Abstract
Moderate weight loss (>5%), which has been associated with improvements in glycemic parameters in patients with dysglycemia, also reduces the presence of other comorbidities, including dyslipidemia and hypertension, culminating in a reduced risk of cardiovascular disease. Lifestyle changes are the recommended preliminary approach to weight loss, with an initial weight-loss goal of 10% of body weight achieved over 6 months at a rate of 1–2 pounds per week selected as an appropriate target to decrease the severity of obesity-related risk factors. Implementing and maintaining the lifestyle changes associated with weight loss can, however, be challenging for many patients. Therefore, additional interventions sometimes may be necessary. Bariatric surgery can also be a highly effective option for weight loss and comorbidity reduction, but surgery carries considerable risks and is still applicable only to selected patients with type 2 diabetes. Thus, attention is turning to the use of weight-loss medications, including 2 recently approved compounds: twice-daily lorcaserin and a once-daily combination of phentermine and topiramate extended-release, both shown to be safe and effective therapies in the management of obesity in patients with type 2 diabetes.
Keywords: Phentermine, Topiramate extended-release, Obesity, Type 2 diabetes, Cardiovascular
1. Introduction
Overweight and obesity are global epidemics affecting about 70% and 35% of the US population, respectively (Flegal, Carroll, Kit, & Ogden, 2012). Both are associated with type 2 diabetes mellitus (T2DM), which itself increases the risk of weight gain (Flegal et al., 2012; Reaven, 2003; Siram, Yanagisawa, & Skamagas, 2010; Tzotzas, Evangelou, & Kiortsis, 2011). Type 2 diabetes mellitus and overweight/obesity both increase risk of cardiovascular disease (CVD) and other comorbidities (American Diabetes Association, 2012; National Heart, Lung, & Blood Institute. The Clinical Guidelines on the Identification, 1998). The American Diabetes Association (ADA) suggests that management of T2DM in overweight/obese patients should focus on control of hyperglycemia (HbA1c goal <7%) via antidiabetic pharmacotherapy, together with moderate weight loss (~7%) (American Diabetes Association, 2012), since the latter is associated with improvements in glycemic parameters in patients with dysglycemia and may subsequently reduce the need for antidiabetic medications (American Diabetes Association, 2012).
While lifestyle changes (dietary modification focused on caloric reduction, increased physical activity, and behavior therapy) (National Heart, Lung, & Blood Institute. The Clinical Guidelines on the Identification, 1998) can produce weight loss in overweight and obese individuals, sustained adherence to these changes and maintenance of weight reduction is often challenging (Greenberg, Stampfer, Schwarzfuchs, Shai, & DIRECT Group, 2009; Mata, Todd, & Lippke, 2010). This review will discuss T2DM and its comorbidities as they relate to obesity and the need for treatment options to address the associated risks. Two compounds that were recently approved by the US Food and Drug Administration (FDA) for the chronic management of obesity or overweight in the presence of ≥1 weight-related comorbidity as adjuncts to a reduced-calorie diet and increased physical activity will be described: twice-daily (BID) lorcaserin (BELVIQ 2012), and once-daily (QD) phentermine and extended-release topiramate (PHEN/TPM ER) (QSYMIA 2012).
2. Obesity-related complications of type 2 diabetes
2.1. Pathophysiology of obesity and T2DM
Although obesity is fundamentally related to an imbalance between energy intake and energy expenditure, it is estimated that genetic variability accounts for around 50% of the variation in body mass within a population (de Ferranti & Mozaffarian, 2008; Lyon & Hirschhorn, 2005) via impact on hormone levels, body composition, and energy metabolism (Lentes et al., 1999). Additional factors leading to alterations in metabolism and/or appetite, such as a lack of sleep, illness, and choice of macronutrients, can also contribute (de Ferranti & Mozaffarian, 2008). The resultant energy imbalance leads to hypertrophy and hyperplasia of adipocytes (de Ferranti & Mozaffarian, 2008). Adipose tissues modulate metabolism by releasing non-esterified fatty acids (NEFAs), glycerol, hormones (e.g., leptin and adiponectin) (Lara-Castro, Fu, Chung, & Garvey, 2007), and proinflammatory cytokines (such as interleukin [IL]-6, and insulin growth factor [IGF]-1) (de Ferranti & Mozaffarian, 2008; Kahn, Hull, & Utzschneider, 2006; Scherer, 2006; Shoelson, Lee, & Goldfine, 2006; Wellen & Hotamisligil, 2005). The expansion of adipose tissue in obese patients is associated with changes in the release of numerous adipokines from adipocytes, as well as from macrophages and other cells that populate adipose tissue.
In particular, NEFAs, which are released from adipocytes, have been shown to induce insulin resistance and impair β-cell function, with increased levels observed in both obesity and T2DM (Boden, 1997; Kahn et al., 2006; Reaven, Hollenbeck, Jeng, Wu, & Chen, 1988). Insulin resistance has also been shown to arise in healthy subjects in response to high plasma NEFA levels induced through diet (Roden et al., 1996), suggesting an association between insulin resistance and obesity (Kahn et al., 2006). A possible mechanism for the β-cell dysfunction associated with obesity (Kahn et al., 2006) that may also contribute to the pathogenesis of T2DM (Schaffer, 2003) is lipid (triglyceride) accumulation in pancreatic β-cells (de Ferranti & Mozaffarian, 2008). In addition, the secretion of an adipokine, adiponectin, which is associated with positive metabolic and vascular effects, is reduced in obesity and may contribute to the pathogenesis of metabolic syndrome, T2DM, and atherosclerosis (Lara-Castro et al., 2007). Finally, it has been suggested that proinflammatory cytokines, such as TNF-α secreted by macrophages present in adipose tissue, play a significant role in obesity-related insulin resistance (Kern, Ranganathan, Li, Wood, & Ranganathan, 2001).
2.2. Comorbidities of T2DM and obesity
The association between obesity and cardiometabolic risk factors, such as dyslipidemia and hypertension (National Heart, Lung, & Blood Institute. The Clinical Guidelines on the Identification, 1998), may lead to further complications in patients with T2DM (American Diabetes Association, 2012; Eckel et al., 2011). For example, it has been reported that even newly diagnosed T2DM is associated with a 25% increase in risk of CVD, emanating from numerous contributors (Wilson & Kannel, 2002). Since obesity often precedes the development of T2DM, common pathogenic mechanisms (Eckel et al., 2011) and comorbidities may already be present (National Heart, Lung, & Blood Institute. The Clinical Guidelines on the Identification, 1998; Wilson & Kannel, 2002).
Obese patients with T2DM have been reported to show significantly increased serum levels of total cholesterol, low-density lipoprotein (LDL) cholesterol, very-low-density lipoprotein (VLDL) cholesterol, and triglycerides and decreased high-density lipoprotein (HDL) cholesterol compared with non-obese patients with T2DM (Saxena, Agrawal, Gautam, Bid, & Banerjee, 2009). Research in monozygotic twins has shown that acquired obesity is associated with increased activity of fibrinogen and coagulation markers, which are strongly correlated with inflammation and insulin resistance and, as such, may increase risk of thrombosis and cardiovascular events (Kaye et al., 2012).
Hypertension is also commonly seen with both T2DM and obesity and is a major risk factor for CVD and microvascular complications. Due to the synergistic effects of hypertension and T2DM, the diagnostic cutoff for hypertension is lower for those with concurrent T2DM (≥130/80 mm Hg) vs those without (≥140/90 mm Hg) (American Diabetes Association, 2012; Chobanian et al., 2003).
3. Effects of intentional weight loss on T2DM
Studies have shown that moderate weight loss in obese patients with T2DM leads to decreased insulin resistance, improvements in glycemic parameters, and reductions in T2DM-related complications as well as in several CVD risk factors, including dyslipidemia and elevated blood pressure (BP) (American Diabetes Association, 2012; National Heart, Lung, & Blood Institute. The Clinical Guidelines on the Identification, 1998). Even modest reductions in risk factors can have a substantial impact on mortality – for example, reductions in systolic BP (SBP) of 5 mm Hg can reduce the risk of mortality from stroke by 14% and coronary heart disease (CHD) by 9% (Fig. 1) (Whelton et al., 2002). In this article, we discuss the relative merits of different approaches to intentional weight loss: lifestyle changes, bariatric surgery, and pharmacotherapy. It is important to remember that some medications used to treat T2DM may induce weight gain (insulin, sulfonylureas, glinides, thiazolidinediones), while others are reported to be weight neutral (DPP-4 inhibitors, acarbose, miglitol, bromocriptine) (Bray & Ryan, 2012). There are also a few antidiabetic medications that may lead to a modest degree of weight loss (metformin, pramlintide, exenatide, liraglutide) (Bray & Ryan, 2012). These effects should be considered when determining treatment options for obese patients with T2DM.
