Background: Behavioral and neurophysiological correlates of methamphetamine and amphetamine differ via unknown mechanisms.
Results: Although extracellular amphetamine produces a higher increase in neuronal firing and inward DAT current, only intracellular methamphetamine prevents dopamine-induced neuronal firing and inward current.
Conclusion: Methamphetamine differently regulates the DAT-mediated conductances and thus the excitability of dopaminergic neuron.
Significance: Results reveal a new mechanism for methamphetamine-induced dysregulation of dopaminergic neurons.
Keywords: Addiction, Dopamine Transporter, Monoamine Transporter, Neurotransmitter Transport, Patch Clamp Electrophysiology, Amphetamine, Methamphetamine
Abstract
The dysregulation of the dopaminergic system is implicated in multiple neurological and neuropsychiatric disorders such as Parkinson disease and drug addiction. The primary target of psychostimulants such as amphetamine and methamphetamine is the dopamine transporter (DAT), the major regulator of extracellular dopamine levels in the brain. However, the behavioral and neurophysiological correlates of methamphetamine and amphetamine administration are unique from one another, thereby suggesting these two compounds impact dopaminergic neurotransmission differentially. We further examined the unique mechanisms by which amphetamine and methamphetamine regulate DAT function and dopamine neurotransmission; in the present study we examined the impact of extracellular and intracellular amphetamine and methamphetamine on the spontaneous firing of cultured midbrain dopaminergic neurons and isolated DAT-mediated current. In dopaminergic neurons the spontaneous firing rate was enhanced by extracellular application of amphetamine > dopamine > methamphetamine and was DAT-dependent. Amphetamine > methamphetamine similarly enhanced DAT-mediated inward current, which was sensitive to isosmotic substitution of Na+ or Cl− ion. Although isosmotic substitution of extracellular Na+ ions blocked amphetamine and methamphetamine-induced DAT-mediated inward current similarly, the removal of extracellular Cl− ions preferentially blocked amphetamine-induced inward current. The intracellular application of methamphetamine, but not amphetamine, prevented the dopamine-induced increase in the spontaneous firing of dopaminergic neurons and the corresponding DAT-mediated inward current. The results reveal a new mechanism for methamphetamine-induced dysregulation of dopaminergic neurons.
Introduction
The monoamine neurotransmitter, dopamine (DA),2 modulates locomotion, motivation, cognition, and reward-associated functions (1, 2). Dysregulation of dopaminergic neurotransmission has been implicated in various pathological conditions such as parkinsonism, schizophrenia, bipolar disorder, attention deficit hyperactivity disorder, and drug addiction (3–7). The amplitude, spatial, and temporal dimensions of the dopaminergic responses in the brain are regulated by the dopamine transporter (DAT) (8, 9). Thus, the elimination of DAT at the synapse or increased in its expression drastically alters synaptic dopamine levels, basal locomotion and psychomotor responses to amphetamine (10, 11).
Although amphetamine and methamphetamine are structural congeners with similar pharmacokinetic profiles, at similar doses methamphetamine is a more potent stimulant compared with amphetamine with longer lasting effects (12, 13). Although the route of methamphetamine administration and its accessibility contributes to the near 4-fold greater lifetime nonmedical use of methamphetamine relative to amphetamine (11, 14–16), recent reports also suggest differences in the mechanisms that underlie the actions of these two drugs on DAT.
Like other neurotransmitters, dopamine is released from synaptic vesicles fused with the plasma membrane after an action potential via what is considered the classical vesicle fusion mechanism (17–21). However, release of dopamine into the extracellular space can also occur via DAT-dependent reverse transport process (dopamine efflux), an action potential-independent mechanism (16). The psychostimulants amphetamine and methamphetamine both increase DAT-mediated dopamine efflux (8, 22–26). These compounds enter dopaminergic neurons either rapidly via DAT or much more slowly by lipophilic diffusion (27, 28). Our recent in vitro and in vivo studies reveal methamphetamine is not just a “stronger amphetamine”; it affects DAT differently. Recently, we demonstrated at equipotent concentrations, methamphetamine evokes a greater dopamine efflux via DAT than amphetamine and at a lower membrane potential (29). This greater effect of methamphetamine on DAT function was supported by in vivo chronomicroamperometry experiments in the nucleus accumbens (29). These findings are consistent with previous reports suggesting that methamphetamine-induced release of [3H]dopamine from pre-loaded COS-7-DAT cells is significantly higher than that induced by amphetamine (23). However, the underlying mechanisms by which methamphetamine regulates DAT activity both in vivo and in vitro is less understood.
DAT, like norepinephrine and serotonin transporters, is a member of the Na+/Cl−-dependent co-transporters. Forward transport and reverse transport of dopamine via DAT is coupled to Na+ and Cl− ions and is electrogenic (i.e. produces measureable current) (30–33). Recent reports suggest the isolated currents elicited by DAT promote excitability of dopamine neurons and thereby may be used as a reliable measure of DAT function. Here we tested the possibility that amphetamine and methamphetamine differentially influence DAT-mediated conductances and thus the excitability of midbrain dopaminergic neurons. Results suggest that, relative to dopamine and methamphetamine, amphetamine induces a greater increase in the frequency of the DAT-dependent spontaneous firing rate of midbrain dopamine neurons and elicits a larger DAT-dependent inward current that is uniquely more sensitive to isosmotic substitution of the external Cl− ions. Although isosmotic substitution of extracellular Na+ ions equally decreased amphetamine- and methamphetamine-mediated inward currents, only the amphetamine-induced inward current was inhibited after removal of extracellular Cl− ions. Furthermore, intracellular amphetamine or methamphetamine equally but less efficiently affected the neuronal firing and DAT-mediated inward current. Surprisingly, only the intracellular administration of methamphetamine prevented the DA-induced enhancement of neuronal firing and selectively inhibited corresponding DA-induced inward current. Because the nature of drug-induced dysregulation of DAT is important in the etiology of drug addiction, these data provide new information for the exploring treatment of methamphetamine addiction.
EXPERIMENTAL PROCEDURES
All reagents and drugs were obtained from Sigma unless otherwise mentioned.
Primary Culture of Mouse Midbrain Dopamine Neurons
All animals were housed in the animal care facility of the McKnight Brain Institute Building, University of Florida, which is accredited by the Association for Assessment and Accreditation of Laboratory Animal Care International (AAALAC). All animal procedures were approved by the Institutional Animal Care and Use Committee of University of Florida. A primary culture of midbrain dopaminergic neurons was obtained and used for the electrophysiology recordings according to our previous reports (34, 35). Mice pups (C57BL/6J) 0 to 3 days of age were anesthetized using isoflurane. The ventral midbrain region was isolated, and the tissue was incubated in dissociating media (116 mm NaCl, 5.4 mm KCl, 26 mm NaHCO3, 25 mm glucose, 2 mm NaH2PO4, 1 mm MgSO4, 1.3 mm cysteine, 0.5 mm EDTA, 0.5 mm kynurenate containing 20 units/ml papain at 34–36 °C) under continuous oxygenation for 2 h. This was followed by trituration of the tissue in glial media (50 % minimum essential medium, 38.5 % heat-inactivated fetal bovine serum, 7.7 % penicillin/streptomycin, 2.9 % d-glucose (45%), and 0.9 % glutamine (200 mm) using fire-polished Pasteur pipettes and then filtration through a 70-μm cell strainer. The subsequent cell suspension was then centrifuged at 500 rpm at 4 °C. The acutely dissociated neuronal suspension was then plated on poly-d-lysine and laminin-treated 12-mm round glass coverslips. One hour after plating the medium was changed to neuronal medium: 2% minimum essential medium, 75% Ham's F-10 medium, 19% heat-inactivated horse serum, 2% heat-inactivated fetal bovine serum, 1.56% d-glucose (45%), 0.04% insulin (0.025g/ml) and 0.4% apotransferrin (50 mg/ml). Neuronal medium was conditioned overnight on cultured glia. The conditioned neuronal medium was supplemented with 1 ng/ml glial cell line-derived neurotrophic factor and 500 μm kynurenate and filter-sterilized before it was added to mesencephalic cultures. For maintaining the cultures, half of the medium was replaced every fourth day.
Cell Lines
Chinese Hamster Ovary (CHO) and human embryonic kidney 293 EM4 cells (HEK) overexpressing human dopamine transporter tagged with yellow fluorescent protein (YFP) used in evaluating DAT-mediated currents were generous gifts from Dr. Jonathan Javitch, Columbia University (23, 29). The N terminus of synthetic human DAT cDNA was fused with the C terminus of the coding region of enhanced YFP from pEYFP-N1 (Clontech) to generate a fusion construct denoted as YFP-DAT, which was then stably overexpressed in CHO and HEK cells (36). Previous experiments from our laboratory and other groups suggest the N-terminal YFP tag does not change substrate-induced DAT activity (36). CHO YFP-DAT cells were maintained in Ham's F-10 medium supplemented with 2 mm l-glutamine, 10% fetal bovine serum at 37 °C in 5% CO2. The HEK 293-YFP-DAT cells were maintained in Dulbecco's modified Eagle's medium supplemented with 2.5% l-glutamine, 2.5% penicillin/streptomycin mix, and 10% fetal bovine serum at 37 °C and in 5% CO2. The YFP-DAT-expressing cell lines used in this study are well characterized and have been frequently used to study DAT activity and its biophysical properties (29, 36–39). Parental non-expressing cells were used for control experiments.
