Background: It is unknown whether SENP1 controls adipocyte differentiation.
Results: SENP1 de-SUMOylates Sharp-1 and promotes PPARγ expression and adipogenesis.
Conclusion: SENP1 regulates adipocyte differentiation.
Significance: SENP1 is a novel regulator in adipocyte differentiation.
Keywords: Adipogenesis, Cell Differentiation, Gene Transcription, Peroxisome Proliferator-activated Receptor (PPAR), SUMOylation
Abstract
Adipocyte differentiation is regulated by a transcriptional cascade that mainly includes CCAAT/enhancer-binding protein family members and the nuclear receptor peroxisome proliferator-activated receptor γ (PPARγ). Here we show the defects in adipocyte differentiation as well as PPARγ expression in Senp1−/− mouse embryonic fibroblast cells induced by adipogenic stimuli. We further determine that SENP1 is a specific de-SUMOylation protease for Sharp-1, a repressor for PPARγ transcription and adipogenesis. SENP1 enhances adipogenesis through de-SUMOylation of Sharp-1, which then releases Sharp-1 repression of PPARγ expression and adipocyte differentiation. These results reveal SENP1 as a novel regulator in adipogenesis.
Introduction
Adipocyte differentiation is regulated by a transcriptional cascade that mainly includes CCAAT/enhancer-binding protein (C/EBP)2 family members (i.e. C/EBPα, C/EBPβ, and C/EBPδ) and the nuclear receptor peroxisome proliferator-activated receptor γ (PPARγ). This transcriptional cascade directs the extensive programming of gene expression required to convert preadipocytes to mature adipocytes (1–3). C/EBPs are early adipogenic transcription factors that are induced within hours of initiation of adipogenesis. C/EBPβ directly binds to the PPARγ2 promoter and activates PPARγ2 expression (4). C/EBPβ and C/EBPδ also activate the expression of another principal adipogenic transcription factor, C/EBPα (5). PPARγ mainly coordinates with C/EBPα in a positive feedback loop to promote the terminal differentiation of adipocytes (6).
It is well documented that the activity of C/EBPs and PPARγ is tightly controlled by distinct regulators in adipocyte differentiation. These regulators include transcriptional coactivators and corepressors, Wnt and FGF signaling, as well as protein posttranslational modification enzymes (3). Sharp-1, a basic helix-loop-helix transcription factor, is one of the transcriptional corepressors modulating C/EBPs and PPARγ activity. Sharp-1 binds to class B E-box sites with high affinity to repress the transcription of target genes and also associates with distinct corepressors, including HDAC1, SIRT1, and the histone methyltransferase G9a, to inhibit gene transcription (7). Taneja and co-workers (8) found that Sharp-1 interacts with and inhibits the transcriptional activity of both C/EBPβ and C/EBPα by retaining HDAC1 and G9a at the C/EBP regulatory sites on the C/EBPα and PPARγ2 promoters to inhibit their expression and, thus, adipogenesis, identifying Sharp-1 function as a negative regulator during adipogenesis.
SUMO (also called Sentrin) is a novel ubiquitin-like protein that can covalently modify a large number of proteins (9, 10). SUMO modification has now emerged as an important regulatory mechanism in many signaling pathways through alteration of the function of target proteins (9, 11, 12). SUMOylation is catalyzed by activating (E1), conjugating (E2), and ligating (E3) enzymes. It is reversed by a family of Sentrin/SUMO-specific proteases (SENPs) (9, 12). In mammalian cells, six SENPs are identified. These six SENPs have substrate specificity and different cellular localization and tissue distribution (9, 12). The SENPs mediating deconjugation play a crucial role in determining protein SUMOylation status (9, 13–17). Interestingly, many transcriptional regulators in adipocyte differentiation are shown as SUMOylated proteins, suggesting that SUMOylation has emerged as a novel regulation mechanism in adipogenesis (18–20).
In this study, Senp1−/− MEF cells show defects in adipocyte differentiation and PPARγ expression induced by adipogenic stimuli. A mechanism study found that SENP1 de-SUMOylates Sharp-1 and releases Sharp-1 inhibition of PPARγ expression and adipogenesis. These results reveal a role of SENP1 in control of Sharp-1 activity and adipocyte differentiation.
EXPERIMENTAL PROCEDURES
Reagents
The Sharp-1-HA, and pGL3.0-PPARγ plasmids were provided by Dr. Bing Sun (Institute Pasteur of Shanghai, Chinese Academy of Sciences, Shanghai, China) and Dr. Zhaoyuan Hou (Shanghai Jiao Tong University School of Medicine, Shanghai, China), respectively. FLAG-SUMO1, FLAG-SENP1, FLAG-SENP1mutant, RGS-SENP1, and RGS-SENP1mutant have been described previously (18–20). We used antibodies against FLAG (M2, Sigma), HA (Covance), RGS (Qiagen), SUMO1 (Cell Signaling Technology), and Sharp-1 (Santa Cruz Biotechnology).
Mutagenesis
To mutate potential SUMOylation residues from lysine to arginine in Sharp-1, the QuikChangeTM site-directed mutagenesis kit (Stratagene) was used. The primers for generating Sharp-1m (K240R)-HA were 5′-CGCGCGGCCGTCAGGCAGGAGCCACCC-3′ and 5′-GGGTGGCTCCTGCCTGACGGCCGCGCG-3′.
