well microtiter plate at 4 °C overnight. The plate was blocked with 3% skim milk in PBS for 1 h at ambient temperature the next day. A series dilution of TBBPA (50 μL/well, 10% MeOH in PBS) was added, followed by the addition of 50 μL of VHH in PBST (0.02 μg μL–1). After incubation at room temperature for 1 h, the plate was washed five times with PBST, and then 100 μL of goat anti-HA tag IgG-HRP (diluted at 1:25000 with PBST) was added. After another incubation step and washing step, 100 μL of TMB solution (400 μL of 0.6% TMB and 100 μL of 1% H2O2 dilution in 25 mL citrate buffer, pH 5.5) was added into the plate, and the reaction was stopped 10 min later by the addition of 50 μL of 2 M H2SO4. The absorbance was read at 450 nm on a microtiter plate reader (Molecular Devices, Sunnyvale, CA). The IC50, an indicator of the assay sensitivity, and the LOD, set as the IC10, were obtained from a four-parameter logistic equation from SigmaPlot 10.0.
Different concentrations of MeOH or dimethyl sulfoxide (DMSO) (5, 10, 20, and 40%) in PBST were used to dilute the TBBPA to optimize the assay performance.
Characteristics of VHH
VHH was diluted with PBST at different pHs (4.0, 5.0, 6.0, 7.0, 8.0, 9.0, 10.0, and 11.0). Correspondingly, the TBBPA was dissolved in the PBST with pH ranging from 4.0 to 11.0. Then calibration curves of ELISA were generated at different pH values.
For the thermal stability study, VHH was incubated at 90 °C for 10, 20, 30, 60, and 90 min, followed by cooling to room temperature. The binding of the VHH to the coating antigen T3-BSA was subsequently evaluated by ELISA.
Cross-Reactivity
The selectivity of the VHH ELISA was evaluated by determining the cross-reactivity (CR) with several TBBPA derivatives and other analogues. For these studies CR was calculated as
Sample Preparation
Soil samples were collected from the plow layer (0–10 cm) at the campus of the University of California at Davis. The soils were dried and sieved through a 100-mesh screen and kept in a sealed container until use. Two grams of soil was weighed and spiked with 10, 100, and 1000 ng g–1 TBBPA. Ethyl acetate (15 mL) was added, and the sample was sonicated for 20 min. The extraction process was repeated twice, and the extracts were combined and centrifuged for 15 min at 3000 × g. The supernatant was evaporated and redissolved with 2 mL of MeOH. The blank fetal bovine serum (FBS) was bought from Gemini Bio Products (Calabasas, CA, U.S.A.). TBBPA was spiked into 1.5 mL of FBS with a final concentration of 10, 100, and 1000 ng mL–1. A solid phase extraction (SPE) HLB cartridge (6 mL, Oasis, Waters) was conditioned with ethyl acetate and MeOH, and equilibrated with H2O. After loading the supernatants, the cartridge was washed with 4 mL of H2O (2% formic acid and 5% MeOH) and eluted with 2 mL of MeOH followed by 2 mL of ethyl acetate. The samples were divided for instrumental and ELISA analysis.
The VHH ELISA was validated with LC–MS/MS (Waters/Micromass, Manchester, UK), which was carried out on a Supelco Discover C18 column (5 cm × 2.1 mm, 5 μm, Sigma). Water (solution A) and acetonitrile containing 0.1% (v/v) acetic acid (solution B) were used as mobile phase with a flow rate of 0.3 mL min–1. The volume of sample injection was 10 μL, running time was 5 min. The mass spectrometry was performed in a negative ESI mode, m/z 417 and 420 being the two daughter ions for quantitative tracing of the target.
