Abstract
Background
Evidence indicates that a transfusion(Tx) trigger hemoglobin(Hgb) value of 8 gm/dL may be safer than a more liberal Tx trigger in cardiac surgery(CS) patients. We hypothesized that weekly physician feedback would improve adherence to such a protocol, but that the public identification of individual physician behavior would have an additive effect.
Methods
We concurrently reviewed all adult CS patients at our institution from 12/1/2010-5/27/2011. We matched any cardiac surgery intensive care unit(CSICU) Tx event (red blood cells) with the Hgb value immediately before Tx. Patients requiring massive transfusions (>10 units/24 hours) were excluded. After all providers agreed upon a Hgb of 8 as the Tx trigger, we studied 3 consecutive time periods: no feedback, weekly feedback of group Tx behavior, and weekly feedback with identification of individual surgeon Tx behavior.
Results
Of the 512 patients who underwent cardiac operations, 144 patients underwent 510 Tx events. Compared to period 1, the unadjusted odds of receiving a Tx above 8 gm/dL decreased by 48% in study period 2(OR: 0.52, p<0.01), and 63% in study period 3(OR: 0.37, p <0.001). Single unit transfusion rates increased from 77% to >90%(p<0.001). In hospital mortality also fell from period 1 to period 3(7.0%-1.5%, p=0.02) with the observed to expected mortality ratio decreasing from 2.19 to 0.51.
Conclusions
Blood transfusion protocol adherence improves when weekly feedback is provided. Identifying individual surgeon behavior improves adherence to a greater degree. Routine presentation of quality metrics with identification of individual physician specific behavior may be the most effective way to accomplish performance improvement.
Keywords: Blood, Outcomes, Postoperative Care, Resuscitation
Introduction
Blood transfusions are not without risks[1-9], and evidence is increasing that a restrictive transfusion policy in intensive care and surgical patients is at least equivalent to a more liberal transfusion policy[4, 6-8]. Nevertheless, transfusion practices remain largely unchanged, demonstrating the inherent difficulty in modifying physician behavior. We undertook this performance improvement project to evaluate whether a restrictive transfusion protocol for cardiac surgery patients could be incorporated into clinical practice. We hypothesized that cardiac surgeons’ behavior could be modified through group feedback and further modified through identification of individual surgeon behavior to the group (peer disclosure).
Material and Methods
Study Design
This study was approved under IRB protocol: NA_00001068 allowing reviews of outcomes of all cardiac surgical procedures at the Johns Hopkins Hospital.
Patient Selection
We concurrently reviewed our prospective database to identify adult patients (≥18) undergoing cardiac surgery between 12/1/2010 and 5/27/2011 and subsequently transferred to the Cardiac Surgical Intensive Care Unit (CSICU). All packed red blood cell (PRBC) transfusions occurring in the CSICU in this population were recorded, as were hemoglobin (Hgb) levels immediately prior to transfusion. Only patients who received >10 units within 24 hours (massive transfusion) were excluded from analysis.
Protocol Selection
Prior to the onset of this study, a consensus was reached among our cardiac surgeons and our cardiac critical care providers that a hemoglobin level of <8 gm/dL would be utilized as our transfusion trigger.
Transfusion Authorization
In our co-managed ICU, both cardiac surgery attendings, cardiac residents, and ICU providers (intensivists, surgery residents and mid-level providers) have the ability to order a transfusion. However, after initiation of the transfusion protocol, only the attending surgeon, cardiac resident or intensivist could order a transfusion. Additionally, intensivists communicated with the cardiac attending or resident prior to administering transfusions. Cardiac resident authorized transfusions were presumed to be due to attending surgeon specifications.
Stratification
We stratified our patient population into 2 groups, transfused and non-transfused. Our study was stratified into 3 periods: Period 1 (week 1 – week 8), cardiac surgical provider transfusion practices were observed without any feedback regarding protocol adherence; Period 2 (week 9 – week 15), providers were given weekly feedback regarding group, but not individual provider, protocol adherence; and Period 3 (week 16 – week 25), providers were given weekly feedback, as a group, but with disclosure of individual cardiac surgeon behavior. All feedback (Periods 2 and 3) was given publically at a weekly cardiac surgical division meeting.
