Background: The wet visual surface of the eye is essentially a sterile environment.
Results: Proteolytic processing of the prosecretory mitogen lacritin in tears releases a fragment that is required for much of the bactericidal activity of tears.
Conclusion: The protease-released C terminus of lacritin is bactericidal under physiological conditions.
Significance: All known lacritin activities are bundled within the same C-terminal region, although at different dose optimum.
Keywords: Bacterial Metabolism, Cornea, Eye, Innate Immunity, Phosphatidylethanolamine, Protein Targeting, Serine Protease, Lacritin, Tears
Abstract
Antimicrobial peptides are important as the first line of innate defense, through their tendency to disrupt bacterial membranes or intracellular pathways and potentially as the next generation of antibiotics. How they protect wet epithelia is not entirely clear, with most individually inactive under physiological conditions and many preferentially targeting Gram-positive bacteria. Tears covering the surface of the eye are bactericidal for Gram-positive and -negative bacteria. Here we narrow much of the bactericidal activity to a latent C-terminal fragment in the prosecretory mitogen lacritin and report that the mechanism combines membrane permeabilization with rapid metabolic changes, including reduced levels of dephosphocoenzyme A, spermidine, putrescine, and phosphatidylethanolamines and elevated alanine, leucine, phenylalanine, tryptophan, proline, glycine, lysine, serine, glutamate, cadaverine, and pyrophosphate. Thus, death by metabolic stress parallels cellular attempts to survive. Cleavage-dependent appearance of the C-terminal cationic amphipathic α-helix is inducible within hours by Staphylococcus epidermidis and slowly by another mechanism, in a chymotrypsin- or leupeptin protease-inhibitable manner. Although bactericidal at low micromolar levels, within a biphasic 1–10 nm dose optimum, the same domain is mitogenic and cytoprotective for epithelia via a syndecan-1 targeting mechanism dependent on heparanase. Thus, the C terminus of lacritin is multifunctional by dose and proteolytic processing and appears to play a key role in the innate protection of the eye, with wider potential benefit elsewhere as lacritin flows from exocrine secretory cells.
Introduction
Antimicrobial peptides protect all classes of life as the first line of innate defense, aided by the continuity of surface epithelium and activation of subepithelial macrophages (1, 2). Although >1900 antibacterial peptides have been identified to date, most (3–5) are individually inactive under normal physiological conditions (6, 7), with electrostatic binding of the anionic bacterial outer membrane a common characteristic. Yet others are primarily hydrophobic. Although membrane disruption is typical with ensuing lysis or pore formation (8), some pass intracellularly to disrupt function (9). Antimicrobial peptides may represent the future of antibiotics, with sensitivity to proteolysis in the gut being a primary weakness.
Different epithelia have evolved distinct protective mechanisms. The surface epithelium of the eye lacks the enhanced cornified barrier of skin, yet is rarely subject to bacterial penetration (7, 10). This property is largely attributable to the bactericidal tear film that covers the surface of the eye with lipids (11), metabolites (12), salts, and at least 1500 different proteins, some only recently characterized (13). Originally, it was thought that tears only immobilized pathogens by salt agglutination for subsequent removal or that salt levels were responsible for tear bactericidal activity because heat was ineffective (14) or, instead, that a moderate heat-resistant activity could be designated as a lysozyme (15). Since then, a variety of antimicrobial factors have been identified in human tear film, including lactoferrin, immunoglobulin A antibodies (IgA), secretory phospholipase A2, mucins, α- and β-defensins, histatins, and cathelicidin LL-37 (7). Gene knock-out studies support antimicrobial roles for lipocalin 2 (16–18), cathelicidin antimicrobial peptide (LL37 (19)), and defensin β1 (20). However, all except lipocalin 2 are individually inactive under physiological conditions (6).
Lacritin is a pleiotropic tear protein (21) that promotes the survival of stressed human corneal epithelial cells (22), basal tearing (23), and corneal epithelial cell proliferation (24). Lacritin is 21% identical (25) with dermcidin, whose cathepsin D-releasable C-terminal domain is processed (26) to the potently bactericidal SSL-25 peptide, the main bactericidal activity in sweat (27). These observations were the rationale for challenging cultures of Escherichia coli, Pseudomonas aeruginosa, and Staphylococcus epidermidis with lacritin and lacritin truncation mutants, peptides, and fragments. We report that cleavage of lacritin releases a potent bactericidal fragment that is distinct from SSL-25 and is active on both Gram-negative and -positive bacteria when applied at low micromolar doses. Activity is retained in 280 mosmol/liter buffer and only slightly diminished at 380 mosmol/liter. Thus, a growth factor with a biphasic dose optimum of 1–10 nm is a potent bactericide at low micromolar levels after proteolytic processing.
EXPERIMENTAL PROCEDURES
Tears and Tear Immunodepletion
Normal human basal tears were collected as described previously (28). The institutional review board at Walter Reed Army Medical Center Department of Clinical Investigation granted approval prior to the initiation of the study. Each participant gave informed consent, and all research adhered to the tenets of the Declaration of Helsinki. Briefly, tears from 0.5% proparacaine-anesthetized eyes were collected onto preweighed wicks and flash-frozen for −70 °C storage. Tears were eluted by immersion of each strip in 30 μl of PBS for 20 min, followed by centrifugation. For immunodepletion, 10-fold diluted tears were incubated overnight (4 °C) or for 1 h at room temperature with protein A beads (0.2 ml, NAb Spin Kit, Peirce/Thermo Scientific) saturated with “anti-N-65 Lac C-term” or preimmune Ig. N-65 is a lacritin truncation mutant lacking 65 N-terminal amino acids (22). The tear flow-through after centrifugation (5000 × g for 1 min) was then assayed for antibacterial activity.
Lacritin Constructs, Purification, Synthetic Peptides, and Mass Spectrometry
Lacritin N-terminal truncations N-55, N-65, N-71, and N-75 (29) were generated by PCR from parent cDNA pLAC, as described previously (29). N-terminal deletions of 80 (N-80) and 86 (N-86) amino acids were generated using forward primers 5′-GGTGGTCATATGAAAGCAGGAAAAGGAATGCACGG-3′ and 5′-GGTGGTCATATGCACGGAGGCGTGCCAGGTGG-3′, respectively, and common reverse primer 5′-GGTGGTCATATGTATATCTCCTTCTTAAAG-3′. All constructs were verified by sequencing. Bacterial protein expression and purification of recombinant lacritin and lacritin truncations were performed as described previously (24). Briefly, cleared cell (ER2566 or BL21-CP) lysates were loaded on chitin columns (IMPACT-CN System; New England Biolabs Inc., Beverly, MA) equilibrated with 50 mm Tris, 0.5 m NaCl (pH 8), followed by 20 column volumes of washing, elution with 50 mm 2-mercaptoethanol for 16 h at room temperature in the same buffer, extensive dialysis against PBS (4 °C), and protein quantitation. Further DEAE purification (22) removed a ∼9-kDa lacritin proteolytic fragment and bacterial contaminants in which lacritin was collected as the flow-through with 140 mm NaCl in phosphate buffer, pH 7.2. Synthetic peptides N-80/C-25, N-94, N-94/C-6, N-94/C-10, N-94/C-15, N-99, and N-104 were synthesized by Genscript (Piscataway, NJ) with acetylated N termini. Purity was >95%. C termini of all were amidated, with the exception of lacritin C-terminal N-94, N-99, and N-104. N-64/C-31 was neither amidated nor acetylated and was synthesized by the University of Virginia Biomolecular Research Facility.
The nature of the lacritin ∼9-kDa fragment was pursued by Western blotting. Briefly, lacritin before and after DEAE separation was separated by SDS-PAGE and then transferred and blotted with anti-Pep Lac N-terminal and anti-N-65 Lac C-terminal antibodies, respectively diluted 1:200 or 1:400 in PBS containing 0.3% Tween 20. Detection was with ECL. For fragment purification, chitin-enriched lacritin was dialyzed against phosphate buffer containing 14 mm NaCl (pH 7.2). Following incubation with DEAE equilibrated in the same buffer, the ∼9-kDa fragment was collected in the flow-through, whereas intact (18 kDa) lacritin was eluted with 140 mm NaCl in phosphate buffer, pH 7.2. After determination of protein concentration (BCA assay), both were aliquoted, lyophilized, and stored at −70 °C. Analysis was by SDS-PAGE on 4–20% gradient gels. The identity of the ∼9-kDa fragment was determined by mass spectrometry.