Results and Discussion
Selection of anti-TBBPA Phage-Displayed VHHs
The phage display VHH library was panned in both homologous format with T5-BSA and heterologous format with T1-BSA or T3-BSA. Both the concentrations of coating antigens and TBBPA were gradually decreased in an attempt to capture high-affinity binders. For all the selected phage clones showing binding to the coating antigens T1-BSA, T3-BSA, and T5-BSA, the 50% inhibition values of TBBPA in the competitive phage ELISA varied in a range of 1.0–10 ng mL–1, <1.0 ng mL–1, and >10 ng mL–1, respectively. Based on the sensitivities, the strongest binders, clones T1–4, T3–15, and T5–10, were chosen from selections using templates T1-BSA, T3-BSA, and T5-BSA, respectively. Since the structure of T3 is similar to the immunizing hapten T5, all positive clones had better binding ability with T3-BSA and T5-BSA than with other coating antigens (T1-, T2-, T4-, and T6-BSA) (Table 1). T5–10 recognized most of the coating antigens except T4-BSA which lacks the bromines while T3–15 only recognized T3-BSA and T5-BSA (Table 1). The binding activity of T5–10 is similar to that of the PAb previously produced with immunogen T5-KLH.16 Hapten T6 with the fragment of TBBPA was only recognized by T5–10. Since T1–4 was isolated from T1-BSA, this clone showed moderate binding ability with both T1-BSA and T2-BSA that varied in linker length.
Table 1. Responses of Three Positive VHHs from Homologous and Heterologous Selections to Different Coating Antigens in the Absence or Presence of TBBPA; the Value Shown Is the Average of Three Replicates and the Standard Deviations.
| VHH |
||||||
|---|---|---|---|---|---|---|
| T1–4 |
T3–15 |
T5–10 |
||||
| coating antigens | A0a | IC50 (ng mL–1) | A0a | IC50 (ng mL–1) | A0a | IC50 (ng mL–1) |
| T1-BSA | 0.97 ± 0.02 | 1.79 ± 0.13 | 0.11 ± 0.01 | NDb | 1.01 ± 0.02 | 7.50 ± 0.12 |
| T2-BSA | 0.63 ± 0.02 | 1.83 ± 0.09 | 0.11 ± 0.01 | NDb | 0.60 ± 0.01 | 6.90 ± 0.62 |
| T3-BSA | 1.75 ± 0.01 | 2.02 ± 0.01 | 1.28 ± 0.03 | 0.41 ± 0.05 | 1.77 ± 0.01 | 6.13 ± 0.09 |
| T4-BSA | 0.15 ± 0.03 | NDb | 0.13 ± 0.01 | NDb | 0.20 ± 0.03 | NDb |
| T5-BSA | 1.21 ± 0.03 | 3.70 ± 0.42 | 0.78 ± 0.03 | 0.54 ± 0.08 | 1.70 ± 0.06 | 4.10 ± 0.01 |
| T6-BSA | 0.13 ± 0.02 | NDb | 0.11 ± 0.01 | NDb | 1.63 ± 0.03 | 6.39 ± 0.23 |
A0: The signal in the absence of TBBPA.
ND: Not detectable
The three VHHs showed different affinities to TBBPA, because the sensitivities of VHH ELISAs (IC50) varied in a range of 0.41–7.5 ng mL–1 (Table 1). Clone T5–10 from the homologous VHH panning system showed less sensitivity than clones T1–4 and T3–15 from the heterologous VHH panning system. It is possible that VHH T5–10 has higher binding ability to the linker of coating antigens than VHH T1–4 and VHH T3–15 and this nonspecific binding is difficult to inhibit by free TBBPA. VHH T5–10 showed better selectivity for TBBPA in the homologous assay with T5-BSA than in the heterologous assays with T1-, T2-, T3-, and T6-BSA (Table 1), probably more nonspecific binding presented in the heterologous format than in the homologous one. VHH T1–4 may have less affinity to both coating antigen T1-BSA and TBBPA because analogues T1 and T5 were different in spatial configuration and linker length. Hapten T3 only being two carbons shorter in the linker than hapten T5 would allow VHHs to recognize TBBPA well with negligible recognition of the linker, and the most sensitivity was observed in the combination of VHH T3–15 and T3-BSA (Table 1). Consequently, the most sensitive assays were primarily determined by the heterologous antigen selection, rather than coating antigens competitor optimization. Therefore, T3-BSA was used as the coating antigen in both panning and testing systems for the remaining studies.
