Background: Cytokines are important inflammatory mediators that can affect cardiac function.
Results: Treatment with IL-1β but not TNFα reduced the density of L-type Ca2+ current.
Conclusion: Mechanisms underlying IL-1β effects implicate oxidative stress and PKCϵ signaling to alter L-type Ca2+ current.
Significance: Elucidating the cytokine signaling pathways are important in understanding their role in heart disease.
Keywords: Calcium Channel, Cardiovascular Disease, Heart Failure, Inflammation, Interleukin 1 (IL-1), Patch Clamp Electrophysiology, Protein Kinase C (PKC), Reactive Oxygen Species (ROS), Tumor Necrosis Factor (TNF), Interleukin-1β
Abstract
Inflammation is now widely recognized as a key component of heart disease. Patients suffering from arrhythmias and heart failure have increased levels of tumor necrosis factor-α (TNFα) and interleukin-1β (IL-1β). Evidence suggests that these cytokines are important mediators of cardiac remodeling; however, their effects on ion channels and arrhythmogenesis remain incompletely understood. The L-type Ca2+ current (ICaL) is a major determinant of the plateau phase of cardiac action potential and has a critical excitation-contraction coupling role. Thus, altering its properties could have detrimental effects on cardiac electrical and contractile functions. Accordingly, the objective of this study was to elucidate the effect of TNFα and IL-1β on ICaL, while exploring the underlying regulatory mechanisms. Neonatal mouse ventricular myocytes were treated with a pathophysiological concentration (30 pg/ml) of TNFα and IL-1β for 24 h. Voltage-clamp recordings showed that TNFα had no effect on ICaL, whereas IL-1β decreased the current density by 36%. Although both IL-1β- and TNFα-treated myocytes showed significant increase in reactive oxidative species (ROS), Western blot experiments revealed that only IL-1β increased PKCϵ membrane translocation. The antioxidant N-acetyl-l-cysteine normalized ROS levels and restored ICaL density. Furthermore, the PKCϵ translocation inhibitor ϵ-V1-2 blocked the effect of IL-1β on ICaL. The reduction of ICaL by IL-1β was also seen in cultured adult ventricular myocytes. Overall, chronic IL-1β treatment decreased ICaL density in cardiomyocytes. These effects implicated ROS signaling and PKCϵ activation. These findings could contribute to explain the role of IL-1β in the development of arrhythmia and heart failure.
Introduction
Pro-inflammatory cytokines are immune system modulators that are secreted in response to an insult and even though on the short term they play a crucial role in the healing process, the prolonged secretion of pro-inflammatory cytokines has many deleterious effects that can lead to the worsening of pathologies by affecting tissue homeostasis (1). In patients suffering from heart failure and arrhythmias, several reports have shown that levels of the pro-inflammatory cytokines tumor necrosis factor-α (TNFα) and interleukin-1β (IL-1β) are elevated in both tissue and plasma (2–5). The fact that cytokine levels were altered in serum from patients with severe heart failure, arrhythmias, or myocardial infarction (2, 6) implied they can play an important role in the pathogenesis of heart disease (7, 8). Accordingly, in an attempt to understand the contribution of these cytokines to the disease state several experimental studies have been conducted. Early reports have shown that TNFα and/or IL-1β can inhibit the β-adrenergic response and depress cardiac contractility (9–12). Furthermore, these cytokines have also been associated with hypertrophic signaling, generation of reactive oxidative species (ROS),4 fibrosis, and other detrimental cardiac effects (13, 14). Nonetheless, to date, the effects of these cytokines on ion channels and electrical properties of the heart and how they contribute to rhythm disturbances is a topic that remains incompletely understood. In a few studies examining the role of TNFα on K+ currents, it was concluded that TNFα reduced the current density and expression of the Ca2+-independent transient outward K+ current (Ito), K+ channel-interacting protein-2 (KChIP-2), and ultrarapid delayed rectifier K+ current (IKur) (15–17). However, most of these studies used a concentration of TNFα in the “ng/ml” range, i.e. 10, 30, or even 1000-fold higher than the reported concentration in patients with heart disease, which is typically in the “pg/ml” range (5, 18, 19). In fact, the use of elevated concentrations of cytokines, varying treatment times, and experimental conditions might be an explanation to why the literature has often yielded contradictory results in the study of the cytokine-dependent regulation of contraction, Ca2+ dynamics, or intracellular signaling or ionic current regulation (7).
Previously, we have reported that a 6-week treatment protocol in mice with recombinant TNFα resulted in a serum concentration of 27.4 ± 5.0 pg/ml (20). We believe that the combination of longer treatment times with pathophysiologically relevant concentrations of cytokines might provide a better means for understanding the effects of pro-inflammatory cytokines compared with an acute cytokine exposure at elevated concentrations. Indeed, the voltage-clamp recordings in isolated cardiomyocyte from TNFα-treated mice demonstrated that Ito and IKur were reduced, however, in contrast to other reports, no change in mRNA or protein expression of the underlying ion channels subunits was noted. Other cytokines such as IL-1β are also important players in the pathogenesis of cardiovascular disease (21). Among the several reported effects, IL-1β was shown to induce vascular resistance and favor atherosclerotic plaque formation (22). Nonetheless, it remains unknown whether and how longer exposure at pathophysiological levels of IL-1β affect cardiomyocyte function and ionic currents in the heart.
Accordingly, in the present study we investigated whether a 24–32-h treatment with 30 pg/ml of IL-1β and TNFα affected the L-type Ca2+ current (ICaL) while exploring their underlying regulatory mechanisms in neonatal mouse ventricular myocytes. Because ICaL is a major determinant of the plateau phase of the action potential and plays a central role in excitation-contraction coupling (23), modifying its properties by cytokines could prove to be detrimental to cardiomyocyte function and warrants further study.
EXPERIMENTAL PROCEDURES
Experimental Animals
Pregnant CD1 mice were purchased from Charles River (St. Constant, Quebec, Canada) and after delivery, neonatal pups aged 1 to 2 days old were used for the experimental work described. All animal protocols were conducted in accordance with the Canadian Council Animal Care guidelines and conformed to the Guide for the Care and Use of Laboratory Animals published by the United States National Institutes of Health (NIH Publication No. 85-23, revised 1996). Experiments were also approved by the Montreal Heart Institute Animal Care Committee (approval reference number 2012-80-01).
Neonatal Mouse Ventricular Myocytes Isolation
Ventricular myocytes were isolated from neonatal mouse heart as previously described (24). Briefly, the pups were decapitated and hearts were rapidly excised and put in cold 95% O2, 5% CO2 bubbled suspension minimal essential medium (SMEM) solution (Invitrogen) containing, 1 mm dl-carnitine. The atria were discarded and the ventricles transferred into the enzymatic solution containing the previous solution supplemented with 1% bovine serine albumin (BSA), 20 mm taurine, and 0.25 mg/ml of collagenase (Yakult, Tokyo, Japan). The ventricles were digested for 45 min during which the enzymatic solution was collected and replaced by a fresh solution every 5 min. The collected solution was added to an inactivating solution containing medium 199 (M199) supplemented with 30% fetal bovine serum (FBS), 1.5% insulin (100 units/ml), and 1% penicillin G/streptomycin (PenG/Strep, 10,000 units/ml, Invitrogen). The inactivating solution was then centrifuged for 5 min at 100 × g and the cells were resuspended in M199 media containing 10% FBS, 1.5% insulin (Novolin, 100 units/ml), and 1% PenG/Strep (10000 units/ml) and preplated for 25 min to remove fibroblasts and other non-cardiomyocyte cells. The myocytes were subsequently plated on coverslips in Petri dishes and were incubated with appropriate drugs and reagents in a water-jacketed 37 °C (5% CO2) incubator for 24–32 h before any experimentation. Each preparation utilized the hearts from 15 to 20 mice.
