Background: Guanine nucleotide exchange factors (GEFs) regulate the activity of small GTPases to control cellular functions.
Results: Gef3p interacts with the GTPase Rho3p at the division site and localizes to the septin ring.
Conclusion: The module Gef3p/Rho3p contributes to downstream events in the secretory pathway.
Significance: The interactions between septins and Rho-GEFs provide a new targeting mechanism for GTPases to direct secretion in cytokinesis.
Keywords: Cytokinesis, Guanine Nucleotide Exchange Factor (GEF), Morphogenesis, Rho GTPases, Yeast Genetics
Abstract
The small GTP-binding proteins of the Rho family and its regulatory proteins play a central role in cytokinetic actomyosin ring assembly and cytokinesis. Here we show that the fission yeast guanine nucleotide exchange factor Gef3p interacts with Rho3p at the division site. Gef3p contains a putative DH homology domain and a BAR/IMD-like domain. The protein localized to the division site late in mitosis, where it formed a ring that did not constrict with actomyosin ring (cytokinetic actomyosin ring) invagination; instead, it split into a double ring that resembled the septin ring. Gef3p co-localized with septins and Mid2p and required septins and Mid2p for its localization. Gef3p interacts physically with the GTP-bound form of Rho3p. Although Gef3p is not essential for cell separation, the simultaneous disruption of gef3+ and Rho3p-interacting proteins, such as Sec8p, an exocyst component, Apm1p, a subunit of the clathrin adaptor complex or For3p, an actin-polymerizing protein, yielded cells with strong defects in septation and polarity respectively. Our results suggest that interactions between septins and Rho-GEFs provide a new targeting mechanism for GTPases in cytokinesis, in this case probably contributing to Rho3p function in vesicle tethering and vesicle trafficking in the later steps of cell separation.
Introduction
Cytokinesis is the final step in the process of cell division, during which two daughter cells are generated. From animal to fungal cells, cytokinesis requires coordinated contractile actomyosin ring closure and plasma membrane extension (1, 2). In addition, fungal cytokinesis requires the synthesis of a special division wall termed septum. Cells of the fission yeast Schizosaccharomyces pombe are rod-shaped and divide by medial fission. In S. pombe cells, cytokinesis starts at late anaphase, triggered by activation of the septation initiation network that promotes contractile actomyosin ring constriction and plasma membrane ingression (3). Both processes guide the deposition of a multilayered division septum composed of a middle disk, called the primary septum, covered on both sides by the secondary septum (4–6). The last step in cytokinesis is cell separation by controlled cell-wall and primary septum degradation. Correct septum formation, and especially cell separation, are critical processes for cell integrity and survival and must be tightly restricted in space and time (7, 8).
Guanine nucleotide-exchange factors (GEFs)4 of the Rho family are key proteins in the process of cytokinesis in mammals and budding yeast (9–11). GEFs are responsible for the activation of Rho-family GTPases in response to diverse stimuli; GEFs are much larger and complex proteins than the GTPases themselves, and they contain protein-protein interaction domains that could be important for GTPase localization, activation, stabilization, and interaction with their effectors (12–15).
S. pombe contains eight proteins with a Rho-GEF domain (Scd1p, Gef1p, Gef2p, Gef3p, Rgf1p, Rgf2p, Rgf3p, and Mug10p) (16–18) and six Rho GTPases (Cdc42p and Rho1p, Rho2p, Rho3p, Rho4p, and Rho5p). Scd1p and Gef1p are Cdc42p-specific GEFs. Gef1p and Scd1p form a ring structure at the cell division site that shrinks during cytokinesis after contraction of the contractile actomyosin ring. Both Scd1p and Gef1p collaborate in the recruitment of active Cdc42p to the septation site (19, 20). Gef2p is involved in division-site and contractile-ring positioning by interacting with the anillin-related protein Mid1p (21). Rgf1p, Rgf2p, and Rgf3p function as GEFs for Rho1p (the regulatory subunit of (1,3)-β-d-glucan synthase). Although Rgf1p and Rgf2p localize to the septum area, to date it has not been shown whether they play a role in cell division (22–25). Rgf3p activates Rho1p specifically in cytokinesis; it localizes exclusively to the middle region of the cell and is essential for maintaining cell integrity during cell separation (25–27). Gef2p, Gef3p, and Mug10p have not been assigned to any known GTPase, and the functions of Gef3p and Mug10p are unknown.
In this study we observed that a putative Rho-GEF, Gef3p, interacted physically with Rho3p. In fission yeast Rho3p has been isolated as a multicopy suppressor of sec8-1, a mutant allele of a component of the exocyst complex (28). The exocyst is highly conserved from yeasts to mammals and is involved in the late stages of exocytosis by targeting and tethering post-Golgi vesicles to the plasma membrane (29, 30). In S. pombe, mutations in exocyst components show defects in cell separation (28, 31, 32). Rho3p restored exocyst localization in a cdc42 thermosensitive mutant with multiple membrane traffic defects (33), and it has been implicated in the regulation of Golgi/endosome trafficking through a functional interaction with adaptin (clathrin-associated adaptor protein-1) and Sip1p (34, 35). Thus, it is possible that Rho3p could stimulate secretion by locally increasing the exocytic apparatus or through Golgi/endosome regulation.
In addition, Rho3p has been implicated in polarized cell growth through both formin For3p (36) and Pob1p function (37). For3p and Pob1p mediate the formation of the actin cables that serve as tracks for the type V myosin-dependent delivery of secretory vesicles to sites of growth (37–40). Here we report that Gef3p interacts physically and functionally with Rho3p and plays a role during cytokinesis. In addition, Gef3p localization to the septin ring might provide a scaffold for septin-mediated Rho3p-directed polarized secretion during septum formation and cell separation.
EXPERIMENTAL PROCEDURES
Media, Reagents, and Genetics
The genotypes of the S. pombe strains used in this study are listed in Table 1. The complete yeast growth medium (YES), selective medium (MM) supplemented with the appropriate requirements, and sporulation medium (MEA) have been described elsewhere (41). Crosses were performed by mixing appropriate strains directly on MEA plates. For overexpression experiments using the nmt1 promoter, cells were grown in selective medium (MM) containing 15 μm thiamine up to the logarithmic phase. Then the cells were harvested, washed 3 times with water, and inoculated in fresh medium without thiamine (−T) at an A600 = 0.01 for 16, 18, or 22 h, depending on the experiment.
TABLE 1.
S. pombe strains used in this work
All strains were generated in this study except as indicated in the footnotes.
| Strains | Genotypes |
|---|---|
| YS64 | h− leu1-32 ade6M210 ura4D-18 his3D1 |
| SM116 | h− gef3::ura4+ leu1-32 ade6M210 ura4D-18 his3D1 |
| NG69 | h− cdc25-22 leu1-32 ura4D18 |
| SM176 | h− gef3::ura4+ cdc25-22 leu1-32 ura4D18 |
| PG153a | h+ gef1::kanMX6 leu1-32 ura4D18 |
| SM143 | h− gef3::ura4+ gef1::kanMX6 leu1-32 ura4D18 |
| CL5 | h− gef2::ura4+ leu1-32 ade6M210 ura4D-18 his3D1 |
| SM172 | h− gef2::ura4+ gef3::his3+ leu1-32 ade6M210 ura4D-18 his3D1 |
| PG154a | h+ scd1::kanMX6 leu1-32 ura4D18 |
| SM157 | h− gef3::ura4+ scd1::kanMX6 leu1-32 ura4D18 |
| VT14 | h− rgf1::his3+ leu1-32 ade6M210 ura4D18 his3d1 |
| SM148 | h− gef3::ura4+ rgf1::his3+ leu1-32 ade6M210 ura4D1 his3d1 |
| PG1 | h− rgf2::ura4+ his3d1 ura4D18 leu1-32 ade6M210 |
| SM150 | h− gef3::ura4+ rgf2::ura4+ his3d1 ura4D18 leu1-32 ade6M210 |
| MS264 | h− ehs2–1 leu1-32 ura4D18 |
| SM152 | h− gef3::ura4+ ehs2–1 leu1-32 ura4D18 |
| SM217 | h− ura4+:GFP-gef3+ leu1-32 ura4D18 |
| SM267 | h− GFP-gef3:KanMX6 rlc-tdTomato:natMX6 ura4D18 |
| SM263 | h− ura4+:tdTomato-gef3+ leu1-32 ura4D18 |
| PPG2772a | h− spn3-GFP:kanMX6 leu1-32 ade6M210 ura4D-18 his3D1 |
| SM197b | h− mid2-GFP:kanMX6 leu1-32 ura4D-18 |
| SM297 | h− spn3-GFP:kanMX6-tdTomato gef3:ura4+ leu1-32 ura4D-18 |
| SM295 | h− mid2-GFP:kanMX6 tdTomato-gef3:ura4+ leu1-32 ura4D-18 |
| SM277c | h− GFP-gef3:KanMX6 spn1–5::ura4+ura4D18 |
| SM253 | h− GFP-gef3:KanMX6 mid2::ura4+ ura4D18 leu1-32 |
| SM273 | h− spn3-GFP:kanMX6 gef3::ura4+ leu1-32 ade6M210 ura4D-18 his3D1 |
| SM229 | h− mid2-GFP:kanMX6 gef3::ura4+ leu1-32 ura4D-18 |
| SM271 | h− GFP-gef3:ura4+ exo70-RFP:kanMX6 leu1-32 ura4D18 |
| SM259 | h− GFP-gef3:KanMX6 exo70::ura4+ ura4D18 leu1-32 |
| SM261 | h− GFP-gef3:KanMX6 sec8–1 ura4D18 leu1-32 |
| MBY797b | h− sec6-GFP:ura4+ leu1-32 ura4D18 |
| MBY856b | h− sec8-GFP:ura4+ leu1-32 ura4D18 |
| SM244 | h− sec6-GFP:ura4+ gef3::KanMX6 leu1-32 ura4D18 |
| SM245 | h− sec8-GFP:ura4+ gef3::KanMX6 leu1-32 ura4D18 |
| SM242a | h− kanMX6-GFP-rho3+ leu1-32 ura4D18 |
| SM594 | h− kanMX6-GFP-rho3+ gef3::ura4+ leu1-32 ura4D18 |
| PPG3740a | h+ rho3::ura4+ leu1-32 ade6M210 ura4D18 |
| EM257 | h− rho3::ura4+ gef3::KanMX6 leu1-32 ade6M210 ura4D18 |
| SM686 | h− kanMX6-GFP-rho3+ sid4-RFP:natMX6 leu1-32 ura4D18 |
| EM83a | h90 leu1:GFP-Psy1 |
| EM256 | h− GFP-Psy: leu gef3::KanMX6 |
| MBY919b | h− exo70::ura4+ leu1-32 ura4D-18 |
| SM243 | h− exo70::ura4+ gef3::KanMX6 leu1-32 ura4D-18 |
| MBY887b | h+ sec8–1 leu1-32 ura4D18 |
| SM226 | h− sec8–1 gef3::ura4+ leu1-32 ura4D18 |
| PPG2567a | h− for3::KanMX1 leu1-32 ura4D18 |
| SM300 | h− for3::KanMX1 gef3::ura4+leu1-32 ura4D18 |
| SM708d | h− apm1::ura4+ leu1-32 ura4D18 |
| SM721 | h− gef3::KanMX6, apm1::ura4+ leu1-32 |
a From P. Pérez, IBFG, University of Salamanca.