Transfused patients were primarily stratified by hemoglobin levels at the time of transfusion. Secondary stratification (in Period 3) was by surgical provider.
Outcomes
Our primary outcome of interest was protocol adherence. Secondary outcomes included the percentage of single unit transfusions, change in creatinine at discharge compared to pre-operative levels, CSICU length of stay (LOS), total hospital LOS, and in-hospital mortality. Additionally, for each study period, the observed mortality to expected mortality ratio was determined.
Statistics
Baseline differences in demographic and operative variables among study periods were compared using the Student’s t test or analysis of variance (ANOVA) for normally distributed continuous variables. These variables are presented as the mean and standard deviation (SD). Continuous variables that were not normally distributed were compared with the Kruskal-Wallis rank test or the rank sum test and presented with median and interquartile ranges (IQR). Chi-square analysis or Fisher’s exact test were utilized for categorical variables. Categorical variables are shown in numbers and percentages. Post-operative outcomes were compared according to study period stratification utilizing Chi-square, Fisher’s exact, Student’s t test, ANOVA, rank sum test or Kruskal-Wallis analysis as appropriate.
To examine protocol adherence, Chi-square and Fisher’s exact analysis were used to assess the number of transfusions given for a hemoglobin > 8 gm/dL, stratified by study period. Additionally, linear regression was utilized to calculate the line of best fit and its associated p value for all transfusions stratified by week. Finally, logistic regression analysis was utilized to calculate the odds ratio(OR) of transfusions given for a hemoglobin > 8 gm/dL when stratified by study period. ORs are presented with 95% confidence intervals (CI). Values of p<0.05 (two-tailed) were deemed significant. Analysis was performed utilizing Stata/SE 12.1 software (StataCorp, College Station, TX).
Results
Cohort Characteristics
During our 25 week study, 524 patients underwent cardiac surgery. Of these, 156 underwent 726 ICU transfusions. After excluding mass transfusions (>10 units in 24 hours), our final cohort consisted of 512 patients, with 144 undergoing 510 PRBC transfusion events.
The mean age of the cohort was 60.2±15.5 years old, 325(63.5%) were male, with 154(30.1%) having diabetes and 338(66.0%) hypertension. Isolated coronary artery bypass(CABG) procedures were performed in 161(31.4%). Mean cardiopulmonary bypass time(CPB) was 110.9±50.1 minutes.
Stratified Cohort Characteristics
In comparing transfused and non-transfused patients for all 3 study periods, transfused patients had a higher pre-operative creatinine (p=0.02), a greater predicted mortality risk (p<0.01), longer bypass times (p=0.02) and more intraoperative transfusions (p=0.02, Table 1).
Table 1.
Demographics and Operative Statistics by Transfusion Status
| Variables | No transfusion (N=368) | Transfusion (N=144) | p valuea |
|---|---|---|---|
| Demographics | |||
| Age (years) | 59.5 ± 15.4 | 62.0 ± 15.8 | 0.10 |
| Male | 226 (61.4%) | 99/140 (70.7%) | 0.05 |