Fig. 1.
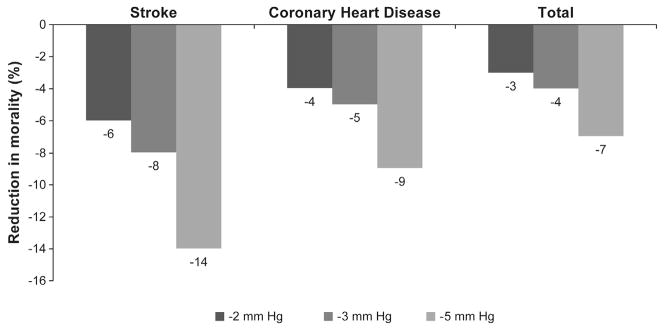
Reductions in mortality by reduction in systolic blood pressure (SBP) (Chobanian et al., 2003; Whelton et al., 2002). Figure adapted from Whelton et al., 2002.
3.1. Lifestyle changes
Lifestyle changes, involving modification of diet, physical activity, and behavior, are the initial approaches to weight-loss therapy recommended by the National Heart, Lung, and Blood Institute (NHLBI) (National Heart, Lung, & Blood Institute. The Clinical Guidelines on the Identification, 1998). The NHLBI guidelines state that an initial weight loss of 10% of body weight achieved over 6 months, at a rate of 1 to 2 pounds per week, is an appropriate target to decrease the severity of obesity-related risk factors (National Heart, Lung, & Blood Institute. The Clinical Guidelines on the Identification, 1998). The ADA also recommends weight loss of 7% or more for all overweight or obese individuals who have or are at risk of developing T2DM, citing caloric reduction, physical activity, and behavior modification as important elements in aiding and maintaining weight loss (American Diabetes Association, 2012).
In the Look AHEAD (Action for Health in Diabetes) study, weight loss of 8.6% with intensive lifestyle intervention (Table 1) was associated with significant improvements in CVD risk factors in 5145 overweight/obese participants with T2DM (Table 2) (Look AHEAD Research Group et al., 2007). When stratifying these patients by degree of weight loss, those achieving weight loss of 5% to <10% experienced significant improvements in CVD risk factors (Wing et al., 2011) compared with participants whose weight remained stable. Specifically, weight loss of 5% to 10% led to a 0.5% reduction in HbA1c, a 5 mm Hg decrease in SBP and diastolic BP (DBP), a 5 mg/dL increase in HDL-cholesterol, and a 40 mg/dL decrease in triglycerides (Wing et al., 2011). For those who lost 10% to 15% of their body weight, the odds of achieving clinically significant improvements in most risk factors were even greater (Wing et al., 2011). In the longer term, intensive lifestyle intervention participants had modest weight loss (4.7% at 4 years), decreased sleep apnea, increased quality of life, and improved CVD risk factors, while requiring fewer medications than the usual care group (Look AHEAD Research Group & Wing, 2010). However, in September 2012, an interim analysis found a lower than expected rate of cardiovascular events in all participants with no significant difference between treatment arms, resulting in the Look AHEAD study being ended prematurely (National Institutes of Health, 2012).
Table 1.
Effects of weight-loss treatment options on body weight.
| Study | Trial design | Weight loss |
|---|---|---|
| Intensive lifestyle intervention | ||
| Look AHEAD (Action for Health in Diabetes) (Look AHEAD Research Group et al., 2007) |
|
Mean change at year 1
|
| Bariatric surgery (Kadera et al., 2009) |
|
Mean percentage of EWL at year 1
|
| SAGB vs LB (Cunneen, 2008) |
|
Mean percentage of EWL at year 3
|
| MOBIL (Morbid Obesity treatment, Bariatric Surgery vs ILI) Study (Hofsø et al., 2011) |
|
Mean weight loss at year 1
|
| Swedish Obese Subjects (SOS) study (Sjöström et al., 2012) |
|
Mean change in body weight after 2, 10, 15, and 20 years
|
| Pharmacotherapies | ||
| Orlistat | ||
| Orlistat Obesity Study Group (Rössner et al., 2000) |
|
Mean weight loss at year 1
|
| XENDOS (XENical in the prevention of diabetes in obese subjects) (Torgerson et al., 2004) |
|
Mean weight loss at 4 years
|
| (Zhou et al., 2012) |
|
Overall weight loss
|
| Lorcaserin | ||
| BLOOM (Behavioral Modification and Lorcaserin for Overweight and Obesity Management) (Smith et al., 2010) |
|
Mean weight loss at year 1
|
| BLOSSOM (Behavioral modification and Lorcaserin Second Study) (Fidler et al., 2011) |
|
Mean weight loss at year 1
|
| BLOOM-DM (O’Neil et al., 2012) |
|
Mean weight loss at year 1
|
| Phentermine/Topiramate ER | ||
| EQUIP (Allison et al., 2012) |
|
LS mean weight loss at year 1
|
| CONQUER (Gadde et al., 2011) |
|
LS mean weight loss at year 1
|
| Naltrexone SR/bupropion SR | ||
| COR-I (Greenway et al., 2010) |
|
Mean weight loss at year 1
|
| COR-II (Rubino et al., 2010) |
|
Mean weight loss at year 1
|
| COR-DM (Hollander et al., 2010) |
|
Mean weight loss at year 1
|
| COR-BMOD (Wadden et al., 2011) |
|
Mean weight loss at year 1
|
| Liraglutide (Astrup et al., 2009) |
|
Mean change from randomization (after a diet and placebo run-in period) to week 20:
|
RCT, randomized, controlled trial; ILI, intensive lifestyle intervention; DSE, Diabetes Support and Education; RYGB, Roux-en-Y gastric bypass; EWL, excess weight loss; SAGB, Swedish Adjustable Gastric Bypass; LB, Lap-Band; NGT, normal glucose tolerance; NB, naltrexone/bupropion; PBO, placebo
Table 2.
Effects of weight-loss treatment options on cardiometabolic risk factors.
| Study | Cardiometabolic risk factors |
|---|---|
| Intensive lifestyle intervention | |
| Look AHEAD (Action for Health in Diabetes) (Look AHEAD Research Group et al., 2007) | Mean change from baseline to 1 year (for ISI vs DSE)
|
| Bariatric surgery (Kadera et al., 2009) |
|
| SAGB vs LB (Cunneen, 2008) |
|
| MOBIL (Morbid Obesity treatment, Bariatric Surgery vs ILI) Study (Hofsø et al., 2011) |
|
| Swedish Obese Subjects (SOS) study (Sjöström et al., 2012) |
|
| Pharmacotherapies | |
| Orlistat (Rössner et al., 2000) | Changes from baseline to year 1 (for orlistat vs PBO)
|
| XENDOS (XENical in the prevention of diabetes in obese subjects) (Torgerson et al., 2004) | Effects from baseline to year 4 (for orlistat vs PBO)
|
| (Zhou et al., 2012) | Effects from baseline to study end (meta-analysis; for orlistat vs PBO)
|
| Lorcaserin | |
| BLOOM (Behavioral Modification and Lorcaserin for Overweight and Obesity Management) (Smith et al., 2010) | Changes from baseline to year 1 (ITT-LOCF; for lorcaserin BID vs PBO)
|
| BLOSSOM (Behavioral modification and Lorcaserin Second Study) (Fidler et al., 2011) | Changes from baseline to year 1 (mITT-LOCF)
|
| BLOOM-DM (O’Neil et al., 2012) | Changes from baseline to year 1 (mITT-LOCF)
|
| Phentermine/Topiramate ER | |
| EQUIP (Allison et al., 2012) | Changes from baseline to year 1 (ITT-LOCF; PHEN/TPM ER vs PBO)
|
| CONQUER (Gadde et al., 2011; Garvey et al., 2010) | Changes from baseline to year 1 in the overall population (ITT-LOCF; PHEN/TPM ER vs PBO)
|
| Naltrexone SR/bupropion SR (investigational, not approved for weight loss) | |
| COR-I (Greenway et al., 2010; Makowski et al., 2011) | Changes from baseline to year 1 (for NB16/NB32 vs PBO)
|
| COR-II (Makowski et al., 2011; Rubino et al., 2010) | Changes from baseline to year 1 (mITT-LOCF; NB32 vs PBO)
|
| COR-DM (Hollander et al., 2010; Makowski et al., 2011) | Changes from baseline to year 1 (mITT-LOCF; NB32 vs PBO)
|
| COR-BMOD (Makowski et al., 2011; Wadden et al., 2011) | Changes from baseline to year 1 (mITT-LOCF; NB32 vs PBO)
|
| Liraglutide (not approved for weight loss) (Astrup et al., 2009) | Mean change from randomization (after a diet and PBO run-in period) to week 20:
|
ILI, intensive lifestyle intervention; DSE, Diabetes Support and Education; FG, fasting glucose; SAGB, Swedish Adjustable Gastric Bypass; LB, Lap-Band; RYGB, Roux-en-Y Gastric Bypass; PBO, placebo; FI, fasting insulin; NB, naltrexone/bupropion.
