Background: The molecular mechanism and key signaling pathways underlying MT expression in response to metal stress remains elusive.
Results: Upon metal stress, PP2A PR110 complexes bind to and dephosphorylate MTF-1 at Thr-254, leading to the transactivation of MTs.
Conclusion: Specific PP2A complexes regulate metal-induced MTs expression.
Significance: Delineate a novel pathway regulating metal-induced cytotoxicity and clarify the role of PP2A in cellular stress response.
Keywords: Cell Biology, Metallothione, Protein Phosphatase 2 (PP2A), Stress Response, Transcription Factor, Cytotoxicity, Metal-responsive Transcription Factor-1, Metallothioneins, Protein Phosphatase 2A
Abstract
Induction of metallothionein (MT) expression is involved in metal homeostasis and detoxification. To identify the key pathways that regulate metal-induced cytotoxicity, we investigate how phosphorylated metal-responsive transcription factor-1 (MTF-1) contributed to induction of MT expression. Immortal human embryonic kidney cells (HEK cells) were treated with seven kinds of metals including cadmium chloride (CdCl2), zinc sulfate (ZnSO4), copper sulfate(CuSO4), lead acetate (PbAc), nickel sulfate (NiSO4), sodium arsenite (NaAsO2), and potassium bichromate (K2Cr2O7). The MT expression was induced in a dose-response and time-dependent manner upon various metal treatments. A cycle of phosphorylation and dephosphorylation was required for translocation of MTF-1 from cytoplasm to nucleus, leading to the up-regulation of MTs expression. Protein phosphatase 2A (PP2A) participated in regulating MT expression through dephosphorylation of MTF-1. A loss-of-function screen revealed that the specific PP2A complexes containing PR110 were involved in metal-induced MT expression. Suppression of PP2A PR110 in HEK cells resulted in the persistent MTF-1 phosphorylation and the disturbance of MTF-1 nuclear translocation, which was concomitant with a significant decrease of MT expression and enhanced cytotoxicity in HEK cells. Notably, MTF-1 was found in complex with specific PP2A complexes containing the PR110 subunit upon metal exposure. Furthermore, we identify that the dephosphorylation of MTF-1 at residue Thr-254 is directly regulated by PP2A PR110 complexes and responsible for MTF-1 activation. Taken together, these findings delineate a novel pathway that determines cytotoxicity in response to metal treatments and provide new insight into the role of PP2A in cellular stress response.
Introduction
Toxic heavy metals such as arsenic, cadmium, lead, and mercury are ubiquitous, have no essential role in maintaining cellular homeostasis, and are known to exert multiple organ toxicities and contribute to a variety of chronic diseases (1–3). To date, environmental heavy metal contamination becomes an increasingly serious threat to human health. Currently, high level exposure to heavy metals in some regions of China remains a serious issue. For example, the Dabaoshan mine in the southeast of Guangdong Province is at high risk of multi-metal pollutant discharge into a local river, Hengshihe, and the surrounding area. Previously, our group reported the high level exposure to cadmium, zinc, and lead in local environmental samples (water and crops) and blood of local residents. In addition, heavy metal exposure was associated with increased risk of behavioral disorders in school-aged children. The epidemiological data revealed that high level exposure to multiple heavy metals within the environment significantly increased the risk of mortality from cancer, such as stomach, esophageal, and lung cancers (4–6). Previous studies have demonstrated that exposure to heavy metals can cause many adverse health effects through the formation of free radicals, DNA damage, lipid peroxidation, and consumption of protein sulfhydryls, etc. (7). However, the molecular mechanism and critical signaling pathways underlying the toxicity of heavy metals still remains elusive.
Previous studies have demonstrated that heavy metal-induced acute toxicity mostly depended upon enzymatic inhibition, antioxidants metabolism, or oxidative stress. However, exposure to heavy metals triggers a number of adaptive responses such as induction of metallothionein (MT),4 which confers cells with resistance to heavy metal-induced toxicities (8). Upon heavy metals stimuli, metallothionein genes are rapidly transcriptionally activated and function in protecting cells from damage (9, 10). MTs are a group of intracellular low molecular (6–7 kDa), cysteine-rich, metal-binding proteins, acting as scavengers of toxic metal ions or reactive oxygen species. MTs have been implicated in the regulation of cell proliferation and apoptosis (11, 12), suggesting a role for MTs in cell survival. MT function in heavy metal detoxification primarily depends on the high affinity binding between the heavy metals and MTs, leading to the sequestration of metals away from critical macromolecules (13, 14). Moreover, the studies conducted in MT transgenic mice or MT-null mice models provide strong evidence that MTs play an essential role in protecting cells from acute heavy metal poisoning (15–18). It is evident that MTs can be a useful biomarker for the prediction of heavy metal toxicity and adverse biological outcome (19, 20).
MT expression can be transcriptionally induced by a variety of environmental stressors such as metals, oxidative stress, or hypoxia (21, 22). Metal-responsive transcription factor 1 (MTF-1) is considered to be a major activator for MT gene expression (22, 23). Previous reports have indicated that MTF-1 activity is mainly regulated by phosphorylation (24, 25). Although protein kinases such as protein kinase C (PKC), c-Jun N-terminal kinase (JNK), or phosphoinositide 3-kinase (PI3K) have been reported to be involved in modifying the MTF-1 signaling pathway (24, 25), the dynamic changes of phosphorylated MTF-1 in transactivation of MT remains to be defined. Because specific dephosphorylation of this transcription factor contributes to its activation (24), it is crucial to identify the specific protein phosphatases involved in transcriptional activation of MTF-1 under heavy metal stress.
Protein phosphatase 2A (PP2A) holoenzymes are ubiquitously expressed serine/threonine phosphatases, each containing a catalytic C subunit, a structural scaffolding A subunit, and a variable B regulatory subunit. The dynamic interaction of the B subunits with the core AC dimer contributes to the target specificity and subcellular localization of individual PP2A holoenzymes (26), and it is evident that specific PP2A complexes mediate particular physiological processes (27, 28). Previous studies have revealed the crucial roles for PP2A in cellular signaling pathways including transcriptional activation, cell cycle progression, apoptosis, DNA damage response, and cell transformation (27, 29–31). Our preliminary results provided evidence that inhibition of PP2A resulted in a down-regulation of MT, suggesting a role for PP2A during this process. Hereby, we speculate that PP2A may regulate cellular responses to metals through modification of the phosphorylation status of key targets such as MTF-1, in turn altering the expression of MT and metal-induced acute cytotoxicity.
In this study, we investigated the role of PP2A in the cellular stress response against the heavy metals and identify specific PP2A complexes containing the PR110 subunit that functions in regulating MT expression through dephosphorylation of MTF-1. Our results indicate the involvement of PP2A in the modulation of cellular response.
EXPERIMENTAL PROCEDURES
Antibodies and Reagents
The following primary antibodies were used: mouse anti-MT (GeneTex), mouse anti-phosphoserine/threonine (BD Biosciences), mouse anti-myc tag, rabbit anti-HA tag, rabbit anti-GAPDH, rabbit anti-Lamin B1 (Cell Signaling Technology), mouse anti-PP2A Cα (1D6; Upstate Biotechnology), rabbit anti-PR110 (Proteintech Group), and rabbit anti-B56β were purchased from Novus Biologicals.
Cadmium chloride (CdCl2), zinc sulfate (ZnSO4), copper sulfate (CuSO4), and nickel sulfate (NiSO4) were purchased from Sigma. Sodium arsenite (NaAsO2) was obtained from Sigma. Lead acetate (PbAc) and potassium bichromate (K2Cr2O7) were purchased from Guangzhou Experiment Reagent (Shanghai, China). All of the chemicals were of >99% purity.
Plasmid Construction and Establishments of Stable Cell Lines
To create an HA epitope-tagged version of PP2A PR110, we performed PCR using the pEGFP-N3 wild type striatin vector (generously provided by Dr. David C. Pallas, Emory University, Atlanta) as a template and subcloned the fragment into a retroviral vector pBabe to generate a retroviral vector pBabe-puro-HA-PR110. The pBabe-puro-HA-MTF-1 and pBabe-puro- HA-B56β were generated by RT-PCR with specific primers (Table 1). The human embryonic kidney cells expressing simian virus 40 LT antigen (LT) and the telomerase catalytic subunit (hTERT) (HEK cells), and shRNAs against each PP2A subunit were generously provided by Dr. William C. Hahn (Dana Farber Cancer Institute, Harvard Medical School, Boston). To generate stable HEK cells, pBabe-HA-MTF-1 or pLKO-shRNAs were introduced into HEK cells by lentiviral infection and selected with puromycin (1 μg/ml).
TABLE 1.