Adult Mouse Ventricular Myocytes Isolation
Single rod-shaped adult mouse ventricular cardiomyocytes were isolated by enzymatic dispersion on a modified Langendorff apparatus using an adaptation of previously published protocols (20, 25). Briefly, after cannulation, the hearts were rapidly perfused with the following solution A for 5 min at 3 ml/min (in mm): 130 NaCl, 15 KCl, 0.6 KH2PO4, 0.6 Na2HPO4, 1.2 MgSO4-7H2O, 10 HEPES, 4.6 NaHCO3, 30 taurine, 5.5 glucose, 0.005 blebbistatin. Next, the digestion buffer consisting of 50 ml of solution A supplemented with 120 mg of collagenase type II (290 units/ml, Worthington, Lakewood, NJ) was used for enzymatic dispersion. pH was adjusted to 7.4 with NaOH. After 7–9 min of digestion, a stopping buffer made from solution A supplemented with 10% FBS and 12.5 μm Ca2+ was used. The ventricles were cut, minced, and triturated to yield individual rod-shaped myocytes. After Ca2+ readaptation, myocytes were centrifuged and resuspended in M199 supplemented with 1% FBS, 1% insulin-transferrin-selenium, 1% PenG/Strep, 2 mm GlutaMAX, 1 mm Na+-pyruvate and plated on laminin-coated coverslips. All media and cell culture reagents were purchased from Invitrogen. Myocytes were split into controls and IL-1β-treated (1 ng/ml) groups and placed 12–16 h in a 5% CO2 incubator at 37 °C before experimentation.
Voltage-clamp Recordings
Whole-cell voltage-clamp techniques were used to record ICaL from cardiomyocytes using a patch clamp amplifier (Axopatch 200B, Molecular Devices, Sunnyvale, CA) and by adapting protocols from a previous study (26). Pipettes were pulled from borosilicate glass (World Precision Instruments, Sarasota, FL) and had resistance of 2–4 MΩ when filled with the following internal solution (in mm): 100 aspartic acid, 70 CsOH, 40 CsCl, 2 MgCl2, 4 MgATP, 10 EGTA, 10 HEPES (pH adjusted to 7.2 with CsOH). The bath was perfused at 36 ± 1 °C with the external solution (in mm): 145 TEA-Cl, 10 CsCl, 2 CaCl2, 0.5 MgCl2, 5 HEPES, 5.5 glucose (pH adjusted to 7.4 with CsOH). Neonatal ventricular myocytes were held at −50 mV and ICaL was obtained by imposing a series of voltage steps from −50 to +60 mV, each lasting 250 ms at a frequency of 0.1 Hz. Voltage-clamp recordings were low-pass filtered at 1 kHz (4-pole Bessel), digitized at 4–10 kHz, and stored using pCLAMP 10.2 software (Molecular Devices). The data were corrected for a −10 mV liquid junction potential. To account for differences in current amplitudes between different cells all currents were normalized to cell capacitance and reported in pA/pF. For all neonatal cardiomyocytes preparations, experiments were performed in the 24–32 h range after treatment with 30 pg/ml of mouse recombinant IL-1β and TNFα (Invitrogen). We used clinically relevant concentrations of cytokines that were observed in heart failure patients, in HIV and TNFα mouse models we previously studied (5, 18, 27, 28). In some experiments, the nonspecific antioxidant N-acetyl-l-cysteine (NAC, Sigma) was added to cell culture media at 100 μm for the entire duration of the cytokine treatments. The protein kinase C (PKC) activator phorbol 12-myristate 13-acetate (PMA, 100 nm, Sigma) was added to cell culture media 10 min before experimentation. The cell-permeable myristoylated PKCϵ peptide inhibitor (ϵ-V1-2, 10 μm) with amino acid sequence EAVSLKPT (AnaSpec) was incubated along with cytokine treatment (29, 30). Recordings from cells with different treatments were all obtained on the same day. A minimum of 3 different cell preparations was required for each I-V curve. Cell culture offers several benefits, however, there are some disadvantages such as inherent variability in primary cultures. From that perspective, the density of ICaL can fluctuate between experiments. To control for this variability, new control data were obtained on every experimental day, which means that the observed cytokine effects on ICaL density was always within the same percent (36–38%) range regardless of the actual control values. This highlights the importance of always having controls under matching conditions while maintaining cautiousness in choice and quality of reagents and media supplements (FBS, cytokines etc.).
Quantitative Polymerase Chain Reaction (qPCR)
The quantitative reverse transcriptase PCR technique was used to study mRNA expression of the α subunit of ICaL, CaV1.2, as described previously (24, 26). Briefly, total RNA isolated from ventricular myocytes cultured for 24 h was made using the RNeasy Fibrous Tissue kit (Qiagen) including treatment with DNase I to prevent contamination by genomic DNA. cDNA was then synthesized with first strand SuperScriptIII (Invitrogen) and primers specific for CaV1.2 and 18 S. The qPCR was performed using the Platinum SYBR Green qPCR Supermix (Invitrogen) in a real-time PCR system (MX3005P QPCR system, Stratagene). The specific CaV1.2 primers were used at a concentration of 300 nm and mRNA expression was normalized to 18 S mRNA.
Reactive Oxidative Species Assay
The ROS assay was performed using the fluorescent 5-(and-6)-chloromethyl-2′,7′-dichlorodihydrofluorescein diacetate, acetyl ester (CM-H2DCFDA) and the Hoechst 33342-based NucBlue nuclear stain (Molecular Probes) and used according to the manufacturer's instructions. Cardiomyocytes were plated in equal numbers into 6-well plates with the different treatments for a minimum of 24 h. Cells were then incubated with an M199 master mix containing the ROS probe and NucBlue nuclear stain following the manufacturer's protocol. After the incubation time, wells were washed 3 times and refilled with M199 (Invitrogen) (no phenol red, at 37 °C, pH 7.3), containing insulin and appropriate drug treatments. The plate was then immediately loaded into the Synergy 2 plate reader (Biotek) with the temperature set to 37 °C. The ROS probe was excited first at 485 ± 20 nm and emission was read at 528 ± 20 nm. Subsequently, the nuclear stain probe was excited at 360 ± 40 nm with emission read at 460 ± 40 nm. Fluorescence reading sensitivity was calibrated to the wells containing antioxidants, where the least signal is expected. The ROS fluorescent signal obtained from each well was normalized to the nuclear probe signal to account for differences in signal intensity that may arise from cell clustering, proliferation, or apoptosis during the long-term cytokine treatment.
Western Blots
The protocols used to isolate the enriched cytosolic and total membrane protein fractions (particulate fraction) as well as the Western blot analysis were previously described (29, 31). Briefly, cells were washed with ice-cold extraction buffer containing a mixture of protease and phosphatase inhibitors (29) before being snap frozen and stored at −80 °C for subsequent use. Cells were scraped from the Petri dishes on ice and thoroughly homogenized in the extraction buffer using 28.5-gauge syringes (2 preparations yielded n = 1). Homogenates were then centrifuged for 30 min at 48,000 × g. The supernatant corresponding to the cytosolic fraction was collected and stored at −80 °C. Pellets that contained total membrane proteins were resuspended in extraction buffer containing 1% detergent, Triton X-100 and placed at 4 °C on a rotating platform for 1 h. Samples were then centrifuged for 30 min at 48,000 × g and the supernatant corresponding to the particulate fraction was collected. Protein concentration for all the samples was determined at once using the standard Bradford assay (Bio-Rad) (31). Protein samples (20 μg/lane) were separated using SDS-PAGE and electrophoretically transferred onto polyvinylidene difluoride membranes. Membranes were blocked in Tris-buffered saline (TBS) containing 5% nonfat dry milk and 1% Tween 20, and then incubated overnight in TBS containing primary antibodies, 3% nonfat dry milk, and 1% Tween 20. The membranes were then washed and hybridized with horseradish peroxidase-conjugated secondary antibody for 1 h in TBS containing 1% milk and 1% Tween 20. Immunoreactive bands were detected using enhanced chemiluminescence reagents (PerkinElmer Life Sciences). Fold-increase in protein translocation was determined by quantifying the intensity of the IL-1β and TNFα particulate band and normalizing it to the control particulate band (QuantityOne, Bio-Rad).
