Abstract
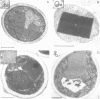
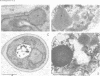
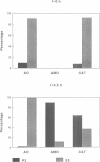
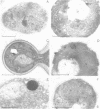
We have identified two temperature-sensitive peroxisome-deficient mutants of Hansenula polymorpha (ts6 and ts44) within a collection of ts mutants which are impaired for growth on methanol at 43 degrees C but grow well at 35 degrees C. In both strains peroxisomes were completely absent in cells grown at 43 degrees C; the major peroxisomal matrix enzymes alcohol oxidase, dihydroxyacetone synthase and catalase were synthesized normally but assembled into the active enzyme protein in the cytosol. As in wild-type cells, these enzymes were present in peroxisomes under permissive growth conditions (< or = 37 degrees C). However, at intermediate temperatures (38-42 degrees C) they were partly peroxisome-bound and partly resided in the cytosol. Genetic analysis revealed that both mutant phenotypes were due to monogenic recessive mutations mapped in the same gene, designated PER13. After a shift of per13-6ts cells from restrictive to permissive temperature, new peroxisomes were formed within 1 h. Initially one--or infrequently a few--small organelles developed which subsequently increased in size and multiplied by fission during prolonged permissive growth. Neither mature peroxisomal matrix nor membrane proteins, which were present in the cytosol prior to the temperature shift, were incorporated into the newly formed organelles. Instead, these proteins remained unaffected (and active) in the cytosol concomitant with further peroxisome development. Thus in H.polymorpha alternative mechanisms of peroxisome biogenesis may be possible in addition to multiplication by fission upon induction of the organelles by certain growth substrates.
Full text
PDF


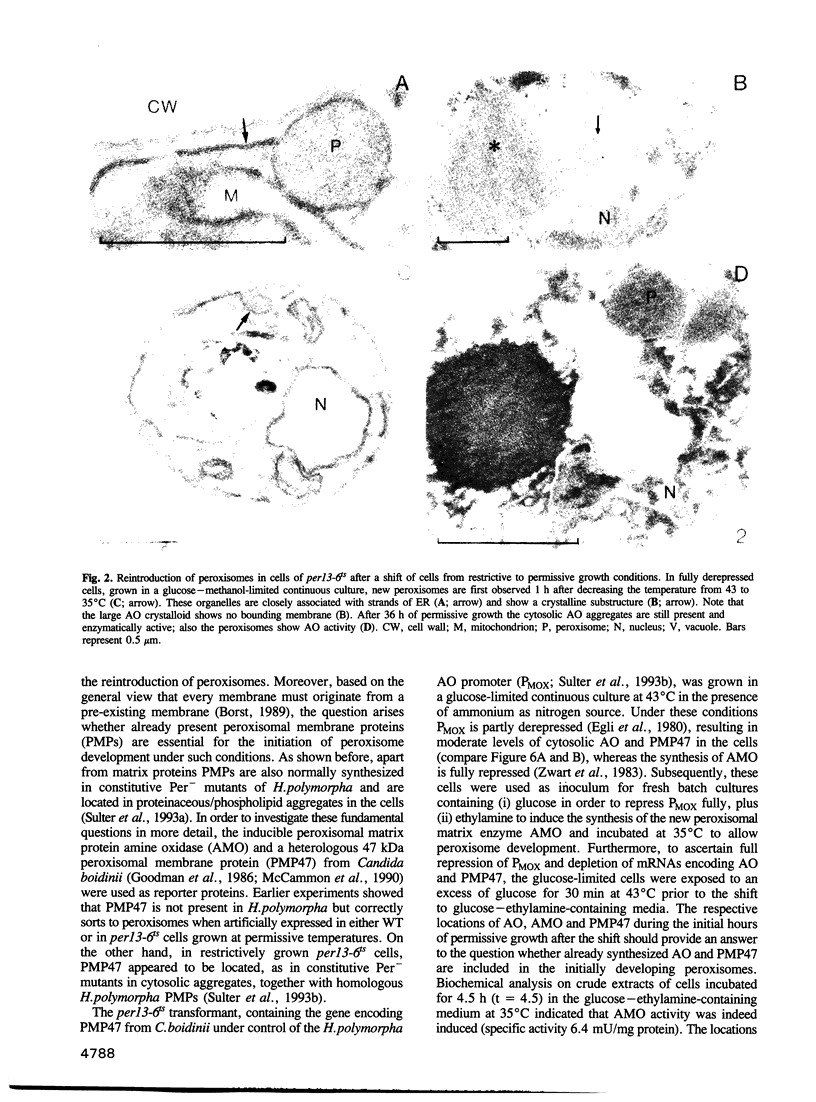
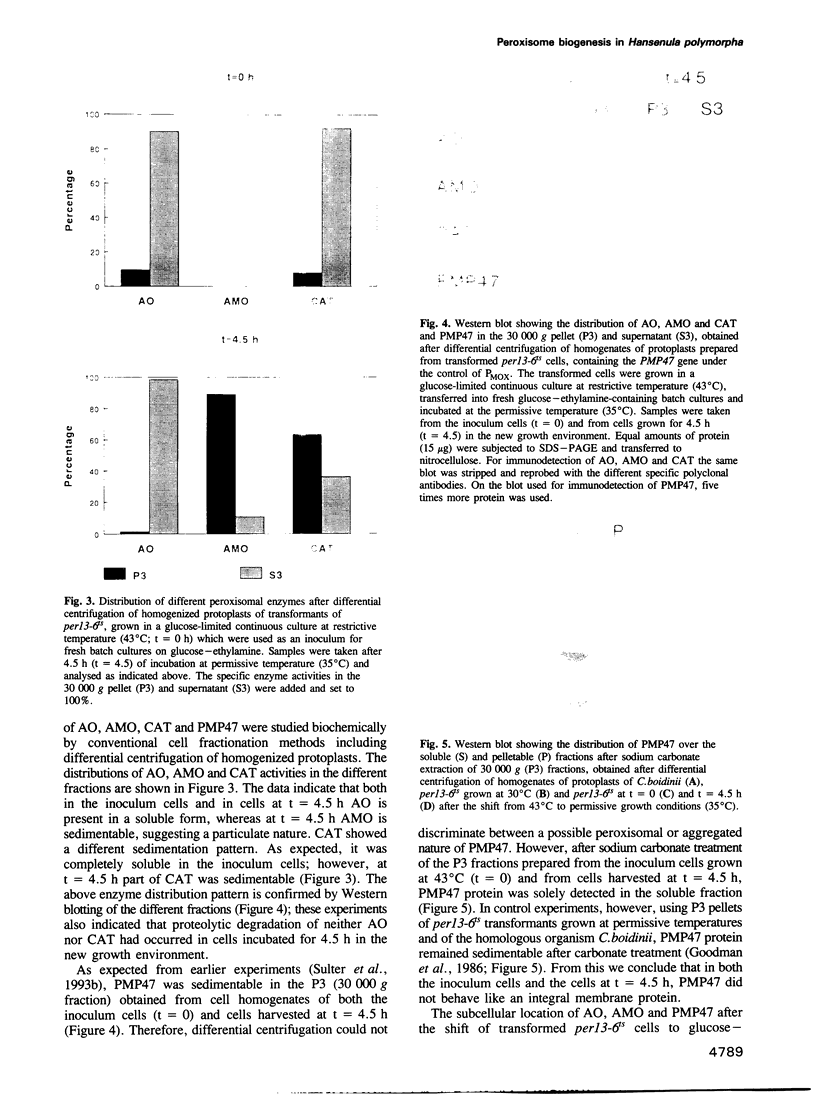
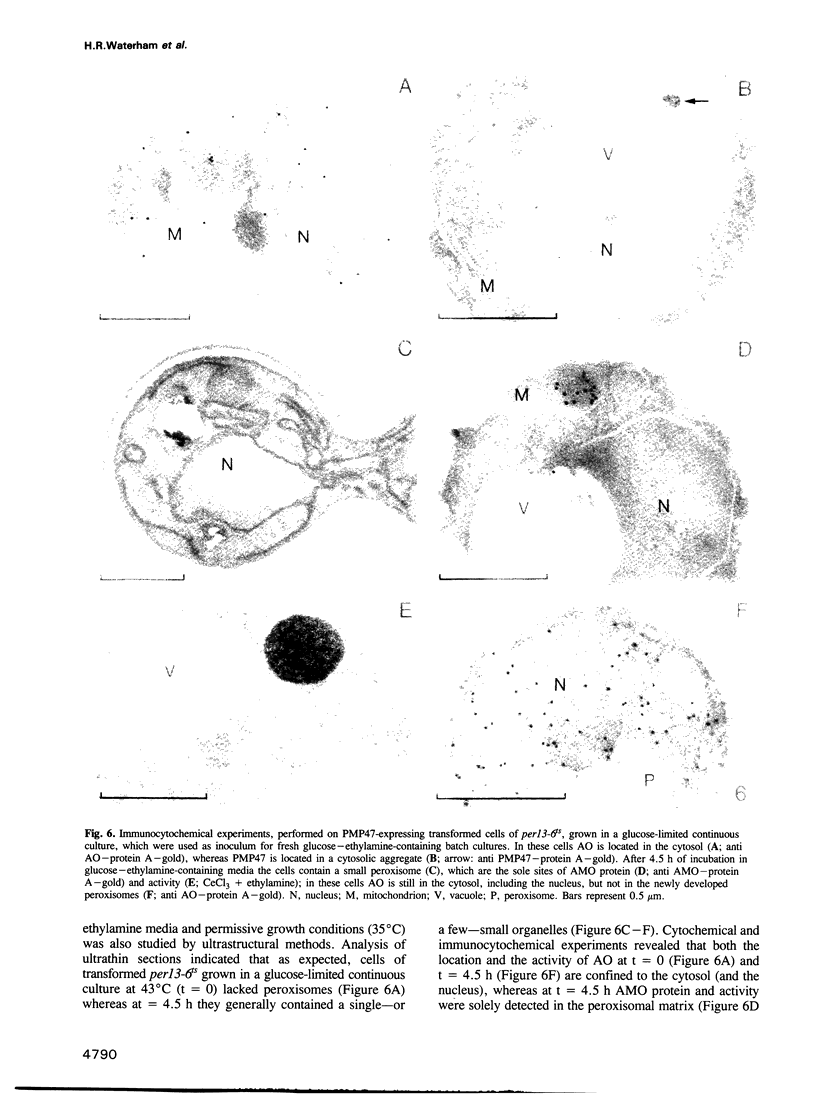