Bacterial Growth, SYTOX Green Assays, and on Column Cleavage
E. coli (ATCC (Manassas VA) catalog no. 10536), S. epidermidis (ATCC catalog no. 12228), and P. aeruginosa (ATCC catalog no. 9027) were grown to mid-log phase in 50 ml of Luria-Bertani (LB) medium and washed three times in phosphate buffer containing 10 mm NaCl (pH 7.2; PB-NaCl) with centrifugation. Pellets were resuspended in 1 ml of PB-NaCl.
For lacritin inhibition assays, 50 μl of bacterial pellets each diluted 1:100 in PB-NaCl were incubated for 1.5 h (37 °C) with 100 μl of lacritin, lacritin truncations, or synthetic peptides at a final concentration of 0.1–6 μm. Mixtures were diluted 1:10 in PB-NaCl before plating 100 μl in quadruplicate on LB agar plates for overnight growth at 37 °C. Colonies were manually counted. In other experiments, mid-log E. coli was treated at 37 °C for 0, 1, 2, or 3 h with 2 μm lacritin or lacritin truncations or with ampicillin (5 μm) or tetracycline (2 μm). After each treatment, 100 μl was centrifuged, resuspended in 1 ml of PB-NaCl, and plated (100 μl) onto LB agar for overnight growth (37 °C) and colony counting.
For salt sensitivity studies, pelleted and washed mid-log phase E. coli, S. epidermidis, or P. aeruginosa were resuspended in 1 ml of PB-NaCl and then treated as above with PB-NaCl or with 3 μm N-65 in 130, 280, or 380 mosmol/liter PB-NaCl for 1.5 h (37 °C). Mixtures were diluted 1:10 in PB-NaCl before plating 100 μl of each in quadruplicate on LB agar plates for overnight growth at 37 °C. Colonies were manually counted.
For bacterial permeability assays, pelleted and washed mid-log phase E. coli were resuspended in 1 ml of PB-NaCl and then treated as above with 3 μm lacritin, N-65, or C-25 or with 10% Triton X-100. Similarly, washed mid-log phase S. epidermidis were resuspended in 1 ml of PB-NaCl and then treated with lacritin or C-25 or a ∼9-kDa purified lacritin fragment. Later, 1 μl of 0.5 mm SYTOX Green was added to each well of 96-well fluorescent microtiter plates. Readings were taken at 5-min intervals at respective excitation and emission wavelengths of 485 and 538 nm using a Fluoroskan Ascent FL fluorometer (Thermo Fisher Scientific). In parallel, SYTOX Green internalization was visualized by confocal microscopy after 1 h of 10% Triton X-100, PB-NaCl, or 3 μm N-65 treatment of washed mid-log phase E. coli.
For cell-free synthesis without glycosylation, full-length lacritin cDNA in pLacSL was PCR-amplified and subcloned into pTXB1 supplied by the manufacturer (New England Biolabs, Ipswich, MA). Cell-free synthesis and subsequent removal of ribosomes, followed by metal affinity resin adsorption of His-tagged factors, was performed as per the manufacturer's instructions (New England Biolabs; PURExpress). Immediately following expression, an aliquot was stored at −60 °C. Other aliquots were incubated at 37 °C for 24 and 48 h. Each was separated by SDS-PAGE, transferred, and blotted with anti-N-65 Lac C-terminal antibodies.
For lacritin cleavage assays, supernatants from saturated 50-ml overnight cultures of S. epidermidis were collected by centrifugation (10 min; 11,000 rpm). Each supernatant was then incubated for 4, 16, and 20 h (37 °C) in PB-NaCl with chitin beads containing lacritin-intein immobilized via N termini. C-terminal cleavage products were collected by PB-NaCl washing, separated by SDS-PAGE, transferred, and blotted with anti-N-65 Lac C-terminal antibodies. In some experiments, supernatants and lysates from overnight cultures of S. epidermidis, Staphylococcus aureus, P. aeruginosa, and E. coli were incubated overnight (37 °C) with lacritin in solution in PB-NaCl. Mixtures were then separated by SDS-PAGE, transferred, and blotted with anti-N-65 Lac C-terminal antibodies. Parallel studies monitored the integrity of chitin-intein-immobilized lacritin in PB-NaCl at 37 °C for 0, 24, 48, and 72 h or for 24 h (37 °C) with 1 μm pepstatin, 10 μm bestatin, 100 μm antipain, 1 mm 4- benzenesulfonyl fluoride hydrochloride, 100 μm chymostatin, 10 μm E64, 100 μm leupeptin, or 10 mm phosphoramidon or for 24 h after boiling for 5 min at 100 °C.
Hemolysis Assay
The method of Cerovský et al. (30) was followed with some modifications. Washed sheep red blood cell pellets (MP Biomedicals, Santa Ana, CA) were suspended for 1 h at 37 °C in 565 μl of PBS plus 100 μl of lacritin, N-55, N-65, N-71, N-75, N-80, or C-25 at a final concentration of 2 μm or with N-65, N-64/C-31, N-80/C-25, N-94, N-94/C-6, N-94/C-10, N-94/C-15, N-99, or N-104 at a final concentration of 6 μm. As respective positive and negative controls, Triton X-100 (final concentration of 5%) or PBS was included in place of lacritin or lacritin fragments. After centrifugation (250 × g; 5 min), the absorbances of supernatants at 540 nm were monitored.
Metabolome Analysis
Washed mid-log E. coli were incubated with 6 μm N-65 or PB-NaCl for 15 min at 37 °C in replicates of six, each at 1 × 108 cells/replicate. Cells were then washed once, and pellets were flash-frozen for storage at −70 °C. Unbiased metabolite analysis was performed by Metabolon Inc. (Durham, NC) using GC/MS and LC/MS/MS. 78 metabolites were identified.
Statistical Analyses
With the exception of the single metabolomic analysis, all experiments were performed at least three times. Statistical analysis of metabolite data was as described previously (22), where raw data values were first log transformed to be closely distributed as a normal distribution and then assessed by a non-parametric Wilcoxon test and two-sample t test. For both tests with p < 0.05, metabolites were considered significantly different and further analyzed by hierarchical clustering for their association patterns. Data are reported as the mean ± S.E.
RESULTS
Lacritin Bactericidal Activity in Tears
Tears protect the surface of the eye against environmental pathogens and are enriched in the prosecretory mitogen lacritin (Fig. 1A), which flows onto the eye during basal and reflex tearing (21, 31). Lacritin is 21% identical to dermcidin, whose proteolytically processed C terminus contributes to the bactericidal activity of human sweat (26, 27, 32). We first sought to validate whether basal human tears (33–35) (Fig. 1B), like reflex tears (36, 37), are bactericidal and, if so, whether lacritin or a lacritin fragment(s) is in part responsible. Indeed, half-diluted basal tears completely blocked E. coli growth (Fig. 1C). E. coli is a significant contributor to bacterial conjunctivitis in the developing world, as is P. aeruginosa (38, 39). We next tested tears that had been passed over immobilized anti-N-65 Lac C-terminal antibodies (ab C-term) to immunodeplete both lacritin and C-terminal lacritin fragments (Fig. 1D, lane 2), or over preimmune Ig (mock-depleted; Fig. 1D, lane 4). Both were diluted 10-fold for dose-dependent challenge of E. coli and P. aeruginosa. Mock-depleted tears suppressed E. coli and P. aeruginosa colonies in a tear volume-dependent manner (Fig. 1, E and F). This contrasted with C-terminal antibody-immunodepleted tears, which were as ineffective as the phosphate buffer negative control (Fig. 1, E and F).
FIGURE 1.

