Abstract
Purpose of review
The current medical model for obesity management is BMI-centric because BMI is the predominant measure used to gauge disease severity, as well as indications for various treatment modalities. Recent advancements in therapy and understanding of the relationship between BMI and obesity-related complications call for a re-examination of this approach.
Recent findings
Advancements in treatment, including the recent approval of two new weight loss medications in the USA, have enabled development of new medical models for management of obesity. On the basis of accumulating data demonstrating the benefits of weight loss regarding multiple obesity-related complications (e.g., diabetes prevention, type 2 diabetes mellitus, cardiovascular disease risk, nonalcoholic steatohepatitis, sleep apnea), a complications-centric model is proposed that employs weight loss as a tool to treat and prevent obesity comorbidities. This model assures that the aggressiveness of therapy is commensurate with disease severity, and that therapy is directed at those obese patients who will benefit most from weight loss therapy. The treatment algorithm is comprehensive in addressing complications and quantitative when possible in the staging of risk or disease severity.
Summary
A complications-centric approach to obesity management identifies patients who will benefit most from weight loss, and optimizes patient outcomes, benefit/risk ratio, and the cost–effectiveness of interventions.
Keywords: cardiometabolic disease, complications, obesity, treatment algorithm, weight loss
INTRODUCTION
Obesity is arguably the most common medical problem seen today in primary care, and is a disease [1▪▪,2–4] that adversely affects mortality, morbidity, and quality of life (QOL) as a result of its associated complications [5,6]. These complications can broadly be categorized as cardiometabolic, mechanical, and lifestyle based. The health risks associated with being overweight and obese include a range of conditions, including diabetes, cardiovascular disease (CVD), hypertension, dyslipidemia, sleep apnea, some cancers, musculoskeletal disease, infertility, disability, dementia, and mortality[7–10]. Moderate weight loss (5–10%) has been associated with improvements in these obesity-related comorbidities [11▪], with lifestyle modification, pharmacotherapy, and bariatric surgery representing the three available treatment options. As per National Heart, Lung, and Blood Institute (NHLBI) guidelines, a comprehensive program of lifestyle modification is the initial option for achieving this goal [5]. In 2012, the US Food and Drug Administration (FDA) approved two new effective medications to be used as adjuncts to lifestyle modification based on the results of placebo-controlled trials [12,13,14▪▪,15]. Bariatric surgery is typically limited to severely obese patients (BMI ≥40 kg/m2) or those with a BMI of at least 35 kg/m2 and weight-related comorbidities and can be a highly effective weight-loss option [16▪▪]. Currently, a BMI-centric approach represents the most commonly employed algorithm for care. We will discuss the limitations of this approach in favor of a complications-centric model for the medical management of obesity. This latter approach emphasizes the identification and staging of complications, and treatment paradigm directed at patients who would derive the most benefit from weight loss.
BMI-CENTRIC APPROACH TO OBESITY MANAGEMENT
Adolphe Quetelet (1796–1874), a Belgian statistician, described the Quetelet Index of relative body weight in 1832, which was the ratio of weight in kilograms divided by the square of height in meters. Ancel Keys (1904–2004) later termed this the BMI in 1972 [17]. In 1985, BMI was adapted as the standard for evaluating overweight and obese patients by the National Institutes of Health [18]. A major shortcoming of BMI as a measure of adiposity is that the numerator (weight) of the index fails to distinguish between lean and fat mass [19–21]. Variables that limit BMI as a comparative measure include aging, sex, physical fitness and muscular build, weight loss with exercise, racial differences, and clinical disease [22–24]. A systematic review found that around 50% of individuals not labeled as obese by BMI might indeed have excess adiposity [25], helping to explain why BMI is a poor discriminator of cardiovascular risk in people with intermediate BMI (below 30) values.
Despite its limitations as a measure of adiposity, BMI is the predominant measure used to gauge the severity of obesity, and the key determinant of treatment indications in current guidelines and algorithms for management [26]. In 1997, the WHO put forth a classification of disease severity for overweight and obesity according to BMI as shown in Table 1. Using the WHO classification as a foundation, the NHLBI published clinical guidelines on the identification, evaluation, and treatment of overweight and obese adults in 1998 as shown in Table 2 [5]. In this algorithm, it is the presenting BMI value that largely dictates indications for lifestyle, medical, and surgical interventions, without reference to the presence or absence of obesity complications.
Table 1.
WHO classification of obesity by BMI, waist circumference, and associated disease risk
| Classification | BMI | Waist circumference | ||
|---|---|---|---|---|
| BMI (kg/m2) | Comorbidity risk | Normal men ≤102 cm (≤40 in); women ≤88 cm (≤35 in) | Elevated men >102 cm (>40 in); women >88 cm (>35 in) | |
| Underweight | <18.5 | Low but there may be other clinical problems | ||
| Normal weight | 18.5–24.9 | Average | ||
| Preobese (Overweight) | 25–29.9 | Increased | Increased | High |
| Obese class I | 30–34.9 | Moderate | High | Very high |
| Obese class II | 35–39.9 | Severe | Very high | Very high |
| Obese class III | ≥40 | Very severe | Extremely high | Extremely high |
World Health Organization. Report of a WHO consultation on obesity. Obesity: preventing and managing the global epidemic. World Health Organization: Geneva, 1998.
Table 2.
National Heart, Lung, and Blood Institute guide to selecting treatment for overweight and obesitya
| Treatment | BMI category | ||||
|---|---|---|---|---|---|
| 25–26.9 | 27–29.9 | 30–34.9 | 35–39.9 | ≥40 | |
| Lifestyle (diet, physical activity, behavior) | Yes | Yes | Yes | Yes | Yes |
| Pharmacotherapy | No | Only with comorbidities | Yes | Yes | Yes |
| Surgeryb | No | No | No. Only LAGB approved with ≥1 comorbidityb | Only with comorbidities | Yes |
LAGB, laparoscopic adjustable gastric banding.
From the Summary of Recommendations in the Clinical Guidelines on the Identification, Evaluation, and Treatment of Overweight and Obesity in Adults, National Institutes of Health/National Heart Lung and Blood Institute, 1998. http://www.nhlbi.nih.gov/guidelines/obesity/ob_gdlns.pdf.
Bariatric surgeries require lifestyle medical follow-up.
US Food and Drug Administration (FDA)-approved Lap Band surgery for patients with BMI of at least 30 and one weight-related medical condition (February 2011).
The relationship between generalized obesity, as measured by the BMI, and its associated comorbidities is complex. Obesity can exacerbate insulin resistance and impel cardiometabolic disease progression to metabolic syndrome, prediabetes, diabetes, and CVD. However, insulin resistance exists largely independent of BMI, and BMI is a poor predictor of CVD when compared with other measurements such as waist/hip ratio [27–32]. Importantly, up to 30% of obese individuals (BMI ≥30) are relatively insulin sensitive and do not have manifestations of cardiometabolic disease (i.e., the metabolically healthy obese), and up to 30% of lean individuals are insulin resistant with cardiometabolic disease manifestations [30,33,34]. Thus, obesity is neither necessary nor sufficient to explain the pathophysiology underlying cardiometabolic disease. Similarly, regarding the mechanical complications of obesity, the presence and severity of obstructive sleep apnea (OSA), osteoarthritis, gastroesophageal reflux disease (GERD), and stress incontinence can be poorly correlated with the BMI level [29]. Despite the poor correlation between baseline BMI, weight loss can be used as a therapeutic tool to treat obesity-related complications. Weight loss whether achieved by lifestyle intervention [35], medications [36,37▪▪,38,39], or bariatric surgery [40,41,42▪] can improve glucose tolerance and prevent progression to type 2 diabetes mellitus (T2DM) in high-risk individuals, ameliorate dyslipidemia, and lower blood pressures. It also improves symptoms related to OSA, GERD, osteoarthritis, and poor QOL. Therapeutic indications based primarily on BMI will fail to consistently identify patients with these obesity-related complications who will most benefit from weight loss therapy.
COMPLICATIONS-CENTRIC APPROACH TO OBESITY MANAGEMENT
In the general approach to the overweight/ obese patient, clinicians must identify patients who will benefit most from therapy, establish therapeutic targets and goals, and identify the modality and intensity of treatment in order to optimize the benefit/risk ratio. As alluded to above, a BMI-centric algorithm is not ideal. Rather, the patients who will benefit most from treatment have obesity-related complications that can be ameliorated by weight loss. A complications-centric medical model, rather than a BMI-centric model, is more rationally designed to achieve these goals. Aggressive and resource-intensive weight management would be directed at those patients with the highest severity of complications that are remediable using weight loss therapy [43,44].