| Race | |||
| White | 261 (70.9%) | 106/140 (75.7%) | |
| Black | 64 (17.4%) | 20/140 (14.3%) | |
| Other | 43 (11.7%) | 14/140 (10.0%) | 0.56 |
| BMI | 29.5 ± 7.8 | 29.3 ± 8.7 | 0.84 |
| Diabetes | 108 (29.4%) | 46/140 (32.9%) | 0.44 |
| Hypertension | 240 (65.2%) | 98/140 (70.0%) | 0.31 |
| Dyslipidemia | 223 (60.6%) | 91/140 (65.0%) | 0.36 |
| Cerebrovascular Disease | 31 (8.4%) | 14/140 (10.0%) | 0.58 |
| Pre-op Creatinine | 0.9 (0.8-1.1) | 1.0 (0.8-1.3) | 0.02 |
| Pre-op Dialysis | 7 (1.9%) | 7/140 (5.0%) | 0.07 |
| Pre-op EF | 50.4 ± 14.7 | 47.7 ± 15.5 | 0.08 |
| Anticoagulated | 253 (68.8%) | 105 (72.9%) | 0.36 |
| STS Risk Score | 0.012 (0.006-0.022) | 0.023 (0.008-0.050) | <0.01 |
| Operative Statistics | |||
| Procedure | |||
| Isolated CABG | 116/351 (33.1%) | 45/131 (34.4%) | |
| Valve | 76/351 (21.7%) | 24/131 (18.3%) | |
| CABG + Valve | 28/351 (8.0%) | 14/131 (10.7%) | |
| Other | 131/351 (37.3%) | 48/131 (36.6%) | 0.71 |
| Bypass time (min) | 107.3 ± 46.6 | 119.8 ± 56.9 | 0.02 |
| Cross-clamp time (min) | 69.6 ± 30.9 | 73.4 ± 36.2 | 0.28 |
| Circulatory Arrest time | 35 (13-38) | 23.5 (4-41) | 0.80 |
| Intra-op transfusions | 2 (1-3) | 2 (1-4) | 0.02 |
BMI=Body Mass Index, CABG=Coronary Artery Bypass Graft, EF=Ejection Fraction, N=Number of Patients
Stratification of the transfused group by study period showed that nearly all variables were evenly distributed. Period 2 had fewer patients who were anticoagulated or received antiplatelet therapy prior to surgery (p=0.02) when compared to periods 1 and 3. Period 3 had fewer patients with cerebrovascular disease (p=0.049). No other significant differences existed between groups.
Outcomes
Analysis of our primary outcome revealed that the incidence of off-protocol transfusions (transfusion trigger ≥8 gm/dL) decreased significantly over the 3 periods (p<0.001; Fig. 1). Logistic regression analysis demonstrated a 48% decrease in the unadjusted odds of receiving a transfusion above a trigger of 8 gm/dL in period 2 and a 63% decrease in period 3 (p=0.003 and <0.001 respectively, Table 2). Furthermore, the odds of receiving a transfusion with a trigger > 9 gm/dL decreased significantly by period 3 (OR: 0.50, p=0.04). The number of transfused patients with a trigger >10 gm/dL showed a decreasing trend, but did not reach significance, almost certainly due to small numbers. Finally, single unit transfusions increased (p<0.001; Fig. 2), although there was no difference between study periods 2 and 3.
Figure 1.
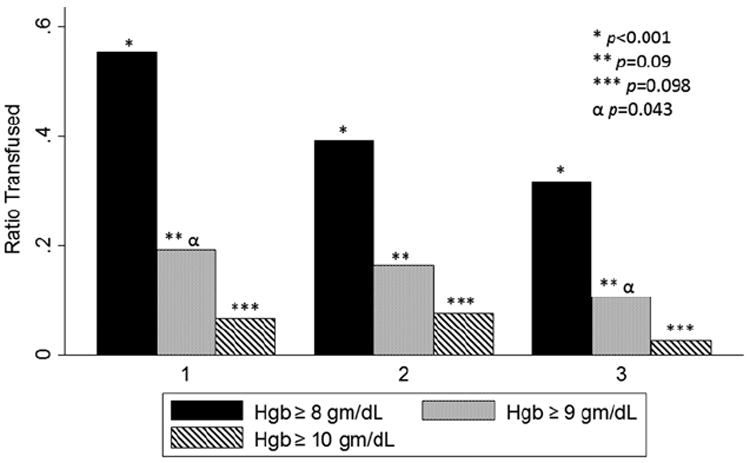
Bar graph demonstrating the ratio of off-protocol transfusion events by study period for all open heart surgery cases performed during the study, excluding patients massively transfused patients. The x-axis represents the study period. The y-axis represents the ratio of transfused units given at or above a Hgb trigger of 8 (solid bar), 9 (dotted bar) and 10 (striped bar) gm/dL, respectively. The single, double and triple asterisks with associated p values denote Chi-square analyses of each ratio across all study periods. The α symbol denotes Chi-square analysis of transfusions over 9 gm/dL between periods 1 and 3 only.
Table 2.