b From C. R. Vázquez de Aldana, IBFG, University of Salamanca.
c From J. Bähler, Wellcome Trust, UK.
d From H. Valdivieso, IBFG, University of Salamanca.
Disruption of the gef3+ Gene
The gef3::ura4+ disruption construct was obtained in a two-step process. The 5′ non-coding region of the gef3+ open reading frame (ORF) (nucleotides (nt −1584 to −40)) was amplified by PCR, inserting the ApaI and SalI sites (one at each end), and was ligated into the same sites of the SK-ura4+ vector. The 3′ flanking region of the gef3+ ORF (nt +1609 to +3577) was amplified by PCR, inserting the PstI and NotI sites as above, and was cloned into the same sites of pSK-ura+ with the 5′ end to yield pSM31. gef3+ gene disruption was accomplished using the 5.2-kb fragment from pSM31 cut with ApaI and NotI and transforming the YS64 haploid strain. Correct deletion of the gef3+ ORF was confirmed by PCR analysis using oligonucleotides: M13 (5′-CTGGTGGCCTTAGGTAA-3′) in the ura4+ gene and Gef3-Ext-5′ (5′-CTCCTAAGAAGCGGGAAG-3′) upstream from nucleotide +1153 and hence external to the disruption cassette.
Plasmid and DNA Manipulations
To make pALgef3+ (pPB7), the gef3+ ORF was obtained from two PCR fragments; a BglII-PstI fragment (nt −80 to +1170) and a PstI-SmaI fragment (nt +1170 to +1645); both fragments were cloned into the same sites of pSM31 (described above) to make pPB7. The gef3+ ORF was confirmed by the sequencing of four different clones of pPB7. To tag Gef3p at the N terminus with enhanced green fluorescent protein (GFP) (engineered with eight alanines at the C terminus) and with tdTomato (Cherry variant; Ref. 42), pAL-gef3+ (pPB7) was modified by site-directed mutagenesis. The NotI site at the multiple cloning sites was destroyed, and site-directed mutagenesis was used to create a NotI site just before the ATG codon (pSM70). The GFP and tdTomato epitopes were inserted in-frame at the NotI site of pSM70 to create pSM71 and pSM74, respectively. Strains with a genomic copy of gef3+ tagged with GFP or tdTomato were obtained by one-step gene replacement. pSM71 and pSM74 were modified to create a XhoI and a BamHI restriction site (nt −1220) before the promoter of the corresponding tagged version of the gef3+ ORF, allowing the insertion of the ura4+ marker at those sites and thus creating pSM86 and pSM87, respectively. An ApaI-XbaI cassette from pSM86 and pSM87 were used to transform strain YS64, respectively, creating the SM217 (GFP-gef3+) and SM263 (tdTomato-gef3+) strains. In addition, pSM89 and pSM90 were created, both containing cassettes (KanMX6-GFP-gef3+) and (KanMX6-tdTomato-gef3+) for kanamycin selection of the tagged gef3+. For gef3+ and GFP-tagged gef3+ overexpression, pPB7 (pAL-gef3+) and pSM71 (pAL-GFP-gef3+) were modified by site-directed mutagenesis, introducing XhoI and SmaI sites flanking the gef3+ ORF. The resulting XhoI-SmaI fragments were cloned into the same sites of pREP3x, thus affording pREP3x-gef3+ and pREP3x-GFP-gef3+. The strain with GFP-Rho3p tagged at the N terminus and plasmid pREP4x-GST-rho3+ were kindly provided by P. Perez (Instituto de Biología Funcional y Genómica (IBFG), Salamanca, Spain). pREP4x-GST-rho3+ was modified by site-directed mutagenesis to replace glycine 22 by valine, creating pREP4x-GST-rho3+G22V.
Microscopy and Image Analysis
Cell samples were visualized using an Olympus IX71 microscope equipped with a personal Delta Vision system and a Photometrics CoolSnap HQ2 monochrome camera. Stacks of seven z-series sections were acquired at 0.2-μm intervals. All fluorescence images are maximum two-dimensional projections of z-series and were analyzed using deconvolution software from Applied Precision. Measurements were made from micrographs using the IMAGEJ (National Institutes of Health) or METAMORPH (Molecular Devices) programs. For Calcofluor (Cfw), cells were harvested (1 ml), washed once, and resuspended in water with 20 μg/ml Cfw at room temperature. For time-lapse imaging, 0.3–0.6 ml of log-phase cells were collected by low speed centrifugation (3000 × g for 1 min), suspended in 0.3 ml of YES media containing Cfw (5 μg/ml final concentration), and placed in a well from a μ-Slide 8 well (80821-Uncoated; Ibidi) previously coated with 10 μl of 1 mg/ml soybean lectin (L1395; Sigma).
Yeast Two-hybrid Analysis
For two-hybrid screenings, the entire ORF of gef3+ w/o intron was modified to introduce BamHI and NcoI flanking sites and was cloned into the same sites of pGADT7 (Clontech) to express the GAL4-activating domain fused to the Gef3p (pSM73). GTPases rho1+ to rho5+ and cdc42+ cloned into pAS2 were kindly provided by Perez and P. M. Coll (IBFG, Salamanca, Spain) (43) and were used as bait against gef3+. The Saccharomyces cerevisiae AH109 strain, which carries the GAL4 recognition sequence and the lacZ and HIS3 reporter genes, was transformed with different combinations of plasmids.
GST Pulldown Assay
For Gef3p/Rho3p GST pulldown assays, the wild-type strain expressing pAL-GFP-gef3+ (pSM107) was transformed with either pREP4x-GST, pREP4x-GST-rho3+, or pREP4x-GST-rho3+ G22V (pSM139). Rho3 protein expression was induced by growing the cells in the absence of thiamine for 15 h at 28 °C. Cell homogenates from 50-ml cultures (absorbance = 0.6) were obtained using 200 μl of lysis buffer (50 mm HCl-Tris, pH 7.5, 200 mm NaCl, 2 mm EDTA, 0.5% Nonidet P-40, containing 1 mm p-amino phenylmethanesulfonyl fluoride, 1 μg/ml leupeptin, 1 μg/ml aprotinin, and 1 μg/ml pepstatin). Extracts were cleared by centrifugation at 2500 × g for 5 min, and ∼1 mg of total protein was used in each immunoprecipitation in a final volume of 600 μl. The extracts were incubated with 20 μl of glutathione-Sepharose beads for 2 h at 4 °C in immunoprecipitation buffer (same as the lysis buffer w/o Nonidet P-40 and with 2% Triton X-100 instead). The beads were washed 3× with immunoprecipitation buffer and 1× with immunoprecipitation buffer containing 500 mm NaCl and then resuspended in sample buffer and separated by 4–12% SDS-PAGE (Invitrogen). Proteins were transferred electrophoretically to an Immobilon-P membrane (Millipore, Bedford, MA) and blotted with α-GFP antibody JL-8 (BD Living Colors) to detect GFP-Gef3p and with conjugated anti-GST-HRP (Amersham Biosciences) to detect GST-Rho3p. Total protein levels were monitored in whole-cell extracts (10 μg of total protein) and used directly for Western blots.
Pulldown Assays for GTP-bound Rho Proteins
The GST-rhotekin binding domain (RBD) fusion protein was obtained as described previously (24). The amount of GTP-bound Rho proteins was analyzed using the Rho-GTP pulldown assay, modified as described in Ren et al. (44). Extracts from 50-ml cultures of cells expressing pREP3X-HArho3+ transformed with pREP4X-HAgef3+ or the empty plasmid grown for 18 h in minimal medium (−T) were obtained using 200 μl of lysis buffer (50 mm Tris, pH 7.5, 20 mm NaCl, 0.5% Nonidet P-40, 10% glycerol, 0.1 mm dithiothreitol, 2 mm MgCl2, containing 100 μm p-amino phenylmethanesulfonyl fluoride, leupeptin, and aprotinin). 100 μg of GST-RBD fusion protein coupled to glutathione-agarose beads was used to immunoprecipitate 1.5 mg of the cell lysates. The extracts were incubated with GST-RBD beads for 2 h at 4 °C. The beads were washed with lysis buffer four times, and bound proteins were blotted against 1:5000-diluted 12CA5 mAb as the primary antibody to detect HA-Rho3p. The total amount of HA-Rho3p was monitored in whole-cell extracts (10 μg of total protein) by Western blot with 12CA5 mAb. Immunodetection was accomplished using the ECL detection kit (Amersham Biosciences).
