Background: The role of CRADD in endothelial cells is unknown.
Results: CRADD attenuates responses to proinflammatory agonists in endothelial cells and stabilizes their barrier function.
Conclusion: CRADD plays a pivotal role in maintaining the integrity of the endothelial barrier.
Significance: Understanding the role of CRADD as a physiologic rheostat of perturbed endothelial cells informs development of CRADD-based measures to stabilize endothelial integrity.
Keywords: Endothelial Cell, Inflammation, Lipopolysaccharide (LPS), Permeability, Thrombin, BCL10, CARMA3 Signalosome, CRADD, Cytokines/Chemokines, Intracellular Delivery
Abstract
A hallmark of inflammation, increased vascular permeability, is induced in endothelial cells by multiple agonists through stimulus-coupled assembly of the CARMA3 signalosome, which contains the adaptor protein BCL10. Previously, we reported that BCL10 in immune cells is targeted by the “death” adaptor CRADD/RAIDD (CRADD), which negatively regulates nuclear factor κB (NFκB)-dependent cytokine and chemokine expression in T cells (Lin, Q., Liu, Y., Moore, D. J., Elizer, S. K., Veach, R. A., Hawiger, J., and Ruley, H. E. (2012) J. Immunol. 188, 2493–2497). This novel anti-inflammatory CRADD-BCL10 axis prompted us to analyze CRADD expression and its potential anti-inflammatory action in non-immune cells. We focused our study on microvascular endothelial cells because they play a key role in inflammation. We found that CRADD-deficient murine endothelial cells display heightened BCL10-mediated expression of the pleotropic proinflammatory cytokine IL-6 and chemokine monocyte chemoattractant protein-1 (MCP-1/CCL2) in response to LPS and thrombin. Moreover, these agonists also induce significantly increased permeability in cradd−/−, as compared with cradd+/+, primary murine endothelial cells. CRADD-deficient cells displayed more F-actin polymerization with concomitant disruption of adherens junctions. In turn, increasing intracellular CRADD by delivery of a novel recombinant cell-penetrating CRADD protein (CP-CRADD) restored endothelial barrier function and suppressed the induction of IL-6 and MCP-1 evoked by LPS and thrombin. Likewise, CP-CRADD enhanced barrier function in CRADD-sufficient endothelial cells. These results indicate that depletion of endogenous CRADD compromises endothelial barrier function in response to inflammatory signals. Thus, we define a novel function for CRADD in endothelial cells as an inducible suppressor of BCL10, a key mediator of responses to proinflammatory agonists.
Introduction
Inflammation represents a fundamental mechanism of diseases caused by microbial, autoimmune, metabolic, and physical insults. For example, the action of microbial insults on endothelial cells in severe microbial infections evolving into sepsis leads to endothelial dysfunction that contributes to major organ failure, disseminated intravascular coagulation, and acute respiratory distress syndrome (1). To counteract the deleterious action of proinflammatory cytokines and chemokines, an intracellular negative feedback system has evolved to limit the duration and strength of proinflammatory signaling. This system is comprised of intracellular physiologic proteins that control excessive inflammatory responses. They include interleukin-1 receptor-associated kinase (IRAK)2-M, an inhibitory member of the IRAK family, the inhibitor of nuclear factor κB (NFκB) transcription factors IκB, the suppressors of cytokine signaling (SOCS) proteins that inhibit activated STAT transcription factors, and the ubiquitin-modifying enzyme A20 (2–5). Recently, we added the “death” adaptor caspase and receptor interacting protein adaptor with death domain/receptor interacting protein-associated ICH-1/CED-3 homologous protein with a death domain (CRADD/RAIDD), hereafter designated as CRADD, to this list. CRADD negatively regulates NFκB-dependent cytokine and chemokine expression in T cells by targeting the NH2-terminal caspase recruitment domain (CARD) of B-cell CLL/Lymphoma 10 (BCL10) (6). In immune cells, the CARD of BCL10 functions as an oligomerization region and interacts with the CARD of the CARD membrane-associated guanylate kinase (CARMA) 1 (6, 7), which is required for activation of the NFκB pathway (8–12). In non-immune cells, such as endothelial cells, a CARMA3 signalosome containing BCL10 and mucosa-associated lymphoid tissue lymphoma translocation protein 1 (MALT1), operates to regulate the NFκB signaling pathway (13, 14). Disruption of the CARMA3 signalosome by genetic deletion of Bcl10 leads to dramatic reduction of vascular inflammation, illustrating that BCL10 is an essential component of the signaling complex (15).
The CARMA3 signalosome also modulates endothelial barrier function in response to proinflammatory agonists that induce increased vascular permeability (16). Induction of vascular permeability causes swelling, one of the four classic signs of inflammation, due to the action of proinflammatory agonists sensed by their cognate receptors expressed on microvascular endothelial cells (17). The CARMA3 signalosome amplifies signaling in response to proinflammatory agonists and mediates stimulus-dependent nuclear reprogramming (13–15, 18), which depends on transcription factors NFκB and AP-1 (13, 16, 18, 19). Thus, the CARMA3 signalosome plays a pivotal role in shifting microvascular endothelial cells from a resting to activated state, integrating signaling pathways evoked by recognition of diverse agonists. This signaling promulgates an inflammatory response, based in part on disruption of endothelial barrier function by altering cell-cell junctions that include adherens junctions and tight junctions (20, 21). These mainstays of endothelial monolayer integrity dynamically guard barrier function in major organs that contain an extensive network of microcirculation, such as lungs, kidneys, liver, and brain. Vascular endothelial cadherin (VE-cadherin) is a strictly endothelial specific cell adhesion molecule and the major determinant of endothelial cell contact integrity. Its adhesive function requires association with the cytoplasmic catenin protein p120 (22). LPS and thrombin induce F-actin reorganization and subsequent reductions in VE-cadherin at endothelial cell junctions, resulting in increased vascular permeability (22–24). The target of CRADD, BCL10, and its effector, NFκB, have been implicated in mediating these changes (25–27).
Here we analyzed the potential role of CRADD in endothelial cell homeostasis by employing three approaches: (i) reduction of CRADD expression in murine endothelial cells with shRNA, (ii) analysis of microvascular endothelial cells isolated from CRADD-deficient mice (6), and (iii) intracellular delivery of a novel recombinant cell-penetrating CRADD protein homolog (CP-CRADD) to CRADD-deficient and sufficient endothelial cells. We documented a protective role for CRADD in maintaining the permeability barrier of primary lung microvascular endothelial cells (LMEC) by demonstrating increased agonist-induced permeability of cradd−/− LMEC monolayers compared with cradd+/+ LMEC monolayers. Moreover, treatment with CP-CRADD restored barrier function in endothelial monolayers of human and murine cells challenged with proinflammatory agonists.
EXPERIMENTAL PROCEDURES
Mice
Wild-type cradd+/+ and knock-out cradd−/− mice were generated and maintained as previously described (6). All work with animals was carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health, and approved by the Vanderbilt University Institutional Animal Care and Use Committee.
Endothelial Cell Culture
Primary human umbilical vein endothelial cells were purchased from ScienCell and cultured in ECM (ScienCell). Primary murine LMEC were isolated from cradd+/+ and cradd−/− mice using a lung dissociation kit and purified by immunomagnetic separation, with anti-CD45-conjugated and anti-CD31-conjugated MicroBeads according to protocols provided by the manufacturer (Miltenyi Biotec), then cultured in collagen-coated tissue culture dishes with EBM-2 (Lonza) supplemented with 5% heat-inactivated FBS, 25 μg/ml of endothelial mitogen (Biomedical Technologies), and 1% penicillin/streptomycin solution (Mediatech). The human lung microvascular endothelial cell line HPMEC-ST1.6R was generously provided by C. J. Kirkpatrick (28) and cultured in M199 (Mediatech) supplemented with 10% heat-inactivated FBS, 25 μg/ml of endothelial mitogen, 50 μg/ml of heparin (Sigma), and 1% penicillin/streptomycin. The endothelial cell line LEII (mouse lung capillary) was a kind gift from T. Maciag (29). EA.hy926, EOMA, and SVEC4–10 cell lines were purchased from ATCC. LEII and cell lines from ATCC were cultured in DMEM (Mediatech) supplemented with 10% heat-inactivated FBS and 1% penicillin/streptomycin.