Electrophysiology
Whole-cell current-clamp recordings of DAT substrate-induced effects on the neuronal firing rate of midbrain dopaminergic neurons were acquired using an Axopatch 200B Amplifier (Molecular Devices, Sunnyvale, CA) sampled at 10 kHz with a low-pass Bessel filter set at 2 kHz and digitized using a Digidata 1440 (Molecular Devices). Data were analyzed off-line using Clampfit 10.4 software (Molecular Devices).
Immediately before recording, glass coverslips containing neurons in primary culture (7–12 days in vitro) were washed twice in standard external solution before being placed in fresh external solution that contained NaCl (146 mm), HEPES (5 mm), KCl (5 mm), dextrose (30 mm), CaCl2·2H2O (2.5 mm), and MgCl2·6H2O (1.2 mm), pH 7.4 (0.1 n NaOH used to adjust the pH), and a final osmolarity of 290–300 mosm. Patch clamp electrodes (3–4 megaohms) were pulled from borosilicate pipettes on a P-2000 puller (Sutter Instruments, Novato, CA) and filled with an internal solution containing potassium gluconate (138 mm), KCl (10 mm), HEPES (10 mm), EGTA (1 mm), Mg-ATP (4 mm), Na-GTP (0.5 mm) CaCl2·2H2O (0.3 mm), and MgCl2·6H2O (1 mm) with pH 7.4 adjusted with KOH and a with final osmolarity of 270–280 mosm. The junction potential was calculated using pClamp 10.2 and corrected offline. Where indicated, 1% neurobiotin (Vector Laboratories), 10 μm amphetamine, or methamphetamine was added directly to the internal solution. All recordings were performed in the presence of D2 receptor antagonist, sulpiride (5 μm).
Dopaminergic neurons were identified both morphologically by their large cell bodies with broad 2–5 first order processes and electrophysiologically. Based on previous findings, dopaminergic neurons with a spontaneously firing frequency of 0.3–4 Hz were selected. After each experiment cultures were subjected to immunostaining for tyrosine hydroxylase (TH) and neurobiotin (see below). 90% of the selected neurons were immunopositive for TH and neurobiotin. Only TH-positive neurons were included in analysis.
The frequency of action potential was measured offline using Clampfit 10.2 software template search. Firing rate histograms were calculated using 5-s bins (time-frame). For calculation, an average response of >30 s before drug application and during the maximum response over a duration of 5–7 min after drug application were considered. To determine the effect of extracellular drug on the neuronal firing rate, the firing rate of each neuron after drug application was normalized to its own base-line spontaneous firing activity before drug application. We determined the effects of intracellular drug application by normalizing the response to the base-line spontaneous neuronal firing rate of each neuron. Similarly, we determined the effect of extracellular dopamine after drug dialysis into the neuron. The firing rate after extracellular dopamine was normalized to the base-line spontaneous firing. The DAT blocker GBR12935 (10 μm) was applied to block DAT-mediated depolarizing inward current at the end of each experiment. Only GBR12935-sensitive neurons were included in the analysis. We and others have shown that after whole-cell patch clamp the pipette solution reaches equilibrium with the intracellular milieu within 5–8 min when the pipette resistance is ∼4–5 megaohms (29, 36, 40, 41). To examine the consequences of intracellular amphetamine and methamphetamine on DAT-mediated current or changes in the neuronal firing rate, the base-line current or voltage recordings were acquired immediately after obtaining the whole cell configuration and 8 min after the initiation of whole-cell patch clamp.
Immunostaining
At the end of each recording cultures were fixed in 3.7% paraformaldehyde in phosphate-buffered saline (PBS) for 15 min at room temperature. Cultures were then washed 3 times for 5 min with PBS and stored at 4 °C. On the day of immunostaining the coverslips were washed 3 times for 5 min with PBS, incubated with 0.2% Triton X-100 for 15 min at room temperature, and washed 3 times for 5 min PBS. Coverslips were blocked with 10% goat serum for 30 min at room temperature. Primary (rabbit polyclonal anti-TH, 1:1000, Millipore) or secondary (Alexa Fluor 633 goat anti-rabbit, 1:500; Invitrogen) antibody was diluted in PBS containing 0.25% bovine serum albumin and 0.05% Triton X-100. In addition to immunostaining for TH, DyLight 488 conjugated streptavidin (1:500, Vector Laboratories) was also used to visualize in neurobiotin-filled neurons. A Nikon A1 confocal microscope with FITC and TRITC filter cubes were used to visualize TH (633 nm) staining in neurobiotin (488 nm) filled neurons, respectively (Fig. 1A).
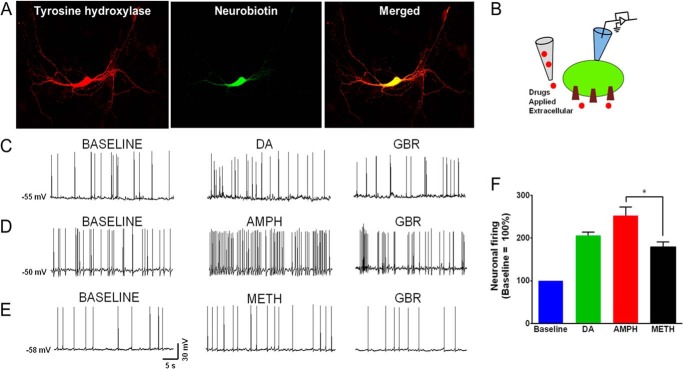
FIGURE 1.
Extracellular amphetamine and methamphetamine differentially affect the DAT-mediated spontaneous firing of dopaminergic neurons. A, a representative image of a TH and neurobiotin immunoreactive dopaminergic neuron obtained from primary culture of midbrain region of 1–2 days old mice. The neurons were selected by morphological (bright cell bodies, long processes) and electrophysiological signature (D2-induced hyperpolarization). The neurons were filled with neurobiotin via the patch pipette during the recording for subsequent staining for TH and neurobiotin. The data obtained from TH and neurobiotin-immunoreactive neurons are reported. Neurobiotin is widely used to identify the neurons from which the recordings were performed and does not affect the electrical properties of neurons (54). The spontaneous firing activity of midbrain dopaminergic neurons was measured in the current clamp mode when D2 receptor is blocked (sulpiride, 5 μm). B, the schematic depicts the experimental configuration showing DAT substrates were applied extracellularly. C–E, representative traces of current clamp recording of midbrain dopaminergic neurons show the basal spontaneous firing activity of dopaminergic neurons at resting membrane potential in the absence of DAT substrates, dopamine, amphetamine, or methamphetamine. The extracellular application of DAT substrates, dopamine (1 μm; n = 5), amphetamine (1 μm; n = 5), or methamphetamine (1 μm; n = 5), increases the firing rate of dopaminergic neurons that is blocked by DAT antagonist, GBR12935 (GBR; 1 μm). F, bar graphs show the spontaneous neuronal firing rate after extracellular drug application. The data are normalized to the spontaneous firing rate at base line before drug application. The reported base line (100%) is not an interleaved base line. Although the amphetamine- and dopamine-induced increases in the spontaneous firing rate of dopaminergic neurons were similar, the methamphetamine-induced changes in the firing rate was significantly lower than amphetamine (*, p < 0.05, one-way ANOVA followed by Tukey's post hoc test).
Measurement of DAT-mediated Whole Cell Currents
Cells were plated at 105 per 35-mm culture dish. Attached cells were washed 3 times with external solution at room temperature. The external solution contained NaCl (130 mm), HEPES (10 mm), dextrose (34 mm), KH2PO4 (1.3 mm), CaCl2·2H2O (1.5 mm), and MgSO4·7H2O (0.5 mm), pH 7.35 (adjusted using 0.1 n NaOH) and final osmolarity of 290–300 mosm. The internal solution contained KCl (120 mm), HEPES (10 mm), dextrose (30 mm), EGTA (1.1 mm), CaCl2·2H2O (0.1 mm), and MgCl2·6H2O (2 mm) with pH 7.35 (adjusted using 0.1N KOH) and final osmolarity 270–280 mosm. In ion substitution experiments, choline was iso-osmotically substituted for Na+ in the external solution to completely remove extracellular Na+, and KOH was used to adjust pH. For Cl− substitution in the external solution, NaNO3 was iso-osmotically substituted for NaCl, and the pH was adjusted with NaOH. In all experiments intracellular Cl− and Na+ concentrations were held constant, Cl− = 124.2 mm, and Na+ = 0 mm. The pH and osmolarity of internal and external solutions were kept constant at 270 mosm and pH 7.35 or 290–300 mosm and pH 7.35, respectively.