RESULTS
gef3Δ Mutant Cells Show Defects in Cell Separation
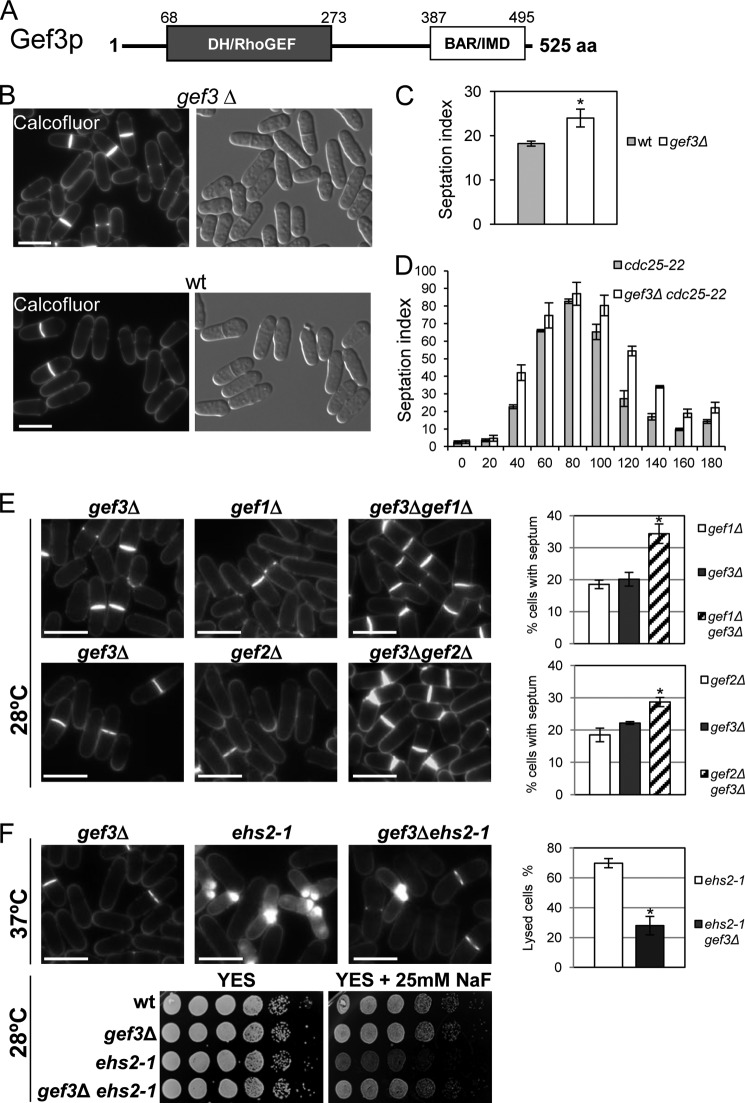
Gef3p was first reported as a GEF by Iwaki et al. (17). Gef3p contains a DH homology domain, also called the Rho-GEF domain, at amino acids 68–273, and a BAR/IMD-like domain at amino acids 387–495, present in proteins involved in membrane dynamics (Fig. 1A). BAR (Bin1/amphiphysin/Rvs167) domains have been characterized by their function in sensing and inducing membrane curvature, but they are also involved in binding to small GTPases (45, 46). To investigate the role of Gef3p in the control of morphology, we deleted gef3+ by replacing the gef3+ ORF with the ura4+ marker. The gef3Δ mutant grew well under standard growth conditions at both 28 and 37 °C and entered the stationary phase at the same time as the wild-type cultures. gef3Δ cells did not exhibit any evident morphological changes as judged by light microscopy (17) (Fig. 1B), but in asynchronous cultures ∼25% of the cells in the gef3Δ mutant showed a septum compared with only 18% of the cells in the wild-type strain (Fig. 1C). To examine septation in more detail, we constructed the double mutant cdc25-22 gef3Δ and synchronized cells in G2 by cdc25-22 arrest and release. In both strains septation peaked after ∼80 min, but the septation index remained high in cdc25-22 gef3Δ cells as compared with the wild-type cdc25-22 cells (Fig. 1D). This suggested that septum formation proceeds normally, but cell separation is slower in the absence of Gef3p.
FIGURE 1.
gef3Δ mutant cells show defects in cell separation. A, schematic representation of the domain structure of Gef3p. The numbers indicate amino acids, beginning with the start methionine. DH/RhoGEF, Dbl-homology domain; BAR/IMD, BAR-like homology domain. aa, amino acids. B, morphology of gef3Δ null cells. Cfw staining and differential interference contrast micrographs of S. pombe gef3Δ (SM116) and wild-type (YS64) cells grown in YES liquid medium at 28 °C. Bar, 10 μm. C, asynchronous cultures of wild-type and gef3Δ cells growing exponentially were stained with Cfw. The septation index is the percentage of septated cells in the culture and was quantified directly by fluorescence microscopy (n = 200). Values are the means from three independent experiments, and S.D. bars are indicated. The asterisk indicates that the gef3Δ value is significantly (p < 0.05) different from that of the wild type. D, cultures of cdc25-22 (NG69) and gef3Δ cdc25-22 (SM176) were synchronized in early G2 and released into mitosis. The septation index was quantified as in C at the indicated times (minutes). E, ef3Δ (SM176), gef1Δ (PG153), gef3Δ gef1Δ (SM143), gef2Δ (CL5), and gef3Δ gef2Δ (SM172) cells growing exponentially were stained with Cfw to visualize septa. Bar, 10 μm. The graphs on the right represent the septation index of each set of mutants (the double mutant and the parental strains). At least 200 cells were scored in three independent experiments. Mean values were plotted with error bars representing the S.D. around the mean. *, p < 0.01versus gef3Δ. F, loss of Gef3p reduces the lysis and abolishes the sensitivity to NaF of the ehs2-1 (rgf3) mutant. gef3Δ (SM176), ehs2-1(MS264), and gef3Δehs2-1 (SM152) cells were stained with Cfw. The graphic represents the percentage of lysis of ehs2-1 and gef3Δehs2-1 cell growth for 4 h at 37 °C. The NaF hypersensitivity of wild type, gef3Δ, ehs2-1, and gef3Δehs2-1 was analyzed on a plate assay. Cells were spotted onto YES plates with or without 25 mm NaF as serial dilutions (8 × 104 cells in the left row and then 4 × 104, 2 × 104, 2 × 103, 2 × 102, and 2 × 101 in each subsequent spot) and incubated at 28 °C for 3 days. *, p < 0.01 versus ehs2-1.
We then wondered whether there was a functional relationship between Gef3p and the other known GEF proteins in fission yeast. Curiously, the septation defect of the cells deleted for gef3+ was exacerbated when combined with a deletion in gef1+ and gef2+ (Fig. 1E). No interaction was seen when combined with null mutations in Scd1p, Rgf1p, and Rgf2p, but we detected a positive interaction with the ehs2-1 mutant (a point mutation in the rgf3+ gene) (27). Deletion of Gef3p suppressed the growth defect of ehs2-1 cells in the presence of NaF and strongly diminished the number of lysed cells in the ehs2-1 mutant grown at 37 °C (Fig. 1F). The gef1, gef2, and ehs2-1 mutants showed defects in cytokinesis, further suggesting that Gef3p is involved in this process.
Gef3p Localizes as a Ring That Splits and Persists until the End of Septation
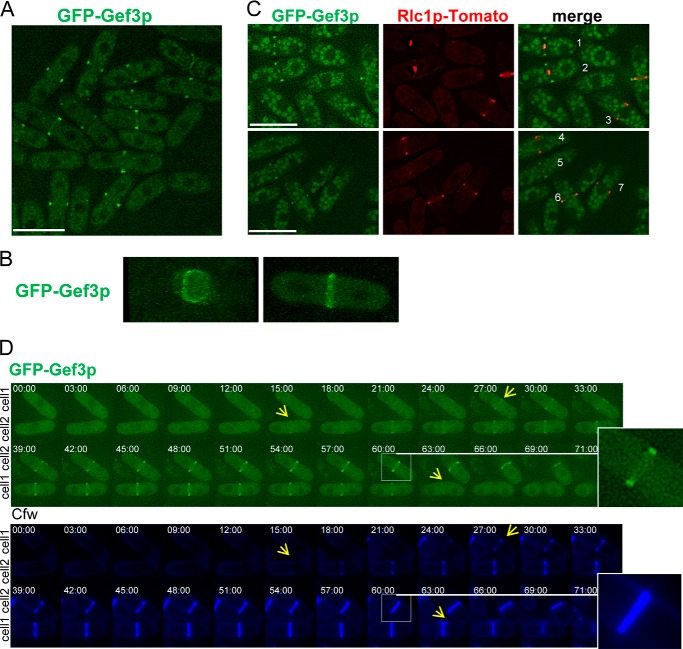
To determine the intracellular location of Gef3p, we replaced the chromosomal gef3+ ORF with the GFP gene and an eight-alanine linker fused in-frame to either the 5′ end or the 3′ end of gef3+. Cells carrying the GFP at the N terminus were morphologically normal and behaved like the wild type in combination with gef1Δ mutants, indicating that the fusion protein was functional. However, we were unable to visualize the protein tagged at the C-end, suggesting that the BAR/IMD-like domain is required for the proper localization of Gef3p (not shown). GFP-Gef3p was detected at the division site in cells undergoing cytokinesis (Fig. 2A; the cells were synchronized to show a high proportion of septated cells). To determine whether Gef3p assembles into a ring structure, we used confocal microscopy and acquired Z sections of cells expressing GFP-Gef3p. The reconstruction of these images in Fig. 2B reveals that Gef3p indeed formed a ring structure; however, we were unable to observe rings of different diameters in cells undergoing cytokinesis (supplemental Movies 1 and 2). To determine the localization pattern of Gef3p during ring contraction, a GFP-Gef3p Rlc1p-tdTomato strain was constructed. The myosin regulatory light chain Rlc1p-tdTomato was used as a marker of contraction (47). The images show that GFP-Gef3p assembled into a ring structure, and both proteins colocalized at the end of mitosis (Fig. 2C, cell 6), but whereas Rlc1p-tdTomato constricted during septation, most of the GFP-Gef3p remained static until the end of cytokinesis (Fig. 2C, cells 1 and 2).