Immunoprecipitation and Immunoblot Analysis
Antibodies to CRADD (Proteintech Group), BCL10 and NFκB p65/RelA (Santa Cruz) were used for immunoblot analyses. GAPDH, β-actin, or TATA-binding protein (TBP) antibodies (Abcam) were used for normalization of cytosolic and nuclear extracts as indicated in the figure legends. Complexes were immunoprecipitated from cell lysates with antibody to IRAK-1 (Santa Cruz) and protein A/G-agarose beads (Thermo) then analyzed by quantitative immunoblotting using antibodies to IRAK-1 and BCL10. All immunoblots were analyzed with the LI-COR Odyssey Infrared Imaging System as previously described (6, 30).
Lentiviral shRNA Knockdown of CRADD and BCL10 in Endothelial Cells
Lentiviral packaging and shRNA transduction were performed as previously described (31). CRADD and BCL10 knockdown (K/D) efficiency was assessed at the transcript and protein level after 96 h, when shRNA-mediated knockdown experiments were performed.
RT-PCR Analysis
Total RNA was isolated for RT-PCR analysis using TRIzol reagent (Invitrogen) and reverse-transcribed using the iScript cDNA synthesis kit (Bio-Rad). Targets were amplified by PCR using PCR Master Mix (Promega) with specific primers listed in Table 1 for the indicated protein mRNAs. PCR products were separated on 1% agarose gels. Ethidium bromide-stained gels were imaged on a Gel Doc EZ Imager (Bio-Rad) and analyzed with Image Lab 5.0 software to quantify bands.
TABLE 1.
Oligonucleotide PCR primer sequences used in the current study
| Gene | Primer sequences (5′ to 3′) | |
|---|---|---|
| hCRADD | Forward | 5-AGTACTCCGCTCACTTCGC-3 |
| Reverse | 5-CTGCAGGCAGGTCGGTCAT-3 | |
| mCRADD | Forward | 5-GAAGAAATGGAAGCCAGAG-3 |
| Reverse | 5-CTGTAGGCAGCTCGGCTG-3 | |
| hBCL10 | Forward | 5-CCCGCTCCGCCTCCTCTCCTT-3 |
| Reverse | 5-GGCGCTTCTTCCGGGTCCG-3 | |
| mBCL10 | Forward | 5-GAGAGCATCCACTGTCATG-3 |
| Reverse | 5-GGAGAAACATCTCACTTGAG-3 | |
| mTNF-α | Forward | 5-GCGACGTGGAACTGGCAGAAG-3 |
| Reverse | 5-GGTACAACCCATCGGCTGGCA-3 | |
| mIL-6 | Forward | 5-TTCCATCCAGTTGCCTTCTTGG-3 |
| Reverse | 5-CTTCATGTACTCCAGGTAG-3 | |
| mIL-1α | Forward | 5-CTCTAGAGCACCATGCTACAGAC-3 |
| Reverse | 5-TGGAATCCAGGGGAAACACTG-3 | |
| mβ-actin | Forward | 5-TTCTTTGCAGCTCCTTCGTTGCCG-3 |
| Reverse | 5-TGGATGGCTACGTACATGGCTGGG-3 |
Cytokine/Chemokine Assays
Cytokines and chemokines in tissue culture media were assayed by cytometric bead array (BD Biosciences) in the Vanderbilt Flow Cytometry Core according to the manufacturer's instructions and as described previously (6). In some experiments, cells were treated with CP-CRADD or non-CP-CRADD before stimulation.
Immunofluorescence Staining and Fluorescence Microscopy
LMEC or LEII cells were plated into Lab-Tek II chamber slides (Thermo Scientific) and stimulated with LPS or thrombin (Sigma) as indicated. After stimulation, cells were fixed in 4% paraformaldehyde (Electron Microscopy Sciences), then washed in PBS and permeabilized with 0.1% Triton X-100 (Invitrogen). For immunofluorescence staining, cells were blocked with 5% normal goat serum (Jackson ImmunoResearch) before overnight incubation at 4 °C with antibodies to NFκB p65/RelA (Abcam) or VE-Cadherin and p120 (Santa Cruz) followed by incubation with Alexa 488- (Invitrogen) or Cy-3-labeled (Jackson ImmunoResearch) secondary antibodies. Alexa 488-labeled phalloidin (Cytoskeleton, Inc.) was used to visualize F-actin polymerization in permeabilized cells. Slides were mounted with ProLong Gold Antifade reagent containing DAPI (Invitrogen) to stain nuclei. Images were captured with MetaMorph software on an Axioplan widefield microscope in the Vanderbilt Cell Imaging Core facility using ×40 or ×63 oil immersion objectives, as indicated.
Design, Preparation, and Intracellular Delivery of Recombinant CRADD Proteins
Design, production, and analysis of recombinant murine CRADD proteins followed our previously published protocols (30, 32). Plasmid constructs for wild type (non-CP-CRADD) and cell penetrating (CP-CRADD) were produced using standard molecular biology techniques. CP-CRADD contains a membrane-translocating motif that enables it to cross the plasma membrane. Proteins were isolated from bacterial inclusion bodies using previously described protocols (30, 32) and dialyzed into DMEM supplemented with 1% penicillin/streptomycin and 66 μm PEG3350. LPS content in all recombinant protein preparations was below the level of detection (0.06 EU/ml) by Limulus assay (Endosafe, Charles River), performed according to the manufacturer's instructions. To confirm intracellular delivery of CP-CRADD, human ST1.6R endothelial cells were treated for 1 h with equimolar concentrations (10 μm) of non-CP-CRADD and CP-CRADD or medium alone. The cells were washed with warm DMEM without serum and treated with 7 μg/ml of proteinase K (Sigma) for 10 min to remove proteins attached to the cell surface, followed by a wash in warm DMEM supplemented with 5% FBS. Pelleted cells were lysed in RIPA buffer supplemented with protease inhibitors (Sigma). Lysates were cleared by centrifugation then analyzed by immunoblotting using antibodies to CRADD and β-actin. Although some membrane-associated non-CP-CRADD is detected by immunoblotting, CP-CRADD is 2–3-fold more abundant when recombinant proteins are normalized to endogenous CRADD or β-actin.
Endothelial Cell Permeability
LMEC (1 × 104, passage 3 or 4) isolated from cradd+/+ and cradd−/− mice, or human ST1.6R cells were seeded onto 24-well Transwell insets (Costar) pre-coated with type I collagen and incubated until confluent. Confluent monolayers were serum-starved in 0.5% heat-inactivated FBS for 24 h, then left unstimulated or stimulated with vascular permeability inducers as indicated. In some experiments, cells were treated with CP-CRADD or non-CP-CRADD before stimulation. Monolayer permeability was assessed by detection of FITC-dextran in the lower chamber at various times after addition of 1 mg/ml of 10-kDa FITC-dextran or the molar equivalent of 70-kDa FITC-dextran (Invitrogen) to the top chamber. We determined that the relative fluorescence of 70-kDa FITC-dextran is ∼6-fold greater than that of 10-kDa FITC-dextran at equal molarities.