Patch electrodes (4–5 megaohms) were pulled from borosilicate pipettes on a P-2000 puller (Sutter Instruments) and filled with the physiological-like internal solution as described above. Whole cell currents were acquired using an Axopatch 200B (Molecular Devices) sampling at 10 kHz with a low-pass Bessel filter set at 2 kHz. Inward currents were generated using a voltage step (500 ms) protocol at −100 and −80 mV from a holding potential of −40 mV. Data were recorded and analyzed off-line using Clampfit 10.2 software (Molecular Devices). The steady-state current at each voltage was calculated as the average current during the final 100 ms of each potential tested. The DAT-mediated whole-cell current was isolated by subtracting the current produced in the presence of GBR12935 from the current produced after bath application of drugs or after intracellular delivery of drugs into the cell via the patch pipette. The % inhibition of current was obtained after Na+ or Cl− substitution was calculated by normalizing these currents with a mean average current with normal Na+ and Cl− in the external milieu.
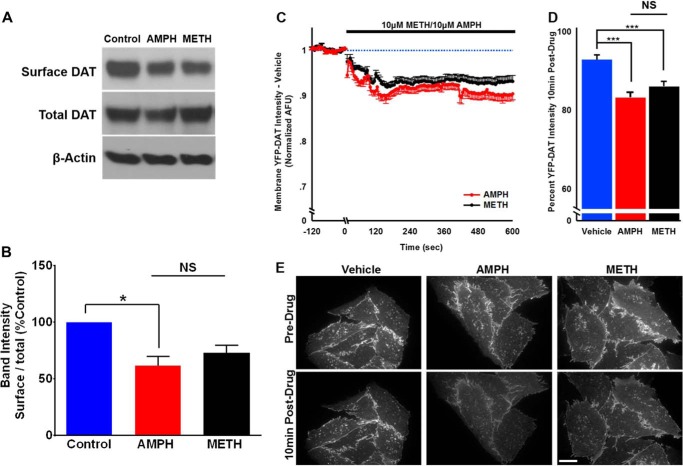
Cell-surface Biotinylation
EM4 cells expressing FLAG-human DAT were plated in 6-well plates and grown to full confluency for 4 days. Cells were pretreated with external solution, 10 μm amphetamine, or 10 μm methamphetamine, for 10 min at 37 °C in external solution. After treatment cells were washed with cold excess PBS, and the subsequent steps were performed on ice to prevent further trafficking. The surface expression of DAT was determined by reacting surface proteins with 1.0 mg/ml sulfo-NHS-SS-biotin (sulfosuccinimidyl-2-(biotinamido)-ethyl-1,3-dithiopropionate) (Thermo Scientific) for 20 min. Excess biotin was quenched by incubating cells with 100 mm glycine in PBS followed by two additional washes in 100 mm glycine. Cells were lysed in radioimmunoprecipitation assay buffer, and soluble protein was isolated. 500 μg of protein was reacted with neutravidin beads (Pierce) overnight to isolate surface protein. Beads were then pelleted and washed 3 times in radioimmunoprecipitation buffer. Protein was eluted in Laemmli sample buffer (Bio-Rad) supplemented with 2-mercaptoethanol. Protein was resolved using SDS-PAGE and transferred to PVDF membrane. Western blot analysis was performed using a rat anti-dopamine transporter antibody (Millipore) and mouse anti-β-actin antibody (Sigma). Data are expressed as the optical density units normalized to external solution-treated (vehicle) control. Statistical significance was determined using one-way ANOVA followed by Tukey's post hoc analysis.
Total Internal Reflection Fluorescence Microscopy
For quantitative TIRF imaging of cell membrane YFP-DAT intensity, CHO-YFP-DAT cells were grown on (no. 1 thickness) 35-mm glass-bottom dishes (Mattek, Inc.) for 48–72 h as described above. Cells were washed twice with external solution before adding fresh external solution immediately before imaging at 37 °C. All total internal reflection fluorescence microscopy imaging of cell surface YFP-tagged DAT was completed on a Nikon Ti Eclipse inverted microscope (Nikon Instruments Inc., Melville, NY). To achieve TIRF, the emission of the 514-nm laser source was guided through a manual XY translator for angle adjustment then through a multiband pass filter cube containing filters centered at 514 nm (excitation) and 535 nm (emission) before passing through a 60 × 1.4 NA objective and contacting the specimen attached to the glass-bottom dish. Images were detected digitally using an attached CoolSNAP HQ2 CCD camera (1392 × 1040 pixels, Photometrics, Tucson, AZ) and stored on a computer hard drive at 5-s intervals. Using NIS-Elements software (Nikon Instruments Inc.), image exposure time was coupled with a YFP stimulation duration of 200 ms, and 514-nm laser intensity was maintained at 40% of maximum intensity. For quantification of fluorescence intensity at the cell surface, experimenter-defined regions of interest (ROIs) were created for each cell in order to include the basal surface of only that cell and exclude overlapping regions from neighboring cells. ROI shape and size were unchanged throughout the duration of each experiment. Background fluorescence was determined using an ROI of comparable size to those used for individual cells positioned in an area devoid of cells. This background intensity was subtracted from all images and from cell ROI values for presentation and analysis. Mean intensity (in arbitrary fluorescent units) over time for each cell ROI was recorded continuously for 5 min before and 10 min after the application of vehicle (external solution, 10 μm amphetamine, or 10 μm methamphetamine in the bathing solution. All quantification is expressed normalized to the mean intensity of each cell over the 1 min before drug application. If the normalized fluorescence intensity changed by >5% during the 3-min base-line acquisition period before adding drug or vehicle, it was not included in analysis.
RESULTS
Extracellular Amphetamine and Methamphetamine Differentially Alter the DAT-mediated Firing Rate of Dopaminergic Neurons
The DAT substrates, dopamine, amphetamine, and methamphetamine interact with the transporter, are transported into the neuron, and elicit an excitatory current that is blocked by DAT antagonist (13, 29, 42). First we sought to investigate the influence of the extracellularly applied (Fig. 1B) dopamine, amphetamine, and methamphetamine on the firing rate of cultured mice midbrain dopaminergic neurons in the presence of dopamine receptor antagonist sulpiride (5 μm), which isolate the excitatory effect of these DAT substrates. The neurons were whole-cell patch-clamped and spontaneous firing rates were recorded in the current clamp mode. The dopamine neurons spontaneously fired at 0.3–4 Hz at the resting membrane potential of 53 ± 5 mV (n = 15). Similar to previous reports (26, 29, 42–45), in the presence of D2 receptor antagonist (sulpiride, 5 μm), the bath application of dopamine (1 μm) increased the firing rate of dopamine neurons (Fig. 1C). Subsequent application of the selective DAT blocker, GBR12935 (1 μm), decreased this dopamine-induced enhancement of the firing of dopamine neurons. Consistent with previous reports, the results suggest that dopamine modulation of the firing rate of dopaminergic neurons depends on the activity of the transporter. Subsequently, all of the current clamp recordings were performed in the presence of D2 receptor blocker, sulpiride (5 μm). Because in cultured mice dopamine neurons as well as mice ventral midbrain slices the dopamine-specific excitatory conductance is maximal at 1 μm dopamine (42), we used this concentration for the neuronal recordings in this study. Using this experimental configuration (Fig. 1B), the firing rate of dopamine neurons significantly increased as compared with the basal firing rate in response to all three DAT substrates (Fig. 1, C–E; p < 0.05, one-way ANOVA followed by Tukey's post hoc test): 1 μm dopamine (105.4 ± 19%; n = 5), 1 μm amphetamine (152 ± 45%; n = 5), and 1 μm methamphetamine (79.1 ± 27%; n = 5). The data are shown as neuronal firing after extracellular application of the drug. These values are normalized to the base-line spontaneous firing. The base-line spontaneous firing activity is defined as the firing rate before drug application. The base-line firing activity of each neuron is set as 100%. Although amphetamine stimulated the greatest increase in the firing rate of dopamine neurons, the effect of methamphetamine on the firing rate of these neurons was significantly smaller than that of amphetamine (Fig. 1F; p < 0.05, one-way ANOVA followed by Tukey's post hoc test). Extracellular dopamine produced an intermediate response (105.4 ± 19%) that under current experimental conditions was not significantly different from either amphetamine or methamphetamine. All three DAT substrates stimulated GBR12935-sensitive increases in the firing rate of dopaminergic neurons, supporting the interpretation that the DAT-mediated conductance affects depolarization of dopaminergic neurons. In addition, these results reveal an unexpected difference between the influence of extracellular amphetamine and methamphetamine on the firing rate of dopaminergic neurons.
