Abstract
Background
Citicoline is a dietary supplement that has been used as a neuroprotective agent for neurological disorders such as stroke and dementia. Citicoline influences acetylcholine, dopamine, and glutamate neurotransmitter systems; serves as an intermediate in phospholipid metabolism; and enhances the integrity of neuronal membranes. Interest has grown in citicoline as a treatment for addiction since it may have beneficial effects on craving, withdrawal symptoms, and cognitive functioning, as well as the ability to attenuate the neurotoxic effects of drugs of abuse.
Objectives
To review the literature on citicoline’s use in addictive disorders.
Methods
Using PubMed we conducted a narrative review of the clinical literature on citicoline related to addictive disorders from the years 1900 to 2013 using the following keywords: citicoline, CDP-choline, addiction, cocaine, alcohol, substance abuse, and substance dependence. Out of approximately 900 first hits, nine clinical studies have been included in this review.
Results
Most addiction research investigated citicoline for cocaine use. The findings suggest that it is safe and well tolerated. Furthermore, citicoline appears to decrease craving and is associated with a reduction in cocaine use, at least at high doses in patients with both bipolar disorder and cocaine dependence. Limited data suggest citicoline may also hold promise for alcohol and cannabis dependence and in reducing food consumption.
Conclusions
Currently, there is limited research on the efficacy of citicoline for addictive disorders, but the available literature suggests promising results. Future research should employ larger sample sizes, increased dosing, and more complex study designs.
Keywords: Citicoline, Addiction, Substance Use, Substance Abuse, Bipolar Disorder, Cocaine, Substance Dependence, Alcohol, Methamphetamine
Introduction
Addictive disorders are significant public health concerns because they are common, associated with poor outcomes, and result in significant health and economic burdens. Substance use disorders have a 14.6% lifetime prevalence rate in the U.S. (1) and over 20% of all deaths in the U.S. are attributable to substance use and addiction (2). The National Institute on Drug Abuse estimates that drug and alcohol abuse in the U.S. result in a combined $366 billion annual cost to society (3).
While much research has been conducted and some promising interventions have been identified, developing effective treatments for addictive disorders remains a significant challenge given that many patients still do not respond to either single or multiple treatment interventions, and effect sizes for these interventions remain modest (4). One potentially promising pharmacological intervention for the addictive disorders population may be citicoline, a dietary supplement widely studied and used in Europe. While its clinical applications have primarily focused on cognitive impairment and stroke recovery populations, the past several years have seen increased interest in citicoline as a novel agent in the treatment of addictive disorders.
Pharmacological Mechanisms of Citicoline
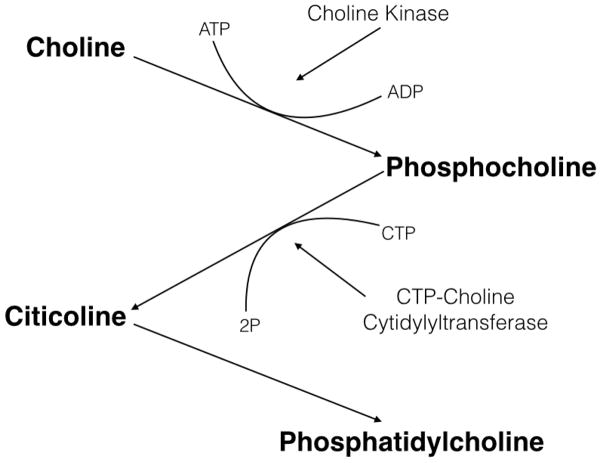
Citicoline (aka CDP-choline) is marketed as a drug in Europe and Japan and as an over-the-counter dietary supplement in the United States. Citicoline is produced endogenously from choline as an intermediate step in the synthesis of cell membrane phospholipids. When administered exogenously, citicoline is thought to be useful in a variety of neurological disorders, possibly because of its ability to enhance the integrity and function of neuronal membranes. Citicoline is a nucleotide composed of cytosine, choline, ribose, and pyrophosphate (see Figure 1). After oral administration, citicoline has a greater than 90% bioavailability and is rapidly metabolized to cytidine and choline (5). Cytidine is metabolized to uridine, crosses the blood-brain barrier, and is then converted to cytidine triphosphate (CTP). The free choline is phosphorylized into phosphocholine, which combines with CTP to form citicoline. Citicoline and diacylglycerol then form phosphatidylcholine, a precursor in the synthesis of phospholipid membranes (6) (see Figure 2). Among other roles in the body, citicoline functions as a metabolite and precursor of the neurotransmitter acetylcholine and is also an essential component of cell membrane phospholipids (7). As a result, it plays an important role in neuronal structure and signaling (8).
FIGURE 1.