Tear bactericidal activity is largely attributable to lacritin, as suggested by immunodepletion using an anti-lacritin C-terminal antibody. A, line diagram of lacritin, drawn together with dermcidin, with which it is 20% identical. In brackets is the N-65 truncation mutant. Bracketed in dermcidin is the anti-bacterial SSL-25 fragment. Rectangles, PSIPRED (version 3.0)-predicted (or validated) (24, 29) α-helices. B, schematic diagram of the eye with tear film. C, washed mid-log E. coli were incubated with phosphate (phos) buffer containing 10 mm NaCl (PB-NaCl) without or with half-diluted human basal tears for 1.5 h (37 °C). The mixture was then diluted and transferred to agar plates for overnight growth (37 °C) and cfu counting. Tears completely inhibit growth. D, 10-fold diluted tears were incubated with immobilized “anti-N-65 Lac C-term” or preimmune Ig. Material not bound (lanes 2 (ab C-term) and 4 (pre-immune)) and starting tears (lanes 1 and 3) were separated by SDS-PAGE, transferred, and blotted for lacritin. E, washed mid-log E. coli were incubated for 1.5 h (37 °C) with phosphate (phos) buffer containing increasing volumes of tear flow-through from anti-N-65 Lac C-terminal or preimmune Ig columns. The mixture was then diluted and transferred to agar plates for overnight growth (37 °C) and cfu counting. F, same as E but with washed mid-log P. aeruginosa.
Lacritin's C Terminus Contains a Bactericidal Domain
Lacritin's C terminus contains three predicted α-helices (Fig. 1A), each validated by circular dichroism (24, 29) (Fig. 2G). The most C-terminal α-helix is amphipathic and targets syndecan-1 as an initiator of corneal epithelial cell proliferation (24) and survival (22), largely via hydrophobic face residues (29). Association of amphipathic α-helices with bacterial membranes can be destabilizing (40). To explore whether these or other lacritin domains are bactericidal, we generated recombinant lacritin and lacritin truncations (24) (Fig. 2A). Each was generated as an intein fusion protein, purified on chitin to also remove the intein tag and then on DEAE to exclude bacterial contaminants. Lacritin and truncations were then assayed in equimolar (2 μm) amounts in the presence of mid-log E. coli, P. aeruginosa, or S. epidermidis. P. aeruginosa is an eye pathogen often responsible for keratitis in contact lens wear (41). S. epidermidis is a common cause of conjunctivitis and keratitis and is abundant in blepharitis (42, 43), an eyelid inflammation associated with slightly altered tear composition, including selectively less lacritin (44). Lacritin without truncation had no effect on the appearance of colonies, with numbers equivalent to the phosphate buffer negative control (Fig. 2, B–D). However, few colonies were apparent with lacritin lacking 65 (N-65) or 80 (N-80) amino acids from the N terminus, an effect completely or partly negated by removing six additional amino acids (N-86) in E. coli (Fig. 2B) or P. aeruginosa (Fig. 2C) but not S. epidermidis (Fig. 2D). Amino acids 81–86 comprise the sequence LAKAGKG, which ClustalW2 (Fig. 2E) and FASTA align with LDGAKKA from potent dermcidin fragment SSL-25 with an amino acid identity of 44%.
FIGURE 2.
Localization of lacritin bactericidal activity to N-104 within N-65. A, line diagram of secreted lacritin with truncation mutants indicated by dashed lines. B–D, washed mid-log E. coli (B), P. aeruginosa (C), and S. epidermidis (D) were incubated with phosphate (phos) buffer containing 10 mm NaCl (PB-NaCl) without or with 2 μm lacritin or 2 μm lacritin N-truncation mutants for 1.5 h (37 °C) before dilution and transfer to agar plates for overnight growth (37 °C) and cfu counting. E, ClustalW2 alignment of lacritin N-65 truncation mutant (C-terminal 53 amino acids) with 51 C-terminal residues of dermcidin. Lacritin synthetic peptides are displayed below and dermcidin SSL-25 peptide above. Red residues are those probably contributing to α-helices. F, washed mid-log E. coli were incubated as in B–D with 4 μm N-65 or synthetic peptides or PB-NaCl (phos), diluted, and then plated. G, circular dichroism of N-64/C-31 in PBS (dashed line) or in 10 mm dodecylphosphocholine (solid line). See Refs. 24 and 29 for circular dichroism of N-94/C-6 and N-94, respectively. H, washed mid-log E. coli were incubated as in B–D with increasing amounts of N-65 or N-104 (inset), diluted, and then plated. I, washed mid-log E. coli were incubated with PB-NaCl (phos) without or with 2 μm N-65, 5 μm ampicillin, or 2 μm tetracycline for 0, 1, 2, or 3 h (37 °C). Cells were then pelleted, resuspended in PB-NaCl, and then plated. J, washed mid-log P. aeruginosa were incubated as in B–D with increasing amounts of N-65 or N-104, diluted, and then plated. Error bars, S.E.
To ask whether the LAKAGKG region was responsible, we generated AKAGKGMHGGVPGG (amino acids 81–94; N-80/C-25), comprising the truncation-narrowed portion of the SSL-25 homologous region. Also generated were partially overlapping LKSIVEKSILLTEQALAKAGKGMH (amino acids 65–88; N-64/C-31) and C-terminal KQFIENGSEFAQKLLKKFSLLKPWA (amino acids 95–119; N-94). Unexpectedly, colonies were abundant with N-80/C-25 and N-64/C-31, whereas few or no colonies were apparent with N-94, a region only 12.5% identical with the C terminus of dermcidin. To narrow this site, we generated synthetic peptides N-94/C-6, N-94/C-10, N-94/C-15, N-99, and N-104 (Fig. 2E). N-94 and N-104 were fully active but not the other peptides, although N-94/C-6 was slightly so (Fig. 2F). N-65 is bactericidal and equipotent to ampicillin (Fig. 2I). In dose-response studies, N-104 was almost as effective as N-65 (Fig. 2, H and J), with a half-maximal inhibition of <1 μm for E. coli (Fig. 2H) and ∼1–1.5 μm for P. aeruginosa (Fig. 2J), a dose range common to antimicrobial peptides.
Antimicrobial Mechanism
To ask whether N-65 was destabilizing the outer bacterial membrane such that small extracellular molecules were gaining entry, we challenged mid-log E. coli with it in the presence of the membrane-impermeable dye SYTOX Green (45). After entry, SYTOX Green binds nucleic acids. We also monitored the release of hemoglobin from sheep red blood cells to control for lysis of mammalian cells under identical incubation conditions. N-65 (Fig. 3, A and B), but not C-25 or lacritin (Fig. 3A), opened E. coli to SYTOX Green with kinetics similar to the 10% Triton X-100 positive control (Fig. 3, A and B). None, including N-104, lysed sheep red blood cells (Fig. 3, C and D). We wondered whether the interaction might involve a cell surface protein(s) because the same region binds a hydrophobic patch within the eukaryotic cell surface proteoglycan syndecan-1 (46). E. coli were biotinylated, lysed, and incubated with immobilized lacritin or C-25. After washing and then exposure to 1 m NaCl, no streptavidin-peroxidase-detectable bands were eluted (not shown), suggesting a lack of high affinity protein binding. N-65 may bind LPS or peptidoglycan. However, binding via LPS would be incompatible with targeting Gram-positive bacteria. Conversely, peptidoglycan is the main component of the cell wall of Gram-positive bacteria, but it is primarily periplasmic in Gram-negative bacteria.
FIGURE 3.