Primer sequences used in cloning
Underlined sequences represent a site for restriction enzyme.
| Plasmid | Vector | Restriction enzyme | Primer (5′-3′) |
|---|---|---|---|
| 4HA | pBabe-puro | BglII | GGAAGATCTATGGCTTACCCATACGATG |
| SnaBI | GGCCTACGTAAGCGTAATCTGGAACGTC | ||
| myc-MTF-1 | pBabe-puro | SnaBI | GGCTACGTAATGGAGCAGAAACTCATCTCTGAAGAGGATCTGGGGGAACACAGT |
| SalI | GGCGGCGTCGACTCACTTGGAGAAGCT | ||
| HA-MTF-1 | pBabe-4HA | SnaBI | GGCGGCTACGTAATGGGGGAACACAGT |
| SalI | GGCGGCGTCGACTCACTTGGAGAAGCT | ||
| HA-PR110 | pBabe-4HA | SnaBI | GGCGGCTACGTAATGGACGAGCAGGCG |
| SalI | GGCGGCGTCGACTCATACAAAGACTTT | ||
| HA-B56β | pBabe-4HA | SnaBI | GGCGGCTACGTAATGGAGACGAAGCTG |
| SalI | GGCGGCGTCGACCTAGCTCTGACC | ||
| MT1A | pGL3 | XhoI | GGCGGCCTCGAGGGTGGCTGTGAATGA |
| HindIII | GGCGGCAAGCTTCCAAGCAAGAAGTTG | ||
| HA-MTF-1-S5A | pBabe-4HA | SnaBI | ATGGGGGAACACGCTCCAGACAAC |
| SalI | GTTGTCTGGAGCGTGTTCCCCCAT | ||
| HA-MTF-1-T252A | pBabe-4HA | SnaBI | AAGCACATTCGAGCTCATACAGGGGAA |
| SalI | TTCCCCTGTATGAGCTCGAATGTGCTT | ||
| HA-MTF-1-T254A | pBabe-4HA | SnaBI | ATTCGAACTCATGCAGGGGAAAAGCCA |
| SalI | TGGCTTTTCCCCTGCATGAGTTCGAAT | ||
| HA-MTF-1-S305A | pBabe-4HA | SnaBI | AGCACTCAATACAGTCTCAAAAGTCAC |
| SalI | GTGACTTTTGAGACTGTATTGAGTGCT | ||
| HA-MTF-1-S620A | pBabe-4HA | SnaBI | CAGATTGGCCTCGCTGTTCCTGTGATC |
| SalI | GATCACAGGAACAGCGAGGCCAATCTG |
Detection of Phosphatase Activity
The protein phosphatase activity in PP2A C immune complexes was determined as previously described (32).
Measurement of Cytotoxicity
HEK cells were seeded in 96-well plates with a density of 8 × 103 per well. After 24 h, the HEK cells were treated with seven kinds of heavy metal for 24 h. The concentrations for each heavy metal compound were CdCl2 (0, 10, 20, 40, 80, and 160 μm); ZnSO4 (0, 25, 50, 100, 200, and 400 μm), CuSO4 (0, 50, 100, 200, 400, and 800 μm), PbAc (0, 50, 100, 200, 400, and 800 μm), NiSO4 (0, 100, 200, 400, 800, and 1600 μm), and NaAsO2 (0, 3.13, 6.25, 12.50, 25.00, and 50.00 μm), and K2Cr2O7 (0, 3.13, 6.25, 12.50, 25.00, and 50.00 μm). Cytotoxicity was measured by modified MTT (3-[4,5-dimethyl-2-thiazolyl]-2,5-diphenyl-2-H-tetrazolium bromide) assay using a cell proliferation kit (WST-1). The IC50 (the concentration that caused 50% growth inhibition) was calculated by the modified Karbers method (33) according to the formula: IC50 = log−1[Xk - i (ΣP - 0.5)], in which Xk represents the logarithm of the highest chemical concentration, i is that of the ratio of adjacent concentration, and ΣP is the sum of the percentage of growth inhibition at various concentrations.
Extraction of Cytoplasmic and Nuclear Fractions
Nuclear extract was prepared by the NE-PER TM Nuclear and Cytoplasmic Extraction Reagents (Thermo Scientific, Rockford, IL). Briefly, harvested cells were suspended in Cytoplasmic Extraction Reagent I and incubated on ice for 5 min before Cytoplasmic Extraction Reagent II was added. After shaking vigorously for 10 s, the homogenate was centrifuged at 16,000 × g for 5 min at 4 °C, and the supernatant was defined as the cytoplasmic fraction. The pellet was resuspended in ice-cold nuclear extraction reagent and rocked vigorously at 4 °C for 40 min. The mixture was centrifuged at 16,000 × g for 10 min, and the supernatant was collected as the nuclear fraction.
Immunoblotting Analysis
HEK cells were suspended in ice-cold lysis buffer (150 mmol/liter NaCl, 1% Triton X-100, 0.5% deoxycholate, 0.1% SDS, and 50 mmol/liter Tris (pH 7.4)) containing protease inhibitors (Roche Applied Science). The lysates were centrifuged at 12,000 × g for 20 min at 4 °C. The supernatant was removed and transferred to a new tube and stored at −80 °C. For analysis of the content of metallothionein, HEK cells were lysed directly on the plate using 2× SDS sample buffer (125 mm Tris-base, 138 mm SDS, 10% β-mercaptoethanol, 20% glycerol, bromphenol blue (pH 6.8)). Soluble proteins (50 μg) were subjected to 8–16% gradient acrylamide gel for SDS-PAGE before immunoblotting.
Co-immunoprecipitation (Co-IP)
For immunoprecipitation, 293FT cells were lysed in a 0.3% CHAPS lysis buffer. Cell lysates (3 mg) were incubated with the HA tag or PP2Ac (clone 1D6) antibody overnight at 4 °C followed by the addition of protein G-Sepharose beads (GE Healthcare) for 2 h at 4 °C. The protein G beads were eluted in 2× SDS sample buffer followed by SDS-PAGE and immunoblotting.
Laser Scanning Confocal Microscopy Analysis
HEK cells were grown overnight on the coverslips. After treatment with 40 μm CdCl2 for 12 h, the cells were fixed in 4% formaldehyde for 15 min, washed in PBS, permeabilized in 0.2% Triton X-100, washed and blocked with PBS containing 0.3% FBS for 1 h, and followed by an incubation with specific antibody against the HA tag overnight. Alexa Fluor 488-conjugated goat anti-rabbit IgG second antibody (1:1000) was incubated for 1 h then counter-stained with 4′,6-diamidino-2-phenylindole (DAPI, 1 μg/ml) and observed with a LSM510 META laser scanning confocal microscope (Carl Zeiss) under oil with ×100 magnification.
Vector Construction and Luciferase Reporter Assay
To create luciferase reporter construct pGL3-MT1A-promoter, cDNA (100 ng) from HEK cells served as a template to amplify MTIA promoter (NC_000016.9) and was cloned into the XhoI and HindIII sites downstream of the luciferase reporter gene in pGL3 plasmid.
For the luciferase reporter assay, HEKSHGFP, HEKSHB56β, and HEKSHPR110 cells expressing HA-tagged MTF-1 were grown in a 96-well plate for 24 h and then transiently co-transfected with 50 ng pGL3-MT1A-promoter and 25 ng of pRL-TK by Lipofectamine 2000 (Invitrogen). pRL-TK was used as an internal control (Promega, Madison, WI). 24 h after transfection, the cells were treated with or without 40 μm CdCl2 for 12 h and analyzed for luciferase activity using the Dual-Luciferase Reporter Assay System (Promega) according to manufacturer's instructions.
Statistical Analysis
The results are presented as the mean ± S.D. for at least three replicate experiments. Differences between treatment groups were analyzed by a one-way analysis of variance followed by LSD multiple comparison tests. Differences were considered statistically significant when p < 0.05.
RESULTS
MT Expression Is Induced by Heavy Metals
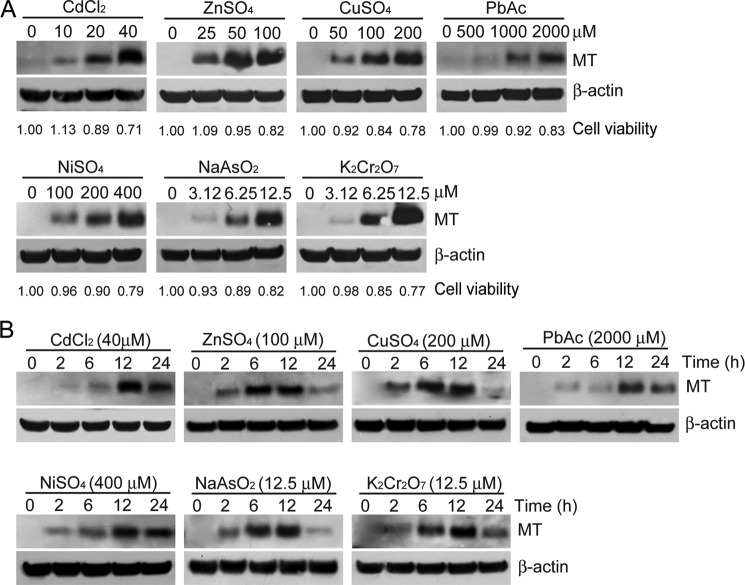
It has been demonstrated that the induction of MT expression increased by several-fold in response to many kinds of heavy metals or oxidative stress (34, 35). We first examined the effect of seven kinds of heavy metal compounds including cadmium chloride (CdCl2), zinc sulfate (ZnSO4), copper sulfate(CuSO4), lead acetate (PbAc), nickel sulfate (NiSO4), sodium arsenite (NaAsO2), and potassium bichromate (K2Cr2O7) on the induction of MT expression. In parallel, we measured the cytotoxicity of these heavy metals in HEK cells using MTT assay. The IC50 values for CdCl2, ZnSO4, CuSO4, PbAc, NiSO4, NaAsO2, and K2Cr2O7 were determined as 72.5, 225.4, 413.8, 463.1, 854.0, 40.2, and 22.6 μm, respectively. The highest concentration of heavy metals used in induction of MT expression was ∼50% of the IC50. As shown in Fig. 1, the levels of MT proteins in HEK cells increased upon exposure to seven kinds of heavy metals in a dose-response and time-dependent manner. Correspondingly, the cell viability displayed dose-dependent effects (p < 0.05) (Fig. 1, A and B). Similar results in MT expression were also observed in HEK cells treated with six other heavy metals (Fig. 1B). These results indicate that the level of MT expression is a good marker for the exposure and the cellular adaptive response to heavy metals.