A 75-g oral glucose tolerance test (OGTT) was performed at baseline and then at every 6 months. Diagnosis of T2DM was based on a single 2-h whole blood glucose measure ≥10 mmol/L. After the first 6 months of the study, by a protocol amendment, patients with a diabetic OGTT underwent a repeat OGTT within 4 weeks. A repeat positive test was based on a 2-h whole blood glucose ≥10 mmol/L, a whole blood fasting glucose ≥6.7 mmol/L, or 2 consecutive fasting whole blood glucose measurements ≥6.7 mmol/L.
Weight loss through lifestyle changes alone is often difficult to implement and maintain (Barte et al., 2010; UKPDS Group, 1990), with almost half of patients failing to maintain ≥75% of their initial weight loss over 52 weeks (Wadden et al., 2010). Therefore, medical interventions, such as surgery or pharmacotherapy, may be a necessary adjunct in some patients.
3.2. Bariatric surgery
Bariatric surgery is recommended for consideration in adults with body mass index (BMI) >35 kg/m2 and T2DM, especially if the T2DM or associated comorbidities are difficult to control with lifestyle and pharmacologic therapy (American Diabetes Association, 2012). Surgery can be a highly effective option for weight and comorbidity reduction, providing medically significant weight loss that is sustained for more than 5 years in most patients (National Heart, Lung, & Blood Institute. The Clinical Guidelines on the Identification, 1998). Bariatric surgery has been shown to improve pancreatic β-cell function (Hofsø et al., 2011) and may reduce the need for medication in obese patients with T2DM and lead to remission of T2DM and hypertension in some cases (Tables 1 and 2) (Cunneen, 2008; Kadera et al., 2009).
The Swedish Obese Subjects (SOS) study, an ongoing, nonrandomized, prospective, controlled study, demonstrated that the risk of cardiovascular death and cardiovascular events, as assessed in 4047 obese subjects (of whom 2010 underwent bariatric surgery and 2037 received conventional treatment), was reduced in those who received bariatric surgery compared with conventional treatment (Tables 1 and 2) (Sjöström et al., 2012). It was found that the benefit of bariatric surgery with respect to cardiovascular events was not related to baseline BMI (P = .58), but was significantly associated with baseline plasma insulin (P < .001), with increasing baseline insulin levels related to increasing treatment benefit, suggesting that insulin resistance, as opposed to general adiposity measured as BMI, may be the driver for the increase in cardiovascular risk (Sjöström et al., 2012).
Prevention of T2DM was assessed in a subanalysis of patients in the SOS study without T2DM at baseline (1658 patients who underwent bariatric surgery and a matched control group of 1771) (Carlsson et al., 2012). During the follow-up period (up to 15 years), 110 participants in the bariatric-surgery group and 392 in the control group developed T2DM, corresponding to incidence rates of 6.8 vs 28.4 cases per 1000 person-years, respectively (adjusted hazard ratio with bariatric surgery, 0.17; 95% confidence interval (CI), 0.13–0.21; P < .001), suggesting that bariatric surgery is an effective adjunct to usual care in the prevention of T2DM in obese patients (Carlsson et al., 2012).
Patients who have undergone bariatric surgery, however, need lifelong lifestyle support and medical monitoring (American Diabetes Association, 2012). It is also important to note that the presence of T2DM is strongly associated with increased morbidity and mortality following bariatric surgery, possibly related to complications, such as impaired wound healing (Keidar, 2011). Therefore, less-invasive methods of inducing weight loss in obese patients with T2DM should also be considered.
3.3. Current weight-loss pharmacotherapies
In recognition of the benefits of weight loss in reducing the need for medication and the risk of long-term complications, attention is now beginning to focus on the use of weight-loss medications in obese patients with T2DM. Four medications, benzphetamine, diethylpropion, phendimetrazine, and phentermine, are approved for the short-term treatment of obesity (Bray & Ryan, 2012). Although longer term weight loss data on phentermine and related changes in BP and heart rate have been published, it is not approved in the United States for use beyond a few weeks and is not approved in Europe (Hendricks, Greenway, Westman, & Gupta, 2011). In addition, pharmacotherapies for chronic weight management in adults are now available, including orlistat and 2 pharmaceuticals recently approved by the US FDA, lorcaserin and PHEN/TPM ER.
3.3.1. Orlistat
Orlistat (XENICAL, 2012), a weight-loss medication approved for long-term treatment of obesity, is a gastrointestinal lipase inhibitor that reduces the absorption of dietary fat. It has been shown in a large, 4-year, double-blind prospective study to result in modest weight loss (5.8 kg vs 3.0 kg for orlistat vs placebo; intent-to-treat population with last observation carried forward [ITT-LOCF]), a reduction in the progression to T2DM compared with placebo (P = .0032), and improvement in cardiometabolic risk factors (XENDOS, Table 1) (Torgerson, Hauptman, Boldrin, & Sjöström, 2004). One- and 2-year studies have also shown significant improvements in lipid profiles, glycemic control, and BP (Table 2) (Rössner, Sjöström, Noack, Meinders, & Noseda, 2000; Torgerson et al., 2004). A recent systematic review and meta-analysis to evaluate the effect of weight-loss pharmacotherapies on cardiometabolic risk found that, along with a reduction in weight of 2.4 kg (95% CI: −3.34 to −1.45), orlistat improved several cardiometabolic parameters, including lipid profile, BP, and fasting glucose (Zhou et al., 2012).
3.3.1.1. Adverse events
During the XENDOS study, the most common adverse events with orlistat were gastrointestinal, including flatus with discharge, oily spotting, and fecal urgency; these were mild to moderate in nature (Davidson et al., 1999; Torgerson et al., 2004). Significant decreases in fat-soluble vitamins (A, D, E, K1) in the orlistat group occurred when compared with placebo (Torgerson et al., 2004); however, the mean level of each vitamin assessed was within the reference range during the 4-year study. Subjects receiving orlistat are instructed to take vitamin supplements ≥2 hours before or after their evening dose (XENICAL 2012).
3.3.2. Lorcaserin
In 2012, the FDA approved the weight-loss drug lorcaserin, a selective serotonin 2C receptor agonist that acts centrally to promote weight loss by reducing food intake and promoting satiety (BELVIQ 2012). The exact mechanism of action is not known. Because lorcaserin acts on serotonergic receptors, there is the potential for serotonin syndrome, which manifests in autonomic, cognitive, and somatic symptoms that can be life-threatening. As a result, extreme caution should be employed when lorcaserin is used in combination with other serotonergically acting drugs, including, but not limited to, selective serotonin-norepinephrine reuptake inhibitors, selective serotonin reuptake inhibitors, triptans, and monoamine oxidase inhibitors (BELVIQ 2012).
Lorcaserin is recommended to be administered at a dose of 10 mg orally BID in conjunction with lifestyle intervention including a reduced-calorie diet and increased physical activity. After 12 weeks, therapy should be evaluated, and if the patient has not lost ≥5% of baseline body weight, lorcaserin therapy should be discontinued (BELVIQ 2012).