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen L. A., Morand O. H., Raetz C. R. Cytoplasmic requirement for peroxisome biogenesis in Chinese hamster ovary cells. Proc Natl Acad Sci U S A. 1989 Sep;86(18):7012–7016. doi: 10.1073/pnas.86.18.7012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borst P. Peroxisome biogenesis revisited. Biochim Biophys Acta. 1989 Jun 1;1008(1):1–13. doi: 10.1016/0167-4781(89)90163-2. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Bruinenberg P. G., Evers M., Waterham H. R., Kuipers J., Arnberg A. C., AB G. Cloning and sequencing of the peroxisomal amine oxidase gene from Hansenula polymorpha. Biochim Biophys Acta. 1989 Jul 7;1008(2):157–167. doi: 10.1016/0167-4781(80)90003-2. [DOI] [PubMed] [Google Scholar]
- Brul S., Wiemer E. A., Westerveld A., Strijland A., Wanders R. J., Schram A. W., Heymans H. S., Schutgens R. B., Van den Bosch H., Tager J. M. Kinetics of the assembly of peroxisomes after fusion of complementary cell lines from patients with the cerebro-hepato-renal (Zellweger) syndrome and related disorders. Biochem Biophys Res Commun. 1988 May 16;152(3):1083–1089. doi: 10.1016/s0006-291x(88)80395-4. [DOI] [PubMed] [Google Scholar]
- Goodman J. M., Maher J., Silver P. A., Pacifico A., Sanders D. The membrane proteins of the methanol-induced peroxisome of Candida boidinii. Initial characterization and generation of monoclonal antibodies. J Biol Chem. 1986 Mar 5;261(7):3464–3468. [PubMed] [Google Scholar]
- Heikoop J. C., van den Berg M., Strijland A., Weijers P. J., Just W. W., Meijer A. J., Tager J. M. Turnover of peroxisomal vesicles by autophagic proteolysis in cultured fibroblasts from Zellweger patients. Eur J Cell Biol. 1992 Apr;57(2):165–171. [PubMed] [Google Scholar]
- Kunau W. H., Hartig A. Peroxisome biogenesis in Saccharomyces cerevisiae. Antonie Van Leeuwenhoek. 1992 Aug;62(1-2):63–78. doi: 10.1007/BF00584463. [DOI] [PubMed] [Google Scholar]
- Kyhse-Andersen J. Electroblotting of multiple gels: a simple apparatus without buffer tank for rapid transfer of proteins from polyacrylamide to nitrocellulose. J Biochem Biophys Methods. 1984 Dec;10(3-4):203–209. doi: 10.1016/0165-022x(84)90040-x. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lazarow P. B., Fujiki Y. Biogenesis of peroxisomes. Annu Rev Cell Biol. 1985;1:489–530. doi: 10.1146/annurev.cb.01.110185.002421. [DOI] [PubMed] [Google Scholar]