FIGURE 2.
Gef3p localizes as a medial ring. A, cells producing GFP-Gef3p (SM217) were synchronized with 12.5 mm hydroxyurea (HU) for 2.5 h, washed 3x, and photographed 1 h after release into fresh medium. Bar, 10 μm. B, cells expressing GFP-Gef3p were imaged for GFP fluorescence using confocal three-dimensional microscopy. Frontal and lateral views of the GFP-Gef3p septum are shown (see supplemental Movies 1 and 2). C, cells (SM267) expressing GFP-Gef3p and Rlc1p-tdTomato were imaged for GFP and RFP fluorescence. During ring contraction, most Gef3p remains as an external ring (cells 1 and 2). Cells 3, 4, and 7 exhibit myosin but not Gef3p staining, whereas cell 5 shows just the opposite. Bar, 10 μm. D, GFP-gef3+ cells were imaged by time-lapse microscopy. The elapse time is shown in minutes. The inset shows that the GFP-Gef3p ring splits into a double ring.
We next analyzed the localization of GFP-Gef3 by time-lapse microscopy in cells stained with Cfw, a dye used to track septum assembly and cell separation in S. pombe cells (48). The time-lapse images revealed that GFP-Gef3p concentrated in a ring detected concomitantly with the appearance of the primary septum (Fig. 2D, cells 1 and 2 with yellow arrow, time 27 and 15 min, respectively). As the septum formed, the GFP-Gef3p ring split into a double ring that persisted along septation and was dissipated after cell cleavage (see enlarged cells and cell 2 marked with a yellow arrow in Fig. 2D). Thus, these data indicate that Gef3p localizes to the division site as a double ring during the later stages of cytokinesis.
Gef3p Colocalizes with Mid2p and Septins and Is Dependent on Mid2p and Septins for Localization
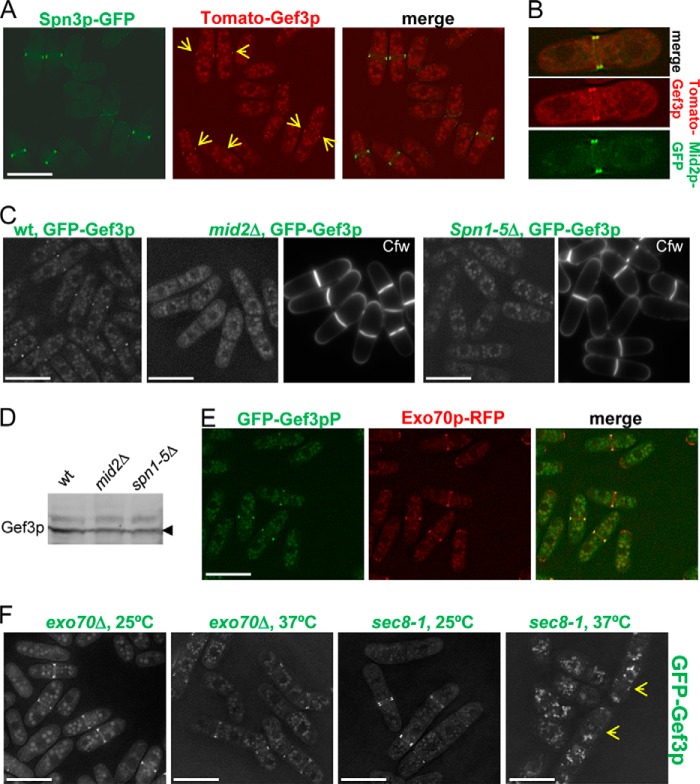
In fission yeast septins appear at the division site, where they form a ring that splits as the septum forms, and this ring does not constrict with actomyosin ring invagination (49–51). The similarities with Gef3p prompted us to analyze a possible relationship between the septins and Gef3p. First, we studied the localization of both proteins in the same cell using a strain that simultaneously expressed Spn3p-GFP and Tomato-Gef3p. We found that Gef3p and Spn3p colocalized during septation (Fig. 3A). Curiously, in >100 cells scored we did not find cells with Tomato-Gef3p present and Spn3p-GFP absent. This result suggests that Spn3p may precede Gef3p localization at the medial ring. Colocalization experiments with Mid2p-GFP (a protein necessary for proper organization of the septin ring) provided similar results (Fig. 3B). We also tested the dependence of Gef3p localization on septins and Mid2p. In mid2Δ cells and spn1–5Δ cells, GFP-Gef3p was not localized to any specific structure (Fig. 3C). A Western blot revealed that GFP-Gef3p was expressed in both mutants (Fig. 3D). As expected, Mid2p and the septins localized normally in gef3Δ cells (not shown). Thus, the results concerning the order and the localization dependence suggest that Gef3p associates with the septin ring for proper localization.
FIGURE 3.
Gef3p colocalizes with septins and Mid2p and its localization is dependent on septins and Mid2p. A, Spn3p and Gef3p colocalize during septum formation. SM297 cells expressing Spn3p-GFP (green) and tdTomato-Gef3p (red) were imaged for GFP and RFP fluorescence. B, cells (SM295) expressing Mid2p-GFP and tdTomato-Gef3p colocalize in a double ring structure. C, Gef3p localization in septin ring mutants. Wild-type, mid2Δ, and spn1–5Δ mutants expressing GFP-Gef3p (SM217, SM253, and SM277, respectively) were treated with Cfw to visualize the septum and imaged for GFP and Cfw fluorescence. D, the amount of GFP-Gef3p expressed from the chromosome in wild type, mid2Δ, and spn1–5Δ mutants. Gef3p levels were measured with Western blots using anti-GFP antibodies. E, Gef3p colocalizes with the exocyst. Wild-type cells expressing GFP-Gef3p and Exo70p-RFP (SM259) were imaged for GFP and RFP fluorescence. F, Gef3p localization in exocyst mutants. exo70Δ (left) and sec8-1 (right) mutants expressing GFP-Gef3p (SM259 and SM261, respectively) were incubated at 25 °C or 37 °C for 4 h and imaged for GFP fluorescence. Bar, 10 μm.
The exocyst is a multiprotein complex involved in polarized exocytosis (52). In fission yeast, the exocyst localizes to a double ring at the site of cell constriction; it is essential for cell separation, and its function is modulated by Rho3p (28, 37). Thus, as above we analyzed the localization of Gef3p-GFP and Exo70-RFP in the same cells. Gef3p localization coincided with that of Exo70p in all cells, except that the exocyst protein also localized to cell tips (Fig. 3E). We, therefore, investigated whether this complex is involved in targeting Gef3p to the septum. Exo70p is conditionally essential; when grown at 37 °C the exo70Δ cells die with a multiseptated phenotype. We found that Gef3p localized properly to the septum of exo70Δ cells both at 25 °C and 37 °C (Fig. 3F). A similar result was obtained with the temperature-sensitive hypomorphic sec8-1 mutant at 37 °C, although in this case the fluorescence was accumulated intracellularly, making it more difficult to detect Gef3p in the septa (Fig. 3F, the arrows point to Gef3p fluorescence). Additionally, the localization of the exocyst components Sec6p-GFP and Sec8p-GFP was independent of the presence or absence of Gef3p (not shown). These results support the notion that septins, but not the exocyst, direct Gef3p to the growing septum.
Gef3p Interacts Physically and Functionally with Rho3p in S. pombe Cells
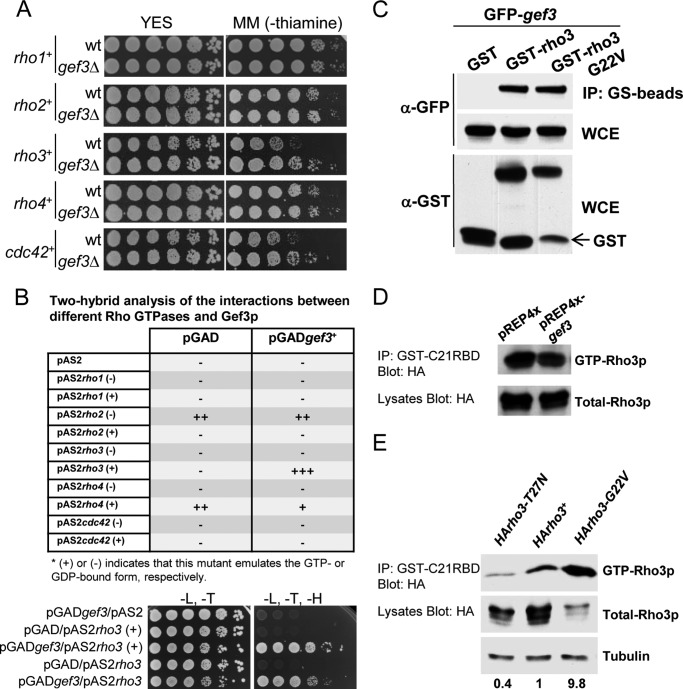
To study the specificity of Gef3p toward the Rho GTPases, we undertook a genetic approach. In wild-type cells the overexpression of Rho proteins is not lethal (except for Rho2p) (53), but in general it causes morphological changes and a loss of cell polarity that interferes with normal growth. Thus, if Gef3p acts through any of the GTPases it could be expected that the overexpression of this particular GTPase would be less dangerous in a gef3Δ background. We found that gef3Δ cells that expressed rho3+ (under the control of the thiamine regulable nmt1 promoter) grew better on a plate assay than the wild-type cells in the same conditions (Fig. 4A). The overexpression of cdc42+ also produced healthier cells in the absence of gef3+. We did not observe appreciable changes in growth among the strains due to the overexpression of the other GTPases (Fig. 4A). These results suggest that Gef3p could act on Rho3p and/or Cdc42p.
FIGURE 4.