The efficacy and safety of lorcaserin were evaluated in 3 separate randomized, double-blind, placebo-controlled phase 3 trials. In the 2-year Behavioral Modification and Lorcaserin for Overweight and Obesity Management (BLOOM) trial, 3182 subjects with a BMI ≥30 to ≤45 kg/m2 or BMI ≥27 to ≤45 kg/m2 and ≥1 weight-related comorbidity were randomized to receive placebo BID or lorcaserin 10 mg BID (Smith et al., 2010). The primary efficacy end points evaluated percent weight loss at 1 year (ITT-LOCF) and weight maintenance through 2 years in subjects achieving at least 5% weight loss at 1 year. The 1-year Behavioral modification and Lorcaserin Second Study for Obesity Management (BLOSSOM) randomized 4008 subjects with a BMI ≥30 to ≤45 kg/m2 or BMI ≥27 to ≤29.9 kg/m2 and ≥1 weight-related comorbidity to placebo, lorcaserin 10 mg QD, or lorcaserin 10 mg BID (Fidler et al., 2011). In BLOOM-DM, a 1-year trial, 604 overweight or obese subjects (BMI ≥27 to ≤45 kg/m2) with T2DM treated with metformin, a sulfonylurea, or both were randomized to placebo, lorcaserin 10 mg QD, or lorcaserin 10 mg BID (O’Neil et al., 2012). Both BLOSSOM and BLOOM-DM efficacy analyses used a modified ITT population with LOCF (mITT-LOCF) imputation for missing data, including all patients who took ≥1 dose of study drug and had ≥1 post-baseline body weight recorded. All subjects were managed to standard of care for their respective comorbidities, including T2DM, and received dietary and lifestyle counseling.
3.3.2.1. Weight loss
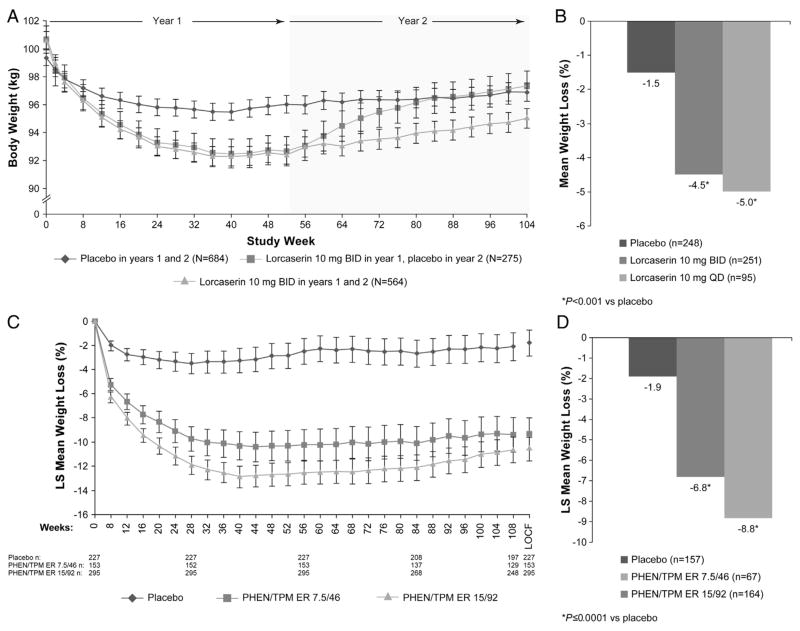
In the BLOOM trial, subjects receiving lorcaserin experienced significant weight loss vs placebo after 1 year (P < .001 vs placebo; Table 1) (Smith et al., 2010). Additionally, more subjects in the lorcaserin group than the placebo group achieved ≥5% weight loss (P < .001 vs placebo) (Smith et al., 2010). In subjects who continued receiving lorcaserin during the second year, mean body weight was lower than in those receiving placebo for 2 years and those receiving lorcaserin in year 1 and placebo in year 2. However, although remaining below baseline, weight steadily increased after week 52 in patients who continued to receive lorcaserin for a second year (Fig. 2A). In the BLOSSOM trial, lorcaserin QD and BID led to significant least-squares (LS) mean percent weight loss compared with placebo (P < .01 vs placebo; mITT-LOCF; Table 1) (Fidler et al., 2011). Similar to the BLOOM study, significantly more subjects receiving lorcaserin 10 mg QD and BID in the BLOSSOM trial achieved ≥5% weight loss vs placebo (P < .0001 vs placebo; mITT-LOCF) (Fidler et al., 2011). After 1 year of treatment in BLOOM-DM, subjects receiving lorcaserin demonstrated significant improvements in LS mean weight change with lorcaserin 10 mg QD and BID, as well as with placebo (P < .001 for both vs placebo; Table 1; Fig. 2B)(O’Neil et al., 2012). Likewise, more subjects achieved ≥5% weight loss with lorcaserin BID or lorcaserin QD (P < .001 vs placebo; mITT-LOCF) (O’Neil et al., 2012). In a phase 2b trial of 469 obese subjects, 31.2% of subjects completing 12 weeks of lorcaserin 10 mg BID treatment experienced weight loss of ≥5%, which the lorcaserin label defines as responders (BELVIQ 2012; Smith et al., 2009).
Fig. 2.
(A) Weight loss over 2 years with lorcaserin (ITT-LOCF; BLOOM), (B) mean percent weight loss with lorcaserin in subjects with T2DM from baseline to 52 weeks (mITT-LOCF; BLOOM-DM), (C) weight loss over 2 years with PHEN/TPM ER (SEQUEL), (D) least-squares mean percent weight loss with PHEN/TPM ER in subjects with T2DM from baseline to 56 weeks (ITT-LOCF; CONQUER) (Garvey et al., 2010; Garvey et al., 2012; O’Neil et al., 2012; Smith et al., 2010). Figures adapted from (Garvey et al., 2010; Garvey et al., 2012; O’Neil et al., 2012; Smith et al., 2010).
3.3.2.2. Cardiometabolic parameters
In the BLOOM trial, glycemic parameters (HbA1c, fasting glucose, and fasting insulin) were improved when compared with placebo (Table 2); however, glucose and insulin tended to increase over year 2 as body weight increased (data not shown) (Smith et al., 2010). Similarly, lipid parameters (total cholesterol, LDL-cholesterol, and triglycerides) were improved when compared with placebo at year 1 (Table 2), but these parameters were increased to levels comparable with those observed in the placebo group by year 2 (Smith et al., 2010). BP was also improved at year 1 (Table 2), with these changes maintained through year 2 (Smith et al., 2010). Subjects without T2DM in the BLOSSOM trial receiving lorcaserin did not experience significant changes in HbA1c, LDL-cholesterol, SBP, or DBP vs placebo after 1 year (Table 2) (Fidler et al., 2011). However, both HDL-cholesterol and triglycerides were reduced vs placebo in lorcaserin-treated patients (P < .05; Table 2) (Fidler et al., 2011). In the BLOOM-DM study, subjects with T2DM receiving lorcaserin QD or BID experienced improvements in HbA1c (P < .001) and fasting glucose (P < .001 vs placebo; Table 2) (O’Neil et al., 2012). However, no statistical differences were seen between groups in terms of SBP or DBP (Table 2) (O’Neil et al., 2012).
3.3.2.3. Adverse events
During the BLOOM and BLOSSOM trials, the most common adverse events were upper respiratory infections, headache, dizziness, nasopharyngitis, and nausea (Fidler et al., 2011; Smith et al., 2010). The rates of FDA-defined valvulopathy after 1 year were equivalent in all groups: 2.7% in the lorcaserin 10 mg BID group vs 2.3% in the placebo group in the BLOOM study and 2.0%, 1.4%, and 2.0% in the lorcaserin 10 mg BID, lorcaserin 10 mg QD, and placebo arms, respectively, in the BLOSSOM study (Fidler et al., 2011; Smith et al., 2010). As with the BLOOM and BLOSSOM trials, the most common adverse events reported by subjects with T2DM in the BLOOM-DM trial were headache, back pain, nasopharyngitis, and nausea (O’Neil et al., 2012). At week 52 in the BLOOM-DM trial, 2.9% and 2.5% of lorcaserin 10 mg BID and lorcaserin 10 mg QD T2DM patients, respectively, reported new valvulopathy, but the rate in placebo-treated patients with T2DM fell to 0.5%; these changes were not significant (O’Neil et al., 2012). In addition, patient-reported hypoglycemia occurred in 29.3% of subjects receiving lorcaserin 10 mg BID and 21.0% of subjects receiving placebo (BELVIQ 2012), with symptomatic hypoglycemia occurring in 7.4% of subjects with T2DM receiving lorcaserin 10 mg BID, 10.5% receiving lorcaserin 10 mg QD, and 6.3% receiving placebo; both were more frequent in subjects receiving sulfonylureas (O’Neil et al., 2012).
