Background: Molecular signaling during chondrogenic differentiation of mesenchymal stem cells (MSC) is poorly understood.
Results: Knockdown of sirtuin-1 (SIRT1) in MSC induced inhibition of chondrogenesis, Sox9 expression, up-regulation of nuclear factor-κB (NF-κB) phosphorylation and acetylation, and NF-κB-dependent pro-inflammatory enzymes.
Conclusion: SIRT1 supports chondrogenic differentiation of MSCs by Sox9 activation and NF-κB deacetylation.
Significance: These findings may be essential for improving cartilage tissue regeneration.
Keywords: Chondrogenesis, Inflammation, Mesenchymal Stem Cells (MSCs), NF-κB, Resveratrol, Sirtuin 1 (SIRT1), Rheumatoid Arthritis, SIRT1-ASO, SOX9
Abstract
Sirtuin-1 (SIRT1), NAD+-dependent deacetylase, has been linked to anabolic effects in cartilage, although the mechanisms of SIRT1 signaling during differentiation of mesenchymal stem cells (MSCs) to chondrocytes are poorly understood. Therefore, we investigated the role of SIRT1-mediated signaling during chondrogenic differentiation of MSCs in vitro. High density and alginate cultures of MSCs were treated with chondrogenic induction medium with/without the SIRT1 inhibitor nicotinamide, antisense oligonucleotides against SIRT1 (SIRT1-ASO), IL-1β, and/or resveratrol. Transient transfection of MSCs with SIRT1-antisense oligonucleotides, nicotinamide, and IL-1β inhibited chondrogenesis-induced down-regulation of cartilage-specific proteins, cartilage-specific transcription factor Sox9, and enhanced NF-κB-regulated gene products involved in the inflammatory and degradative processes in cartilage (MMP-9, COX-2, and caspase-3), and NF-κB phosphorylation, acetylation, and activation of IκBα kinase. In contrast, the SIRT1 activator resveratrol or BMS-345541 (inhibitor of IKK) inhibited IL-1β- and NAM-induced suppression of cartilage-specific proteins, Sox9, and up-regulation of NF-κB-regulated gene products. Moreover, SIRT1 was found to interact directly with NF-κB and resveratrol-suppressed IL-1β and NAM but not SIRT1-ASO-induced NF-κB phosphorylation, acetylation, and activation of IκBα kinase. Knockdown of SIRT1 by mRNA abolished the inhibitory effects of resveratrol on inflammatory and apoptotic signaling and Sox9 expression, suggesting the essential role of this enzyme. Finally, the modulatory effects of resveratrol were found to be mediated at least in part by the association between SIRT1 and Sox9. These results indicate for the first time that SIRT1 supports chondrogenic development of MSCs at least in part through inhibition/deacetylation of NF-κB and activation of Sox9.
Introduction
Mesenchymal stem cells (MSCs)2 are multipotent cells and can differentiate into different specialized cell types, like chondrocytes, osteocytes, or adipocytes in vitro (1–3). Adult MSCs are easy to isolate from bone marrow or adipose tissue and expand in vitro, and they provide a suitable source of cells for tissue engineering and regenerative medicine (2, 4). Moreover, adult MSCs are a practicable alternative and an ethically accepted source of multipotent cells compared with the limited use of embryonic or fetal stem cells (5, 6). Development of cartilage tissue from MSCs commences with condensation and differentiation (7). Chondrocytes then produce the cartilage-specific extracellular matrix (ECM) proteins that provide this tissue with its unique mechanical properties required for resistance to adequate loading.
Several other investigations and our laboratory have clearly demonstrated that three-dimensional culture models of mesenchyme-derived cells are suitable to develop and differentiate MSCs toward chondrocytes as they closely replicate the extensive cell-matrix interactions that occur in vivo (2, 8–10). We have already adopted high density and alginate cultures as in vitro models to culture MSCs derived from mouse limb buds, adult MSCs, and chondrocytes and to develop cartilage tissue (2, 10–14). However, age-related arthritic disease, osteoarthritis, and rheumatoid arthritis are involved in the degenerative changes in the joint, leading to loss of function, pain, and significant disability (15). It is known that articular cartilage is an avascular, alymphatic, and aneural tissue with bradytrophic characteristics and a very poor capacity for self-repair and regeneration (16, 17). This weakness in cartilage repair capacity highlights the need for novel treatments using tissue engineering and regenerative medicine and new regenerative strategies involving stimulation of articular cartilage repair in vivo (18). Indeed, it has been shown that adult MSC-like progenitors also exist in the cartilage tissue and that their abundance in arthritic cartilage is elevated (19). The lack of regeneration in cartilage can be due to the ongoing inflammatory microenvironment that occurs during the course of osteoarthritis and rheumatoid arthritis. It is therefore important to block the pro-inflammatory cytokine-induced cartilage degeneration and at the same time create a more suitable microenvironment for the chondrogenesis of MSC-like progenitors (20).
Resveratrol (3,5,4′-trihydroxy-trans-stilbene) is a phytoalexin (polyphenolic compound) found in the skin of red grapes, cranberries, peanuts, and root extracts of the weed Polygonum cuspidatum (21). Several reports have demonstrated that resveratrol has anti-inflammatory, antioxidant, and antitumor activity in cancer cell lines derived from human and animal tumors (22–24). One of the most important and functional novel molecular targets of resveratrol is sirtuin-1 (SIRT1), a member of the sirtuin family of nicotinamide adenine dinucleotide (NAD)-dependent deacetylases, which is found to be an anti-aging gene (25, 26). SIRT1 is able to de-acetylate many different transcription factors in the nucleus such as p53, NF-κB, myogenic differentiation, high mobility group I, E2F transcription factor, and forkhead box O, thus playing an essential role in cell differentiation, cell survival, tumorigenesis, inflammation, and metabolism (27–31). Moreover, SIRT1 targets chromatin (histones) as well as nonchromatin proteins in the cells, has been linked to transcriptional silencing, and appears to play a key role in inflammation (32, 33). More recently, several reports have shown that normal cartilage homeostasis requires enzymatically active SIRT1 protein in vivo (34–36).
In the past, it has been shown that SIRT1 plays an essential role in a variety of tissue development and diseases. However, still little is known about its role in MSC differentiation. The purpose of this study was therefore to examine whether SIRT1, at least in part, regulates differentiation of MSCs to chondrocytes in vitro.
EXPERIMENTAL PROCEDURES
Antibodies
Acetylated lysine (Ac-K-103) antibody was purchased from Cell Signaling Technology (Danvers, MA). Monoclonal and polyclonal antibody to SIRT1 was purchased from Abcam PLC (Cambridge, UK). Polyclonal anti-collagen type II, monoclonal anti-adult cartilage-specific proteoglycan (CSPGs), and alkaline phosphatase-linked sheep anti-mouse and sheep anti-rabbit secondary antibodies for immunoblotting were from Millipore (Schwalbach, Germany). Antibodies to active caspase-3 and MMP-9 were from R&D Systems, Inc. (Heidelberg, Germany). Cyclo-oxygenase-2 antibody was obtained from Cayman Chemical (Ann Arbor, MI). Anti-phospho-specific p65 (NF-κB)/(Ser-536) and anti-phospho-specific IκBα (Ser-32/36) were obtained from Cell Technology, Inc. (Beverly, MA). Anti-IKK-α and anti-IKK-β antibodies were obtained from Imgenex (Hamburg, Germany). Monoclonal anti-Sox9, monoclonal anti-CD105, monoclonal anti-CD90, monoclonal anti-CD45, and monoclonal anti-CD34 were purchased from Acris Antibodies GmbH (Hiddenhausen, Germany). Monoclonal anti-β-actin was purchased from Sigma. Secondary antibodies used for fluorescence labeling were purchased from Dianova (Hamburg, Germany). All antibodies were used at concentrations and dilutions recommended by the manufacturer.
Growth Media, Chemicals, Cytokines
Whole cell culture growth medium consisting of DMEM/Ham's F-12 1:1, 10% FBS, 1% partricin solution, 1% penicillin/streptomycin solution (10,000 IU/10,000 IU), 75 μg/ml ascorbic acid, 1% essential amino acids, and 1% glutamine were purchased from Seromed (Munich, Germany). BMS-345541, NAM, and resveratrol were purchased from Sigma. Resveratrol was prepared as a 1000 μm solution in ethanol and then further diluted in cell culture medium. The final concentration of ethanol did not, in any case, exceed 0.5%. Further dilutions were made in cell culture medium to achieve the final working concentrations. Interleukin-1β (IL-1β) was purchased from Acris antibodies GmbH (Herold, Germany).
Chondrocyte and Mesenchymal Stem Cell Culture
Primary human chondrocytes (catalog no. 121 0211) and human mesenchymal stem cells (catalog no. 121 0911 HMSC-bm) were obtained from Provitro (Berlin, Germany). Specimens of knee joint cartilage and human bone marrow were obtained with total informed consent and approval of the ethics committee of the Charité-University Medical School Berlin (Germany). Cells were seeded at a density of 300,000 cells/T75 flask, cultured in whole cell culture medium, and passaged at 70–80% confluence. Passages 3–4 were used for the experiments. Characterization of MCS was performed by immunofluorescent evaluation of a set of defined markers (CD105+, CD90+, CD45−, and CD34−) (37). To quantify the population for each marker, the number of cells with positive labeling was determined by scoring 300 cells from 10 different microscopic fields within the stained slides.