Univariate Logistic Regression Analysis by Study Period for Risk of Transfusion above a Defined Hgb Trigger
| Variable | Period 1 | Period 2 | Period 3 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| OR | CI | P valuea | OR | CI | p valuea | OR | CI | P valuea | |
| Threshold > 8 gm/dL | 1 (Reference) | 0.52 | 0.34-0.79 | 0.003 | 0.37 | 0.24-0.59 | <0.001 | ||
| Threshold > 9 gm/dL | 1 (Reference) | 0.84 | 0.49-1.44 | 0.52 | 0.50 | 0.27-0.95 | 0.04 | ||
| Threshold > 10 gm/dL | 1 (Reference) | 1.15 | 0.51-2.60 | 0.74 | 0.37 | 0.11-1.17 | 0.09b | ||
CI=95% Confidence Interval, OR=Odds Ratio
p values determined by univariate logistic regression analysis
Period 3 is significantly different than Period 2 by univariate analysis (OR: 0.32 [0.10-0.99], p=0.048)
Figure 2.
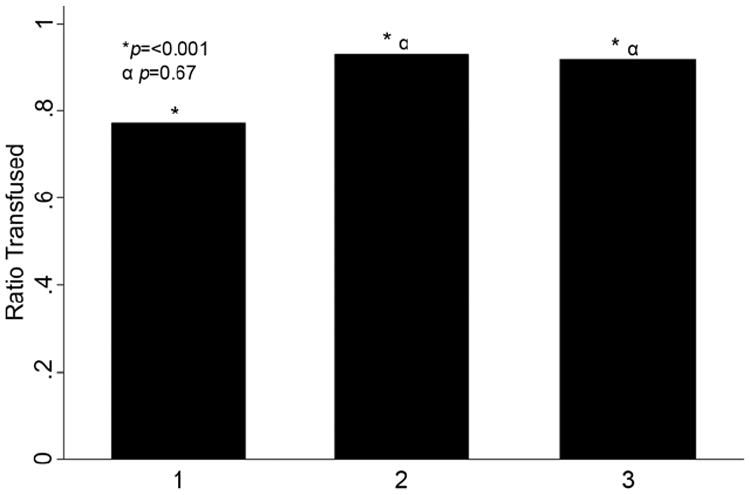
Bar graph demonstrating the number of single unit transfusions by study period for all open heart surgery cases performed during the study. The x-axis represents the study period while the y-axis represents the ratio of single unit transfusions. The single asterisk marks comparison of all ratios using Chi-square analysis. The α symbol denotes a sub-group comparison of period 2 and period 3 using logistic regression analysis. The associated p values are indicated.
To determine whether harm was associated with protocol adherence, we assessed in hospital mortality, change in creatinine, and LOS across study periods. In-hospital mortality did not increase, but decreased during the study., with a gross mortality of 7.0%, 2.2%, and 1.5% for study periods 1, 2 and 3 respectively (p=0.02; (Table 3). In the transfused cohort, mortality also decreased (p=0.02) and, in fact, was responsible for the difference in mortality seen across the study periods. There was no significant difference in mortality in the non-transfused cohorts during the study (p=0.39; Table 4). The observed to expected mortality (O/E) across the study periods revealed a non-significant decrease, falling from 2.19 to 0.51 (p=0.15; Table 3), with no significant differences in change in creatinine, ICU LOS or overall LOS across study periods in either cohort (Table 4).
Table 3.
Total cohort mortality
| Period 1 (N=172) | Period 2 (N=138) | Period 3 (N=202) | p valuea | |
|---|---|---|---|---|
| Observed In hospital mortality | 12 (7.0 %) | 3/134 (2.2%) | 3 (1.5%) | 0.02 |
| Mean Expected In hospital mortality | 3.19% | 2.52% | 2.96% | 0.77 |
| Observed/expected ratio | 2.19 | 0.87 | 0.51 | 0.15 |
p values determined by Fisher’s exact test, rank sum or Chi-square analysis as appropriate
Table 4.