3.3.3. Phentermine and topiramate extended-release
In 2012, PHEN/TPM ER was approved by the FDA for chronic weight management to be taken QD in the morning. It is a combination formulation with multiple mechanisms of action (QSYMIA 2012). Phentermine hydrochloride, a sympathomimetic amine anorectic, likely induces weight loss through a release of catecholamines in the hypothalamus, resulting in reduced appetite and decreased food consumption (QSYMIA 2012). Topiramate, a sulfamate-substituted monosaccharide antiepileptic drug, may lead to weight loss through its effects on both appetite suppression and satiety enhancement, induced by a combination of pharmacologic effects, including augmenting the activity of the neurotransmitter gamma-aminobutyrate, modulation of voltage-gated ion channels, inhibition of AMPA/kainite excitatory glutamate receptors, or inhibition of carbonic anhydrase (QSYMIA 2012). The topiramate component of PHEN/TPM ER is an extended-release formulation, which allows for QD dosing and a lower Cmax than the immediate-release formulation of topiramate and improves tolerability. There is a teratogenic risk associated with topiramate therapy; therefore, PHEN/TPM ER was initially available only through mail order, but is expanding to retail pharmacies in summer 2013 under the Risk Evaluation and Mitigation Strategy (REMS), which provides patient information with each prescription or refill (QSYMIA 2012).
A treatment algorithm has been proposed to maximize patient benefit-risk. Treatment should start with PHEN 3.75 mg/TPM ER 23 mg (3.75/23) QD for 2 weeks (14 days) and then increase to PHEN 7.5 mg/TPM ER 46 mg (7.5/46) QD for 12 weeks (QSYMIA 2012). Weight loss should be evaluated after 12 weeks on the 7.5/46 dose, and if the patient has not lost ≥3% of body weight, treatment should be discontinued or the dose increased to PHEN 15 mg/TPM ER 92 mg (15/92) in a 2-step process, increasing to PHEN 11.25 mg/TPM ER 69 mg QD for 2 weeks followed by 15/92 QD for 12 weeks. If after 12 additional weeks of treatment with 15/92 a patient has not lost ≥5% of baseline body weight, treatment should be discontinued as directed.
The efficacy and safety of PHEN/TPM ER were evaluated in two 1-year, randomized, double-blind, placebo-controlled phase 3 trials. The EQUIP study evaluated 1267 obese adults (BMI ≥35 kg/m2) randomized to placebo, 3.75/23, or 15/92 (Allison et al., 2012). The CONQUER study evaluated 2487 overweight and obese adults (BMI ≥27 kg/m2 and ≤45 kg/m2) with ≥2 weight-related comorbidities, including T2DM and cardiovascular disease (Gadde et al., 2011). Subjects were randomized to placebo, 7.5/46, or 15/92. After the initial 56 weeks of treatment in CONQUER, 676 subjects at a subset of sites continued into the SEQUEL extension study, which evaluated subjects for an additional 52 weeks while maintaining their original blinded randomization (Garvey et al., 2012). All subjects were managed to standard of care for their respective comorbidities, including T2DM, and received dietary and lifestyle counseling based on the LEARN program (Brownell, 2000).
3.3.3.1. Weight loss
In the EQUIP trial, use of PHEN/TPM ER led to significant weight loss over 56 weeks when compared with placebo, and more subjects achieved weight loss of ≥5% at 1 year than did those receiving placebo (P < .0001 vs placebo; ITT-LOCF; Table 1) (Allison et al., 2012). After 1 year in the CONQUER study, LS mean percent weight loss in the overall population was significantly greater than with placebo (P < .0001; ITT-LOCF; Table 1) (Gadde et al., 2011) and was sustained through 2 years in the SEQUEL trial (P < .0001; ITT-LOCF; Table 1; Fig. 2C) (Garvey et al., 2012). In the CONQUER trial, more subjects receiving PHEN/TPM ER achieved weight loss of ≥5% at 1 year than did those receiving placebo (P < .0001 vs placebo; ITT-LOCF; Table 1) (Gadde et al., 2011), and this was also maintained through 2 years in the SEQUEL trial (P < .0001 vs placebo; ITT-LOCF; Table 1) (Garvey et al., 2012). Similarly, those subjects who had T2DM at baseline experienced significant LS mean percent weight loss after 1 year of treatment (P < .0001 vs placebo; ITT-LOCF; Fig. 2D) (Gadde et al., 2011; Garvey, Peterson, & Troupin, 2010). A pooled analysis of clinical trial results found that 83.5% of subjects completing 12 weeks of 7.5/46 treatment were responders (≥3% weight loss at 12 weeks), as defined in the label (Dvorak, Peterson, & Day, 2012; QSYMIA 2012).
3.3.3.2. Cardiometabolic parameters
The weight loss experienced by subjects receiving PHEN/TPM ER was associated with improvements in several cardiometabolic parameters. In EQUIP, obese subjects experienced significant improvements in fasting glucose, blood pressure, and lipid parameters in the 15/92 arm at year 1 (P < .05 vs placebo; ITT-LOCF; Table 2) (Allison et al., 2012). Additionally, in the overall CONQUER population, significant improvements vs placebo in these parameters were also experienced at year 1 (Gadde et al., 2011).
As in the overall population, subjects with T2DM at baseline receiving PHEN/TPM ER had greater improvements vs placebo in glycemic parameters at 1 year in the CONQUER study (Table 2) (Gadde et al., 2011). These improvements in glycemic parameters were greater with PHEN/TPM ER than with placebo, and fewer of these actively managed subjects receiving PHEN/TPM ER required an increase in concomitant antidiabetic medications (Gadde et al., 2011): 12.1% of subjects with T2DM in the placebo group required a net increase in antidiabetic medications, compared with only 1.5% and 0.6% of subjects in the 7.5/46 and 15/92 groups, respectively (Garvey et al., 2010).
3.3.3.3. Adverse events
In the EQUIP study, the most common adverse events were paraesthesia, dry mouth, constipation, headache, dysgeusia, and insomnia (Allison et al., 2012); in the CONQUER study, dry mouth, paraesthesia, constipation, insomnia, dizziness, and dysgeusia. Similarly, the types of adverse events occurring between years 1 and 2 in the SEQUEL study were similar to those reported in the overall CONQUER sample from baseline to year 1, although the incidence of individual treatment-emergent adverse events was markedly lower in the second year (weeks 56–108) (Garvey et al., 2012). In EQUIP and CONQUER, there were small increases in mean heart rate: EQUIP, −0.2, −0.3, and 1.2 bpm with placebo, 3.75/23, and 15/92, respectively (Allison et al., 2012); CONQUER, −0.1, 0.1, and 1.7 bpm with placebo, 7.5/46, and 15/92, respectively (Gadde et al., 2011; Garvey et al., 2012). However, mean BP was decreased with PHEN/TPM ER treatment vs placebo and there were no adverse events reported that were associated with changes in heart rate (Allison et al., 2012; Gadde et al., 2011; Garvey et al., 2012).
3.4. Investigational weight-loss pharmacotherapies
3.4.1. Naltrexone sustained-release and bupropion sustained-release
An additional therapy under investigation for the treatment of obesity is a combination of naltrexone sustained-release (SR) and bupropion SR. The Contrave Obesity Research (COR) Program consisted of four 1-year, phase 3 trials of overweight/obese patients: COR-I (Greenway et al., 2010), COR-II (Rubino et al., 2010), and COR-Behavioral Modification (COR-BMOD) (Wadden et al., 2011), which excluded participants with T2DM; and COR-Diabetes Mellitus (CORDM) (Hollander et al., 2010), which included participants with T2DM (Makowski, Gwinn, & Hurren, 2011). Participants in COR-I, COR-II, and COR-DM received lifestyle counseling, including decreased energy consumption by 500 kcal/day and increased physical activity, at baseline and every 12 weeks (Greenway et al., 2010; US Food & Drug Administration, 2012). Alternatively, participants in the COR-BMOD trial attended 90-minute multidisciplinary group visits weekly for 16 weeks and monthly thereafter and were prescribed 1200 to 2000 kcal/day diets and encouraged to engage in physical activity for 180 min/week for the first 6 months, increasing to 360 min/week thereafter (Wadden et al., 2011).