The titer of the input phage with T3-BSA selection in each cycle was 1013 cfu mL–1, while the titer of output phage gradually increased from 9.6 × 107 to 1.3 × 109 cfu mL–1 (Table S-1 in Supporting Information [SI]), showing an enrichment of the specific phages. After the fourth round of panning, 16 clones were selected, and all showed inhibition by TBBPA in the phage ELISA. The DNA was isolated, and sequencing revealed five groups of amino acid sequences. The VHH diversity38 partly resulted from the extension of CDR1 and CDR3, antigen binding loops, frequency of mutation, and the paratope diversity.24 Although there are only 12 amino acids or fewer in the CDR3 of T1–4, T3–15, and T5–10 in our study (Figure S-1 in SI) compared to the average 16 amino acid length observed in llama VHH,39 the VHH showed a complex repertoire and high inhibition by TBBPA. Showing the highest sensitivity in the positive phage ELISAs, the phagemid vector containing VHH T3–15 was transferred to top 10 F′ cells, and the expressed VHH was purified with a Ni-NTA affinity column. The size and purity of VHH T3–15 were verified on a 15% SDS-PAGE gel with one major band at MW 17 kD.
VHH Competitive ELISA for TBBPA
TBBPA is a highly lipophilic (log Kow = 4.5) compound and a water-miscible cosolvent is necessary in the assay buffer for analysis. MeOH and DMSO were reported to be efficient cosolvents for analyte–antibody reactions40 and the effects of these solvents on the VHH T3–15 assay performance were studied (Figure S-2 in SI). The increase of MeOH concentration from 5% to 40% made the IC50 vary between 0.42 and 1.33 ng mL–1 (Figure S-2A in SI) and the maximal signal A0 declined from 0.94 to 0.76. The most sensitive assay (IC50 = 0.42 ng mL–1) was observed at 10% MeOH with a reasonable A0 (0.9) and the least sensitive (IC50 = 1.33 ng mL–1) was at 40% MeOH, with a lower A0 (0.76). These data suggest that a high concentration of MeOH may interfere with the VHH binding with both the coating antigen and the target. The A0 of the assay declined from 1.22 to 1.07 with the increasing of DMSO. The sensitivities in 5–40% of DMSO were in a range of 1.66–2.41 ng mL–1 (Figure S-2B in SI), lower than those in MeOH. Thus, 10% MeOH, not only compatible with the VHH affinity but also showing efficient solubility of TBBPA, was chosen to optimize competitive VHH ELISAs.