Evaluation of the obese patient
In a complications-centric model, the existence and severity of complications at baseline is more important than the baseline BMI itself in determining the treatment modality and intensity for obesity [44–46]. Therefore, the first step is to evaluate the patient for the presence and severity of obesity complications in order to develop an appropriate therapeutic strategy. In patients with cardiometabolic disease or risk factors, the objective of weight loss therapy is to reduce risk of future T2DM and CVD, and to treat patients with overt diabetes, hypertension, and dyslipidemia. This includes insulin-resistant patients with traits that comprise the diagnosis of metabolic syndrome [elevated waist circumference, fasting glucose, blood pressure, and triglycerides, and low high-density lipoprotein (HDL) cholesterol], patients with prediabetes, and those who have progressed to type 2 diabetes or CVD. The clinician should evaluate patients for the metabolic syndrome [47▪▪] and prediabetes [48], as this effectively identifies individuals at high risk for future diabetes and CVD. However, the metabolic syndrome and prediabetes have high specificity but low sensitivity for identifying patients with insulin resistance and cardiometabolic disease [11▪,49], and these entities alone will not identify significant proportions of at-risk patients. The initial evaluation should also screen for other disease entities that will benefit from weight loss, including nonalcoholic fatty liver disease (NAFLD) and sleep apnea. Finally, obese patients should be evaluated for mechanical complications such as problematic degenerative joint disease, GERD, stress incontinence, and immobility/disability.
Current obesity staging systems
There are two paradigms that have been developed for comprehensive clinical staging of obesity according to the severity of comorbidities that can be used to guide the modality and intensity of therapy.
Edmonton obesity staging system
A staging system for obesity was proposed by Sharma and Kushner in 2009 [43] as a guide to treatment intensity for weight loss. As shown in Table 3, Edmonton obesity staging system (EOSS) establishes five stages (0 through 4) that integrate the severity of obesity-related complications together with an assessment of the adverse functional impact imposed by complications on the well being and functional status of the patient. EOSS was the first cogent complications-centric strategy that went beyond BMI level and emphasized obesity-related complications as a basis for the intensity of weight loss therapy.
Table 3.
Edmonton obesity staging system
| Stage | Cardiometabolic and mechanical disease complications | Functional impact |
|---|---|---|
| 0 | No risk factors | No functional impairments or impairments in well-being |
| 1 | ‘Subclinical risk factors’: prediabetes, metabolic syndrome, NAFLD, borderline hypertension, dyspnea on moderate exertion | Mild functional limitations and impairment of well-being, mild psychopathology, occasional aches and pains |
| 2 | Established chronic disease: T2DM, hypertension, sleep apnea, PCOS, osteoarthritis, GERD | Moderate limitations in activities of daily living, moderate impairment of well-being, and/or moderate psychopathology (e.g., anxiety disorder) |
| 3 | Established end organ damage: myocardial infarction, heart failure, stroke, diabetes vascular complications, incapacitating osteoarthritis | Significant functional limitations and/or impairment of well-being |
| 4 | Severe end-stage disabilities | Severe limitations and impairment of well-being, severe disabling psychopathology |
The EOSS is a valuable guideline for obesity management as it integrates an evaluation of the severity of both cardiometabolic disease and mechanical complications together with an assessment of functional impairment. However, there are two limitations. First the assessments are not quantitative and staging depends largely on clinical judgment. Second, it lacks granularity for cardiometabolic disease staging (CMDS). All patients with insulin resistance, prediabetes, metabolic syndrome, elevated hepatic transaminases, borderline hypertension, moderate dyspnea on exertion, and mild impairment of well-being are included within stage 1, which encompasses a wide range of risk for future diabetes and all-cause and CVD mortality. This lack of granularity is highlighted in the CMDS system described below wherein three stages (1, 2, and 3) differentiate a broad range of cardiometabolic disease risk that is encompassed within the single stage 1 of EOSS.
Cardiometabolic disease staging system
Garvey and coworkers [44,46] have recently proposed CMDS as a guide for treatment of obesity or other interventions intended to treat or prevent diabetes and CVD risk. CMDS is a single staging system that provides a quantitative assessment of risk for both future diabetes and all-cause and CVD mortality. CMDS assigns patients to one of five risk categories using quantitative parameters readily available to the clinician, including waist circumference, SBP and DBP, fasting blood levels of glucose, triglycerides, and HDL-C, as well as the 2-h oral glucose tolerance test (OGTT) value. With advancement from stage 0 to stage 4, there are significant increments in risk and adjusted hazard ratio for diabetes as validated using the Coronary Artery Risk Development in Young Adults (CARDIA) study national cohort, as well as increased risk and hazard ratios for both all-cause and CVD-related mortality in the National Health and Nutrition Examination Survey (NHANES) cohort [46]. This staging system provides a strong predictor of diabetes, CVD mortality, and all-cause mortality independent of BMI.
As shown in Table 4, individuals in stage 0 have no risk factors (i.e., metabolically healthy obese) and exhibit minimal rates of incident diabetes and all-cause and CVD mortality. Patients with one or two risk factors (waist, blood pressure, HDL, or triglycerides) comprise stage 1; these patients do not meet criteria for either metabolic syndrome or prediabetes, but exhibit increased risk of future diabetes. In stage 2, patients meet criteria for only one of the following: metabolic syndrome (three or four of the following risk factors: waist circumference, blood pressure, HDL, triglycerides), or impaired fasting glucose (IFG), or impaired glucose tolerance (IGT). Stage 3 describes patients who meet criteria for any two out of three: metabolic syndrome, IFG, and IGT. Several studies show that patients who meet criteria for metabolic syndrome and prediabetes, or IFG and IGT are at substantially higher risk for T2DM than patients who satisfy criteria for only one of these diagnoses [50–56]. Stage 4 represents the highest severity stage of CMDS and includes patients with overt T2DM and/or CVD, and considers T2DM as CVD equivalent due to the high risk of future CVD events conferred by T2DM even in the absence of known CVD [56]. With advancement of CMDS from stages 0 to 4, there is a progression of risk for both diabetes and all-cause and CVD mortality.
Table 4.
Cardiometabolic disease staging
| Stage | Descriptor | Criteria |
|---|---|---|
| 0 | Metabolically healthy | No risk factors |
| 1 | One or two risk factors | Have one or two of the following risk factors:
|
| 2 | Metabolic syndrome or prediabetes | Have only one of the following three conditions in isolation:
|
| 3 | Metabolic syndrome and prediabetes | Have any two of the following three conditions:
|
| 4 | T2DM and/or CVD | Have T2DM and/or CVD:
|
CVD, cardiovascular disease; HDL-C, high-density lipoprotein cholesterol; IFG, impaired fasting glucose; IGT, impaired glucose tolerance; T2DM, type 2 diabetes mellitus.
OBESITY-RELATED COMPLICATIONS AND WEIGHT LOSS THERAPY
Obesity-related complications, which can be ameliorated by weight loss therapy, can be broadly categorized as cardiometabolic, mechanical, and lifestyle factors.
Cardiometabolic disease
Cardiometabolic disease includes prediabetes, diabetes, and cardiovascular disease.
Prediabetes, diabetes, and cardiovascular disease
The spectrum of cardiometabolic disease begins with relative insulin resistance, which is a trait that is expressed early in life, detectable in children with origins perhaps in utero. Insulin resistance becomes associated with other metabolic traits and cardiovascular risk factors as patients age, and progresses to the clinically identifiable high-risk states of prediabetes [57] and metabolic syndrome [58], which then culminates in T2DM, or CVD, or both in individual patients. Thus, the consequences of cardiometabolic disease are severe, with T2DM being associated with elevated risk for morbidity and mortality [49], and CVD being the leading cause of death in Western societies. Although obesity can exacerbate insulin resistance, the relationship between generalized obesity, as measured by the BMI (kg/m2), and cardiometabolic disease is complex [46]. In short, generalized obesity can worsen cardiometabolic disease by exacerbating insulin resistance, but is neither necessary nor sufficient to impel the progression of this disease as a cause of diabetes and CVD.