FIGURE 1.
MT expression is induced by metals. A, dose-dependent induction of MT expression by metals. HEK cells were treated with CdCl2, ZnSO4, CuSO4, PbAc, NiSO4, NaAsO2, and K2Cr2O7 at the concentrations indicated for 12 h and followed by immunoblotting analysis with an antibody against MT. Cytotoxicity was measured by using MTT assay at the indicated concentrations. The relative cell viability corresponding to the amount of MT is calculated as the ratio of each dose of a particular metal to that observed in control cells treated with a vehicle and is indicated under each lane. B, time-dependent induction of MT proteins. HEK cells were treated with metals at the indicated concentrations for 2, 6, 12, or 24 h, and the cell lysates isolated at each time point were subjected to immunoblotting analysis using the antibodies indicated.
MTF-1 Activity Determines MT Expression through Dephosphorylation and Nuclear Translocation
MTF-1 is the key trans-activating factor for MT expression (22, 24, 36). To examine whether the amount of phosphorylated MTF-1 (p-MTF-1) determines the transactivation of MT, we transfected 293FT cells with a vector encoding HA-MTF-1. 48 h after transfection, 293FT cells were treated with diverse heavy metals for 2 h before harvesting. The cell lysates were co-immunoprecipitated with an antibody against the HA tag and followed by detection of the amount of p-MTF-1 with an antibody against Ser/Thr-phosphorylated protein. As a result, we found that these metals with the exception of CuSO4 could induce phosphorylation of MTF-1, indicating a role in regulation of MT expression (Fig. 2A). Given the result that CuSO4 had no impact on level of p-MTF-1, we speculated that there was an alternative way for copper-responsive MTs induction, which was consistent with previous findings showing that copper did not activate MT-1 transcription in Nrf-null cells (37). Because the treatment of CdCl2 and ZnSO4 exhibits profound effects on induction of MT expression in a relatively low concentration (Fig. 1A), we chose them as the representative heavy metals for the following experiments. Next, we revealed that p-MTF-1 exhibited a dynamic change in response to 40 μm CdCl2 and 100 μm ZnSO4 treatments. Phosphorylated MTF-1 peaked at the 2 h time and then gradually declined to a basal level at 12 h (Fig. 2, B and C). In contrast, MT expression was up-regulated gradually with the time and reached at the highest level at 12 h upon CdCl2 treatment, which was inversely correlated with the reduction of p-MTF-1 (Fig. 2, B and C). These observations are in agreement with the previous findings (24) that the dephosphorylation of MTF-1 plays a central role in regulation of heavy metal-induced MT expression.
FIGURE 2.
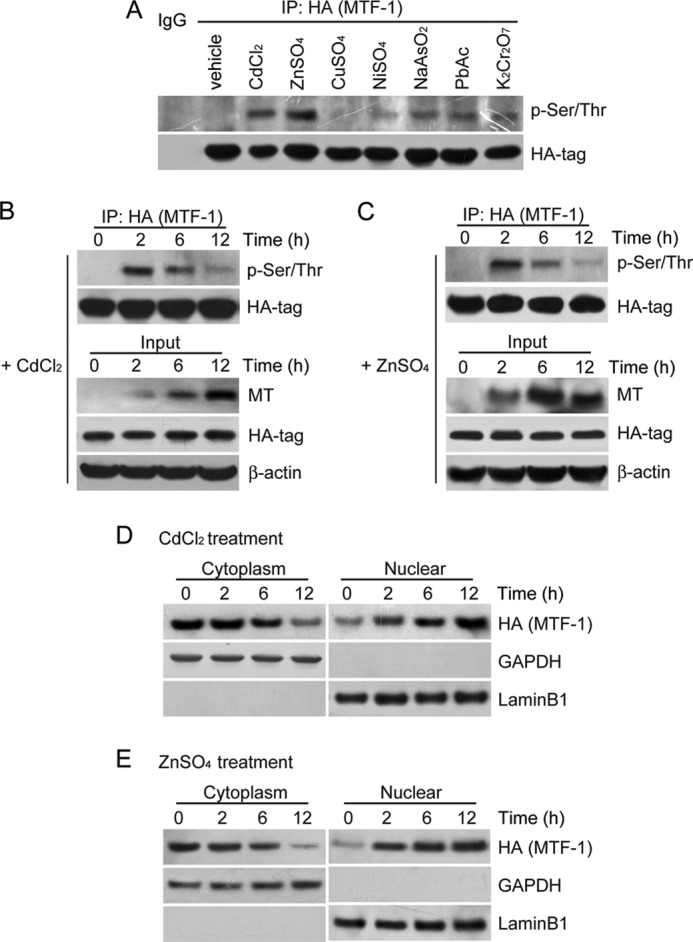
MTF-1 activity regulates MT expression. 293FT cells were transfected with a vector encoding HA-tagged MTF-1 for 48 h and followed by the treatment of 40 μm CdCl2, 100 μm ZnSO4, 200 μm CuSO4, 400 μm NiSO4, 12.5 μm NaAsO2, 2000 μm PbAc, and 12.5 μm K2Cr2O7 for 2 h (A) or 40 μm CdCl2 (B) and 100 μm ZnSO4 (C) for different time intervals (2, 6, and 12 h) before harvested. The co-IP assay was performed with an antibody against the HA tag and followed by immunoblotting using antibodies indicated. The lower panels show the input of each protein indicated at the different time points. A retroviral vector encoding HA-tagged MTF-1 was introduced into HEK cells to generate stable HEK-MTF-1 cells. The HEK cells were treated with 40 μm CdCl2 (D) or 100 μm ZnSO4 (E) for different time intervals (2, 6, and 12 h). Cytoplasmic and nuclear fractions were isolated, and the protein expression was examined by immunoblotting. GAPDH and Lamin B1 were used as the loading controls for cytoplasmic and nuclear fractions, respectively.
It has been revealed that MTF-1 primarily localizes in the cytoplasm and translocates to the nucleus to activate MT gene expression upon zinc or cadmium stimuli (38, 39). Next, we determine whether the nuclear translocation of MTF-1 can be induced by heavy metals. To this end, we established the HEK cell lines stably expressing HA-tagged MTF-1 (named as HEKHA-MTF-1 cells) and isolated cytoplasmic and nuclear fractions from cells exposed to 40 μm CdCl2 at different time points (2, 6, and 12 h). Immunoblotting analysis revealed that the amount of MTF-1 in the nuclear fraction increased by 1.5-fold at 6 h and was retained at a high level at 12 h (Fig. 2D). Accordingly, the level of MTF-1 in the cytoplasmic fraction declined at 6 h after CdCl2 treatment. A similar pattern was observed by exposing cells to 100 μm ZnSO4 (Fig. 2E). Consistent with these findings, MT expression gradually increased and reached the highest level at 12 h in response to CdCl2 or ZnSO4 treatments (Fig. 2, B and C). Taken together, these observations suggest that the reduction of phosphorylated MTF-1 and subsequent nuclear import of MTF-1 are required for the induction of MT expression.
PP2A Is Involved in Dephosphorylation of MTF-1
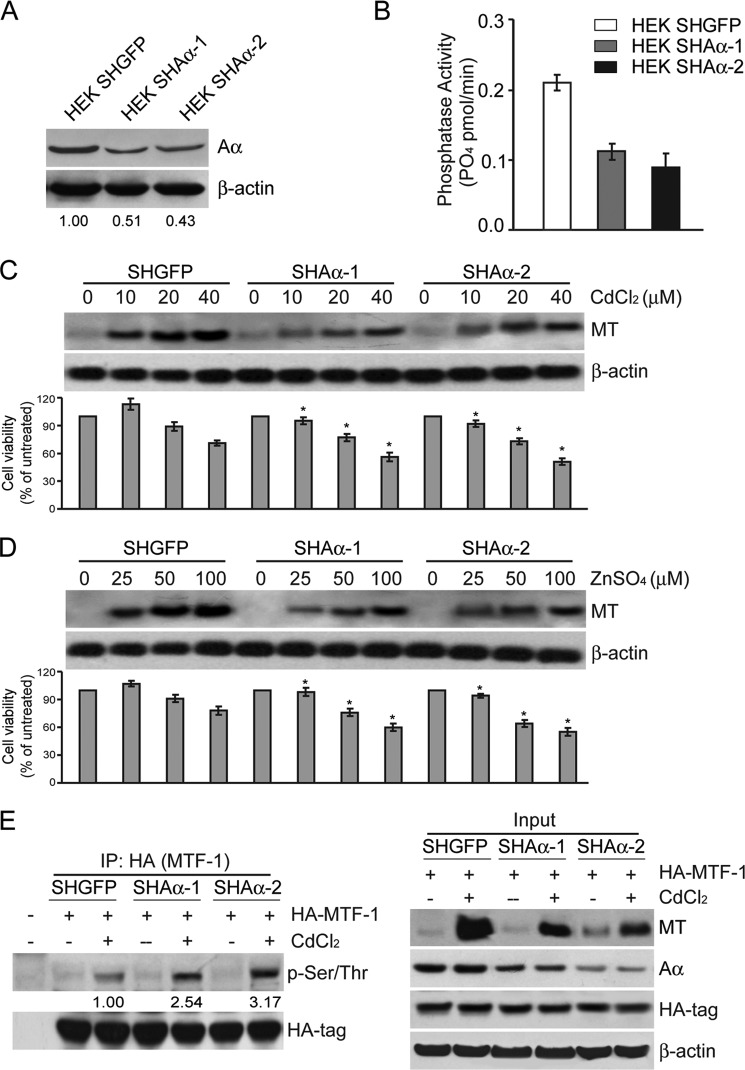
Because the dephosphorylation of MTF-1 is responsible for up-regulation of MTs expression, we assess whether the MTF-1 dephosphorylation is mediated by PP2A, a large family of holoenzymes that accounts for the majority of Ser/Thr phosphatase activity in eukaryotic cells. We first examined whether MT expression was affected by PP2A activity in HEK cells stably expressing two independent PP2A Aα-specific shRNAs (named as HEKSHAα-1 and HEKSHAα-2 cells). Two shRNAs decreased Aα expression by ∼50% compared with the control cells (HEKSHGFP) as determined by immunoblotting analysis (Fig. 3A), resulting in a significant reduction of the PP2A-attributable phosphatase activity by 46.5% and 54.3%, respectively (Fig. 3B). Notably, suppression of Aα led to a decrease in MT induction in HEK cells treated with CdCl2 or ZnSO4 in a dose-dependent manner (Fig. 3, C and D). In line with the MT expression, cell viability in HEK cells expressing two independent shAα showed a 17.0 ± 3.3 or 16.2 ± 6.1% decrease compared with the control cells with the treatment of different concentrations of CdCl2 or ZnSO4 (p < 0.05) (Fig. 3, C and D). These findings indicate that suppression of PP2A activity sensitized cells to heavy metal-induced cytotoxicity.