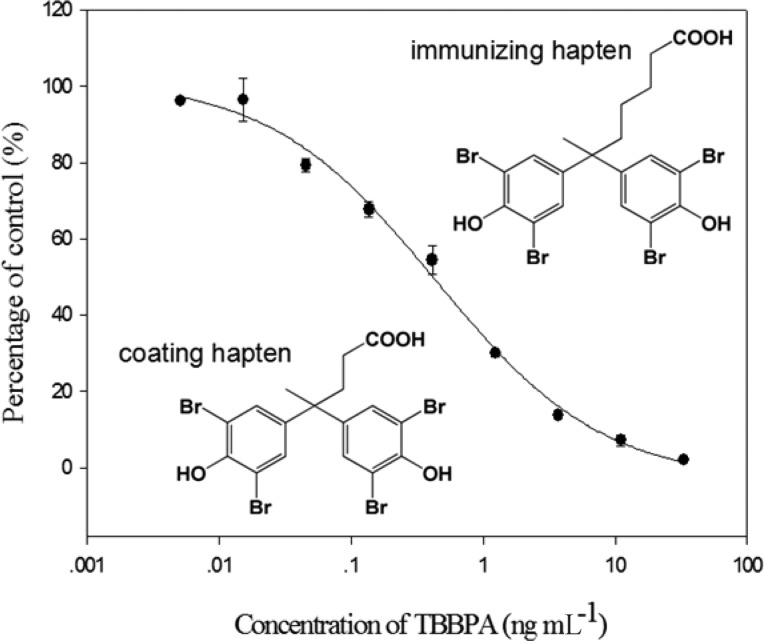
The optimal concentrations of coating antigen T3-BSA and VHH T3–15 were determined by a checkerboard titration. Figure 2 is a typical calibration curve of competitive VHH ELISA for TBBPA under optimized conditions (10% MeOH, pH 7.4). The assay has a linear range (IC20–IC80) of 0.06–2.53 ng mL–1, with an IC50 value of 0.40 ng mL–1 and an IC10 value of 0.02 ng mL–1. Compared to the heterologous competitive ELISA with alpaca antiserum (IC50 = 500 ng mL–1), the sensitivity was increased around 1000-fold in the VHH ELISA. In contrast with ELISAs based on PAbs16 and mAbs,18 the sensitivities were increased approximately 2-fold and 9-fold, respectively. Instead of using the solubilized VHH in the ELISA, displaying the VHH on phage particles showed a similar sensitivity (IC50 = 0.46 ng mL–1) to VHH ELISA, but a narrower linear range (0.19–1.07 ng mL–1) than the latter.
Figure 2.
Calibration curve of competitive VHH ELISA for TBBPA. Each value is the average of three replicates and the standard deviations. The day to day variability of the IC50 was 5%.
Cross-Reactivity
The specificity of the VHH T3–15 ELISA was evaluated by comparing the IC50 of TBBPA with those of several TBBPA derivatives, including 2,2′,6,6′-tetrabromobisphenol A diallyl ether (TBBPA-bAE) and tetrabromobisphenol A bis(2-hydroxyethyl) ether (TBBPA-bOHEE)s or other commonly used BFRs: hexabromocyclododecane (HBCD), PBDEs (BDE-47, BDE-99, BDE-100, BDE-153, BDE-154, and BDE-183), hydroxylated BDE-47 metabolites (5-OH-BDE-47 and 6-OH-BDE-47), 1,2-bis(pentabromodiphenyl) ethane (DBDPE), and bisphenol A (BPA) (Figure S-3 in SI). As shown in Table 2, the VHH assay is very selective for TBBPA because low cross-reactivities with the other tested compounds were observed (<0.1%). The high selectivity of the assay is useful to detect TBBPA which always coexists with its nonbrominated metabolite BPA as well as HBCDs and PBDEs in environmental matrices.10,13,14
Table 2. Cross-Reactivity of VHH T3-15 with TBBPA Structural Analogues.
| TBBPA analogues | cross-reactivity (%) |
|---|---|
| TBBPA | 100 |
| TBBPA-bAE | <0.1 |
| TBBPA-bOHEE | <0.1 |
| DBDPE | <0.1 |
| HBCD | <0.1 |
| BDE-47 | <0.1 |
| BDE-99 | <0.1 |
| BDE-100 | <0.1 |
| BDE-153 | <0.1 |
| BDE-154 | <0.1 |
| BDE-183 | <0.1 |
| 5-OH-BDE 47 | <0.1 |
| 6-OH-BDE 47 | <0.1 |
| BPA | <0.1 |
VHH Characterization
It is reported that thermal stability is one of the most extraordinary features of VHH.41 In this study, the VHH–antigen binding signal retained about 80% and 20% of the unheated control signal after heating at 90 °C for 10 and 90 min, respectively (Figure S-4A in SI). It was reported that conventional mAbs lost all binding activities within 5 min at 100 °C.41 The thermal stability of VHH could be explained by its extreme plasticity allowing refolding from the denatured state.42 Another reason for this property is cysteines forming in this case a disulfide bond, thus increasing thermal and conformational stabilities.43 The thermal stability of VHH makes it preferable to conventional antibodies for on-site analysis.