Translocation experiments for various PKC isozymes were made from the same set of membranes that were stripped with 0.2% NaOH and probed with different PKC isozyme antibodies. Primary rabbit monoclonal anti-PKCα (1:2000) and anti-PKCϵ (1:2000) antibodies were purchased from Cell Signaling (29). Polyclonal rabbit anti-PKCβI and anti-PKCβII (1:1000) were purchased from Santa Cruz Biotechnology (32). Horseradish peroxidase-conjugated AffiniPure goat anti-rabbit IgG secondary antibodies were purchased from Jackson ImmunoResearch.
Statistical Analysis
Data were expressed as mean ± S.E. and statistical analysis were performed by Origin 8.0 (OriginLab, MA). Unpaired Student's t test or analysis of variance with a Tukey post hoc test was used to compare data sets when appropriate p values less than 0.05 were considered statistically significant.
RESULTS
TNFα Does Not Affect ICaL
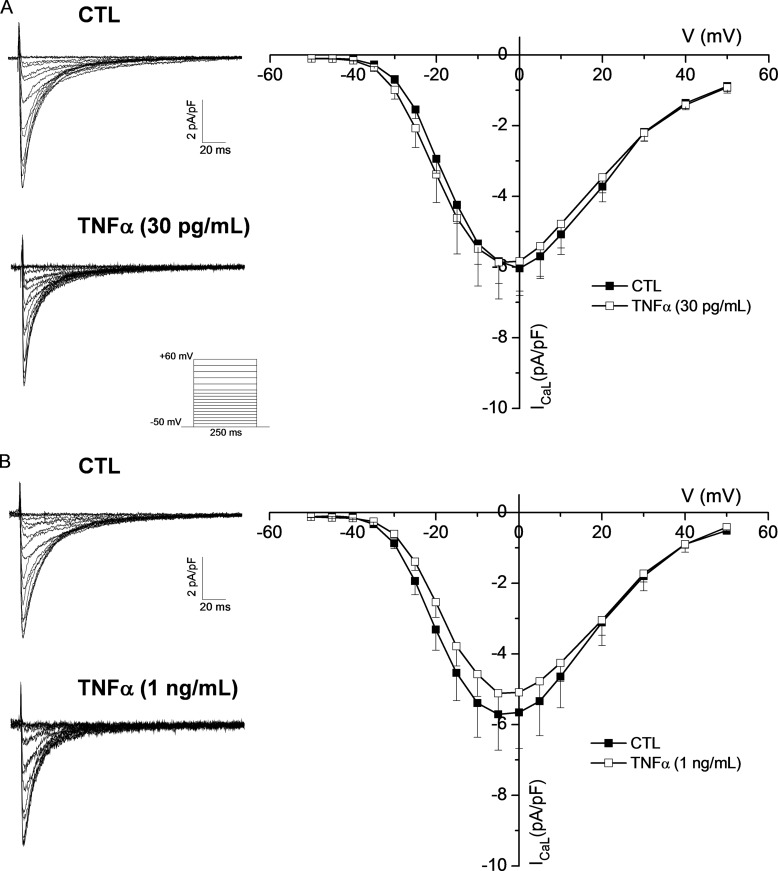
Because the cytokine TNFα is elevated in patients suffering from cardiac arrhythmia and heart failure, we investigated the potential effects it might have on ICaL. Voltage-clamp data from Fig. 1A shows that ICaL was not affected by TNFα (at 0 mV: CTL, −6.0 ± 0.6 pA/pF; TNFα, −5.8 ± 1.0 pA/pF). Furthermore, data presented in Fig. 1B shows that increasing the treatment concentration to 1 ng/ml failed to produce any effect (at 0 mV: CTL, −5.7 ± 1.0 pA/pF; TNFα, −5.08 ± 0.61 pA/pF), demonstrating that TNFα does not alter ICaL.
FIGURE 1.
TNFα treatment does not affect ICaL. A, left, representative traces of ventricular ICaL obtained with the protocol shown in the inset from an untreated myocyte (CTL) and a myocyte exposed to pathophysiological concentration (30 pg/ml) of TNFα. Right, IV curve showing the mean data from all recorded currents for control (CTL, n = 7) and TNFα-treated (TNFα, n = 7) cells. B, left, representative traces of ventricular ICaL recorded from a control and a myocyte exposed to 1 ng/ml of TNFα. Right, IV curve showing the mean data from all the recorded currents from control (CTL, n = 11) and TNFα-treated cells (TNFα, n = 7). All recordings were performed in a 24–32-h range after cytokine treatment in this and subsequent figures.
IL-1β Reduces the Density of ICaL
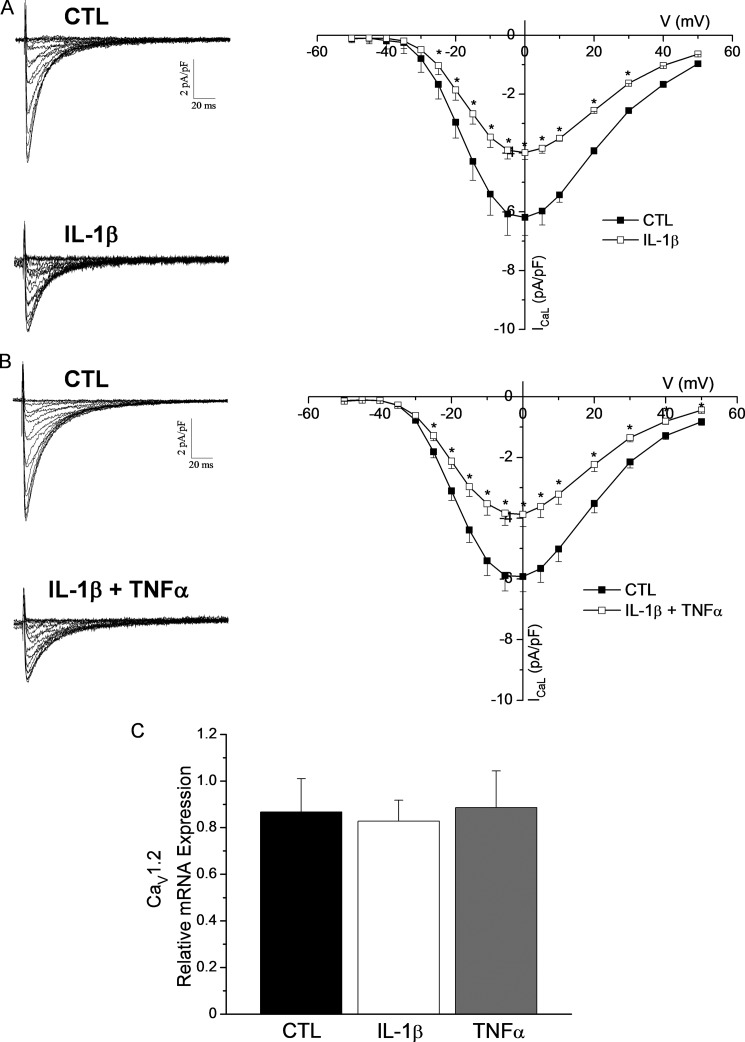
We then treated cardiomyocytes with a pathophysiological concentration (30 pg/ml) of IL-1β for 24–32 h and subsequently recorded ICaL using whole-cell voltage-clamp recordings. Our results demonstrate that the density of ICaL was significantly reduced. Indeed, Fig. 2A indicates that IL-1β reduced ICaL density by 36% (at 0 mV: CTL, −6.2 ± 0.5 pA/pF; IL-1β, −4.0 ± 0.5 pA/pF). Of note, the reduction in current density was independent of hypertrophy because cellular capacitance was comparable between untreated and IL-1β-treated myocytes.