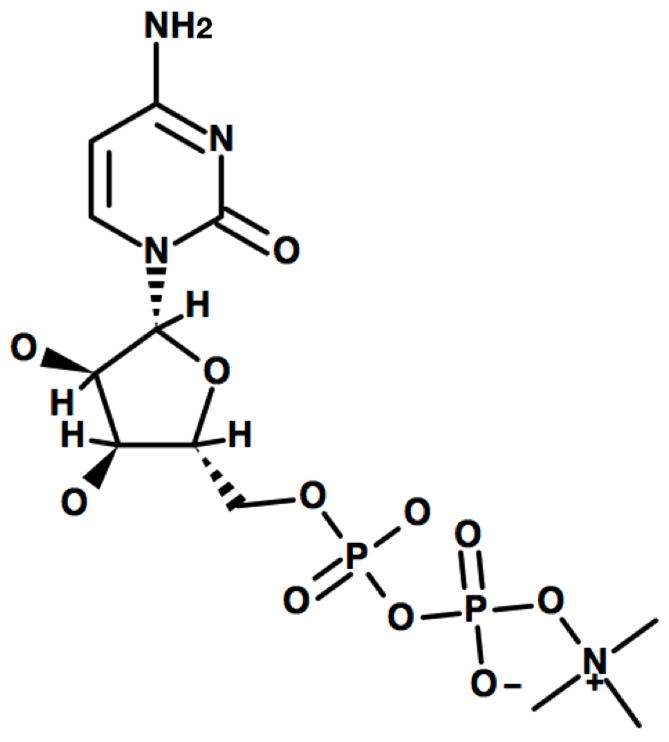
Citicoline
FIGURE 2.
Citicoline Metabolism Pathway
Citicoline is thought to act as a phosphatidylcholine precursor (9). Because the brain preferentially uses citicoline for acetylcholine synthesis, the amount of available citicoline for the production of phosphatidylcholine production can be limited, and as a result, phospholipids in neuronal membranes are often catabolized to supply the necessary choline (5). However, if administered exogenously citicoline may help to preserve the integrity of the neuronal membrane (10) and enhance synthesis of structural phospholipids (11).
Citicoline affects neurotransmitter levels primarily through modulation of catecholaminergic neurotransmission (12, 13). Citicoline increases norepinephrine in the cerebral cortex and hypothalamus as well as dopamine in the corpus striatum. Additionally, it has been shown to increase serotonin in the cerebral cortex, striatum, and hypothalamus, as well as acetylcholine in the hippocampus and neocortex (5, 14, 15). Citicoline may reduce brain glutamate activity by increasing expression of excitatory amino acid transporter-2 (EAAT2) (16). Finally, citicoline increases tyrosine levels in the striatum and also stimulates tyrosine hydroxylase activity and dopamine release (13, 17).
Citicoline in Neurological Diseases
Citicoline is most widely used as a neuroprotective agent in neurological disorders, including Parkinson’s Disease, dementia, traumatic brain injury, glaucoma, and stroke (8). A Cochrane review concluded that citicoline was more effective than placebo for cognitive impairment in vascular dementia and that it was well tolerated, with a trend towards better tolerability than placebo (14). A study of citicoline therapy in elderly patients with mild cognitive impairment showed greater improvements in verbal memory compared to placebo (18). However, another trial showed non-significant effects for citicoline vs. placebo in functional outcomes following traumatic brain injury (19). While a recent study found citicoline effective for cognitive impairment following stroke (20), another trial of citicoline in acute stroke patients confirmed safety and tolerability of the medication but did not find significant differences compared to placebo on the primary outcome of global recovery (21). A drug surveillance study of citicoline in 4,191 stroke patients by Cho & Kim (22) reported that more favorable results were observed in patients given high doses of citicoline (2000–4000 mg/day) for six weeks. The dose-dependency of the citicoline findings in this study was striking, indicating that higher dosing was associated with significant improvements in both the National Institutes of Health Stroke Scale (23) and the Barthel Index (24) of daily living and that tolerability was good regardless of dosing.
Citicoline in Addictive Disorders
Eight studies examined efficacy or potential mechanisms for citicoline in addictive disorders while one (discussed below) focused on safety (Table 1). Cocaine use disorders have been investigated most frequently in the literature on citicoline for addictive disorders, with five studies published to date. This literature includes several small studies in cocaine dependent patients that are suggestive of a possible efficacy signal. In a small pilot study, Renshaw et al. (26) demonstrated that cocaine users who were treated for 14 days with citicoline (N=6 for citicoline and N=8 for placebo) reported increased control over their cocaine use and decreased urges for cocaine. Licata et al. (27) conducted an eight-week double-blind, placebo-controlled study of citicoline (1000 mg/day) in 29 cocaine-dependent individuals. While citicoline did not significantly reduce cocaine use or craving compared to placebo in this small study, it decreased concomitant cannabis and alcohol use. Braken et al. (28) found no associations between citicoline use and changes in the sleep/wake cycle in 12 cocaine-dependent volunteers given citicoline (1,000 mg/day) in a double-blind, placebo-controlled study.
TABLE 1.