Intracellular Methamphetamine Prevents Dopamine-induced Stimulation of Dopaminergic Neurons
Next we studied the underlying mechanism of the disparity between the effect of amphetamine and methamphetamine on the excitability of dopaminergic neurons. We asked whether the transport step mediates the differences in the enhancement of the excitability of the neurons. Therefore, to bypass the transport step, amphetamine or methamphetamine was dialyzed into the neuron as described under “Experimental Procedures.” Unlike the extracellular applications of these psychostimulants, when either 1 μm methamphetamine (n = 5) or 1 μm amphetamine (n = 4) was applied intracellularly, only a modest and similar increase in the neuronal firing rate was measured (37.16 ± 26% for amphetamine and 36.43 ± 12% for methamphetamine; Fig. 2, B–D). The firing rate after dialysis of each drug into the neuron was normalized to the base-line firing rates. The base-line spontaneous firing activity was defined as the firing rate before drug dialysis. The base line of each neuron was set as 100%.
FIGURE 2.
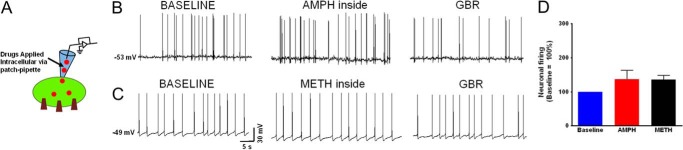
Intracellular amphetamine or methamphetamine less effectively modulates the firing rate of dopaminergic neurons. A, schematic depicts the experimental configuration showing DAT substrates were applied intracellularly. B and C, current clamp recording of midbrain dopaminergic neurons showing the spontaneous firing activity at the endogenous resting membrane potential when DAT substrates, amphetamine (1 μm, n = 4), or methamphetamine (1 μm, n = 5) were dialyzed into the neuron to bypass the forward transport step. At the resting membrane potential the spontaneous firing activity of the neurons immediately after achieving whole-cell configuration reflects the base-line firing activity of the neuron. GBR, GBR12935. D, intracellular amphetamine or methamphetamine equally but only modestly affected the firing rate of dopaminergic neurons (black and red bar). The bar graphs show the spontaneous firing activity of the neurons when amphetamine or methamphetamine was dialyzed into the neuron. The data are normalized to the base-line spontaneous firing rate of each experiment. The change in firing rate was blocked by extracellular application of a DAT antagonist, GBR12935 (1 μm).
To determine whether inward transport was necessary to account for this disparity between extracellularly and intracellularly applied psychostimulants, after intracellular dialysis with methamphetamine (n = 5) or amphetamine (n = 4), 1 μm dopamine was applied extracellularly (Fig. 3A). The increase in firing rate in response to extracellular dopamine with the presence of intracellular amphetamine (94.2 ± 22.5%) did not significantly differ from the increase in firing rate in response to extracellular dopamine alone (105.4 ± 19.5%; Fig. 3, B and D). However, intracellular methamphetamine significantly attenuated (44.9 ± 34.7%) the extracellular dopamine-induced increase in firing rate of dopaminergic neurons relative to the application of dopamine alone or in the presence of intracellular amphetamine Fig. 3, B–D). The firing rates of the neurons after extracellular application of dopamine are normalized to the spontaneous firing rate when physiological-like internal solution, amphetamine, or methamphetamine was dialyzed into the neurons. These data suggest that, unlike amphetamine, intracellular methamphetamine prevents dopamine-induced excitation of dopaminergic neurons and DAT-mediated inward depolarizing current. Thus, compared to amphetamine, methamphetamine differentially influences the firing of dopaminergic neurons (Fig. 4).
FIGURE 3.
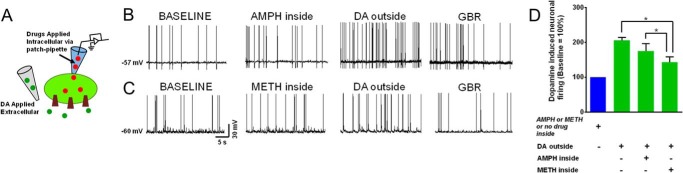
Intracellular methamphetamine attenuates the dopamine-induced increase in the firing rate of dopaminergic neurons. A, schematic depicts the experimental configuration showing DAT substrates were applied intracellularly and dopamine was applied extracellularly. B and C, current clamp recording from midbrain dopaminergic neurons shows that dopamine (1 μm, n = 5) increases the spontaneous firing activity of dopaminergic neurons. Intracellular methamphetamine (1 μm, n = 5) but not amphetamine (1 μm, n = 4) attenuates the influence of dopamine on the spontaneous firing activity of dopaminergic neurons. The increase in the firing was blocked by DAT blocker GBR12935 (GBR; 1 μm). D, bar graph compares the firing activity of the neurons after extracellular application of dopamine. The data are normalized to the firing rate when physiological-like internal solution, amphetamine, or methamphetamine was dialyzed into the neurons (one-way ANOVA followed by Tukey's post hoc test; *, p < 0.05).
FIGURE 4.
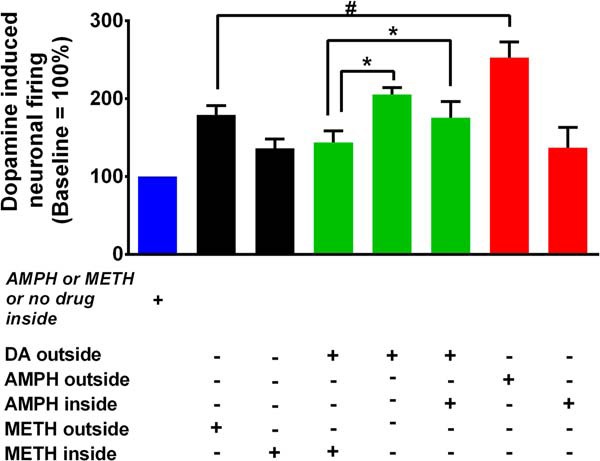
Comparison of the firing activity of dopaminergic neurons across all treatment groups. The bar graph compares dopamine, amphetamine, or methamphetamine regulation of the firing activity of dopaminergic neurons when the drug is applied extra- or intracellularly (#, *; p < 0.05).
Methamphetamine Induces Less DAT-mediated Inward Current Than Amphetamine
The significant contribution of DAT-mediated inward current on the excitability of dopamine neurons is widely supported in the literature (30, 42). To determine the underlying mechanism of the disparity between the effect amphetamine and methamphetamine on the excitability of dopaminergic neurons, we examined their effect on isolated DAT-mediated inward currents in mammalian expression systems stably expressing YFP-DAT.
For these experiments we focused on the substrate- (dopamine, amphetamine, and methamphetamine) regulation of DAT-mediated inward currents at −100 and −80 mV. The DAT-mediated currents were measured when the cells were voltage-clamped at −40 mV and then stepped to −80 mV or −100 mV for 500 ms. Consistent with the previous reports, bath application of dopamine (10 μm; n = 6), amphetamine (10 μm; n = 6), or methamphetamine (10 μm; n = 6) induced GBR12935-sensitive inward currents at −80 and −100 mV (10 μm). Extracellular amphetamine consistently stimulated the greatest GBR12935-senstive inward current, whereas methamphetamine produced an intermediate amplitude of GBR12935-senstive inward currents (Fig. 5; dopamine, −25.9 ± 2.6 pA at −100 mV and −21.1 ± 5.8 pA at −80mV; methamphetamine, −32.6 ± 4.2 pA at −100mV and −23 ± 3.1 pA at −80mV; amphetamine, −40 ± 5.6 pA at −100 mV and −32.5 ± 5.3 pA at −80mV). At both voltage steps, amphetamine induced a significantly greater DAT-mediated inward current than methamphetamine (p < 0.05, one-way ANOVA followed by Tukey's post hoc test; Fig. 5B). These data indicate a positive correlation between the amplitude of substrate-induced DAT-mediated inward current and the substrate-induced increase in the firing rate of dopamine neurons.
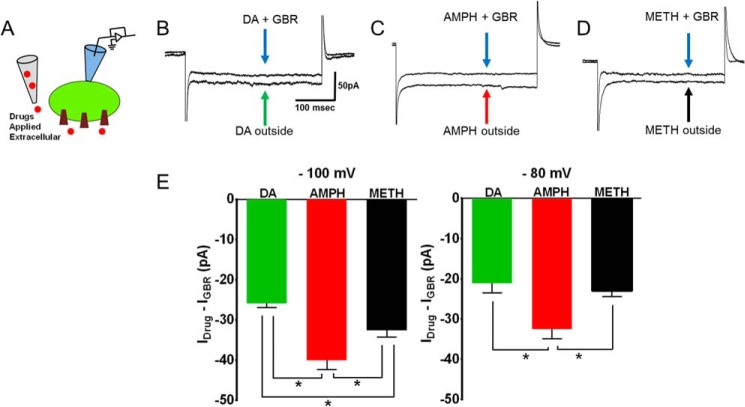
FIGURE 5.