Statistical Analyses
Data analysis and statistical calculations were performed using Prism (GraphPad). Cytokine and chemokine levels in cultured cell supernatants, and nuclear levels of p65/RelA were compared using an unpaired t test with Welch's correction for unequal standard deviations. Quantification of RT-PCR bands was used to calculate the fold-change in transcripts compared with non-transduced cells stimulated with LPS or thrombin and statistical differences were determined by Student's t test. For permeability experiments, the p values shown compare the area under the curve calculated for each condition, analyzed by an unpaired t test with Welch's correction for unequal standard deviations. Additional evaluation of permeability curves by repeated measures two-way analysis of variance resulted in a p value of <0.0001 for all indicated comparisons. In all experiments, a p value of <0.05 was considered significant.
RESULTS
The outcome of inflammation depends on the balance between proinflammatory mediators and anti-inflammatory suppressors. Our prior studies in immune cells (T lymphocytes) established that CRADD inhibits pro-inflammatory signaling at the level of BCL10-dependent NFκB activation (6, 7). We investigated the possibility of a similar function for CRADD in non-immune cells (endothelial cells) in which BCL10 plays a pivotal role in the CARMA3 signalosome-dependent activation of the NFκB pathway.
Expression of CRADD in Endothelial Cells
We hypothesized that CRADD could negatively regulate BCL10, an essential component of the CARMA3 signalosome assembled in endothelial cells following their response to proinflammatory stimuli. To test this hypothesis, we first examined expression of CRADD mRNA and protein in primary human endothelial cells, primary murine LMEC, and human and murine endothelial cell lines. We show by RT-PCR (Fig. 1A) and immunoblot analysis (Fig. 1B) that the human umbilical vein endothelial cell, LMEC, and endothelial cell lines constitutively express CRADD.
FIGURE 1.

Expression of CRADD and BCL10 in human and mouse endothelial cells and association of BCL10 with IRAK-1 in the proinflammatory TLR4 signaling pathway induced by LPS. CRADD mRNA and protein expression in endothelial cells was assessed by RT-PCR (A) and immunoblot analysis (B). C, BCL10 mRNA was assessed by RT-PCR in endothelial cells. In RT-PCR analyses, human negative control (HNC) and mouse negative control (MNC) reactions were performed using human or mouse primers, respectively, without cDNA. In immunoblot analyses, mouse spleen and liver extracts derived from cradd+/+ and cradd−/− mice served as positive (+/+) and negative (−/−) controls for CRADD protein, respectively, and β-actin served as a cellular protein loading control. D, co-immunoprecipitation of BCL10 with IRAK-1 is stimulus- and time-dependent. Primary cradd+/+ LMEC cells or human ST1.6 R endothelial cells were stimulated with 1 μg/ml of LPS for the indicated times. Protein complexes precipitated with anti-IRAK-1 (IP) from cell lysates were immunoblotted (IB) with antibodies to the indicated proteins. All gels and blots shown are representative of three independent experiments.
The Anti-inflammatory Action of CRADD Is Dependent on BCL10
We previously identified BCL10 as a direct target of CRADD responsible for suppression of T cell receptor agonist-evoked signaling in T lymphocytes (6). This new function of CRADD is dependent on its CARD domain, which binds to BCL10 and impedes its interaction with CARMA1 (6). Thus, the CRADD-BCL10 axis prevents formation of a complete CARMA1 signalosome required for activation of the NFκB signaling pathway in immune cells (6). BCL10 is expressed in endothelial cells (Fig. 1C), consistent with other reports that also documented expression of CARMA3 and MALT1 (15, 16, 18). BCL10 has been identified as an important mediator of NFκB activation, and is recruited to Toll-like receptor 4 (TLR4) signaling complexes in response to LPS stimulation by interacting with IRAK-1 (33, 34). We confirmed this interaction in LPS-stimulated LMEC from cradd+/+ mice and human lung microvascular endothelial HPMEC-ST1.6R cells by showing stimulus- and time-dependent association of BCL10 with IRAK-1 (Fig. 1D). We chose HPMEC-ST1.6R cells because they display the major constitutive and inducible endothelial cell characteristics and show an angiogenic response on Matrigel similar to that of primary human endothelial cells isolated from umbilical vein (HUVEC), lung (HPMEC), and skin (HDMEC) (28).
Subsequently, we demonstrated that the regulatory action of CRADD depends on BCL10 in stimulated endothelial cells by employing shRNA knockdown of CRADD and/or BCL10 (Fig. 2, A and B). Upon stimulation with the TLR4 agonist LPS, or the proteinase-activated receptor 1 (PAR-1) agonist thrombin, CRADD K/D LEII cells display significantly increased transcripts for cytokines TNF-α, IL-6, and IL-1α (Fig. 3). Consistent with increased expression of IL-6 mRNA transcripts, IL-6 protein expression was also increased in response to LPS and thrombin (Fig. 2, C and D, right). Thus, endothelial production of this pleotropic cytokine and permeability inducer (35) is negatively controlled by CRADD, regulating signaling pathways evoked by two distinct agonists, LPS and thrombin, in endothelial cells. Although CRADD K/D cells produced more IL-6 in response to LPS or thrombin, BCL10 K/D endothelial cells displayed the opposite effect (Fig. 2, C and D, left). Simultaneous reduction in CRADD and BCL10 expression (CRADD/BCL10 K/D) abrogated the increased IL-6 expression observed in CRADD-deficient cells stimulated with LPS and thrombin (Fig. 2, C and D, right). Thus, increased IL-6 expression in CRADD-depleted cells depends on BCL10. This enhancement of LPS-induced signaling to the nucleus in CRADD K/D LEII cells resulted in predicted downstream activation events as demonstrated by elevated levels of nuclear NFκB RelA/p65 (p65) in LPS-stimulated CRADD K/D cells compared with LPS-stimulated control cells (Fig. 2E).
FIGURE 2.
Expression of IL-6 induced by proinflammatory agonists in CRADD-depleted endothelial cells is dependent on BCL10. CRADD (A) and BCL10 (B) protein knockdown (K/D) induced by shRNA transduction in LEII cells was assessed by immunoblot analysis after 96 h. Shown are immunoblots and mean ± S.D. of proteins from at least 3 independent immunoblots normalized to β-actin and GAPDH cellular protein loading controls for CRADD and BCL10, respectively, with calculation of percent suppression of CRADD and BCL10. C and D, LEII cells were transduced with control, CRADD, and/or BCL10 shRNA as indicated for 96 h then treated with 100 ng/ml of LPS (C) or 1.5 units/ml of thrombin (D). IL-6 in culture media was measured 24 h after stimulation. Results are presented as mean ± S.D. from three independent experiments performed in duplicate (***, p < 0.0001 by t test). E, LEII cells were transduced with control, or CRADD shRNA as indicated for 96 h then treated with 10 ng/ml of LPS for 1 h. Nuclear translocation of NFκB p65/RelA (p65) was assessed by immunofluorescence staining and immunoblot analysis of nuclear extracts. Shown are immunofluorescence and immunoblot images representative of at least 3 independent experiments. Quantification of immunoblots is based on analysis of 6 lanes and shown as mean ± S.D. of proteins normalized to TBP nuclear protein loading control in that lane. Magnification ×40, scale bars = 5 μm. (*, p < 0.05 by t test.)
FIGURE 3.