Citicoline Clinical Trials.
| Authors | Substance | N | Design | Dosing | Findings of interest |
|---|---|---|---|---|---|
| Chinchilla et al., 1995 | Alcohol | 20 | Double-blind, placebo-controlled | 1,000 mg/day | Improved attention, concentration, and orientation in the citicoline group. |
| Renshaw et al., 1999 | Cocaine | 14 | Double-blind, placebo-controlled | 500 mg/day | Increased control and decreased craving with citicoline compared to placebo. |
| Lukas et al., 2001 | Cocaine | 8 | Double-blind, placebo-controlled, crossover | 1,000 mg/day | Citicoline safe and well tolerated but no changes in cocaine use. |
| Brown et al., 2007 | Cocaine | 44 | Double-blind, placebo-controlled | 2,000 mg/day | Citicoline associated with less cocaine use, increased declarative memory performance, and increased treatment retention. |
| Yoon et al., 2010 | Methamphetamine | 31 | Double-blind, placebo-controlled | Up to 2,000 mg/day | Decrease in positive UAs with citicoline; increased NAA and Cho with citicoline. |
| Killgore et al., 2010 | Food | 16 | Double-blind, placebo-controlled | 500 vs 2,000 mg/day | Greater declines in appetite ratings and increased functional brain response to food stimuli in 2,000 mg/day group compared to 500 mg/day group. |
| Bracken et al., 2011 | Cocaine | 12 | Double-blind, placebo-controlled | 1,000 mg/day | No association between citicoline and sleep/wake disturbances. |
| Licata et al., 2011 | Cocaine | 29 | Double-blind, placebo-controlled | 1,000 mg/day | Citicoline safe and well tolerated but no changes in cocaine craving. |
| Brown &Gabrielson, 2012 | Methamphetamine | 48 | Double-blind, placebo-controlled | 2,000 mg/day | Citicoline group had a reduction in depressive symptoms, increased treatment retention, but no changes in methamphetamine use. |
To date, the largest study of citicoline for cocaine use was in outpatients with both bipolar disorders and cocaine dependence. Brown et al. (29) conducted a 12-week, placebo-controlled, proof-of-concept study in 44 individuals with bipolar disorder and comorbid cocaine dependence. Citicoline as an add-on therapy (up to 2,000 mg/day) was associated with reduced likelihood of a cocaine-positive urine at exit, with a covariate-adjusted odds ratio estimate of 6.41, suggesting that participants taking placebo had a 6.41-times higher likelihood of testing positive for cocaine than those taking citicoline. Citicoline also improved declarative memory performance. Study completion rates were twice as high for the citicoline group compared to placebo (39.1% vs. 19.0%).
Two studies investigated the effects of citicoline in methamphetamine dependence. In a double-blind, placebo-controlled study of 31 methamphetamine-dependent volunteers, Yoon et al. (30) administered citicoline (n=16) and placebo (n=15) to determine if citicoline was associated with increased levels of prefrontal N-acetyl-aspartate (NAA), a marker of neuronal viability (31) assessed using magnetic resonance spectroscopy, and whether these changes were associated with an increase in negative urine samples. Their findings demonstrated a significant association between citicoline administration (2,000 mg/day) and changes in both NAA and choline-containing compound (Cho) levels. Changes in NAA, but not Cho, levels were associated with negative urine samples. Brown &Gabrielson(32) conducted a double-blind, placebo-controlled trial of citicoline (2,000 mg/day) for bipolar and unipolar depression with comorbid methamphetamine dependence in 48 volunteers over 12 weeks. Participants receiving citicoline (n=28) had a significantly greater reduction in depressive symptoms but not methamphetamine use compared to placebo (n=20). Citicoline was also associated with significantly greater treatment retention and study completion compared to those who received placebo.
One study investigated citicoline in an alcohol dependent population. Chinchilla et al. (33) randomized 20 volunteers with alcohol dependence to citicoline or placebo. After 60 days the group receiving citicoline showed greater improvement than placebo on measures of attention, concentration and temporo-spatial orientation, and also had a 143 point decrease in gamma-glutamyltransferase (GGT) levels as compared to 38 points for the placebo group.
Finally, while not formally classified as an addictive disorder, Killgore et al. (34) recently investigated the effects of citicoline on appetite and cortico-limbic responses to images of high calorie foods. Over the course of six weeks, they compared 500 mg/day (n=8) versus 2,000 mg/day (n=8) doses of citicoline in healthy adult volunteers. After six weeks there was no difference in weight. However, significant declines in appetite ratings and increases in functional brain responses to food stimuli in the amygdala, insula, and lateral orbital cortex as measured by functional magnetic resonance imaging were present in the 2000 mg/day group but not the 500 mg/day group. Notably, increased activation in those brain regions was significantly associated with decreases in appetite ratings.
Safety and Tolerability
The side effect profile for citicoline has been shown to be favorable. A Cochrane review concluded that citicoline tended to be associated with fewer adverse effects than placebo in elderly patients with cerebral disorders (14). In a large drug surveillance study using citicoline(35) with 2817 cases, no potential side effects were observed in 95% of patients. Of the remaining patients, 4% experienced digestive symptoms (nausea, stomach pain, and diarrhea), and <1% experienced cardiovascular symptoms (low blood pressure or slow or tachycardia). Safety has also been demonstrated in studies of citicoline in neurological conditions such as chronic cerebral disorder (14), acute ischemic stroke (21), and traumatic brain injury (19). In a large study in stroke patients Cho and Kim (22) found good tolerability at doses up to 4,000 mg.