Cell Culture
The generation of Senp1+/+ and Senp1−/− MEF cells has been described previously (18). MEF, 293T, and 3T3-L1 cells were cultured in DMEM (Hyclone) supplemented with 10% fetal bovine serum (Invitrogen) and 1% antibiotics (penicillin/streptomycin) (Invitrogen).
Adipocyte Differentiation and Oil Red O Staining
MEF and 3T3-L1 cells were cultured in DMEM (Hyclone) containing 10% FBS (Invitrogen) and 1% antibiotics until reaching full confluence. Two days later (day 2), differentiation was induced by addition of insulin (5 μg/ml, Sigma), dexamethasone (1 μm, Sigma), isobutyl-1-methylxanthine (0.5 mm, Sigma), and rosiglitazone (1 μm, Sigma). On day 2, the medium was replaced with the same medium containing 5 μg/ml insulin and 1 μm rosiglitazone. This medium was changed every 2 days until the end of differentiation. Oil Red O (Sigma) staining and quantification were performed as described previously (8).
Transfection
Plasmids were transfected into 293T cells using LipofectamineTM 2000 (Invitrogen). MEF and 3T3-L1 cells were transfected with plasmids by electroporation with a NEPA21 system (NEPA GENE) according to the instructions of the manufacturer. Briefly, 1 × 106 MEF and 3T3-L1 cells (100 μl of Opti-MEM/10 μg of a plasmid/cuvette) were electroporated under the most optimal conditions (140 V, 10 ms, twice and 275 V, 2 ms, twice, respectively). Cells were then plated on 6-well plates (3 × 105 cells/well) and treated with differentiation mixture for analysis.
Real-time Quantitative PCR
Total RNA was isolated with a TRIzol kit (Roche). RNA was treated with DNase (Promega). Complementary DNA was synthesized using a cDNA synthesis kit (Takara) according to the instructions of the manufacturer. Fluorescence real-time RT-PCR was performed with Power SYBR Green PCR Master Mix (Applied Biosystems) using the ABI 7500 Fast system (Applied Biosystems). PCR was done in triplicate and standard deviations representing experimental errors were calculated. The pairs of PCR primers used for amplification of the target genes were as follows: SENP1, 5′-TTGGCCAGAGTGCAAATGG-3′ (forward) and 5′-TCGGCTGTTTCTTGATTTTTGTAA-3′ (reverse); PPARγ, 5′-AACTCTGGGAGATTCTCCTGTTGA-3′ (forward) and 5′-TGGTAATTTCTTGTGAAGTGCTCATA-3′ (reverse); aP2, 5′-ACACCGAGATTTCCTTCAAACTG-3′ (forward) and 5′-CCATCTAGGGTTATGATGCTCTTCA-3′ (reverse); adiponectin, 5′-GCACTGGCAAGTTCTACTGCAA-3′ (forward) and 5′-GTAGGTGAAGAGAACGGCCTTGT-3′ (reverse); LPL, 5′-TCCAGCCAGGATGCAACA-3′ (forward) and 5′-CCACGTCTCCGAGTCCTCTCT-3′ (reverse); 18 S, 5′-AGTCCCTGCCCTTTGTACACA-3′ (forward) and 5′-CGATCCGAGGGCCTCACTA-3′ (reverse).
Immunoprecipitation
Cells were collected 48 h after transfection and lysed in the presence of 10 mm N-ethylmaleimide using ice-cold radioimmune precipitation assay buffer (50 mm Tris-HCl (pH 7.4), 400 mm NaCl, 1% Triton X-100, 0.1% SDS, 1 mm PMSF, and protease inhibitors (Roche)). Cell lysis was performed for 30 min on ice, and the DNA in the sample was sheared with a 22-gauge needle. After centrifugation at 20,000 × g for 10 min at 4 °C, the supernatants were added to the appropriate antibody coupled to 20 μl of protein A/G-Sepharose beads (Amersham Biosciences). The bead suspensions were rotated for 3 h at 4 °C. The beads were then washed five times with radioimmune precipitation assay buffer. The immunoprecipitates were treated with 30 μl of 2% SDS solution containing 5% β-mercaptoethanol and analyzed by Western blotting.
Luciferase Assay
293T cells in a 24-well plate were transiently transfected with expression plasmids. The cells were incubated for 24 h before luciferase was assayed using the Dual-Luciferase reporter assay system (Promega). Luc-PPARγ was used for construction of the mouse PPARγ promoter (−628∼+32 bp), which was ligated between the KpnI and XhoI sites to plasmid pGL3.0-Basic. Renilla luciferase activity was used as an internal control.
Statistical Analysis
Error bars indicate mean ± S.D. Statistical analysis was performed using Student's t test, and p < 0.01 was considered to be statistically significant.