In each of these trials, naltrexone SR/bupropion SR produced greater weight loss and improvements in cardiometabolic parameters when compared with placebo (Table 1) (Greenway et al., 2010; Makowski et al., 2011; US Food & Drug Administration, 2012; Wadden et al., 2011). In the CORDM study, among patients with T2DM using oral or no antidiabetic medications, naltrexone SR/bupropion SR was found to induce significant weight loss and improvements in HbA1c as well as improvements in waist circumference, triglycerides, and HDL-cholesterol (Table 2) (Hollander et al., 2010; Makowski et al., 2011; US Food & Drug Administration, 2012). In all trials, after 56 weeks of treatment, the placebo groups experienced greater decreases in SBP and DBP than did the naltrexone SR/bupropion SR groups (Makowski et al., 2011; US Food & Drug Administration, 2012). In the phase 3 trials, the most common adverse events were nausea, constipation, headache, vomiting, and dizziness. Further, at 56 weeks, heart rate was increased by an average of 0.3 bpm with naltrexone SR/bupropion SR vs −0.98 bpm with placebo (Makowski et al., 2011; US Food & Drug Administration, 2012).
In January 2011, the FDA issued Orexigen, the manufacturer of naltrexone SR/bupropion SR, a complete response letter in which a cardiovascular outcomes trial (CVOT; The Light Study) was requested. Orexigen initiated enrollment in the CVOT in May 2012. The estimated completion date of the CVOT is July 2017. Completion date will be dependent on the time needed to accrue enough events to statistically assess hazard ratios from an interim analysis. The primary outcome measure will be time from treatment-period randomization to the first confirmed occurrence of major adverse cardiovascular event (MACE), with secondary end points including time from treatment-period randomization to the first confirmed cardiovascular death, myocardial infarction (fatal/nonfatal), stroke (fatal/nonfatal), and nonfatal unstable angina requiring hospitalization (Cardiovascular outcomes, 2012). Subjects will include overweight and obese (BMI ≥27 and ≤50 kg/m2) adults ≥45 years old who are at a high risk of experiencing these events due to T2DM and/or other cardiovascular risk factors; participants will be randomized to placebo or naltrexone SR 32 mg/buproprion SR 360 mg/day.
3.4.2. Liraglutide
Liraglutide (Novo Nordisk A/S, Bagsvaerd, Denmark), a glucagon-like peptide-1 analog, is currently approved for the treatment of T2DM and has demonstrated weight-loss properties (Astrup et al., 2009). In a double-blind, placebo controlled, 20-week, phase 2 trial, with open-label orlistat comparator, 564 obese adults (≤65 years, BMI ≥30 to ≤40 kg/m2) were randomized to placebo QD, liraglutide QD (1.2 mg, 1.8 mg, 2.4 mg, or 3.0 mg), or orlistat TID (120 mg). After 20 weeks, liraglutide use led to significant weight loss vs placebo and vs orlistat (P < .01; Table 1). Reductions in BP and increases in heart rate of up to 4 bpm were also observed (Table 2) (Astrup et al., 2009). The most common adverse events were nausea and vomiting. Phase 3 studies in overweight and obese patients with comorbidities are currently underway or completed but not reported in the literature; primary end points include change in body weight, percentage of patients achieving ≥5% or ≥10% weight loss, and proportion of subjects progressing to T2DM (Clinicaltrials.gov, 2012).
4. Conclusion
As the rates of obesity and T2DM continue to increase, the need for effective weight-loss strategies becomes more urgent. While lifestyle interventions and bariatric surgery have been shown to reduce body weight and improve risk factors related to T2DM and CVD, lifestyle interventions alone are difficult to maintain, and bariatric surgery, which is available to a limited number of patients, can be associated with greater risks. Currently available orlistat has demonstrated only moderate weight loss, and adherence is limited by tolerability. Lorcaserin and PHEN/TPM ER were recently approved by the FDA, and clinical trials demonstrated both significant, durable weight loss compared with placebo and improvements in glycemic parameters and cardiometabolic risk factors in overweight/obese patients with and without T2DM. Both lorcaserin and PHEN/TPM ER were well-tolerated in clinical studies and will be followed for long-term safety. These results suggest that lorcaserin or PHEN/TPM ER, when used in conjunction with lifestyle modifications, may represent a safe and effective therapy for the management of obesity in patients with T2DM.
Acknowledgments
We would like to acknowledge and thank The Lockwood Group for editorial assistance (funding was provided by VIVUS, Inc.), and VIVUS, Inc. internal contributors.
Footnotes
Dr Henry has served as an advisor for VIVUS, Inc. Dr Garvey has served as an advisor and investigator for VIVUS, Inc., and is also a stockholder of VIVUS, Inc. Dr Chilton is an advisor and speaker for VIVUS, Inc.
Grant Support: none.
Funding: VIVUS, Inc., for manuscript assistance.
References
- Allison DB, Gadde KM, Garvey WT, Peterson CA, Schwiers ML, Najarian T, et al. Controlled-release phentermine/topiramate in severely obese adults: a randomized controlled trial (EQUIP) Obesity (Silver Spring, Md ) 2012;20:330–342. doi: 10.1038/oby.2011.330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Diabetes Association. Standards of medical care in diabetes – 2012. Diabetes Care. 2012;35(Suppl 1):S11–S63. doi: 10.2337/dc12-s011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Astrup A, Rössner S, Van Gaal L, Rissanen A, Niskanen L, Al Hakim M, et al. NN8022–1807 Study Group. Effects of liraglutide in the treatment of obesity: a randomised, double-blind, placebo-controlled study. The Lancet. 2009;374:1606–1616. doi: 10.1016/S0140-6736(09)61375-1. [DOI] [PubMed] [Google Scholar]
- Barte JC, ter Bogt NC, Bogers RP, Teixeira PJ, Blissmer B, Mori TA, Bemelmans WJ. Maintenance of weight loss after lifestyle interventions for overweight and obesity, a systematic review. Obesity Reviews. 2010;11:899–906. doi: 10.1111/j.1467-789X.2010.00740.x. [DOI] [PubMed] [Google Scholar]
- BELVIQ [package insert] Woodcliff Lake, NJ: Eisai Inc; 2012. [Google Scholar]
- Boden G. Role of fatty acids in the pathogenesis of insulin resistance and NIDDM. Diabetes. 1997;46:3–10. [PubMed] [Google Scholar]
- Bray GA, Ryan DH. Medical therapy for the patient with obesity. Circulation. 2012;125:1695–1703. doi: 10.1161/CIRCULATIONAHA.111.026567. [DOI] [PubMed] [Google Scholar]
- Brownell K. The LEARN program for weight management. Dallas, TX: The Life Style Company; 2000. [Google Scholar]
- Cardiovascular outcomes study of naltrexone SR/bupropion SR in overweight and obese subjects with cardiovascular risk factors (The Light Study) 2012 Retrieved December 7 2012, from http://clinicaltrials.gov/ct2/show/NCT01601704?term=naltrexone+bupropion&rank=11.NCT01601704.