FIGURE 3.
PP2A regulates MT expression through dephosphorylation of MTF-1. A, lentiviral vectors encoding two independent shRNAs targeting Aα were introduced into HEK cells, generating stable cell lines, HEKSHAα-1 and HEKSHAα-2. The value under each band indicates -fold change of the Aα level normalized to β-actin expression relative to control cells. B, PP2A-attributable activity was detected in HEKSHGFP, HEKSHAα-1, and HEKSHAα-2 cells. Shown is the immunoblotting analysis of MT expression in HEKSHGFP, HEKSHAα-1, and HEKSHAα-2 cells exposed to CdCl2 at concentrations of 0, 10, 20, and 40 μm, respectively, for 12 h (C) or ZnSO4 at concentrations of 0, 25, 50, and 100 μm, respectively, for 12 h (D). The corresponding cell viability at indicated concentrations was measured by MTT assay and is indicated under each lane. *, p < 0.05 compared with the SHGFP control group. E, HA-tagged MTF-1 was co-expressed with two independent shRNAs that targets Aα subunit (shAα-1 and shAα-2) in 293FT cells. The 293FT cells were treated with 40 μm CdCl2 for 12 h. 3 mg of the cell lysates were subjected to co-IP with an antibody against HA tag and followed by immunoblotting analysis with specific antibodies indicated. The value under each band indicates -fold change of p-MTF-1 level normalized to HA-tag expression relative to SHGFP cells.
To explore whether PP2A participates in MTF-1 dephosphorylation, we analyzed the amount of p-MTF-1 in 293FT cells expressing two shRNAs against Aα (named as shAα-1 and shAα-2 cells). As shown in Fig. 3E, we found that the levels of p-MTF-1 in shAα-1 or shAα-2 cells were elevated by 2.3∼2.5-fold, respectively. Consistent with these findings, we observed that the induction of MT reduced by 50% and 75%, respectively (Fig. 3E). These results suggest that PP2A activity may be involved in regulation of MT expression through dephosphorylation of MTF-1.
Identification of Specific PP2A Complexes in Regulation of MT Expression
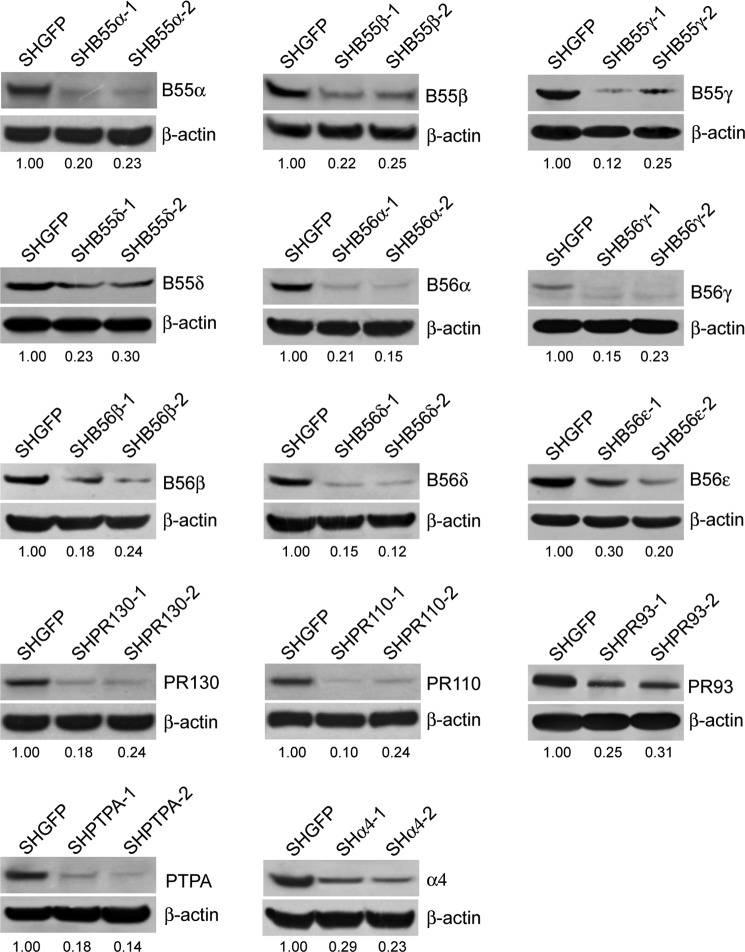
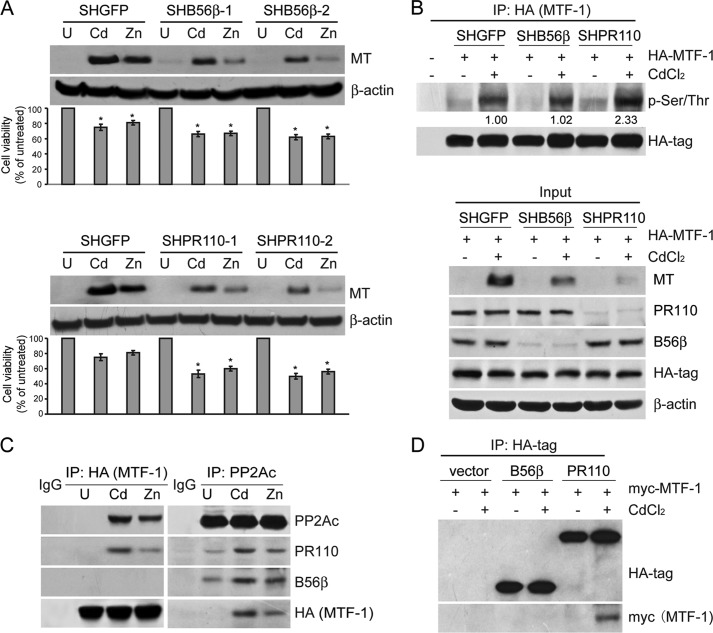
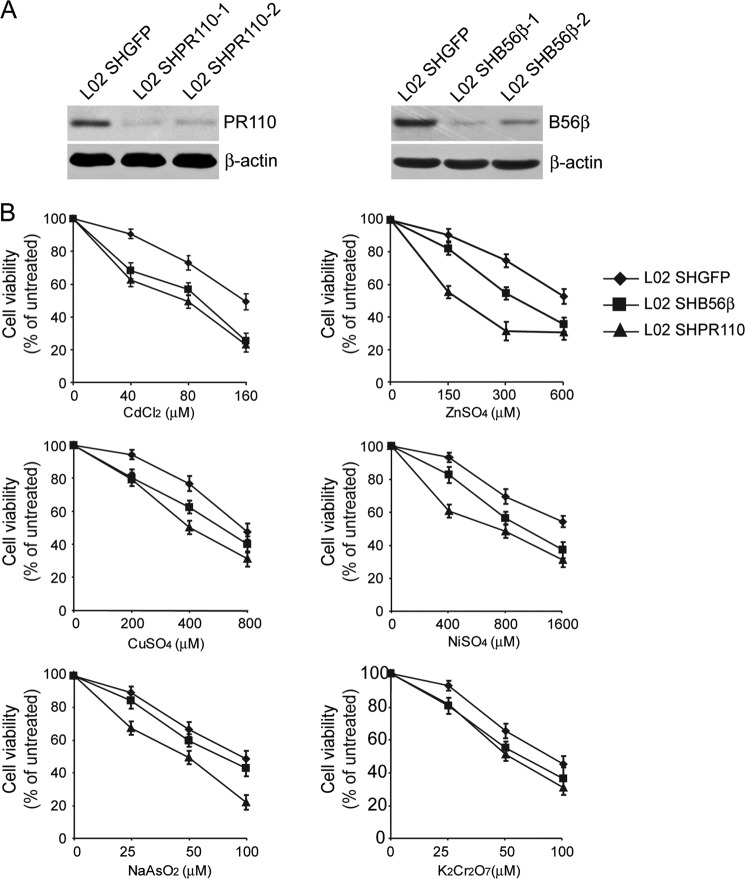
To identify which PP2A complexes participated in the regulation of MT expression, we performed a loss-of-function screen in HEK cells expressing shRNAs against each of the PP2A regulatory subunits. Two independent shRNAs that targeted different sequences of each PP2A regulatory subunit were chosen to eliminate the off-target effects. The effects of gene suppression by introduction of shRNAs were examined by immunoblotting analysis (Fig. 4). In addition, we analyzed cytotoxicity in HEK cells expressing shRNAs treated with heavy metals by MTT assay and found that suppressing the expression of PP2A B56β, B56δ, PR130, or PR110 subunit in two independent cell lines resulted in a reduced cell viability compared with HEKSHGFP cells. Immunoblotting analysis of MT in HEK cells expressing shRNAs after heavy metal treatments showed that suppressing the expression of PP2A B56δ or PR130 led to an increase in MT expression in response to ZnSO4, suggesting that B56δ or PR130 regulated heavy metal-induced cytotoxicity in a MT-independent manner. However, we observed that PP2A B56β or PR110 suppression resulted in a 62.5 ± 6.4 or 78.6 ± 4.2% declines in MT expression in HEK cells treated with 40 μm CdCl2 or 100 μm ZnSO4, respectively (Fig. 5A). Consistent with these observations, we found that PP2A B56β and PR110 suppression led to a 13.5 ± 3.7 or 23.3 ± 2.1% reduction in cell viability (p < 0.05) compared with HEKSHGFP cells treated with either 40 μm CdCl2 or 100 μm ZnSO4. Similar results were obtained when we measured cytotoxicity in a human hepatic L02 cells expressing shB56β or shPR110 (Fig. 6). Taken together, PP2A holoenzyme containing B56β or PR110 may be putatively involved in regulating heavy metal-induced cytotoxicity through modification of MT expression.