RESULTS
SENP1 Deficiency Decreases Adipogenesis in Senp1−/− MEF Cells
To determine whether SENP1 is involved in the regulation of adipogenesis, we first monitored the expression of SENP1 during adipogenesis. MEF cells were induced to differentiate by dexamethasone, isobutyl-1-methylxanthine, insulin, and rosiglitazone (referred to as “DMIR”). Analysis of the messenger RNA of SENP1 showed that SENP1 expression increased in the early stage (peak at day 2 after induction) and then went down to normal levels (Fig. 1A) during differentiation induced by DMIR. This result indicates that SENP1 might be involved in the regulation of the initiation event of adipogenesis. We further compared the adipogenic ability of Senp1+/+ and Senp1−/− MEF cells. Senp1+/+ and Senp1−/− MEF cells were induced by the adipocyte differentiation stimulant DMIR for 8 days. The differentiated adipocytes were stained with Oil Red O. As shown in Fig. 1, B and C, there was less red staining in Senp1−/− MEF cells than that in the Senp1+/+ control. The lipid droplet formation in Senp1−/− MEF cells was also much less than that in Senp1+/+ MEF cells. These data suggest an essential role of SENP1 in adipocyte differentiation.
FIGURE 1.
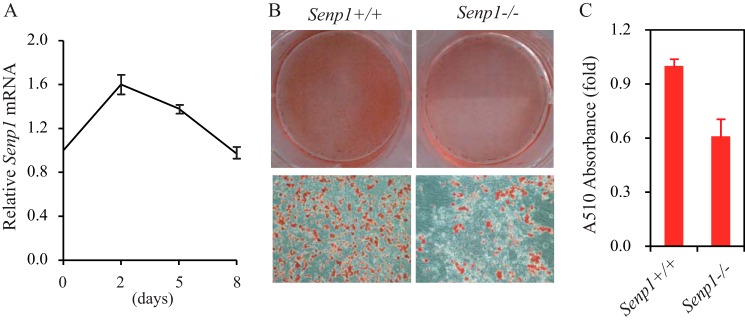
SENP1 deficiency decreases adipocyte differentiation in Senp1−/− MEF cells. A, the expression of SENP1 was analyzed in DMIR-treated MEF cells. Data are mean ± S.D. of three independent experiments. Differences between days 2 or 5 and day 0 were significant (p < 0.01, Student's t test). B, Senp1+/+ and Senp1−/− MEF cells were stained by Oil Red O on day 8 after DMIR treatment. C, the Oil Red O staining in B was quantitatively measured by A510 absorbance analysis. Data are mean ± S.D. of three independent experiments. Differences between Senp1+/+ and Senp1−/−MEF cells were significant (p < 0.01, Student's t test).
Adipogenically Related Genes Are Down-regulated in Senp1−/− MEF Cells during Adipocyte Differentiation
To understand the molecular basis in SENP1 regulation of adipogenesis, we analyzed the expression of adipogenically related genes in Senp1+/+ and Senp1−/− MEF cells induced by DMIR. The expression of C/EBPα/β and PPARγ, the master regulators and transcription factors during adipogenesis, was down-regulated significantly in Senp1−/− MEF cells compared with Senp1+/+ MEFs (Fig. 2, A–C). We also examined the expression of aP2, adiponectin, and LPL, which are indicators of the differentiated adipocyte and PPARγ target genes, and found that SENP1 deficiency significantly reduced the expression of these genes during adipogenesis (Fig. 2, D–F). These data reveal an essential role of SENP1 in the regulation of C/EBPα/β and PPARγ expression in adipocyte differentiation.
FIGURE 2.
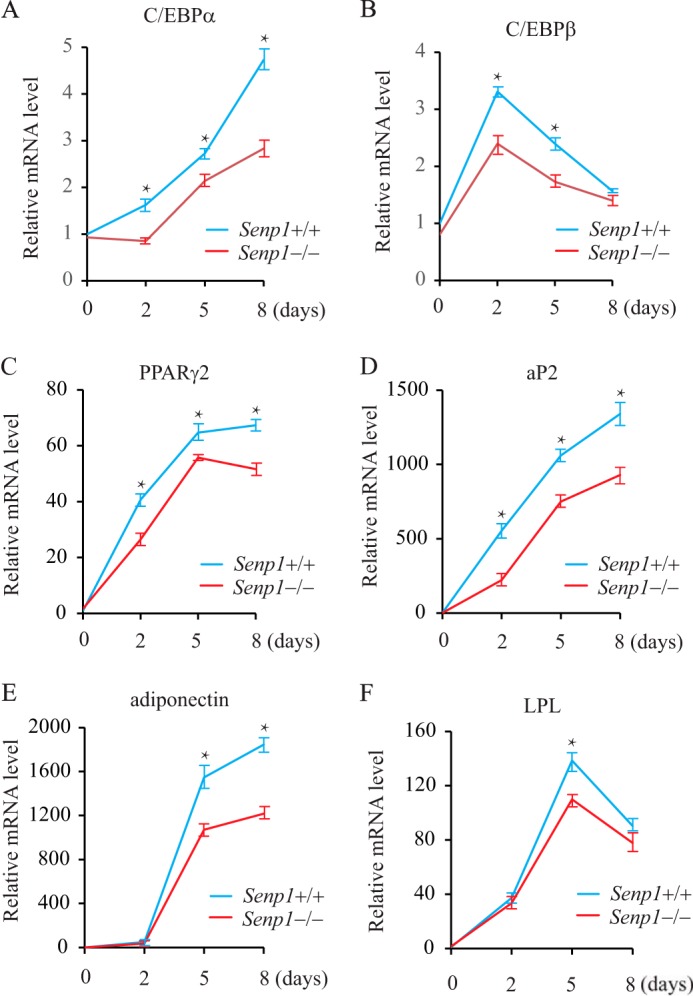
The expression of adipogenically related genes in DMIR-treated Senp1+/+ and Senp1−/− MEF cells. The expression of the adipogenically related genes C/EBPα (A), C/EBPβ (B), PPARγ2 (C), aP2 (D), adiponectin (E) and LPL(F) was measured in Senp1+/+ and Senp1−/− MEF cells at different time points after DMIR treatment. Data are mean ± S.D. of three independent experiments. *, p < 0.01; Student's t test; difference between Senp1+/+ and Senp1−/− MEF cells.