Chondrogenic Differentiation in High Density and Alginate Bead Culture
Chondrogenic differentiation of MSC was performed in high density culture and in alginate bead culture as described previously (2, 10). We have used alginate bead culture complementary to high density culture, because in alginate bead culture the cells have strong chondrogenic potential; additionally it is fairly easy to retrieve the cells from alginate to perform additional experiments such as immunoprecipitation, immunofluorescence, and MTT assays. For high density culture, a 10-μl drop of ∼1 million cells was plated on a cellulose filter on top of a steel mesh bridge. Cell culture medium reached the filter medium interface, and cells were nurtured through diffusion. This model allows the cells to aggregate, and after 1 day in culture, the cells formed a three-dimensional pellet on the filter. For alginate bead culture, ∼2 million cells/ml alginate were encapsulated in sterile alginate (2.5% in 0.15 m NaCl solution) by pipetting single drops into a 100 mm CaCl2 solution. At contact with the CaCl2 solution, the alginate drops polymerized forming a round and stable bead. To ensure adequate polymerization, beads were incubated with 100 mm CaCl2 solution for 10 min. Subsequently, beads were rinsed three times in 0.15 m NaCl solution followed by washing two times with complete cell culture medium to remove excess NaCl/CaCl2 solution and were transposed to a new Petri dish. Chondrogenic differentiation medium was prepared as described by Pittenger et al. (1) consisting of DMEM base medium, d-(+)-glucose 0.35 g/100 ml, ITS + 1 liquid media supplement (10 μg/ml insulin, 5.5 μg/ml transferrin, 5 ng/ml selenium, 0.5 mg/ml bovine albumin, 4.7 μg/ml linoleic acid (Sigma, catalog no. I-2521)), 0.1 mm ascorbate 2-phosphate (Sigma catalog no. A-8960), 10−7 m dexamethasone (Sigma catalog no. D-8893), penicillin/streptomycin solution (10,000 IU/10,000 IU/100 ml). Ten ng/ml human TGFβ1 (Acris Antibodies GmbH, Germany) was added freshly to the medium before each medium change, and medium changes were made three times/week. The cultures were incubated for 14 days in a humidified incubator at 37 °C in an atmosphere of 95% air and 5% CO2 before further evaluation.
Antisense and Lipofectin-mediated Transfection
Transient transfection of primary human chondrocytes, chondrogenic differentiated MSCs, and MSCs undergoing chondrogenesis was performed as described previously (38). Phosphorothioated antisense oligonucleotide derived from mRNA nucleotide sequence of sirtuin-1 gene (SIRT1-ASO) (sequence 5′-GTATTCCACATGAAACAGACA-3′) and control sense oligonucleotides (SIRT1-SO) (sequence 5′-TGTCTGTTTCATGTGGAATAC-3′) used in the experiments were synthesized by Eurofins (MWG/Operon, Ebersberg, Germany). SIRT1-ASO and SIRT1-SO were phosphorothioate-modified to protect them from the cell nucleases. Cells in monolayer culture were transfected by incubation with 0.5 μm SIRT1-ASO or SIRT1-SO and 10 μl/ml Lipofectin transfection reagent (Invitrogen) in serum-starved medium (3% FCS) for 24 h before starting the respective experiments. All monolayer transfection experiments were carried out on 50–60% confluent monolayer cultures. For transfection of high density and alginate bead cultures, MSCs (1 × 106) were either untreated or pretreated in slurry with resveratrol (5 μm) for 4 h in serum-starved medium. After this treatment, whole cells were transferred to high density or alginate cultures and either served as controls (no treatment) or were transfected with various concentrations (0.1, 0.5, 1, and 5 μm) of SIRT1-ASO or SIRT1-SO in the presence of Lipofectin (10 μl/ml) transfection reagent in chondrogenic induction medium for 14 days. Culture medium with SIRT1-ASO or SIRT1-SO was changed every 3 days.
Electron Microscopic Evaluation
To evaluate chondrogenic ultrastructure, transmission electron microscopy was performed as described previously in detail (39). Briefly, cultures were fixed for 1 h in Karnovsky fixative, post-fixed in 1% OsO4 solution, dehydrated in serial alcohol dilutions, and embedded in Epon (Plano, Germany). Following this, ultrathin cuts were made on a Reichert-Ultracut E, contrasted with a mixture of 2% uranyl acetate/lead citrate, and evaluated with a Zeiss 10 transmission electron microscope (Institute of Pharmacology, Berlin, Germany).
Quantification of Apoptotic Cell Death
Ultrathin sections of the cultures were prepared and evaluated with a transmission electron microscope. To quantify the apoptotic cells, the number of cells with morphological features of apoptotic cell death was determined by scoring 100 cells from 25 different microscopic fields.
Western Blot Analysis
Western blotting of whole cell lysates was performed as described previously (40, 41). Briefly, whole cell lysate proteins were extracted with lysis buffer (50 mm Tris-HCl, pH 7.2, 150 mm NaCl, 1% (v/v) Triton X-100, 1 mm sodium orthovanadate, 50 mm sodium pyrophosphate, 100 mm sodium fluoride, 0.01% (v/v) aprotinin, 4 μg/ml pepstatin A, 10 μg/ml leupeptin, 1 mm phenylmethylsulfonyl fluoride, PMSF) on ice for 30 min, and total protein content was measured. Subsequently, samples were reduced with 2-mercaptoethanol, and total protein concentrations were adjusted. After separation of proteins by SDS-PAGE under reducing conditions, samples were blotted onto a membrane using a transblot apparatus (Bio-Rad) and blocked for 2 h in 5% (w/v) skimmed milk powder in phosphate-buffered saline, 0.1% Tween 20. Membranes were incubated overnight with the primary antibody at a 1:1000 dilution in blocking buffer at 4 °C on a shaker. Incubation with the secondary antibody conjugated with alkaline phosphatase was performed for 90 min. After washing the membranes three times in 0.1 m Tris, pH 9.5, containing 0.05 m MgCl2 and 0.1 m NaCl, specific antigen-antibody complexes were detected using nitro blue tetrazolium and 5-bromo-4-chloro-3-indoylphosphate (p-toluidine salt; Pierce) as substrates for alkaline phosphatase.
Immunoprecipitation of SIRT1/p65 Acetylation and SIRT1/Sox9 Assay
To evaluate the effect of resveratrol on IL-1β-, NAM-, and SIRT1-ASO-induced p65 acetylation and Sox9 expression, endogenous protein interactions from high density and alginate cultures were evaluated by co-immunoprecipitation experiments using SIRT1, anti-acetyl-lysine, and Sox9 antibodies. After 14 days, whole cell extracts were washed and lysed to prepare whole cell lysates. Whole cell extracts were precleared by incubating with 25 μl of either normal rabbit IgG serum or mouse IgG serum and protein A/G-Sepharose beads. The precleared whole cell extract was incubated with primary antibodies (anti-SIRT1 or anti-Sox9) and diluted in wash buffer (0.1% Tween 20, 150 mm NaCl, 50 mm Tris-HCl, pH 7.2, 1 mm CaCl2, 1 mm MgCl2, and 1 mm PMSF) for 2 h at 4 °C and finally with protein A/G-Sepharose beads for 1 h at 4 °C. After incubation, immunocomplexes were washed with lysis buffer, boiled with SDS sample buffer for 5 min, resolved on SDS-PAGE, and subjected to Western blot analysis using an anti-acetyl-lysine, anti-SIRT1, or anti-Sox9 antibodies. The original samples were also detected using antibodies against poly(ADP-ribose) polymerase or β-actin.
Immune Complex Kinase Assay
To investigate the interplay between SIRT1 and IκBα activation during chondrogenesis, an immune complex kinase assay was performed. The IKK complex was immunoprecipitated from whole cell lysates with antibodies against IKK-α and IKK-β and subsequently incubated with protein A/G-agarose beads (Pierce). After 2 h of incubation, the beads were washed with lysis buffer, resuspended in a kinase assay solution containing 50 mm HEPES, pH 7.4, 20 mm MgCl2, 2 mm dithiothreitol, 10 mm unlabeled ATP, and 2 mg of substrate IκBα (amino acids 1–54), incubated at 30 °C for 30 min, and boiled in SDS-PAGE sample buffer for 5 min. SDS-PAGE was performed under reducing conditions as described above. Phosphorylation of IκBα was assessed using a specific antibody against phosphorylated IκBα (Ser-32/36). To demonstrate the total amounts of IKK-α and IKK-β in each sample, whole cell lysates were transferred to a nitrocellulose membrane after SDS-PAGE was performed as described above. Detection of phosphorylated IκBα, IKK-α, and IKK-β was performed by immunoblotting.
Immunofluorescence Analysis
For double immunofluorescence detection of collagen type II/CSPGs and of SIRT1/Sox9, MSC-derived chondrocytes were retrieved from alginate beads and cultured in monolayers on glass plates for 24 h. After methanol fixation and additional cell nucleus permeabilization with Triton X-100 (2% in PBS) for 5 min, cells were rinsed three times with PBS and overlaid with bovine serum albumin (1% BSA in PBS) for 1 h. The first primary antibody incubation was performed overnight at 4 °C in a humid chamber, followed by incubation with rhodamine-coupled secondary antibodies (diluted 1:80 in PBS) for 2 h at ambient temperature. After additional washing with 1% BSA in PBS, cells were incubated with the second primary antibody followed by an additional FITC-coupled secondary antibody as described above. Finally counter-staining was performed with DAPI (Sigma) to visualize cell nuclei, and slides were then covered with FluoromountTM mountant and examined under a fluorescent microscope (Leica, Germany).
Cell Viability Assay
The cell viability was evaluated by the MTT uptake method as described previously (42). Briefly, MSCs in alginate culture were either left untreated or were treated with resveratrol (5 μm), NAM (10 mm), Lipofectin (10 μm/ml), or were transfected with SIRT1-SO or SIRT1-ASO (0.5 μm in the presence of 10 μl/ml Lipofectin) for 14 days, or cells were pretreated with resveratrol (5 μm) for 4 h followed by co-treatment with either NAM (10 mm) or transfected with 0.5 μm SIRT1-SO or SIRT1-ASO in chondrogenic induction medium for 14 days. Cells were released from alginate by dissolving the beads in 55 mm sodium citrate solution (1618 g of sodium citrate in 100 ml of 0.15 m NaCl) for 20 min. Cells were washed twice with Hanks' balanced salt solution to remove excess alginate and resuspended in modified cell culture medium (DMEM without phenol red, without vitamin C, and only 3% FBS) on a 96-well plate. 10 μl of MTT solution (5 mg/ml) was immediately added to each well, and the plate was incubated for 4 h at 37 °C. Finally, MTT solubilization solution (10% Triton X-100/acidic isopropyl alcohol) was added, and the cells were incubated overnight at 37 °C. Absorbance was measured at 550 nm using Revelation 96-well multiscanner plate reader (Bio-Rad). The data obtained were calculated and were represented as percentage of survival relative to controls. This experiment was repeated three times independently, and statistical analysis was done to obtain the final values.
Statistical Analysis
Each experiment was performed three times as individual experiments with three replicates. For statistical analysis, a Wilcoxon-Mann-Whitney test was applied. Score values for image quality and presence of artifacts were compared for each sequence. A p value of <0.05 was considered to establish statistically significant differences.