FIGURE 4.
Establishment of stable HEK cells expressing shRNAs targeting respective PP2A subunits. HEK cells were infected with two independent shRNAs that target a specific subunit of PP2A to generate stable cell lines indicated. The suppression of PP2A subunits was confirmed by immunoblotting with the specific antibodies indicated.
FIGURE 5.
Identification of specific PP2A complexes in regulation of MT expression. A, immunoblotting analysis of MT expression in HEK cells expressing shRNA that targets GFP or expressing two independent shRNAs that target B56β or PR110 with the treatment of 40 μm CdCl2 or 100 μm ZnSO4. A MTT assay was performed to measure the cytotoxicity at the indicated concentrations. The corresponding cell viability is indicated under each lane. *, p < 0.05 compared with the SHGFP control cells. B, HA-tagged MTF-1 was co-expressed with shRNAs targeting GFP, B56β, and PR110 in 293FT cells and followed by a treatment of 40 μm CdCl2 for 12 h. 3 mg of the cell lysates were co-immunoprecipitated with an antibody against the HA tag and followed by immunoblotting analysis with specific antibodies indicated. The lower panel shows the input of each protein indicated corresponding to the IP assay. The value under each band indicates -fold change of p-MTF-1 level normalized to HA-tag expression relative to SHGFP cells. C, 293FT cells were transfected with vectors encoding HA-tagged MTF-1 for 48 h and treated with 40 μm CdCl2 or 100 μm ZnSO4 for 6 h before harvesting. 3 mg of the cell lysates was subjected to co-IP using antibodies against the HA tag and PP2Ac and followed by immunoblotting using antibodies against B56β, PR110, PP2Ac, and the HA tag. D, Myc-tagged MTF-1 was co-expressed with HA-tagged vectors encoding B56β or PR110 in 293FT cells for 48 h and followed by a treatment of 40 μm CdCl2 for 6 h. The co-IP assay was performed with an antibody against the HA tag and followed by immunoblotting using specific antibodies against the myc tag and the HA tag.
FIGURE 6.
The effects of suppression of PP2A B56β or PR110 on cytotoxicity induced by various metals. A, L02 cells were infected with vectors encoding shRNAs targeting B56β and PR110 to generate stable cell lines indicated. Immunoblotting assay was performed to detect the gene suppression. B, the cell viability was measured upon various metal treatments at different concentrations in L02 cells with PP2A B56β or PR110 suppression. Data are presented as the mean ± S.E. from three experiments.
MTF-1 Is a Direct Target of PP2A PR110 Complexes
To determine whether suppression of expression of PP2A B56β or PR110 subunit has an impact on dephosphorylation of MTF-1, we co-transfected 293FT cells with shRNA vector targeting GFP, B56β, or PR110 and a vector encoding HA epitope-tagged MTF-1. 48 h after transfection, we treated 293FT cells with 40 μm CdCl2 for 6 h. The co-IP was performed with an antibody against HA tag. As a result, we found that the suppression of PR110 resulted in a 158.4 ± 63.2% elevation in p-MTF-1 after CdCl2 treatment (Fig. 5B). In concert with this observation, we detected a remarkable decrease in MT expression (Fig. 5B), reinforcing the notion that dephosphorylation of MTF-1 is required for transactivation of MT. Although the MT suppression was also presented in 293FT cells expressing shB56β, we failed to detect a direct interaction between MTF-1 and B56β and an increase in p-MTF-1, indicating there were alternative pathways involved in control of MT expression (Fig. 5B).
To address whether the direct interaction existed between PP2A complexes and MTF-1, we transfected 293FT cells with a retroviral vector encoding HA-MTF-1 for 48 h and followed by 40 μm CdCl2 or 100 μm ZnSO4 treatment for 6 h. The co-IP results revealed that MTF-1 was in complex with PP2Ac catalytic subunit in 293FT cells upon exposure to CdCl2 or ZnSO4 (Fig. 5C). Notably, we detected PR110 subunit presenting in the MTF-1 complex (Fig. 5, C and D), indicating a role of PP2A PR110 on dephosphorylation of MTF-1.
PP2A PR110 Complexes Are Involved in the Regulation of MTF-1 Activity
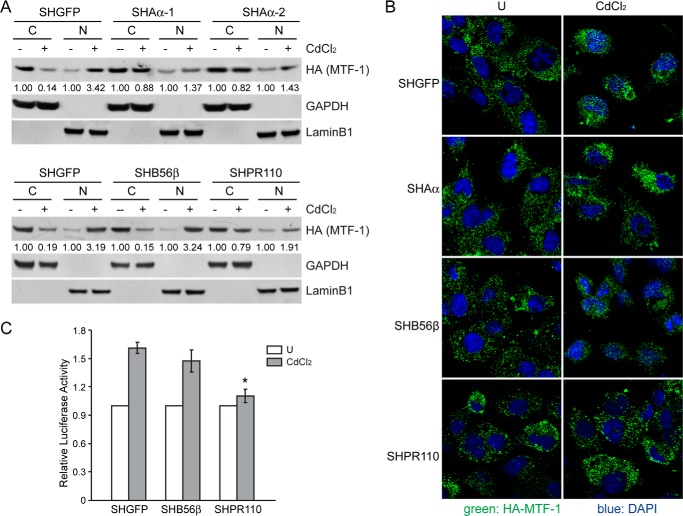
Prior studies reveal that the translocation of MTF-1 from cytoplasm to the nucleus is a prerequisite for activation of MT (36, 40). To assess whether dephosphorylation of MTF-1 by PP2A PR110 complexes mediated the nuclear import of MTF-1, we generated HEK cell lines stably expressing HA-MTF-1 in HEKSHGFP, HEKSHAα-1, HEKSHAα-2, HEKSHB56β, or HEKSHPR110 cells and visualized the localization of MTF-1 upon heavy metal treatment. Cytoplasmic and nuclear fractions of HEK cells were isolated after exposing cells to 40 μm CdCl2 for 12 h. As shown in Fig. 7A, in addition to Aα, the suppression of PP2A PR110 resulted in a 63.0 ± 7.4% decrease in the amount of MTF-1 translocation upon CdCl2 treatment. Moreover, we visualized the MTF-1 translocation under the laser scan confocal microscopy and found that the MTF-1 primarily localized in the cytoplasm in HEKSHGFP cells treated with a vehicle. However, the treatment of CdCl2 resulted in a translocation of HA-MTF-1 from the cytoplasm to the nucleus. Notably, we found that the suppression of PP2A Aα or PR110 subunit disturbed this translocation (Fig. 7B), indicating that PP2A activity and PP2A PR110 complexes were indispensible for activation of MTF-1. In contrast, B56β suppression had no impact on the translocation of MTF-1 (Fig. 7, A and B). Consistent with these observations, the luciferase reporter assay results revealed that PR110 deficiency suppressed the transcriptional activity of MTF-1 by 34.1 ± 7.6% compared with the SHGFP cells (p < 0.05) upon CdCl2 treatment. No difference was observed in the cells expressing shB56β (Fig. 7C). These observations indicate that PP2A PR110 complexes specifically regulate the activity and cellular translocation of MTF-1.
FIGURE 7.
Dephosphorylation of MTF-1 is directly regulated by PP2A PR110 complexes. A retroviral vector encoding HA-tagged MTF-1 was introduced into HEKSHGFP, HEKSHB56β, and HEKSHPR110 cells. A, HEK cells generated were treated with 40 μm CdCl2 for 12 h. Immunoblotting analysis was performed on cytoplasmic (indicated as C) and nuclear fractions (indicated as N) using specific antibodies indicated. The value under each band indicates the -fold change of MTF-1 level normalized to GAPDH or LaminB1 expression relative to vehicle control. B, HEK cells generated were treated with 40 μm CdCl2 for 12 h. U, untreated. Immunofluorescence analysis was conducted using an antibody against HA-tag (green), and the representative images were taken under a laser-scanning confocal microscopy. The nuclei (blue) were stained with DAPI. C, these cells were co-transfected with pGL3-MT1A-promoter and pRL-TK for 24 h and treated with 40 μm CdCl2 for an additional 12 h. Relative luciferase activity was measured. *, p < 0.05 compared with the SHGFP control cells.