SENP1 Is a Positive Regulator of PPARγ Transcription
Because SENP1 functions in the initiation of adipogenesis, we determined PPARγ expression in MEF cells at the first day of induction by DMIR. As shown in Fig. 3A, PPARγ mRNA increased abruptly in Senp1+/+ MEFs after induction. However, only a mild increase in PPARγ expression was detected in the induced Senp1−/− cells, suggesting that SENP1 is essential for PPARγ expression in the initiation stage of adipocyte differentiation. By using PPARγ promoter-driven luciferase, we confirmed the role of SENP1 in promoting PPARγ transcription (Fig. 3B). Importantly, the SENP1 catalytic mutant could not activate PPARγ expression, suggesting that de-SUMOylation activity is essential for SENP1 regulation of PPARγ transcription. To further demonstrate the de-SUMOylation activity of SENP1 in the regulation of the expression of adipogenically related genes, we generated SENP1 wild-type- or SENP1 catalytic mutant-transfected Senp1−/− MEF cells. SENP1 up-regulated the expression of PPARγ and its targets aP2, adiponectin, and LPL, as shown in SENP1-transfected cells. However, the mutation of the catalytic domain abolished the SENP1 induction of these genes (Fig. 3C). These data suggest that SENP1 is a positive regulator of PPARγ expression through de-SUMOylation.
FIGURE 3.
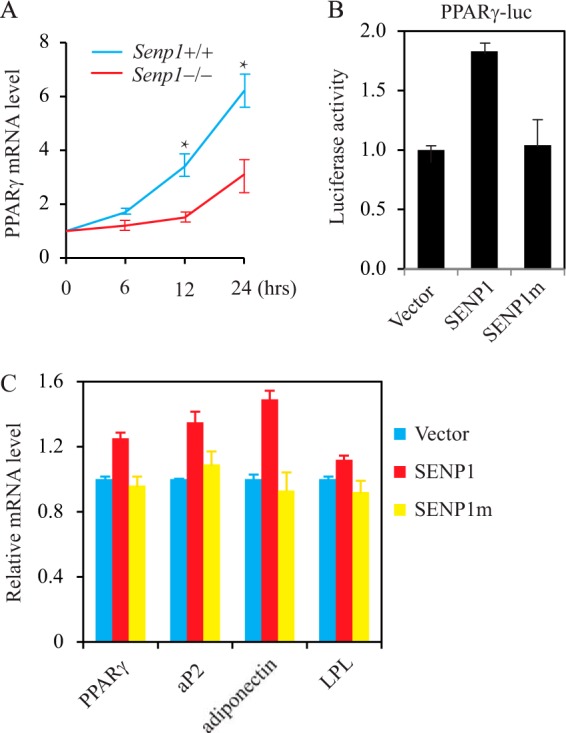
SENP1 positively regulates PPARγ transcription. A, PPARγ mRNA was measured in Senp1+/+ and Senp1−/− MEF cells treated with DMIR. Data are mean ± S.D. of three independent experiments. *, p < 0.01; Student's t test; difference between Senp1+/+ and Senp1−/− MEF cells. B, PPARγ transcription was analyzed in 293 cells transfected with PPARγ-luciferase plus SENP1 or SENP1mut. Data are mean ± S.D. of three independent experiments. Differences between SENP1 versus vector or SENP1 versus SENP1m were significant (p < 0.01, Student's t test). C, the expression of PPARγ, aP2, adiponectin, and LPL in Senp1−/− MEF cells transfected with FLAG-SENP1 or FLAG-SENP1m. Data are mean ± S.D. of three independent experiments. Differences between SENP1 versus Vector, or SENP1 versus SENP1m were significant (p < 0.01, Student's t test).