Despite the complex relationship with generalized obesity, weight loss is highly effective in the treatment of cardiometabolic disease. Moderate weight loss (~10%) is sufficient to lower fasting glucose and insulin, enhance insulin sensitivity, reduce blood pressure, lower triglycerides, raise HDL cholesterol, decrease levels of hepatic transaminases, prevent progression to diabetes, lower HbA1c in patients with T2DM, and improve biomarkers of cardiovascular risk such as C-reactive protein, fibrinogen, and adiponectin [14▪▪,18,29,32–36,45]. Perhaps, the greatest potential benefit of a complications-centric approach, in terms of public health and containment of heath care costs, is the use of weight loss to prevent diabetes in high-risk individuals [5]. For patients with T2DM, weight loss, whether induced by diet and exercise [35], bariatric surgery [42▪], or medications [36,37▪▪, 38,39], can improve control of glycemia, blood pressure, and lipids, while reducing the need for medications being used to specifically treat these metabolic abnormalities. The clinical trials programs for phentermine/topiramate extended-release [14▪▪,37▪▪,38] and lorcaserin [12,13,36] included studies on T2DM, and consistently demonstrated lower HbA1c with medication-assisted weight loss, together with the reduced need for medications in actively managed patients, when compared with patients treated with lifestyle modification alone. It could be argued that weight loss medications should be considered for any overweight or obese patient with overt T2DM who fail to achieve moderate weight loss (i.e., ~10%) with lifestyle modification.
Nonalcoholic fatty liver disease
NAFLD encompasses a spectrum of clinico-pathologic entities characterized by hepatic steatosis in the absence of significant alcohol use, and is associated with insulin resistance and the metabolic syndrome. It is the most common form of chronic liver disease in the USA and in many parts of the world. The spectrum of NAFLD ranges from simple hepatic steatosis with a generally benign course, to steatosis with nonspecific inflammation, to inflammation and fibrosis referred to as nonalcoholic steatohepatitis (NASH) that can progress to cirrhosis, hepatic failure, and hepatocellular carcinoma [59]. Unexplained elevations in liver enzymes affect up to 23% of American adults in the NHANES III cohort [60–62], presumably attributable in large part to NAFLD [59,63]. This prevalence is much higher in patients with metabolic syndrome and T2DM (63%), and even higher (96%) in the morbidly obese [64–66], wherein it contributes to excess deaths from cirrhosis and hepatic failure [67,68].
Weight loss resulting from very low calorie diets, gastric band surgery, and hypocaloric feeding in combination with increased physical activity has induced significant (up to 40%) reductions in mean liver fat based on radiological imaging [69–71]. Several studies using the drug orlistat have shown improvements in biochemical, histological, and radiological profiles of NASH in obese patients following at least 10% weight loss [72–74]. Similarly, surgical literature also shows significant improvement in the histological features of NASH, including resolution of disease in the majority of the patients who underwent roux-en-Y gastric bypass surgery [75,76].
Mechanical complications
Most common mechanical complications associated with obesity include obstructive sleep apnea, gastroesophageal reflux disease, and osteoarthritis.
Obstructive sleep apnea
OSA continues to be underdiagnosed in the general population [77], and even more so in the obese. With weight gain, even a 10% increase in body weight is associated with significant risk of developing OSA [78]. The prevalence of OSA is particularly high in obese patients with diabetes in whom prevalence rates as high as 86 percent have been reported [79]. OSA has been associated with CVDs, metabolic disorders, insulin resistance, and diabetes, and, therefore, its associated comorbidities result in a large population-level burden of morbidity [80].
Weight loss improves OSA. Lifestyle intervention with weight reduction has been shown to be a feasible and low-cost treatment for the vast majority of patients with mild OSA [81]. In recent clinical trials, the use of an intensive lifestyle approach, bariatric surgery, and medications to achieve weight loss significantly improved OSA among obese participants [82,83▪,84,85].
Gastroesophageal reflux disease
GERD-related complications include erosive esophagitis, Barrett esophagus, and esophageal adenocarcinoma [86], and have been increasing in frequency over the last several decades [87]. There is a strong positive association between increasing obesity and GERD symptoms and esophageal erosions [88].
Weight loss can dramatically improve GERD symptomatology. Complete resolution of symptoms in the majority of women with weight loss of 5–10%, and in men with more than 10% weight reduction, was observed in a clinical trial employing a structured weight loss program [88]. Bariatric procedures have also been shown to result in near normalization of GERD-related symptoms [89–91].
Osteoarthritis
Osteoarthritis and its complications are common in most persons older than 65 years, and contribute to increasing economic and social burdens [92,93]. Obesity is strongly linked to osteoarthritis as a risk factor and weight loss is considered the treatment of choice, particularly with osteoarthritis of the knee [94,95]. For prevention, as little as 5.1 kg reduction over a 10-year period decreases the likelihood of developing knee osteoarthritis by 50% [96]. A systematic review and meta-analysis showed clinically significant reductions in pain and improvements in function as a result of weight loss in obese patients diagnosed with osteoarthritis [97].
Other complications
Weight loss has been shown to ameliorate and/ or prevent multiple other obesity-related complications including cancers, musculoskeletal disease, infertility, stress incontinence, disability, dementia, and mortality [8,9,98–106].
Lifestyle factors
QOL and health-related QOL (HRQOL) encompass a person’s experiences, beliefs, and expectations with respect to physical, psychological, and social domains of health, and reflect an individual’s subjective evaluation of health and illness [107–109]. There are several obesity-specific QOL instruments: Impact of Weight on Quality of Life (IWQOL), IWQOL-Lite, Obesity Specific Quality of Life (OSQOL), Obesity Related Well Being, Short-Form Health Survey (SF-36), and Sickness Impact Profile. Important domains of these instruments include health, social/interpersonal, work, mobility, self-esteem, sexual life, activities of daily living, bodily pain, general mental health, and relationship with food. There is growing evidence in favor of using these instruments as reliable and valid outcome measurement tools to assess obesity-specific QOL [110].
Obese persons experience significant impairments in HRQOL, which worsen with increasing body weight and the number of comorbid illnesses [6,109]. However, there is poor correlation between anthropometric measures and health, and BMI provides little insight into functionality, QOL, or other prognostic factors in an individual [43]. Weight loss, however, improves QOL and HRQOL. Several studies have reported significant improvements in QOL outcomes after surgical treatment of obesity [111–114]. In several double-blinded, randomized, controlled trials, moderate weight loss (5–10%) was associated with improved HRQOL [115,116]. Furthermore, two studies have demonstrated the importance of baseline assessment of QOL as it relates to compliance to a lifestyle intervention and helping identify patients who may require additional support and reinforcement [117,118].
Psychological and behavioral factors
The lifestyle assessment of obese patients should include a history of psychological, behavioral, and social factors that may contribute to obesity and impact weight loss therapy [2]. Multiple variables assessed at baseline include weight-specific history, prior weight loss attempts, motivations, eating behavior/disorders, depression, anxiety, body image, readiness for change, and QOL [119]. These assessments most importantly identify individual patients who may require more intensive or specialized programs for lifestyle modification. These factors should also be used to determine whether patients are appropriate candidates for bariatric surgery. Although the relationship between obesity and psychopathology remains unclear, several studies have shown possible links between obesity and depression and anxiety [120–124].
In particular, it is important to evaluate patients for binge eating disorder (BED), which is prevalent in obese individuals and is characterized by loss of control while eating unusually large amounts of food [125▪]. BED has been established as a formal diagnosis in Diagnostic and Statistical Manual of Mental Disorders, fifth edition (DSM-V) [126]. A recent study found cognitive-behavioral therapy (CBT), an established treatment of choice for BED [127], and weight loss to be effective in treating BED, and that remission of binge-eating was associated with significantly greater weight loss [128]. A systematic review of randomized controlled trials indicated that combination therapies (CBT and medication) for BED improve both binge eating and weight loss [129].
A COMPREHENSIVE COMPLICATIONS-CENTRIC ALGORITHM FOR TREATING OBESITY
A comprehensive complications-centric obesity treatment algorithm (COTA) is shown in Fig. 1. In evaluating patients for obesity-related complications, we have endeavored to be comprehensive in addressing both cardiometabolic and biomechanical complications, and to highlight psychological and behavioral factors that call for more intensive or specialized programs for lifestyle modification. Furthermore, we have been quantitative in the staging of the severity of complications when possible. In this way, we have incorporated the comprehensive approach pioneered by the EOSS and addressed shortcomings regarding lack of quantification and granularity for differentiating complication severity and disease risk. In addition, we have incorporated CMDS as a quantitative approach to assessing severity of cardiometabolic disease and the attendant risk of T2DM and CVD, together with the broad-based evaluation of all other key complications that can be treated with weight loss. COTA builds upon the recent algorithm from the American Association of Clinical Endocrinologists [47▪▪] by providing a comprehensive approach to evaluation of obesity-related complications.