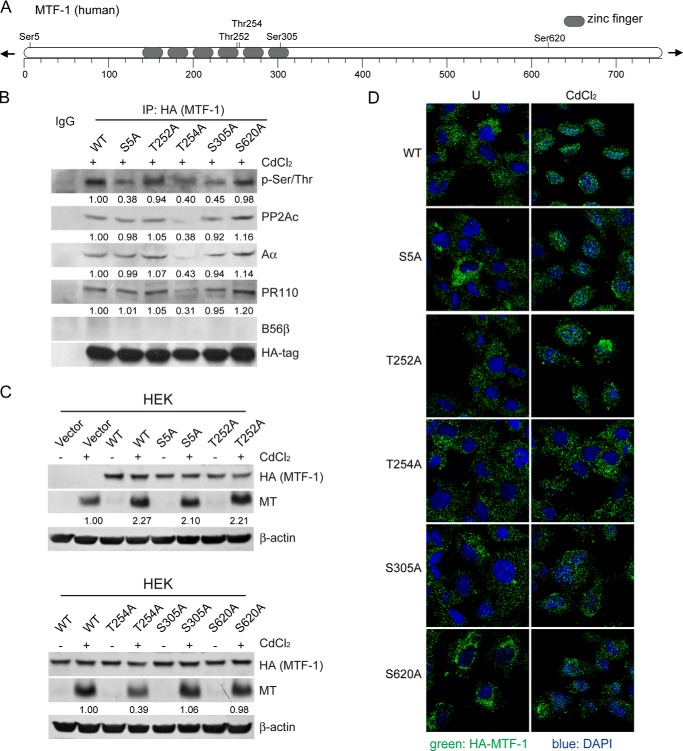
MTF-1 Thr-254 Is Responsible for MTF-1 Activation
It has been reported that MTF-1 can be phosphorylated at multiple sites (41). To determine which phosphorylated serine/threonine residue of MTF-1 interacts with PP2Ac, we generated vectors encoding wild type HA-MTF-1 (WT) or mutants HA-MTF-1 at S5A, T252A, T254A, S305A, and S620A (Fig. 8A). We expressed these vectors in 293FT cells and performed co-IP assays with an antibody against the HA tag. As a result, we found that the levels of p-MTF-1 reduced by 55∼62% in Mut-MTF-1-bound complexes at residue S5A, T254A, or S305A (Fig. 8B). However, we only observed a 64.3 ± 5.9% decline in the amount of PP2A PR110 complexes interacting with Mut-MTF-1 T254A upon CdCl2 treatment (Fig. 8B). These results imply that other phosphatases may be involved in dephosphorylation of MTF-1.
FIGURE 8.
The dephosphorylation of MTF-1 Thr-254 is responsible for MTF-1 activation. A, schematic display of serine/threonine sites of the MTF-1 predicted using PhosphoSitePlus software. B, 293FT cells were transfected with vectors encoding wild type HA-tagged MTF-1 (HA-MTF-1 WT) or each HA-MTF-1 mutant at residue S5A, T252A, T254A, S305A, and S620A for 48 h followed by 40 μm CdCl2 treatment. Co-IP was performed with an antibody against HA tag followed by immunoblotting using specific antibodies indicated. Vectors encoding HA-MTF-1 (WT) or mutants HA-MTF-1 (Mut-HA-MTF-1) S5A, T252A, T254A, S305A, and S620A were introduced into HEK cells, respectively. These cells were treated with 40 μm CdCl2 for 12 h. The value under each band indicates the -fold change of the indicated protein levels normalized to HA-tag expression relative to WT cells. C, immunoblotting analysis was performed using antibodies indicated. The value under each band indicates the -fold change of MT level normalized to β-actin expression relative to Vector or WT cells. D, immunofluorescence analysis was performed using an antibody against the HA tag (green), and the images were visualized under the laser-scanning confocal microscopy. The nuclei (blue) were stained with DAPI.
To further address whether p-MTF-1 Thr-254 played a critical role in regulation of MT transactivation, we generated HEK cells stably expressing HA-MTF-1 (WT) or mutants HA-MTF-1 (Mut-MTF-1) at residue S5A, T252A, T254A, S305A, and S620A, respectively. We analyzed MT expression on these cells treated with 40 μm CdCl2 for 12 h. As shown in Fig. 8C, the overexpression of HA-MTF-1 (WT) in HEK cells resulted in a 25% increase in MT expression. In accord with HA-MTF-1 (WT), expression of Mut-MTF-1 at residue S5A, T252A, S305A, or S620A in HEK cells led to an up-regulation of MT by 20∼30%, suggesting that the defective phosphorylation of MTF-1 at these residues did not affect MT induction. However, we failed to observe an additional transactivation of MT in HEK cells expressing Mut-MTF-1 T254A, indicating that this mutant was functionally defective in MT induction. Consistent with these results, the laser scan confocal microscopy analysis revealed that the disturbance of MTF-1 nuclear translocation only occurred in HEK cells expressing Mut-MTF-1 T254A (Fig. 8D). Taken together, these findings indicate that the phosphorylation of MTF-1 at residue Thr-254 is responsible for MTF-1 activation and subsequent MT induction.
DISCUSSION
The understanding of how environmental chemicals affect cellular responses and toxicity pathways will lead to a better prediction of toxicity and adverse health outcome. In this study, we identify particular PP2A complexes containing PR110 that participate in regulation of MT expression through direct dephosphorylation of MTF-1 at Thr-254. The perturbation of this regulatory pathway sensitizes cells to heavy metal-induced cytotoxicity. Our findings uncover a key event mediated by protein phosphatases 2A in determination of cellular response to heavy metals.
Cellular response to various stresses may change the gene expression profile, which allows cells to repair the damage. MTs, a group of stress response proteins induced at a high level by reactive oxygen species or heavy metals, is generally considered to be a critical defense mechanism in several organisms (35). Previous studies have demonstrated the important roles of MTs in numerous biological effects including zinc and copper homeostasis (42, 43), metal detoxification (14, 44), and oxygen radical scavenging (45, 46) and promote carcinogenesis (47, 48). Moreover, the amount of MTs is considered as a potential biomarker for monitoring heavy metal exposure and predicting the toxic effects based on the strong correlation between MT expression and environmental heavy metal burden (20, 49). In an effort to clarify the molecular mechanism that triggers the induction of MT expression, we identify a novel pathway that is specifically involved in regulation of MT expression and plays an important role in control of cytotoxicity. A critical event in this pathway is the dephosphorylation of MTF-1 by the specific PP2A complexes. In response to heavy metal stress, alternative phosphorylation/dephosphorylation of MTF-1 triggers the translocation of MTF-1 from cytoplasm to nucleus and the following MT transactivation. This proposed regulatory model is supported by the evidence that suppressing a particular PP2A subunit PR110 or mutation at a specific residue, Thr-254, of MTF-1 abolishes the MTF-1 nuclear import and up-regulation of MT expression. Importantly, we identify that PP2A PR110 complexes directly bind to and dephosphorylate MTF-1 at Thr-254.
MTF-1 has been considered as the major transcription factor in the induction of MT by heavy metals, hypoxia, or reactive oxygen species (24, 36). The dysfunction of MTF-1 confers cells highly sensitive to the heavy metal-induced toxic effects (36, 50). Upon heavy metal exposure or stress stimuli, MTF-1 translocates from the cytoplasm into the nucleus. Nuclear MTF-1 binds to specific DNA sequences termed as metal response elements (MREs) and in turn activates MT transcription (22, 24). It has been reported that MTF-1 activation requires stress-induced posttranslational modifications including phosphorylation (25). Although previous study demonstrates that the dephosphorylation of MTF-1 leads to its activation (24), the dynamic regulatory pattern remains unknown. In this study, we reveal that the phosphorylation and coupled dephosphorylation of MTF-1 at Thr-254 occur in response to heavy metal stress and are critical for MTF-1 activation. At an early stage, the action of phosphorylation is predominant and may reach a plateau at a certain time point. Afterward, the overwhelming dephosphorylation of MTF-1 allows the nuclear translocation of MTF-1. Moreover, we showed that T254A mutant attenuated the phosphorylation and activation of MTF-1. Similarly, in an effort to assess whether the phosphomimetic mutant T254E led to the induction of MT, we failed to observe an interaction between PP2Ac and MTF-1 and transactivation of MT (data not shown), indicating that the dephosphorylation of p-MTF-1 at Thr-254 was prerequisite for MTF-1 activation. Based on our observations, we speculate that the dynamic phosphorylation/dephosphorylation provide a signal for MTF-1 nuclear translocation. Without a signal, the unphosphorylated MTF-1 will remain in the cytoplasm and has no impact in MT induction.