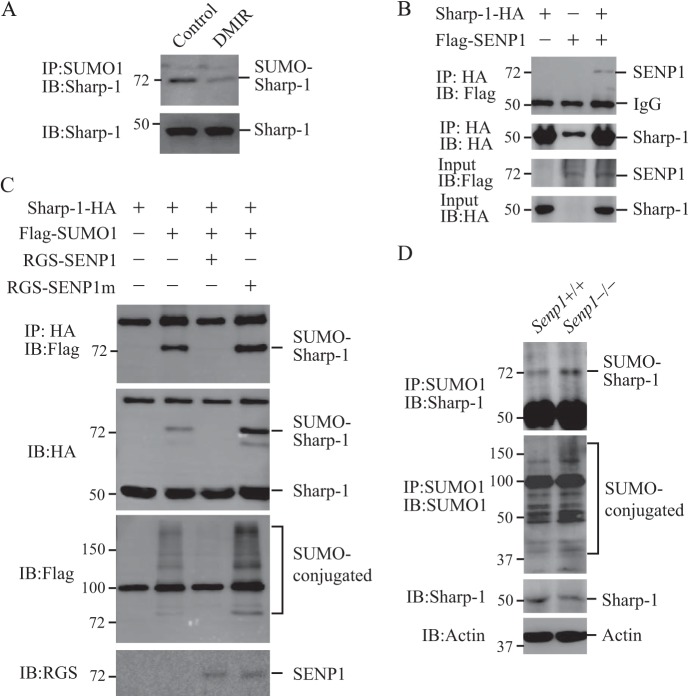
SENP1 De-SUMOylates Sharp-1
At the initiation of adipogenesis, C/EBP binds directly to the PPARγ promoter and induces PPARγ. PPARγ also turns on the expression of C/EBP and then further induces PPARγ expression. This self-reinforcing regulatory loop is critical for PPARγ function in the initiation of adipogenesis (1–3). However, this regulation is modulated by negative regulators such as Sharp-1, a member of the transcriptional repressor subfamily of basic helix-loop-helix transcription factors (7). Sharp-1 has been shown to be a corepressor to retain HDAC1 and G9a on the C/EBPα and PPARγ2 promoter to inhibit PPARγ expression in adipogenesis (8). Sharp-1 has been reported to be a SUMOylated protein (21). Interestingly, Sharp-1 SUMOylation was decreased in NIH3T3-L1 cells treated with DMIR (Fig. 4A), which coincides with the up-regulation of SENP1 expression. Therefore, we proposed that SENP1 could de-SUMOylate Sharp-1, which would be essential to activate PPARγ expression and adipogenesis. We first tested whether SENP1 could bind to Sharp-1. As shown in Fig. 4B, Sharp-1 pulled down SENP1 from 293T cell cotransfected with Sharp-1 and SENP1. We then tested whether SENP1 could de-SUMOylate Sharp-1 in a cotransfection system. SUMOylated Sharp-1 was readily detected in the cotransfection of Sharp-1 and SUMO-1. This SUMOylated band disappeared after overexpression of the SENP1 wild type but not the catalytic mutant (Fig. 4C). To further determine the specificity of SENP1 in the de-SUMOylation of Sharp-1, we immunoprecipitated the endogenous SUMO-conjugated proteins from Senp1+/+ or Senp1−/− MEF cells and detected the SUMOylated Sharp with an anti-Sharp-1 antibody. As shown in Fig. 4D, SUMO-Sharp-1 in Senp1−/− cells was shown to be much more than that in Senp1+/+ cells, suggesting that SENP1 deficiency results in the accumulation of SUMOylated Sharp-1.
FIGURE 4.
SENP1 de-SUMOylates Sharp-1. A, Sharp-1 SUMOylation in NIH3T3-L1 cells was decreased after DMIR treatment. SUMO1-conjugated proteins in NIH3T3-L1 cells with or without DMIR treatment for 2 days were immunoprecipitated (IP) with anti-SUMO1 antibody, and SUMO-Sharp-1 proteins were blotted with anti-Sharp-1. Cell lysate was immunoblotted (IB) with anti-Sharp-1 antibody. B, 293T cells were transfected with Sharp-1-HA and FLAG-SENP1 as indicated. Sharp-1-HA proteins were pulled down by HA beads from these cell lysates. Bound SENP1 protein was blotted with anti-FLAG (top panel). Cell lysate was immunoblotted with anti-HA antibody and anti-FLAG antibody. C, 293T cells were transfected with FLAG-SUMO1, Sharp-1-HA, RGS-SENP1, or RGS-SENP1m as indicated. Sharp-1-HA proteins were pulled down by HA beads from these cell lysates. Bound proteins were blotted with anti-FLAG (top panel). Cell lysate was immunoblotted with anti-HA antibody (second panel), anti-FLAG antibody (third panel), or anti-RGS antibody (fourth panel). D, SUMOylated Sharp-1 is accumulated Senp1−/− MEF cells. SUMO1-conjugated proteins were immunoprecipitated with anti-SUMO1 antibody, and SUMO-Sharp-1 proteins were blotted with anti-Sharp-1. Cell lysate was immunoblotted with anti-Sharp-1 antibody and anti-actin antibody.
SUMOylation Contributes to Sharp-1 Repression of PPARγ Transcription
To test whether SUMOylation could modulate Sharp-1 activity in PPARγ expression, we compared the effect of Sharp-1 or the Sharp-1 SUMOylation mutant on PPARγ transcription. As shown in Fig. 5A, SUMOylation-deficient Sharp-1 showed much less repressive activity in PPARγ transcription than the Sharp-1 wild type, suggesting that SUMOylation could enhance Sharp-1 repression of PPARγ expression. This phenotype was further confirmed by coexpression of SENP1, which reduced the Sharp-1 repression of PPARγ luciferase activity (Fig. 5, A and B). Importantly, the SENP1 catalytic mutant could not affect Sharp-1 repression (Fig. 5B), suggesting that SENP1 regulation of PPARγ transcription is through de-SUMOylation of Sharp-1.
FIGURE 5.