Citicoline was also shown to be safe and well-tolerated in a range of addictive disorder studies. In a double-blind, crossover, placebo-controlled study of eight occasional cocaine users, Lukas et al. (25) administered a small dose of intranasal cocaine on three separate visits and monitored the participants for 3.5 hours. During either the second or third visit, participants were administered a four-day pretreatment consisting of 1,000 mg/day of citicoline. Citicoline administration did not alter the physiological or subjective effects of cocaine. Similarly, Licata et al. (27) found citicoline to be safe during short-term treatment and to reduce scores on a somatic symptoms scale over 12 weeks. In a study by Brown et al. (29) of patients with bipolar disorder and comorbid cocaine dependence they found that citicoline was well-tolerated and also associated with significantly fewer somatic symptoms than the placebo group. Brown &Gabrielson(32) investigated citicoline for patients with bipolar or unipolar depression and comorbid methamphetamine dependence, and similarly found that citicoline was well-tolerated, with few mild side effects and no patients withdrawing from the study or decreasing medication dosage due to side effects.
Discussion
Citicoline has been reasonably well studied in neurological disorders and appears to have potential beneficial effects in a range of conditions including dementia, glaucoma, and stroke (36–38). However, the literature on citicoline in addictive disorders is more limited.
The majority of research on citicoline in addictive disorders has focused on cocaine use. The studies of citicoline in cocaine dependence alone have had very small sample sizes and have generally not focused on reduction in drug use. However, the findings suggest excellent tolerability, with no changes in sleep patterns, and possibly a reduction in craving. A larger study of citicoline was conducted in patients with bipolar disorder and cocaine dependence with positive findings on cocaine use but not mood outcomes (29). These findings suggest that either larger trials of citicoline may be warranted in patients with cocaine use disorders alone or that citicoline may have specific effects in patients with bipolar disorder and cocaine addiction that are not mediated through changes in mood. Because the study of citicoline in bipolar disorder and cocaine dependence was relatively small (n=44) for a trial focusing on mood symptoms, and both manic and depressive symptom severity were relatively modest at baseline, another possibility is that citicoline is effective for mood symptoms, but the study did not have sufficient power to detect this effect. It is noteworthy that the trial of citicoline in a mixed sample of bipolar and unipolar depressed patients with methamphetamine dependence (32) observed improvement in depressive symptoms but not methamphetamine use with citicoline. The studies of citicoline in dual diagnosis populations both used a 2000 mg/day dose, while many of the studies in cocaine use without these comorbidities used 500 mg/day - 1000 mg/day doses.
Both studies of citicoline in patients with bipolar disorder and stimulant use disorders observed greater treatment retention with citicoline than placebo. The citicoline study in patients with bipolar disorder and cocaine dependence (29) suggest that citicoline may have fewer side effects than placebo. Thus, it seems unlikely that side effects compromised the integrity of the blind resulting in greater retention because participants suspected they were on active medication. An alternative and highly speculative possibility is that subtle improvements in mood, well-being, or physical symptoms with citicoline might result in greater treatment retention.
One study of citicoline in cocaine dependence did not observe significant differences compared to placebo on cocaine use, but did demonstrate a trend toward less alcohol and cannabis use with citicoline(27). In addition, a small study of citicoline in patients with alcohol dependence reported promising findings on cognitive measures and GGT levels (33). Thus, additional research, with larger sample sizes, on citicoline in patients with alcohol or cannabis use disorders is also needed.
A small open label study comparing 500 mg/day vs. 2000 mg/day of citicoline for food craving found that self-rated appetite declined significantly in participants given the 2000 mg/day dose of citicoline(34). This finding suggests that higher doses of citicoline may be necessary in order to detect significant differences in effects. Furthermore, the authors of the study speculate that citicoline administration may facilitate dopamine release in several brain areas associated with reward including the amygdala. As a result, increased amygdala activation might lead to more aversive reactions to perceived food stimuli and therefore negatively influence craving ratings.
The mechanism by which citicoline may act in addictive diseases is not clear. Given the potent effects of citicoline on cholinergic systems, citicoline may attenuate cravings and withdrawal symptoms via increased acetylcholine and subsequent dopaminergic system modulation. While drug addiction is thought to be a disease of brain reward system dysfunction – with a particular focus on dopaminergic neurotransmitter system – the role of the cholinergic system in the processes underlying drug addiction has garnered increased interest in the study of addiction (39). Recent research into the mechanisms underlying the influence of the dopaminergic system on drug-taking behavior has identified several cholinergic pathways that appear to play important modulating roles on this system (40). Acetylcholine connections from the laterodorsal tegmental nucleus to the ventral tegmental area are thought to form a loop with dopamine cells in the midbrain and the pons and prefrontal cortex. As a result, acetylcholine input into dopamine bodies in the ventral tegmental area might serve an important gating function for the information traveling between mesocorticolimbic and mesostriatal systems (41). Additionally, cholinergic and dopaminergic systems are thought to coordinate reward functioning in the striatum, with various dopamine receptor subtypes controlling acetylcholine transmission as well striatal cholinergic output modulating dopamine output (42). Further evidence for the modulating effect of citicoline on the cholinergic system comes from magnetic resonance spectroscopy research showing that citicoline administration is associated with increased Cho levels in the brain, although changes in Cho did not correlate with changes in drug use (30).
Citicoline may decrease initiation and maintenance of drug use, as well as increase treatment adherence and relapse prevention via increased cognitive function mediated by increased acetylcholine release. The literature on citicoline in neurological disorders suggests that it may improve cognition. In addition, the trial of citicoline in patients with bipolar disorder and cocaine dependence suggested improvement in some cognitive domains (29). Similarly, the study of citicoline for alcohol dependence found that citicoline administration was associated with significant increases in attention, concentration and temporo-spatial orientation (33).