In this study, we identify a PP2A holoenzyme containing PR110 as a key regulator in the cellular stress in response to heavy metals. PR110 subunit is classified as one of the PP2A regulatory B‴ subunits, also named striatin. To date, the function of the PP2A PR110 complexes and their regulatory targets remain largely unknown. A previous study showed that PP2A bound directly to and regulated the activity of estrogen receptor α (51). In the course of regulation, PR110 subunit functioned as critical molecular anchors targeting estrogen receptor to the cell membrane and served as a scaffold for the assembly of proteins, facilitating estrogen-induced activation of endothelial NO synthase (eNOS) (52). Several lines of evidence also suggest that PP2A PR110 complexes are involved in regulating vesicular trafficking (53, 54) and the remodeling of cellular cytoskeleton (55). Here, we revealed a novel role of PP2A PR110 complexes in regulation of MT expression by directly dephosphorylating MTF-1. Given the results that suppression of PR110 expression leads to abolishment of MTF-1 nuclear translocation, we conclude that MTF-1 dephosphorylation and relocation are a prerequisite for the transcriptional activation of MT.
In this study, we also showed that PP2A B56β suppression led to a down-regulation of MTs and enhanced heavy metal-induced cytotoxicity. However, we fail to find the impact of B56β on the phosphorylation of MTF-1, suggesting that PP2A B56β regulated MT expression in a MTF-1-independent manner. Consistent with these observations, a previous study reported that the activation of PI3K/AKT pathway was involved in suppression of MT expression in primary hepatocellular carcinoma (HCC) cells through inhibition of glycogen synthase kinase-3/C/EBPα signaling (56). PP2A B56β has been implicated in the dephosphorylation of AKT (57, 58). Because glycogen synthase kinase-3 and C/EBPα are the downstream targets of PP2A, which plays a role in the regulation of cell growth and survival (59, 60), we thus speculate that PP2A B56β may affect MT expression via PI3K/AKT pathway.
In summary, we discover a novel signaling pathway in which PP2A is involved in response to the regulation of heavy metals stress. The suppression of PP2A PR110-sensitized cells to heavy metal-induced cytotoxicity is attributable to the down-regulation of MT expression. The dephosphorylation of MTF-1 at residue Thr-254 is responsible for its translocation and activation. Our study also revealed that suppression of other PP2A regulatory subunits including PR130 and B56δ subunits led to an enhanced cytotoxicity of heavy metals, indicating that alternative pathways are involved in regulation of cellular toxicity independent of MTs expression. Further study is required to elucidate the comprehensive mechanism by which PP2A contribute to cellular stress response.
Acknowledgment
We thank Jonathan Crowther for critical reading of the manuscript.
This work was supported by the National Key Basic Research and Development Program (2010CB912803 and 2012CB525003), the National Nature Science Foundation of China (NSFC) (81325017, 81072284, 30901211, 81172603, and 81273127), the National “Twelfth Five-Year” Plan for Science and Technology (2014BAI12B02), the Guangdong Province Universities and Colleges Pearl River Scholar Funded Scheme GDUPS (2010), and the Natural Science Foundation of Guangdong Province (S2012040007713).
- MT
- metallothionein
- MTF-1
- metal-responsive transcription factor-1
- p-MTF-1
- phosphorylated MTF-1
- PP2A
- protein phosphatase 2A
- MTT
- 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
- co-IP
- co-immunoprecipitation.
REFERENCES
- 1. Järup L. (2003) Hazards of heavy metal contamination. Br. Med. Bull. 68, 167–182 [DOI] [PubMed] [Google Scholar]
- 2. Jomova K., Valko M. (2011) Advances in metal-induced oxidative stress and human disease. Toxicology 283, 65–87 [DOI] [PubMed] [Google Scholar]
- 3. Alissa E. M., Ferns G. A. (2011) Heavy metal poisoning and cardiovascular disease. J. Toxicol. 2011, 870125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wang M., Song H., Chen W. Q., Lu C., Hu Q., Ren Z., Yang Y., Xu Y., Zhong A., Ling W. (2011) Cancer mortality in a Chinese population surrounding a multi-metal sulphide mine in Guangdong province: an ecologic study. BMC Public Health 11, 319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wang M., Xu Y., Pan S., Zhang J., Zhong A., Song H., Ling W. (2011) Long-term heavy metal pollution and mortalitioy in a Chinese population: an ecologic study. Biol. Trace Elem. Res. 142, 362–379 [DOI] [PubMed] [Google Scholar]
- 6. Bao Q. S., Lu C. Y., Song H., Wang M., Ling W., Chen W. Q., Deng X. Q., Hao Y. T., Rao S. (2009) Behavioural development of school-aged children who live around a multi-metal sulphide mine in Guangdong province, China: a cross-sectional study. BMC Public Health 9, 217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Valko M., Morris H., Cronin M. T. (2005) Metals, toxicity, and oxidative stress. Curr. Med. Chem. 12, 1161–1208 [DOI] [PubMed] [Google Scholar]
- 8. Durnam D. M., Palmiter R. D. (1981) Transcriptional regulation of the mouse metallothionein-I gene by heavy metals. J. Biol. Chem. 256, 5712–5716 [PubMed] [Google Scholar]
- 9. Giedroc D. P., Chen X., Apuy J. L. (2001) Metal response element (MRE)-binding transcription factor-1 (MTF-1): structure, function, and regulation. Antioxid. Redox Signal. 3, 577–596 [DOI] [PubMed] [Google Scholar]
- 10. Miura N., Koizumi S. (2007) Heavy metal responses of the human metallothionein isoform genes. Yakugaku Zasshi 127, 665–673 [DOI] [PubMed] [Google Scholar]
- 11. Jin R., Chow V. T., Tan P. H., Dheen S. T., Duan W., Bay B. H. (2002) Metallothionein 2A expression is associated with cell proliferation in breast cancer. Carcinogenesis 23, 81–86 [DOI] [PubMed] [Google Scholar]
- 12. Abdel-Mageed A., Agrawal K. C. (1997) Antisense down-regulation of metallothionein induces growth arrest and apoptosis in human breast carcinoma cells. Cancer Gene Ther. 4, 199–207 [PubMed] [Google Scholar]
- 13. Klaassen C. D., Liu J., Choudhuri S. (1999) Metallothionein: an intracellular protein to protect against cadmium toxicity. Annu. Rev. Pharmacol. Toxicol. 39, 267–294 [DOI] [PubMed] [Google Scholar]
- 14. Huang P. C., Morris S., Dinman J., Pine R., Smith B. (1987) Role of metallothionein in detoxification and tolerance to transition metals. Experientia Suppl. 52, 439–446 [DOI] [PubMed] [Google Scholar]
- 15. Goering P. L., Klaassen C. D. (1983) Altered subcellular distribution of cadmium following cadmium pretreatment: possible mechanism of tolerance to cadmium-induced lethality. Toxicol. Appl. Pharmacol. 70, 195–203 [DOI] [PubMed] [Google Scholar]
- 16. Park J. D., Liu Y., Klaassen C. D. (2001) Protective effect of metallothionein against the toxicity of cadmium and other metals (1). Toxicology 163, 93–100 [DOI] [PubMed] [Google Scholar]
- 17. Conrad C. C., Walter C. A., Richardson A., Hanes M. A., Grabowski D. T. (1997) Cadmium toxicity and distribution in metallothionein-I and -II deficient transgenic mice. J. Toxicol. Environ. Health 52, 527–543 [DOI] [PubMed] [Google Scholar]
- 18. Brandão R., Santos F. W., Farina M., Zeni G., Bohrer D., Rocha J. B., Nogueira C. W. (2006) Antioxidants and metallothionein levels in mercury-treated mice. Cell Biol. Toxicol. 22, 429–438 [DOI] [PubMed] [Google Scholar]
- 19. Shariati F., Esaili Sari A., Mashinchian A., Pourkazemi M. (2011) Metallothionein as potential biomarker of cadmium exposure in Persian sturgeon (Acipenser persicus). Biol. Trace Elem. Res. 143, 281–291 [DOI] [PubMed] [Google Scholar]
- 20. Knapen D., Reynders H., Bervoets L., Verheyen E., Blust R. (2007) Metallothionein gene and protein expression as a biomarker for metal pollution in natural gudgeon populations. Aquat. Toxicol. 82, 163–172 [DOI] [PubMed] [Google Scholar]
- 21. Haq F., Mahoney M., Koropatnick J. (2003) Signaling events for metallothionein induction. Mutat. Res. 533, 211–226 [DOI] [PubMed] [Google Scholar]
- 22. Murphy B. J., Andrews G. K., Bittel D., Discher D. J., McCue J., Green C. J., Yanovsky M., Giaccia A., Sutherland R. M., Laderoute K. R., Webster K. A. (1999) Activation of metallothionein gene expression by hypoxia involves metal response elements and metal transcription factor-1. Cancer Res. 59, 1315–1322 [PubMed] [Google Scholar]
- 23. Andrews G. K. (2000) Regulation of metallothionein gene expression by oxidative stress and metal ions. Biochem. Pharmacol. 59, 95–104 [DOI] [PubMed] [Google Scholar]
- 24. Saydam N., Adams T. K., Steiner F., Schaffner W., Freedman J. H. (2002) Regulation of metallothionein transcription by the metal-responsive transcription factor MTF-1: identification of signal transduction cascades that control metal-inducible transcription. J. Biol. Chem. 277, 20438–20445 [DOI] [PubMed] [Google Scholar]