N-65 permeabilizes bacterial membranes without hemolysis and is effective at 380 mosmol/liter. A, washed mid-log E. coli were incubated (37 °C) with PB-NaCl (phos) without or with 3 μm lacritin, N-65, or C-25 or with 10% Triton X-100 in the presence of 5 μm SYTOX Green in microtiter plates. Fluorescent readings were monitored at 485 nm (excitation) and 538 nm (emission). B, fluorescent confocal microscopic visualization of washed mid-log E. coli 1 h after treatment (37 °C) with 5 μm SYTOX Green in the presence of PB-NaCl (phos) without or with 10% Triton X-100 or 3 μm N-65. Double staining with DAPI is shown. C and D, washed sheep red blood cells were incubated (37 °C) for 1 h with PB-NaCl (phos) without or with 1 μm lacritin or truncations (C) or with 6 μm N-65 or synthetic peptides (D). Others were similarly incubated with 5% Triton X-100. The A540 nm of supernatants after centrifugation was measured. E–G, pelleted and washed mid-log phase E. coli (E), P. aeruginosa (F), or S. epidermidis (G) were resuspended in 1 ml of PB-NaCl and then treated as above with PB-NaCl or with 3 μm N-65 in 130, 280, or 380 mosmol/liter PB-NaCl for 1.5 h (37 °C). The mixtures were then diluted and transferred to agar plates for overnight growth (37 °C) and cfu counting. Colonies were manually counted. Error bars, S.E.
An alternative possibility is that the interaction might be largely electrostatic between the cationic C terminus and anionic phospholipids that predominate in bacterial membranes. However, electrostatic binding renders cationic antimicrobial proteins, such as cathelicidins and most defensins, inactive in physiological solutions (3–5). Tear osmolarity is ∼302 (47) to 318 mosmol/liter (48), rising to ∼324 mosmol/liter or more in tears from dry eye patients (49). We therefore challenged all three bacteria with 2 μm N-65 in 130, 280, or 380 mosmol/liter (pH 7.2) buffer (Fig. 3, E–G). N-65 was effective against P. aeruginosa (Fig. 3F) and S. epidermidis (Fig. 3G) in all three buffers and against E. coli at 130 and 280 mosmol/liter (Fig. 3E; 380 mosmol/liter buffer alone was toxic for E. coli). Thus, lacritin C-terminal bactericidal activity is largely insensitive to osmolarity. Electrostatic capture of bacteria by N-65 may thus be substantially strengthened by mutual hydrophobic interaction, should hydrophobic residues penetrate the membrane possibly to form pores, as implied by SYTOX Green studies.
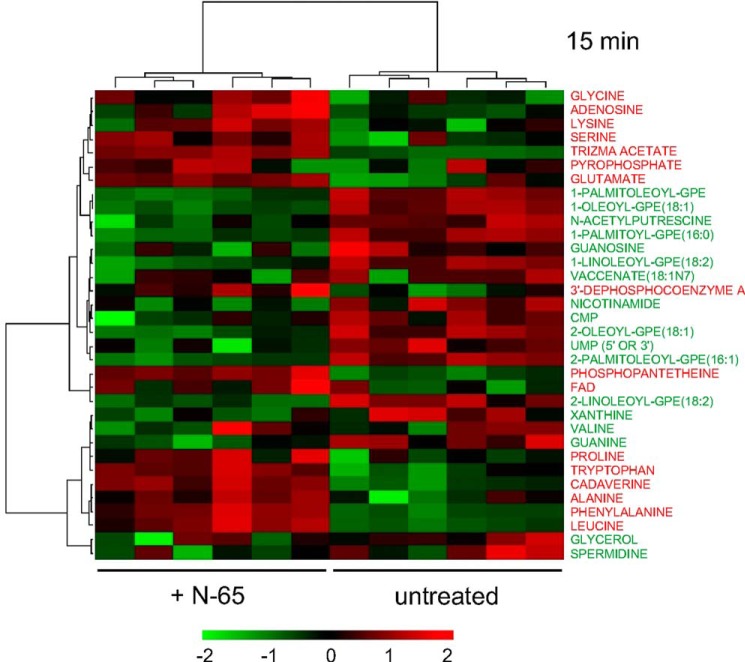
Although compromised membrane integrity may be sufficient to promote death, additional mechanisms could be in play either prior to or as a consequence of putative pore formation. We monitored 80 E. coli metabolites 15 min after treatment without or with 6 μm N-65 and observed significant changes in 34 (Fig. 4). Notably, N-65-treated cells displayed less dephosphocoenzyme A, spermidine, putrescine, and phosphatidylethanolamines. Dephosphocoenzyme A is the immediate precursor of widely employed cofactor coenzyme A (50). Spermidine and putrescine counter damage from reactive oxygen species (51), and phosphatidylethanolamine is the primary bacterial membrane phospholipid (52) that proportionally decreases in osmotic stress and is necessary for transport protein function (53). Thus, N-65 appears to widely compromise cellular metabolic capacity, protection against reactive oxygen species that are elevated by antibiotics, and perhaps integral transmembrane transport processes. We also observed an accumulation of alanine, leucine, phenylalanine, tryptophan, proline, glycine, lysine, serine, and glutamate (Fig. 4) and a decrease of valine, as also occurs in cold- or heat-stressed E. coli (54). Other changes included more cadaverine and pyrophosphate. Generation of cadaverine from lysine decarboxylation is a mechanism by which E. coli counters acid stress (55), and pyrophosphate is beneficial to bacterial growth (56). Thus, stress from N-65 disruption of the bacterial membrane and the availability of dephosphocoenzyme A, spermidine, putrescine, and phosphatidylethanolamines appears to go hand in hand with mechanisms attempting to counteract it.
FIGURE 4.
Metabolomic heat map of mid-log phase E. coli revealing N-65-triggered changes at 15 min versus no treatment control. Washed mid-log E. coli were incubated with PB-NaCl (untreated) without or with 6 μm N-65 for 15 min at 37 °C. Cells were washed, flash-frozen, and subjected to metabolite analysis using GC/MS and LC/MS/MS. Raw data values were first log-transformed to be closely distributed as a normal distribution and then assessed by non-parametric Wilcoxon test and two-sample t test. For both tests with p < 0.05, metabolites were considered significantly different and further analyzed by hierarchical clustering for their association patterns.