FIGURE 2.
IL-1β treatment reduces ICaL density. A, left, representative traces of ICaL recorded from a control ventricular myocyte and an IL-1β-treated myocyte (30 pg/ml). Right, the mean I-V curve shows a 36% reduction in ICaL density in IL-1β-treated myocytes (IL-1β, n = 17) compared with controls (CTL, n = 19) (*, p < 0.05 versus CTL). B, left, representative traces of ventricular ICaL obtained from CTL myocytes and IL-1β + TNFα-treated myocytes (30 pg/ml). Right, the mean I-V curve shows ICaL density in treated cells (IL-1β + TNFα, n = 24) was reduced similarly to IL-1β-treated myocytes when compared with controls (CTL, n = 18) (*, p < 0.05 versus CTL). C, mean qPCR data shows that CaV1.2 mRNA expression was not altered by IL-1β (n = 3) or TNFα (n = 3) and was comparable with controls (CTL, n = 3).
Because some studies have suggested that IL-1β and TNFα can act synergistically to alter cardiomyocyte function (33, 34) we also investigated whether administration of both cytokines to cardiomyocytes would result in a more pronounced effect on the density of ICaL. Data in Fig. 2B shows that ICaL density of IL-1β + TNFα-treated myocytes was similar to the density measured when cardiomyocytes were treated with IL-1β alone thus, suggesting that the observed reduction in ICaL is IL-1β-dependent and is not altered by TNFα.
IL-1β Does Not Alter CaV1.2 mRNA Expression
Several reports have shown that pro-inflammatory cytokines can modulate the gene expression of multiple ion channels and proteins (15, 17, 35, 36); we therefore examined whether IL-1β could alter gene expression of CaV1.2. The qPCR results shown in Fig. 2C demonstrate that the transcript levels of CaV1.2 were unaffected by IL-1β. Not surprisingly, data also showed that TNFα did not alter CaV1.2 mRNA abundance either. These findings are consistent with our previous reports showing that pathophysiological concentrations of cytokines do not affect gene expression of ion channels (20, 28).
IL-1β Induces Intracellular ROS Production
Several studies have shown that pro-inflammatory cytokines are potent inducers of oxidative stress in heart disease and various cell types (14, 37). Thus, we tested whether IL-1β, the cytokine that significantly depressed ICaL, could induce ROS in a manner that would affect ICaL. Using the nonspecific ROS-sensitive probe H2DCFDA in a plate reader assay we were able to measure intracellular ROS levels in live cardiomyocytes. Fig. 3A indicates that IL-1β treatment caused a significant increase in ROS levels compared with untreated cardiomyocytes (CTL). Furthermore, the use of the non-selective antioxidant NAC (100 μm) along with IL-1β prevented the increase in intracellular ROS, whereas NAC had no effect on basal ROS levels in untreated cells (CTL + NAC).
FIGURE 3.
ROS is increased in IL-1β-treated cardiomyocytes. A, microplate ROS assay shows a significant increase in ROS production in cells treated with IL-1β (IL-1β, n = 16) compared with controls (CTL, n = 14). IL-1β increased in ROS was reversed with NAC treatment (IL-1β + NAC, n = 9). NAC did not have an effect on the basal level of ROS in control myocytes (CTL + NAC, n = 4). Positive control group (50 μm H2O2, 30 min, n = 4) saturated the signal intensity (*, p < 0.05 versus CTL; †, p < 0.05 versus IL-1β). B, left, typical examples of superimposed ICaL recordings at 0 mV are shown for the different experimental groups. Right, mean I-V curve data shows that administration of NAC with IL-1β (IL-1β + NAC, n = 13) prevented the inhibitory effects of IL-1β on ICaL (IL-1β, n = 19) and mean current density was comparable with untreated control cells (CTL, n = 14) and NAC-treated controls (CTL + NAC, n = 17) (*, p < 0.05 versus CTL; †, p < 0.05 versus IL-1β + NAC).
Increases in IL-1β-induced ROS Reduce ICaL Density
Increases in intracellular ROS levels have been associated with the deterioration of heart failure by affecting multiple intracellular pathways including mitochondrial function, stimulation of hypertrophy, and apoptosis but also directly impact myocyte contraction (9, 38). However, it remains incompletely understood whether cytokine-induced ROS could affect ion channels, notably ICaL. Accordingly, the next series of experiments was designed to test whether IL-1β-induced ROS affected the density of ICaL. Fig. 3B shows that treatment of myocytes concomitantly with IL-1β (30 pg/ml) and NAC (100 μm) prevented the effects of IL-1β on ICaL. The current density of ICaL was indistinguishable from controls (at 0 mV: CTL, −9.1 ± 0.9 pA/pF; IL-1β, −5.6 ± 0.6 pA/pF; IL-1β + NAC, −8.6 ± 0.7 pA/pF; CTL + NAC, −8.9 ± 1.06 pA/pF). Overall, these last two sets of experiments show that IL-1β significantly increases intracellular ROS that can be normalized using the antioxidant NAC, these changes were paralleled in electrophysiological studies where ICaL density, reduced by IL-1β, was also restored by administration of NAC. These data indicate that IL-1β alters ICaL in a ROS-dependent manner.
PKC Is Implicated in IL-1β-induced Reduction in ICaL
Recent studies have shown that low levels of oxidative species can act as signaling molecules with highly specific downstream targets, a divergent mechanism from the indiscriminately damaging nature of excessive ROS (38, 39). Thus, we hypothesized that IL-1β increases intracellular ROS levels to activate a specific redox-signaling pathway. The L-type calcium channel is known to be highly affected by intracellular signaling and interacts with multiple kinases including the Ca2+/calmodulin-dependent protein kinase II, PKA, and PKC (40). Because PKC is well recognized to be highly implicated in ROS signaling in different cell types and diseases (41), we examined whether PKC activation had any effect on ICaL. Data of Fig. 4A shows that when we rapidly activated PKC in control cardiomyocytes with the nonspecific PKC activator PMA this resulted in a significant reduction in ICaL density similarly to IL-1β. As expected, PKC activation is also accompanied by an increase in PKC translocation to the particulate fraction. All PKC isoforms activated by PMA translocated to the membrane. This is exemplified in Fig. 4B showing significant PKCϵ translocation. These findings indicate that activation of PKC by PMA leads to a decrease in ICaL current density and that this reduction was also associated with PKC translocation to membrane fractions where it could exert an inhibitory effect on ICaL. To verify whether a similar PKC activation occurred after chronic treatment with IL-1β we assayed PKC translocation in Western blots experiments reported in Fig. 5. Data shows that of the four PKC isozymes tested (PKCα, PKCβI, PKCβII, and PKCϵ) only PKCϵ witnessed a 2-fold increase in the particulate fraction, whereas none of the other isoforms translocated significantly. Because translocation of PKC to the membrane fractions is required for their activation and subsequent phosphorylation of downstream targets (29, 42), our result indicates that IL-1β activates PKCϵ. To verify whether the activated PKCϵ in IL-1β-treated myocytes had an actual effect on ICaL, we co-incubated these treated cells with a selective cell-permeable PKCϵ translocation inhibitor ϵ-V1-2 (44). The patch-clamp data presented in Fig. 6 demonstrate that when the cells were treated concomitantly with IL-1β and ϵ-V1-2, the reduction in ICaL density was prevented and that effect of IL-1β was effectively blocked by ϵ-V1-2 (at 0 mV: CTL, −9.2 ± 0.9 pA/pF; IL-1β: −6.0 ± 0.8 pA/pF; IL-1β + ϵ-V1-2, −10.1 ± 0.9 pA/pF; CTL + ϵ-V1-2, −10.3 ± 1.33 pA/pF).