Endothelial CRADD suppresses mRNA expression of cytokines TNFα, IL-6, and IL-1α in response to proinflammatory agonists. Murine lung capillary endothelial LEII cells were left non-transduced (N), or were transduced with shRNA for CRADD knockdown (KD), or with scrambled control shRNA (C). After 96 h, control and CRADD K/D LEII cells were left unstimulated (U) or stimulated with 1 μg/ml of LPS (L) or with 10 units/ml of thrombin (T) for 24 h. Some CRADD K/D cells were treated with CP-CRADD (CP) or non-CP-CRADD (non-CP) for 2 h before stimulation. β-Actin was used as a control for RT-PCR. A, in unstimulated cells, CRADD expression is reduced by shRNA targeting CRADD but not by non-target scrambled shRNA. LPS (B) and thrombin (C) stimulation increased transcripts for TNFα, IL-6, and IL-1α in cells not transduced with shRNA. Knockdown of CRADD with shRNA targeting CRADD further increases mRNA expression. Treatment with CP-CRADD reduces expression to that of cells without CRADD knockdown. Gels shown are representative of 3 independent experiments. Graphs represent quantification of bands from three gels. Values from unstimulated samples were set as background and NL or NT bands were set to 1. Fold-change from stimulated non-transduced samples (NL or NT) are shown as ±S.D. from three independent experiments (NS, not significant; *, p < 0.05; **, p < 0.01; ***, p < 0.001 by t test).
Proinflammatory Agonist-induced Cytokine Expression Is Suppressed by Replenishing Endogenous CRADD with a Novel Recombinant Cell Penetrating (CP) Protein, CP-CRADD
We reasoned that by increasing the intracellular content of CRADD in endothelial cells we can attenuate their responses to proinflammatory agonists. Consistent with our prior evidence with recombinant cell penetrating SOCS1 and -3 that inhibited inflammation and apoptosis (30, 32, 36), we developed a novel recombinant CP-CRADD protein (Fig. 4A) to restore CRADD protein in CRADD-deficient endothelial cells and analyze its regulatory function. Purity and yields of the recombinant CRADD protein homologs were comparable (Fig. 4B). Intracellular delivery of CP-CRADD was verified in human and murine endothelial cells by immunoblot analysis (Fig. 4C) before use in functional assays, which ultimately provided proof of CP-CRADD intracellular activity.
FIGURE 4.

Design, purity, and intracellular delivery of recombinant CP-CRADD. A, schematic representation of full-length wild-type CRADD, showing different functional domains of the protein including the CARD domain (green) and Death domain (blue). Non-CP-CRADD lacks the membrane translocating motif but contains an N-terminal His6 tag (black), whereas CP-CRADD contains the N-terminal His6 tag (black), followed by a 12-amino acid membrane-translocating motif (red). B, protein staining with Coomassie Blue displays recombinant non-CP-CRADD (25 kDa) and CP-CRADD (26 kDa) proteins. BSA is shown between them for size reference. C, tracking intracellular delivery of CP-CRADD by protease resistance and quantitative immunoblotting. Left, lysates from ST1.6R cells incubated for 1 h with equimolar doses (10 μm) of non-CP-CRADD or CP-CRADD, then treated with proteinase K to remove extracellular proteins. Right, recombinant protein preparations added to cells. All samples were run on the same gel and immunoblotted with an anti-CRADD antibody that recognizes both endogenous and recombinant CRADD in cell lysates as indicated. Immunoblot is representative of two independent experiments performed in triplicate.
As final evidence of the negative regulatory function of CRADD in endothelial cells, CP-CRADD protein delivery to CRADD-depleted LEII cells (CRADD K/D) significantly suppressed both LPS- and thrombin-induced IL-6 expression (Fig. 5A). Consistent with the changes in protein expression, increased mRNA transcripts for TNF-α, IL-6, and IL-1α in CRADD K/D cells were reduced by treatment with CP-CRADD to the levels displayed by CRADD-sufficient LEII cells after stimulation with LPS or thrombin (see Fig. 3, B and C). Moreover, treatment with CP-CRADD significantly reduced the IL-6 protein in CRADD-sufficient LEII control cells stimulated with LPS, although a similar reduction in IL-6 induced by thrombin in the control LEII cells was not apparent (Fig. 5A).
FIGURE 5.
Intracellular delivery of CP-CRADD suppresses agonist-induced IL-6 and MCP-1 expression in wild-type and CRADD-deficient endothelial cells. A, after a 2-h treatment of control or CRADD K/D LEII cells with equimolar doses (6 μm) of non-CP-CRADD or CP-CRADD, cells were stimulated with 100 ng/ml of LPS (left) or 1.5 units/ml of thrombin (right). B, LMEC were isolated from cradd+/+ and cradd−/− mice. Cells were stimulated with 100 ng/ml of LPS. C, LMEC isolated from cradd+/+ and cradd−/− mice were treated for 3 h with equimolar doses (11 μm) of non-CP-CRADD or CP-CRADD, then stimulated with 100 ng/ml of LPS. D, human ST1.6R endothelial cells were treated for 3 h with equimolar doses (11 μm) of non-CP-CRADD or CP-CRADD, then stimulated as in A. IL-6 and MCP-1 in culture media were measured 24 h after stimulation. Results are presented as mean ± S.D. from three independent experiments performed in duplicate (*, p < 0.05; **, p < 0.01; ***, p < 0.0001 by t test).
We next compared the inflammatory response to LPS in LMEC derived from previously characterized cradd−/− and wild-type cradd+/+ control mice (6). As shown in Fig. 1B, LMEC isolated from cradd−/− mice are deficient in CRADD protein, whereas LMEC from wild-type cradd+/+ mice contain endogenous CRADD. Concordant with results obtained in LEII cells, primary LMEC isolated from cradd−/− mice also displayed an enhanced response to LPS stimulation compared with LMEC from wild-type cradd+/+ mice (Fig. 5B). Treatment with CP-CRADD suppressed IL-6 production of LMEC from cradd−/− mice by 43%, and, significantly, in LMEC from cradd+/+ mice, CP-CRADD supplemented endogenous CRADD to reduce their IL-6 production in response to LPS by 35% (Fig. 5C). This beneficial effect of CRADD augmentation was further explored in the human lung microvascular endothelial cell line HPMEC-ST1.6R. The enhanced production of IL-6 and monocyte chemoattractant protein-1 (MCP-1/CCL2) in response to LPS was counteracted by treatment with CP-CRADD, reducing their expression by 40 and 47%, respectively (Fig. 5D). Additionally, thrombin-induced IL-6 and MCP-1 were reduced by 63 and 64%, respectively. The chemokine MCP-1 is known to induce reorganization of tight junctions proteins and increase endothelial permeability (37). Thus, intracellular delivery of recombinant CP-CRADD complemented the negative regulation of cytokine/chemokine expression by endogenous CRADD. The contrast between the results from ST1.6R cells and control LEII cells (Fig. 5A) may be attributed to low expression of IL-6 by LEII cells in response to thrombin.