Proteolysis of Lacritin
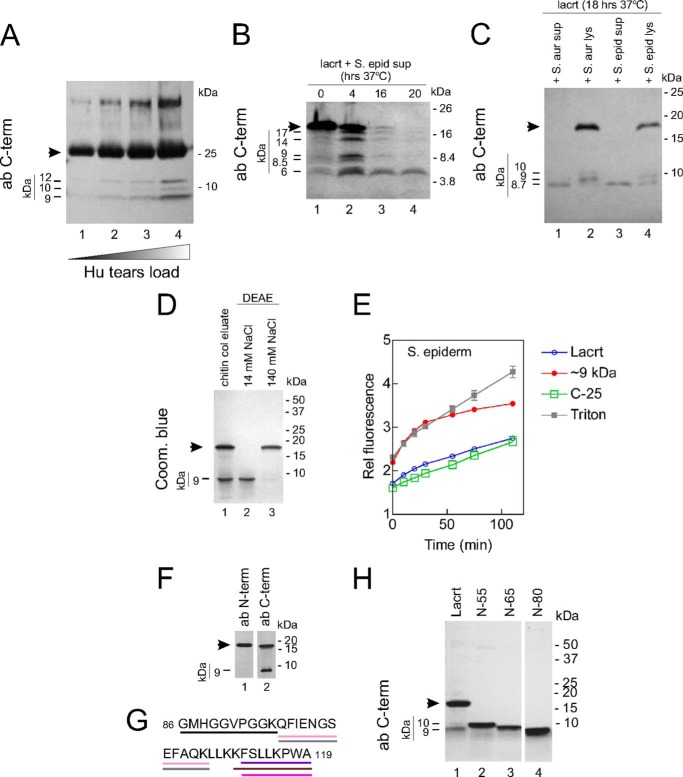
If this bactericidal mechanism is available in basal tears, as suggested by lacritin immunodepletion experiments (Fig. 1, E and F), C-terminal fragments must be available at a sufficiently high micromolar level. Levels may be enhanced by bacteria-dependent cleavage, for which there is plenty of lacritin available. Basal human tears appear to contain ∼27 μm lacritin antigen, as implied by an ELISA estimate of 4.2 ng of lacritin per 100 ng of total tear protein (28) and a suggested basal tear protein concentration of ∼8 mg/ml (57). We therefore searched for evidence of lacritin C-terminal processing in normal tears and in manipulations of recombinant lacritin without or with bacterial supernatant. Monomer is ∼25 kDa, with dimer and trimer at 50 and 75 kDa, respectively. Blotting of normal basal tears with anti-C-terminal lacritin antibodies detected all three, as well as immunoreactive bands of ∼12, 10, and 9 kDa, suggesting that C-terminal fragments are natural constituents of basal tears (Fig. 5A). Quantities of these fragments appear to be relatively low. To ask whether lacritin is subject to bacteria-dependent cleavage, we incubated recombinant lacritin (∼18 kDa) with S. epidermidis supernatant. This promoted the appearance of ∼17, 14, 9, 8.5, and 6 kDa bands within 4 h at 37 °C that resolved to a single ∼6 kDa (Fig. 5B) or ∼8.7 kDa (Fig. 5C) anti-C-terminal detectable band by 18–20 h. Supernatants from other species were tested. S. aureus supernatant also gave rise to a ∼8.7 kDa anti-C-terminal detectable band (Fig. 5C). P. aeruginosa and E. coli supernatants were less effective over 18 h, with monomer largely left intact but with some ∼8.7-kDa fragment detectable (not shown). Purification of recombinant lacritin has often yielded a second ∼9 kDa band after DEAE purification (Fig. 5D) that promotes entry of SYTOX Green (Fig. 5E); is detectable with anti-C-terminal, but not anti-N-terminal, specific antibodies (Fig. 5F); and was validated as a lacritin C-terminal fragment by mass spectrometry (Fig. 5G). Its migration in SDS-PAGE is similar to that of N-80 (Fig. 5H). The same band also slowly developed with time of lacritin incubation alone at 37 °C (Fig. 6A), suggesting that an E. coli proteolytic enzyme co-purifies with lacritin, as is not uncommon with recombinant proteins. It is unlikely but possible that lacritin has self-cleavage activity (58). To consider this possibility, we generated lacritin using a cell-free translation system. Lacritin was detectable initially as a doublet (Fig. 6B), which decreased substantially by 24 h at 37 °C and was barely apparent at 48 h. No C-terminal fragment was detected (Fig. 6B). To address the nature of the C-terminal fragment generating proteolytic activity, we subjected E. coli recombinant lacritin to a panel of proteolytic inhibitors and discovered that processing was inhibitable with chymostatin, with leupeptin, or by boiling but not with 4-(2-aminoethyl) benzenesulfonyl fluoride hydrochloride, antipain, bestatin, E64, pepstatin, or phosphoramidon (Fig. 6C). Thus, a serine protease-like activity may be responsible.
FIGURE 5.
Lacritin C-terminal bactericidal fragment is detectable in tears, is a product of recombinant lacritin production in E. coli, and is generated by S. epidermidis- and S. aureus-dependent proteolysis. A, increasing tear volumes were separated by SDS-PAGE, transferred, and blotted with anti-N-65 Lac C-terminal antibodies (ab C-term), revealing lacritin C-terminal fragments of 9, 10, and 12 kDa, glycosylated lacritin monomer (arrowhead), and higher molecular weight lacritin polymer (69). B, saturated overnight cultures of S. epidermidis were centrifuged, and supernatants were collected. Each supernatant was then incubated for 4, 16, and 20 h (37 °C) in PB-NaCl with chitin beads containing lacritin-intein immobilized via the N terminus. C-terminal cleavage products (arrowhead and below) were collected by PB-NaCl washing, separated by SDS-PAGE, transferred, and blotted with anti-N-65 Lac C-terminal antibodies. C, centrifugation of saturated overnight cultures of S. aureus and S. epidermidis separated supernatants from lysates. Supernatants and lysates were incubated for 18 h (37 °C) in PB-NaCl with purified lacritin in solution and then separated by SDS-PAGE, transferred, and blotted with anti-N-65 Lac C-terminal antibodies. D, recombinant lacritin eluted from chitin columns was separated by SDS-PAGE and stained with Coomassie Blue. The arrowhead indicates unglycosylated monomer. Shown also is a ∼9-kDa fragment (lane 1). Lane 2, chitin-enriched lacritin was dialyzed against phosphate buffer containing 14 mm NaCl and passed over DEAE, and the flow-through was collected to thereby purify the ∼9-kDa fragment. Lane 3, 140 mm NaCl elution of lacritin 18-kDa monomer. E, the lacritin ∼9-kDa fragment purified in D, lacritin, C-25, or 10% Triton X-100 was incubated (37 °C) with washed mid-log phase S. epidermidis. Fluorescent readings were collected in the presence of 5 μm SYTOX Green at 485 nm (excitation) and 538 nm (emission). F, chitin-enriched lacritin was separated by SDS-PAGE, transferred, and blotted with N-terminal antibodies (“anti-Pep Lac N-term”; lane 1) or C-terminal antibodies (lane 2). G, tandem mass spectrometry analysis of the ∼9-kDa fragment with lines indicating hits. All are contained within N-85. H, N-55, N-65, N-80, and chitin-enriched lacritin (Lacrt) were separated by SDS-PAGE, transferred, and blotted with C-terminal antibodies. Error bars, S.E.
FIGURE 6.
Lacritin C-terminal bactericidal activity is exposed at slower kinetics in a serine protease-inhibitable manner when recombinant lacritin is incubated alone. A, DEAE-purified lacritin (18 kDa) after incubation for different times in PB-NaCl, stained with Coomassie Blue. B, cell-free translated and purified lacritin retained at −60 °C (0 h; lane 1) or incubated at 37 °C for 24 h (lane 2) or 48 h (lane 3). C, DEAE-purified lacritin (18 kDa) before (4 °C; lane 12) or after 37 °C incubation for 24 h in PB-NaCl in the presence of proteolytic inhibitors or no inhibitor. Some lacritin was also preboiled at 100 °C (lane 1). Mixtures were separated by SDS-PAGE, transferred, and blotted with C-terminal antibodies. Inhibitors were as follows: 1 μm pepstatin, 10 μm bestatin, 100 μm antipain, 1 mm 4- benzenesulfonyl fluoride hydrochloride (AEBSF), 100 μm chymostatin, 10 μm E64, 100 μm leupeptin, 10 mm phosphoramidon.
DISCUSSION
Antimicrobial peptides protect all classes of life as the first line of innate defense. The surface epithelium of the eye lacks the enhanced cornified barrier of skin and yet is rarely subject to bacterial penetration (7, 10), a property largely attributed to the bactericidal tear film. Tears are enriched in the prosecretory mitogen lacritin (21) that flows onto the eye during basal and reflex tearing. Here we discover that lacritin is subject to C-terminal proteolytic processing and that the amphipathic α-helix-containing fragment appears to account for much of the bactericidal activity of normal basal tears by creating pores without hemolysis and a rapid form of bacterial death that may be regulated.
Our rationale for exploring whether lacritin might be bactericidal was its 21% identity with dermcidin (Figs. 1A and 2E), whose proteolytically processed C terminus contributes to the bactericidal activity of human sweat (26, 27, 31) and is in tears (13). Tear bactericidal activity has been the subject of much curiosity for over a century (7), including the original discovery of lysozyme (15) and a variety of other tear antimicrobial factors, particularly lactoferrin, lipocalin 2, immunoglobulin A antibodies (IgA), secretory phospholipase A2, mucins, α- and β-defensins (20), histatins, and cathelicidin LL-37 (7, 19, 37), and recently cytokeratin fragments (59). However, few are individually insensitive to the ionic strength (6) of normal or dry eye tears. This might be overcome in combination (7), or there could be other contributors. Seeking clarity, we took advantage of a tear immunodepletion strategy (22) with anti-C lacritin terminal specific antibodies, thereby providing confirmation of a C-terminal bactericidal lacritin fragment resident in normal tears that is salt-resistant.