Secondary Outcomes by Transfusion Status and Study Period
| Cohort | Variables | Period 1 | Period 2 | Period 3 | p valuea |
|---|---|---|---|---|---|
| Non-Transfused (N=368) | Change in | 0.2 (0.1-0.4) | 0.2 (0.0-0.4) | 0.2 (0.0-0.3) | 0.78 |
| CSICU LOS (days) | 1.8 (1.1-2.6) | 1.7 (1.0-2.9) | 1.3 (1.0-2.6) | 0.21 | |
| Total hospital LOS | 8 (6-12) | 8 (6-13) | 7 (5-11) | 0.11 | |
| In hospital mortality | 4/127 (3.2%) | 2/94 (2.1%) | 1/147 (0.7%) | 0.39 | |
| Transfusion (N=144) | Change in | 0.4 (0.2-0.9) | 0.4 (0.1-0.8) | 0.3 (0.0-0.6) | 0.3 |
| CSICU LOS (days) | 4 (2.7-8) | 2.6 (1.9-5.8) | 4.0 (2.3-7.2) | 0.22 | |
| Total hospital LOS | 15 (9-21) | 12 (9-19) | 16 (11-27) | 0.36 | |
| In hospital mortality | 8/45 (17.8%) | 1/40 (2.5%)b | 2/55 (3.6%) | 0.02 |
CSICU=Cardiac Surgical Intensive Care Unit, LOS=Length of Stay, N=Number of Patients
p values determined by Fisher’s exact test or Kruskal Wallis analysis as appropriate
Mortality data missing on 4 patients
Comment
Both the incidence of off-protocol transfusions and the univariate odds of giving a transfusion for a Hgb trigger ≥ 8gm/dL decreased as the study progressed. Additionally, single unit transfusions increased. More restrictive behavior did not adversely affect patients, but rather was associated with a decrease in in-hospital mortality from study period 1 to period 3.
The documented risks of blood product transfusions include fever, lung injury, circulatory overload, sepsis, hemolytic transfusion reactions and infectious complications.[1-5, 7-12] Furthermore, transfused patients appear to suffer an increased mortality compared to non-transfused patients, with mortality curves continuing to separate through 5 years of follow up.[3, 13, 14] Although many strategies aim at reducing the risks associated with these transfusions, the most effective manner of avoiding a transfusion complication is to avoid the transfusion.[10] Although the risks associated with transfusions are well known, consensus on the most appropriate Hgb trigger for a transfusion is not uniformly agreed upon despite evidence that restrictive strategies appear as safe as liberal ones.
Only 4 randomized controlled trials have investigated the subject of restrictive strategies in blood transfusions. In perhaps the most well-known, the TRICC trial of 1999, Hébert et al. analyzed 838 ICU patients, both medical and surgical, and randomized them to blood transfusions based on either a Hgb trigger of 7 or 10 gm/dL. They found equivalent all-cause 30-day mortalities between groups, with decreased in-hospital mortality in the restrictive cohort. Subgroup analyses showed that younger patients, as well as patients with lower APACHE II scores did worse with a liberal transfusion strategy, with significant increases in 30-day mortality.[6] Recently, the FOCUS trial examined 2016 hip surgery patients, with an average age of 82 and cardiovascular risk factors. Patients were randomized to transfusion triggers of 8 and 10 gm/dL respectively. No difference in mortality or ability to ambulate was seen between groups at 30 or 60 days, though the liberal transfusion group received 1,200 more blood transfusions.[8] In the most recent randomized trial of a restrictive vs. a liberal transfusion trigger (7 vs 9 gm/dL) in patients with upper gastrointestinal bleeds, the restrictive strategy had a higher 45 day survival (95 vs 90%) as well as a lower rate of further bleeding.[15] There is only one randomized control trial in cardiac surgery examining transfusion triggers. In that study from 2010, Hajjar et al. examined 512 consecutive open heart surgery patients, including coronary bypass, valve surgery, and combined procedures. Patients were randomized to a hematocrit transfusion trigger of 24 vs. 30%. They found no difference in 30 day mortality or the incidence of severe postoperative morbidity. However, the lower transfusion trigger value led to a decrease in the transfusion rate from 78% to 47% and an overall decrease in red blood cell utilization by over 50%. Additionally, the number of transfusions remained a significant hazard for mortality and complications, with each transfusion increasing the risk of death at 30 days by 20%.[7]
Taking into consideration the risks and benefits of transfusion, the Society of Thoracic Surgeons revised their clinical practice guidelines for blood transfusions in 2011, noting that transfusions with Hgb values >10 gm/dL are unlikely to be useful, while transfusions for Hgb values <7gm/dL are likely reasonable.[13] Despite these recommendations, the transfusion behavior of cardiac surgery programs throughout this country are widely disparate.[2, 5, 9] In an attempt to standardize practice, there has been a suggestion that transfusion rates be made public, as a measure of quality of care.[2]