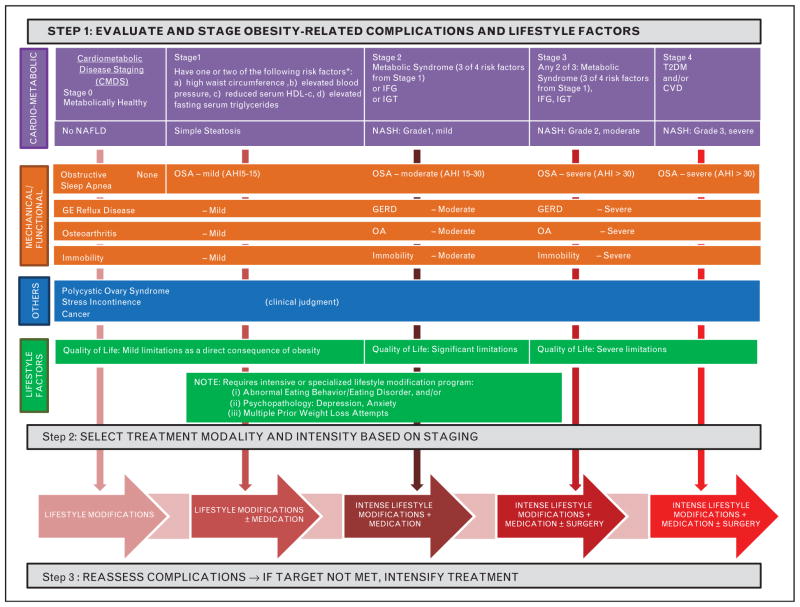
FIGURE 1.
Comprehensive obesity treatment algorithm (COTA). The figure illustrates the three steps of a complications-centric model for the management of obesity. In step 1, a comprehensive approach to the identification and staging of obesity-related complications is depicted using quantitative measures wherever possible. Step 2 indicates that weight loss therapy can be intensified, whether involving lifestyle modification or medications or bariatric surgery options, to achieve the targeted improvements in complications. Step 3 reflects the observation that there is a dose–response relationship between the amount of weight loss and the degree of improvement for multiple complications. GERD, gastroesophageal reflux disease; OSA, obstructive sleep apnea.
Step 1 involves the assessment of patients for the presence and severity of obesity-related complications. COTA delineates assessment of complications in the following categories: cardiometabolic, mechanical, others, and lifestyle factors. Clinical assessment will include medical history, examination, laboratory data, diagnostic procedures (e.g., ECG, 2-h OGTT), and assessment of psychosocial factors and functional limitations. CMDS, as described above, is used to quantitatively assess risk for diabetes, and all-cause and CVD mortality. NAFLD is included as a component of cardiometabolic disease pathophysiology, and staging incorporates histological markers of disease severity if liver biopsy data are available. Mechanical complications include OSA that can be quantitatively assessed using the apnea-hypopnea index. The severity of other mechanical complications, such as GERD, osteoarthritis, and immobility, are best ascertained by clinical judgment. The other category encompasses polycystic ovary syndrome (PCOS), stress incontinence, and cancer prevention, which, again, are best assessed as indications for weight loss therapy based on clinical judgment. Lifestyle factors include QOL, which can be targeted for improvement by weight loss, as well as factors such as abnormal eating behaviors and psychopathologies that are important in identifying patients who will generally require more intensified or specialized programs for lifestyle modification therapy.
In step 2, the clinician selects the modality and intensity of therapy (Table 5) [5,130–133] based on the initial evaluation and staging, as well as objective targets for improvements in the complications. The aggressiveness of therapy is determined to achieve the amount of weight loss that is sufficient to improve complications to the desired target. Lifestyle modification is the cornerstone of obesity management and its effective application is essential for optimal outcomes in all patients. Medications are used as an adjunct to lifestyle modification [47▪▪]. Lifestyle modifications are also needed for optimal outcomes following bariatric surgery to promote weight loss following the procedure [especially after laparoscopic adjustable gastric banding (LAGB)], to prevent weight regain over time, and to assure required intake of nutrients. Weight loss therapy must be individualized based on patient attributes such as age, access, cost, safety, adherence, readiness for change, psychological and behavioral factors, and other individual circumstances. There is a dose–response between the amount of weight loss and improvements in various obesity-related complications, and weight loss therapy can be intensified over a broad range to obtain the desired improvements [134].
Table 5.
Therapeutic options for weight loss
| Modality (references) | Description |
|---|---|
| Lifestyle modification [5,130] | Diet, exercise, and behavioral therapy |
| Intense lifestyle modification [131] | Diet, exercise, and behavioral therapy +weekly visit (individual and group sessions) +behavior modification curriculum ± meal replacement ± pharmacotherapy |
| Medication [132] | New: phentermine/topiramate extended release, lorcaserin Previous: orlistat, phentermine, benzphetamine, diethylpropion, and phendimetrazine |
| Surgery [133] | Vertical banded gastroplasty roux-en-Y gastric bypass Laparoscopic adjustable gastric banding Sleeve gastrectomy Biliopancreatic diversion and duodenal switch |
Step 3 involves reassessment of the patient with respect to complications after equilibrium weight loss is achieved. If complications have not been optimally improved to target, then intensification of weight loss therapy is required together with or without treatment with agents that are specifically designed to treat individual complications (e.g., diabetes, lipid-lowering, or hypertension medications).
CONCLUSION
Advancements in treatment modalities for obesity have enabled development of medical models for management [44]. We have made the argument that a complications-centric model that employs weight loss as a tool to treat and prevent obesity comorbidities will assure that the aggressiveness of therapy is commensurate with disease severity. This approach is designed to identify patients who will most benefit from weight loss, and optimize patient outcomes, the benefit/risk ratio, and the cost–effectiveness of interventions. We have proposed a comprehensive COTA that is comprehensive in addressing complications (as in EOSS), and quantitative when possible in the staging of risk or disease severity (as in CMDS).
KEY POINTS.
Overview of obesity staging systems.
Obesity and related complications (cardiometabolic, mechanical, lifestyle, and others).
A comprehensive and complications-centric obesity treatment algorithm to help clinicians identify patients who will benefit most from weight loss therapy.
Review of current modalities for treatment of obesity (lifestyle modification, medications, and surgery).
Footnotes
Acknowledgements
None.
Conflicts of interest
This work was supported by the Merit Review program of the Department of Veterans Affairs, the National Institutes of Health (DK-038765 and DK-083562), and the UAB Diabetes Research Center (P60-DK079626).
W.T.G. is a speaker for Merck, Amylin, and Liposcience. He is on the advisory boards of Daiichi-Sankyo, Vivus, Alkermes, Eisai, Liposcience, Tethys Bioscience, and Janssen. He receives research support from Merck, Amylin, and Weight Watchers. S.D. is a speaker for Vivus. T.S. is a speaker for Vivus.
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the annual period of review, have been highlighted as:
▪ of special interest
▪▪ of outstanding interest Additional references related to this topic can also be found in the Current World Literature section in this issue (pp. 495–496).