Surprisingly, dermcidin primary sequence homology was not the source of lacritin activity. Only 40.7% identity is shared between dermcidin's bactericidal SSL-25 peptide and the homologous lacritin region that as a synthetic peptide was inactive. Instead, lacritin N-104 fragment with 7% dermcidin identity embodies the core activity, a hybrid domain consisting of an N-terminal amphipathic α-helix and hydrophobic C-terminal coiled coil tail, together appropriate for bacterial membrane contact and insertion, as was apparent by rapid entry of membrane-impermeable SYTOX Green in N-65-treated cells. Yet, dermcidin's SSL-25 peptide also forms an amphipathic α-helix (60) and, together with an adjoining C-terminal α-helix (60), binds artificial 1-palmitoyl-2-oleoyl-sn-glycero-3-phospho-(1-rac-glycerol)-rich membranes to form Zn2+-dependent toroidal pores (61). If N-104 or -65 create pores, as per SYTOX Green entry and metabolomic changes incompatible with lysis, how is pore formation linked to death? The answer is not clear, although levels of some cellular elements fell, such as phosphatidylethanolamine, the primary bacterial membrane phospholipid (52) necessary for transport protein function (53). Also lower were spermidine and putrescine to potentially diminish cellular repair from reactive oxygen species damage. Not yet addressed is the question of whether lacritin or N-104 affects redox potential. Dephosphocoenzyme A, the immediate precursor of essential cofactor coenzyme A necessary for key metabolic processes (50), was also lower. Spermidine and putrescine are notably essential for virulence in Salmonella gallinarum (62) and in P. aeruginosa, where spermidine is necessary for expression of the type III secretion system that delivers virulence proteins into eukaryotic cells (63). This differs from S. aureus, which cannot produce spermine or spermidine or the precursors agmatine and putrescine (64). Guanosine was lower. Synthesis of guanosine penta- or tetraphosphate is necessary for the bacterial “stringent response” to nutritional stress (65). Other metabolites rose, some apparently in a failed attempt to restore homeostasis. Examples include the increase of glycine, cadaverine, and pyrophosphate. Cellular importation of dimethylglycine protects Bacillus subtilis from osmotic stress (66). Cadaverine is the proton consuming product of lysine decarboxylase (55). The exogenous addition of pyrophosphate increases catabolic and anabolic processes for bacterial growth (56). Thus, cleavage of lacritin, like dermcidin, releases an amphipathic α-helical bactericidal fragment with poorly conserved primary sequence that nonetheless promotes metabolic stress leading to rapid death.
That this cleavage may be regulated by a serine protease, as per proteolytic inhibitor sensitivity, is in keeping with in silico analysis by the Protease Specificity Prediction Server (67), which predicts serine protease sensitivity after phenylalanine 96, leaving intact the complete C-terminal amphipathic α-helix. Dermcidin and cathelicidin are primarily processed by cathepsin D (26) and proteinase 3 (68), respectively. Whether lacritin is partially cleaved within tears as a consequence of or as an innate defense mechanism against infection or, alternatively, is in part processed intracellularly is not known. Most lacritin in normal human tears is uncleaved in monomeric or polymeric forms, the latter from tissue transglutaminase cross-linking (69), but some C-terminal fragment is resident. Curiously, manufacture in E. coli persistently yields a contaminating C-terminal fragment. Fragment abundance increases with time of affinity tag-purified lacritin at 37 °C, suggesting that an E. coli serine protease co-purifies with lacritin or that lacritin has self-cleavage activity, as per the SEA module of MUC1 (58), or generation of the active N-terminal domain from the hedgehog protein (70), among other examples. Loss of detectable cell-free translated lacritin with incubation leaves open the possibility that lacritin may be self-cleaving, yet instability when dilute could be an alternative explanation. That the same C-terminal region also supports lacritin's mitogenic (24) and prosurvival (22) activities, although at a much lower dose optimum, is an interesting example of pleiotropic conservation.
Acknowledgments
We thank Jeffrey Romano for preparing synthetic peptides and analysis of fragment molecular weights and Shannon Beck for circular dichroism analysis.
Footnotes
This work was supported, in whole or in part, by National Institutes of Health Grants RO1 EY013143 and EY018222 (to G. W. L.). G. W. L. is co-founder of TearSolutions, LLC, which seeks to take a lacritin C-terminal peptide through phase II human trials for treating dry eye.
REFERENCES
- 1. Yang D., Biragyn A., Hoover D. M., Lubkowski J., Oppenheim J. J. (2004) Multiple roles of antimicrobial defensins, cathelicidins, and eosinophil-derived neurotoxin in host defense. Annu. Rev. Immunol. 22, 181–215 [DOI] [PubMed] [Google Scholar]
- 2. Zugasti O., Ewbank J. J. (2009) Neuroimmune regulation of antimicrobial peptide expression by a noncanonical TGF-beta signaling pathway in Caenorhabditis elegans epidermis. Nat. Immunol. 10, 249–256 [DOI] [PubMed] [Google Scholar]
- 3. Ellison R. T., 3rd, Giehl T. J. (1991) Killing of Gram-negative bacteria by lactoferrin and lysozyme. J. Clin. Invest. 88, 1080–1091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Nagaoka I., Hirota S., Yomogida S., Ohwada A., Hirata M. (2000) Synergistic actions of antibacterial neutrophil defensins and cathelicidins. Inflamm. Res. 49, 73–79 [DOI] [PubMed] [Google Scholar]
- 5. Veldhuizen E. J., Rijnders M., Claassen E. A., van Dijk A., Haagsman H. P. (2008) Porcine β-defensin 2 displays broad antimicrobial activity against pathogenic intestinal bacteria. Mol. Immunol. 45, 386–394 [DOI] [PubMed] [Google Scholar]
- 6. Strömstedt A. A., Pasupuleti M., Schmidtchen A., Malmsten M. Oligotryptophan-tagged antimicrobial peptides and the role of the cationic sequence. Biochim. Biophys. Acta 1788, 1916–1923 [DOI] [PubMed] [Google Scholar]
- 7. McDermott A. M. (2013) Antimicrobial compounds in tears. Exp. Eye Res. 117, 53–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Imura Y., Choda N., Matsuzaki K. (2008) Magainin 2 in action: distinct modes of membrane permeabilization in living bacterial and mammalian cells. Biophys. J. 95, 5757–5765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sochacki K. A., Barns K. J., Bucki R., Weisshaar J. C. (2011) Real-time attack on single Escherichia coli cells by the human antimicrobial peptide LL-37. Proc. Natl. Acad. Sci. U.S.A. 108, E77–E81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Brown-Elliott B. A., Mann L. B., Hail D., Whitney C., Wallace R. J., Jr. (2012) Antimicrobial susceptibility of nontuberculous mycobacteria from eye infections. Cornea 31, 900–906 [DOI] [PubMed] [Google Scholar]
- 11. Butovich I. A. (2013) Tear film lipids. Exp. Eye Res. 117, 4–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chen L., Zhou L., Chan E. C., Neo J., Beuerman R. W. (2011) Characterization of the human tear metabolome by LC-MS/MS. J. Proteome Res. 10, 4876–4882 [DOI] [PubMed] [Google Scholar]