FIGURE 4.
PKC activation by PMA reduced ICaL similarly to IL-1β. A, PMA was used as a non-selective PKC activator. Left, representative traces of ventricular ICaL before (CTL) and after PMA exposure (10 min, 100 nm). Right, I-V curve showing the mean data from all the current recordings from control (n = 10) and PMA-treated cells (n = 15) (*, p < 0.05 versus CTL). B, left, the acute PMA treatment also resulted in significant translocation of PKC, including PKCϵ, to the particulate fraction as shown on Western blot. Right, bar graph with mean data quantification of PKCϵ translocation (n = 3, for both conditions, *, p < 0.05 versus CTL).
FIGURE 5.
IL-1β increases membrane translocation of PKCϵ. Western blots for PKCα, PKCβI, PKCβII, and PKCϵ under control (CTL) and IL-1β-treated (IL-1β) myocytes are shown on the upper left panel. Soluble cytosolic protein (Cytosol) and insoluble particulate fractions (Membrane) were loaded on the gels and fold-increase in membrane fraction, corresponding to PKC translocation, was quantified for all the PKC isozymes (bar graphs). Only PKCϵ was significantly increased in the particulate fraction after IL-1β treatment (n = 3 for both groups, *, p < 0.05 versus CTL).
FIGURE 6.
PKCϵ is implicated in the IL-1β-induced reduction in ICaL density. The implication of PKCϵ was assessed using the specific ϵ-V1-2 translocation inhibitor in voltage-clamp experiments. A, superimposed representative current recordings obtained from the different experimental groups are shown. B, mean I-V curve generated from all the obtained recordings showing that IL-1β treatment significantly reduced current density (IL-1β, n = 11) compared with controls (CTL, n = 13), whereas addition of ϵ-V1-2 to culture media blocked the IL-1β effect (IL-1β + ϵ-V1-2, n = 10) and restored current density to the level of CTL + ϵ-V1-2 (n = 5) (*, p < 0.05 versus CTL; †, p < 0.05 versus IL-1β + ϵ-V1-2).
PKCϵ Is Downstream of ROS Activation
The fact that both NAC and ϵ-V1-2 fully restored ICaL density in electrophysiological experiments (Figs. 3 and 6) suggests that ROS and PKCϵ are both implicated in IL-1β regulation of ICaL. Furthermore, data reported in Fig. 7A shows that ROS levels remained elevated when cells were co-incubated with IL-1β + ϵ-V1-2 suggesting that PKCϵ is downstream of ROS activation. Altogether, these series of experiments strongly suggest that IL-1β, after signaling through its receptor, produces ROS, which leads to PKCϵ activation and reduction of ICaL density. Conceivably, membrane-bound PKCϵ could interact with L-type Ca2+ channels by phosphorylating serine-threonine residues and affect overall current density by reducing channel open probability (43).
FIGURE 7.
IL-1β but not TNFα causes ROS-dependent PKCϵ activation. A, microplate ROS assay shows a significant increase in ROS levels in IL-1β (n = 16) and IL-1β + ϵ-V1-2 (n = 4). Additionally, TNFα also significantly increases ROS levels (*, p < 0.05 versus CTL; †, p < 0.05 versus IL-1β). B, PKCϵ translocation assay in TNFα-treated cells is shown in the Western blot example and mean data are reported in the bar graph below. PKCϵ fails to translocate after TNFα treatment despite significant increase in ROS (n = 4 for each group).
TNFα Increases ROS but Fails to Activate PKCϵ
Interestingly, data reported in Fig. 7A also shows that for the same concentration, TNFα generated more ROS than IL-1β demonstrating its potent biological activity. Importantly, data on Fig. 7B indicates that, unlike IL-1β, long-term treatment of cardiomyocytes with TNFα does not cause any PKCϵ activation, as assayed by its membrane translocation, despite the increased ROS level caused by this cytokine. This finding supports our previous observation that the reduction in ICaL is indeed specific to IL-1β and that no effect on the current could be obtained with TNFα (Fig. 1) despite the fact that both cytokines produce significant ROS. These results are consistent with recent studies that offer support for a well defined and specific role for ROS in intracellular signaling in contrast to the widespread view of it being erratically damaging.
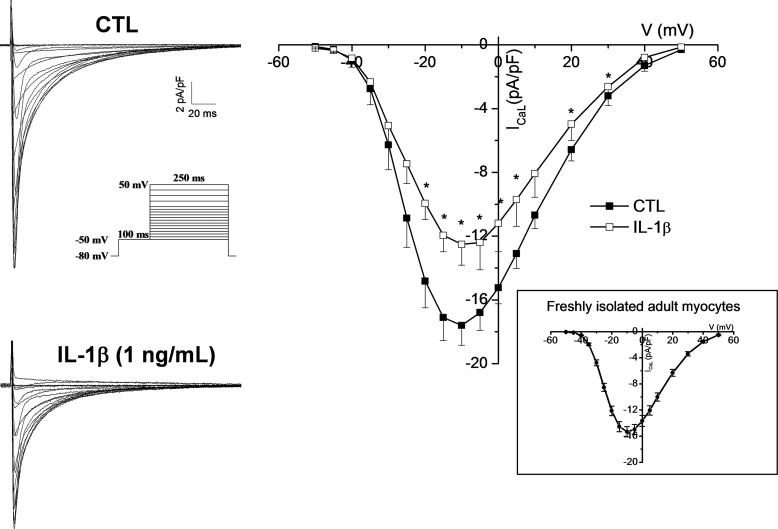
IL-1β Reduces ICaL in Adult Ventricular Myocytes
Even though both neonatal and adult cardiomyocytes express the L-type Ca2+ channel (45, 46) the neonatal myocyte has limited T-tubules development and the excitation-contraction coupling mechanism rely mostly on sarcoplasmic reticulum function rather than a Ca2+-induced Ca2+ release mechanism (47). Because cellular structure, morphology, and shape can intricately be linked to function (e.g. excitation-contraction coupling), it is therefore important to determine whether IL-1β could affect ICaL in the adult myocyte, where heart failure and arrhythmias occur. Even though addressing this point by long-term culture of adult ventricular myocytes sounds appealing, it is not technically feasible. In fact, within 24 h of culture there is significant loss of T-tubules, structural reorganization, and profound ion channel remodelling in adult myocytes (48). Therefore, to limit these effects we adapted an experimental protocol that yielded highly viable rod-shaped cultures of mouse ventricular myocytes that were only incubated for 12–16 h with an elevated (1 ng/ml) concentration of IL-1β to enhance or accelerate the cytokine effect due to culture time restraints. Our electrophysiology data shows first that the density of ICaL was comparable with that from freshly isolated myocytes (Fig. 8) and more importantly, ICaL was significantly reduced by IL-1β, albeit to a slightly lower extent than the neonatal myocytes. This is most likely due to the shorter incubation time. Specifically, reduction in peak current density was 29% (CTL, 17.6 ± 1.2 pA/pF; IL-1β, 12.5 ± 1.3 pA/pF). The data obtained in this experiment therefore provides a proof of principle that IL-1β exerts effects not only on neonatal but also on the adult myocytes.
FIGURE 8.