- 25. LaRochelle O., Gagné V., Charron J., Soh J. W., Séguin C. (2001) Phosphorylation is involved in the activation of metal-regulatory transcription factor 1 in response to metal ions. J. Biol. Chem. 276, 41879–41888 [DOI] [PubMed] [Google Scholar]
- 26. McCright B., Rivers A. M., Audlin S., Virshup D. M. (1996) The B56 family of protein phosphatase 2A (PP2A) regulatory subunits encodes differentiation-induced phosphoproteins that target PP2A to both nucleus and cytoplasm. J. Biol. Chem. 271, 22081–22089 [DOI] [PubMed] [Google Scholar]
- 27. Janssens V., Goris J. (2001) Protein phosphatase 2A: a highly regulated family of serine/threonine phosphatases implicated in cell growth and signalling. Biochem. J. 353, 417–439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Eichhorn P. J., Creyghton M. P., Bernards R. (2009) Protein phosphatase 2A regulatory subunits and cancer. Biochim. Biophys. Acta 1795, 1–15 [DOI] [PubMed] [Google Scholar]
- 29. Yorimitsu T., He C., Wang K., Klionsky D. J. (2009) Tap42-associated protein phosphatase type 2A negatively regulates induction of autophagy. Autophagy 5, 616–624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Arroyo J. D., Hahn W. C. (2005) Involvement of PP2A in viral and cellular transformation. Oncogene 24, 7746–7755 [DOI] [PubMed] [Google Scholar]
- 31. Virshup D. M. (2000) Protein phosphatase 2A: a panoply of enzymes. Curr Opin Cell Biol. 12, 180–185 [DOI] [PubMed] [Google Scholar]
- 32. Chen W., Possemato R., Campbell K. T., Plattner C. A., Pallas D. C., Hahn W. C. (2004) Identification of specific PP2A complexes involved in human cell transformation. Cancer Cell 5, 127–136 [DOI] [PubMed] [Google Scholar]
- 33. Ma T., Zhu Z. G., Ji Y. B., Zhang Y., Yu Y. Y., Liu B. Y., Yin H. R., Lin Y. Z. (2004) Correlation of thymidylate synthase, thymidine phosphorylase, and dihydropyrimidine dehydrogenase with sensitivity of gastrointestinal cancer cells to 5-fluorouracil and 5-fluoro-2′-deoxyuridine. World J. Gastroenterol. 10, 172–176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Yamada H., Koizumi S. (1991) Metallothionein induction in human peripheral blood lymphocytes by heavy metals. Chem. Biol. Interact. 78, 347–354 [DOI] [PubMed] [Google Scholar]
- 35. Ghoshal K., Jacob S. T. (2001) Regulation of metallothionein gene expression. Prog. Nucleic Acid Res. Mol. Biol. 66, 357–384 [DOI] [PubMed] [Google Scholar]
- 36. Heuchel R., Radtke F., Georgiev O., Stark G., Aguet M., Schaffner W. (1994) The transcription factor MTF-1 is essential for basal and heavy metal-induced metallothionein gene expression. EMBO J. 13, 2870–2875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Song M. O., Mattie M. D., Lee C. H., Freedman J. H. (2014) The role of Nrf1 and Nrf2 in the regulation of copper-responsive transcription. Exp. Cell Res. 322, 39–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Saydam N., Georgiev O., Nakano M. Y., Greber U. F., Schaffner W. (2001) Nucleo-cytoplasmic trafficking of metal-regulatory transcription factor 1 is regulated by diverse stress signals. J. Biol. Chem. 276, 25487–25495 [DOI] [PubMed] [Google Scholar]
- 39. Smirnova I. V., Bittel D. C., Ravindra R., Jiang H., Andrews G. K. (2000) Zinc and cadmium can promote rapid nuclear translocation of metal response element-binding transcription factor-1. J. Biol. Chem. 275, 9377–9384 [DOI] [PubMed] [Google Scholar]
- 40. Radtke F., Heuchel R., Georgiev O., Hergersberg M., Gariglio M., Dembic Z., Schaffner W. (1993) Cloned transcription factor MTF-1 activates the mouse metallothionein I promoter. EMBO J. 12, 1355–1362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Jiang H., Fu K., Andrews G. K. (2004) Gene- and cell-type-specific effects of signal transduction cascades on metal-regulated gene transcription appear to be independent of changes in the phosphorylation of metal-response-element-binding transcription factor-1. Biochem. J. 382, 33–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. De Lisle R. C., Sarras M. P., Jr., Hidalgo J., Andrews G. K. (1996) Metallothionein is a component of exocrine pancreas secretion: implications for zinc homeostasis. Am. J. Physiol. 271, C1103–C1110 [DOI] [PubMed] [Google Scholar]
- 43. Pearce L. L., Wasserloos K., St Croix C. M., Gandley R., Levitan E. S., Pitt B. R. (2000) Metallothionein, nitric oxide and zinc homeostasis in vascular endothelial cells. J. Nutr. 130, 1467S–1470S [DOI] [PubMed] [Google Scholar]
- 44. Ecker D. J., Butt T. R., Sternberg E. J., Neeper M. P., Debouck C., Gorman J. A., Crooke S. T. (1986) Yeast metallothionein function in metal ion detoxification. J. Biol. Chem. 261, 16895–16900 [PubMed] [Google Scholar]
- 45. Li X., Chen H., Epstein P. N. (2004) Metallothionein protects islets from hypoxia and extends islet graft survival by scavenging most kinds of reactive oxygen species. J. Biol. Chem. 279, 765–771 [DOI] [PubMed] [Google Scholar]
- 46. Anderson R. S., Patel K. M., Roesijadi G. (1999) Oyster metallothionein as an oxyradical scavenger: implications for hemocyte defense responses. Dev. Comp. Immunol. 23, 443–449 [DOI] [PubMed] [Google Scholar]
- 47. Hart B. A., Voss G. W., Vacek P. M. (1993) Metallothionein in human lung carcinoma. Cancer Lett. 75, 121–128 [DOI] [PubMed] [Google Scholar]
- 48. Mao J., Yu H., Wang C., Sun L., Jiang W., Zhang P., Xiao Q., Han D., Han D., Saiyin H., Zhu J., Chen T., Roberts L. R., Huang H., Yu L. (2012) Metallothionein MT1M is a tumor suppressor of human hepatocellular carcinomas. Carcinogenesis 33, 2568–2577 [DOI] [PubMed] [Google Scholar]
- 49. Papetti P., Rossi G. (2009) Heavy metals in the fishery products of low Lazio and the use of metallothionein as a biomarker of contamination. Environ. Monit. Assess. 159, 589–598 [DOI] [PubMed] [Google Scholar]
- 50. Palmiter R. D. (1994) Regulation of metallothionein genes by heavy metals appears to be mediated by a zinc-sensitive inhibitor that interacts with a constitutively active transcription factor, MTF-1. Proc. Natl. Acad. Sci. U.S.A. 91, 1219–1223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Lu Q., Surks H. K., Ebling H., Baur W. E., Brown D., Pallas D. C., Karas R. H. (2003) Regulation of estrogen receptor α-mediated transcription by a direct interaction with protein phosphatase 2A. J. Biol. Chem. 278, 4639–4645 [DOI] [PubMed] [Google Scholar]
- 52. Lu Q., Pallas D. C., Surks H. K., Baur W. E., Mendelsohn M. E., Karas R. H. (2004) Striatin assembles a membrane signaling complex necessary for rapid, nongenomic activation of endothelial NO synthase by estrogen receptor α. Proc. Natl. Acad. Sci. U.S.A. 101, 17126–17131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Castets F., Bartoli M., Barnier J. V., Baillat G., Salin P., Moqrich A., Bourgeois J. P., Denizot F., Rougon G., Calothy G., Monneron A. (1996) A novel calmodulin-binding protein, belonging to the WD-repeat family, is localized in dendrites of a subset of CNS neurons. J. Cell Biol. 134, 1051–1062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Baillat G., Moqrich A., Castets F., Baude A., Bailly Y., Benmerah A., Monneron A. (2001) Molecular cloning and characterization of phocein, a protein found from the Golgi complex to dendritic spines. Mol. Biol. Cell 12, 663–673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Moreno C. S., Lane W. S., Pallas D. C. (2001) A mammalian homolog of yeast MOB1 is both a member and a putative substrate of striatin family-protein phosphatase 2A complexes. J. Biol. Chem. 276, 24253–24260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Datta J., Majumder S., Kutay H., Motiwala T., Frankel W., Costa R., Cha H. C., MacDougald O. A., Jacob S. T., Ghoshal K. (2007) Metallothionein expression is suppressed in primary human hepatocellular carcinomas and is mediated through inactivation of CCAAT/enhancer binding protein α by phosphatidylinositol 3-kinase signaling cascade. Cancer Res. 67, 2736–2746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Padmanabhan S., Mukhopadhyay A., Narasimhan S. D., Tesz G., Czech M. P., Tissenbaum H. A. (2009) A PP2A regulatory subunit regulates C. elegans insulin/IGF-1 signaling by modulating AKT-1 phosphorylation. Cell 136, 939–951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Rodgers J. T., Vogel R. O., Puigserver P. (2011) Clk2 and B56β mediate insulin-regulated assembly of the PP2A phosphatase holoenzyme complex on Akt. Mol. Cell 41, 471–479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Lin C. F., Chen C. L., Chiang C. W., Jan M. S., Huang W. C., Lin Y. S. (2007) GSK-3β acts downstream of PP2A and the PI 3-kinase-Akt pathway and upstream of caspase-2 in ceramide-induced mitochondrial apoptosis. J. Cell Sci. 120, 2935–2943 [DOI] [PubMed] [Google Scholar]
- 60. Wang G. L., Iakova P., Wilde M., Awad S., Timchenko N. A. (2004) Liver tumors escape negative control of proliferation via PI3K/Akt-mediated block of C/EBP α growth inhibitory activity. Genes Dev. 18, 912–925 [DOI] [PMC free article] [PubMed] [Google Scholar]