RESULTS
In this study, we evaluated the effect of resveratrol/SIRT1 on chondrogenic differentiation of mesenchymal stem cells in an in vitro model of chondrogenesis. The concentration of resveratrol or ethanol applied in our study and the time of exposure had no effect on cell viability.
Characterization of MSCs
Human-derived MSCs exhibited a polymorphic fibroblast-like phenotype (Fig. 1A, panel a). To examine that the human MSCs (Fig. 1A) are indeed MSCs, immunofluorescence was used to confirm positive expression of the stem cell-specific markers (CD90+ and CD105+) (Fig. 1A, panels b and c). In contrast to this, they were clearly labeled negative for the hematopoietic stem cell markers CD45− and CD34− (Fig. 1A, panels d and e). Statistical evaluation of the labeled cultures displayed the stem cell-specific marker-positive cell population by counting 300 cells from 10 microscopic fields within the stained slides. As shown in Fig. 1B, this confirmed the results in Fig. 1A and revealed that positive expression of the stem cell-specific markers CD105+ and CD90+ and negative expression of the hematopoietic lineage markers CD45− and CD34− compared with controls (Fig. 1B).
FIGURE 1.
Characterization of MSCs. A, in monolayer culture the human MSCs (panel a) showed a polymorphic, fibroblast-like morphology. The MSCs expressed positive stem cell-specific markers CD90+ (panel b) and CD105+ (panel c) and were negative for the hematopoietic stem cell markers CD45− (panel d) and CD34− (panel e). Magnification: ×10 (panel a), ×40 (panels b–e), and bar, 1 μm. B, to quantify the stem cell marker-positive cells, the labeled cultures were examined by counting 300 cells from 10 microscopic fields. The examination was performed in triplicate, and the results are provided as the mean values with S.D. from three independent experiments. Values were compared with the control, and statistically significant values with p < 0.05 are designated by a star, and p < 0.01 is designated by two stars. Co., without primary antibody, followed by incubation with rhodamine-coupled secondary antibodies and counterstaining with DAPI to visualize the cell nuclei.
Knockdown of SIRT1 with ASO Inhibits Differentiation of MSCs to Chondrocytes in Vitro
To investigate whether the specific inhibition of SIRT1 mRNA and protein expression by ASO blocks the differentiation of MSCs to chondrocytes, we examined ultrastructural cell morphology by transmission electron microscopy (Fig. 2A). As positive control, high density cultures of primary chondrocytes were performed and prepared for transmission electron microscopy (Fig. 2A, panel a). After 14 days in high density cultures, chondrocytes showed well developed cartilage nodules with viable cells and well developed and organized cell organelles. The cells were embedded in an extensive fine fibrillar matrix tightly attached to the cytoplasmic membrane (Fig. 2A, panel a). Untreated (Fig. 2A, panel b) or Lipofectin-treated (data not shown) MSCs cultured in chondrogenic induction medium for 14 days resulted in chondrogenesis; cells exhibited high levels of nuclear euchromatin and large numbers of morphologically normal cellular organelles, and the cells were embedded in a well organized extracellular matrix (Fig. 2A, panel b). Treatment of MSC cultures with the chondrogenic induction medium and SIRT1-SO in various concentrations (0.1, 0.5, 1, and 5 μm) in the presence of Lipofectin (10 μl/ml) induced chondrogenesis (Fig. 2A, panels c–f). However, no significant differences in chondrogenesis were seen at the ultrastructural level between SIRT1-SO-treated and -untreated MSC cultures. In contrast, transfection of MSCs with SIRT1-ASO in various concentrations (0.1, 0.5, 1, and 5 μm) in the presence of Lipofectin for 14 days revealed inhibition of chondrogenesis. The results showed a dose-dependent increase in morphological signs of degeneration and degradation of MSCs (Fig. 2A, panels g–j). Furthermore, treatment with 0.5 μm or more SIRT1-ASO led to changes such as multiple vacuoles, swelling, and degeneration of cell organelles, and MSCs underwent apoptosis, with nuclear damage and formation of apoptotic bodies (Fig. 2A, panels h–j). Statistical evaluation of the ultrastructural data of cells displaying severe apoptosis was performed by counting 100 cells from 25 microscopic fields. As shown in Fig. 2B, specific SIRT1-ASO effectively induced cell death in a dose-dependent manner with 85% apoptotic cells at 5 μm, 83% at 1 μm, 81% at 0.5 μm and 28% at 0.1 μm SIRT1-ASO compared with untreated cells.
FIGURE 2.
Effects of SIRT1-ASO on chondrogenic differentiation of MCS in vitro. A, primary human chondrocytes (PCH) in high density were left untreated (panel a). MSCs were either cultured only with chondrogenic induction medium (panel b) or with chondrogenic induction medium and simultaneously transfected with different concentrations (0.1, 0.5, 1, 5 μm) of SIRT1-SO (panels c–f) or SIRT1-ASO (panels g–j) in the presence of Lipofectin (10 μl/ml). Ultrastructural morphology of high density cultures was evaluated after 14 days by transmission electron microscope. Control cultures of chondrocytes or MSCs or treatment of MSCs with SIRT1-SO showed well developed chondrocytes (Ch) embedded in a well developed ECM and the formation of cartilage nodules (panels a–f). Treatment with SIRT1-ASO resulted in matrix breakdown and cell lysis and apoptosis (arrows) (panels g–j). Micrographs shown are representative of three individual experiments. Magnification: ×5000, bar, 1 μm. B, to quantify degenerative changes, high density cultures of A were examined for apoptosis by counting 100 cells from 25 microscopic fields. The examination was performed in triplicate, and the results are provided as the mean values with S.D. from three independent experiments. Values were compared with the control and statistically significant values with p < 0.05 were designated by a star and p < 0.01 were designated by a two stars.
Specific Inhibition of SIRT1 by SIRT1-ASO Reduces SIRT1 Protein Expression and Suppresses Cartilage-specific ECM Proteins and Chondrocyte Transcription Factor Sox9 Expression during Chondrogenesis of MSC in Vitro
Primary human chondrocytes were left untreated, and MSCs were either cultured only with chondrogenic induction medium or with chondrogenic induction medium and transfected with different concentrations (0.1, 0.5, 1, and 5 μm) of SIRT1-SO or SIRT1-ASO in the presence of Lipofectin (10 μl/ml) for 14 days. Whole cell lysates were fractionated and analyzed by immunoblotting using anti-SIRT1 (A), anti-collagen type II (B), anti-cartilage specific proteoglycans (C), anti-cartilage-specific transcription factor Sox9 (D), and anti-β-actin (Fig. 3). SIRT1 control peptide was used as a control for antibody specificity. As shown in Western blot analysis and densitometric evaluation, treatment with SIRT1-ASO clearly down-regulated the expression of SIRT1, collagen type II, CSPG proteins, and Sox9 in a dose-dependent manner. The dosages of 1–5 μm SIRT1-ASO almost completely suppressed the expression of SIRT1 and Sox9 proteins (Fig. 3, A–D). In contrast, Lipofectin (data not shown) and SIRT1-SO treatment had no effect on SIRT1, collagen type II, CSPG, and Sox9 protein levels (Fig. 3, A–D). They remained comparable with the untreated basal control (primary human chondrocytes), thereby highlighting the specificity of SIRT1-ASO, suggesting that there is a connection between SIRT1 protein and chondrogenesis of MSCs.
FIGURE 3.
Effects of SIRT1-ASO on SIRT1 expression, ECM protein production, and cartilage-specific transcription factor Sox9 during chondrogenesis of MSC in vitro. Primary human chondrocytes (PCH) in high density were left untreated. MSCs in high density were either cultured only with chondrogenic induction medium or with chondrogenic induction medium and transfected with different concentrations of SIRT1-SO or SIRT1-ASO (0.1, 0.5, 1, and 5 μm) in the presence of Lipofectin (10 μl/ml) for 14 days. Whole cell lysates were prepared and analyzed by Western blotting with antibodies against SIRT1 (A), collagen type II (B), cartilage-specific proteoglycans (C), and cartilage-specific transcription factor Sox9 (D). Densitometric evaluation of protein expression as revealed by Western blot analysis was performed in triplicate. Bars represent the mean values (± S.D.) for collagen type II of the 216-, 137-, and 110-kDa bands and for cartilage-specific proteoglycans of the 140-, 127-, and 33-kDa bands. SIRT1 control peptide (Co. Pep) was used as a control for SIRT1 antibody specificity. The results shown are representative of three independent experiments. Housekeeping protein β-actin served as a loading control in all experiments. The arrows in the right margin indicate the relative position of the proteins and molecular mass markers (left margin) are indicated in kDa. Values were compared with the control and statistically significant values with p < 0.05. Significant values are marked with a star.
Resveratrol Suppresses Down-regulation of ECM Proteins and Sox9 Induced by IL-1β and NAM but Not by SIRT1-ASO during Chondrogenesis of MSCs
Primary human chondrocytes in high density culture were left untreated as basal control. MSCs in high density culture were either left untreated or were treated with resveratrol (5 μm), IL-1β (10 ng/ml), NAM (10 mm), or BMS-345541 (5 μm) or were transfected with SIRT1-SO or SIRT1-ASO (0.5 μm in the presence of Lipofectin 10 μl/ml) for 14 days, or cells were pretreated with BMS-345541 (5 μm) for 4 h followed by co-treatment with IL-1β (10 ng/ml) for 14 days, or cells were pretreated with resveratrol (5 μm) for 4 h followed by co-treatment with either IL-1β (10 ng/ml), NAM (10 mm), or BMS-345541 (5 μm), or transfected with 0.5 μm SIRT1-SO or SIRT1-ASO in chondrogenic induction medium for 14 days. Whole cell lysates were fractionated and analyzed by Western blotting with antibodies against collagen type II (Fig. 4A, panel I), CSPGs (panel II), and Sox9 (panel III) (Fig. 4A). As shown in Fig. 4A, treatment of MSC with IL-1β, SIRT1 inhibitor NAM, or SIRT1-ASO alone markedly suppressed collagen type II, CSPG production, and cartilage-specific transcription factor Sox9 during chondrogenesis of MSCs in vitro. The basal levels of cartilage-specific matrix expression (collagen type II and CSPGs) and Sox9 expression were not significantly changed after incubation with BMS-345541, resveratrol, or SIRT1-SO (Fig. 4A). However, expression of cartilage-specific ECM and Sox9 was significantly increased in MSC cultures pretreated with resveratrol comparable with control cultures but not in SIRT1-ASO treated MSCs. This indicates that SIRT1 suppression on mRNA levels is not reversible by resveratrol and highlights the crucial role of SIRT1 during chondrogenesis of MSCs in vitro (Fig. 4A). Moreover, these findings suggest that there is a functional link between SIRT1 protein and cartilage-specific transcription factor Sox9 and that resveratrol is able to enhance chondrogenic MSC differentiation, at least in part, by up-regulation of Sox9 via SIRT1.