Gef3p interacts with Rho3p. A, wild-type cells (YS64) or gef3Δ cells (SM116) were transformed with pREP3x-rho1+, pREP3x-rho2+, pREP3x-rho3+, pREP3x-rho4+, and pREP3x-cdc42+. Cells were spotted onto YES plates or MM (without thiamine) as serial dilutions and incubated at 28 °C for 4 days. B, two-hybrid analysis of the interactions between different Rho GTPases and Gef3p (top). Gef3p binds directly to the GTP-bound form of Rho3p (rho3G22V) and to the wild-type Rho3p (not modified). Growth in −Leu (L)/−Trp (T)/−His (H) media of yeast cotransformed with pGADgef3 and the empty vector pAS2 (first row), pAS2rho3(+), and the empty vector pGAD (second row), pGADgef3 and pAS2rho3(+) (third row), pAS2rho3 and pGAD (fourth row), or pAS2rho3 and pGADgef3 (fifth row) in yeast two-hybrid screenings. The results are representative of three separate co-transformation experiments. C, GST pulldown assay showing the interaction of Gef3p and Rho3p. Cell extracts from cells expressing GST and GFP-Gef3p, GST-Rho3p and GFP-Gef3p, and GST-Rho3p (G22V) and GFP-Gef3p were precipitated with glutathione beads and blotted against anti-GFP antibody. A Western blot with anti-GFP and anti-GST was performed on total extracts to visualize total GFP-Gef3p and GST-Rho3p levels (WCE). D, wild-type (YS64) cells expressing pREP4X or pREP4X-gef3 were transformed with pREP3xHA-rho3. GTP-Rho3p was pulled down from the cell extracts with GST-C21RBD and blotted against 12CA5, anti-HA monoclonal antibody. Total HA-Rho3p was visualized by Western blot. IP, immunoblot. E, wild-type (MS38) cells were transformed with pREP42X-HArho3-T27N, pREP42X-rho3+, and pREP42X-HArho3-G22V. GTP-Rho3p was pulled down from the cell extracts with GST-C21RBD and blotted against 12CA5, anti-HA monoclonal antibody. Numbers indicate the relationship between active Rho3p and total Rho3p normalized regarding to wild-type plasmid pREP42X-rho3+.
To confirm these results, the ORF of Gef3p (amino acids 1–525) was fused in-frame to the Gal4 transcriptional activation domain in plasmid pGAD and used as bait to screen for the interaction(s) with the Rho family proteins in a two-hybrid assay (see “Experimental Procedures”). For each Rho protein, a point mutation that theoretically trapped the GTPase in the GTP-bound form (+) or the GDP-bound form (−) fused to the DNA binding domain was assayed (27, 43). As shown in Fig. 4B, Gef3p specifically interacted with the GTP-bound form of Rho3p (rho3G22V) but not with the GDP-bound form of Rho3p (rho3T27N) or with Cdc42p or indeed any of the other Rho proteins. The wild-type form of Rho3p also showed binding to Gef3p (Fig. 4B). In addition, we tested two different deletion constructs of Gef3p for interaction with Rho3p, pGADgef3DHΔ that lacks the DH domain and pGADgef3BARΔ that lacks the BAR/IMD-like domain. Both mutant constructs showed interaction with Rho3p in a two-hybrid assay (not shown).
Next we examined whether there was interaction between Gef3p and Rho3p by co-precipitation experiments. To this end, we co-expressed GFP-epitope-tagged Gef3 protein (GFP-Gef3p) together with GST, GST-Rho3p, or GST-Rho3p (G22V) (an allele of Rho3p permanently bound to GTP) in S. pombe cells. As shown in Fig. 4C, GFP-Gef3p was found to be associated with both the wild-type Rho3p and with the GTP-bound form of Rho3p. Moreover, in a separate set of pulldown experiments using GST-RBD, we found that the amount of active Rho3p (GTP-bound Rho3p) did not increase in the strain that overexpressed Gef3p (expressed from the nmt1 promoter, a thiamine-regulable and high expression-rate promoter) (55) as compared with the wild-type strain (Fig. 4D). Because GST-RBD was described to affinity-precipitate cellular GTP-RhoA (44), we further demonstrated that Rho3p binds to GST-RBD in the same way as Rho1p (the counterpart of mammalian RhoA in S. pombe). Extracts from wild-type cells transformed with pREP-HA-rho3T27N (the dominant-negative allele), pREP-HA-rho3+ (wild-type), and pREP-HA-rho3G22V (the constitutively active allele) were incubated with GST-C21RBD. The experiment showed that the amount of affinity-precipitated GTP-Rho3p was different depending on the activation state. As expected the dominant negative mutant showed very little GTP-Rho3p, whereas the constitutively active allele that is always bound to GTP showed the highest levels (Fig. 4E). Taken together, these results suggest that Gef3p preferentially binds to the active form of Rho3p.
We then tested whether the lack of Gef3p might influence the localization and/or stability of Rho3p. Our experiments were performed with the GFP-Rho3p expressed chromosomally under its endogenous promoter.5 As shown previously (36, 56), in wild-type cells GFP-Rho3p localized to the plasma membrane and was more intense at the division site (Fig. 5A). Interestingly, in gef3Δ cells GFP-Rho3p still localized to the plasma membrane and to the septum area (Fig. 5A). However, it also formed small invaginations on one or both sides of the incomplete septum (Fig. 5A, n = 100, 15% of the cells showed aberrant GFP-Rho3p). Some cells also showed similar bubbles of GFP-Rho3p at different places on the cell periphery. To ascertain whether this defect was a characteristic of GFP-Rho3p localization in gef3Δ cells or whether it was due to a general defect in membrane organization, we tested the localization of Psy1p-GFP in gef3Δ cells. The fission yeast psy1+ gene encodes a mammalian syntaxin (t-SNARE) homolog that localizes to the plasma membrane during vegetative growth (57). Psy1p-GFP was properly localized to the plasma membrane of gef3Δ cells (Fig. 5B). Together, these results indicate that Gef3p may act as a scaffold for Rho3p, perhaps being required to concentrate active Rho3p during the early stages of septum formation when plasma membrane invagination takes place.
FIGURE 5.
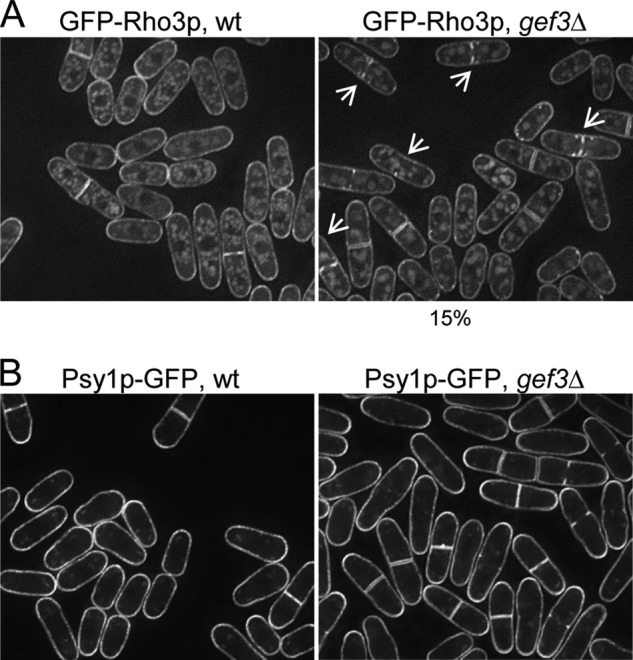
The localization of Rho3p to the septum is disturbed in gef3Δ cells. A, wild-type (SM242) and gef3Δ (SM594) cells expressing a functional, chromosomal GFP-Rho3p from the endogenous promoter were grown in YES medium at 28 °C and imaged for fluorescence. Maximum projection images are shown. B, wild-type (EM83) and gef3Δ (EM256) cells expressing a functional, chromosomal Psy1p-GFP were grown in YES medium at 28 °C and imaged for fluorescence. Maximum projection images are shown.
Loss of Gef3p Shows Genetic Interactions with Exocyst Mutants, apm1Δ Mutants, and Formin Mutants
The above results suggested that Gef3p was more related to Rho3p than to any of the other GTPases of the Rho family. Accordingly, we deleted gef3+ in rho3Δ cells to conduct epistasis experiments. The double mutant gef3Δrho3Δ grew as well as the single mutant rho3Δ in a temperature range between 25 °C and 35 °C, whereas neither of them was able to grow at 37 °C (Fig. 6A), suggesting that Gef3p and Rho3p could act in the same pathway. We then wondered which of the Rho3p functions might be specifically affected by Gef3p. In S. pombe, the overproduction of Rho3p compensates the abnormal elongated multicellular phenotype of sec8-1 and exo70Δ exocyst mutants, and rho3Δ shows strong negative interactions with sec8-1 and exo70Δ. Accordingly, we analyzed whether the exocyst was functionally related to Gef3p. The overproduction of Gef3p did not suppress the thermosensitive phenotype of the sec8-1 mutant (not shown). However, the sec8-1 gef3Δ double-mutant cells showed a severe growth defect. They did not form colonies detectable at 35 °C, a cell growth-permissive temperature for each single mutant (Fig. 6B). Although gef3Δ and sec8-1 showed a mild cell separation defect at 25 °C (Fig. 6B), this phenotype was exacerbated in the sec8-1 gef3Δ double mutant, most cells arresting with multiple septa (Fig. 6B) and similar to the sec8-1 phenotype at 37 °C (28). In addition, a genetic interaction was found between gef3Δ and exo70Δ (not shown). Rho3p regulates secretion by influencing membrane trafficking in cooperation with Apm1p (34). Therefore, we also entertained the possibility of the existence of a functional link between Gef3p and genes encoding adaptin. We found that gef3Δapm1Δ cells grew at 25 °C but did not form colonies detectable at 35 °C (a cell growth-permissive temperature for each single mutant) (Fig. 6C). Moreover, even at 25 °C >90% of the cells showed 1 septum. Thus, Gef3p and Apm1p interact genetically to promote cell growth at high temperature.
FIGURE 6.