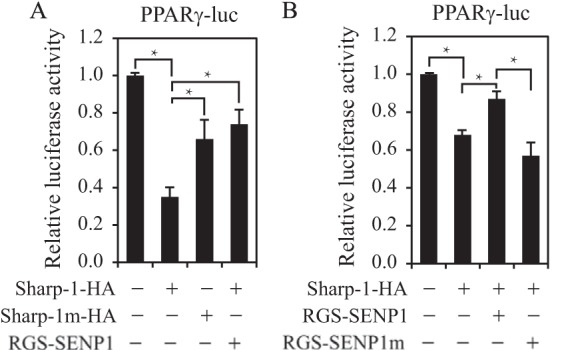
SUMOylation contributes to Sharp-1 repression of PPARγ transcription. A, 293T cells were transfected with PPARγ-luciferase and Sharp-1-HA, Sharp-1m-HA, and Sharp-1-HA plus FLAG-SENP1 as indicated. Luciferase activity is presented as mean ± S.D. of three independent experiments. *, p < 0.01; Student's t test; differences between Sharp-1 versus Control, Sharp-1 versus Sharp-1m, or Sharp-1 versus Sharp-1 + SENP1. B, 293T cells were transfected with PPARγ-luciferase and Sharp-1-HA, Sharp-1-HA plus SENP1, or SENP1m as indicated. Luciferase activity is presented as mean ± S.D. of three independent experiments. *, p < 0.01; Student's t test; differences between Sharp-1 versus Control, Sharp-1 versus Sharp-1 + SENP1, or Sharp-1 + SENP1 versus Sharp-1 + SENP1m.
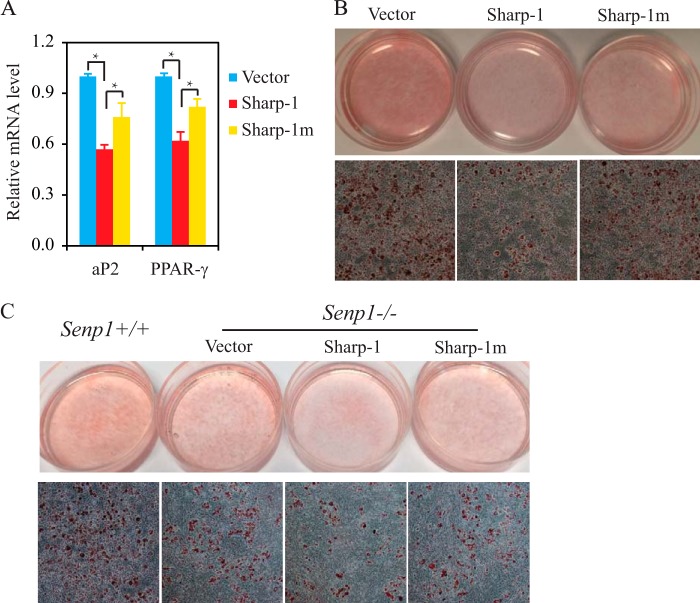
Deficiency in SUMOylation Reduces Sharp-1 Suppression of Adipogenesis
To determine whether SUMOylation has an effect on Sharp-1 regulation of adipogenesis, we generated Sharp-1 wild-type or Sharp-1 SUMOylation mutant-transfected 3T3-L1 cell lines. We first measured the expression of aP2 and PPARγ in these cells on day 2 after DMIR induction. Overexpression of Sharp-1 markedly reduced the expression of these genes compared with the vector control. However, Sharp-1 repression of these genes was reduced significantly in Sharp-1 SUMOylation mutant cells (Fig. 6A). We then stained these cells with Oil Red O on day 6 after DMIR induction. The Sharp-1 wild-type cells showed less red staining compared with the vector. However, the Sharp-1 SUMOylation mutant had more red color than the Sharp-1 wild type. The lipid droplet formation in the Sharp-1 cells was also much less than that in the vector cells and in Sharp-1 mutant cells were more than in the Sharp-1 wild type (Fig. 6B). To further confirm the contribution of Sharp-1 SUMOylation in SENP1 regulation of adipogenesis, we stably transfected the Sharp-1 wild type and the Sharp-1 SUMOylation mutant into Senp1−/− MEF cells and compared their ability for adipogenesis. As shown in Fig. 6C, the Sharp-1-Senp1−/− cells showed less red staining compared with the vector-Senp1−/− cells. However, Sharp-1m-Senp1−/− cells had more red color than Sharp-1-Senp1−/− cells. The lipid droplet formation in the Sharp-1-Senp1−/− cells was also much less than in the vector-Senp1−/− cells. However, in Sharp-1 mutant cells, it was more than in Sharp-1-Senp1−/− MEF cells. These data suggest that SUMOylation contributes to Sharp-1 repression of adipogenesis.
FIGURE 6.
Deficiency in SUMOylation reduces Sharp-1 suppression of adipogenesis. A, expression of PPARγ and aP2 in Sharp-1 and Sharp-1m-transfected 3T3-L1 cells was measured on day 2 after DMIR treatment. *, p < 0.01; Student's t test; differences between Sharp-1 versus control or Sharp-1 versus Sharp-1m. B, vector control, Sharp-1, or Sharp-1 mutant-transfected 3T3-L1 cells were stained with Oil Red O on day 6 after DMIR treatment. C, Senp1+/+ MEF cells and vector, Sharp-1, or Sharp-1 mutant-transfected Senp1−/− MEF cells were stained with Oil Red O on day 8 after DMIR treatment.