FIGURE 4.
Effects of resveratrol, IL-1β, NAM, BMS-345541, and SIRT1-ASO on ECM protein, Sox9, and NF-κB-dependent proinflammatory, matrix-degrading, and apoptotic gene products during chondrogenesis of MSC in vitro. A, primary human chondrocytes (PCH) in high density culture were left untreated as control. MSCs in high density culture were either left untreated or treated with resveratrol (5 μm), IL-1β (10 ng/ml), NAM (10 mm), BMS-345541 (5 μm) or were transfected with SIRT1-SO or SIRT1-ASO (0.5 μm) in the presence of Lipofectin (10 μl/ml) for 14 days, or cells were pretreated with BMS-345541 (5 μm) for 4 h followed by co-treatment with IL-1β (10 ng/ml) for 14 days. Other cultures were pretreated with resveratrol (5 μm) for 4 h followed by co-treatment with IL-1β (10 ng/ml), NAM (10 mm), or BMS-345541 (5 μm) or transfected with 0.5 μm SIRT1-SO, SIRT1-ASO in chondrogenic induction medium for 14 days. Whole cell lysates (500 ng of protein/lane) were prepared and analyzed by Western blotting with antibodies against collagen type II (panel I), cartilage-specific proteoglycans (panel II), and Sox9 (panel III). B, same MSC cultures as in A were subsequently also probed for expression of COX-2 (panel I), MMP-9 (panel II), and cleavage of caspase-3 (panel III). Densitometric evaluation of protein expression as revealed by Western blot analysis was performed in triplicate. Housekeeping protein β-actin served as a loading control in all experiments. Values were compared with the control and statistically significant values with p < 0.05. Significant values are marked a star.
Down-regulation of SIRT1 with ASO Modulates NF-κB-mediated Pro-inflammatory, Matrix-degrading, and Apoptotic Gene Products during Chondrogenesis of MSC
We next focused on the causal relationship between SIRT1 and NF-κB signaling pathways. Therefore, we used BMS-345541, which is a potent and specific IKK complex inhibitor and can significantly block NF-κB activation induced by diverse stimuli (43). It has been shown that BMS-345541 is a highly selective inhibitor of IκB kinase that binds at an allosteric site of the enzyme and blocks the NF-κB-dependent signal transduction pathway in mice (43, 44). Primary human chondrocytes in high density culture were left untreated as control. MSCs in high density culture were either left untreated or treated as described above. IL-1β, NAM, or SIRT1-ASO induced the expression of NF-κB-dependent matrix-degrading enzymes (COX-2 and MMP-9) and pro-apoptotic signaling (cleavage of caspase-3). Treatment with resveratrol suppressed the expression of the mentioned proteins in all combinations, except with SIRT1-ASO (Fig. 4B). Furthermore, treatment with BMS-345541 alone, pretreatment with resveratrol followed by treatment with BMS-345541, and pretreatment with BMS-345541 followed by treatment with IL-1β strongly stimulated the production of cartilage-specific ECM components (Fig. 4A) and significantly decreased NF-κB-mediated degradation of ECM, as well as expression of pro-inflammatory, matrix-degrading, and apoptotic gene products in MSCs during chondrogenesis to levels similar to control cultures (Fig. 4B). Taken together, these results demonstrate that the IKK inhibitor (BMS-345541) suppresses, in a similar way to resveratrol, the destructive effects of IL-1β and that IKK, at least in part, is one of the kinases that play an important role in resveratrol/SIRT1 signaling pathways during chondrogenesis.
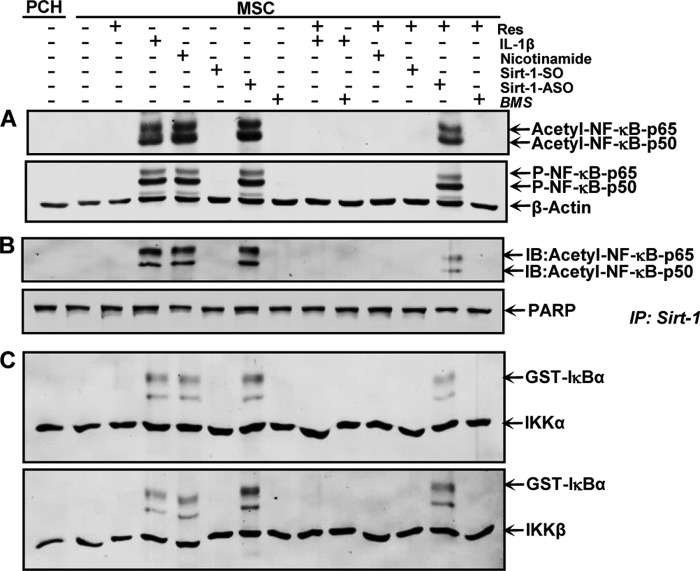
Knockdown of SIRT1 with ASO Enhances NF-κB Acetylation and Phosphorylation during Chondrogenesis of MSCs in Vitro
Acetylation and phosphorylation of p50/p65 play an important role in IκBα-mediated activation of NF-κB transcriptional activity (45, 46); therefore, we investigated the effect of resveratrol on the induction of NF-κB acetylation and phosphorylation by IL-1β, NAM, and SIRT1-ASO. Primary human chondrocytes in high density culture were left untreated as control. MSCs in high density culture were either left untreated or treated as described above. Immunoblotting of whole cell lysates was performed using anti-acetylated lysine and anti-phosphorylated NF-κB (Fig. 5). As shown in Fig. 5A, treatment with IL-1β, NAM, or SIRT1-ASO alone markedly increased acetylation and phosphorylation of the NF-κB-p65 subunit in MSCs during chondrogenesis in vitro. In contrast to this, pretreatment with resveratrol blocked NF-κB acetylation and phosphorylation during chondrogenesis of MSCs in all groups, except in cells treated with SIRT1-ASO. These findings indicate that down-regulation of SIRT1 on the protein level by the SIRT1 inhibitor NAM or on the mRNA level by ASO induces an equal inflammatory response as shown by stimulation with pro-inflammatory cytokine IL-1β. Furthermore, resveratrol can block this inflammation signaling through activation of SIRT1 if down-regulation of SIRT1 was induced at the protein level but not at the gene level. Additionally, treatment with the specific NF-κB pathway inhibitor BMS-345541 alone or pretreatment with resveratrol or BMS-345541 followed by stimulation with IL-1β resulted in suppression of cytokine-induced effects on NF-κB acetylation and phosphorylation during chondrogenesis of MSCs. These results indicate that IKK is the one of the upstream kinases involved in resveratrol-mediated signaling to inhibit NF-κB activation (Fig. 5).
FIGURE 5.
Effects of resveratrol, IL-1β, NAM, BMS-345541, and SIRT1-ASO on NF-κB signaling pathway during chondrogenesis of MSC in vitro. The same primary human chondrocytes (PCH) and MSC cultures as in Fig. 4A were subsequently subjected to Western blotting with antibodies against acetyl-lysine, p-NF-κB, and β-actin (A). Whole cell lysates were immunoprecipitated (IP) for SIRT1 and then subjected to Western blotting with antibodies against acetyl-lysine. The original samples were probed with an antibody to anti-poly(ADP-ribose) polymerase (PARP) as a loading control (B). Whole cell lysates were immunoprecipitated for IKK and analyzed by an immune complex kinase assay as described under “Experimental Procedures” (C). The level of activation of IKK proteins was then determined by Western blotting using anti-IKK-α, anti-IKK-β, and anti-phospho-specific IκBα antibodies. The results shown are representative of three independent experiments. IB, immunoblot.
SIRT1 Associates and De-acetylates NF-κB-p65 during Chondrogenesis of MSCs in Vitro
The above data show that stimulation of SIRT1 enzymatic activity correlated with increased de-acetylation of NF-κB, suggesting that both proteins interact together during chondrogenesis of MSCs in vitro. Therefore, next we performed a co-immunoprecipitation assay to evaluate association of SIRT1 and NF-κB during chondrogenesis of MSCs in vitro (Fig. 5B). Primary human chondrocytes in high density culture were left untreated as control. MSCs in high density culture were either left untreated or treated as described above. Whole cell extracts were immunoprecipitated with anti-SIRT1 and then probed for anti-acetyl-lysine by Western blotting. As shown in Fig. 5B, SIRT1 was strongly co-immunoprecipitated with acetylated NF-κB in cultures treated with IL-1β, NAM, or SIRT1-ASO. In contrast, pretreatment of cultures with resveratrol followed by co-treatment with IL-1β, NAM, or SIRT1-ASO strongly inhibited acetylation of NF-κB subunits (p50/p65) in IL-1β and NAM but weaker in SIRT1-ASO cultures, suggesting that resveratrol effectively blocks activation of NF-κB signaling pathways by modulating acetylation. Taken together, these findings indicate that SIRT1 inhibition on mRNA levels is not reversible by resveratrol, highlighting the crucial role of SIRT1 in inhibiting the NF-κB pathway. Interestingly, in resveratrol-pretreated cultures followed by treatment with SIRT1-ASO, marginal SIRT1 co-immunoprecipitation with acetylated NF-κB was observed. To determine whether BMS-345541, the specific inhibitor of IKK, can inhibit the NF-κB pathway like resveratrol, cells were treated with BMS-345541 alone or pretreated with resveratrol or BMS-345541 followed by stimulation with IL-1β. This resulted in an inhibition of acetylation of NF-κB subunits (p50/p65) (Fig. 5B). These findings demonstrate that the two proteins (NF-κB and SIRT1) interact with each other and that NF-κB may be a substrate for SIRT1 deacetylase in MSCs during MSC chondrogenesis. Taken together, these results indicate that resveratrol-induced SIRT1 forms a complex with NF-κB, de-acetylating NF-κB, thereby suppressing NF-κB-mediated production of inflammatory gene end products and enhancing chondrogenesis of MSCs in vitro.