Methamphetamine elicits smaller DAT-mediated inward current compared with amphetamine. A, schematic depicts the experimental configuration. The DAT-mediated inward current was measured when drugs are applied extracellularly. The substrate-induced, DAT-mediated current is defined as the GBR12935-subtracted current. The DAT-mediated, amphetamine- or methamphetamine-induced current was measured when the cells were voltage-clamped in whole-cell configuration. B–D, representative traces at −100 mV. E, the bar graph shows the average DAT-mediated current after bath application of dopamine (10 μm; n = 6), methamphetamine (10 μm; n = 6), and amphetamine (10 μm; n = 6) at −100 mV and −80 mV. The amphetamine-induced, DAT-mediated inward currents were significantly larger than methamphetamine-induced or dopamine-induced inward currents (*, p < 0.05, one-way ANOVA followed by Tukey's post hoc test).
Intracellular Amphetamine or Methamphetamine Produced Similar DAT-mediated Inward Current
As shown in Fig. 2, intracellular amphetamine or methamphetamine similarly but modestly affected the excitability of dopaminergic neurons. Therefore, due to the correlation observed with depolarization and isolate DAT-mediated inward currents, we examined whether a similar relationship exists between the DAT-mediated inward current and the excitability of dopaminergic neurons when these compounds are dialyzed into the cytoplasm, interacting with the intracellular face of the transporter. Dialysis of methamphetamine (10 μm; n = 5) or amphetamine (10 μm; n = 5) via the whole cell pipette induced a similar but smaller DAT-mediated inward current that was not significantly different from one another (p > 0.05, t test; Fig. 6, A and B; methamphetamine, −18.4 ± 4.4 pA at −100 mV and −14 ± 8.2 pA at −80 mV; amphetamine, −18.2 ± 6.5 pA at −100 mV and −11.5 ±.7 pA at −80 mV).
FIGURE 6.
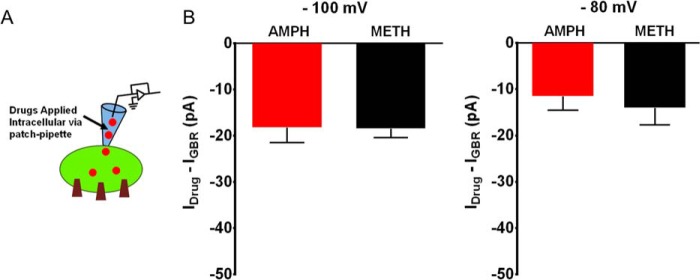
Intracellular amphetamine and methamphetamine elicit similar DAT-mediated current. A, the schematic depicts the experimental configuration. The CHO-YFP-DAT cells were patch-clamped in whole-cell configuration. Amphetamine (10 μm; n = 5) or methamphetamine (10 μm; n = 5) was dialyzed into the cell via the patch pipette. B, the bar graph compares the DAT-mediated inward current at −100 mV and −80 mV after intracellular delivery of the drugs. There is no difference between amphetamine- or methamphetamine-induced DAT-mediated inward currents at the examined membrane potentials.
Intracellular Methamphetamine, but Not Amphetamine, Prevents the Dopamine-evoked Inward Current
Although intracellular amphetamine and methamphetamine similarly affected the excitability of dopamine neurons and DAT-mediated inward current (Figs. 2B and 6B), this similarity did not persist in response to extracellular dopamine when these drugs exist intracellularly (Fig. 7A). Therefore, we asked whether stimulation of forward transport by bath application of dopamine affects the DAT-mediated inward current when methamphetamine or amphetamine is present intracellularly (Fig. 1A). The DAT-mediated inward current in response to extracellular dopamine application alone or in the presence of intracellular amphetamine was similar, whereas intracellular methamphetamine significantly reduced the GBR12935-sensitive, dopamine-evoked current by comparison (p < 0.05, one-way ANOVA followed by Tukey's post hoc test) (Fig. 7, B--E). The dopamine-induced inward current in the presence of methamphetamine inside the cell at −100 and −80 mV were −18 ± 4.2 pA and −11.3 ± 6.2 pA respectively, whereas in the presence of intracellular amphetamine the inward currents at −100 and −80 mV were 26 ± 2 pA and 20 ± 2 pA, respectively. These findings suggest that amphetamine versus methamphetamine uniquely regulates the DAT activity.
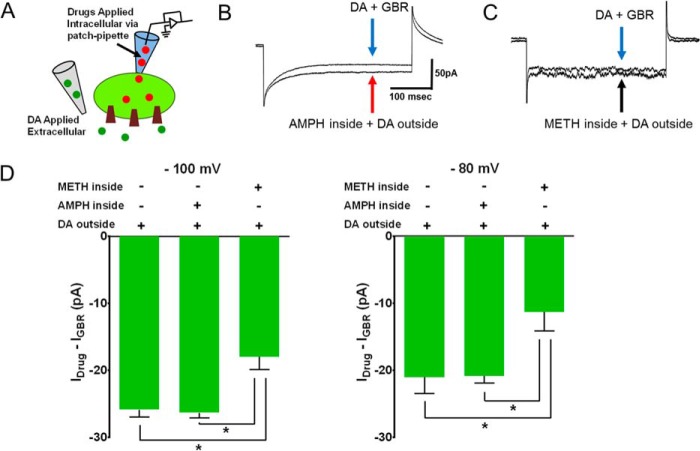
FIGURE 7.
Intracellular methamphetamine prevents the dopamine-induced inward current. A, schematic depicts the experimental configuration. The dopamine-induced inward current was measured in CHO-YFP-DAT cells when internal solution or internal solution containing amphetamine (10 μm; n = 4), or methamphetamine (10 μm; n = 5) was dialyzed into the cell. Dopamine (10 μm) was bath-applied. The experiments were performed as described in Fig. 6. B and C, representative traces at −100mV. D, the bar graph compares the dopamine-induced inward current at −100 mV and −80 mV after intracellular delivery of methamphetamine (10 μm; n = 5) or amphetamine (10 μm; n = 4). Intracellular methamphetamine, but not amphetamine, decreases the dopamine-induced, DAT-mediated inward current. *, p < 0.05, one-way ANOVA followed by Tukey's post hoc test.
Amphetamine- and Methamphetamine-induced DAT-mediated Inward Currents Are Equally Na+-dependent, Whereas Amphetamine-induced DAT-mediated Inward Current Is Preferentially Cl−-dependent
DAT uses the electrochemical gradients of the cell to drive transport (13, 30, 42, 46). The DAT-mediated current is coupled to the translocation of Na+ and Cl− ions (30–32, 42, 46). These results and the previous reports support the notion that amphetamine and methamphetamine differentially regulate the activity of the transporter. Therefore, we investigated whether the substrate-specific ionic transport is the underlying mechanism. Na+ or Cl− ions were iso-osmotically replaced by inclusion of choline-Cl or NaNO3 for NaCl in the external solution, respectively. The internal solution was kept unchanged. Compared with the inward current in the presence of normal Na+ ion concentration, removal of Na+ ion significantly decreased DAT-mediated inward current in response to amphetamine by 87.5% (n = 5) and methamphetamine by 87.7% (n = 5), (p < 0.05; Fig. 8, B, D, and F). In contrast, removal of Cl− ion preferentially reduced amphetamine-induced DAT-mediated inward current by 70.16 ± 2% relative to the 42.44 ± 2.4% reduction of the methamphetamine response (Fig. 8, A, C, and E; p < 0.05, n = 2–3). These results suggest that amphetamine-induced DAT-mediated inward current is preferentially dependent on external Cl− ion. The side by side comparison of amphetamine- and methamphetamine-induced DAT-mediated inward current at different Cl− concentrations is depicted in Fig. 9.
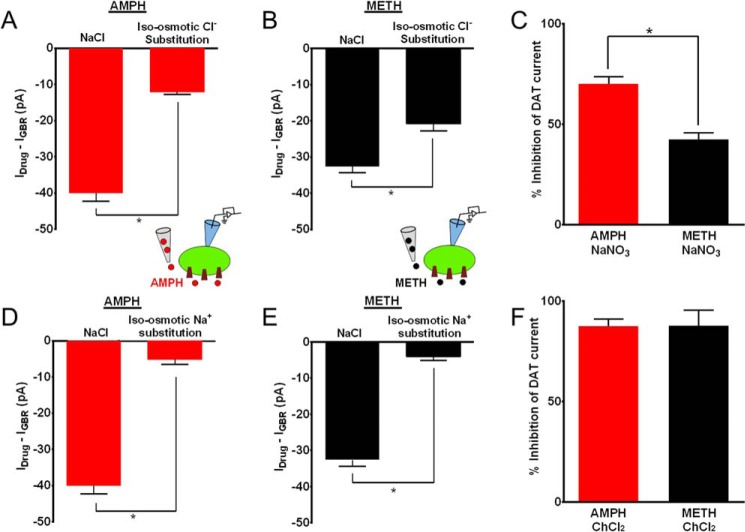
FIGURE 8.
Removal of extracellular Cl− preferentially blocks amphetamine-induced DAT-mediated current relative to methamphetamine. A and B, bar graphs show the amphetamine- or methamphetamine-induced DAT current at −100 mV after removal of Cl− ions in the external solution. C, substitution of Cl− ions in the external solution blocks 70.16 ± 2.0% of amphetamine-induced DAT current but only 42.44 ± 2.4% of methamphetamine-induced current. D and E, bar graphs show amphetamine- or methamphetamine-induced inward current at −100 mV upon removal of Na+ ions in the external solution. F, substitution of Na+ ions in the external solution equally blocks the amphetamine- and methamphetamine-induced DAT currents (*; p < 0.05).