- 13. Karnati R., Laurie D. E., Laurie G. W. (2013) Lacritin and the tear proteome as natural replacement therapy for dry eye. Exp. Eye Res. 117, 39–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bernheim J. (1893) Ueber die antisepsis des bindehautsackes und die bakterienfeindliche eigenschaft der tränen. Beitr. z. Augenheilk. von Deutschmann. 1. Bd. [Google Scholar]
- 15. Fleming A. (1922) On a remarkable bacteriolytic element found in tissues and secretions. Proc. R. Soc. London 93, 303–317 [Google Scholar]
- 16. Flo T. H., Smith K. D., Sato S., Rodriguez D. J., Holmes M. A., Strong R. K., Akira S., Aderem A. (2004) Lipocalin 2 mediates an innate immune response to bacterial infection by sequestrating iron. Nature 432, 917–921 [DOI] [PubMed] [Google Scholar]
- 17. Berger T., Togawa A., Duncan G. S., Elia A. J., You-Ten A, Wakeham A., Fong H. E., Cheung C. C., Mak T. W. (2006) Lipocalin 2-deficient mice exhibit increased sensitivity to Escherichia coli infection but not to ischemia-reperfusion injury. Proc. Natl. Acad. Sci. U.S.A. 103, 1834–1839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chan Y. R., Liu J. S., Pociask D. A., Zheng M., Mietzner T. A., Berger T., Mak T. W., Clifton M. C., Strong R. K., Ray P., Kolls J. K. (2009) Lipocalin 2 is required for pulmonary host defense against Klebsiella infection. J. Immunol. 182, 4947–4956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Nizet V., Ohtake T., Lauth X., Trowbridge J., Rudisill J., Dorschner R. A., Pestonjamasp V., Piraino J., Huttner K., Gallo R. L. (2001) Innate antimicrobial peptide protects the skin from invasive bacterial infection. Nature 414, 454–457 [DOI] [PubMed] [Google Scholar]
- 20. Moser C., Weiner D. J., Lysenko E., Bals R., Weiser J. N., Wilson J. M. (2002) β-Defensin 1 contributes to pulmonary innate immunity in mice. Infect. Immun. 70, 3068–3072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sanghi S., Kumar R., Lumsden A., Dickinson D., Klepeis V., Trinkaus-Randall V., Frierson H. F., Jr., Laurie G. W. (2001) cDNA and genomic cloning of lacritin, a novel secretion enhancing factor from the human lacrimal gland. J. Mol. Biol. 310, 127–139 [DOI] [PubMed] [Google Scholar]
- 22. Wang N., Zimmerman K., Raab R. W., McKown R. L., Hutnik C. M., Talla V., Tyler M. F., 4th, Lee J. K., Laurie G. W. (2013) Lacritin rescues stressed epithelia via rapid forkhead box O3 (FOXO3)-associated autophagy that restores metabolism. J. Biol. Chem. 288, 18146–18161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Samudre S., Lattanzio F. A., Jr., Lossen V., Hosseini A., Sheppard J. D., Jr., McKown R. L., Laurie G. W., Williams P. B. (2011) Lacritin, a novel human tear glycoprotein, promotes sustained basal tearing and is well tolerated. Invest. Ophthalmol. Vis. Sci. 52, 6265–6270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wang J., Wang N., Xie J., Walton S. C., McKown R. L., Raab R. W., Ma P., Beck S. L., Coffman G. L., Hussaini I. M., Laurie G. W. (2006) Restricted epithelial proliferation by lacritin via PKCα-dependent NFAT and mTOR pathways. J. Cell Biol. 174, 689–700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Porter D., Weremowicz S., Chin K., Seth P., Keshaviah A., Lahti-Domenici J., Bae Y. K., Monitto C. L., Merlos-Suarez A., Chan J., Hulette C. M., Richardson A., Morton C. C., Marks J., Duyao M., Hruban R., Gabrielson E., Gelman R., Polyak K. (2003) A neural survival factor is a candidate oncogene in breast cancer. Proc. Natl. Acad. Sci. U.S.A. 100, 10931–10936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Baechle D., Flad T., Cansier A., Steffen H., Schittek B., Tolson J., Herrmann T., Dihazi H., Beck A., Mueller G. A., Mueller M., Stevanovic S., Garbe C., Mueller C. A., Kalbacher H. (2006) Cathepsin D is present in human eccrine sweat and involved in the postsecretory processing of the antimicrobial peptide DCD-1L. J. Biol. Chem. 281, 5406–5415 [DOI] [PubMed] [Google Scholar]
- 27. Schittek B., Hipfel R., Sauer B., Bauer J., Kalbacher H., Stevanovic S., Schirle M., Schroeder K., Blin N., Meier F., Rassner G., Garbe C. (2001) Dermcidin: a novel human antibiotic peptide secreted by sweat glands. Nat. Immunol. 2, 1133–1137 [DOI] [PubMed] [Google Scholar]
- 28. Seifert K., Gandia N. C., Wilburn J. K., Bower K. S., Sia R. K., Ryan D. S., Deaton M. L., Still K. M., Vassilev V. C., Laurie G. W., McKown R. L. (2012) Tear lacritin levels by age, sex, and time of day in healthy adults. Invest. Ophthalmol. Vis. Sci. 53, 6610–6616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Zhang Y., Wang N., Raab R. W., McKown R. L., Irwin J. A., Kwon I., van Kuppevelt T. H., Laurie G. W. (2013) Targeting of heparanase-modified syndecan-1 by prosecretory mitogen lacritin requires conserved core GAGAL plus heparan and chondroitin sulfate as a novel hybrid binding site that enhances selectivity. J. Biol. Chem. 288, 12090–12101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Cerovský V., Hovorka O., Cvacka J., Voburka Z., Bednárová L., Borovicková L., Slaninová J., Fucík V. (2008) Melectin: a novel antimicrobial peptide from the venom of the cleptoparasitic bee Melecta albifrons. ChemBioChem 9, 2815–2821 [DOI] [PubMed] [Google Scholar]
- 31. Morimoto-Tochigi A., Walkup R. D., Nakajima E., Shearer T. R., Azuma M. (2010) Mechanism for carbachol-induced secretion of lacritin in cultured monkey lacrimal acinar cells. Invest. Ophthalmol. Vis. Sci. 51, 4395–4406 [DOI] [PubMed] [Google Scholar]
- 32. Rieg S., Seeber S., Steffen H., Humeny A., Kalbacher H., Stevanovic S., Kimura A., Garbe C., Schittek B. (2006) Generation of multiple stable dermcidin-derived antimicrobial peptides in sweat of different body sites. J. Invest. Dermatol. 126, 354–365 [DOI] [PubMed] [Google Scholar]
- 33. Ford L. C., DeLange R. J., Petty R. W. (1976) Identification of a nonlysozymal bactericidal factor (β lysin) in human tears and aqueous humor. Am. J. Ophthalmol. 81, 30–33 [DOI] [PubMed] [Google Scholar]
- 34. Friedland B. R., Anderson D. R., Forster R. K. (1972) Non-lysozyme antibacterial factor in human tears. Am. J. Ophthalmol. 74, 52–59 [DOI] [PubMed] [Google Scholar]
- 35. Janssen P. T., Muytjens H. L., van Bijsterveld O. P. (1984) Nonlysozyme antibacterial factor in human tears. Fact or fiction? Invest. Ophthalmol. Vis. Sci. 25, 1156–1160 [PubMed] [Google Scholar]
- 36. Thompson R., Gallardo E. (1941) The antibacterial action of tears on staphylococci. Am. J. Ophthalmol. 24, 635–640 [Google Scholar]
- 37. Fleiszig S. M., Kwong M. S., Evans D. J. (2003) Modification of Pseudomonas aeruginosa interactions with corneal epithelial cells by human tear fluid. Infect. Immun. 71, 3866–3874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Malhotra S., Mehta D. K., Kumar P. (2005) Spectrum and antibiotic susceptibility pattern of bacterial isolates from conjunctival swabs. Indian J. Pathol. Microbiol. 48, 538–541 [PubMed] [Google Scholar]
- 39. Iwalokun B. A., Oluwadun A., Akinsinde K. A., Niemogha M. T., Nwaokorie F. O. (2011) Bacteriologic and plasmid analysis of etiologic agents of conjunctivitis in Lagos, Nigeria. J. Ophthalmic Inflamm. Infect. 1, 95–103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Tossi A., Sandri L., Giangaspero A. (2000) Amphipathic, α-helical antimicrobial peptides. Biopolymers 55, 4–30 [DOI] [PubMed] [Google Scholar]