The effect of the assay buffer pH on the VHH ELISA was evaluated (Figure S-4B in SI). The VHH ELISA was more susceptible to low pH (≤6.0) than to high pH (7.4–11.0), because low A0 values were shown at pH 4.0 and 5.0 (0.2 and 0.3, respectively) and a very high IC50 (8.2 ng mL–1) was shown at pH 6.0, which may partly cause the denaturing of VHH. The protonated state of the protein at low pH might contribute to protein unfolding, or change surface charge or the antibody paratope. The isoelectric point (pI) of VHH T3–15 was estimated to be 9.56 according to the protein sequence and ExPASy (http://web.expasy.org/compute_pi/). When the pH of assay buffer was close to the pI of VHH T3–15, some antibodies may have precipitated; thus, the A0 declined somewhat at pH 7.4–11.0 but the IC50 varied in a narrow range of 0.48–0.98 ng mL–1. The optimal pH of assay buffer was 7.4, but this assay was able to be performed at pH ranging from 8.0 to 11.0, with little sensitivity loss.
Assay Validation
Matrix effect, inevitable in sample analysis, may cause some false positive results in the format used and can be eliminated with the dilution of the extract. However, dilution generally leads to a loss of assay sensitivity. For soil and FBS extracts, a 100-fold dilution and a 10-fold dilution with PBS were needed to minimize the matrix effect, respectively.
Extraction of TBBPA from soil by sonication was a simple, effective and time-saving method.6 MeOH, dichloromethane (DCM), ethyl acetate, and DCM/acetone (1:1, v/v) were initially tested. Ethyl acetate was proven to be an ideal extraction solvent for TBBPA from soil due to the acceptable recovery (Table 3). A reversed-phase solid phase extraction cartridge was used to remove the hydrophilic contaminants in FBS and extract the TBBPA from FBS with reasonable recoveries (Table 3).
Table 3. Recoveries of TBBPA from Spiked Samples by the VHH ELISA and the LC–MS/MS; Each Assay Was Carried out Three Times on the Same Day.
| average recovery (%) ± CV(%) (n = 3) |
|||
|---|---|---|---|
| sample | spiked TBBPA (ng g–1/ng mL–1) | ELISA | LC–MS/MS |
| soil | 10 | 102.0 ± 5.2 | 95.4 ± 0.3 |
| 100 | 103.2 ± 3.5 | 96.6 ± 6.7 | |
| 1000 | 110.7 ± 4.8 | 92.6 ± 10.6 | |
| fetal bovine serum (FBS) | 10 | 90.3 ± 6.8 | 90.8 ± 8.2 |
| 100 | 93.3 ± 5.6 | 92.5 ± 6.0 | |
| 1000 | 95.6 ± 9.4 | 92.3 ± 3.4 | |
The VHH ELISA for the spiked samples was validated by comparing the results of ELISA with those of an instrumental method LC–MS/MS. The recoveries of TBBPA from soil and FBS determined by VHH ELISA were in a range of 102.0–110.7% and 90.3–95.6%, respectively, and by LC–MS/MS in a range of 92.6–96.6% and 90.8–92.5%, respectively (Table 3). Recoveries in a range of 75–125% are usually acceptable for spiked samples.44 Both methods showed good recoveries and correlated well with each other (Figure S-5 in SI). The VHH ELISA was demonstrated to be a valid method to detect TBBPA in soil and serum.