- 1▪▪.Mechanick JI, Garber AJ, Handelsman Y, Garvey WT. American Association of Clinical Endocrinologists’ position statement on obesity and obesity medicine. Endocr Pract. 2012;18:642–648. doi: 10.4158/EP12160.PS. This article articulates that the American Association of Clinical Endocrinologists considers obesity to be a disease and provides supportive rationale. These arguments led the American Medical Association to subsequently designate obesity as a disease in 2013. [DOI] [PubMed] [Google Scholar]
- 2.Kushner RF, Sarwer DB. Medical and behavioral evaluation of patients with obesity. Psychiatr Clin N Am. 2011;34:797–812. doi: 10.1016/j.psc.2011.07.002. [DOI] [PubMed] [Google Scholar]
- 3.Flegal KM, Carroll MD, Ogden CL, et al. Prevalence and trends in obesity among US adults, 1999–2008. JAMA. 2010;303:235–241. doi: 10.1001/jama.2009.2014. [DOI] [PubMed] [Google Scholar]
- 4.Ogden CL, Carroll MD, Curtin LR, et al. Prevalence of high body mass index in US children and adolescents, 2007–2008. JAMA. 2010;303:242–249. doi: 10.1001/jama.2009.2012. [DOI] [PubMed] [Google Scholar]
- 5.NHLBI Obesity Education Initiative Expert Panel on the Identification, Evaluation, and Treatment of Overweight and Obesity in Adults. Clinical Guidelines on the Identification, Evaluation, and Treatment of Overweight and Obesity in Adults: the evidence report. Obes Res. 1998;6:51S–209S. [PubMed] [Google Scholar]
- 6.Kolotkin RL, Meter K, Williams GR. Quality of life and obesity. Obes Rev. 2001;2:219–229. doi: 10.1046/j.1467-789x.2001.00040.x. [DOI] [PubMed] [Google Scholar]
- 7.Abdullah A, Peeters A, de Courten M, Stoelwinder J. The magnitude of association between overweight and obesity and the risk of diabetes: a meta-analysis of prospective cohort studies. Diabetes Res Clin Pract. 2010;89:309–319. doi: 10.1016/j.diabres.2010.04.012. [DOI] [PubMed] [Google Scholar]
- 8.Berrington de Gonzalez A, Hartge P, Cerhan JR, et al. Body mass index and mortality among 1. 46 million white adults. N Engl J Med. 2010;363:2211–2219. doi: 10.1056/NEJMoa1000367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.World Health Organization. Obesity: preventing and managing the global epidemic. Geneva, Switzerland: World Health Organization; 1998. [PubMed] [Google Scholar]
- 10.Malnick SDH, Knobler H. The medical complications of obesity. Q J Med. 2006;99:565–579. doi: 10.1093/qjmed/hcl085. [DOI] [PubMed] [Google Scholar]
- 11▪.Henry RR, Chilton R, Garvey WT. New options for the treatment of obesity and type 2 diabetes mellitus (narrative review) J Diabetes Complications. 2013 doi: 10.1016/j.jdiacomp.2013.04.011. In Press. This paper summarizes data on the benefits of weight loss in type 2 diabetes, and supports the concept that weight loss therapies should be considered integral in treatment paradigms for diabetes. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Smith SR, Weissman NJ, Anderson CM, et al. Behavioral Modification and Lorcaserin for Overweight and Obesity Management (BLOOM) Study Group. Multicenter, placebo-controlled trial of lorcaserin for weight management. N Engl J Med. 2010;363:245–256. doi: 10.1056/NEJMoa0909809. [DOI] [PubMed] [Google Scholar]
- 13.Fidler MC, Sanchez M, Raether B, et al. BLOSSOM Clinical Trial Group. A one-year randomized trial of lorcaserin for weight loss in obese and overweight adults: the BLOSSOM trial. J Clin Endocrinol Metab. 2011;96:3067–3077. doi: 10.1210/jc.2011-1256. [DOI] [PubMed] [Google Scholar]
- 14▪▪.Allison DB, Gadde KM, Garvey WT, et al. Controlled-release phentermine/topiramate in severely obese adults: a randomized controlled trial (EQUIP) Obesity. 2012;20:330–342. doi: 10.1038/oby.2011.330. This paper presents data from a key phase III clinical trial supporting FDA approval of phentermine/topiramate extended-release, one of the new effective weight loss medications now available for treating obesity. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gadde KM, Allison DB, Ryan DH, et al. Effects of low-dose, controlled-release, phentermine plus topiramate combination on weight and associated comorbidities in overweight and obese adults (CONQUER): a randomized, placebo-controlled, phase 3 trial. Lancet. 2011;377:1341–1352. doi: 10.1016/S0140-6736(11)60205-5. [DOI] [PubMed] [Google Scholar]
- 16▪▪.Sjostrom L, Peltonen M, Jacobson P, et al. Bariatric surgery and long-term cardiovascular events. JAMA. 2012;307:56–65. doi: 10.1001/jama.2011.1914. This paper presents long-term outcome data following bariatric surgery procedures in Sweden. It demonstrates a significant decrease in mortality in patients with surgically assisted weight loss. [DOI] [PubMed] [Google Scholar]
- 17.Eknoyan G. Adolphe Quetelet (1796–1874): the average man and indices of obesity. Nephrol Dial Transplant. 2008;23:47–51. doi: 10.1093/ndt/gfm517. [DOI] [PubMed] [Google Scholar]
- 18.National Institutes of Health Consensus Development Conference Statement. Health implications of obesity. Ann Intern Med. 1985;103:1073–1077. [PubMed] [Google Scholar]
- 19.Behnke A, Wilmore J. Evaluation and regulation of body build and composition. Englewood Cliffs, NJ: Prentice Hall; 1974. [Google Scholar]
- 20.Wellens RI, Roche AF, Khamis HJ, et al. Relationships between the body mass index and body composition. Obes Res. 1996;4:35–44. doi: 10.1002/j.1550-8528.1996.tb00510.x. [DOI] [PubMed] [Google Scholar]
- 21.Kontogianni MD, Panagiotakos DB, Skopouli FN. Does body mass index reflect adequately the body fat content in perimenopausal women? Maturitas. 2005;51:307–313. doi: 10.1016/j.maturitas.2004.08.014. [DOI] [PubMed] [Google Scholar]
- 22.Prentice AM, Jebb SA. Beyond body mass index. Obes Rev. 2001;2:141–147. doi: 10.1046/j.1467-789x.2001.00031.x. [DOI] [PubMed] [Google Scholar]
- 23.The Asia-Pacific Perspective: redefining obesity and its treatment. Health Communications Australia Pty Ltd; Sydney: 2000. [Google Scholar]
- 24.Banerji MA, Faridi N, Atluri R, et al. Body composition, visceral fat, leptin, and insulin resistance in Asian Indian men. J Clin Endocrinol Metab. 1999;84:137–144. doi: 10.1210/jcem.84.1.5371. [DOI] [PubMed] [Google Scholar]
- 25.Okorodudu DO, Jumean MF, Montori VM, et al. Diagnostic performance of body mass index to identify obesity as defined by body adiposity: a systematic review and meta-analysis. Int J Obesity. 2010;34:791–799. doi: 10.1038/ijo.2010.5. [DOI] [PubMed] [Google Scholar]
- 26.Clinical Guidelines on the Identification, Evaluation, and Treatment of Overweight and Obesity in Adults. The Evidence Report. 1998 NIH Publication No. 98-4083. [PubMed] [Google Scholar]
- 27.Welborn TA, Dhaliwal SS, Bennett SA. Waist-hip ratio is the dominant risk factor predicting cardiovascular death in Australia. Med J Aust. 2003;179:580–585. doi: 10.5694/j.1326-5377.2003.tb05704.x. [DOI] [PubMed] [Google Scholar]
- 28.Lawlor DA, Davey Smith G, Ebrahim S. Life course influences on insulin resistance: findings from the British Women’s Heart and Health Study. Diabetes Care. 2003;26:97–103. doi: 10.2337/diacare.26.1.97. [DOI] [PubMed] [Google Scholar]
- 29.Janssen I, Katzmarzyk PT, Ross R. Body mass index, waist circumference, and health risk: evidence in support of current National Institutes of Health guidelines. Arch Intern Med. 2002;162:2074–2079. doi: 10.1001/archinte.162.18.2074. [DOI] [PubMed] [Google Scholar]
- 30.Liao Y, Kwon S, Shaughnessy S, et al. Critical evaluation of adult treatment panel III criteria in identifying insulin resistance with dyslipidemia. Diabetes Care. 2004;27:978–983. doi: 10.2337/diacare.27.4.978. [DOI] [PubMed] [Google Scholar]
- 31.Lara-Castro C, Garvey WT. Diet, insulin resistance, and obesity: zoning in on data for Atkins dieters living in South Beach. J Clin Endocrinol Metab. 2004;89:4197–4205. doi: 10.1210/jc.2004-0683. [DOI] [PubMed] [Google Scholar]