- 41. Zaidi T. S., Priebe G. P., Pier G. B. (2006) A live-attenuated Pseudomonas aeruginosa vaccine elicits outer membrane protein-specific active and passive protection against corneal infection. Infect. Immun. 74, 975–983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Graham J. E., Moore J. E., Jiru X., Moore J. E., Goodall E. A., Dooley J. S., Hayes V. E., Dartt D. A., Downes C. S., Moore T. C. (2007) Ocular pathogen or commensal: a PCR-based study of surface bacterial flora in normal and dry eyes. Invest. Ophthalmol. Vis. Sci. 48, 5616–5623 [DOI] [PubMed] [Google Scholar]
- 43. Karimian F., Zarei-Ghanavati S., A B. R., Jadidi K., Lotfi-Kian A. (2011) Microbiological evaluation of chronic blepharitis among Iranian veterans exposed to mustard gas: a case-controlled study. Cornea 30, 620–623 [DOI] [PubMed] [Google Scholar]
- 44. Koo B. S., Lee D. Y., Ha H. S., Kim J. C., Kim C. W. (2005) Comparative analysis of the tear protein expression in blepharitis patients using two-dimensional electrophoresis. J. Proteome Res. 4, 719–724 [DOI] [PubMed] [Google Scholar]
- 45. Roth B. L., Poot M., Yue S. T., Millard P. J. (1997) Bacterial viability and antibiotic susceptibility testing with SYTOX green nucleic acid stain. Appl. Environ. Microbiol. 63, 2421–2431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Ma P., Beck S. L., Raab R. W., McKown R. L., Coffman G. L., Utani A., Chirico W. J., Rapraeger A. C., Laurie G. W. (2006) Heparanase deglycanation of syndecan-1 is required for binding of the epithelial-restricted prosecretory mitogen lacritin. J. Cell Biol. 174, 1097–1106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Gilbard J. P., Farris R. L., Santamaria J., 2nd (1978) Osmolarity of tear microvolumes in keratoconjunctivitis sicca. Arch. Ophthalmol. 96, 677–681 [DOI] [PubMed] [Google Scholar]
- 48. Benjamin W. J., Hill R. M. (1983) Human tears: osmotic characteristics. Invest. Ophthalmol. Vis. Sci. 24, 1624–1626 [PubMed] [Google Scholar]
- 49. Farris R. L., Stuchell R. N., Mandel I. D. (1986) Tear osmolarity variation in the dry eye. Trans. Am. Ophthalmol. Soc. 84, 250–268 [PMC free article] [PubMed] [Google Scholar]
- 50. O'Toole N., Barbosa J. A., Li Y., Hung L. W., Matte A., Cygler M. (2003) Crystal structure of a trimeric form of dephosphocoenzyme A kinase from Escherichia coli. Protein Sci. 12, 327–336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Tkachenko A. G., Akhova A. V., Shumkov M. S., Nesterova L. Y. (2012) Polyamines reduce oxidative stress in Escherichia coli cells exposed to bactericidal antibiotics. Res. Microbiol. 163, 83–91 [DOI] [PubMed] [Google Scholar]
- 52. Wikström M., Kelly A. A., Georgiev A., Eriksson H. M., Klement M. R., Bogdanovm M., Dowhanm W., Wieslander A. (2009) Lipid-engineered Escherichia coli membranes reveal critical lipid headgroup size for protein function. J. Biol. Chem. 284, 954–965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Romantsov T., Guan Z., Wood J. M. (2009) Cardiolipin and the osmotic stress responses of bacteria. Biochim. Biophys. Acta 1788, 2092–2100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Jozefczuk S., Klie S., Catchpole G., Szymanski J., Cuadros-Inostroza A., Steinhauser D., Selbig J., Willmitzer L. (2010) Metabolomic and transcriptomic stress response of Escherichia coli. Mol. Syst. Biol. 6, 364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Haneburger I., Fritz G., Jurkschat N., Tetsch L., Eichinger A., Skerra A., Gerland U., Jung K. (2012) Deactivation of the E. coli pH stress sensor CadC by cadaverine. J. Mol. Biol. 424, 15–27 [DOI] [PubMed] [Google Scholar]
- 56. Biville F., Laurent-Winter C., Danchin A. (1996) In vivo positive effects of exogenous pyrophosphate on Escherichia coli cell growth and stationary phase survival. Res. Microbiol. 147, 597–608 [DOI] [PubMed] [Google Scholar]
- 57. Sitaramamma T., Shivaji S., Rao G. N. (1998) HPLC analysis of closed, open, and reflex eye tear proteins. Indian J. Ophthalmol. 46, 239–245 [PubMed] [Google Scholar]
- 58. Levitin F., Stern O., Weiss M., Gil-Henn C., Ziv R., Prokocimer Z., Smorodinsky N. I., Rubinstein D. B., Wreschner D. H. (2005) The MUC1 SEA module is a self-cleaving domain. J. Biol. Chem. 280, 33374–33386 [DOI] [PubMed] [Google Scholar]
- 59. Tam C., Mun J. J., Evans D. J., Fleiszig S. M. (2012) Cytokeratins mediate epithelial innate defense through their antimicrobial properties. J. Clin. Invest. 122, 3665–3677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Steffen H., Rieg S., Wiedemann I., Kalbacher H., Deeg M., Sahl H. G., Peschel A., Götz F., Garbe C., Schittek B. (2006) Naturally processed dermcidin-derived peptides do not permeabilize bacterial membranes and kill microorganisms irrespective of their charge. Antimicrob. Agents Chemother. 50, 2608–2620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Paulmann M., Arnold T., Linke D., Özdirekcan S., Kopp A., Gutsmann T., Kalbacher H., Wanke I., Schuenemann V. J., Habeck M., Bürck J., Ulrich A. S., Schittek B. (2012) Structure-activity analysis of the dermcidin-derived peptide DCD-1L, an anionic antimicrobial peptide present in human sweat. J. Biol. Chem. 287, 8434–8443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Schroll C., Christensen J. P., Christensen H., Pors S. E., Thorndahl L., Jensen P. R., Olsen J. E., Jelsbak L. (2014) Polyamines are essential for virulence in Salmonella enterica serovar Gallinarum despite evolutionary decay of polyamine biosynthesis genes. Vet. Microbiol. 170, 144–150 [DOI] [PubMed] [Google Scholar]
- 63. Zhou L., Wang J., Zhang L. H. (2007) Modulation of bacterial Type III secretion system by a spermidine transporter dependent signaling pathway. PLoS One 2, e1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Joshi G. S., Spontak J. S., Klapper D. G., Richardson A. R. (2011) Arginine catabolic mobile element encoded speG abrogates the unique hypersensitivity of Staphylococcus aureus to exogenous polyamines. Mol. Microbiol. 82, 9–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Geiger T., Kästle B., Gratani F. L., Goerke C., Wolz C. (2014) Two small (p)ppGpp synthases in Staphylococcus aureus mediate tolerance against cell envelope stress conditions. J. Bacteriol. 196, 894–902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Bashir A., Hoffmann T., Smits S. H., Bremer E. (2014) Dimethylglycine provides salt and temperature stress protection to Bacillus subtilis. Appl. Environ. Microbiol. 80, 2773–2785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Song J., Tan H., Perry A. J., Akutsu T., Webb G. I., Whisstock J. C., Pike R. N. (2012) PROSPER: an integrated feature-based tool for predicting protease substrate cleavage sites. PLoS One 7, e50300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Sørensen O. E., Follin P., Johnsen A. H., Calafat J., Tjabringa G. S., Hiemstra P. S., Borregaard N. (2001) Human cathelicidin, hCAP-18, is processed to the antimicrobial peptide LL-37 by extracellular cleavage with proteinase 3. Blood 97, 3951–3959 [DOI] [PubMed] [Google Scholar]
- 69. Velez V. F., Romano J. A., McKown R. L., Green K., Zhang L., Raab R. W., Ryan D. S., Hutnik C. M., Frierson H. F., Jr., Laurie G. W. (2013) Tissue transglutaminase is a negative regulator of monomeric lacritin bioactivity. Invest. Ophthalmol. Vis. Sci. 54, 2123–2132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Chen X., Tukachinsky H., Huang C. H., Jao C., Chu Y. R., Tang H. Y., Mueller B., Schulman S., Rapoport T. A., Salic A. (2011) Processing and turnover of the Hedgehog protein in the endoplasmic reticulum. J. Cell Biol. 192, 825–838 [DOI] [PMC free article] [PubMed] [Google Scholar]