FIGURE 9.
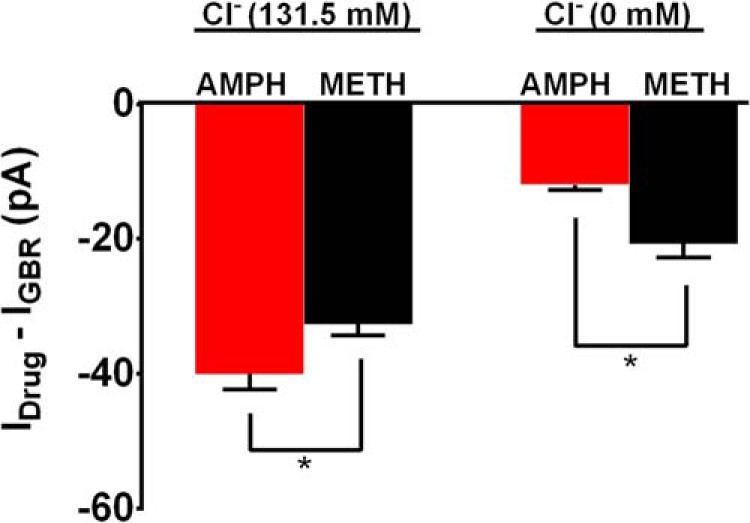
Side by side comparison of the effects of amphetamine versus methamphetamine-induced DAT-mediated inward currents. The bar graph shows a side by side comparison of the effects of amphetamine versus methamphetamine-induced DAT-mediated inward currents at (−100 mV holding potential) at two different concentrations of extracellular chloride ions (Cl−). We observed that at 0 mm Cl− ion concentration the inward current induced by amphetamine was significantly blocked and to a higher extent compared with methamphetamine, whereas at 131.5 mm extracellular Cl− ion concentration amphetamine produced significantly higher inward current compared with methamphetamine.
Amphetamine- and Methamphetamine-induced Cell Surface Redistribution of DAT Are Not Different
We investigated the possibility that the higher DAT-mediated inward current induced by amphetamine compared with methamphetamine might be due to methamphetamine causing a much faster internalization of the transporter. We, therefore, measured surface DAT levels after 10 min of amphetamine or methamphetamine exposure. Using biotinylation followed by Western blot analysis, we observed that amphetamine- and methamphetamine-induced cell surface redistributions of DAT are not significantly different from each other (Fig 10, A and B). These findings were confirmed via live-cell TIRF microscopy showing that both psychostimulants similarly affected the cell surface redistribution of the transporter within the time frame (10 min) studied (Fig 10, C, D, and E).
FIGURE 10.
Amphetamine-induced reduction in surface DAT levels was not different from methamphetamine. FLAG-DAT HEK cells were treated with amphetamine (10 μm) or methamphetamine (10 μm) for 10 min followed by treatment with sulfo-NHS-SS-biotin to detected surface DAT. A, representative immunoblots showing surface DAT, total DAT, and the loading control, β-actin. B, the bar graph shows after amphetamine treatment there was a significant reduction in the surface DAT compared with the control, whereas after 10 min of methamphetamine treatment surface DAT density was not significantly (NS) different from control or amphetamine. C, plot of membrane YFP-DAT intensity (arbitrary fluorescent units (AFU)) after exposure to amphetamine (10 μm) or methamphetamine (10 μm) normalized to the average intensity for each cell during the 15 s before solution change. The normalized response to vehicle (external solution) has been accounted for in the normalized values, correcting for fluorophore bleaching. D, the bar graph shows average normalized intensity after 10min of drug application. Both psychostimulants similarly and significantly internalized the dopamine transporter (***, p < 0.001; *, p < 0.05; one-way ANOVA followed by Tukey's post hoc test). E, representative images showing amphetamine- and methamphetamine-induced reduction in the surface YFP-DAT intensity.
DISCUSSION
Long-standing evidence supports the idea that amphetamine and methamphetamine differentially affect dopamine neurotransmission in the brain, but the underlying mechanism of this differential effect is less understood. In this study we examined the effect of these two compounds on the firing activity of dopaminergic neurons and DAT-mediated current. In this study we examined the effect of these two compounds on the firing activity of dopaminergic neurons and on the DAT-mediated current. We found that although all three DAT substrates increase spontaneous firing of dopaminergic neurons and DAT-mediated inward current, methamphetamine is the least effective. In addition, unlike extracellular application of these drugs, intracellular amphetamine and methamphetamine are less effective in modulating the spontaneous firing of dopaminergic neurons. Importantly, intracellular methamphetamine prevents the dopamine-induced enhancement of neuronal firing and inward current. Consistent with the literature, the isosmotic Na+ substitution in the external solution eliminated DAT-mediated inward current elicited by these substrates. However, to our surprise the substitution of Cl− ions in the external solution only partially inhibited the methamphetamine-induced DAT current.
Dopamine, amphetamine, and methamphetamine are all DAT substrates. They interact and are subsequently transported into the DAT+ neurons in a Na+/Cl−-dependent mechanism. The transport process generates current and increases the excitability of dopaminergic neurons that can be measured via classical electrophysiology experiment. It is not known whether these compounds influence DAT activity once they have entered into the neuron or whether the presence of intracellular drug can influence the response to the extracellular dopamine. Determining DAT activity under these conditions is important because these drugs compete with inward transport of dopamine; they enter into the neuron, whereas dopamine remains outside. Therefore, we designed three complementary experimental configurations to determine the effect of these compounds on the excitability of dopaminergic neurons and DAT-mediated inward current when amphetamine or methamphetamine exist 1) only outside the neuron, 2) only inside the neurons, or 3) when the drugs were directly dialyzed into the cell and the native substrate, dopamine, was applied extracellularly. These experimental configurations provide a unique approach to mechanistically determine why these compounds differentially affect DAT activity, have different central effects, and abuse potential.
DAT substrates differentially alter the DAT-mediate inward current and neuronal firing. As shown in Fig. 1, we found extracellular amphetamine is the most effective compound in increasing the firing rate of dopaminergic neurons. This was paralleled by a consistently larger amphetamine-induced DAT-mediated inward current. The literature supports a direct correlation between the DAT-mediated substrate-induced inward current and the excitability of dopaminergic neurons, albeit with a less understood mechanism (16, 42, 47). To address the mechanism, we directly dialyzed each drug into the neuron bypassing the binding and transport step. Under this condition neither the excitability of dopaminergic neurons nor DAT-mediated inward current was different when either amphetamine or methamphetamine was directly dialyzed into the neurons or DAT cells. This suggests that the differential response in the excitability of dopaminergic neurons might be due to substrate-specific extracellular binding and subsequent transport into the neurons.
It is possible that the underlying mechanism for amphetamine-induced increase in the excitability of dopaminergic neurons and DAT-mediated inward current is due to specific regulation of forward transport step. We tested this hypothesis by bath application of dopamine, whereas amphetamine or methamphetamine exists inside the neuron or DAT cell. Unlike intracellular methamphetamine, the presence of amphetamine inside the neuron did not decrease the dopamine-induced inward current or its enhancement of neuronal excitability. This finding is consistent with an interpretation that intracellular amphetamine facilitates a conformational state of the transporter, which supports the interaction of dopamine with DAT, leading to dopamine-mediated inward current and neuronal excitability. This is consistent with a recent report demonstrating that amphetamine induces a persistent inward current by increasing the Na+ conductance of the transporter via a molecular stent mechanism (46).
As shown in Figs. 2 and 6, surprisingly, when we dialyzed methamphetamine inside the neuron, it significantly decreased both dopamine-induced DAT-mediated inward current and neuronal excitability. Although the current data do not delineate the exact underlying mechanism, it is possible that intracellular methamphetamine induces a state of the transporter that is less sensitive to the effect of extracellular dopamine on the activity of the transporter. Studies by Kahlig et al. (35) suggest another possible mechanism. The authors eloquently have shown that extracellular dopamine decreases the channel-like mode of DAT activity. Therefore, the presence of intracellular methamphetamine may further accentuate the effect of extracellular dopamine (35, 47).
We considered the possibility that the differences in the magnitude of inward current induced by amphetamine versus methamphetamine is due to their differential influences on DAT trafficking. However, biotinylation and live cell TIRF microscopy experiments suggest that amphetamine-induced reduction of TIRF surface DAT levels was not different from methamphetamine-induced cell surface redistribution of DAT. The live-cell TIRF microscopy experiments support this conclusion showing that amphetamine and methamphetamine induce a significant and similar level of cell surface redistribution of DAT within the time frame of these experiments. Thus substrate-induced DAT internalization is not responsible for the measured differential substrates-induced DAT-mediated inward current.