IL-1β reduces ICaL in adult ventricular myocytes. Typical ICaL recordings from adult ventricular myocytes in CTL (n = 10) and IL-1β (1 ng/ml; n = 9) treated myocytes. Inset shows that overnight culture had no significant effect on myocytes because ICaL density in cultured cells was comparable with the one from freshly isolated myocytes. The exposure 12–16 h exposure to high dose of IL-1β caused a 29% reduction in ICaL in adult myocytes (*, p < 0.05 versus CTL).
DISCUSSION
In this study we showed that a 24–32-h treatment of ventricular myocytes with pathophysiologically relevant concentrations of IL-1β but not TNFα caused a significant reduction in the density of ICaL. Mechanistically, the reduction in current density was not associated with a classical hypertrophic response but implicated ROS and PKC signaling. Specifically, intracellular ROS level and PKCϵ translocation were both increased. Blocking either ROS with a non-selective antioxidant, NAC, or the specific PKCϵ translocation inhibitor ϵ-V1-2 resulted in complete prevention of IL-1β effects on ICaL. Compared with the role of TNFα, which has been extensively studied in the context of heart failure, attention to the effects of IL-1β has been somewhat less encompassing and mainly evaluated in the contexts of atherosclerosis and vascular diseases (21) with only a few studies evaluating the influence of IL-1β on cardiomyocyte function.
In one study investigating the effects of IL-1β on intracellular Ca2+ dynamics and contraction it was observed that 3 h incubation with IL-1β alone was not able to affect cardiomyocyte function, whereas combining IL-1β with TNFα resulted in a synergistic effect where contraction, Ca2+ transients, and sarcoplasmic reticulum Ca2+ content were reduced (34). In that study, it is very likely that no effect of IL-1β was noted due to the short exposure time to the cytokine. Consistent with this idea, in initial experiments we also examined the effect of 3-h exposure to IL-1β and found that ICaL was unaffected. In contrast, in another study on the effect of chronic (3 days) IL-1β on cardiomyocyte function, it was noted that contractility, Ca2+ transients, response to isoproterenol, and expression of several excitation-contraction coupling proteins were reduced (49). These effects were independent of nitric oxide (NO) signaling but were attributed to a reduction in response to isoproterenol and cAMP production. This finding is in accordance with early reports showing that IL-1β is implicated in uncoupling the β-adrenergic response in cardiomyocyte (11). In our study, we show that a chronic exposure to IL-1β significantly reduced the density of ICaL. This is consistent with the previously reported reductions in contractility and Ca2+ transient (49), considering ICaL is the primary trigger for Ca2+-induced Ca2+ release from the sarcoplasmic reticulum. Even though reduced cAMP could explain these results, our data, however, strongly suggests that PKCϵ plays a major role in mediating the effects of IL-1β on cardiomyocyte electrical and contractile functions. Consistent with our findings, another study has shown that selective activation of PKCϵ with the ϵ-V1-7 peptide leads to a significant reduction in the density of ICaL in cardiomyocytes (50). Furthermore, when transgenic mice with a cardiac-specific constitutively active PKCϵ were studied the density of ICaL was also found to be reduced and the adrenergic response blunted (51). In line with our study, these results indicate that PKCϵ plays an important role in L-type Ca2+ channel regulation and cardiomyocyte contractile function. Additionally, in our study we showed that chronic IL-1β induces PKCϵ translocation thus, these results can provide additional mechanistic insight on how IL-1β can dampen the β-adrenergic response, affect contraction and intracellular Ca2+ dynamics, as previously reported (7, 11, 49).
Interestingly, data from TNFα-treated myocytes also supports the notion that oxidative species, within a certain range, can act as signaling molecules with specific downstream targets because even though TNFα induced ROS, it failed to activate PKCϵ translocation. Therefore suggesting that not all ROS is equal and conceivably, it is the specific ROS species and or source that is activating a defined signaling pathway rather than overall quantity of ROS that is central in mediating distinct effects on cellular function.
Considering that pro-inflammatory cytokines activate the inducible nitric-oxide synthase (iNOS) and favor the generation of NO (52) and that NO has been implicated in the reduction of basal contractility it is possible that peroxynitrite is the species being accumulated after exposure to IL-1β and is contributing to the observed effects. In support of this notion, Balafanova et al. (53) have shown that NO donors are capable of nitrating PKCϵ and effectively facilitate its translocation and subsequent activation via an NO or peroxynitrite-dependent mechanism. Nonetheless, Combes et al. (49) argue against NO-dependent mechanism of IL-1β-induced impairment of contractility and attribute the effects to lower cAMP production. Although lower cAMP could explain a lower ICaL, it remains to be elucidated whether cAMP or NO levels are changed after IL-1β treatment.
A reduced ICaL density in the heart could have important repercussions on electrophysiological and mechanical properties of the heart in a way that would increase susceptibility to arrhythmias or alter contractile function and favor hypertrophy (46). Yet the mechanisms underlying ion channel regulation by pro-inflammatory cytokines remain incompletely understood. Although experimental evidences from animal models and some clinical observations suggested that these cytokines might be implicated in inducing adverse cardiac structural remodelling, such as hypertrophy and fibrosis, but also induce ROS, alter contractile machinery and calcium homeostasis and dampening the β-adrenergic response (8, 9, 11, 13, 34). It remains unclear whether the observed electrical remodelling is only secondary to structural remodelling. In particular, it has not been completely established whether cytokines are able to directly induce changes in the cardiac electrical properties by altering ion channel function. In previous studies, we have shown that a transgenic HIV mouse model where pro-inflammatory cytokines (TNFα and IL-1β) are elevated, severe electrophysiological remodelling occurred. These mice had normal cardiac function assayed by echocardiography, however, the electrical activity of the heart and ionic currents were severely affected (27, 28).
As mentioned earlier, in many of the published experimental studies the concentration of cytokines used was between 10- and 1000-fold higher than serum cytokine levels in patients suffering from heart disease. Additionally, the effects were frequently reported in acute (minutes to few hours) cytokine exposure. Even though these findings are relevant, large concentrations of cytokines can induce several nonspecific effects and thus, they are unlikely to recapitulate the signaling pathways that are activated during disease. We therefore hypothesized that pro-inflammatory cytokines can indeed alter the properties of ion channels independently of structural remodelling and other nonspecific effects when administered for longer periods of time at pathophysiologically relevant concentrations. Subsequently, we demonstrated that a chronic TNFα resulted in a serum concentration comparable with that of heart failure patients. In these mice there was a significant reduction in the density of the K+ currents Ito and IKur without any noticeable hypertrophy or other structural changes (20). These changes were also independent of mRNA and protein expression alterations.
Overall, our study shows that a chronic exposure to pathophysiological concentrations of IL-1β but not TNFα reduced the density of ICaL without inducing myocyte hypertrophy. The effect of IL-1β was not limited to neonatal myocytes but extended to the adult myocytes where change in ICaL may have even more pronounced repercussions. Even though TNFα increased ROS, the signaling pathways activated by IL-1β were divergent from those of TNFα. The mechanism of IL-1β signaling is independent of transcription but implicated a pathway dependent on ROS and PKCϵ activation. Although our study does not rule out the involvement of other intermediates in the signaling cascades, the outcomes associated with ICaL reduction, ROS and PKCϵ activation in cardiomyocytes could include alterations of the electrical and contractile properties of the heart. Thus, these findings could provide a potential mechanistic explanation to the underlying increased arrhythmia susceptibility and contractile problems in patients suffering from heart failure and other heart diseases where pro-inflammatory cytokine levels are elevated.
Acknowledgments
We express thanks to N. Ethier and A. Douillette for excellent technical assistance. We are also grateful to Dr. A. Maguy for advice concerning the PKC experiments.
This work was supported in part by Canadian Institutes of Health Research Operating Grant CIHR-MOP-89934 (to C. F.).