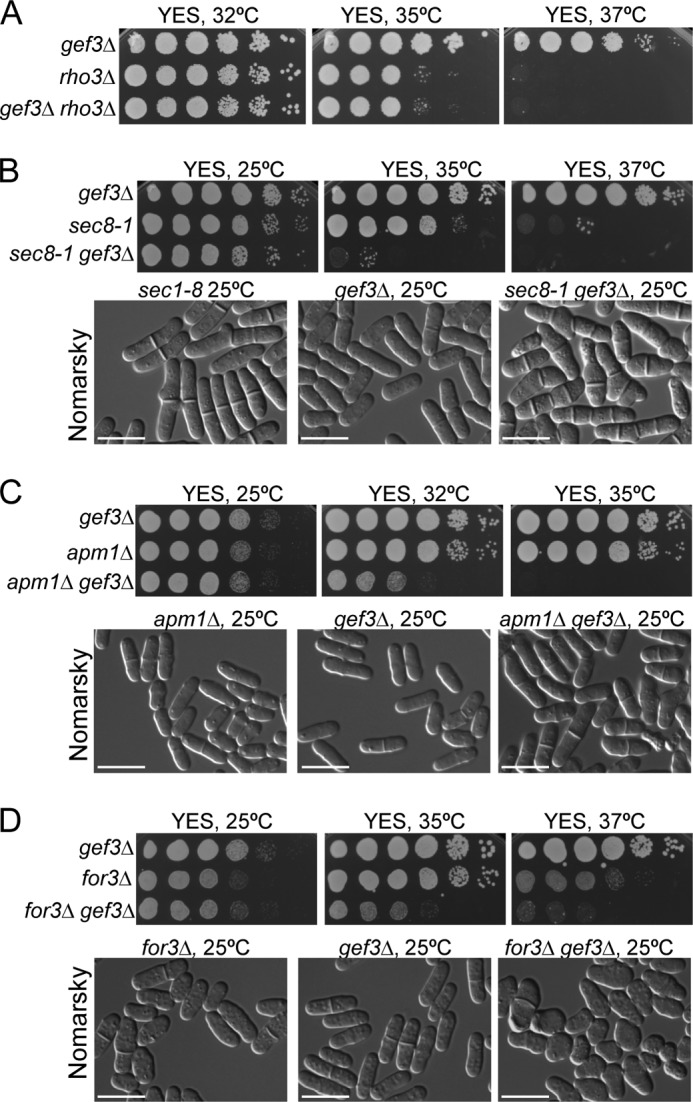
Loss of Gef3p shows genetic interactions with exocyst mutants, adaptin mutants, and formin mutants. A, gef3Δrho3Δ behaved like rho3Δ cells in their ability to grow at high temperatures. To compare the relative growth rates, equal numbers of gef3Δ (SM176), rho3Δ (PPG3740), and gef3Δrho3Δ (EM257) cells were diluted (8 × 104 cells in the left rows and then 4 × 104, 2 × 104, 2 × 103, 2 × 102, and 2 × 101 in each subsequent spot) and spotted onto YES plates. Colony formation was analyzed after 3 days at 32, 35, and 37 °C. B, the gef3Δ sec8-1 double-mutant strain was unable to form colonies at 35 °C. To compare relative growth rates, gef3Δ (SM176), sec8-1 (MBY887), and gef3Δ sec8-1 (SM226) cells were spotted onto YES plates as above and incubated at 25, 35, and 37 °C for 3 days (upper panel). gef3Δ, sec8-1, and gef3Δsec8-1 cells were observed directly in YES liquid medium at 25 °C by differential interference-contrast microscopy (lower panel). C, loss of both gef3+ and apm1+ results in failure to grow at 32 and 35 °C. To compare the growth rates, gef3Δ (SM176), apm1Δ (SM708), and gef3Δapm1Δ (SM721), cells were spotted onto YES plates and incubated for 3 days at different temperatures (upper panels). Differential interference-contrast photographs of gef3Δ, apm1Δ, and gef3Δ apm1Δ cells grown in YES liquid medium at 25 °C (lower panels). D, gef3Δfor3Δ double-mutant cells fail to polarize growth. gef3Δ (SM176), for3Δ (PPG2567), and gef3Δ for3Δ (SM300) cells were diluted, spotted onto YES plates, and incubated at 25, 35, and 37 °C (upper panels). Differential interference-contrast photographs of gef3Δ, for3Δ, and gef3Δfor3Δ cells grown at 25 °C (lower panels). Bar, 10 μm.
Finally, we explored whether Gef3p might be involved in the Rho3p function in polarity. Rho3p binds to For3p, which is the S. pombe ortholog of formin, and the rho3Δfor3Δ double-null cells showed defects that were more severe than those seen in each of the single null cells; that is, round cells appeared (36). Because Gef3p binds to the active form of Rho3p, we speculated that in gef3Δ cells active Rho3p is not properly concentrated and that polarity would be compromised if actin cables were absent due to the deletion of For3p. To examine this possibility, For3p was deleted from the gef3Δ mutant, and the phenotype of the resulting double mutant was assessed. A synthetic cell growth defect was observed at 35 °C, and this was more severe at 37 °C (Fig. 6D). Moreover, the double-mutant cells frequently showed a lemon-like shape, similar to rho3Δfor3Δ cells and swelled from the middle even at 25 °C (Fig. 6D). This phenotype like the one reported for pob1-664 mutant cells at the restrictive temperature (37, 40). Thus, the results suggest that the function of Gef3p is important for Rho3p to control secretion, especially if one of the two molecular systems involved in vesicle-trafficking, namely vesicle tethering and vesicle transport, is also affected.
DISCUSSION
GEF activation of Rho GTPases occurs through the serial formation of macromolecular complexes at precise points in time and space that are currently incompletely understood. Here we studied a Rho-GEF, termed Gef3p, which like other Rho-GEFs contains a DH but lacks the pleckstrin homology domain that is required for most Rho-GEFs to localize to the membrane (59). Instead, at the C terminus Gef3p carries a putative BAR/IMD-like domain. The IMD (IRSp53 and MIM (missing in metastases) homology domain) is an I-BAR or inverse BAR domain that binds to the membrane through its convex surface. IMD domains are involved in plasma membrane deformation, producing protrusions (filopodia and lamellipodia) (60, 61).
In this study we assigned a function to Gef3p that was directly related to the assembly of Rho3p GTPase to the septum. Gef3p interacts functionally with Rho3p, and the loss of Gef3p aggravates the phenotypes of exocyst mutants and formin mutants, similar to what has been described previously for the loss of Rho3p (28, 36). Biochemical and genetic evidence suggests that Gef3p interacts with Rho3p, although it does not seem to promote nucleotide exchange of the GTPase. Gef3p bound to the active form of Rho3p but not to other Rho proteins (either GDP- or GTP-bound) in a two-hybrid assay. Moreover, wild-type Rho3p and GTP-bound Rho3p coprecipitated with Gef3p. The GDP/GTP cycle is highly regulated by GEFs, which induce the release of the bound GDP for replacement by the more abundant GTP. As a result, GEFs usually accelerate the exchange reaction by several orders of magnitude (12, 13). Despite this, here we observed that high levels of Gef3p did not raise the level of GTP-Rho3p in vivo. We also tested the effect of the overexpression of Gef3p on the GTP-bound levels of the other GTPases, from Rho1p to Rho4p and Cdc42p, with negative results. Thus, Gef3p apparently does not activate Rho3p, which could explain why the overexpression of Rho3p, but not that of Gef3p, suppressed the thermosensitive phenotype of the sec8-1 mutants (36) or amp1Δ mutants (34) (not shown). Rho3p binds to Gef3p lacking its DH domain in a two-hybrid assay, suggesting again that Gef3p is not working as a GEF for Rho3p. From our results it is likely that both, the DH and BAR/IMD-like domains, could participate in binding. In this sense, a series of BAR domain-containing proteins have been characterized that are linked to Rho GTPase signaling pathways (62). BAR domains play important roles in the targeting of proteins to specific regions within the plasma membrane where actin remodeling is necessary. At these sites, BAR-domain proteins can control Rho GTPase activity either by regulating the activation status of Rho GTPases or by linking Rho GTPases to their upstream activators or to their downstream effectors (45, 61).
The interaction Gef3p/Rho3p is not unique; in certain circumstances allosteric interactions of the GEF with either the GTPase itself or with its effectors can create an environment that seems to sustain the GTPase activity without modifying the nucleotide binding state of the GTPase. The Gef3p/Rho3p interaction might be required for stabilization of the active form of Rho3p at the septum. GFP-Rho3p showed a uniform membrane distribution, and we failed to observe important differences between the wild-type and gef3Δ cells in the localization of Rho3p to the septum. However, in gef3Δ, GFP-Rho3p was often invaginated in the wrong place, surrounding immature septa. This suggests that Gef3p binding to active Rho3p might stabilize the protein, promoting its accumulation at the contractile actomyosin ring/invaginated membrane interface. Alternatively, Gef3p may convey active Rho3p to a specific platform to become part of a higher-order complex. Here, we noted that Gef3p localized to the cell division site after septins had been deposited and that it required septins for localization. Septins are filament-forming (GTP-binding) proteins that assemble at the division site. In many systems they appear to serve as scaffolds and diffusion barriers for other proteins, and they are important for cytokinesis (50, 63–65). Recent findings suggest that septins regulate membrane traffic or membrane stability (66, 67). Septins could cross-link and corral membrane domains enriched in particular phosphoinositides, blocking the lateral diffusion of membrane proteins and deforming or rigidifying the cell membrane (54, 68, 69). In fission yeast septins are not essential for cell viability, and mutants display a variable delay in separation of the daughter cells, suggesting that septins play some role(s) in the proper completion of the septum or in subsequent processes necessary for cell separation (50, 54, 58, 68). Rho3p is not essential for viability, but cells depleted of Rho3p were elongated and multiseptated, and they accumulated putative secretory vesicles at high temperature (28, 34, 36, 37). Thus, the loss of function of both pathways produces similar cytokinetic defects. In mid2Δ and spn mutants, failure in septin ring assembly impairs the formation of the Gef3p ring and may culminate in perturbing Rho3p function in the medial region. Consistent with this idea, we found that a combination of a defective septin ring (mid2Δ) with a mutation in the adaptin complex (apm1Δ), an effector of Rho3p, was lethal above 32 °C; this phenotype is almost identical to the one seen in gef3Δapm1Δ cells (Fig. 6C). As expected, mid2Δgef3Δ cells grew well at all temperatures. Together, these results suggest that one of the functions of the septin ring in S. pombe could be to act as a positional cue for the targeting of active Rho3p.