It has been shown that Akt, through a Ca2+/calmodulin-dependent kinase II, can regulate the activity of the transporter after exposure to psychostimulants (48–50). It is possible that these two compounds differentially affect the activity of Akt in a manner that does not influence the trafficking of the transporter per se, but it facilitates a conformational state of the transporter to regulate the current. This possibility will be the focus of our future studies.
In an elegant review, Zhu and Reith (51) proposed an intriguing model regarding the substrate regulation of DAT oligomerization. DAT at the cell surface is distributed between oligomers and monomers, where DAT monomers are internalized (Zhu and Reith) after amphetamine (51). Consistent with this idea, data Fleckenstein and co-workers (52) suggest the possibility that methamphetamine induces oligomerization of the transporter, whereas, a report by Reith group has shown that amphetamine promotes the formation of DAT monomers (55). Taken together, it is possible that the methamphetamine-induced oligomerization of DAT differentially affects the DAT-mediated ionic conductance.
The DAT-mediated inward current is dependent upon transporting of Na+ and Cl− ions. This is supported by recently resolved crystal structure of the Drosophila melanogaster dopamine transporter showing separate binding sites for Na+ and Cl− ions (53). Consistent with the identification of a substrate binding pocket on the transporter, it is well established that the DAT substrates amphetamine and methamphetamine bind to the transporter and mediate GBR12935-sensitive inward current (53). In this study we found the DAT substrates, amphetamine, and methamphetamine elicit different amounts of inward current and induce profoundly different magnitudes of neuronal firing. Therefore, we examine the possibility that these two compounds may be differentially coupled to the co-transport of Na+ and Cl− ions. To address this hypothesis, while keeping the composition of the internal solution intact, we isosmotically substituted either Na+ or Cl− ions in the external solution. Although the substitution of Na+ ions in the external solution equally affected amphetamine- and methamphetamine-induced DAT currents, the substitution of Cl− ions in the external solution more effectively blocks the amphetamine-induced DAT current. The Na+ and Cl− ionic dependence of amphetamine-induced current is consistent with the existing data in the literature that removal of either Na+, Cl−, or both ions from the external solution decreases DAT activity (13). Similarly, Erreger et al. (13) have elegantly shown that amphetamine-mediated inward current significantly decreases after the substitution of Na+ or Cl− ions in the external milieu. Furthermore, Ingram et al. (42) demonstrated that Cl− conductance through the transporter is important in amphetamine regulation of excitability of dopaminergic neurons as well as amphetamine-mediated inward current through DAT. These reports explain the Na+ and Cl− ionic dependence of amphetamine-mediated current, but they do not explain why Cl− ionic substitution partially inhibits the methamphetamine-induced DAT current. Future studies should consider determining the mechanism responsible for the decreased sensitivity of DAT to methamphetamine in the absence of Cl− ion in the external solution. Nonetheless, these results provide critical information to further efforts toward designing effective therapeutic approaches when dopamine neurotransmission is dysregulated.
Acknowledgments
We thank Dr. Satya Kalra, Dr. Rahul Khanna, and Niousha Ahmari or critical reviews of the manuscript.
This work was supported, in whole or in part, by National Institutes of Health Grants DA026947 and NS071122.
- DA
- dopamine
- DAT
- dopamine transporter
- TH
- tyrosine hydroxylase
- TRITC
- tetramethylrhodamine isothiocyanate
- ROIs
- regions of interest
- AMPH
- amphetamine
- METH
- methamphetamine
- ANOVA
- analysis of variance
- TIRF
- total internal reflection fluorescence.
REFERENCES
- 1. Iversen S. D., Iversen L. L. (2007) Dopamine: 50 years in perspective. Trends Neurosci. 30, 188–193 [DOI] [PubMed] [Google Scholar]
- 2. Grace A. A., Floresco S. B., Goto Y., Lodge D. J. (2007) Regulation of firing of dopaminergic neurons and control of goal-directed behaviors. Trends Neurosci. 30, 220–227 [DOI] [PubMed] [Google Scholar]
- 3. Bernheimer H., Birkmayer W., Hornykiewicz O., Jellinger K., Seitelberger F. (1973) Brain dopamine and the syndromes of Parkinson and Huntington. Clinical, morphological, and neurochemical correlations. J. Neurol. Sci. 20, 415–455 [DOI] [PubMed] [Google Scholar]
- 4. Hornykiewicz O., Kish S. J., Becker L. E., Farley I., Shannak K. (1986) Brain neurotransmitters in dystonia musculorum deformans. N. Engl. J. Med. 315, 347–353 [DOI] [PubMed] [Google Scholar]
- 5. Dawson T. M., Dawson V. L. (2002) Neuroprotective and neurorestorative strategies for Parkinson's disease. Nat. Neurosci. 5, 1058–1061 [DOI] [PubMed] [Google Scholar]
- 6. Wise C. D., Stein L. (1973) Dopamine-β-hydroxylase deficits in the brains of schizophrenic patients. Science 181, 344–347 [DOI] [PubMed] [Google Scholar]
- 7. Snyder S. H., Meyerhoff J. L. (1973) How amphetamine acts in minimal brain dysfunction. Ann.Ann. N.Y. Acad. Sci. 205, 310–320 [DOI] [PubMed] [Google Scholar]
- 8. Wall S. C., Gu H., Rudnick G. (1995) Biogenic amine flux mediated by cloned transporters stably expressed in cultured cell lines: amphetamine specificity for inhibition and efflux. Mol. Pharmacol. 47, 544–550 [PubMed] [Google Scholar]
- 9. Giros B., Jaber M., Jones S. R., Wightman R. M., Caron M. G. (1996) Hyperlocomotion and indifference to cocaine and amphetamine in mice lacking the dopamine transporter. Nature 379, 606–612 [DOI] [PubMed] [Google Scholar]
- 10. Salahpour A., Ramsey A. J., Medvedev I. O., Kile B., Sotnikova T. D., Holmstrand E., Ghisi V., Nicholls P. J., Wong L., Murphy K., Sesack S. R., Wightman R. M., Gainetdinov R. R., Caron M. G. (2008) Increased amphetamine-induced hyperactivity and reward in mice overexpressing the dopamine transporter. Proc. Natl. Acad. Sci. U.S.A. 105, 4405–4410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Keefe K. A., Zigmond M. J., Abercrombie E. D. (1992) Extracellular dopamine in striatum: influence of nerve impulse activity in medial forebrain bundle and local glutamatergic input. Neuroscience 47, 325–332 [DOI] [PubMed] [Google Scholar]
- 12. (2006) National Institute on Drug Abuse in NIDA Research Report, NIDA, National Institutes of Health, Bethesda, MD [Google Scholar]
- 13. Erreger K., Grewer C., Javitch J. A., Galli A. (2008) Currents in response to rapid concentration jumps of amphetamine uncover novel aspects of human dopamine transporter function. J. Neurosci. 28, 976–989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Keefe K. A., Zigmond M. J., Abercrombie E. D. (1993) In vivo regulation of extracellular dopamine in the neostriatum: influence of impulse activity and local excitatory amino acids. J. Neural Transm. Gen. Sect. 91, 223–240 [DOI] [PubMed] [Google Scholar]
- 15. Nieoullon A., Cheramy A., Glowinski J. (1977) Release of dopamine in vivo from cat substantia nigra. Nature 266, 375–377 [DOI] [PubMed] [Google Scholar]
- 16. Leviel V. (2011) Dopamine release mediated by the dopamine transporter, facts and consequences. J. Neurochem. 118, 475–489 [DOI] [PubMed] [Google Scholar]
- 17. Zhou Z., Misler S. (1995) Action potential-induced quantal secretion of catecholamines from rat adrenal chromaffin cells. J. Biol. Chem. 270, 3498–3505 [PubMed] [Google Scholar]
- 18. Bertorello A. M., Hopfield J. F., Aperia A., Greengard P. (1990) Inhibition by dopamine of (Na+ + K+)ATPase activity in neostriatal neurons through D1 and D2 dopamine receptor synergism. Nature 347, 386–388 [DOI] [PubMed] [Google Scholar]
- 19. Beckstead M. J., Grandy D. K., Wickman K., Williams J. T. (2004) Vesicular dopamine release elicits an inhibitory postsynaptic current in midbrain dopamine neurons. Neuron 42, 939–946 [DOI] [PubMed] [Google Scholar]
- 20. Sara Y., Virmani T., Deák F., Liu X., Kavalali E. T. (2005) An isolated pool of vesicles recycles at rest and drives spontaneous neurotransmission. Neuron 45, 563–573 [DOI] [PubMed] [Google Scholar]
- 21. Rizzoli S. O., Betz W. J. (2005) Synaptic vesicle pools. Nat. Rev. Neurosci. 6, 57–69 [DOI] [PubMed] [Google Scholar]