Conclusion
This study presents a novel heterologous antigen selection procedure for VHHs against TBBPA and the resulting highly sensitive and selective VHH-based immunoassay for TBBPA. Concern over TBBPA in the environmental community is increasing rapidly due to its high-volume use, high levels of human exposure, and resistance to both environmental degradation and metabolism in animals. Thus, high throughput laboratory assays and rugged field portable assays are needed. VHHs assays are increasing in popularity as the basis of analytical procedures for proteins. The monoclonal character, low cost, high selectivity and sensitivity, and recombinant nature of VHH make them very attractive tools. However, very few VHH-based assays have been developed for small-molecule analysis.
Using a heterologous coating antigen was in this case to be more efficient in the selection of a sensitive and selective VHH than using a homologous antigen. In general in small-molecule analysis heterologous assays prove to be more sensitive, but the monoclonal character of VHH-based assays raised the question if heterology was important for VHH assays. The most sensitive VHH ELISA was based on the clone T3–15 selected from T3-BSA, with an IC50 of 0.4 ng mL–1. This assay was highly selective for TBBPA with negligible cross-reactivities to TBBPA derivatives and other BFR compounds, which is suitable for determining TBBPA even at lower levels in complicated environmental samples. The VHH-based ELISA for TBBPA spiked in soil and FBS samples showed good correlation with LC–MS/MS therefore can be used as a supplemental or alternative method for chemical detection. Taking advantage of its small size, solubility, thermal stability, pH tolerance, and ease of production, the VHH would be a useful tool to monitor TBBPA in the abiotic and biotic samples.
Acknowledgments
This work was supported in part by the National Institute of Environmental Health Sciences Superfund Research Program, P42ES04699, the National Institute of Occupational Safety and Health, 2U50OH007550, and the Ph.D. Programs Foundation of Ministry of Education of China, 20130008110019.
Supporting Information Available
Additional information as noted in text. This material is available free of charge via the Internet at http://pubs.acs.org.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the funding agencies.
The authors declare no competing financial interest.
Funding Statement
National Institutes of Health, United States
Supplementary Material
References
- BSEF. TBBPA factsheet: http://www.bsef.com/our-substances/tbbpa/about-tbbpa. 2012.
- Tada Y.; Fujitani T.; Yano N.; Takahashi H.; Yuzawa K.; Ando H.; Kubo Y.; Nagasawa A.; Ogata A.; Kamimura H. Food Chem. Toxicol. 2006, 44, 1408–1413. [DOI] [PubMed] [Google Scholar]
- Kitamura S.; Jinno N.; Ohta S.; Kuroki H.; Fujimoto N. Biochem. Biophys. Res. Commun. 2002, 293, 554–559. [DOI] [PubMed] [Google Scholar]