DISCUSSION
In this study, we reveal the role of SENP1 as a novel positive regulator in adipogenesis on the basis of the following evidence. First, we observed a phenotype of Senp1−/− MEF cells showing defects in adipogenesis. Second, SENP1 can enhance the expression of PPARγ, a master regulator of adipogenesis. Third, SENP1 can de-SUMOylate Sharp-1, a repressor for PPARγ transcription as well as for adipogenesis. Therefore, we propose a model in which SENP1 enhances adipogenesis through de-SUMOylation of Sharp-1, which then releases Sharp-1 repression of PPARγ function as well as adipocyte differentiation.
It is well documented that adipogenesis is controlled by a tightly regulated transcriptional cascade where the transcription factors activate or repress the expression of each other in a sequential manner. C/EBP and PPARγ, as master transcription factors in adipogenesis, play a critical role in regulation of the expression of adipocyte differentiation-related genes. Adipogenic stimuli activate C/EBP, which directly binds to the PPARγ promoter and induces PPARγ. PPARγ also turns on the expression of C/EBP and then further induces PPARγ expression. This self-reinforcing regulatory loop is critical for PPARγ function in adipogenesis. Recently, many regulators have been identified that control C/EBP and PPARγ activity. Sharp-1, a member of the transcriptional repressor subfamily of basic helix-loop-helix transcription factors, has been shown to be one of the negative regulators in control of C/EBP and PPARγ activity. Taneja and co-workers (8) reported that Sharp-1, as a corepressor, inhibits C/EBP activity by retaining HDAC1 and G9a on the C/EBPα and PPARγ2 promoter to inhibit PPARγ expression and adipogenesis. Furthermore, Sharp-1 has been shown to be a SUMOylated protein. However, it is unknown whether SUMOylation contributes to Sharp-1 suppression of adipogenesis. In this study, we show that SUMOylation enhances Sharp-1 repression of PPARγ expression and adipocyte differentiation. SENP1, as a de-SUMOylation protease, reduces Sharp-1 inhibition of PPARγ expression.
SUMOylation has emerged as a novel regulatory mechanism in adipogenesis. C/EBP and PPARγ are SUMOylated proteins. Dutchak et al. (22) reported that FGF21 knockout mice have a marked increase in the SUMOylation of PPARγ and decreased body fat, indicating that SUMOylation of PPARγ modulates adipogenesis. Liu et al. (23) found that PIAS1 promotes C/EBPβ SUMOylation and inhibits adipogenesis. Chung et al. (24) found that a de-SUMOylation protease, SENP2, can directly modulate C/EBPβ SUMOylation and its stability and, consequently, is essential for adipogenesis. Here we provide more evidence for SUMOylation in the regulation of adipogenesis by showing that SENP1, another member of SENP family, is also essential for adipogenesis. SENP1 de-SUMOylates Sharp-1, not C/EBPβ, a target of SENP2 in adipogenesis. Interestingly, Chung et al. (24) didn't detect any change of SENP1 expression in 3T3-L1 induced by DMI. However, in our study, we clearly demonstrated the increase of SENP1 in MEF cells when induced by DMIR. It is unknown whether the discrepancy results from the addition of rosiglitazone to our adipogenic stimuli. Overall, we reveal that SENP1 deconjugates SUMOylated Sharp-1, releases Sharp-1 suppression of PPARγ expression, and, thus, promotes adipocyte differentiation.
Acknowledgments
We thank Dr. Bing Sun (Institute Pasteur of Shanghai, Chinese Academy of Sciences, Shanghai, China) and Zhaoyuan Hou (Shanghai Jiao Tong University School of Medicine, Shanghai, China) for plasmids.
This work was supported by National Basic Research Program of China (973 Program) Grants 2010CB912104 (to J. C.) and 2012CB910102 (to Y. Z.), by National Natural Science Foundation of China Grants 91019021 (to J. C.) and 81302296 (to W. M.), and by Doctoral Innovation Fund of the Shanghai Jiao Tong University School of Medicine Grant BXJ201207 (to B. L.).
- C/EBP
- CCAAT/enhancer-binding protein
- PPARγ
- peroxisome proliferator-activated receptor γ
- SUMO
- small ubiquitin-like modifier
- SENP1
- Sentrin/SUMO-specific protease
- LPL
- lipoprotein lipase
- Luc
- luciferase
- DMIR
- dexamethasone, isobutyl-1-methylxanthine, insulin, and rosiglitazone
- MEF
- mouse embryonic fibroblast.