The role of memory and cognition in the addiction process has drawn increased attention in recent years, with some going as far as to call addiction a disease of learning and memory (43). Specifically, tonic firing of acetylcholine interneurons in the striatum is temporarily depressed in response to rewarding and aversive environmental stimuli (44), and they appear finely tuned towards context recognition, movement control and stimulus detection (39). Furthermore, acute administration of drugs of abuse such as cocaine and amphetamine increase acetylcholine levels in the hippocampus (45), potentially leading to increased memory consolidation of drug-taking behavior and effects.
However, over time and with chronic administration, acetylcholine receptors may be reduced in number, especially during withdrawal. Consequently, cholinergic agonists such as citicoline may be useful in increasing the acquisition of more adaptive behaviors by strengthening the salience of stimuli not associated with substance abuse (39). Citicoline has also been shown to improve cognition in early-onset patients with Alzheimer’s disease (46) and improved performance on some neuropsychological and cognitive tests such as the Mini Mental State Examination (47) in patients with cognitive decline (48). Furthermore, through its effects on phospholipid synthesis, citicoline has been associated with improved memory performance and verbal learning in humans (49). Finally, impulsive behavior and poor decision-making are common in addictions (50), and citicoline-facilitated improvements in cognition may contribute to decreased rates of relapse in addictive disorders.
Citicoline may also be neuroprotective against the neurotoxic effects of some drugs of abuse. Chronic use of drugs such as cocaine or methamphetamine can result in ischemic injury, in part mediated through glutamate release (51). Citicoline has been shown to be neuroprotective in both animal and human studies of ischemia, and may therefore buffer against some of the neurotoxic effects of chronic substance abuse. This neuroprotective effect may arise through several mechanisms, including repairing neuronal membranes through increased phosphatidylcholine production, repairing damaged cholinergic neurons through increased acetylcholine production, and reduced free fatty acid accumulation (5). Citicoline is thought to be neuroprotective through its ability to decrease extracellular glutamate accumulation in various conditions such as ischemia (16), amyotrophic lateral sclerosis (52), and central nervous system injury (53). Evidence from relapse models in cocaine abuse suggests that the glutamatergic system is critically involved in this process. Nucleus accumbens glutamate levels increase during cocaine reinstatement, and glutamate receptor activation is necessary for the reinstatement of drug-seeking behavior (54). As a result, attenuation of glutamate release via citicoline administration may prove useful as a novel pharmacotherapy for the reduction of drug-seeking behavior.
In summary, citicoline appears to be a promising potential treatment for addictive disorders. It is generally safe and well tolerated, even at higher doses. Initial evidence suggests that citicoline may be useful in reducing cocaine use and possibly alcohol and cannabis use. Some studies, however, have reported negative results. Importantly, the study finding the strongest positive results also included the largest sample and the highest dosing of citicoline, suggesting that future studies ought to consider these factors carefully in order to ensure adequate power to detect significant effects. Issues of insufficient sample sizes and conservative dosing will certainly need to be addressed if the literature is to progress, but there are several other directions that we suggest future research might take.
As is evident from the above discussion, the pharmacodynamics of citicolineare complex and multifaceted. Future research will need to investigate more closely how citicoline administration interacts with different neurotransmitter systems given specific circumstances and conditions. For instance, how might citicoline differentially act on cocaine or methamphetamine addiction compared to alcohol addiction? A more detailed understanding of the mechanisms of action underlying the effects of citicoline might lead to a better understanding of its potential spectrum of efficacy.
Another important area for future research is to test the effects of citicoline in better defined and controlled populations. Several of the larger studies of citicoline in addiction employed dual-diagnosis patients with co-morbid mood disorders. While this patient population is clinically important given that a significant number of people suffering from an addiction also have co-morbid psychiatric conditions, future studies will need to accurately and comprehensively assess psychiatric status in order to better understand the effects of citicoline and addictive disorders in the context of psychiatric conditions. Similarly, accurate documentation of participants’ concomitant psychopharmacological treatments is essential.
Finally, future research may benefit from investigating the effects of citicoline in various stages of addiction. For example, as mentioned previously, because of its potential cognitively enhancing properties, citicoline may be especially efficacious during the relapse prevention stage of treatment. The importance of designing studies that are targeted and specific cannot be overstated, particularly with an agent whose actions are likely complex.