- McCammon M. T., Dowds C. A., Orth K., Moomaw C. R., Slaughter C. A., Goodman J. M. Sorting of peroxisomal membrane protein PMP47 from Candida boidinii into peroxisomal membranes of Saccharomyces cerevisiae. J Biol Chem. 1990 Nov 25;265(33):20098–20105. [PubMed] [Google Scholar]
- Santos M. J., Imanaka T., Shio H., Lazarow P. B. Peroxisomal integral membrane proteins in control and Zellweger fibroblasts. J Biol Chem. 1988 Jul 25;263(21):10502–10509. [PubMed] [Google Scholar]
- Santos M. J., Imanaka T., Shio H., Small G. M., Lazarow P. B. Peroxisomal membrane ghosts in Zellweger syndrome--aberrant organelle assembly. Science. 1988 Mar 25;239(4847):1536–1538. doi: 10.1126/science.3281254. [DOI] [PubMed] [Google Scholar]
- Sulter G. J., Vrieling E. G., Harder W., Veenhuis M. Synthesis and subcellular location of peroxisomal membrane proteins in a peroxisome-deficient mutant of the yeast Hansenula polymorpha. EMBO J. 1993 May;12(5):2205–2210. doi: 10.1002/j.1460-2075.1993.tb05868.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulter G. J., Waterham H. R., Vrieling E. G., Goodman J. M., Harder W., Veenhuis M. Expression and targeting of a 47 kDa integral peroxisomal membrane protein of Candida boidinii in wild type and a peroxisome-deficient mutant of Hansenula polymorpha. FEBS Lett. 1993 Jan 11;315(3):211–216. doi: 10.1016/0014-5793(93)81166-w. [DOI] [PubMed] [Google Scholar]
- Titorenko V. I., Waterham H. R., Cregg J. M., Harder W., Veenhuis M. Peroxisome biogenesis in the yeast Hansenula polymorpha is controlled by a complex set of interacting gene products. Proc Natl Acad Sci U S A. 1993 Aug 15;90(16):7470–7474. doi: 10.1073/pnas.90.16.7470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veenhuis M. Peroxisome biogenesis and function in Hansenula polymorpha. Cell Biochem Funct. 1992 Sep;10(3):175–184. doi: 10.1002/cbf.290100307. [DOI] [PubMed] [Google Scholar]
- Veenhuis M., Van Dijken J. P., Harder W. The significance of peroxisomes in the metabolism of one-carbon compounds in yeasts. Adv Microb Physiol. 1983;24:1–82. doi: 10.1016/s0065-2911(08)60384-7. [DOI] [PubMed] [Google Scholar]