gef3Δ cells grew similarly to wild-type cells. However, a deletion of Gef3p caused a small delay in cell-cell separation, suggesting a role for the protein in cytokinesis. Curiously, the interaction with other GEFs may help to understand its role in septum dynamics. The gef3Δ cytokinesis phenotype was aggravated by the deletion of Gef1p (a Cdc42-GEF), producing highly multiseptated cells (Fig. 1E). However, the elimination of Gef3p suppressed hypersensitivity to NaF and lysis in ehs2-1 cells mutated in Rgf3p (a Rho1p-GEF). Thus, the most attractive hypothesis to explain the data is that Gef3p would be involved in a pathway whose function normally complements that of Cdc42p. In addition, the Gef3p/Rho3p pathway might negatively modulate Rho1p signaling, and hence its absence would raise the level of Rho1p, which would help to correct the damage. Our results are consistent with the possibility that Gef3p/Rho3p could function redundantly with the Cdc42p pathway in cytokinesis. Cdc42p is likely to form the most upstream polarizing cue and activates the formin For3p for the assembly of polarized actin cables. This interaction is facilitated by Pob1p (36, 38–40). The pob1-664 mutant shows an accumulation of secretory vesicles and a loss of functional actin cables, and both phenotypes are suppressed by Rho3p (37).
Our results suggest that Gef3p functions by stabilizing active Rho3p and facilitating the interaction of Rho3p with its effectors during the early steps of septum synthesis. This is shown by the fact that a lack of Gef3p produced defects similar to the deletion of Rho3p when combined with mutants in the exocyst and formins (31). The simultaneous mutation of gef3+ and sec8+ and gef3+ and apm1+, both genes encoding proteins involved in secretion, afforded cells with strong defects in septation. In addition, a severe synthetic cell growth defect in polarity was observed in the gef3Δfor3Δ double mutants. These cells frequently showed a lemon-like shape similar to pob1-664 and misslocalized septa (Fig. 6D). As the Gef3p and Rho3p are both required for polar growth in the absence of actin cables (this work and Ref. 36), this complex could provide key spatial information to identify zones of growth, a redundant function related to the master role of Cdc42p in polarized exocytosis. It is possible that the septins that localize Gef3p also play a role in this process.
In conclusion, we observed that the putative Rho-GEF Gef3p binds to active Rho3p and localizes to the septin ring, providing a platform for GTPase function during septum assembly and/or degradation. The Gef3p/Rho3p interaction contributes to downstream events in the secretory pathway redundantly with the two autonomous “morphogenetic modules”: the exocyst and the actin cable machinery.
Supplementary Material
Acknowledgments
We thank P. Perez, P. M. Coll, H. Valdivieso, J. C. Ribas, and C. Rodriguez-Vazquez de Aldana from the IBFG, Spain, F. Chang from Columbia University, M. Balasubramanian from the Mechanobiology Institute, Singapore, and J. Bähler from Wellcome Trust UK for providing plasmids and strains. We recognize P. Bolaños for initiating this work. Text was revised by N. Skinner. Instituto de Biología Funcional y Genómica acknowledges the institutional support granted by the Ramón Areces Foundation during 2011 and 2012.
This work was supported by Grants BFU2008-00963/BMC and BFU2011-24683/BMC from the Comisión Interministerial de Ciencia y Tecnología, Spain and GR231 from the Junta de Castilla y León.

This article contains supplemental Movies 1 and 2.
M. A. Villar-Tajadura and P. Perez, unpublished data.
- GEF
- guanine nucleotide-exchange factor
- IBFG
- Instituto de Biología Funcional y Genómica
- YES
- yeast growth medium
- Cfw
- Calcofluor
- RBD
- rhotekin-binding domain
- nt
- nucleotide(s)
- RFP
- red fluorescent protein
- BAR
- Bin1/amphiphysin/Rvs167
- IMD
- IRSp53, and MIM (missing in metastases) homology domain.
REFERENCES
- 1. Pollard T. D. (2010) Mechanics of cytokinesis in eukaryotes. Curr. Opin. Cell Biol. 22, 50–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Balasubramanian M. K., Srinivasan R., Huang Y., Ng K.-H. (2012) Comparing contractile apparatus-driven cytokinesis mechanisms across kingdoms. Cytoskeleton 69, 942–956 [DOI] [PubMed] [Google Scholar]
- 3. Krapp A., Simanis V. (2008) An overview of the fission yeast septation initiation network (SIN). Biochem. Soc. Trans. 36, 411–415 [DOI] [PubMed] [Google Scholar]
- 4. Bathe M., Chang F. (2010) Cytokinesis and the contractile ring in fission yeast: towards a system-level understanding. Trends Microbiol. 18, 38–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Goyal A., Takaine M., Simanis V., Nakano K. (2011) Dividing the spoils of growth and the cell cycle: the fission yeast as a model for the study of cytokinesis. Cytoskeleton 68, 69–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Pollard T. D., Wu J. Q. (2010) Understanding cytokinesis: lessons from fission yeast. Nat. Rev. Mol. Cell Biol. 11, 149–155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Roncero C., Sánchez Y. (2010) Cell separation and the maintenance of cell integrity during cytokinesis in yeast: the assembly of a septum. Yeast 27, 521–530 [DOI] [PubMed] [Google Scholar]
- 8. Sipiczki M. (2007) Splitting of the fission yeast septum. FEMS Yeast Res. 7, 761–770 [DOI] [PubMed] [Google Scholar]
- 9. Wolfe B. A., Glotzer M. (2009) Single cells (put a ring on it). Genes Dev. 23, 896–901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Yoshida S., Bartolini S., Pellman D. (2009) Mechanisms for concentrating Rho1 during cytokinesis. Genes Dev. 23, 810–823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Yoshida S., Kono K., Lowery D. M., Bartolini S., Yaffe M. B., Ohya Y., Pellman D. (2006) Polo-like kinase Cdc5 controls the local activation of Rho1 to promote cytokinesis. Science 313, 108–111 [DOI] [PubMed] [Google Scholar]
- 12. Bos J. L., Rehmann H., Wittinghofer A. (2007) GEFs and GAPs: critical elements in the control of small G proteins. Cell 129, 865–877 [DOI] [PubMed] [Google Scholar]
- 13. Buchsbaum R. J. (2007) Rho activation at a glance. J. Cell Sci. 120, 1149–1152 [DOI] [PubMed] [Google Scholar]
- 14. Rossman K. L., Der C. J., Sondek J. (2005) GEF means go: turning on Rho GTPases with guanine nucleotide-exchange factors. Nat. Rev. Mol. Cell Biol. 6, 167–180 [DOI] [PubMed] [Google Scholar]
- 15. Schmidt A., Hall A. (2002) Guanine nucleotide exchange factors for Rho GTPases: turning on the switch. Genes Dev. 16, 1587–1609 [DOI] [PubMed] [Google Scholar]
- 16. García P., Tajadura V., García I., Sánchez Y. (2006b) Role of Rho GTPses and Rho-GEFs in the regulation of cell shape and integrity in fission yeast. Yeast 23, 1031–1043 [DOI] [PubMed] [Google Scholar]
- 17. Iwaki N., Karatsu K., Miyamoto M. (2003) Role of guanine nucleotide exchange factors for Rho family GTPases in the regulation of cell morphology and actin cytoskeleton in fission yeast. Biochem. Biophys. Res. Commun. 312, 414–420 [DOI] [PubMed] [Google Scholar]
- 18. Perez P., Rincón S. A. (2010) Rho GTPases: regulation of cell polarity and growth in yeasts. Biochem. J. 426, 243–253 [DOI] [PubMed] [Google Scholar]
- 19. Hirota K., Tanaka K., Ohta K., Yamamoto M. (2003) Gef1p and Scd1p, the two GDP-GTP exchange factors for Cdc42p, form a ring structure that shrinks during cytokinesis in Schizosaccharomyces pombe. Mol. Biol. Cell 14, 3617–3627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Coll P. M., Rincon S. A., Izquierdo R. A., Perez P. (2007) Hob3p, the fission yeast ortholog of human BIN3, localizes Cdc42p to the division site and regulates cytokinesis. EMBO J. 26, 1865–1877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ye Y., Lee I.-J., Runged K. W., Wu J.-Q. (2012) Roles of putative Rho-GEF Gef2 in division-site positioning and contractile-ring function in fission yeast cytokinesis. Mol. Biol. Cell 23, 1181–1195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. García P., García I., Marcos F., de Garibay G. R., Sánchez Y. (2009b) Fission yeast Rgf2p is a Rho1p guanine nucleotide exchange factor required for spore wall maturation and for the maintenance of cell integrity in the absence of Rgf1p. Genetics 181, 1321–1334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. García P., Tajadura V., García I., Sánchez Y. (2006a) Rgf1p is a specific Rho1-GEF that coordinates cell polarization with cell wall biogenesis in fission yeast. Mol. Biol. Cell 17, 1620–1631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Garcia P., Tajadura V., Sanchez Y. (2009a) The Rho1p exchange factor Rgf1p signals upstream from the Pmk1 mitogen-activated protein kinase pathway in fission yeast. Mol. Biol. Cell 20, 721–731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mutoh T., Nakano K., Mabuchi I. (2005) Rho1-GEFs Rgf1 and Rgf2 re involved in formation of cell wall and septum, while Rgf3 is involved in cytokinesis in fission yeast. Genes Cells 10, 1189–1202 [DOI] [PubMed] [Google Scholar]
- 26. Morrell-Falvey J. L., Ren L., Feoktistova A., Haese G. D., Gould K. L. (2005) Cell wall remodeling at the fission yeast cell division site requires the Rho-GEF Rgf3p. J. Cell Sci. 118, 5563–5573 [DOI] [PubMed] [Google Scholar]