- 32.Emerging Risk Factors Collaboration. . Wormser D, Kaptoge S, Di Angelantonio E, et al. Separate and combined associations of body-mass index and abdominal adiposity with cardiovascular disease: collaborative analysis of 58 prospective studies. Lancet. 2011;377:1085–1095. doi: 10.1016/S0140-6736(11)60105-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wildman RP, Muntner P, Reynolds K, et al. The obese without cardiometabolic risk factor clustering and the normal weight with cardiometabolic risk factor clustering: prevalence and correlates of 2 phenotypes among the US population (NHANES 1999–2004) Arch Intern Med. 2008;168:1617–1624. doi: 10.1001/archinte.168.15.1617. [DOI] [PubMed] [Google Scholar]
- 34.Ferrannini E, Balkau B, Coppack SW, et al. RISC Investigators. Insulin resistance, insulin response, and obesity as indicators of metabolic risk. J Clin Endocrinol Metab. 2007;92:2885–2892. doi: 10.1210/jc.2007-0334. [DOI] [PubMed] [Google Scholar]
- 35.Belalcazar LM, Haffner SM, Lang W, et al. Lifestyle intervention and/or statins for the reduction of C-reactive protein in type 2 diabetes: from the look AHEAD study. Obesity (Silver Spring) 2013;21:944–950. doi: 10.1002/oby.20431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Smith SR, Weissman NJ, Anderson CM, et al. Multicenter, placebo-controlled trial of lorcaserin for weight management. N Engl J Med. 2010;363:245–256. doi: 10.1056/NEJMoa0909809. [DOI] [PubMed] [Google Scholar]
- 37▪▪.Garvey WT, Ryan DH, Look M, et al. Two-year sustained weight loss and metabolic benefits with controlled-release phentermine/topiramate in obese and overweight adults (SEQUEL): a randomized, placebo-controlled, phase 3 extension study. Am J Clin Nutr. 2012;95:297–308. doi: 10.3945/ajcn.111.024927. A clinical trial showing that the substantial weight loss produced by phentermine/ topiramate extended-release is maintained over 2 years and leads to marked decrease in the development of diabetes. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gadde KM, Allison DB, Ryan DH, et al. Effects of low-dose, controlled-release, phentermine plus topiramate combination on weight and associated comorbidities in overweight and obese adults (CONQUER): a randomized, placebo-controlled, phase 3 trial. Lancet. 2011;377:1341–1352. doi: 10.1016/S0140-6736(11)60205-5. [DOI] [PubMed] [Google Scholar]
- 39.Greenway FL, Fujioka K, Plodkowski RA, et al. Effect of naltrexone plus bupropion on weight loss in overweight and obese adults (COR-I): a multicenter, randomized, double-blind, placebo-controlled, phase 3 trial. Lancet. 2010;376:595–605. doi: 10.1016/S0140-6736(10)60888-4. [DOI] [PubMed] [Google Scholar]
- 40.O’Brien PE, Macdonald L, Anderson M, et al. Long-term outcomes after bariatric surgery: fifteen-year follow-up of adjustable gastric banding and a systematic review of the bariatric surgical literature. Ann Surg. 2013;257:87–94. doi: 10.1097/SLA.0b013e31827b6c02. [DOI] [PubMed] [Google Scholar]
- 41.Adams TD, Davidson LE, Litwin SE, Hunt SC. Gastrointestinal surgery: cardiovascular risk reduction and improved long-term survival in patients with obesity and diabetes. Curr Atheroscler Rep. 2012;14:606–615. doi: 10.1007/s11883-012-0286-4. [DOI] [PubMed] [Google Scholar]
- 42▪.Heneghan HM, Nissen S, Schauer PR. Gastrointestinal surgery for obesity and diabetes: weight loss and control of hyperglycemia. Curr Atheroscler Rep. 2012;14:579–587. doi: 10.1007/s11883-012-0285-5. This paper demonstrates the substantial metabolic and clinical benefits of surgically assisted weight loss in type 2 diabetes. [DOI] [PubMed] [Google Scholar]
- 43.Sharma AM, Kushner RF. A proposed clinical staging system for obesity. Int J Obesity. 2009;33:289–295. doi: 10.1038/ijo.2009.2. [DOI] [PubMed] [Google Scholar]
- 44.Garvey WT. New tools for weight loss therapy enable a more robust medical model for obesity treatment: rationale for a complications-centric model. Endocrine Practice. 2013 doi: 10.4158/EP13263.RA. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Padwal RS, Pajewski NM, Allison DB, Sharma AM. Using the Edmonton obesity staging system to predict mortality in a population-representative cohort of people with overweight and obesity. CMAJ. 2011;183:E1059–E1066. doi: 10.1503/cmaj.110387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Guo F, Garvey WT. A new cardiometabolic risk staging system to guide treatment for obesity using a complications-centric approach: validation using CARDIA and NHANES data. American Association of Clinical Endocrinologists 22nd Annual Scientific and Clinical Congress; p. Abstract #603. http://am.aace.com/2013-AACE-Abstracts. [Google Scholar]
- 47▪▪.Garber AJ, Abrahamson MJ, Barzilay JI, et al. American Association of Clinical Endocrinologists. AACE Comprehensive Diabetes Management Algorithm Endocr Pract. 2013;19:327–336. doi: 10.4158/endp.19.2.a38267720403k242. As a component of the Comprehensive Diabetes Management Algorithm, AACE has proposed a complications-centric algorithm for obesity, and integrated weight loss therapies, including medically-assisted weight loss, into the paradigms for treatment of prediabetes and diabetes. [DOI] [PubMed] [Google Scholar]
- 48.Klein S, Burke LE, Bray GA, et al. Clinical implications of obesity with specific focus on cardiovascular disease: a statement for professionals from the American Heart Association Council on Nutrition, Physical Activity, and Metabolism: endorsed by the American College of Cardiology Foundation. Circulation. 2004;110:2952–2967. doi: 10.1161/01.CIR.0000145546.97738.1E. [DOI] [PubMed] [Google Scholar]
- 49.Hu FB. Obesity and mortality: watch your waist, not just your weight. Arch Intern Med. 2007;167:875–876. doi: 10.1001/archinte.167.9.875. [DOI] [PubMed] [Google Scholar]
- 50.National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). . Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002;106:3143–8763421. [PubMed] [Google Scholar]
- 51.Hadaegh F, Shafiee G, Ghasemi A, et al. Impact of metabolic syndrome, diabetes and prediabetes on cardiovascular events: Tehran lipid and glucose study. Diabetes Res Clin Pract. 2010;87:342–347. doi: 10.1016/j.diabres.2009.11.010. [DOI] [PubMed] [Google Scholar]
- 52.Wilson PW, D’Agostino RB, Parise H, et al. Metabolic syndrome as a precursor of cardiovascular disease and type 2 diabetes. Circulation. 2005;112:3066–3072. doi: 10.1161/CIRCULATIONAHA.105.539528. [DOI] [PubMed] [Google Scholar]
- 53.Lakka HM, Laaksonen DE, Laaka TA, et al. The metabolic syndrome and total and cardiovascular disease mortality in middle-aged men. JAMA. 2002;288:2709–2716. doi: 10.1001/jama.288.21.2709. [DOI] [PubMed] [Google Scholar]
- 54.Lorenzo C, Okoloise M, Williams K, et al. San Antonio Heart Study. The metabolic syndrome as a predictor of type 2 diabetes: the San Antonio Heart Study. Diabetes Care. 2003;26:3153–3159. doi: 10.2337/diacare.26.11.3153. [DOI] [PubMed] [Google Scholar]
- 55.de Vegt F, Dekker JM, Jager A, et al. Relation of impaired fasting glucose and postload glucose with incident type 2 diabetes in a Dutch population: the Hoorn Study. JAMA. 2011;285:2109–2113. doi: 10.1001/jama.285.16.2109. [DOI] [PubMed] [Google Scholar]
- 56.Haffner SM, Lehto S, Ronnemaa T, et al. Mortality from coronary heart disease in subjects with type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction. N Engl J Med. 1998;339:229–234. doi: 10.1056/NEJM199807233390404. [DOI] [PubMed] [Google Scholar]
- 57.American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2013;36 (Suppl 1):S67–S74. doi: 10.2337/dc13-S067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Alberti KG, Eckel RH, Grundy SM, et al. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009;120:1640–1645. doi: 10.1161/CIRCULATIONAHA.109.192644. [DOI] [PubMed] [Google Scholar]
- 59.Ong JP, Younossi ZM. Epidemiology and natural history of NAFLD and NASH. Clin Liver Dis. 2007;11:1–16. doi: 10.1016/j.cld.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 60.Clark JM, Brancati FL, Diehl AM. The prevalence and etiology of elevated aminotransferase levels in the United States. Am J Gastroenterol. 2003;98:960–967. doi: 10.1111/j.1572-0241.2003.07486.x. [DOI] [PubMed] [Google Scholar]