Several techniques have been attempted to modify physician behavior in the literature, including physician education, feedback (including peer-disclosure), and systems initiatives (e.g. prompts in the electronic medical record or use of check lists).[16-23] Regionally, the Center for Medicare and Medicaid Services has leveraged the publication of hospital performance metrics to the lay public, presuming that inter-hospital comparisons on websites such as www.hospitalcompare.hhs.gov, would improve physician adherence to known evidence based practice. Furthermore, incentivizing hospitals to improve performance is an ongoing effort.[24] Though the results of these interventions vary widely, with many demonstrating both poor short and long term results, the most favorable results are generally produced in studies which incorporate multiple intervention modalities that are repeated regularly.[20, 21] Additionally, studies which utilized peer-disclosure appeared to have larger short-term gains and sustained long-term outcomes.[16, 20]
In our study, we attempted to modify physician transfusion behavior first by education, at which time a consensus was reached regarding an acceptable transfusion trigger. Despite agreement on a trigger value of 8 gm/dL, initial protocol adherence was extremely poor. However, upon weekly presentation of adherence data to the surgeons and providers, presented as group behavior, adherence to the protocol improved. Upon withdrawal of the anonymity of the data in period 3, specifically revealing the transfusion behavior associated with each individual surgeon, we saw further significant improvement in protocol adherence.
Perhaps more significantly, our data demonstrate that no harm occurred to patients when a more restrictive transfusion strategy was observed. When normalized for expected mortality, there was a trend for decreased mortality across the study, with a significant decrease in gross mortality. While a decrease in in-hospital mortality is consistent with data from Hébert et al,[6] the observed effect in this study is most likely due to the relatively small sample size. Therefore, although it is difficult to ascribe a benefit in mortality to the restrictive strategy, we believe that it is very clear that no harm was caused by withholding transfusions. Moreover, in our non-transfused patients stratified by study period, no significant differences in mortality or other secondary outcomes over the course of the study were observed as a result of withholding a transfusion, emphasizing that a restrictive policy carries no harm.
Additionally, our data demonstrated an increased incidence in single unit transfusions as the study progressed. Studies have demonstrated that elevated transferrin saturation is associated with acute kidney injury, suggesting that transfusion-related iron overload may be implicated in renal dysfunction.[25] Although no clinical difference in renal function was identified among cohorts during this study, theoretically renal injury may be minimized with single vs multiple unit transfusions.
Limitations
There are several limitations to our study. First, transfusion indications were not addressed by our study. It stands to reason that although a Hemoglobin of 8 may be an appropriate value for a protocol driven approach to transfusion, off protocol transfusions (when a Hgb is over 8) may be indicated, e.g. ongoing bleeding or end organ ischemia. Aiming for a protocol variance of zero might lead to patient harm. Nevertheless, as evidenced by this study, diminishing off protocol transfusion rates from over 50% to approximately 25% did not jeopardize patient outcome. Understanding the reasons for the variance seen in 1 of 4 transfusions in period 3 could give insight into when the transfusion trigger of 8 should be abandoned. Secondly, this study does not contain any post-intervention follow-up data and as such can make no comment on post discharge outcomes as a result of discharging patients with potentially more significant anemia. Third, our assessment of transfusion behavior did not extend to the post-ICU surgical floor. It is possible that transfusions were withheld in the ICU, only to be given later on the floor, potentially biasing in hospital mortality and LOS findings. Finally, this study does not account for the possibility that time in and of itself might have been associated with improved transfusion protocol adherence, and that weekly disclosure of transfusion behavior by group or by individual was coincidental to the improvement, not causative. To address this possibility, we divided study periods 2 and 3 into two equal length periods and found no difference in protocol adherence during the first half of each study compared to the second half, although the small n in each group makes this comparison vulnerable to a type II error. Nevertheless, this analysis suggests that the duration of the study had less to do with improved protocol compliance than the methodological differences intrinsic to each study period.