Agonist-induced Endothelial Monolayer Permeability Is Enhanced in CRADD-deficient Endothelial Cells
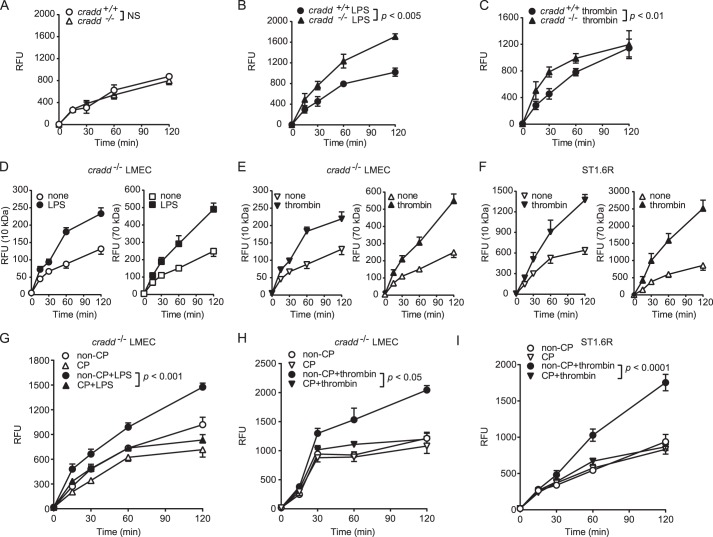
We analyzed the role of CRADD in maintaining endothelial barrier function by first comparing permeability of LMEC monolayers from cradd+/+ and cradd−/− mice. This assay was based on monitoring the passage of FITC-labeled dextran through the monolayer. In the absence of stimulation, there was no difference in permeability between monolayers from cradd−/− and cradd+/+ LMEC (Fig. 6A). We then tested the barrier function of primary LMEC in agonist-induced permeability assays. Proinflammatory agonists LPS and thrombin induced significantly increased permeability in cradd−/− LMEC monolayers as compared with cradd+/+ LMEC monolayers (Fig. 6, B and C). Notably, agonist-induced permeability of endothelial monolayers to both small and large tracers (10-kDa FITC-dextran and 70-kDa FITC-dextran, respectively) was similar (Fig. 6, D–F). CP-CRADD treatment restored barrier function to cradd−/− LMEC (Fig. 6, G and H) stimulated with either LPS or thrombin, providing definitive proof of the negative regulatory function of CRADD in endothelial cells. Moreover, in CRADD-sufficient human ST1.6R cell monolayers, thrombin-induced permeability was reduced by supplementation with CP-CRADD (Fig. 6I), suggesting that supplementation of endogenous CRADD with CP-CRADD may stabilize endothelial barrier function during protracted inflammatory signaling.
FIGURE 6.
Endothelial permeability of CRADD-deficient primary murine LMEC and CRADD-sufficient human ST1. 6R endothelial monolayers is regulated by CP-CRADD. A–C, LMEC isolated from cradd+/+ and cradd−/− mice were grown to confluence on Transwell inserts and left unstimulated (A) or stimulated with 1 μg/ml of LPS for 24 h (B) or 10 units/ml of thrombin for 6 h (C). D–F, permeability of 10-kDa compared with 70-kDa FITC-dextran. LMEC isolated from cradd−/− mice (D and E) were stimulated with LPS (D) or thrombin (E) as in B and C. F, human ST1.6R cells were stimulated with 30 units/ml of thrombin for 20 min. G and H, LMEC isolated from cradd−/− mice were treated for 3 h with equimolar doses (12 μm) of non-CP-CRADD or CP-CRADD, then stimulated with LPS (G) or thrombin (H) as in B and C. I, human ST1.6R cells were stimulated with thrombin as in F. To assess permeability, FITC-dextran (10 kDa in A–C and G–I) was added to each insert and fluorescence in the lower chamber was measured at the indicated times. Results are presented as mean ± S.D. in relative fluorescence units (RFU) from three independent experiments performed in duplicate (p values shown were determined by t test of the area under the curves from 3 independent experiments).
F-Actin Polymerization and Adherens Junctions Are Altered in CRADD-deficient Cells
Stimulation of endothelial cell monolayers with agonists triggers morphological changes through reorganization of the actin cytoskeleton, leading to increased permeability (25, 38–40). We explored the mechanism of increased agonist-induced permeability in CRADD-deficient endothelial monolayers by investigating changes in F-actin organization (Fig. 7) and the adherens junction protein VE-cadherin and its adaptor p120 (Fig. 8) in cradd+/+ and cradd−/− LMEC stimulated with the permeability inducers LPS or thrombin.
FIGURE 7.

F-actin polymerization and cellular contraction are enhanced in cradd−/− as compared with cradd+/+ LMEC monolayers after stimulation with LPS or thrombin. LMEC isolated from cradd+/+ and cradd−/− mice were grown to confluence, then left unstimulated (A and B) or stimulated with 1 μg/ml of LPS for 24 h (C and D) or 10 units/ml of thrombin for 6 h (E and F). Cells were stained with Alexa 488-labeled phalloidin (green) to visualize F-actin. Nuclei are counterstained with DAPI (blue). Arrows indicate gaps in the monolayer caused by cellular retraction in stimulated cells. Images are representative of at least 3 independent experiments. Magnification ×63, scale bars = 1 μm.
FIGURE 8.

CRADD deficiency exacerbates the loss of monolayer integrity in LPS- or thrombin-stimulated LMEC monolayers analyzed by immunofluorescence of VE-cadherin and p120. LMEC isolated from cradd+/+ and cradd−/− mice were grown to confluence, then left unstimulated (A and B) or stimulated with 1 μg/ml of LPS for 24 h (C and D) or 10 units/ml of thrombin for 6 h (E and F). Cells were immunostained with p120 (red) and VE-cadherin (green). Nuclei are counterstained with DAPI (blue). Arrows indicate disruption of both proteins in cell membranes of stimulated cradd−/− LMEC. Images are representative of at least 3 independent experiments. Magnification ×63, scale bars = 1 μm.
Unstimulated LMEC from both cradd+/+ and cradd−/− mice stained with fluorescent phalloidin exhibited an actin cytoskeleton with thin F-actin fibers sparsely crossing the body of cells (Fig. 7, A and B). LPS and thrombin stimulation induced changes in F-actin organization (Fig. 7, C–F), displaying a strong pattern of polymerized actin with prominently thick F-actin fiber bundles. However, loss of peripheral F-actin and more prominent retraction of the cell mass toward the center were evident in cradd−/− LMEC, leading to increased gaps in the monolayer (indicated by arrows) (Fig. 7, D and F).
Immunostaining for the adherens junction protein VE-cadherin and its adaptor p120 produced a strong signal, which appeared as a contiguous line of varied thickness along cell borders in quiescent endothelial cells (Fig. 8, A and B). Please note that unstimulated cradd−/− endothelial cells displayed a spindle-like shape rather than the typical cobblestone pattern of cradd+/+ endothelial cells. LPS and thrombin stimulation triggered a striking distortion of the VE-cadherin/p120 contiguous border pattern with a visible reduction of VE-cadherin/p120 peripheral staining (Fig. 8, C–F). Stimulated cradd−/− LMEC displayed a more dramatic disturbance in the border pattern compared with cradd+/+ LMEC, evidenced by areas of deficient staining surrounding the gaps formed in the initially integral monolayer (indicated by arrows) (Fig. 8, D and F). These results indicate that BCL10-mediated F-actin disorganization (27) and adherens junction disruption are controlled by CRADD.
DISCUSSION
Here we show that the intracellular adaptor CRADD is expressed in non-immune cells. In primary human and murine endothelial cells, CRADD is involved in maintaining their integrity by acting as a negative regulator of the inflammatory response. We document that this physiologic action of CRADD is dependent on its interaction with BCL10, a key component of the CARMA3 signalosome in microvascular endothelial cells. This anti-inflammatory function of CRADD in endothelial cells is consistent with its role as a negative regulator of the BCL10-containing CARMA1 signalosome in immune cells, as documented in our initial study of CRADD (6). BCL10 interacts not only with CARMA1, which is restricted to cells of the immune system, but also with its close homolog, CARMA3, which has a much broader expression pattern including endothelial and epithelial cells (13–16, 18, 41, 42). Therefore, expression of CRADD in endothelial and other cell types, e.g. epithelial cells,3 follows that of BCL10 and may also provide an anti-inflammatory CRADD-BCL10 axis in those cells. CRADD interaction with BCL10 transcends its engagement in the CARMA3 signalosome as BCL10 is known to interact with IRAK-1 (34). CRADD negatively regulates LPS-triggered signaling to the nucleus mediated by NFκB (see Fig. 2E), a process that depends on TLR4-evoked activation of IRAK-1 that binds BCL10 (34). Hence CRADD targeting of BCL10 may reduce the outcome of LPS action on endothelial cells.