Resveratrol Suppresses IκBα Kinase Activation Induced by IL-1β and NAM but Not by SIRT1-ASO during Chondrogenesis of MSC
Next, we evaluated the effect of SIRT1 on IκB kinase (IKK) activation, which is required for phosphorylation of IκBα and subsequently NF-κB signaling pathway activation. Primary human chondrocytes in high density culture were left untreated as control. MSCs in high density culture were either left untreated or treated as described above (Fig. 4A). Whole cell extracts were immunoprecipitated with an antibody against IκB kinase (IKK-α and IKK-β) and then analyzed by an immune complex kinase assay for phosphorylation of IκBα. As shown in Fig. 5C, IKK-α and IKK-β were equally detected in all samples, proving that immunoprecipitation was successful. Treatment with IL-1β, NAM, or SIRT1-ASO led to marked activation of IκBα. No phosphorylated IκBα was found in basal control (primary human chondrocytes), resveratrol-, and SIRT1-SO-treated cultures. Co-treatment with the SIRT1 activator resveratrol, similar to control cultures, blocked IκBα activation in IL-1β- or NAM-treated but not in SIRT1-ASO-treated cells, suggesting that SIRT1 suppression on mRNA levels is not reversible by resveratrol. Pretreatment of cultures with BMS-345541 or resveratrol followed by stimulation with IL-1β resulted in an inhibition of activation of IKK (Fig. 5C). IL-1β, BMS-345541, and resveratrol had no direct effect on the expression of IKK-α and IKK-β proteins. These findings indicate that SIRT1 inhibits the IκBα phosphorylation/NF-κB signaling pathway and highlights the important functional role of SIRT1 in inhibiting NF-κB pathway.
Interaction of SIRT1 with the Master Chondrogenic Transcription Factor Sox9 Is Stimulated by Resveratrol during Chondrogenesis of MSCs in Vitro
It has been reported that resveratrol is a specific potent activator of histone deacetylase SIRT1 (47). To examine the downstream signaling pathway during MSC chondrogenesis, we investigated whether SIRT1 associates with the chondrogenic transcription factor Sox9 and subsequently stimulates chondrogenesis. To this end, primary human chondrocytes (served as control) or MSCs in alginate culture were either left untreated or were treated with resveratrol (5 μm) for 4 h alone or were transfected with 0.5 μm SIRT1-SO or SIRT1-ASO and cultured with chondrogenic induction medium (Fig. 6). After 14 days, whole cell extracts were immunoprecipitated with anti-SIRT1 and analyzed by Western blotting with antibodies against Sox9. Interestingly, immunoprecipitates from chondrocytes or MSCs in untreated controls, SIRT1-SO- or resveratrol-treated cultures, but not from SIRT1-ASO-treated cultures, revealed co-immunoprecipitation of SIRT1 protein with the chondrogenic transcription factor Sox9 (Fig. 6A). Furthermore, treatment with the natural SIRT1 activator resveratrol markedly increased interaction and co-immunoprecipitation of SIRT1 and Sox9 in MSCs during chondrogenesis as well as in chondrocyte controls as shown by densitometric analysis. Moreover, the same samples were immunoprecipitated with anti-Sox9 and analyzed by Western blotting with antibodies against SIRT1 and showed a complex formation between SIRT1 and Sox9 (Fig. 6B). Taken together, these findings indicate that resveratrol activates SIRT1 and induces SIRT-Sox9 complex formation, which may activate the chondrogenic differentiation pathway in MSCs.
FIGURE 6.
Association of SIRT1 proteins with Sox9 during chondrogenic differentiation of MSC in vitro. Primary human chondrocytes (PCH) or MSC in alginate culture were either left untreated (Co.) or treated for 4 h with resveratrol (5 μm), or transfected with 0.5 μm SIRT1-SO or SIRT1-ASO in the presence of Lipofectin (10 μl/ml), and cultured with chondrogenic induction medium. After 14 days, whole cell extracts were lysed and immunoprecipitated (IP) with SIRT1 (A), or with Sox9 (B); subsequently the immunoprecipitates were separated by SDS-PAGE and analyzed by immunoblotting (IB) using anti-Sox9 (A) and anti-SIRT1 (B). Densitometric evaluation of protein expression as revealed by Western blot analysis was performed in triplicate. The original samples were probed with an antibody to β-actin as a loading control. Each experiment was performed in triplicate, and mean values and standard deviations are indicated. Values were compared with the control and statistically significant values with p < 0.05 were designated by a star. M, marker for molecular weights. IgH, immunglobulin heavy chain.
Co-localization of SIRT1 and Sox9 Is Required during Chondrogenesis of MSCs in Vitro
Based on the immunoprecipitation and Western blotting results (Fig. 6) and to confirm them, we performed immunofluorescence analysis. MSCs were induced toward the chondrogenic lineage in three-dimensional alginate cultures with chondrogenic induction medium for 14 days. Newly formed chondrocytes were dissolved from alginate and cultivated in monolayer culture. Chondrogenic phenotype of the differentiated MSCs was confirmed by double immunofluorescent labeling with anti-collagen type II and anti-CSPGs (Fig. 7I, panels a and b). To examine whether inhibition of SIRT1 and/or Sox9 expression with specific SIRT1-ASO can be visualized by immunofluorescence, newly differentiated chondrocytes in monolayer culture were either left untreated or treated with resveratrol (5 μm), NAM (10 mm), or with SIRT1-SO or SIRT1-ASO (0.5 μm) in the presence of Lipofectin for 24 h, or the cells were pretreated with resveratrol (5 μm) for 1 h followed by co-treatment with NAM (10 mm) for 1 h, SIRT1-SO (data not shown), or SIRT1-ASO (0.5 μm) in the presence of Lipofectin for 24 h (Fig. 7II). Double immunolabeling was performed with primary antibodies to SIRT1 and Sox9, followed by incubation with rhodamine- or FITC-coupled secondary antibodies. Counterstaining was performed with DAPI to visualize the cell nuclei. Double immunofluorescence analysis showed that in untreated cultures (control culture, co) or cultures treated with SIRT1-SO (data not shown), marked expression of SIRT1 and Sox9 and nuclear co-localization of these two proteins were observed (Fig. 7II, panels a–c). Treatment with natural SIRT1 activator resveratrol, SIRT1-SO alone (data not shown), or resveratrol in combination with SIRT1-SO (data not shown) strongly induced SIRT1 and Sox9 activation in the nucleus (Fig. 7II, panels d–f). In contrast to this, treatment with NAM (Fig. 7II, panels g–i) or SIRT1-ASO (Fig. 7II, panels j–l) reduced the levels of SIRT1 and Sox9 expression and nuclear localization. However, co-treatment of resveratrol and NAM recovered SIRT1 and Sox9 nuclear expression and co-localization (Fig. 7II, panels m–o). Interestingly, in cultures treated with SIRT1-ASO and resveratrol, co-treatment could marginally restore SIRT1 and Sox9 expression as seen by sporadic nuclear co-localization of SIRT1/Sox9 (Fig. 7II, panels p–r). Furthermore, merged images clearly demonstrate nuclear co-localization of SIRT1/Sox9, underlining the importance of SIRT1/Sox9 nuclear co-localization during chondrogenic signaling in MSCs in vitro. Quantification and statistical analysis of the immunomorphological data highlight the prominent effects of specific SIRT1 inhibition and/or activation on Sox9 expression in MSC-derived chondrocytes in vitro (Fig. 7III).
FIGURE 7.
Effects of resveratrol, NAM, and SIRT1-ASO on the co-localization of SIRT1 and Sox9 in MSC-derived chondrocytes revealed by immunofluorescence microscopy. Chondrogenic differentiation of MSC was performed in three-dimensional alginate cultures with chondrogenic induction medium for 14 days. Differentiated chondrocytes were dissolved from alginate and transferred to monolayer culture for additional treatment. I, chondrocytes were double-immunolabeled with primary antibodies to collagen type II (panel a) and CSPGs (panel b) to confirm chondrogenic lineage. Magnification ×400; bars, 30 nm. II, chondrocytes were either left untreated (panels a–c) or treated with 5 μm resveratrol (panels d–f), 10 mm NAM (panels g–i) alone for 1 h, 0.5 μm SIRT1-SO (data not shown), or SIRT1-ASO (panels j–l) in the presence of Lipofectin (10 μl/ml) for 24 h or cells were pretreated with resveratrol (5 μm) for 1 h followed by co-treatment with 10 mm NAM (panels m–o) for 1 h, 0.5 μm SIRT1-SO (data not shown), or SIRT1-ASO (panels p–r) in the presence of Lipofectin (10 μl/ml) for 24 h. Double immunolabeling was performed for SIRT1 and Sox9. Magnification ×400; bars, 30 nm. III, to quantify co-localization of SIRT1 and Sox9, monolayer cultures were examined for nucleus positive cells by counting 100 cells randomly and evaluating the number of positively stained cells. The results are provided as mean values with standard deviations from at least three independent experiments. Values were compared with the control and statistically significant values with p < 0.05. Significant values are marked with star.
Resveratrol Suppresses Down-regulation of Specific MSC Chondrogenic Differentiation Induced by NAM but Not by SIRT1-ASO in Alginate Culture
Studies from our laboratory have previously shown that in alginate culture only cells with chondrogenic potential can survive and alginate functions as a selective filter station separating vital from nonvital chondrocytes and fibroblasts (10, 14, 48). To assess the potential of MSC chondrogenic differentiation, the inhibition of this differentiation by specific SIRT1 inhibitors, the inductive function of resveratrol in this process, and the viability of the survived cells, MSCs in alginate culture were either left untreated or were treated with resveratrol (5 μm), NAM (10 mm), Lipofectin (10 μm/ml), or transfected with SIRT1-SO or SIRT1-ASO (0.5 μm in the presence of Lipofectin 10 μm/ml) for 14 days. Additionally, in another set of experiments, cells were pretreated with resveratrol (5 μm) for 4 h followed by transfection with 0.5 μm SIRT1-SO or SIRT1-ASO or co-treatment with NAM (10 mm) in chondrogenic induction medium. After 14 days of culture, cells were dissolved from alginate, and cell viability was measured with the MTT method. As shown in Fig. 8, treatment of MSC with the SIRT1 inhibitor NAM or SIRT1-ASO alone markedly reduced MSC viability during chondrogenesis of MSCs compared with controls or with resveratrol-treated cells. However, pretreatment with resveratrol blocked inhibition of MSC chondrogenic differentiation and viability induced by NAM, but not by SIRT1-ASO (Fig. 8). Taken together, MSCs treated with SIRT1 inhibitors (NAM or SIRT1-ASO), as opposed to controls or with resveratrol-treated MSCs, were not able to survive in alginate cultures, and they underwent cell death.