- 61.Ruhl CE, Everhart JE. Determinants of the association of overweight with elevated serum alanine aminotransferase activity in the United States. Gastroenterology. 2003;124:71–79. doi: 10.1053/gast.2003.50004. [DOI] [PubMed] [Google Scholar]
- 62.Clark JM, Brancati FL, Diehl AM. Nonalcoholic fatty liver disease. Gastroenterology. 2002;122:1649–1657. doi: 10.1053/gast.2002.33573. [DOI] [PubMed] [Google Scholar]
- 63.Browning JD, Szczepaniak LS, Dobbins R, et al. Prevalence of hepatic steatosis in an urban population in the United States: impact of ethnicity. Hepatology. 2004;40:1387–1395. doi: 10.1002/hep.20466. [DOI] [PubMed] [Google Scholar]
- 64.Ong JP, Elariny H, Collantes R, et al. Predictors of nonalcoholic steatohepatitis and advanced fibrosis in morbidly obese patients. Obes Surg. 2005;15:310–315. doi: 10.1381/0960892053576820. [DOI] [PubMed] [Google Scholar]
- 65.Kemmer NM, McKinney KH, Xiao SY, et al. High prevalence of NASH among Mexican-American females with type II diabetes mellitus. Philadelphia: W.B. Saunders; 2001. p. A117. [Google Scholar]
- 66.Dixon JB, Bhathal PS, O’Brien PE. Nonalcoholic fatty liver disease: predictors of nonalcoholic steatohepatitis and liver fibrosis in the severely obese. Gastroenterology. 2001;121:91–100. doi: 10.1053/gast.2001.25540. [DOI] [PubMed] [Google Scholar]
- 67.Calle EE, Rodriguez C, Walker-Thurmond K, et al. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med. 2003;348:1625–1638. doi: 10.1056/NEJMoa021423. [DOI] [PubMed] [Google Scholar]
- 68.El-Serag HB, Tran T, Everhart JE. Diabetes increases the risk of chronic liver disease and hepatocellular carcinoma. Gastroenterology. 2004;126:460–468. doi: 10.1053/j.gastro.2003.10.065. [DOI] [PubMed] [Google Scholar]
- 69.Lewis MC, Phillips ML, Slavotinek JP, et al. Change in liver size and fat content after treatment with Optifast very low calorie diet. Obes Surg. 2006;16:697–701. doi: 10.1381/096089206777346682. [DOI] [PubMed] [Google Scholar]
- 70.Thomas EL, Brynes AE, Hamilton G, et al. Effect of nutritional counseling on hepatic, muscle and adipose tissue fat content and distribution in non-alcoholic fatty liver disease. World J Gastroenterol. 2006;12:5813–5819. doi: 10.3748/wjg.v12.i36.5813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Larson-Meyer DE, Newcomer BR, Heilbronn LK, et al. Effect of 6-month calorie restriction and exercise on serum and liver lipids and markers of liver function. Obesity (Silver Spring) 2008;16:1355–1362. doi: 10.1038/oby.2008.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Harrison SA, Fincke C, Helinski D, et al. A pilot study of orlistat treatment in obese, nonalcoholic steatohepatitis patients. Aliment Pharmacol Ther. 2004;20:623–628. doi: 10.1111/j.1365-2036.2004.02153.x. [DOI] [PubMed] [Google Scholar]
- 73.Assy N, Hussein O, Abassi Z. Weight loss induced by orlistat reverses fatty infiltration and improves hepatic fibrosis in obese patients with nonalcoholic steatohepatitis. Gut. 2007;56:443–444. doi: 10.1136/gut.2006.106021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zelber-Sagi S, Kessler A, Brazowsky E, et al. A double-blind randomized placebo-controlled trial of orlistat for treatment of nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol. 2006;4:639–644. doi: 10.1016/j.cgh.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 75.Barker KB, Palekar NA, Bowers SP, et al. Nonalcoholic steatohepatitis: effect of roux-en-Y gastric bypass surgery. Am J Gastroenterol. 2006;101:368–373. doi: 10.1111/j.1572-0241.2006.00419.x. [DOI] [PubMed] [Google Scholar]
- 76.Liu X, Lazenby AJ, Clements RH, et al. Resolution of nonalcoholic steatohepatitis after gastric bypass surgery. Obes Surg. 2007;17:486–492. doi: 10.1007/s11695-007-9086-2. [DOI] [PubMed] [Google Scholar]
- 77.Young T, Evans L, Finn L, Palta M. Estimation of the clinically diagnosed proportion of sleep apnea syndrome in middle-aged men and women. Sleep. 1997;20:705–706. doi: 10.1093/sleep/20.9.705. [DOI] [PubMed] [Google Scholar]
- 78.Peppard P, Young T, Palta M, et al. Longitudinal study of moderate weight change and sleep-disordered breathing. JAMA. 2000;284:3015–3302. doi: 10.1001/jama.284.23.3015. [DOI] [PubMed] [Google Scholar]
- 79.Foster GD, Sanders MH, Millman R, et al. Sleep AHEAD Research Group. Obstructive sleep apnea among obese patients with type 2 diabetes. Diabetes Care. 2009;32:1017–1019. doi: 10.2337/dc08-1776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Young T, Peppard PE, Gottlieb DJ. Epidemiology of obstructive sleep apnea. A population health perspective. Am J Respir Crit Care Med. 2002;165:1217–1239. doi: 10.1164/rccm.2109080. [DOI] [PubMed] [Google Scholar]
- 81.Tuomilehto HP, Seppä JM, Partinen MM, et al. Kuopio Sleep Apnea Group. Lifestyle intervention with weight reduction: first-line treatment in mild obstructive sleep apnea. Am J Respir Crit Care Med. 2009;179:320–327. doi: 10.1164/rccm.200805-669OC. [DOI] [PubMed] [Google Scholar]
- 82.Foster GD, Borradaile KE, Sanders MH, et al. for the Sleep AHEAD Research Group of the Look AHEAD Research Group. A randomized study on the effect of weight loss on obstructive sleep apnea among obese patients with type 2 diabetes: the Sleep AHEAD study. Arch Intern Med. 2009;169:1916–1926. doi: 10.1001/archinternmed.2009.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83▪.Kuna ST, Reboussin DM, Borradaile KE, et al. Sleep AHEAD Research Group of the Look AHEAD Research Group. Long-term effect of weight loss on obstructive sleep apnea severity in obese patients with type 2 diabetes. Sleep. 2013;36:641–649. doi: 10.5665/sleep.2618. An important paper demonstrating the common occurrence of OSA in obese patients with type 2 diabetes, and the benefits of weight loss in the treatment of this common but serious obesity complication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Fritscher LG, Canani S, Mottin CC, et al. Bariatric surgery in the treatment of obstructive sleep apnea in morbidly obese patients. Respiration. 2007;74:647–652. doi: 10.1159/000107736. [DOI] [PubMed] [Google Scholar]
- 85.Winslow DH, Bowden CH, DiDonato KP, McCullough PA. A randomized, double-blind, placebo-controlled study of an oral, extended-release formulation of phentermine/topiramate for the treatment of obstructive sleep apnea in obese adults. Sleep. 2012;35:1529–1539. doi: 10.5665/sleep.2204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hampel H, Abraham NS, El-Serag HB. Meta-analysis: obesity and the risk for gastroesophageal reflux disease and its complications. Ann Intern Med. 2005;143:199–211. doi: 10.7326/0003-4819-143-3-200508020-00006. [DOI] [PubMed] [Google Scholar]
- 87.El-Serag HB, Sonnenberg A. Opposing time trends of peptic ulcer and reflux disease. Gut. 1998;43:327–333. doi: 10.1136/gut.43.3.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Mandeep Singh, Jaehoon Lee, Neil Gupta, et al. Weight loss can lead to resolution of gastroesophageal reflux disease symptoms: a prospective intervention trial. Obesity. 2013;21:284–290. doi: 10.1002/oby.20279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ekelund M, Oberg S, Peterli R, et al. Gastroesophageal reflux after vertical banded gastroplasty is alleviated by conversion to gastric bypass. Obes Surg. 2012;22:851–854. doi: 10.1007/s11695-011-0540-9. [DOI] [PubMed] [Google Scholar]
- 90.Tutuian R. Obesity and GERD: pathophysiology and effect of bariatric surgery (review) Curr Gastroenterol Rep. 2011;13:205–212. doi: 10.1007/s11894-011-0191-y. [DOI] [PubMed] [Google Scholar]
- 91.Woodman G, Cywes R, Billy H, et al. Effect of adjustable gastric banding on changes in gastroesophageal reflux disease (GERD) and quality of life. Curr Med Res Opin. 2012;28:581–589. doi: 10.1185/03007995.2012.666962. [DOI] [PubMed] [Google Scholar]