Conclusion
Evidence advocating a restrictive blood transfusion policy is growing. In order to practice evidenced-based medicine, surgeons must adjust their transfusion behavior appropriately. In this study, we assessed the effect of weekly feedback of transfusion behavior, first showing group behavior and then assessing the benefit of publically disclosing individual surgeon behavior. During each study period, transfusion protocol adherence improved, suggesting that a) repetitive group feedback improves physician behavior, and b) disclosing physician specific behavior at the time of group feedback has a significant, incremental effect on changing physician behavior.
Acknowledgments
Drs. Beaty and Arnaoutakis are the Irene Piccinini Investigators in Cardiac Surgery. Dr. George is the Hugh R. Sharp Cardiac Surgery Research Fellow. This research was supported in part by a National Institutes of Health Grant: T32CA126607. The content is the responsibility of the authors alone and does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. government.
Footnotes
Presentation: The contents of this manuscript were presented at the 26th Annual Meeting of the European Association for Cardio-Thoracic Surgery in CCIB, Barcelona, Spain on October 27, 2012.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Veenith T, et al. Survival and length of stay following blood transfusion in octogenarians following cardiac surgery. Anaesthesia. 2010;65(4):331–6. doi: 10.1111/j.1365-2044.2009.06225.x. [DOI] [PubMed] [Google Scholar]
- 2.Shander AS, Goodnough LT. Blood transfusion as a quality indicator in cardiac surgery. JAMA. 2010;304(14):1610–1. doi: 10.1001/jama.2010.1483. [DOI] [PubMed] [Google Scholar]
- 3.Murphy GJ, et al. Increased mortality, postoperative morbidity, and cost after red blood cell transfusion in patients having cardiac surgery. Circulation. 2007;116(22):2544–52. doi: 10.1161/CIRCULATIONAHA.107.698977. [DOI] [PubMed] [Google Scholar]
- 4.Moskowitz DM, et al. The impact of blood conservation on outcomes in cardiac surgery: is it safe and effective? Ann Thorac Surg. 2010;90(2):451–8. doi: 10.1016/j.athoracsur.2010.04.089. [DOI] [PubMed] [Google Scholar]
- 5.Likosky DS, et al. Effect of the perioperative blood transfusion and blood conservation in cardiac surgery clinical practice guidelines of the Society of Thoracic Surgeons and the Society of Cardiovascular Anesthesiologists upon clinical practices. Anesth Analg. 2010;111(2):316–23. doi: 10.1213/ANE.0b013e3181e329f1. [DOI] [PubMed] [Google Scholar]
- 6.Hebert PC, et al. A multicenter, randomized, controlled clinical trial of transfusion requirements in critical care. Transfusion Requirements in Critical Care Investigators, Canadian Critical Care Trials Group. N Engl J Med. 1999;340(6):409–17. doi: 10.1056/NEJM199902113400601. [DOI] [PubMed] [Google Scholar]
- 7.Hajjar LA, et al. Transfusion requirements after cardiac surgery: the TRACS randomized controlled trial. JAMA. 2010;304(14):1559–67. doi: 10.1001/jama.2010.1446. [DOI] [PubMed] [Google Scholar]
- 8.Carson JL, et al. Liberal or restrictive transfusion in high-risk patients after hip surgery. N Engl J Med. 2011;365(26):2453–62. doi: 10.1056/NEJMoa1012452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bennett-Guerrero E, et al. Variation in use of blood transfusion in coronary artery bypass graft surgery. JAMA. 2010;304(14):1568–75. doi: 10.1001/jama.2010.1406. [DOI] [PubMed] [Google Scholar]