FIGURE 8.

Effects of resveratrol, NAM, and SIRT1-ASO on the chondrogenic differentiation of MSC and chondrocyte viability in alginate culture. MSCs in alginate were either left untreated or treated with 5 μm resveratrol, 10 mm NAM, or 10 μl/ml Lipofectin alone or were transfected with 0.5 μm SIRT1-SO or SIRT1-ASO in the presence of Lipofectin (10 μl/ml), or cells were pretreated with 5 μm resveratrol for 4 h followed by co-treatment with 10 mm NAM or were transfected with 0.5 μm SIRT1-SO or SIRT1-ASO in chondrogenic induction medium. After 14 days of culture, cells were dissolved from alginate and cell viability was measured with the MTT method. The results are provided as mean values with standard deviations from at least three independent experiments. Values were compared with the control and statistically significant values with p < 0.05. Significant values are marked with star.
DISCUSSION
The goal of this study was to examine the role of SIRT1-mediated signaling during chondrogenic differentiation of mesenchymal stem cells in an in vitro model of chondrogenesis.
Cartilage tissue has a very limited capacity for spontaneous healing or repair after articular cartilage defects (micro- or macrotrauma), presenting an increasing problem for humans and animals, which necessitates the development of novel and improved therapeutic strategies (49). Moreover, cartilage tissue engineering from primary chondrocytes is to date unsatisfactory, mainly due to the fact that the long culturing period leads to dedifferentiation of chondrocytes. Therefore, using MSCs as an important source for cartilage tissue engineering has become a highly investigated area of biology and regenerative medicine (4). MSCs are multipotent cells with a stable phenotype and self-renewal capacity that can differentiate into different specialized cell lineages (i.e. osteoblasts, chondroblasts, myoblasts, adipocytes, etc.) (1, 2). Furthermore, they can be easily isolated and expanded several times without losing their specific properties. MSCs have been transferred into osteochondral defects, integrated into the subchondral plate, and produced proper ECMs in a rabbit model (50). However, little is known about the biological behavior of MSCs during chondrogenic differentiation. Therefore, we investigated the potential role of resveratrol (natural SIRT1 activator) on chondrogenic differentiation of MSCs through its effects on SIRT1-mediated cellular responses in three-dimensional models of chondrogenesis in vitro. Because resveratrol is a component of a wide variety of vegetables and fruits actively consumed by most people, it is preferable to examine the mechanism of action of resveratrol. Although more potent activators of SIRT1 have become available, they are excellent for understanding the mechanism but may not be suitable to be consumed every day.
In this study, SIRT1-ASO, like NAM or IL-1β, enhanced cartilage-specific ECM breakdown, down-regulated the expression and activation of SIRT1 protein and of the cartilage-specific transcription factor Sox9, and up-regulated NF-κB-regulated pro-inflammatory and pro-apoptotic proteins during chondrogenic differentiation of MSCs. However, pretreatment of MSCs with resveratrol or BMS-345541 led to the recovery of chondrogenic MSC differentiation, increased production of ECM, and expression of Sox9 and inhibited pro-inflammatory proteins in IL-1β- or NAM-stimulated MSCs but not in with SIRT1-ASO-treated cultures. Thus, SIRT1 and NF-κB appear to be important modulators during chondrogenic differentiation of MSC. In fact, several lines of evidence have shown that resveratrol is a suppressor of the NF-κB transcription factor (51, 52), a potent activator of SIRT1 in chondrocytes (47, 53). SIRT1 has regulatory effects on cell differentiation, proliferation, survival, and organism longevity (47, 54–56). Furthermore, our laboratory and others have previously reported that resveratrol is an inhibitor of the pro-inflammatory cytokine IL-1β, highlighting that resveratrol suppressed IL-1β-induced activation of NF-κB and NF-κB-dependent gene products (51, 57, 58). Indeed, we have also shown that resveratrol inhibited IL-1β-induced apoptosis in tenocytes, and this was linked to changes in the expression of p53, Bax, and caspase-3 (38). However, the mechanisms regulating the suppressive signaling of resveratrol on IL-1β-induced NF-κB transcriptional activity have not yet been fully understood. Opposite to this, several lines of evidence have also reported that resveratrol induces apoptotic cell death in several tumor cell lines through the stimulation of caspase-3, accumulation of p53 and p21, and cleavage of poly(ADP-ribose) polymerase (51, 59, 60). There may be different and multiple reasons for these diverse findings. It should be considered that phytochemicals can interact differently in diverse cell types, species, and stimulants, various cellular stress responses, and many others. Furthermore, the concentration of resveratrol and the period of time the cells are exposed to resveratrol should be considered as this additionally may cause variable effects.
We further found that during chondrogenic differentiation of MSCs, knockdown of SIRT1 by ASO, like NAM or IL-1β, caused NF-κB activation, and this was mediated through stimulation of IKKs, which led to up-regulation of phosphorylated Iκ-Bα. However, pretreatment with resveratrol inhibited IKK activation by NAM or IL-1β but not by SIRT1-ASO. Indeed, activation of NF-κB is primarily regulated by the interaction of inhibitory proteins, such as IκBα and IKKs (61), and activation of IKKs leads to activation of NF-κB independent of NF-κB-inducing kinase (62). Our results further demonstrated that MSC pretreatment with the specific IKK inhibitor BMS-345541, which was followed by stimulation with IL-1β, revealed a significant increase in cartilage-specific matrix expression and Sox9 expression and exhibited a significant decrease in NF-κB-related gene end products (MMP-9, COX-2 expression, and caspase-3 cleavage) in a similar way to resveratrol. These findings showed that IKK-mediated NF-κB activation is stimulated by SIRT1-ASO, IL-1β, and NAM, indicating that IKK is one of the main upstream stimulatory kinases that may be modulated by SIRT1 and that could play a major role in resveratrol/SIRT1-mediated signaling pathways during chondrogenic differentiation of MSCs in vitro. Moreover, inhibition of SIRT1 during differentiation of MSCs to chondrocytes by all inhibitors used in this study stimulated activation of IKK, phosphorylation of IκBα, p65 phosphorylation, and acetylation. This was correlated with up-regulation of NF-κB-regulated gene products involved in degradation, inflammation, and apoptosis. Resveratrol recovered the IL-1β- or NAM-induced up-regulation of various NF-κB-regulated gene products but not the effects of SIRT1-ASO. These data further suggest that resveratrol suppresses NAM-induced NF-κB acetylation through SIRT1 activation. Moreover, these findings strongly indicate that down-regulation of SIRT1 by mRNA interference abrogates its suppressive effects on NF-κB, indicating the essential role and function of SIRT1 for chondrogenic differentiation and survival of MSC. Furthermore, several reports have shown that SIRT1 deacetylates lysine residues of histone proteins and other nuclear proteins and transcription factors linked with apoptosis and inflammation, such as p53 (38, 63) and NF-κB subunit p65 (38, 64), thereby regulating transcriptional activity of target proteins. Interestingly, it has been reported that deacetylation of NF-κB by SIRT1 suppresses DNA binding that leads to a loss of transactivation potential (45). However, to our knowledge, this result is the first evaluation that shows the effects of SIRT1-ASO on NF-κB activation in MSC during chondrogenesis.
We also showed that the positive effect of resveratrol on chondrogenic differentiation of MSC was, at least in part, regulated by activation of Sox9 as a marker for chondrogenic viability. Indeed, immunoprecipitation, Western blotting, and immunofluorescence microscopy results clearly revealed functional and physical interactions between Sox9 and SIRT1, highlighting that this interaction may play an important role in regulating resveratrol-activated SIRT1 during MSC chondrogenesis. Thus, Sox9 appears to be a substrate to SIRT1, and in this way SIRT1 might contribute, at least in part, to the specific induction of MSC differentiation to chondrocytes and maintenance of MSC phenotypes by regulation of the transcription factors such as Sox9.
Supporting our data, a recent report showed that SIRT1 interacts directly with the master cartilage-specific transcription factor Sox9 in differentiated chondrocytes, stimulating transcriptional activity of the collagen promoter in a Sox9-dependent fashion (65). Moreover, SIRT1 plays an important role in the regulation of genome architecture and gene expression (29, 66). Interestingly, MSC derived from SIRT1 knock-out mice showed clearly reduced chondrogenic potential in vitro (67). Indeed, these results are consistent with earlier reports demonstrating that SIRT1 is able to interact with other transcription factors, like Runx2 in osteoblasts or scleraxis in tenocytes (38, 62, 68) or p53, NF-κB, myogenic differentiation, high mobility group I, E2F transcription factor, peroxisome proliferator-activated receptor-γ, and forkhead box O (27, 28, 30, 69).
In this study, we also found that knockdown of SIRT1 by SIRT1-ASO, like NAM, opposite the control or with resveratrol-treated MSCs, inhibited the chondrogenic differentiation of MSCs in alginate beads as was demonstrated by reduced cell viability in MTT assay. With these results in the alginate culture system, we could show additionally the specific differentiation of MSCs to chondrocytes, and here, only the cells with chondrogenic potential survive. These results indicate the suitability of the alginate culture as an adequate microenvironment for chondrogenic differentiation of MSCs, and MSCs that survived during chondrogenic differentiation in the presence of different agents in alginate can only be chondrocytes. Indeed, the alginate culture system employed here has been successfully used as a model for chondrogenic differentiation, and alginate acts as a selective filter station separating vital from nonvital chondrocytes and fibroblasts (10, 14, 48).