Although direct involvement of the CARMA3 signalosome in the LPS/TLR4 pathway is unclear, previous studies have reported that BCL10 and MALT1 play major roles by mediating NFκB activation via the IRAK-1-BCL10-MALT1-TRAF6-TAK1 cascade in the LPS/TLR4 pathway (43, 44). An investigation of Bcl10/Malt1-mediated NFκB signaling in non-immune cells showed that IL-6 production was blocked in the absence of BCL10 (45). Our results, based on co-immunoprecipitation analysis (Fig. 1D) and BCL10 silencing (Fig. 2), also demonstrate BCL10 involvement in the LPS/TLR4 signaling pathway. This pathway is negatively regulated by CRADD and its novel recombinant cell-penetrating homolog CP-CRADD.
Thrombin/PAR-1 signaling to mobilize NFκB for nuclear translocation depends on initial protein kinase C activation, with subsequent steps mediated by BCL10 to engage the canonical NFκB machinery and shift endothelial function toward an “activated” phenotype (46–50). As documented in our current study, thrombin dramatically increased cytokine and chemokine production and significantly induced permeability of the endothelial monolayer. The CARMA3 signalosome links PAR-1-evoked signaling to activation of the IκB kinase signaling complex assembled around TRAF6 through ubiquitination of the NFκB essential modulator (NEMO/IKKγ), the regulatory subunit of the IκB kinase complex (51). In immune cells, BCL10 activates the NFκB pathway through the NFκB essential modulator (52). Although there are significant differences in how the CARMA1 and CARMA3 signalosomes communicate with PAR-1 and other receptors, such as the choice of 3-phosphoinositide-dependent protein kinase 1 and β-arrestin 2, the CARMA3 signalosome shares its positive regulator BCL10 with the CARMA1 signalosome found in lymphocytes (18). Hence BCL10 presents itself as an easy target for CRADD negative regulation of both CARMA1 and CARMA3 signalosomes in agonist-stimulated immune and non-immune cells, respectively.
BCL10 has emerged as a key positive mediator of inflammatory signals, as it has been reported to interact with other CARD domain-containing proteins, including CARD 9, 10, 11, and 14, which are thought to function as upstream regulators in NFκB signaling (43–45, 53). Independent of NFκB signaling, BCL10 is also linked to remodeling of F-actin (27), which is connected to transmembrane junctional proteins that control the barrier function of endothelial cells (26, 40). As the non-immune mainstays of the blood-tissue barrier, endothelial cells are connected by highly regulated tight and adherens junctions, which control paracellular leakage of plasma fluid and proteins that contribute to increased endothelial permeability (54, 55). LPS induces F-actin remodeling through activation of a Src family kinase and TNF receptor-associated factor 6 (TRAF6) (56–58). As shown in Fig. 7, CRADD-deficient cells demonstrate an altered pattern of F-actin polymerization in response to LPS and thrombin stimulation compared with CRADD-sufficient cells. Therefore, CRADD, by targeting BCL10, plays an important role in the negative regulation of BCL10-mediated F-actin polymerization. CRADD deficiency thereby increases inducible but not constitutive permeability of endothelial monolayers compared with CRADD-sufficient endothelium.
Proinflammatory agonist-induced stress fiber formation overlaps with redistributed VE-cadherin (59), an endothelium-specific member of the cadherin family of adhesion proteins found in adherens junctions. Inside the cell, VE-cadherin interacts with its adaptor p120. As shown in Fig. 8, the VE-cadherin/p120 system was highly perturbed in CRADD-deficient cells upon their stimulation with proinflammatory agonists. Thus, the evidence presented here points to another important role for endothelial CRADD as an inducible physiologic regulator of vascular permeability, a cardinal sign of inflammation.
Previous studies have demonstrated that the proinflammatory cytokine IL-6 (35) and chemokine MCP-1 (37) contribute to increased endothelial permeability. This study shows significantly increased IL-6 and MCP-1 expression by CRADD-deficient endothelial cells in response to LPS and thrombin, suggesting that depletion of endogenous CRADD by inflammatory signaling may contribute to the enhanced action of IL-6 and MCP-1 as endothelial permeability inducers. We show that the changes induced by inflammatory signaling are sufficient to allow permeability to large molecules (Fig. 6, D–F), such as plasma proteins. In turn, reduction of BCL10-mediated inflammatory signaling by a novel recombinant protein, CP-CRADD, offers a new strategy to control vascular inflammatory responses not only in non-immune cells (endothelial cells) as demonstrated in this study but also in immune cells. CRADD is also expressed in human brain microvascular endothelial cells (60), suggesting its potential anti-inflammatory function there as well.
In summary, this study provides new evidence that CRADD plays a pivotal role in maintaining the integrity of endothelial monolayers. A better appreciation of the role of CRADD in endothelium may contribute to a deeper understanding of endothelial dysfunction and inform the development of a novel treatment for inflammatory vascular disorders.
Acknowledgments
Fluorescence microscopy and analysis was performed in part through the use of the VUMC Cell Imaging Shared Resource. Cell Imaging and other Core Services were funded in part through Vanderbilt University Medical Center's Digestive Disease Research Center supported by National Institutes of Health Grant P30DK058404 and the Vanderbilt Ingram Cancer Center supported by National Institutes of Health Grant P30CA068485.
This work was supported, in whole or in part, by National Institutes of Health Grants HL069452, HL085833, and AA015752 (to J. H.) from the United States Public Health Service, Ruth Kirschstein Institutional National Service Award T32 HL069765 (to H. Q.), the Vanderbilt Immunotherapy Program and Department of Medicine, and Vanderbilt Clinical and Translational Science Award UL1TR000445.
H. Qiao and J. Hawiger, unpublished data.
- IRAK
- interleukin-1 receptor-associated kinase
- BCL10
- B-cell CLL/lymphoma 10
- CARD
- caspase-recruitment domain
- CARMA
- CARD membrane-associated guanylate kinase
- CP
- cell-penetrating
- CRADD
- caspase and receptor interacting protein adaptor with Death domain
- K/D
- knockdown
- LMEC
- lung microvascular endothelial cells
- MALT1
- mucosa-associated lymphoid tissue lymphoma translocation protein 1
- MCP-1
- monocyte chemoattractant protein-1
- NFκB
- nuclear factor κB
- PAR-1
- protease-activated receptor 1
- RAIDD
- receptor interacting protein-associated ICH-1/CED-3 homologous protein with a Death domain
- SOCS
- suppressor of cytokine signaling
- TBP
- TATA-binding protein
- TLR4
- Toll-like receptor 4
- TRAF6
- TNF receptor-associated factor 6
- VE-cadherin
- vascular endothelial cadherin.