- ROS
- reactive oxidative species
- NAC
- N-acetyl-l-cysteine
- PMA
- phorbol 12-myristate 13-acetate
- qPCR
- quantitative PCR
- CM-H2DCFDA
- 5-(and-6)-chloromethyl-2′,7′-dichlorodihydrofluorescein diacetate, acetyl ester
- CTL
- control.
REFERENCES
- 1. Medzhitov R. (2010) Inflammation 2010: new adventures of an old flame. Cell 140, 771–776 [DOI] [PubMed] [Google Scholar]
- 2. Levine B., Kalman J., Mayer L., Fillit H. M., Packer M. (1990) Elevated circulating levels of tumor necrosis factor in severe chronic heart failure. N. Engl. J. Med. 323, 236–241 [DOI] [PubMed] [Google Scholar]
- 3. Francis S. E., Holden H., Holt C. M., Duff G. W. (1998) Interleukin-1 in myocardium and coronary arteries of patients with dilated cardiomyopathy. J. Mol. Cell. Cardiol. 30, 215–223 [DOI] [PubMed] [Google Scholar]
- 4. Aukrust P., Yndestad A., Damås J. K., Gullestad L. (2004) Inflammation and chronic heart failure-potential therapeutic role of intravenous immunoglobulin. Autoimmun. Rev. 3, 221–227 [DOI] [PubMed] [Google Scholar]
- 5. Testa M., Yeh M., Lee P., Fanelli R., Loperfido F., Berman J. W., LeJemtel T. H. (1996) Circulating levels of cytokines and their endogenous modulators in patients with mild to severe congestive heart failure due to coronary artery disease or hypertension. J. Am. Coll. Cardiol. 28, 964–971 [DOI] [PubMed] [Google Scholar]
- 6. Guillén I., Blanes M., Gómez-Lechon M. J., Castell J. V. (1995) Cytokine signaling during myocardial infarction: sequential appearance of IL-1β and IL-6. Am. J. Physiol. 269, R229–R235 [DOI] [PubMed] [Google Scholar]
- 7. Prabhu S. D. (2004) Cytokine-induced modulation of cardiac function. Circ. Res. 95, 1140–1153 [DOI] [PubMed] [Google Scholar]
- 8. Hohensinner P. J., Niessner A., Huber K., Weyand C. M., Wojta J. (2011) Inflammation and cardiac outcome. Curr. Opin. Infect. Dis. 24, 259–264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Finkel M. S., Oddis C. V., Jacob T. D., Watkins S. C., Hattler B. G., Simmons R. L. (1992) Negative inotropic effects of cytokines on the heart mediated by nitric oxide. Science 257, 387–389 [DOI] [PubMed] [Google Scholar]
- 10. Murray D. R., Freeman G. L. (1996) Tumor necrosis factor-α induces a biphasic effect on myocardial contractility in conscious dogs. Circ. Res. 78, 154–160 [DOI] [PubMed] [Google Scholar]
- 11. Gulick T., Chung M. K., Pieper S. J., Lange L. G., Schreiner G. F. (1989) Interleukin 1 and tumor necrosis factor inhibit cardiac myocyte beta-adrenergic responsiveness. Proc. Natl. Acad. Sci. U.S.A. 86, 6753–6757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Schulz R., Panas D. L., Catena R., Moncada S., Olley P. M., Lopaschuk G. D. (1995) The role of nitric oxide in cardiac depression induced by interleukin-1β and tumour necrosis factor-α. Br. J. Pharmacol. 114, 27–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Saba S., Janczewski A. M., Baker L. C., Shusterman V., Gursoy E. C., Feldman A. M., Salama G., McTiernan C. F., London B. (2005) Atrial contractile dysfunction, fibrosis, and arrhythmias in a mouse model of cardiomyopathy secondary to cardiac-specific overexpression of tumor necrosis factor α. Am. J. Physiol. Heart Circ. Physiol. 289, H1456–H1467 [DOI] [PubMed] [Google Scholar]
- 14. Nakamura K., Fushimi K., Kouchi H., Mihara K., Miyazaki M., Ohe T., Namba M. (1998) Inhibitory effects of antioxidants on neonatal rat cardiac myocyte hypertrophy induced by tumor necrosis factor-α and angiotensin II. Circulation 98, 794–799 [DOI] [PubMed] [Google Scholar]
- 15. Petkova-Kirova P. S., Gursoy E., Mehdi H., McTiernan C. F., London B., Salama G. (2006) Electrical remodeling of cardiac myocytes from mice with heart failure due to the overexpression of tumor necrosis factor-α. Am. J. Physiol. Heart Circ. Physiol. 290, H2098–H2107 [DOI] [PubMed] [Google Scholar]
- 16. Kawada H., Niwano S., Niwano H., Yumoto Y., Wakisaka Y., Yuge M., Kawahara K., Izumi T. (2006) Tumor necrosis factor-α downregulates the voltage gated outward K+ current in cultured neonatal rat cardiomyocytes: a possible cause of electrical remodeling in diseased hearts. Circ. J. 70, 605–609 [DOI] [PubMed] [Google Scholar]
- 17. Fernández-Velasco M., Ruiz-Hurtado G., Hurtado O., Moro M. A., Delgado C. (2007) TNF-α downregulates transient outward potassium current in rat ventricular myocytes through iNOS overexpression and oxidant species generation. Am. J. Physiol. Heart Circ. Physiol. 293, H238–H245 [DOI] [PubMed] [Google Scholar]
- 18. Orús J., Roig E., Perez-Villa F., Paré C., Azqueta M., Filella X., Heras M., Sanz G. (2000) Prognostic value of serum cytokines in patients with congestive heart failure. J. Heart Lung Transplant. 19, 419–425 [DOI] [PubMed] [Google Scholar]
- 19. Roig E., Orús J., Paré C., Azqueta M., Filella X., Perez-Villa F., Heras M., Sanz G. (1998) Serum interleukin-6 in congestive heart failure secondary to idiopathic dilated cardiomyopathy. Am. J. Cardiol. 82, 688–690 [DOI] [PubMed] [Google Scholar]
- 20. Grandy S. A., Fiset C. (2009) Ventricular K+ currents are reduced in mice with elevated levels of serum TNFα. J. Mol. Cell. Cardiol. 47, 238–246 [DOI] [PubMed] [Google Scholar]
- 21. Sims J. E., Smith D. E. (2010) The IL-1 family: regulators of immunity. Nat. Rev. Immunol. 10, 89–102 [DOI] [PubMed] [Google Scholar]
- 22. Chamberlain J., Francis S., Brookes Z., Shaw G., Graham D., Alp N. J., Dower S., Crossman D. C. (2009) Interleukin-1 regulates multiple atherogenic mechanisms in response to fat feeding. PLoS One 4, e5073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bers D. M. (2002) Cardiac excitation-contraction coupling. Nature 415, 198–205 [DOI] [PubMed] [Google Scholar]
- 24. Grandy S. A., Trépanier-Boulay V., Fiset C. (2007) Postnatal development has a marked effect on ventricular repolarization in mice. Am. J. Physiol. Heart Circ. Physiol. 293, H2168–H2177 [DOI] [PubMed] [Google Scholar]
- 25. O'Connell T. D., Rodrigo M. C., Simpson P. C. (2007) Isolation and culture of adult mouse cardiac myocytes. Methods Mol. Biol. 357, 271–296 [DOI] [PubMed] [Google Scholar]
- 26. Rivard K., Grandy S. A., Douillette A., Paradis P., Nemer M., Allen B. G., Fiset C. (2011) Overexpression of type 1 angiotensin II receptors impairs excitation-contraction coupling in the mouse heart. Am. J. Physiol. Heart Circ. Physiol. 301, H2018–H2027 [DOI] [PubMed] [Google Scholar]
- 27. Brouillette J., Grandy S. A., Jolicoeur P., Fiset C. (2007) Cardiac repolarization is prolonged in CD4C/HIV transgenic mice. J. Mol. Cell. Cardiol. 43, 159–167 [DOI] [PubMed] [Google Scholar]