- Veenhuis M., van Dijken J. P., Harder W. Cytochemical studies on the localization of methanol oxidase and other oxidases in peroxisomes of methanol-grown Hansenula polymorpha. Arch Microbiol. 1976 Dec 1;111(1-2):123–135. doi: 10.1007/BF00446559. [DOI] [PubMed] [Google Scholar]
- Walton P. A., Gould S. J., Feramisco J. R., Subramani S. Transport of microinjected proteins into peroxisomes of mammalian cells: inability of Zellweger cell lines to import proteins with the SKL tripeptide peroxisomal targeting signal. Mol Cell Biol. 1992 Feb;12(2):531–541. doi: 10.1128/mcb.12.2.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waterham H. R., Keizer-Gunnink I., Goodman J. M., Harder W., Veenhuis M. Development of multipurpose peroxisomes in Candida boidinii grown in oleic acid-methanol limited continuous cultures. J Bacteriol. 1992 Jun;174(12):4057–4063. doi: 10.1128/jb.174.12.4057-4063.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiemer E. A., Brul S., Just W. W., Van Driel R., Brouwer-Kelder E., Van Den Berg M., Weijers P. J., Schutgens R. B., Van Den Bosch H., Schram A. Presence of peroxisomal membrane proteins in liver and fibroblasts from patients with the Zellweger syndrome and related disorders: evidence for the existence of peroxisomal ghosts. Eur J Cell Biol. 1989 Dec;50(2):407–417. [PubMed] [Google Scholar]
- Zoeller R. A., Allen L. A., Santos M. J., Lazarow P. B., Hashimoto T., Tartakoff A. M., Raetz C. R. Chinese hamster ovary cell mutants defective in peroxisome biogenesis. Comparison to Zellweger syndrome. J Biol Chem. 1989 Dec 25;264(36):21872–21878. [PubMed] [Google Scholar]
- van Dijken J. P., Veenhuis M., Vermeulen C. A., Harder W. Cytochemical localization of catalase activity in methanol-grown Hansenula polymorpha. Arch Microbiol. 1975 Nov 7;105(3):261–267. doi: 10.1007/BF00447145. [DOI] [PubMed] [Google Scholar]
- van der Klei I. J., Harder W., Veenhuis M. Methanol metabolism in a peroxisome-deficient mutant of Hansenula polymorpha: a physiological study. Arch Microbiol. 1991;156(1):15–23. doi: 10.1007/BF00418181. [DOI] [PubMed] [Google Scholar]