Acknowledgments
Supported, in part, by NIH grant DA022460
References
- 1.Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Archives of general psychiatry. 2005;62 (6):593–602. doi: 10.1001/archpsyc.62.6.593. [DOI] [PubMed] [Google Scholar]
- 2.University T.N.C.o.A.a.S.A.a.C, editor. Annual Report 2012. 2012. [Google Scholar]
- 3.Drug Abuse and Addiction. NIDA; http://www.drugabuse.gov/sites/default/files/addictionscience.ppt. [Google Scholar]
- 4.van den Brink W. Evidence-based pharmacological treatment of substance use disorders and pathological gambling. Current drug abuse reviews. 2012;5 (1):3–31. doi: 10.2174/1874473711205010003. [DOI] [PubMed] [Google Scholar]
- 5.D'Orlando KJ, Sandage BW., Jr Citicoline (CDP-choline): mechanisms of action and effects in ischemic brain injury. Neurological research. 1995;17 (4):281–284. doi: 10.1080/01616412.1995.11740327. [DOI] [PubMed] [Google Scholar]
- 6.Silveri MM, Dikan J, Ross AJ, Jensen JE, Kamiya T, Kawada Y, Renshaw PF, Yurgelun-Todd DA. Citicoline enhances frontal lobe bioenergetics as measured by phosphorus magnetic resonance spectroscopy. NMR in biomedicine. 2008;21 (10):1066–1075. doi: 10.1002/nbm.1281. [DOI] [PubMed] [Google Scholar]
- 7.Tayebati SK, Amenta F. Choline-containing phospholipids: relevance to brain functional pathways. Clinical chemistry and laboratory medicine : CCLM / FESCC. 2013;51 (3):513–521. doi: 10.1515/cclm-2012-0559. [DOI] [PubMed] [Google Scholar]
- 8.Arenth PM, Russell KC, Ricker JH, Zafonte RD. CDP-choline as a biological supplement during neurorecovery: a focused review. PM & R : the journal of injury, function, and rehabilitation. 2011;3 (6 Suppl 1):S123–131. doi: 10.1016/j.pmrj.2011.03.012. [DOI] [PubMed] [Google Scholar]
- 9.de la Morena E. Efficacy of CDP-choline in the treatment of senile alterations in memory. Annals of the New York Academy of Sciences. 1991;640:233–236. doi: 10.1111/j.1749-6632.1991.tb00224.x. [DOI] [PubMed] [Google Scholar]
- 10.Thorne Research I. Citicoline. Alternative Medicine Review. 2008;13(1) [Google Scholar]
- 11.Adibhatla RM, Hatcher JF. Cytidine 5'-diphosphocholine (CDP-choline) in stroke and other CNS disorders. Neurochemical research. 2005;30 (1):15–23. doi: 10.1007/s11064-004-9681-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Agut J, Ortiz JA, Wurtman RJ. Cytidine (5')diphosphocholine modulates dopamine K(+)-evoked release in striatum measured by microdialysis. Annals of the New York Academy of Sciences. 2000;920:332–335. doi: 10.1111/j.1749-6632.2000.tb06944.x. [DOI] [PubMed] [Google Scholar]
- 13.Secades JJ, Frontera G. CDP-choline: pharmacological and clinical review. Methods and findings in experimental and clinical pharmacology. 1995;17 (Suppl B):1–54. [PubMed] [Google Scholar]
- 14.Fioravanti M, Yanagi M. Cytidinediphosphocholine (CDP-choline) for cognitive and behavioural disturbances associated with chronic cerebral disorders in the elderly. The Cochrane database of systematic reviews. 2005;(2):CD000269. doi: 10.1002/14651858.CD000269.pub3. [DOI] [PubMed] [Google Scholar]
- 15.Fioravanti M, Buckley AE. Citicoline (Cognizin) in the treatment of cognitive impairment. Clinical interventions in aging. 2006;1 (3):247–251. doi: 10.2147/ciia.2006.1.3.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hurtado O, Moro MA, Cardenas A, Sanchez V, Fernandez-Tome P, Leza JC, Lorenzo P, Secades JJ, Lozano R, Davalos A, Castillo J, Lizasoain I. Neuroprotection afforded by prior citicoline administration in experimental brain ischemia: effects on glutamate transport. Neurobiology of disease. 2005;18 (2):336–345. doi: 10.1016/j.nbd.2004.10.006. [DOI] [PubMed] [Google Scholar]
- 17.Martinet M, Fonlupt P, Pacheco H. Effects of cytidine-5' diphosphocholine on norepinephrine, dopamine and serotonin synthesis in various regions of the rat brain. Archives internationales de pharmacodynamie et de therapie. 1979;239 (1):52–61. [PubMed] [Google Scholar]
- 18.Spiers PA, Myers D, Hochanadel GS, Lieberman HR, Wurtman RJ. Citicoline improves verbal memory in aging. Archives of neurology. 1996;53 (5):441–448. doi: 10.1001/archneur.1996.00550050071026. [DOI] [PubMed] [Google Scholar]
- 19.Zafonte RD, Bagiella E, Ansel BM, Novack TA, Friedewald WT, Hesdorffer DC, Timmons SD, Jallo J, Eisenberg H, Hart T, Ricker JH, Diaz-Arrastia R, Merchant RE, Temkin NR, Melton S, Dikmen SS. Effect of citicoline on functional and cognitive status among patients with traumatic brain injury: Citicoline Brain Injury Treatment Trial (COBRIT) JAMA : the journal of the American Medical Association. 2012;308 (19):1993–2000. doi: 10.1001/jama.2012.13256. [DOI] [PubMed] [Google Scholar]