- 22. Fischer J. F., Cho A. K. (1976) Properties of dopamine efflux from rat striatal tissue caused by amphetamine and p-hydroxyamphetamine. Proc. West. Pharmacol. Soc. 19, 179–182 [PubMed] [Google Scholar]
- 23. Eshleman A. J., Henningsen R. A., Neve K. A., Janowsky A. (1994) Release of dopamine via the human transporter. Mol. Pharmacol. 45, 312–316 [PubMed] [Google Scholar]
- 24. Sitte H. H., Huck S., Reither H., Boehm S., Singer E. A., Pifl C. (1998) Carrier-mediated release, transport rates, and charge transfer induced by amphetamine, tyramine, and dopamine in mammalian cells transfected with the human dopamine transporter. J. Neurochem. 71, 1289–1297 [DOI] [PubMed] [Google Scholar]
- 25. Jones S. R., Gainetdinov R. R., Jaber M., Giros B., Wightman R. M., Caron M. G. (1998) Profound neuronal plasticity in response to inactivation of the dopamine transporter. Proc. Natl. Acad. Sci. U.S.A. 95, 4029–4034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Jones S. R., Gainetdinov R. R., Wightman R. M., Caron M. G. (1998) Mechanisms of amphetamine action revealed in mice lacking the dopamine transporter. J. Neurosci. 18, 1979–1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Obianwu H. O., Stitzel R., Lundborg P. (1968) Subcellular distribution of [3H]amphetamine and [3H]guanethidine and their interaction with adrenergic neurons. J. Pharm. Pharmacol. 20, 585–594 [DOI] [PubMed] [Google Scholar]
- 28. Wong D. T., van Frank R. M., Horng J. S., Fuller R. W. (1972) Accumulation of amphetamine and p-chloroamphetamine into synaptosomes of rat brain. J. Pharm. Pharmacol. 24, 171–173 [DOI] [PubMed] [Google Scholar]
- 29. Goodwin J. S., Larson G. A., Swant J., Sen N., Javitch J. A., Zahniser N. R., De Felice L. J., Khoshbouei H. (2009) Amphetamine and methamphetamine differentially affect dopamine transporters in vitro and in vivo. J. Biol. Chem. 284, 2978–2989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sonders M. S., Zhu S. J., Zahniser N. R., Kavanaugh M. P., Amara S. G. (1997) Multiple ionic conductances of the human dopamine transporter: the actions of dopamine and psychostimulants. J. Neurosci. 17, 960–974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Krueger B. K. (1990) Kinetics and block of dopamine uptake in synaptosomes from rat caudate nucleus. J. Neurochem. 55, 260–267 [DOI] [PubMed] [Google Scholar]
- 32. McElvain J. S., Schenk J. O. (1992) A multisubstrate mechanism of striatal dopamine uptake and its inhibition by cocaine. Biochem. Pharmacol. 43, 2189–2199 [DOI] [PubMed] [Google Scholar]
- 33. Gu H., Wall S. C., Rudnick G. (1994) Stable expression of biogenic amine transporters reveals differences in inhibitor sensitivity, kinetics, and ion dependence. J. Biol. Chem. 269, 7124–7130 [PubMed] [Google Scholar]
- 34. Fog J. U., Khoshbouei H., Holy M., Owens W. A., Vaegter C. B., Sen N., Nikandrova Y., Bowton E., McMahon D. G., Colbran R. J., Daws L. C., Sitte H. H., Javitch J. A., Galli A., Gether U. (2006) Calmodulin kinase II interacts with the dopamine transporter C terminus to regulate amphetamine-induced reverse transport. Neuron 51, 417–429 [DOI] [PubMed] [Google Scholar]
- 35. Kahlig K. M., Binda F., Khoshbouei H., Blakely R. D., McMahon D. G., Javitch J. A., Galli A. (2005) Amphetamine induces dopamine efflux through a dopamine transporter channel. Proc. Natl. Acad. Sci. U.S.A. 102, 3495–3500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Khoshbouei H., Wang H., Lechleiter J. D., Javitch J. A., Galli A. (2003) Amphetamine-induced dopamine efflux. A voltage-sensitive and intracellular Na+-dependent mechanism. J. Biol. Chem. 278, 12070–12077 [DOI] [PubMed] [Google Scholar]
- 37. Kahlig K. M., Galli A. (2003) Regulation of dopamine transporter function and plasma membrane expression by dopamine, amphetamine, and cocaine. Eur. J. Pharmacol. 479, 153–158 [DOI] [PubMed] [Google Scholar]
- 38. Hastrup H., Karlin A., Javitch J. A. (2001) Symmetrical dimer of the human dopamine transporter revealed by cross-linking Cys-306 at the extracellular end of the sixth transmembrane segment. Proc. Natl. Acad. Sci. U.S.A. 98, 10055–10060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Hastrup H., Sen N., Javitch J. A. (2003) The human dopamine transporter forms a tetramer in the plasma membrane: cross-linking of a cysteine in the fourth transmembrane segment is sensitive to cocaine analogs. J. Biol. Chem. 278, 45045–45048 [DOI] [PubMed] [Google Scholar]
- 40. Swant J., Goodwin J. S., North A., Ali A. A., Gamble-George J., Chirwa S., Khoshbouei H. (2011) α-Synuclein stimulates a dopamine transporter-dependent chloride current and modulates the activity of the transporter. J. Biol. Chem. 286, 43933–43943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Bukauskas F. F., Kempf C., Weingart R. (1992) Cytoplasmic bridges and gap junctions in an insect cell line (Aedes albopictus). Exp. Physiol. 77, 903–911 [DOI] [PubMed] [Google Scholar]
- 42. Ingram S. L., Prasad B. M., Amara S. G. (2002) Dopamine transporter-mediated conductances increase excitability of midbrain dopamine neurons. Nat. Neurosci. 5, 971–978 [DOI] [PubMed] [Google Scholar]
- 43. Paladini C. A., Robinson S., Morikawa H., Williams J. T., Palmiter R. D. (2003) Dopamine controls the firing pattern of dopamine neurons via a network feedback mechanism. Proc. Natl. Acad. Sci. U.S.A. 100, 2866–2871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Fon E. A., Pothos E. N., Sun B. C., Killeen N., Sulzer D., Edwards R. H. (1997) Vesicular transport regulates monoamine storage and release but is not essential for amphetamine action. Neuron 19, 1271–1283 [DOI] [PubMed] [Google Scholar]
- 45. Pierce R. C., Kalivas P. W. (1997) Repeated cocaine modifies the mechanism by which amphetamine releases dopamine. J. Neurosci. 17, 3254–3261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Rodriguez-Menchaca A. A., Solis E., Jr., Cameron K., De Felice L. J. (2012) S(+)amphetamine induces a persistent leak in the human dopamine transporter: molecular stent hypothesis. Br. J. Pharmacol. 165, 2749–2757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Carvelli L., McDonald P. W., Blakely R. D., Defelice L. J. (2004) Dopamine transporters depolarize neurons by a channel mechanism. Proc. Natl. Acad. Sci. U.S.A. 101, 16046–16051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Wei Y., Williams J. M., Dipace C., Sung U., Javitch J. A., Galli A., Saunders C. (2007) Dopamine transporter activity mediates amphetamine-induced inhibition of Akt through a Ca2+/calmodulin-dependent kinase II-dependent mechanism. Mol. Pharmacol. 71, 835–842 [DOI] [PubMed] [Google Scholar]
- 49. Garcia B. G., Wei Y., Moron J. A., Lin R. Z., Javitch J. A., Galli A. (2005) Akt is essential for insulin modulation of amphetamine-induced human dopamine transporter cell-surface redistribution. Mol. Pharmacol. 68, 102–109 [DOI] [PubMed] [Google Scholar]
- 50. Rau T. F., Kothiwal A., Zhang L., Ulatowski S., Jacobson S., Brooks D. M., Cardozo-Pelaez F., Chopp M., Poulsen D. J. (2011) Low dose methamphetamine mediates neuroprotection through a PI3K-AKT pathway. Neuropharmacology 61, 677–686 [DOI] [PubMed] [Google Scholar]
- 51. Zhu J., Reith M. E. (2008) Role of the dopamine transporter in the action of psychostimulants, nicotine, and other drugs of abuse. CNS Neurol. Disord. Drug Targets 7, 393–409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Baucum A. J., 2nd, Rau K. S., Riddle E. L., Hanson G. R., Fleckenstein A. E. (2004) Methamphetamine increases dopamine transporter higher molecular weight complex formation via a dopamine- and hyperthermia-associated mechanism. J. Neurosci. 24, 3436–3443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Penmatsa A., Wang K. H., Gouaux E. (2013) X-ray structure of dopamine transporter elucidates antidepressant mechanism. Nature 503, 85–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. McCutcheon J. E., Conrad K. L., Carr S. B., Ford K. A., McGehee D. S., Marinelli M. (2012) Dopamine neurons in the ventral tegmental area fire faster in adolescent rats than in adults. J. Neurophysiol. 108, 1620–1630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Chen N., Reith M. E. (2008) Substrates dissociate dopamine transporter oligomers. J. Neurochem. 105, 910–920 [DOI] [PMC free article] [PubMed] [Google Scholar]