In summary, the results obtained strongly suggest that SIRT1, at least in part, may inhibit NF-κB signaling via deacetylation and IKK inhibition, demonstrating a novel role for resveratrol in chondrogenic differentiation of MSCs. Furthermore, this study identified that during chondrogenic differentiation of MSC, Sox9 and SIRT1 directly interact together and revealed a positive cooperation by inhibiting inflammatory signaling and enhancing expression of cartilage-specific ECM in vitro. Further studies will be required to examine the full diversity of the effects of resveratrol/SIRT1-mediated signaling and its important role for the differentiation of MSCs to chondrocytes, and these results may reveal new insights for cartilage tissue regeneration.
Acknowledgments
We gratefully acknowledge the excellent technical assistance provided by Patricia Kraehe, Zahra Kamyabi-Moghaddam, Ursula Schwikowski, and Dr. Andreas Eimannsberger of Ludwig Maximilian University of Munich.
Footnotes
- MSC
- mesenchymal stem cell
- CSPG
- cartilage-specific proteoglycan
- ECM
- extracellular matrix
- IκBα
- inhibitor of κB
- IKK
- IκB kinase
- MMP
- matrix metalloproteinase
- MTT
- 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
- NAM
- nicotinamide
- NF-κB
- nuclear factor κB
- SIRT1
- sirtuin-1
- SIRT1-ASO
- sirtuin-1-antisense oligonucleotides
- SIRT1-SO
- sirtuin-1-sense oligonucleotides
- Sox9
- SRY (sex-determining region Y)-box 9.
REFERENCES
- 1. Pittenger M. F., Mackay A. M., Beck S. C., Jaiswal R. K., Douglas R., Mosca J. D., Moorman M. A., Simonetti D. W., Craig S., Marshak D. R. (1999) Multilineage potential of adult human mesenchymal stem cells. Science 284, 143–147 [DOI] [PubMed] [Google Scholar]
- 2. Csaki C., Matis U., Mobasheri A., Ye H., Shakibaei M. (2007) Chondrogenesis, osteogenesis and adipogenesis of canine mesenchymal stem cells: a biochemical, morphological and ultrastructural study. Histochem. Cell Biol. 128, 507–520 [DOI] [PubMed] [Google Scholar]
- 3. Jaiswal N., Haynesworth S. E., Caplan A. I., Bruder S. P. (1997) Osteogenic differentiation of purified, culture-expanded human mesenchymal stem cells in vitro. J. Cell. Biochem. 64, 295–312 [PubMed] [Google Scholar]
- 4. Csaki C., Schneider P. R., Shakibaei M. (2008) Mesenchymal stem cells as a potential pool for cartilage tissue engineering. Ann. Anat. 190, 395–412 [DOI] [PubMed] [Google Scholar]
- 5. Lee E. H., Hui J. H. (2006) The potential of stem cells in orthopaedic surgery. J. Bone Joint Surg. Br. 88, 841–851 [DOI] [PubMed] [Google Scholar]
- 6. Leo A. J., Grande D. A. (2006) Mesenchymal stem cells in tissue engineering. Cells Tissues Organs 183, 112–122 [DOI] [PubMed] [Google Scholar]
- 7. DeLise A. M., Fischer L., Tuan R. S. (2000) Cellular interactions and signaling in cartilage development. Osteoarthritis Cartilage 8, 309–334 [DOI] [PubMed] [Google Scholar]
- 8. Yeh H. Y., Lin T. Y., Lin C. H., Yen B. L., Tsai C. L., Hsu S. H. (2013) Neocartilage formation from mesenchymal stem cells grown in type II collagen-hyaluronan composite scaffolds. Differentiation 86, 171–183 [DOI] [PubMed] [Google Scholar]
- 9. Ko J. Y., Kim K. I., Park S., Im G. I. (2014) In vitro chondrogenesis and in vivo repair of osteochondral defect with human induced pluripotent stem cells. Biomaterials 35, 3571–3581 [DOI] [PubMed] [Google Scholar]
- 10. Shakibaei M., De Souza P. (1997) Differentiation of mesenchymal limb bud cells to chondrocytes in alginate beads. Cell Biol. Int. 21, 75–86 [DOI] [PubMed] [Google Scholar]
- 11. Zimmermann B., Wachtel H. C., Vormann J. (1992) Kinetics of β-glycerophosphate-induced endochondral mineralization in vitro. Calcium accumulation, alkaline phosphatase activity, and effects of levamisole. Calcif. Tissue Int. 51, 54–61 [DOI] [PubMed] [Google Scholar]
- 12. Shakibaei M., Schröter-Kermani C., Merker H. J. (1993) Matrix changes during long-term cultivation of cartilage (organoid or high density cultures). Histol. Histopathol. 8, 463–470 [PubMed] [Google Scholar]
- 13. Shakibaei M. (1998) Inhibition of chondrogenesis by integrin antibody in vitro. Exp. Cell Res. 240, 95–106 [DOI] [PubMed] [Google Scholar]
- 14. Schulze-Tanzil G., de Souza P., Villegas Castrejon H., John T., Merker H. J., Scheid A., Shakibaei M. (2002) Redifferentiation of dedifferentiated human chondrocytes in high density cultures. Cell Tissue Res. 308, 371–379 [DOI] [PubMed] [Google Scholar]
- 15. Sandell L. J., Aigner T. (2001) Articular cartilage and changes in arthritis. An introduction: cell biology of osteoarthritis. Arthritis Res. 3, 107–113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hunziker E. B. (1999) Articular cartilage repair: are the intrinsic biological constraints undermining this process insuperable? Osteoarthritis Cartilage 7, 15–28 [DOI] [PubMed] [Google Scholar]
- 17. Buckwalter J. A., Brown T. D. (2004) Joint injury, repair, and remodeling: roles in post-traumatic osteoarthritis. Clin. Orthop. Relat. Res. 423, 7–16 [PubMed] [Google Scholar]
- 18. Goepfert C., Slobodianski A., Schilling A. F., Adamietz P., Pörtner R. (2010) Cartilage engineering from mesenchymal stem cells. Adv. Biochem. Eng. Biotechnol. 123, 163–200 [DOI] [PubMed] [Google Scholar]
- 19. Alsalameh S., Amin R., Gemba T., Lotz M. (2004) Identification of mesenchymal progenitor cells in normal and osteoarthritic human articular cartilage. Arthritis Rheum. 50, 1522–1532 [DOI] [PubMed] [Google Scholar]
- 20. Buhrmann C., Mobasheri A., Matis U., Shakibaei M. (2010) Curcumin mediated suppression of nuclear factor-κB promotes chondrogenic differentiation of mesenchymal stem cells in a high density co-culture microenvironment. Arthritis Res. Ther. 12, R127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Baolin L., Inami Y., Tanaka H., Inagaki N., Iinuma M., Nagai H. (2004) Resveratrol inhibits the release of mediators from bone marrow-derived mouse mast cells in vitro. Planta Med. 70, 305–309 [DOI] [PubMed] [Google Scholar]
- 22. Gautam S. C., Xu Y. X., Dumaguin M., Janakiraman N., Chapman R. A. (2000) Resveratrol selectively inhibits leukemia cells: a prospective agent for ex vivo bone marrow purging. Bone Marrow Transplant. 25, 639–645 [DOI] [PubMed] [Google Scholar]
- 23. Surh Y. J., Hurh Y. J., Kang J. Y., Lee E., Kong G., Lee S. J. (1999) Resveratrol, an antioxidant present in red wine, induces apoptosis in human promyelocytic leukemia (HL-60) cells. Cancer Lett. 140, 1–10 [DOI] [PubMed] [Google Scholar]
- 24. Tsan M. F., White J. E., Maheshwari J. G., Bremner T. A., Sacco J. (2000) Resveratrol induces Fas signalling-independent apoptosis in THP-1 human monocytic leukaemia cells. Br. J. Haematol. 109, 405–412 [DOI] [PubMed] [Google Scholar]
- 25. Knutson M. D., Leeuwenburgh C. (2008) Resveratrol and novel potent activators of SIRT1: effects on aging and age-related diseases. Nutr. Rev. 66, 591–596 [DOI] [PubMed] [Google Scholar]
- 26. Milne J. C., Lambert P. D., Schenk S., Carney D. P., Smith J. J., Gagne D. J., Jin L., Boss O., Perni R. B., Vu C. B., Bemis J. E., Xie R., Disch J. S., Ng P. Y., Nunes J. J., Lynch A. V., Yang H., Galonek H., Israelian K., Choy W., Iffland A., Lavu S., Medvedik O., Sinclair D. A., Olefsky J. M., Jirousek M. R., Elliott P. J., Westphal C. H. (2007) Small molecule activators of SIRT1 as therapeutics for the treatment of type 2 diabetes. Nature 450, 712–716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Chen Lf, Fischle W., Verdin E., Greene W. C. (2001) Duration of nuclear NF-κB action regulated by reversible acetylation. Science 293, 1653–1657 [DOI] [PubMed] [Google Scholar]
- 28. Gu W., Roeder R. G. (1997) Activation of p53 sequence-specific DNA binding by acetylation of the p53 C-terminal domain. Cell 90, 595–606 [DOI] [PubMed] [Google Scholar]
- 29. Haigis M. C., Guarente L. P. (2006) Mammalian sirtuins–emerging roles in physiology, aging, and calorie restriction. Genes Dev. 20, 2913–2921 [DOI] [PubMed] [Google Scholar]
- 30. Martínez-Balbás M. A., Bauer U. M., Nielsen S. J., Brehm A., Kouzarides T. (2000) Regulation of E2F1 activity by acetylation. EMBO J. 19, 662–671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Shakibaei M., Buhrmann C., Mobasheri A. (2011) Resveratrol-mediated SIRT1 interactions with p300 modulate receptor activator of NF-κB ligand (RANKL) activation of NF-κB signaling and inhibit osteoclastogenesis in bone-derived cells. J. Biol. Chem. 286, 11492–11505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kotas M. E., Gorecki M. C., Gillum M. P. (2013) Sirtuin-1 is a nutrient-dependent modulator of inflammation. Adipocyte 2, 113–118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hwang J. W., Yao H., Caito S., Sundar I. K., Rahman I. (2013) Redox regulation of SIRT1 in inflammation and cellular senescence. Free Radic. Biol. Med. 61C, 95–110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Gabay O., Oppenhiemer H., Meir H., Zaal K., Sanchez C., Dvir-Ginzberg M. (2012) Increased apoptotic chondrocytes in articular cartilage from adult heterozygous SIRT1 mice. Ann. Rheum. Dis. 71, 613–616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Gabay O., Sanchez C., Dvir-Ginzberg M., Gagarina V., Zaal K. J., Song Y., He X. H., McBurney M. W. (2013) Sirtuin 1 enzymatic activity is required for cartilage homeostasis in vivo in a mouse model. Arthritis Rheum. 65, 159–166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Gabay O., Zaal K. J., Sanchez C., Dvir-Ginzberg M., Gagarina V., Song Y., He X. H., McBurney M. W. (2013) SIRT1-deficient mice exhibit an altered cartilage phenotype. Joint Bone Spine 80, 613–620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Dominici M., Le Blanc K., Mueller I., Slaper-Cortenbach I., Marini F., Krause D., Deans R., Keating A., Prockop Dj, Horwitz E. (2006) Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 8, 315–317 [DOI] [PubMed] [Google Scholar]