- 20.Alvarez-Sabin J, Maisterra O, Santamarina E, Kase CS. Factors influencing haemorrhagic transformation in ischaemic stroke. Lancet neurology. 2013;12 (7):689–705. doi: 10.1016/S1474-4422(13)70055-3. [DOI] [PubMed] [Google Scholar]
- 21.Davalos A, Alvarez-Sabin J, Castillo J, Diez-Tejedor E, Ferro J, Martinez-Vila E, Serena J, Segura T, Cruz VT, Masjuan J, Cobo E, Secades JJ International Citicoline Trial on acUte Stroke trial i. Citicoline in the treatment of acute ischaemic stroke: an international, randomised, multicentre, placebo-controlled study (ICTUS trial) Lancet. 2012;380 (9839):349–357. doi: 10.1016/S0140-6736(12)60813-7. [DOI] [PubMed] [Google Scholar]
- 22.Cho HJ, Kim YJ. Efficacy and safety of oral citicoline in acute ischemic stroke: drug surveillance study in 4,191 cases. Methods and findings in experimental and clinical pharmacology. 2009;31 (3):171–176. doi: 10.1358/mf.2009.31.3.1364241. [DOI] [PubMed] [Google Scholar]
- 23.Richardson J, Murray D, House CK, Lowenkopf T. Successful implementation of the National Institutes of Health Stroke Scale on a stroke/neurovascular unit. The Journal of neuroscience nursing : journal of the American Association of Neuroscience Nurses. 2006;38 (4 Suppl):309–315. doi: 10.1097/01376517-200609000-00007. [DOI] [PubMed] [Google Scholar]
- 24.Mahoney FI, Barthel DW. Functional Evaluation: The Barthel Index. Maryland state medical journal. 1965;14:61–65. [PubMed] [Google Scholar]
- 25.Lukas SE, Kouri EM, Rhee C, Madrid A, Renshaw PF. Effects of short-term citicoline treatment on acute cocaine intoxication and cardiovascular effects. Psychopharmacology. 2001;157 (2):163–167. doi: 10.1007/s002130100824. [DOI] [PubMed] [Google Scholar]
- 26.Renshaw PF, Daniels S, Lundahl LH, Rogers V, Lukas SE. Short-term treatment with citicoline (CDP-choline) attenuates some measures of craving in cocaine-dependent subjects: a preliminary report. Psychopharmacology. 1999;142 (2):132–138. doi: 10.1007/s002130050871. [DOI] [PubMed] [Google Scholar]
- 27.Licata SC, Penetar DM, Ravichandran C, Rodolico J, Palmer C, Berko J, Geaghan T, Looby A, Peters E, Ryan E, Renshaw PF, Lukas SE. Effects of daily treatment with citicoline: a double-blind, placebo-controlled study in cocaine-dependent volunteers. Journal of addiction medicine. 2011;5 (1):57–64. doi: 10.1097/ADM.0b013e3181d80c93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bracken BK, Penetar DM, Rodolico J, Ryan ET, Lukas SE. Eight weeks of citicoline treatment does not perturb sleep/wake cycles in cocaine-dependent adults. Pharmacology, biochemistry, and behavior. 2011;98 (4):518–524. doi: 10.1016/j.pbb.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brown ES, Gorman AR, Hynan LS. A randomized, placebo-controlled trial of citicoline add-on therapy in outpatients with bipolar disorder and cocaine dependence. Journal of clinical psychopharmacology. 2007;27 (5):498–502. doi: 10.1097/JCP.0b013e31814db4c4. [DOI] [PubMed] [Google Scholar]
- 30.Yoon SJ, Lyoo IK, Kim HJ, Kim TS, Sung YH, Kim N, Lukas SE, Renshaw PF. Neurochemical alterations in methamphetamine-dependent patients treated with cytidine-5'-diphosphate choline: a longitudinal proton magnetic resonance spectroscopy study. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2010;35 (5):1165–1173. doi: 10.1038/npp.2009.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tsai G, Coyle JT. N-acetylaspartate in neuropsychiatric disorders. Progress in neurobiology. 1995;46 (5):531–540. doi: 10.1016/0301-0082(95)00014-m. [DOI] [PubMed] [Google Scholar]
- 32.Brown ES, Gabrielson B. A randomized, double-blind, placebo-controlled trial of citicoline for bipolar and unipolar depression and methamphetamine dependence. Journal of affective disorders. 2012;143 (1–3):257–260. doi: 10.1016/j.jad.2012.05.006. [DOI] [PubMed] [Google Scholar]
- 33.Chinchilla A, Lopez-Ibor M, Camarero Vegay M. CDP-colina en la evolucion de las funciones mentales en el sindrome de abstinencia alcoholica. Psiquiatria Biologica. 2(5):171–175. [Google Scholar]
- 34.Killgore WD, Ross AJ, Kamiya T, Kawada Y, Renshaw PF, Yurgelun-Todd DA. Citicoline affects appetite and cortico-limbic responses to images of high-calorie foods. The International journal of eating disorders. 2010;43 (1):6–13. doi: 10.1002/eat.20658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lozano Fernandez R. Efficacy and safety of oral CDP-choline. Drug surveillance study in 2817 cases. Arzneimittel-Forschung. 1983;33 (7A):1073–1080. [PubMed] [Google Scholar]
- 36.Alvarez XA, Sampedro C, Lozano R, Cacabelos R. Citicoline protects hippocampal neurons against apoptosis induced by brain beta-amyloid deposits plus cerebral hypoperfusion in rats. Methods and findings in experimental and clinical pharmacology. 1999;21 (8):535–540. doi: 10.1358/mf.1999.21.8.794835. [DOI] [PubMed] [Google Scholar]