REFERENCES
- 1. Rosen E. D., Walkey C. J., Puigserver P., Spiegelman B. M. (2000) Transcriptional regulation of adipogenesis. Genes Dev. 14, 1293–1307 [PubMed] [Google Scholar]
- 2. Farmer S. R. (2006) Transcriptional control of adipocyte formation. Cell Metab. 4, 263–273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Rosen E. D., MacDougald O. A. (2006) Adipocyte differentiation from the inside out. Nat. Rev. Mol. Cell Biol. 7, 885–896 [DOI] [PubMed] [Google Scholar]
- 4. Lefterova M. I., Zhang Y., Steger D. J., Schupp M., Schug J., Cristancho A., Feng D., Zhuo D., Stoeckert C. J., Jr., Liu X. S., Lazar M. A. (2008) PPARγ and C/EBP factors orchestrate adipocyte biology via adjacent binding on a genome-wide scale. Genes Dev. 22, 2941–2952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wu Z., Rosen E. D., Brun R., Hauser S., Adelmant G., Troy A. E., McKeon C., Darlington G. J., Spiegelman B. M. (1999) Cross-regulation of C/EBP α and PPAR γ controls the transcriptional pathway of adipogenesis and insulin sensitivity. Mol. Cell 3, 151–158 [DOI] [PubMed] [Google Scholar]
- 6. Rosen E. D., Hsu C. H., Wang X., Sakai S., Freeman M. W., Gonzalez F. J., Spiegelman B. M. (2002) C/EBPα induces adipogenesis through PPARγ: a unified pathway. Genes Dev. 16, 22–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Azmi S., Sun H., Ozog A., Taneja R. (2003) mSharp-1/DEC2, a basic helix-loop-helix protein functions as a transcriptional repressor of E box activity and Stra13 expression. J. Biol. Chem. 278, 20098–20109 [DOI] [PubMed] [Google Scholar]
- 8. Gulbagci N. T., Li L., Ling B., Gopinadhan S., Walsh M., Rossner M., Nave K. A., Taneja R. (2009) SHARP1/DEC2 inhibits adipogenic differentiation by regulating the activity of C/EBP. EMBO Rep. 10, 79–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Yeh E. T. (2009) SUMOylation and De-SUMOylation: wrestling with life's processes. J. Biol. Chem. 284, 8223–8227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hay R. T. (2005) SUMO: a history of modification. Mol. Cell 18, 1–12 [DOI] [PubMed] [Google Scholar]
- 11. Billon N., Kolde R., Reimand J., Monteiro M. C., Kull M., Peterson H., Tretyakov K., Adler P., Wdziekonski B., Vilo J., Dani C. (2010) Comprehensive transcriptome analysis of mouse embryonic stem cell adipogenesis unravels new processes of adipocyte development. Genome Biol. 11, R80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zhao X., Huang Z., Liu X., Chen Y., Gong W., Yu K., Qin L., Chen H., Mo D. (2013) The switch role of the Tmod4 in the regulation of balanced development between myogenesis and adipogenesis. Gene 532, 263–271 [DOI] [PubMed] [Google Scholar]
- 13. Wang Q. A., Tao C., Gupta R. K., Scherer P. E. (2013) Tracking adipogenesis during white adipose tissue development, expansion and regeneration. Nat. Med. 19, 1338–1344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Vogel G., Richard S. (2012) Emerging roles for Sam68 in adipogenesis and neuronal development. RNA Biol. 9, 1129–1133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Yan X., Huang Y., Zhao J. X., Rogers C. J., Zhu M. J., Ford S. P., Nathanielsz P. W., Du M. (2013) Maternal obesity downregulates microRNA let-7g expression, a possible mechanism for enhanced adipogenesis during ovine fetal skeletal muscle development. Int. J. Obes. 37, 568–575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Scroyen I., Frederix L., Lijnen H. R. (2012) Axl deficiency does not affect adipogenesis or adipose tissue development. Obesity 20, 1168–1173 [DOI] [PubMed] [Google Scholar]
- 17. Van Hul M., Bauters D., Himmelreich U., Kindt N., Noppen B., Vanhove M., Lijnen H. R. (2012) Effect of gelatinase inhibition on adipogenesis and adipose tissue development. Clin. Exp. Pharmacol. Physiol. 39, 49–56 [DOI] [PubMed] [Google Scholar]
- 18. Cheng J., Kang X., Zhang S., Yeh E. T. (2007) SUMO-Specific protease 1 is essential for stabilization of HIF1 α during hypoxia. Cell 131, 584–595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kamitani T., Nguyen H. P., Kito K., Fukuda-Kamitani T., Yeh E. T. (1998) Covalent modification of PML by the sentrin family of ubiquitin-like proteins. J. Biol. Chem. 273, 3117–3120 [DOI] [PubMed] [Google Scholar]
- 20. Gong L., Millas S., Maul G. G., Yeh E. T. (2000) Differential regulation of sentrinized proteins by a novel sentrin-specific protease. J. Biol. Chem. 275, 3355–3359 [DOI] [PubMed] [Google Scholar]
- 21. Wang Y., Shankar S. R., Kher D., Ling B. M., Taneja R. (2013) Sumoylation of the basic helix-loop-helix transcription factor sharp-1 regulates recruitment of the histone methyltransferase G9a and function in myogenesis. J. Biol. Chem. 288, 17654–17662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Dutchak P. A., Katafuchi T., Bookout A. L., Choi J. H., Yu R. T., Mangelsdorf D. J., Kliewer S. A. (2012) Fibroblast growth factor-21 regulates PPARγ activity and the antidiabetic actions of thiazolidinediones. Cell 148, 556–567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Liu Y., Zhang Y. D., Guo L., Huang H. Y., Zhu H., Huang J. X., Liu Y., Zhou S. R., Dang Y. J., Li X., Tang Q. Q. (2013) Protein inhibitor of activated STAT 1 (PIAS1) is identified as the SUMO E3 ligase of CCAAT/enhancer-binding protein β (C/EBPβ) during adipogenesis. Mol. Cell Biol. 33, 4606–4617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Chung S. S., Ahn B. Y., Kim M., Choi H. H., Park H. S., Kang S., Park S. G., Kim Y. B., Cho Y. M., Lee H. K., Chung C. H., Park K. S. (2010) Control of adipogenesis by the SUMO-specific protease SENP2. Mol. Cell Biol. 30, 2135–2146 [DOI] [PMC free article] [PubMed] [Google Scholar]