REFERENCES
- 1. Hawiger J., Musser J. M. (2011) How to approach genome wars in sepsis? Crit. Care 15, 1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Alexander W. S., Hilton D. J. (2004) The role of suppressors of cytokine signaling (SOCS) proteins in regulation of the immune response. Annu. Rev. Immunol. 22, 503–529 [DOI] [PubMed] [Google Scholar]
- 3. Liew F. Y., Xu D., Brint E. K., O'Neill L. A. (2005) Negative regulation of toll-like receptor-mediated immune responses. Nat. Rev. Immunol. 5, 446–458 [DOI] [PubMed] [Google Scholar]
- 4. Rakesh K., Agrawal D. K. (2005) Controlling cytokine signaling by constitutive inhibitors. Biochem. Pharmacol. 70, 649–657 [DOI] [PubMed] [Google Scholar]
- 5. Coornaert B., Carpentier I., Beyaert R. (2009) A20: central gatekeeper in inflammation and immunity. J. Biol. Chem. 284, 8217–8221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lin Q., Liu Y., Moore D. J., Elizer S. K., Veach R. A., Hawiger J., Ruley H. E. (2012) Cutting edge: the “death” adaptor CRADD/RAIDD targets BCL10 and suppresses agonist-induced cytokine expression in T lymphocytes. J. Immunol. 188, 2493–2497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Paul S., Schaefer B. C. (2013) A new look at T cell receptor signaling to nuclear factor-κB. Trends Immunol. 34, 269–281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Egawa T., Albrecht B., Favier B., Sunshine M. J., Mirchandani K., O'Brien W., Thome M., Littman D. R. (2003) Requirement for CARMA1 in antigen receptor-induced NF-κB activation and lymphocyte proliferation. Curr. Biol. 13, 1252–1258 [DOI] [PubMed] [Google Scholar]
- 9. Gaide O., Favier B., Legler D. F., Bonnet D., Brissoni B., Valitutti S., Bron C., Tschopp J., Thome M. (2002) CARMA1 is a critical lipid raft-associated regulator of TCR-induced NF-κB activation. Nat. Immunol. 3, 836–843 [DOI] [PubMed] [Google Scholar]
- 10. Hara H., Wada T., Bakal C., Kozieradzki I., Suzuki S., Suzuki N., Nghiem M., Griffiths E. K., Krawczyk C., Bauer B., D'Acquisto F., Ghosh S., Yeh W. C., Baier G., Rottapel R., Penninger J. M. (2003) The MAGUK family protein CARD11 is essential for lymphocyte activation. Immunity 18, 763–775 [DOI] [PubMed] [Google Scholar]
- 11. Jun J. E., Wilson L. E., Vinuesa C. G., Lesage S., Blery M., Miosge L. A., Cook M. C., Kucharska E. M., Hara H., Penninger J. M., Domashenz H., Hong N. A., Glynne R. J., Nelms K. A., Goodnow C. C. (2003) Identifying the MAGUK protein Carma-1 as a central regulator of humoral immune responses and atopy by genome-wide mouse mutagenesis. Immunity 18, 751–762 [DOI] [PubMed] [Google Scholar]
- 12. Newton K., Dixit V. M. (2003) Mice lacking the CARD of CARMA1 exhibit defective B lymphocyte development and impaired proliferation of their B and T lymphocytes. Curr. Biol. 13, 1247–1251 [DOI] [PubMed] [Google Scholar]
- 13. Sun J. (2010) CARMA3: A novel scaffold protein in regulation of NF-kappaB activation and diseases. World J. Biol. Chem. 1, 353–361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Jiang C., Lin X. (2012) Regulation of NF-κB by the CARD proteins. Immunol. Rev. 246, 141–153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. McAllister-Lucas L. M., Jin X., Gu S., Siu K., McDonnell S., Ruland J., Delekta P. C., Van Beek M., Lucas P. C. (2010) The CARMA3-Bcl10-MALT1 signalosome promotes angiotensin II-dependent vascular inflammation and atherogenesis. J. Biol. Chem. 285, 25880–25884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Martin D., Galisteo R., Gutkind J. S. (2009) CXCL8/IL8 stimulates vascular endothelial growth factor (VEGF) expression and the autocrine activation of VEGFR2 in endothelial cells by activating NFκB through the CBM (Carma3/Bcl10/Malt1) complex. J. Biol. Chem. 284, 6038–6042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kenne E., Lindbom L. (2011) Imaging inflammatory plasma leakage in vivo. Thromb. Haemost. 105, 783–789 [DOI] [PubMed] [Google Scholar]
- 18. Delekta P. C., Apel I. J., Gu S., Siu K., Hattori Y., McAllister-Lucas L. M., Lucas P. C. (2010) Thrombin-dependent NF-κB activation and monocyte/endothelial adhesion are mediated by the CARMA3·Bcl10·MALT1 signalosome. J. Biol. Chem. 285, 41432–41442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Blonska M., Lin X. (2009) CARMA1-mediated NF-κB and JNK activation in lymphocytes. Immunol. Rev. 228, 199–211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Colgan O. C., Ferguson G., Collins N. T., Murphy R. P., Meade G., Cahill P. A., Cummins P. M. (2007) Regulation of bovine brain microvascular endothelial tight junction assembly and barrier function by laminar shear stress. Am. J. Physiol. Heart Circ. Physiol. 292, H3190–H3197 [DOI] [PubMed] [Google Scholar]
- 21. Goldenberg N. M., Steinberg B. E., Slutsky A. S., Lee W. L. (2011) Broken barriers: a new take on sepsis pathogenesis. Sci. Transl. Med. 3, 88ps25. [DOI] [PubMed] [Google Scholar]
- 22. Vestweber D. (2008) VE-cadherin: the major endothelial adhesion molecule controlling cellular junctions and blood vessel formation. Arterioscler. Thromb. Vasc. Biol. 28, 223–232 [DOI] [PubMed] [Google Scholar]
- 23. Herwig M. C., Tsokos M., Hermanns M. I., Kirkpatrick C. J., Müller A. M. (2013) Vascular endothelial cadherin expression in lung specimens of patients with sepsis-induced acute respiratory distress syndrome and endothelial cell cultures. Pathobiology 80, 245–251 [DOI] [PubMed] [Google Scholar]
- 24. Garcia J. G., Verin A. D., Schaphorst K. L. (1996) Regulation of thrombin-mediated endothelial cell contraction and permeability. Semin. Thromb. Hemost. 22, 309–315 [DOI] [PubMed] [Google Scholar]
- 25. Wang Y., Chen H., Li H., Zhang J., Gao Y. (2013) Effect of angiopoietin-like protein 4 on rat pulmonary microvascular endothelial cells exposed to LPS. Int. J. Mol. Med. 32, 568–576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Marion S., Mazzolini J., Herit F., Bourdoncle P., Kambou-Pene N., Hailfinger S., Sachse M., Ruland J., Benmerah A., Echard A., Thome M., Niedergang F. (2012) The NF-κB signaling protein Bcl10 regulates actin dynamics by controlling AP1 and OCRL-bearing vesicles. Dev. Cell 23, 954–967 [DOI] [PubMed] [Google Scholar]
- 27. Rueda D., Gaide O., Ho L., Lewkowicz E., Niedergang F., Hailfinger S., Rebeaud F., Guzzardi M., Conne B., Thelen M., Delon J., Ferch U., Mak T. W., Ruland J., Schwaller J., Thome M. (2007) Bcl10 controls TCR- and FcγR-induced actin polymerization. J. Immunol. 178, 4373–4384 [DOI] [PubMed] [Google Scholar]
- 28. Unger R. E., Krump-Konvalinkova V., Peters K., Kirkpatrick C. J. (2002) In vitro expression of the endothelial phenotype: comparative study of primary isolated cells and cell lines, including the novel cell line HPMEC-ST1.6R. Microvasc. Res. 64, 384–397 [DOI] [PubMed] [Google Scholar]
- 29. Schreiber A. B., Kenney J., Kowalski W. J., Friesel R., Mehlman T., Maciag T. (1985) Interaction of endothelial cell growth factor with heparin: characterization by receptor and antibody recognition. Proc. Natl. Acad. Sci. U.S.A. 82, 6138–6142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Fletcher T. C., DiGiandomenico A., Hawiger J. (2010) Extended anti-inflammatory action of a degradation-resistant mutant of cell-penetrating suppressor of cytokine signaling 3. J. Biol. Chem. 285, 18727–18736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Qiao H., May J. M. (2012) Interaction of the transcription start site core region and transcription factor YY1 determine ascorbate transporter SVCT2 exon 1a promoter activity. PloS One 7, e35746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Jo D., Liu D., Yao S., Collins R. D., Hawiger J. (2005) Intracellular protein therapy with SOCS3 inhibits inflammation and apoptosis. Nat. Med. 11, 892–898 [DOI] [PubMed] [Google Scholar]