- 28. Grandy S. A., Brouillette J., Fiset C. (2010) Reduction of ventricular sodium current in a mouse model of HIV. J. Cardiovasc. Electrophysiol. 21, 916–922 [DOI] [PubMed] [Google Scholar]
- 29. Makary S. (2011) Differential protein kinase C isoform regulation and increased constitutive activity of acetylcholine-regulated potassium channels in atrial remodeling. Circ. Res. 109, 1031–1043 [DOI] [PubMed] [Google Scholar]
- 30. Brandman R., Disatnik M. H., Churchill E., Mochly-Rosen D. (2007) Peptides derived from the C2 domain of protein kinase Cϵ (ϵPKC) modulate ϵPKC activity and identify potential protein-protein interaction surfaces. J. Biol. Chem. 282, 4113–4123 [DOI] [PubMed] [Google Scholar]
- 31. Lizotte E., Tremblay A., Allen B. G., Fiset C. (2005) Isolation and characterization of subcellular protein fractions from mouse heart. Anal. Biochem. 345, 47–54 [DOI] [PubMed] [Google Scholar]
- 32. Ferreira J. C., Koyanagi T., Palaniyandi S. S., Fajardo G., Churchill E. N., Budas G., Disatnik M. H., Bernstein D., Brum P. C., Mochly-Rosen D. (2011) Pharmacological inhibition of βIIPKC is cardioprotective in late-stage hypertrophy. J. Mol. Cell. Cardiol. 51, 980–987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kumar A., Thota V., Dee L., Olson J., Uretz E., Parrillo J. E. (1996) Tumor necrosis factor α and interleukin 1β are responsible for in vitro myocardial cell depression induced by human septic shock serum. J. Exp. Med. 183, 949–958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Duncan D. J., Yang Z., Hopkins P. M., Steele D. S., Harrison S. M. (2010) TNF-α and IL-1β increase Ca2+ leak from the sarcoplasmic reticulum and susceptibility to arrhythmia in rat ventricular myocytes. Cell Calcium 47, 378–386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. McTiernan C. F., Lemster B. H., Frye C., Brooks S., Combes A., Feldman A. M. (1997) Interleukin-1β inhibits phospholamban gene expression in cultured cardiomyocytes. Circ. Res. 81, 493–503 [DOI] [PubMed] [Google Scholar]
- 36. Thaik C. M., Calderone A., Takahashi N., Colucci W. S. (1995) Interleukin-1β modulates the growth and phenotype of neonatal rat cardiac myocytes. J. Clin. Invest. 96, 1093–1099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Suematsu N., Tsutsui H., Wen J., Kang D., Ikeuchi M., Ide T., Hayashidani S., Shiomi T., Kubota T., Hamasaki N., Takeshita A. (2003) Oxidative stress mediates tumor necrosis factor-α-induced mitochondrial DNA damage and dysfunction in cardiac myocytes. Circulation 107, 1418–1423 [DOI] [PubMed] [Google Scholar]
- 38. Tsutsui H., Kinugawa S., Matsushima S. (2011) Oxidative stress and heart failure. Am. J. Physiol. Heart Circ. Physiol. 301, H2181–H2190 [DOI] [PubMed] [Google Scholar]
- 39. Finkel T. (2003) Oxidant signals and oxidative stress. Curr. Opin. Cell Biol. 15, 247–254 [DOI] [PubMed] [Google Scholar]
- 40. Kamp T. J., Hell J. W. (2000) Regulation of cardiac L-type calcium channels by protein kinase A and protein kinase C. Circ. Res. 87, 1095–1102 [DOI] [PubMed] [Google Scholar]
- 41. Giorgi C., Agnoletto C., Baldini C., Bononi A., Bonora M., Marchi S., Missiroli S., Patergnani S., Poletti F., Rimessi A., Zavan B., Pinton P. (2010) Redox control of protein kinase C: cell- and disease-specific aspects. Antioxid. Redox Signal. 13, 1051–1085 [DOI] [PubMed] [Google Scholar]
- 42. Rybin V. O., Guo J., Sabri A., Elouardighi H., Schaefer E., Steinberg S. F. (2004) Stimulus-specific differences in protein kinase Cδ localization and activation mechanisms in cardiomyocytes. J. Biol. Chem. 279, 19350–19361 [DOI] [PubMed] [Google Scholar]
- 43. Chahine M., Qu Y., Mancarella S., Boutjdir M. (2008) Protein kinase C activation inhibits α1D L-type Ca channel: a single-channel analysis. Pflugers Arch. 455, 913–919 [DOI] [PubMed] [Google Scholar]
- 44. Johnson J. A., Gray M. O., Chen C. H., Mochly-Rosen D. (1996) A protein kinase C translocation inhibitor as an isozyme-selective antagonist of cardiac function. J. Biol. Chem. 271, 24962–24966 [DOI] [PubMed] [Google Scholar]
- 45. Nuss H. B., Marban E. (1994) Electrophysiological properties of neonatal mouse cardiac myocytes in primary culture. J. Physiol. 479, 265–279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Goonasekera S. A., Hammer K., Auger-Messier M., Bodi I., Chen X., Zhang H., Reiken S., Elrod J. W., Correll R. N., York A. J., Sargent M. A., Hofmann F., Moosmang S., Marks A. R., Houser S. R., Bers D. M., Molkentin J. D. (2012) Decreased cardiac L-type Ca2+ channel activity induces hypertrophy and heart failure in mice. J. Clin. Invest. 122, 280–290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Tanaka H., Sekine T., Nishimaru K., Shigenobu K. (1998) Role of sarcoplasmic reticulum in myocardial contraction of neonatal and adult mice. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 120, 431–438 [DOI] [PubMed] [Google Scholar]
- 48. Louch W. E., Sheehan K. A., Wolska B. M. (2011) Methods in cardiomyocyte isolation, culture, and gene transfer. J. Mol. Cell. Cardiol. 51, 288–298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Combes A., Frye C. S., Lemster B. H., Brooks S. S., Watkins S. C., Feldman A. M., McTiernan C. F. (2002) Chronic exposure to interleukin 1β induces a delayed and reversible alteration in excitation-contraction coupling of cultured cardiomyocytes. Pflugers Arch. 445, 246–256 [DOI] [PubMed] [Google Scholar]
- 50. Hu K., Mochly-Rosen D., Boutjdir M. (2000) Evidence for functional role of ϵPKC isozyme in the regulation of cardiac Ca2+ channels. Am. J. Physiol. Heart Circ. Physiol. 279, H2658–H2664 [DOI] [PubMed] [Google Scholar]
- 51. Yue Y., Qu Y., Boutjdir M. (2004) β- and α-Adrenergic cross-signaling for L-type Ca current is impaired in transgenic mice with constitutive activation of ϵPKC. Biochem. Biophys. Res. Commun. 314, 749–754 [DOI] [PubMed] [Google Scholar]
- 52. Kröncke K. D., Fehsel K., Kolb-Bachofen V. (1998) Inducible nitric oxide synthase in human diseases. Clin. Exp. Immunol. 113, 147–156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Balafanova Z., Bolli R., Zhang J., Zheng Y., Pass J. M., Bhatnagar A., Tang X. L., Wang O., Cardwell E., Ping P. (2002) Nitric oxide (NO) induces nitration of protein kinase Cepsilon (PKCϵ), facilitating PKCϵ translocation via enhanced PKCϵ-RACK2 interactions: a novel mechanism of no-triggered activation of PKCϵ. J. Biol. Chem. 277, 15021–15027 [DOI] [PubMed] [Google Scholar]