- Gosavi R. A.; Knudsen G. A.; Birnbaum L. S.; Pedersen L. C. Environ. Health Persp. 2013, 121, 1194–1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang G.; Ying G.; Liang Y.; Zhao J.; Yang B.; Liu S.; Liu Y. Comp. Biochem. Physiol. 2013, 157, 344–351. [DOI] [PubMed] [Google Scholar]
- Sánchez-Brunete C.; Miguel E.; Tadeo J. L. J. Chromatogr. A 2009, 1216, 5497–5503. [DOI] [PubMed] [Google Scholar]
- Zhang X.; Luo X.; Chen S.; Wu J.; Mai B. Environ. Pollut. 2009, 157, 1917–1923. [DOI] [PubMed] [Google Scholar]
- Sellstrom U.; Jansson B. Chemosphere 1995, 31, 3085–3092. [Google Scholar]
- Guerra P.; Eljarrat E.; Barcelo D. Anal. Bioanal. Chem. 2010, 397, 2817–2824. [DOI] [PubMed] [Google Scholar]
- Gorga M.; Martinez E.; Ginebreda A.; Eljarrat E.; Barcelo D. Sci. Total Environ. 2013, 444, 51–59. [DOI] [PubMed] [Google Scholar]
- Takigami H.; Suzuki G.; Hirai Y.; Sakai S. Chemosphere 2009, 76, 270–277. [DOI] [PubMed] [Google Scholar]
- Shi Z.; Jiao Y.; Hu Y.; Sun Z.; Zhou X.; Feng J.; Li J.; Wu Y. Sci. Total Environ. 2013, 452, 10–18. [DOI] [PubMed] [Google Scholar]
- Carignan C. C.; Abdallah M. A. E.; Wu N.; Heiger-Bernays W.; McClean M. D.; Harrad S.; Webster T. F. Environ. Sci. Technol. 2012, 46, 12146–12153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakobsson K.; Thuresson K.; Rylander L.; Sjodin A.; Hagmar L.; Bergman A. Chemosphere 2002, 46, 709–716. [DOI] [PubMed] [Google Scholar]
- Morris S.; Allchin C. R.; Zegers B. N.; Haftka J. J. H.; Boon J. P.; Belpaire C.; Leonards P. E. G.; Van Leeuwen S. P. J.; De Boer J. Environ. Sci. Technol. 2004, 38, 5497–5504. [DOI] [PubMed] [Google Scholar]
- Xu T.; Wang J.; Liu S. Z.; Lu C.; Shelver W. L.; Li Q. X.; Li J. Anal. Chem. Acta 2012, 751, 119–127. [DOI] [PubMed] [Google Scholar]
- Bu D.; Zhuang H.; Zhou X.; Yang G. Talanta 2014, 120, 40–46. [DOI] [PubMed] [Google Scholar]
- Xu C.; Ou J.; Cui Y.; Wang L.; Lv C.; Liu K.; Wang B.; Xu T.; Li Q. X.; Liu S. Monoclonal Antibodies Immunodiagn. Immunother. 2013, 32, 113–118. [DOI] [PubMed] [Google Scholar]
- Hamerscasterman C.; Atarhouch T.; Muyldermans S.; Robinson G.; Hamers C.; Songa E. B.; Bendahman N.; Hamers R. Nature 1993, 363, 446–448. [DOI] [PubMed] [Google Scholar]
- Muyldermans S. J. Biotechnol. 2001, 74, 277–302. [DOI] [PubMed] [Google Scholar]
- De Groeve K.; Deschacht N.; De Koninck C.; Caveliers V.; Lahoutte T.; Devoogdt N.; Muyldermans S.; De Baetselier P.; Raes G. J. Nucl. Med. 2010, 51, 782–789. [DOI] [PubMed] [Google Scholar]
- Chiu N. H. L.; Christopoulos K. T.. Advances in Immunoassay Technology; InTech: Croatia, 2012. [Google Scholar]
- Harmsen M. M.; De Haard H. J. Appl. Microbiol. Biotechnol. 2007, 77, 13–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muyldermans S.; Baral T. N.; Retarnozzo V. C.; De Baetselier P.; De Genst E.; Kinne J.; Leonhardt H.; Magez S.; Nguyen V. K.; Revets H.; Rothbauer U.; Stijemans B.; Tillib S.; Wernery U.; Wyns L.; Hassanzadeh-Ghassabeh G.; Saerens D. Vet. Immunol. Immunopathol. 2009, 128, 178–183. [DOI] [PubMed] [Google Scholar]
- Pleschberger M.; Saerens D.; Weigert S.; Sleytr U. B.; Muyldermans S.; Sara M.; Egelseer E. M. Bioconjugate. Chem. 2004, 15, 664–671. [DOI] [PubMed] [Google Scholar]