- 33. Bhattacharyya S., Borthakur A., Dudeja P. K., Tobacman J. K. (2010) Lipopolysaccharide-induced activation of NF-κB non-canonical pathway requires BCL10 serine 138 and NIK phosphorylations. Exp. Cell Res. 316, 3317–3327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Dong W., Liu Y., Peng J., Chen L., Zou T., Xiao H., Liu Z., Li W., Bu Y., Qi Y. (2006) The IRAK-1-BCL10-MALT1-TRAF6-TAK1 cascade mediates signaling to NF-κB from Toll-like receptor 4. J. Biol. Chem. 281, 26029–26040 [DOI] [PubMed] [Google Scholar]
- 35. Desai T. R., Leeper N. J., Hynes K. L., Gewertz B. L. (2002) Interleukin-6 causes endothelial barrier dysfunction via the protein kinase C pathway. J. Surg. Res. 104, 118–123 [DOI] [PubMed] [Google Scholar]
- 36. DiGiandomenico A., Wylezinski L. S., Hawiger J. (2009) Intracellular delivery of a cell-penetrating SOCS1 that targets IFN-γ signaling. Sci. Signal. 2, ra37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Stamatovic S. M., Keep R. F., Kunkel S. L., Andjelkovic A. V. (2003) Potential role of MCP-1 in endothelial cell tight junction “opening”: signaling via Rho and Rho kinase. J. Cell Sci. 116, 4615–4628 [DOI] [PubMed] [Google Scholar]
- 38. He F., Peng J., Deng X. L., Yang L. F., Wu L. W., Zhang C. L., Yin F. (2011) RhoA and NF-κB are involved in lipopolysaccharide-induced brain microvascular cell line hyperpermeability. Neuroscience 188, 35–47 [DOI] [PubMed] [Google Scholar]
- 39. Bogatcheva N. V., Zemskova M. A., Kovalenkov Y., Poirier C., Verin A. D. (2009) Molecular mechanisms mediating protective effect of cAMP on lipopolysaccharide (LPS)-induced human lung microvascular endothelial cells (HLMVEC) hyperpermeability. J. Cell Physiol. 221, 750–759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. van der Heijden M., van Nieuw Amerongen G. P., van Bezu J., Paul M. A., Groeneveld A. B., van Hinsbergh V. W. (2011) Opposing effects of the angiopoietins on the thrombin-induced permeability of human pulmonary microvascular endothelial cells. PloS One 6, e23448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Wegener E., Krappmann D. (2007) CARD-Bcl10-Malt1 Signalosomes: missing link to NF-κB. Sci. STKE 2007, pe21. [DOI] [PubMed] [Google Scholar]
- 42. Fraser C. C. (2008) G protein-coupled receptor connectivity to NF-κB in inflammation and cancer. Int. Rev. Immunol. 27, 320–350 [DOI] [PubMed] [Google Scholar]
- 43. Bhattacharyya S., Borthakur A., Pant N., Dudeja P. K., Tobacman J. K. (2007) Bcl10 mediates LPS-induced activation of NF-κB and IL-8 in human intestinal epithelial cells. Am. J. Physiol. Gastrointest. Liver Physiol. 293, G429–G437 [DOI] [PubMed] [Google Scholar]
- 44. Wang D., You Y., Lin P. C., Xue L., Morris S. W., Zeng H., Wen R., Lin X. (2007) Bcl10 plays a critical role in NF-κB activation induced by G protein-coupled receptors. Proc. Natl. Acad. Sci. U.S.A. 104, 145–150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Klemm S., Zimmermann S., Peschel C., Mak T. W., Ruland J. (2007) Bcl10 and Malt1 control lysophosphatidic acid-induced NF-κB activation and cytokine production. Proc. Natl. Acad. Sci. U.S.A. 104, 134–138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Coughlin S. R. (2000) Thrombin signalling and protease-activated receptors. Nature 407, 258–264 [DOI] [PubMed] [Google Scholar]
- 47. Minami T., Sugiyama A., Wu S. Q., Abid R., Kodama T., Aird W. C. (2004) Thrombin and phenotypic modulation of the endothelium. Arterioscler. Thromb. Vasc. Biol. 24, 41–53 [DOI] [PubMed] [Google Scholar]
- 48. Major C. D., Santulli R. J., Derian C. K., Andrade-Gordon P. (2003) Extracellular mediators in atherosclerosis and thrombosis: lessons from thrombin receptor knockout mice. Arterioscler. Thromb. Vasc. Biol. 23, 931–939 [DOI] [PubMed] [Google Scholar]
- 49. Hirano K. (2007) The roles of proteinase-activated receptors in the vascular physiology and pathophysiology. Arterioscler. Thromb. Vasc. Biol. 27, 27–36 [DOI] [PubMed] [Google Scholar]
- 50. Martorell L., Martínez-González J., Rodríguez C., Gentile M., Calvayrac O., Badimon L. (2008) Thrombin and protease-activated receptors (PARs) in atherothrombosis. Thromb. Haemost. 99, 305–315 [DOI] [PubMed] [Google Scholar]
- 51. Chen Z. J. (2012) Ubiquitination in signaling to and activation of IKK. Immunol. Rev. 246, 95–106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Zhou H., Wertz I., O'Rourke K., Ultsch M., Seshagiri S., Eby M., Xiao W., Dixit V. M. (2004) Bcl10 activates the NF-κB pathway through ubiquitination of NEMO. Nature 427, 167–171 [DOI] [PubMed] [Google Scholar]
- 53. McAllister-Lucas L. M., Ruland J., Siu K., Jin X., Gu S., Kim D. S., Kuffa P., Kohrt D., Mak T. W., Nuñez G., Lucas P. C. (2007) CARMA3/Bcl10/MALT1-dependent NF-κB activation mediates angiotensin II-responsive inflammatory signaling in nonimmune cells. Proc. Natl. Acad. Sci. U.S.A. 104, 139–144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Aghajanian A., Wittchen E. S., Allingham M. J., Garrett T. A., Burridge K. (2008) Endothelial cell junctions and the regulation of vascular permeability and leukocyte transmigration. J. Thromb. Haemost. 6, 1453–1460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Vandenbroucke E., Mehta D., Minshall R., Malik A. B. (2008) Regulation of endothelial junctional permeability. Ann. N.Y. Acad. Sci. 1123, 134–145 [DOI] [PubMed] [Google Scholar]
- 56. Bannerman D. D., Goldblum S. E. (1999) Direct effects of endotoxin on the endothelium: barrier function and injury. Lab. Investig. 79, 1181–1199 [PubMed] [Google Scholar]
- 57. Gong P., Angelini D. J., Yang S., Xia G., Cross A. S., Mann D., Bannerman D. D., Vogel S. N., Goldblum S. E. (2008) TLR4 signaling is coupled to SRC family kinase activation, tyrosine phosphorylation of zonula adherens proteins, and opening of the paracellular pathway in human lung microvascular endothelia. J. Biol. Chem. 283, 13437–13449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Liu A., Gong P., Hyun S. W., Wang K. Z., Cates E. A., Perkins D., Bannerman D. D., Puché A. C., Toshchakov V. Y., Fang S., Auron P. E., Vogel S. N., Goldblum S. E. (2012) TRAF6 protein couples Toll-like receptor 4 signaling to Src family kinase activation and opening of paracellular pathway in human lung microvascular endothelia. J. Biol. Chem. 287, 16132–16145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Yuan D., He P. (2012) Vascular remodeling alters adhesion protein and cytoskeleton reactions to inflammatory stimuli resulting in enhanced permeability increases in rat venules. J. Appl. Physiol. 113, 1110–1120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Londoño D., Carvajal J., Strle K., Kim K. S., Cadavid D. (2011) IL-10 Prevents apoptosis of brain endothelium during bacteremia. J. Immunol. 186, 7176–7186 [DOI] [PubMed] [Google Scholar]