- 27. Tajadura V., García B., García I., García P., Sánchez Y. (2004) Schizosaccharomyces pombe Rgf3p is a specific Rho1 GEF that regulates cell wall β-glucan biosynthesis through the GTPase Rho1p. J. Cell Sci. 117, 6163–6174 [DOI] [PubMed] [Google Scholar]
- 28. Wang H., Tang X., Balasubramanian M. K. (2003) Rho3p regulates cell separation by modulating exocyst function in Schizosaccharomyces pombe. Genetics 164, 1323–1331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. TerBush D. R., Maurice T., Roth D., Novick P. (1996) The exocyst a multiprotein complex required for exocytosis in Saccharomyces cerevisiae. EMBO J. 15, 6483–6494 [PMC free article] [PubMed] [Google Scholar]
- 30. Heider M. R., Munson M. (2012) Exorcising the exocyst complex. Traffic 13, 898–907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bendezú F. O., Martin S. G. (2011) Actin cables and the exocyst form two independent morphogenesis pathways in the fission yeast. Mol. Biol. Cell 22, 44–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bendezú F. O., Vincenzetti V., Martin S. G. (2012) Fission yeast Sec3 and Exo70 are transported on actin cables and localize the exocyst complex to cell poles. Plos ONE 7, e40248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Estravís M., Rincón S. A., Santos B., Pérez P. (2011) Cdc42 regulates multiple membrane traffic events in fission yeast. Traffic 12, 1744–1758 [DOI] [PubMed] [Google Scholar]
- 34. Kita A., Li C., Yu Y., Umeda N., Doi A., Yasuda M., Ishiwata S., Taga A., Horiuchi Y., Sugiura R. (2011) Role of the small GTPase Rho3 in Golgi/endosome trafficking through functional interaction with adaptin in Fission Yeast. Plos ONE 6, e16842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Yu Y., Li C., Kita A., Katayama Y., Kubouchi K., Udo M., Imanaka Y., Ueda S., Masuko T., Sugiura R. (2013) Sip1, an AP-1 accessory protein in fission yeast, is required for localization of Rho3 GTPase. Plos ONE 8, e68488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Nakano K., Imai J., Arai R., Toh-E A., Matsui Y., Mabuchi I. (2002) The small GTPase Rho3 and the diaphanous/formin For3 function in polarized cell growth in fission yeast. J. Cell Sci. 115, 4629–4639 [DOI] [PubMed] [Google Scholar]
- 37. Nakano K., Toya M., Yoneda A., Asami I. Y., Yamashita A., Kamasawa N., Osumi M., Yamamoto M. (2011) Pob1 ensures cylindrical cell shape by coupling two distinct Rho signaling events during secretory vesicle targeting. Traffic 12, 726–739 [DOI] [PubMed] [Google Scholar]
- 38. Feierbach B., Chang F. (2001) Roles of the fission yeast formin For3 in cell polarity, actin cable formation, and symmetric cell division. Curr. Biol. 11, 1656–1665 [DOI] [PubMed] [Google Scholar]
- 39. Martin S. G., Rincón S. A., Basu R., Pérez P., Chang F. (2007) Regulation of the formin for3p by cdc42p and bud6p. Mol. Biol. Cell 18, 4155–4167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Rincón S. A., Ye Y., Villar-Tajadura M. A., Santos B., Martin S. G., Pérez P. (2009) Pob1 participates in the Cdc42 regulation of fission yeast actin cytoskeleton. Mol. Biol. Cell 20, 4390–4399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Moreno S., Klar A., Nurse P. (1991) Molecular genetic analysis of fission yeast Schizosaccharomyces pombe. Methods Enzymol. 194, 795–823 [DOI] [PubMed] [Google Scholar]
- 42. Shaner N. C., Steinbach P. A., Tsien R. Y. (2005) A guide to choosing fluorescent proteins. Nat. Methods 2, 905–909 [DOI] [PubMed] [Google Scholar]
- 43. Coll P. M., Trillo Y., Ametzazurra A., Perez P. (2003) Gef1p, a new guanine nucleotide exchange factor for Cdc42p, regulates polarity in Schizosaccharomyces pombe. Mol. Biol. Cell 14, 313–323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Ren X. D., Kiosses W. B., Schwartz M. A. (1999) Regulation of the small GTP-binding protein Rho by cell adhesion and the cytoskeleton. EMBO J. 18, 578–585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Habermann B. (2004) The BAR-domain family of proteins: a case of bending and binding? EMBO Rep. 5, 250–255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Aspenström P. (2009) Roles of F-BAR/PCH proteins in the regulation of membrane dynamics and actin reorganization. Int. Rev. Cell Mol. Biol. 272, 1–31 [DOI] [PubMed] [Google Scholar]
- 47. Laporte D., Coffman V. C, Lee I. -J., Wu J.-Q. (2011) Assembly and architecture of precursor nodes during fission yeast cytokinesis. J. Cell Biol. 192, 1005–1021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Cortés J. C., Konomi M., Martins I. M., Muñoz J., Moreno M. B., Osumi M., Durán A., Ribas J. C. (2007) The (1,3)β-d-glucan synthase subunit Bgs1p is responsible for the fission yeast primary septum formation. Mol. Microbiol. 65, 201–217 [DOI] [PubMed] [Google Scholar]
- 49. Berlin A., Paoletti A., Chang F. (2003) Mid2p stabilizes septin rings during cytokinesis in fission yeast. J. Cell Biol. 160, 1083–1092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Longtine M. S., DeMarini D. J., Valencik M. L., Al-Awar O. S., Fares H., De Virgilio C., Pringle J. R. (1996) The septins: roles in cytokinesis and other processes. Curr. Opin. Cell Biol. 8, 106–119 [DOI] [PubMed] [Google Scholar]
- 51. Tasto J. J., Morrell J. L., Gould K. L. (2003) An anillin homologue, Mid2p, acts during fission yeast cytokinesis to organize the septin ring and promote cell separation. J. Cell Biol. 160, 1093–1103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. He B., Guo W. (2009) The exocyst complex in polarized exocytosis. Curr. Opin. Cell Biol. 21, 537–542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Hirata D., Nakano K., Fukui M., Takenaka H., Miyakawa T., Mabuchi I. (1998) Genes that cause aberrant cell morphology by overexpression in fission yeast: a role for a small GTP-binding protein Rho2 in cell morphogenesis. J. Cell Sci. 111, 149–159 [DOI] [PubMed] [Google Scholar]
- 54. Martín-Cuadrado A. B., Morrell J. L., Konomi M., An H., Petit C., Osumi M., Balasubramanian M., Gould K. L., Del Rey F., de Aldana C. R. (2005) Role of septins and the exocyst complex in the function of hydrolytic enzymes responsible for fission yeast cell separation. Mol. Biol. Cell 16, 4867–4881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Forsburg S. L. (1993) Comparison of Schizosaccharomyces pombe expression systems. Nucleic Acids Res. 21, 2955–2956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Wang H., Tang X., Liu J., Trautmann S., Balasundaram D., McCollum D., Balasubramanian M. K. (2002) The multiprotein exocyst complex is essentail for cell separation in Schizosaccharomyces pombe. Mol. Biol. Cell 13, 515–529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Nakamura T., Nakamura-Kubo M., Hirata A., Shimoda C. (2001) The Schizosaccharomyces pombe spo3+ gene is required for assembly of the forespore membrane and genetically interacts with psy1+-encoding syntaxin-like protein. Mol. Biol. Cell 12, 3955–3972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Wu J.-Q., Ye Y., Wang N., Pollard T. D., Pringle J. R. (2010) Cooperation between the septins and the actomyosin ring and role of a cell-integrity pathway during cell division in fission yeast. Genetics 186, 897–915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Rossman K. L., Sondek J. (2005b) Larger than Dbl: new structural insights into RhoA activation. Trends Biochem. Sci. 30, 163–165 [DOI] [PubMed] [Google Scholar]
- 60. Suetsugu S., Toyooka K., Senju Y. (2010) Subcellular membrane curvature mediated by the BAR domain superfamily proteins. Semin. Cell Dev. Biol. 21, 340–349 [DOI] [PubMed] [Google Scholar]
- 61. Frost A., Unger V. M., De Camilli P. (2009) The BAR domain superfamily: membrane-molding macromolecules. Cell 137, 191–196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. de Kreuk B. J., Hordijk P. L. (2012) Control of Rho GTPase function by BAR-domains. Small GTPases 3, 45–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Caudron F., Barral Y. (2009) Septins and the lateral compartmentalization of eukaryotic membranes. Dev. Cell 16, 493–506 [DOI] [PubMed] [Google Scholar]
- 64. Gladfelter A. S., Pringle J. R., Lew D. J. (2001) The septin cortex at the yeast mother-bud neck. Curr. Opin. Microbiol. 4, 681–689 [DOI] [PubMed] [Google Scholar]
- 65. Barral Y. (2008) Yeast septins: a cortical organizer. Chapter 4, in The Septins (Hall P. A., Russell S. E. H., Pringle J. R., eds) pp. 101–125, Wiley Blackwell, London [Google Scholar]
- 66. Joo E., Tsang C. W., Trimble W. S. (2005) Septins: traffic control at the cytokinesis intersection. Traffic 6, 626–634 [DOI] [PubMed] [Google Scholar]
- 67. Spiliotis E. T., Gladfelter A. S. (2012) Spatial guidance of cell asymmetry: septin GTPases show the way. Traffic 13, 195–203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. An H., Morrell J. L., Jennings J. L., Link A. J., Gould K. L. (2004) Requirements of fission yeast septins for complex formation, localization, and function. Mol. Biol. Cell 15, 5551–5564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Zhang J., Kong C., Xie H., McPherson P. S., Grinstein S., Trimble W. S. (1999) Phosphatidylinositol polyphosphate binding to the mammalian septin H5 is modulated by GTP. Curr. Biol. 9, 1458–1467 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.