- Carlsson LM, Peltonen M, Ahlin S, Anveden Å, Bouchard C, Carlsson B, et al. Bariatric surgery and prevention of type 2 diabetes in Swedish obese subjects. The New England Journal of Medicine. 2012;367:695–704. doi: 10.1056/NEJMoa1112082. [DOI] [PubMed] [Google Scholar]
- Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jr, et al. National Heart, Lung, and Blood Institute Joint National Committee on Prevention, Detection, Evaluation, & Treatment of High Blood Pressure, & National High Blood Pressure Education Program Coordinating Committee. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003;289:2560–2572. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- ClinicalTrials.gov search for “liraglutide weight loss”+“Phase 3” yielded 4 studies as of Dec. 7, 2012. These can be viewed at http://clinicaltrials.gov/ct2/results?term=liraglutide+weight+loss&recr=&rslt=&type=&cond=&intr=&titles=&outc=&spons=&lead=&id=&state1=&cntry1=&state2=&cntry2=&state3=&cntry3=&locn=&gndr=&phase=2&rcv_s=&rcv_e=&lup_s=&lup_e=
- Cunneen SA. Review of meta-analytic comparisons of bariatric surgery with a focus on laparoscopic adjustable gastric banding. Surgery for Obesity and Related Diseases. 2008;4(3 suppl):S47–S55. doi: 10.1016/j.soard.2008.04.007. [DOI] [PubMed] [Google Scholar]
- Davidson MH, Hauptman J, DiGirolamo M, Foreyt JP, Halsted CH, Heber D, et al. Weight control and risk factor reduction in obese subjects treated for 2 years with orlistat: a randomized controlled trial. JAMA. 1999;281:235–242. doi: 10.1001/jama.281.3.235. [DOI] [PubMed] [Google Scholar]
- de Ferranti S, Mozaffarian D. The perfect storm: obesity, adipocyte dysfunction, and metabolic consequences. Clinical Chemistry. 2008;54:945–955. doi: 10.1373/clinchem.2007.100156. [DOI] [PubMed] [Google Scholar]
- Dvorak RV, Peterson CA, Day WW. Application of proposed treatment algorithm (PTA) benefit/risk profile of phentermine plus extended-release topiramate (PHEN/TPM ER). Poster 34-LB-P. The Obesity Society. 30th Annual Scientific Meeting; September 20–24, 2012; San Antonio, Texas, USA. 2012. [Google Scholar]
- Eckel RH, Kahn SE, Ferrannini E, Goldfine AB, Nathan DM, Schwartz MW, et al. Endocrine Society, American Diabetes Association, & European Association for the Study of Diabetes. (2011). Obesity and type 2 diabetes: what can be unified and what needs to be individualized? Diabetes Care. 34:1424–1430. doi: 10.2337/dc11-0447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fidler MC, Sanchez M, Raether B, Weissman NJ, Smith SR, Shanahan WR, Anderson CM BLOSSOM Clinical Trial Group. A one-year randomized trial of lorcaserin for weight loss in obese and overweight adults: the BLOSSOM trial. The Journal of Clinical Endocrinology and Metabolism. 2011;96:3067–3077. doi: 10.1210/jc.2011-1256. [DOI] [PubMed] [Google Scholar]
- Flegal KM, Carroll MD, Kit BK, Ogden CL. Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999–2010. JAMA. 2012;307:491–497. doi: 10.1001/jama.2012.39. [DOI] [PubMed] [Google Scholar]
- Gadde KM, Allison DB, Ryan DH, Peterson CA, Troupin B, Schwiers ML, Day WW. Effects of low-dose, controlled-release, phentermine plus topiramate combination on weight and associated comorbidities in overweight and obese adults (CONQUER): a randomised, placebo-controlled, phase 3 trial. The Lancet. 2011;377:1341–1352. doi: 10.1016/S0140-6736(11)60205-5. [DOI] [PubMed] [Google Scholar]
- Garvey WT, Peterson CA, Troupin B. Low-dose controlled-release phentermine/topiramate (PHEN/TPM CR) for weight loss and management of type 2 diabetes mellitus (T2DM). Abstract 492-P. The Obesity Society. 28th Annual Scientific Meeting. October 8–12, 2010. San Diego, California, USA. Obesity (Silver Spring, Md ) 2010;18(Suppl 2):S154. [Google Scholar]
- Garvey WT, Ryan DH, Look M, Gadde KM, Allison DB, Peterson CA, et al. Two-year sustained weight loss and metabolic benefits with controlled-release phentermine/topiramate in obese and overweight adults (SEQUEL): a randomized, placebo-controlled, phase 3 extension study. The American Journal of Clinical Nutrition. 2012;95:297–308. doi: 10.3945/ajcn.111.024927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg I, Stampfer MJ, Schwarzfuchs D, Shai I DIRECT Group. Adherence and success in long-term weight loss diets: the dietary intervention randomized controlled trial (DIRECT) Journal of the American College of Nutrition. 2009;28:159–168. doi: 10.1080/07315724.2009.10719767. [DOI] [PubMed] [Google Scholar]
- Greenway FL, Fujioka K, Plodkowski RA, Mudaliar S, Guttadauria M, Erickson J, et al. Effect of naltrexone plus bupropion on weight loss in overweight and obese adults (COR-I): a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. The Lancet. 2010;376:595–605. doi: 10.1016/S0140-6736(10)60888-4. [DOI] [PubMed] [Google Scholar]
- Hendricks EJ, Greenway FL, Westman EC, Gupta AK. Blood pressure and heart rate effects, weight loss and maintenance during long-term phentermine pharmacotherapy for obesity. Obesity (Silver Spring, Md ) 2011;19:2351–2360. doi: 10.1038/oby.2011.94. [DOI] [PubMed] [Google Scholar]
- Hofsø D, Jenssen T, Bollerslev J, Ueland T, Godang K, Stumvoll M, et al. Beta cell function after weight loss: a clinical trial comparing gastric bypass surgery and intensive lifestyle intervention. European Journal of Endocrinology/European Federation of Endocrine Societies. 2011;164:231–238. doi: 10.1530/EJE-10-0804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollander P, Plodkowski R, Gupta AK, Guttadauria M, Erickson J, Kim D, Dunayevich E. COR-Diabetes: naltrexone SR/bupropion SR combination therapy led to significant and sustained weight loss and improved HbA1c in overweight/obese subjects with type 2 diabetes. Abstract 56-OR. ADA 70th Scientific Sessions; June 25–29, 2010; Orlando, Florida. 2010. Retrieved July 13, 2012, from. http://professional.diabetes.org/Abstracts_Display.aspx?TYP=1&CID=79004. [Google Scholar]
- Kadera BE, Lum K, Grant J, Pryor AD, Portenier DD, DeMaria EJ. Remission of type 2 diabetes after Roux-en-Y gastric bypass is associated with greater weight loss. Surgery for Obesity and Related Diseases: Official Journal of the American Society for Bariatric Surgery. 2009;5:305–309. doi: 10.1016/j.soard.2009.02.007. [DOI] [PubMed] [Google Scholar]
- Kahn SE, Hull RL, Utzschneider KM. Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature. 2006;444:840–846. doi: 10.1038/nature05482. [DOI] [PubMed] [Google Scholar]
- Kaye SM, Pietilainen KH, Kotronen A, Joutsi-Korhonen L, Kaprio J, Yki-Jarvinen H, et al. Obesity-related derangements of coagulation and fibrinolysis: a study of obesity-discordant monozygotic twin pairs. Obesity (Silver Spring, Md ) 2012;20:88–94. doi: 10.1038/oby.2011.287. [DOI] [PubMed] [Google Scholar]
- Keidar A. Bariatric surgery for type 2 diabetes reversal: the risks. Diabetes Care. 2011;34(Suppl 2):S361–S366. doi: 10.2337/dc11-s254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kern PA, Ranganathan S, Li C, Wood L, Ranganathan G. Adipose tissue tumor necrosis factor and interleukin-6 expression in human obesity and insulin resistance. American Journal of Physiology Endocrinology and Metabolism. 2001;280:E745–E751. doi: 10.1152/ajpendo.2001.280.5.E745. [DOI] [PubMed] [Google Scholar]
- Lara-Castro C, Fu Y, Chung BH, Garvey WT. Adiponectin and the metabolic syndrome: mechanisms mediating risk for metabolic and cardiovascular disease. Current Opinion in Lipidology. 2007;18:263–270. doi: 10.1097/MOL.0b013e32814a645f. [DOI] [PubMed] [Google Scholar]
- Lentes KU, Tu N, Chen H, Winnikes U, Reinert I, Marmann G, Pirke KM. Genomic organization and mutational analysis of the human UCP2 gene, a prime candidate gene for human obesity. Journal of Receptor and Signal Transduction Research. 1999;19:229–244. doi: 10.3109/10799899909036648. [DOI] [PubMed] [Google Scholar]
- Look AHEAD Research Group. Pi-Sunyer X, Blackburn G, Brancati FL, Bray GA, Bright R, et al. Reduction in weight and cardiovascular disease risk factors in individuals with type 2 diabetes: one-year results of the Look AHEAD trial. Diabetes Care. 2007;30:1374–1383. doi: 10.2337/dc07-0048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wing RR Look AHEAD Research Group. Long-term effects of a lifestyle intervention on weight and cardiovascular risk factors in individuals with type 2 diabetes mellitus: four-year results of the Look AHEAD trial. Archives of Internal Medicine. 2010;170:1566–1575. doi: 10.1001/archinternmed.2010.334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyon HN, Hirschhorn JN. Genetics of common forms of obesity: a brief overview. The American Journal of Clinical Nutrition. 2005;82(1 suppl):215S–217S. doi: 10.1093/ajcn/82.1.215S. [DOI] [PubMed] [Google Scholar]
- Makowski CT, Gwinn KM, Hurren KM. Naltrexone/bupropion: an investigationalcombinationforweightlossandmaintenance. ObesityFacts. 2011;4:489–494. [Google Scholar]
- Mata J, Todd PM, Lippke S. When weight management lasts. Lower perceived rule complexity increases adherence. Appetite. 2010;54:37–43. doi: 10.1016/j.appet.2009.09.004. [DOI] [PubMed] [Google Scholar]
- National Heart, Lung, and Blood Institute. The Clinical Guidelines on the Identification, Evaluation, and Treatment of Overweight and Obesity in Adults: The Evidence Report. National Institutes of Health; Bethesda: 1998. NIH Publication No. 98-4083. [PubMed] [Google Scholar]
- National Institutes of Health. Weight loss does not lower heart disease risk from type 2 diabetes. 2012 Retrieved December 7, 2012, from http://www.nih.gov/news/health/oct2012/niddk-19.htm.