- 10.Vamvakas EC, Blajchman MA. Blood still kills: six strategies to further reduce allogeneic blood transfusion-related mortality. Transfus Med Rev. 2010;24(2):77–124. doi: 10.1016/j.tmrv.2009.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vamvakas EC, Blajchman MA. Transfusion-related mortality: the ongoing risks of allogeneic blood transfusion and the available strategies for their prevention. Blood. 2009;113(15):3406–17. doi: 10.1182/blood-2008-10-167643. [DOI] [PubMed] [Google Scholar]
- 12.Gilliss BM, Looney MR, Gropper MA. Reducing noninfectious risks of blood transfusion. Anesthesiology. 2011;115(3):635–49. doi: 10.1097/ALN.0b013e31822a22d9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ferraris VA, et al. 2011 update to the Society of Thoracic Surgeons and the Society of Cardiovascular Anesthesiologists blood conservation clinical practice guidelines. Ann Thorac Surg. 2011;91(3):944–82. doi: 10.1016/j.athoracsur.2010.11.078. [DOI] [PubMed] [Google Scholar]
- 14.Bhaskar B, et al. Impact of blood product transfusion on short and long-term survival after cardiac surgery: more evidence. Ann Thorac Surg. 2012;94(2):460–7. doi: 10.1016/j.athoracsur.2012.04.005. [DOI] [PubMed] [Google Scholar]
- 15.Villanueva C, et al. Transfusion strategies for acute upper gastrointestinal bleeding. N Engl J Med. 2013;368(1):11–21. doi: 10.1056/NEJMoa1211801. [DOI] [PubMed] [Google Scholar]
- 16.Winickoff RN, et al. Improving physician performance through peer comparison feedback. Med Care. 1984;22(6):527–34. doi: 10.1097/00005650-198406000-00003. [DOI] [PubMed] [Google Scholar]
- 17.Wang TJ, et al. A utilization management intervention to reduce unnecessary testing in the coronary care unit. Arch Intern Med. 2002;162(16):1885–90. doi: 10.1001/archinte.162.16.1885. [DOI] [PubMed] [Google Scholar]
- 18.Neilson EG, et al. The impact of peer management on test-ordering behavior. Ann Intern Med. 2004;141(3):196–204. doi: 10.7326/0003-4819-141-3-200408030-00008. [DOI] [PubMed] [Google Scholar]
- 19.Calderon-Margalit R, et al. An administrative intervention to improve the utilization of laboratory tests within a university hospital. Int J Qual Health Care. 2005;17(3):243–8. doi: 10.1093/intqhc/mzi025. [DOI] [PubMed] [Google Scholar]
- 20.Bunting PS, Van Walraven C. Effect of a controlled feedback intervention on laboratory test ordering by community physicians. Clin Chem. 2004;50(2):321–6. doi: 10.1373/clinchem.2003.025098. [DOI] [PubMed] [Google Scholar]
- 21.Axt-Adam P, van der Wouden JC, van der Does E. Influencing behavior of physicians ordering laboratory tests: a literature study. Med Care. 1993;31(9):784–94. doi: 10.1097/00005650-199309000-00003. [DOI] [PubMed] [Google Scholar]
- 22.Whitman G, et al. Prophylactic antibiotic use: hardwiring of physician behavior, not education, leads to compliance. J Am Coll Surg. 2008;207(1):88–94. doi: 10.1016/j.jamcollsurg.2008.01.023. [DOI] [PubMed] [Google Scholar]
- 23.Kucher N, et al. Electronic alerts to prevent venous thromboembolism among hospitalized patients. N Engl J Med. 2005;352(10):969–77. doi: 10.1056/NEJMoa041533. [DOI] [PubMed] [Google Scholar]
- 24.Lindenauer PK, et al. Public reporting and pay for performance in hospital quality improvement. N Engl J Med. 2007;356(5):486–96. doi: 10.1056/NEJMsa064964. [DOI] [PubMed] [Google Scholar]
- 25.Karkouti K, et al. Advance targeted transfusion in anemic cardiac surgical patients for kidney protection: an unblinded randomized pilot clinical trial. Anesthesiology. 2012;116(3):613–21. doi: 10.1097/ALN.0b013e3182475e39. [DOI] [PubMed] [Google Scholar]