- 37.Warach S, Pettigrew LC, Dashe JF, Pullicino P, Lefkowitz DM, Sabounjian L, Harnett K, Schwiderski U, Gammans R. Effect of citicoline on ischemic lesions as measured by diffusion-weighted magnetic resonance imaging. Citicoline 010 Investigators. Annals of neurology. 2000;48 (5):713–722. [PubMed] [Google Scholar]
- 38.Parisi V, Coppola G, Centofanti M, Oddone F, Angrisani AM, Ziccardi L, Ricci B, Quaranta L, Manni G. Evidence of the neuroprotective role of citicoline in glaucoma patients. Progress in brain research. 2008;173:541–554. doi: 10.1016/S0079-6123(08)01137-0. [DOI] [PubMed] [Google Scholar]
- 39.Williams MJ, Adinoff B. The role of acetylcholine in cocaine addiction. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2008;33 (8):1779–1797. doi: 10.1038/sj.npp.1301585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hoebel BG, Avena NM, Rada P. Accumbens dopamine-acetylcholine balance in approach and avoidance. Current opinion in pharmacology. 2007;7 (6):617–627. doi: 10.1016/j.coph.2007.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mark GP, Shabani S, Dobbs LK, Hansen ST. Cholinergic modulation of mesolimbic dopamine function and reward. Physiology & behavior. 2011;104 (1):76–81. doi: 10.1016/j.physbeh.2011.04.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang W, Yamada M, Gomeza J, Basile AS, Wess J. Multiple muscarinic acetylcholine receptor subtypes modulate striatal dopamine release, as studied with M1-M5 muscarinic receptor knock-out mice. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2002;22 (15):6347–6352. doi: 10.1523/JNEUROSCI.22-15-06347.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hyman SE. Addiction: a disease of learning and memory. The American journal of psychiatry. 2005;162 (8):1414–1422. doi: 10.1176/appi.ajp.162.8.1414. [DOI] [PubMed] [Google Scholar]
- 44.Apicella P, Ravel S, Deffains M, Legallet E. The role of striatal tonically active neurons in reward prediction error signaling during instrumental task performance. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2011;31 (4):1507–1515. doi: 10.1523/JNEUROSCI.4880-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Imperato A, Obinu MC, Gessa GL. Effects of cocaine and amphetamine on acetylcholine release in the hippocampus and caudate nucleus. European journal of pharmacology. 1993;238 (2–3):377–381. doi: 10.1016/0014-2999(93)90869-j. [DOI] [PubMed] [Google Scholar]
- 46.Caamano J, Gomez MJ, Franco A, Cacabelos R. Effects of CDP-choline on cognition and cerebral hemodynamics in patients with Alzheimer's disease. Methods and findings in experimental and clinical pharmacology. 1994;16 (3):211–218. [PubMed] [Google Scholar]
- 47.Cockrell JR, Folstein MF. Mini-Mental State Examination (MMSE) Psychopharmacology bulletin. 1988;24 (4):689–692. [PubMed] [Google Scholar]
- 48.Garcia-Cobos R, Frank-Garcia A, Gutierrez-Fernandez M, Diez-Tejedor E. Citicoline, use in cognitive decline: vascular and degenerative. Journal of the neurological sciences. 2010;299 (1–2):188–192. doi: 10.1016/j.jns.2010.08.027. [DOI] [PubMed] [Google Scholar]
- 49.Babb SM, Wald LL, Cohen BM, Villafuerte RA, Gruber SA, Yurgelun-Todd DA, Renshaw PF. Chronic citicoline increases phosphodiesters in the brains of healthy older subjects: an in vivo phosphorus magnetic resonance spectroscopy study. Psychopharmacology. 2002;161 (3):248–254. doi: 10.1007/s00213-002-1045-y. [DOI] [PubMed] [Google Scholar]
- 50.Adinoff B, Rilling LM, Williams MJ, Schreffler E, Schepis TS, Rosvall T, Rao U. Impulsivity, neural deficits, and the addictions: the “oops” factor in relapse. Journal of addictive diseases. 2007;26 (Suppl 1):25–39. doi: 10.1300/J069v26S01_04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kosten TR. Pharmacotherapy of cerebral ischemia in cocaine dependence. Drug and alcohol dependence. 1998;49 (2):133–144. doi: 10.1016/s0376-8716(97)00158-0. [DOI] [PubMed] [Google Scholar]
- 52.Matyja E, Taraszewska A, Naganska E, Grieb P, Rafalowska J. CDP-choline protects motor neurons against apoptotic changes in a model of chronic glutamate excitotoxicity in vitro. Folia neuropathologica / Association of Polish Neuropathologists and Medical Research Centre, Polish Academy of Sciences. 2008;46 (2):139–148. [PubMed] [Google Scholar]
- 53.Mir C, Clotet J, Aledo R, Durany N, Argemi J, Lozano R, Cervos-Navarro J, Casals N. CDP-choline prevents glutamate-mediated cell death in cerebellar granule neurons. Journal of molecular neuroscience : MN. 2003;20 (1):53–60. doi: 10.1385/JMN:20:1:53. [DOI] [PubMed] [Google Scholar]
- 54.Uys JD, LaLumiere RT. Glutamate: the new frontier in pharmacotherapy for cocaine addiction. CNS & neurological disorders drug targets. 2008;7 (5):482–491. doi: 10.2174/187152708786927868. [DOI] [PubMed] [Google Scholar]