- Wesolowski J.; Alzogaray V.; Reyelt J.; Unger M.; Juarez K.; Urrutia M.; Cauerhff A.; Danquah W.; Rissiek B.; Scheuplein F.; Schwarz N.; Adriouch S.; Boyer O.; Seman M.; Licea A.; Serreze D. V.; Goldbaum F. A.; Haag F.; Koch-Nolte F. Med. Microbiol. Immun. 2009, 198, 157–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Transue T. R.; De Genst E.; Ghahroudi M. A.; Wyns L.; Muyldermans S. Proteins 1998, 32, 515–522. [DOI] [PubMed] [Google Scholar]
- Makvandi-Nejad S.; Fjaellman T.; Arbabi-Ghahroudi M.; MacKenzie C. R.; Hall J. C. J. Immunol. Methods. 2011, 373, 8–18. [DOI] [PubMed] [Google Scholar]
- Tabares-da Rosa S.; Rossotti M.; Carleiza C.; Carrion F.; Pritsch O.; Ahn K. C.; Last J. A.; Hammock B. D.; Gonzalez-Sapienza G. Anal. Chem. 2011, 83, 7213–7220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H. J.; McCoy M. R.; Majkova Z.; Dechant J. E.; Gee S. J.; Tabares-da Rosa S.; Gonzalez-Sapienza G. G.; Hammock B. D. Anal. Chem. 2012, 84, 1165–1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clackson T.; Hoogenboom H. R.; Griffiths A. D.; Winter G. Nature 1991, 352, 624–628. [DOI] [PubMed] [Google Scholar]
- Goodrow M. H.; Hammock B. D. Anal. Chem. Acta 1998, 376, 83–91. [Google Scholar]
- Zhang Q.; Wang L.; Ahn K. C.; Sun Q.; Hu B.; Wang J.; Liu F. Anal. Chem. Acta 2007, 596, 303–311. [DOI] [PubMed] [Google Scholar]
- Guo Y.; Sanders M.; Galvita A.; Heyerick A.; Deforce D.; Bracke M.; Eremin S.; Saeger S. D. World Mycotoxin J. 2014, 10.3920/WMJ2013.1668. [DOI] [Google Scholar]
- Muldoon M. T.; Holtzapple C. K.; Deshpande S. S.; Beier R. C.; Stanker L. H. J. Agric. Food Chem. 2000, 48, 537–544. [DOI] [PubMed] [Google Scholar]
- Wang C.; Li X.; Liu Y.; Guo Y.; Xie R.; Gui W.; Zhu G. J. Agric. Food Chem. 2010, 58, 5658–5663. [DOI] [PubMed] [Google Scholar]
- Barbas C. F.; Burton D. R.; Scott J. K.; Silverman G. J.. Phage Display: A Laboratory Manual; Cold Spring Harbor: New York, 2001. [Google Scholar]
- Ghassabeh G. H.; Muyldermans S.; Saerens D. In Current Trends in Monoclonal Antibody Development and Manufacturing; Shire S. J., Gombotz W., Bechtold-Peters K., Andya J., Eds.; Springer: New York, 2010; Vol. 11, pp 29–48. [Google Scholar]
- Vu K. B.; Ghahroudi M. A.; Wyns L.; Muyldermans S. Mol. Immunol. 1997, 34, 1121–1131. [DOI] [PubMed] [Google Scholar]
- Ahn K. C.; Gee S. J.; Tsai H.-J.; Bennett D.; Nishioka M. G.; Blum A.; Fishman E.; Hammock B. D. Environ. Sci. Technol. 2009, 43, 7784–7790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman E. R.; Anderson G. P.; Liu J. L.; Delehanty J. B.; Sherwood L. J.; Osborn L. E.; Cummins L. B.; Hayhurst A. Anal. Chem. 2006, 78, 8245–8255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ewert S.; Cambillau C.; Conrath K.; Pluckthun A. Biochemistry 2002, 41, 3628–3636. [DOI] [PubMed] [Google Scholar]
- Saerens D.; Conrath K.; Govaert J.; Muyldermans S. J. Mol. Biol. 2008, 377, 478–488. [DOI] [PubMed] [Google Scholar]
- Gustavo Gonzalez A.; Angeles Herrador M.; Asuero A. G. Talanta 1999, 48, 729–736. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.