- 38. Busch F., Mobasheri A., Shayan P., Stahlmann R., Shakibaei M. (2012) SIRT1 is required for the inhibition of apoptosis and inflammatory responses in human tenocytes. J. Biol. Chem. 287, 25770–25781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Shakibaei M., De Souza P., Merker H. J. (1997) Integrin expression and collagen type II implicated in maintenance of chondrocyte shape in monolayer culture: an immunomorphological study. Cell Biol. Int. 21, 115–125 [DOI] [PubMed] [Google Scholar]
- 40. Shakibaei M., John T., De Souza P., Rahmanzadeh R., Merker H. J. (1999) Signal transduction by β1 integrin receptors in human chondrocytes in vitro: collaboration with the insulin-like growth factor-I receptor. Biochem. J. 342, 615–623 [PMC free article] [PubMed] [Google Scholar]
- 41. Shakibaei M., Schulze-Tanzil G., de Souza P., John T., Rahmanzadeh M., Rahmanzadeh R., Merker H. J. (2001) Inhibition of mitogen-activated protein kinase kinase induces apoptosis of human chondrocytes. J. Biol. Chem. 276, 13289–13294 [DOI] [PubMed] [Google Scholar]
- 42. Csaki C., Keshishzadeh N., Fischer K., Shakibaei M. (2008) Regulation of inflammation signalling by resveratrol in human chondrocytes in vitro. Biochem. Pharmacol. 75, 677–687 [DOI] [PubMed] [Google Scholar]
- 43. Burke J. R., Pattoli M. A., Gregor K. R., Brassil P. J., MacMaster J. F., McIntyre K. W., Yang X., Iotzova V. S., Clarke W., Strnad J., Qiu Y., Zusi F. C. (2003) BMS-345541 is a highly selective inhibitor of IκB kinase that binds at an allosteric site of the enzyme and blocks NF-κB-dependent transcription in mice. J. Biol. Chem. 278, 1450–1456 [DOI] [PubMed] [Google Scholar]
- 44. Wu L., Shao L., An N., Wang J., Pazhanisamy S., Feng W., Hauer-Jensen M., Miyamoto S., Zhou D. (2011) IKKβ regulates the repair of DNA double-strand breaks induced by ionizing radiation in MCF-7 breast cancer cells. PLoS One 6, e18447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Kiernan R., Brès V., Ng R. W., Coudart M. P., El Messaoudi S., Sardet C., Jin D. Y., Emiliani S., Benkirane M. (2003) Post-activation turn-off of NF-κB-dependent transcription is regulated by acetylation of p65. J. Biol. Chem. 278, 2758–2766 [DOI] [PubMed] [Google Scholar]
- 46. Miyamoto S., Maki M., Schmitt M. J., Hatanaka M., Verma I. M. (1994) Tumor necrosis factor α-induced phosphorylation of IκBα is a signal for its degradation but not dissociation from NF-κB. Proc. Natl. Acad. Sci. U.S.A. 91, 12740–12744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Howitz K. T., Bitterman K. J., Cohen H. Y., Lamming D. W., Lavu S., Wood J. G., Zipkin R. E., Chung P., Kisielewski A., Zhang L. L., Scherer B., Sinclair D. A. (2003) Small molecule activators of sirtuins extend Saccharomyces cerevisiae lifespan. Nature 425, 191–196 [DOI] [PubMed] [Google Scholar]
- 48. Schulze-Tanzil G., Mobasheri A., de Souza P., John T., Shakibaei M. (2004) Loss of chondrogenic potential in dedifferentiated chondrocytes correlates with deficient Shc-Erk interaction and apoptosis. Osteoarthritis Cartilage 12, 448–458 [DOI] [PubMed] [Google Scholar]
- 49. Cao L., Yang F., Liu G., Yu D., Li H., Fan Q., Gan Y., Tang T., Dai K. (2011) The promotion of cartilage defect repair using adenovirus mediated Sox9 gene transfer of rabbit bone marrow mesenchymal stem cells. Biomaterials 32, 3910–3920 [DOI] [PubMed] [Google Scholar]
- 50. Yan H., Yu C. (2007) Repair of full-thickness cartilage defects with cells of different origin in a rabbit model. Arthroscopy 23, 178–187 [DOI] [PubMed] [Google Scholar]
- 51. Gupta S. C., Sundaram C., Reuter S., Aggarwal B. B. (2010) Inhibiting NF-κB activation by small molecules as a therapeutic strategy. Biochim. Biophys. Acta 1799, 775–787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Manna S. K., Mukhopadhyay A., Aggarwal B. B. (2000) Resveratrol suppresses TNF-induced activation of nuclear transcription factors NF-κB, activator protein-1, and apoptosis: potential role of reactive oxygen intermediates and lipid peroxidation. J. Immunol. 164, 6509–6519 [DOI] [PubMed] [Google Scholar]
- 53. Kim H. J., Braun H. J., Dragoo J. L. (2014) The effect of resveratrol on normal and osteoarthritic chondrocyte metabolism. Bone Joint Res. 3, 51–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Elmali N., Baysal O., Harma A., Esenkaya I., Mizrak B. (2007) Effects of resveratrol in inflammatory arthritis. Inflammation 30, 1–6 [DOI] [PubMed] [Google Scholar]
- 55. Sauve A. A., Wolberger C., Schramm V. L., Boeke J. D. (2006) The biochemistry of sirtuins. Annu. Rev. Biochem. 75, 435–465 [DOI] [PubMed] [Google Scholar]
- 56. Tseng P. C., Hou S. M., Chen R. J., Peng H. W., Hsieh C. F., Kuo M. L., Yen M. L. (2011) Resveratrol promotes osteogenesis of human mesenchymal stem cells by upregulating RUNX2 gene expression via the SIRT1/FOXO3A axis. J. Bone Miner. Res. 26, 2552–2563 [DOI] [PubMed] [Google Scholar]
- 57. Shakibaei M., Csaki C., Nebrich S., Mobasheri A. (2008) Resveratrol suppresses interleukin-1β-induced inflammatory signaling and apoptosis in human articular chondrocytes: potential for use as a novel nutraceutical for the treatment of osteoarthritis. Biochem. Pharmacol. 76, 1426–1439 [DOI] [PubMed] [Google Scholar]
- 58. Shakibaei M., Mobasheri A., Buhrmann C. (2011) Curcumin synergizes with resveratrol to stimulate the MAPK signaling pathway in human articular chondrocytes in vitro. Genes Nutr. 6, 171–179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Estrov Z., Shishodia S., Faderl S., Harris D., Van Q., Kantarjian H. M., Talpaz M., Aggarwal B. B. (2003) Resveratrol blocks interleukin-1β-induced activation of the nuclear transcription factor NF-κB, inhibits proliferation, causes S-phase arrest, and induces apoptosis of acute myeloid leukemia cells. Blood 102, 987–995 [DOI] [PubMed] [Google Scholar]
- 60. Gupta S. C., Kannappan R., Reuter S., Kim J. H., Aggarwal B. B. (2011) Chemosensitization of tumors by resveratrol. Ann. N.Y. Acad. Sci. 1215, 150–160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Häcker H., Karin M. (2006) Regulation and function of IKK and IKK-related kinases. Sci. STKE 2006, re13. [DOI] [PubMed] [Google Scholar]
- 62. Shakibaei M., Shayan P., Busch F., Aldinger C., Buhrmann C., Lueders C., Mobasheri A. (2012) Resveratrol-mediated modulation of SIRT1/Runx2 promotes osteogenic differentiation of mesenchymal stem cells: potential role of Runx2 deacetylation. PLoS One 7, e35712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Vaziri H., Dessain S. K., Ng Eaton E., Imai S. I., Frye R. A., Pandita T. K., Guarente L., Weinberg R. A. (2001) hSIR2(SIRT1) functions as an NAD-dependent p53 deacetylase. Cell 107, 149–159 [DOI] [PubMed] [Google Scholar]
- 64. Yeung F., Hoberg J. E., Ramsey C. S., Keller M. D., Jones D. R., Frye R. A., Mayo M. W. (2004) Modulation of NF-κB-dependent transcription and cell survival by the SIRT1 deacetylase. EMBO J. 23, 2369–2380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Dvir-Ginzberg M., Gagarina V., Lee E. J., Hall D. J. (2008) Regulation of cartilage-specific gene expression in human chondrocytes by SIRT1 and nicotinamide phosphoribosyltransferase. J. Biol. Chem. 283, 36300–36310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Imai S., Armstrong C. M., Kaeberlein M., Guarente L. (2000) Transcriptional silencing and longevity protein Sir2 is an NAD-dependent histone deacetylase. Nature 403, 795–800 [DOI] [PubMed] [Google Scholar]
- 67. Simic P., Zainabadi K., Bell E., Sykes D. B., Saez B., Lotinun S., Baron R., Scadden D., Schipani E., Guarente L. (2013) SIRT1 regulates differentiation of mesenchymal stem cells by deacetylating β-catenin. EMBO Mol. Med. 5, 430–440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Busch F., Mobasheri A., Shayan P., Lueders C., Stahlmann R., Shakibaei M. (2012) Resveratrol modulates interleukin-1β-induced phosphatidylinositol 3-kinase and nuclear factor κB signaling pathways in human tenocytes. J. Biol. Chem. 287, 38050–38063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Bäckesjö C. M., Li Y., Lindgren U., Haldosén L. A. (2006) Activation of SIRT1 decreases adipocyte formation during osteoblast differentiation of mesenchymal stem cells. J. Bone Miner. Res. 21, 993–1002 [DOI] [PubMed] [Google Scholar]