- O’Neil PM, Smith SR, Weissman NJ, Fidler MC, Sanchez M, Zhang J, et al. Randomized placebo-controlled clinical trial of lorcaserin for weight loss in type 2 diabetes mellitus: The BLOOM-DM Study. Obesity (Silver Spring, Md ) 2012;20:1426–1436. doi: 10.1038/oby.2012.66. [DOI] [PubMed] [Google Scholar]
- QSYMIA [package insert] Mountain View, CA: VIVUS, Inc; 2012. [Google Scholar]
- Reaven GM. Importance of identifying the overweight patient who will benefit the most by losing weight. Annals of Internal Medicine. 2003;138:420–423. doi: 10.7326/0003-4819-138-5-200303040-00012. [DOI] [PubMed] [Google Scholar]
- Reaven GM, Hollenbeck C, Jeng CY, Wu MS, Chen YD. Measurement of plasma glucose, free fatty acid, lactate, and insulin for 24 h in patients with NIDDM. Diabetes. 1988;37:1020–1024. doi: 10.2337/diab.37.8.1020. [DOI] [PubMed] [Google Scholar]
- Roden M, Price TB, Perseghin G, Petersen KF, Rothman DL, Cline GW, Shulman GI. Mechanism of free fatty acid-induced insulin resistance in humans. The Journal of Clinical Investigation. 1996;97:2859–2865. doi: 10.1172/JCI118742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rössner S, Sjöström L, Noack R, Meinders AE, Noseda G. Weight loss, weight maintenance, and improved cardiovascular risk factors after 2 years treatment with orlistat for obesity. European Orlistat Obesity Study Group. Obesity Research. 2000;8:49–61. doi: 10.1038/oby.2000.8. [DOI] [PubMed] [Google Scholar]
- Rubino D, Apovian C, Still C, Mignon L, Burns C, Harris-Collazo R, et al. Naltrexone SR/bupropion SR combination (NB) therapy shifts subjects from the obese to the non-obese body mass index (BMI) classes: From the COR-II phase 3, double-blind, placebo-controlled, 56-week study. Abstract 499-P. Obesity (Silver Spring, Md ) 2010;18(Suppl 2):S156. [Google Scholar]
- Saxena M, Agrawal CG, Gautam S, Bid HK, Banerjee M. Overt diabetic complications in obese type 2 diabetes mellitus patients from North India. Archives of Applied Science Research. 2009;1:57–66. [Google Scholar]
- Schaffer JE. Lipotoxicity: when tissues overeat. Current Opinion in Lipidology. 2003;14:281–287. doi: 10.1097/00041433-200306000-00008. [DOI] [PubMed] [Google Scholar]
- Scherer PE. Adipose tissue: from lipid storage compartment to endocrine organ. Diabetes. 2006;55:1537–1545. doi: 10.2337/db06-0263. [DOI] [PubMed] [Google Scholar]
- Shoelson SE, Lee J, Goldfine AB. Inflammation and insulin resistance. The Journal of Clinical Investigation. 2006;116:1793–1801. doi: 10.1172/JCI29069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siram AT, Yanagisawa R, Skamagas M. Weight management in type 2 diabetes mellitus. The Mount Sinai Journal of Medicine, New York. 2010;77:533–548. doi: 10.1002/msj.20208. [DOI] [PubMed] [Google Scholar]
- Sjöström L, Peltonen M, Jacobson P, Sjöström CD, Karason K, Wedel H, et al. Bariatric surgery and long-term cardiovascular events. JAMA. 2012;307:56–65. doi: 10.1001/jama.2011.1914. [DOI] [PubMed] [Google Scholar]
- Smith SR, Prosser WA, Donahue DJ, Morgan ME, Anderson CM, Shanahan WR ADP356-004 Study Group. Lorcaserin (APD356), a selective 5-HT(2C) agonist, reduces body weight in obese men and women. Obesity (Silver Spring, Md ) 2009;17:494–503. doi: 10.1038/oby.2008.537. [DOI] [PubMed] [Google Scholar]
- Smith SR, Weissman NJ, Anderson CM, Sanchez M, Chuang E, Stubbe S, et al. Behavioral Modification and Lorcaserin for Overweight and Obesity Management (BLOOM) Study Group. Multicenter, placebo-controlled trial of lorcaserin for weight management. The New England Journal of Medicine. 2010;363:245–256. doi: 10.1056/NEJMoa0909809. [DOI] [PubMed] [Google Scholar]
- Torgerson JS, Hauptman J, Boldrin MN, Sjöström L. XENical in the prevention of diabetes in obese subjects (XENDOS) study: a randomized study of orlistat as an adjunct to lifestyle changes for the prevention of type 2 diabetes in obese patients. Diabetes Care. 2004;27:155–161. doi: 10.2337/diacare.27.1.155. [DOI] [PubMed] [Google Scholar]
- Tzotzas T, Evangelou P, Kiortsis DN. Obesity, weight loss and conditional cardiovascular risk factors. Obesity Reviews: an Official Journal of the International Association for the Study of Obesity. 2011;12:e282–e289. doi: 10.1111/j.1467-789X.2010.00807.x. [DOI] [PubMed] [Google Scholar]
- UKPDS Group. UK Prospective Diabetes Study 7: response of fasting plasma glucose to diet therapy in newly presenting type II diabetic patients. Metabolism. 1990;39:905–912. [PubMed] [Google Scholar]
- US Food and Drug Administration. Contrave (naltrexone SR/bupropion SR combination): Advisory Committee briefing document. NDA200063, Orexigen Therapeutics. 2012 Retrieved December 7, 2012, from www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/EndocrinologicandMetabolicDrugsAdvisoryCommittee/UCM235672.pdf.
- Wadden TA, Foreyt JP, Foster GD, Hill JO, Klein S, O’Neil PM, et al. Weight loss with naltrexone SR/bupropion SR combination therapy as an adjunct to behavior modification: the COR-BMOD trial. Obesity (Silver Spring, Md ) 2011;19:110–120. doi: 10.1038/oby.2010.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wadden TA, Fujioka K, Toubro S, Gantz I, Erondu NE, Chen M, et al. A randomized trial of lifestyle modification and taranabant for maintaining weight loss achieved with a low-calorie diet. Obesity (Silver Spring, Md ) 2010;18:2301–2310. doi: 10.1038/oby.2010.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellen KE, Hotamisligil GS. Inflammation, stress, and diabetes. The Journal of Clinical Investigation. 2005;115:1111–1119. doi: 10.1172/JCI25102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whelton PK, He J, Appel LJ, Cutler JA, Havas S, Kotchen TA, et al. Primary prevention of hypertension: clinical and public health advisory from The National High Blood Pressure Education Program. JAMA. 2002;288:1882–1888. doi: 10.1001/jama.288.15.1882. [DOI] [PubMed] [Google Scholar]
- Wilson PW, Kannel WB. Obesity, diabetes, and risk of cardiovascular disease in the elderly. The American Journal of Geriatric Cardiology. 2002;11(119–123):125. doi: 10.1111/j.1076-7460.2002.00998.x. [DOI] [PubMed] [Google Scholar]
- Wing RR, Lang W, Wadden TA, Safford M, Knowler WC, Bertoni AG, et al. Look AHEAD Research Group. Benefits of modest weight loss in improving cardiovascular risk factors in overweight and obese individuals with type 2 diabetes. Diabetes Care. 2011;34:1481–1486. doi: 10.2337/dc10-2415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- XENICAL [package insert] South San Francisco, CA: Genentech USA, Inc; 2012. [Google Scholar]
- Zhou YH, Ma XQ, Wu C, Lu J, Zhang SS, Guo J, et al. Effect of anti-obesity drug on cardiovascular risk factors: a systematic review and meta-analysis of randomized controlled trials. PLoS One. 2012;7:e39062. doi: 10.1371/journal.pone.0039062. [DOI] [PMC free article] [PubMed] [Google Scholar]