- 92.Zhang Y, Jordan JM. Epidemiology of osteoarthritis. Clin Geriatr Med. 2010;26:355–369. doi: 10.1016/j.cger.2010.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Gordon T, Engel A. Osteoarthritis in U.S. adults. In: Bennett PH, Wood PHN, editors. Sponsored by the National Institute of Arthritis and Metabolic Diseases; Population Studies of the Rheumatic Diseases: Proceedings of the Third International Symposium; New York. June 5th to lOth, 1966; Amsterdam: Excerpta Medica Foundation; 1968. pp. 391–397. (International Congress Series; no. 148) [Google Scholar]
- 94.Felson DT, Anderson JJ, Naimark A, et al. Obesity and knee osteoarthritis. The Framingham Study. Ann Intern Med. 1988;109:18–24. doi: 10.7326/0003-4819-109-1-18. [DOI] [PubMed] [Google Scholar]
- 95.Christensen R, Astrup A, Bliddal H. Weight loss: the treatment of choice for knee osteoarthritis? A randomized trial. Osteoarthritis Cartilage. 2005;13:20–27. doi: 10.1016/j.joca.2004.10.008. [DOI] [PubMed] [Google Scholar]
- 96.Felson DT, Zhang Y, Anthony JM, et al. Weight loss reduces the risk for symptomatic knee osteoarthritis in women. The Framingham Study. Ann Intern Med. 1992;116:535–539. doi: 10.7326/0003-4819-116-7-535. [DOI] [PubMed] [Google Scholar]
- 97.Christensen R, Bartels EM, Astrup A, Bliddal H. Effect of weight reduction in obese patients diagnosed with knee osteoarthritis: a systematic review and meta-analysis. Ann Rheum Dis. 2007;66:433–439. doi: 10.1136/ard.2006.065904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Abdullah A, Peeters A, de Courten M, et al. The magnitude of association between overweight and obesity and the risk of diabetes: a meta-analysis of prospective cohort studies. Diabetes Res Clin Pract. 2010;89:309–319. doi: 10.1016/j.diabres.2010.04.012. [DOI] [PubMed] [Google Scholar]
- 99.Pasquali R, Gambineri A, Biscotti D, et al. Effect of long-term treatment with metformin added to hypocaloric diet on body composition, fat distribution, and androgen and insulin levels in abdominally obese women with and without the polycystic ovary syndrome. J Clin Endocrinol Metab. 2000;85:2767–2774. doi: 10.1210/jcem.85.8.6738. [DOI] [PubMed] [Google Scholar]
- 100.Norman RJ, Davies MJ, Lord J, Moran LJ. The role of lifestyle modification in polycystic ovary syndrome. Trends Endocrinol Metab. 2002;13:251–257. doi: 10.1016/s1043-2760(02)00612-4. [DOI] [PubMed] [Google Scholar]
- 101.Gambineri A, Pelusi C, Vicennati V, et al. Obesity and the polycystic ovary syndrome. Int J Obesity. 2002;26:883–896. doi: 10.1038/sj.ijo.0801994. [DOI] [PubMed] [Google Scholar]
- 102.Panidis D, Farmakiotis D, Rousso D, et al. Obesity, weight loss, and the polycystic ovary syndrome: effect of treatment with diet and orlistat for 24 weeks on insulin resistance and androgen levels. Fertil Steril. 2008;89:899–906. doi: 10.1016/j.fertnstert.2007.04.043. [DOI] [PubMed] [Google Scholar]
- 103.Burgio KL, Richter HE, Clements RH, et al. Changes in urinary and fecal incontinence symptoms with weight loss surgery in morbidly obese women. Obstet Gynecol. 2007;110:1034–1040. doi: 10.1097/01.AOG.0000285483.22898.9c. [DOI] [PubMed] [Google Scholar]
- 104.Subak LL, Wing R, West DS, et al. Weight loss to treat urinary incontinence in overweight and obese women. N Engl J Med. 2009;360:481–490. doi: 10.1056/NEJMoa0806375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med. 2003;348:1625–1638. doi: 10.1056/NEJMoa021423. [DOI] [PubMed] [Google Scholar]
- 106.Vucenik I, Stains JP. Obesity and cancer risk: evidence, mechanisms, and recommendations. Ann NY Acad Sci. 2012;1271:37–43. doi: 10.1111/j.1749-6632.2012.06750.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Testa MA, Simonson DC. Assessment of quality of life outcomes. N Engl J Med. 1996;334:833–840. doi: 10.1056/NEJM199603283341306. [DOI] [PubMed] [Google Scholar]
- 108.Fontaine KR, Bartlett SJ. Estimating health-related quality of life in obese individuals. Dis Manage Health Outcomes. 1998;3:61–70. [Google Scholar]
- 109.Kushner RF, Foster GD. Obesity and quality of life. Nutrition. 2000;16:947–952. doi: 10.1016/s0899-9007(00)00404-4. [DOI] [PubMed] [Google Scholar]
- 110.Forhan M, Vrkljan B, MacDermid J. A systematic review of the quality of psychometric evidence supporting the use of an obesity-specific quality of life measure for use with persons who have class III obesity. Obes Rev. 2010;11:222–228. doi: 10.1111/j.1467-789X.2009.00612.x. [DOI] [PubMed] [Google Scholar]
- 111.Karlsson J, Sjostrom L, Sullivan M. Swedish obese subjects (SOS): an intervention study of obesity. Two-year-follow-up of health-related quality of life (HRQL) and eating behavior after gastric surgery for severe obesity. Int J Obesity Rel Metab Disord. 1998;22:113. doi: 10.1038/sj.ijo.0800553. [DOI] [PubMed] [Google Scholar]
- 112.Narbro K, Agren G, Jonsson E, et al. Sick leave and disability pension before and after treatment for obesity: a report from the Swedish Obese Subjects (SOS) study. Int J Obesity Rel Metab Disord. 1999;23:619. doi: 10.1038/sj.ijo.0800890. [DOI] [PubMed] [Google Scholar]
- 113.Choban PS, Onyejekwe J, Burge JC, Flancbaum L. A health status assessment of the impact of weight loss following roux-en-Y gastric bypass for clinically severe obesity. J Am Coll Surg. 1999;188:491. doi: 10.1016/s1072-7515(99)00030-7. [DOI] [PubMed] [Google Scholar]
- 114.van Gemert WG, Adang EM, Greve JW, Soeters PB. Quality of life assessment of morbidly obese patients: effect of weight-reducing surgery. Am J Clin Nutr. 1998;67:197. doi: 10.1093/ajcn/67.2.197. [DOI] [PubMed] [Google Scholar]
- 115.Samsa GP, Kolotkin RL, Williams GR, et al. Effect of moderate weight loss on health-related quality of life: an analysis of combined data from 4 randomized trials of sibutramine vs placebo. Am J Manag Care. 2001;7:875–883. [PubMed] [Google Scholar]
- 116.McMahon FG, Fujioka K, Singh BN, et al. Efficacy and safety of sibutramine in obese white and African American patients with hypertension: a 1-year, double-blind, placebo-controlled, multicenter trial. Arch Intern Med. 2000;160:2185–2191. doi: 10.1001/archinte.160.14.2185. [DOI] [PubMed] [Google Scholar]
- 117.Clark MM, Niaura R, King TK, Pera V. Depression, smoking, activity level, and health status: pretreatment predictors of attrition in obesity treatment. Addict Behav. 1996;21:509–513. doi: 10.1016/0306-4603(95)00081-x. [DOI] [PubMed] [Google Scholar]
- 118.Kirk A, De Feo P. Strategies to enhance compliance to physical activity for patients with insulin resistance. Appl Physiol Nutr Metab. 2007;32:549–556. doi: 10.1139/H07-023. [DOI] [PubMed] [Google Scholar]
- 119.Teixeira PJ, Going SB, Houtkooper LB, et al. Pretreatment predictors of attrition and successful weight management in women. Int J Obes Relat Metab Disord. 2004;28:1124–1133. doi: 10.1038/sj.ijo.0802727. [DOI] [PubMed] [Google Scholar]
- 120.Sullivan M, Karlsson J, Sjostrom L, et al. Swedish Obese Subjects (SOS): an intervention study of obesity. Baseline evaluation of health and psychosocial functioning in the first 1743 subjects examined. Int J Obes Relat Metab Disord. 1993;17:503–512. [PubMed] [Google Scholar]
- 121.Tuthill A, Slawik H, O’Rahilly S, Finer N. Psychiatric co-morbidities in patients attending specialist obesity services in the UK. QJM. 2006;99:317–325. doi: 10.1093/qjmed/hcl041. [DOI] [PubMed] [Google Scholar]
- 122.Britz B, Siegfried W, Ziegler A, et al. Rates of psychiatric disorders in a clinical study group of adolescents with extreme obesity and in obese adolescents ascertained via a population based study. Int J Obes Relat Metab Disord. 2000;24:1707–1714. doi: 10.1038/sj.ijo.0801449. [DOI] [PubMed] [Google Scholar]
- 123.Black DW, Goldstein RB, Mason EE. Prevalence of mental disorder in 88 morbidly obese bariatric clinic patients. Am J Psychiatry. 1992;149:227–234. doi: 10.1176/ajp.149.2.227. [DOI] [PubMed] [Google Scholar]
- 124.Legenbauer T, De Zwaan M, Benecke A, et al. Depression and anxiety: their predictive function for weight loss in obese individuals. Obes Facts. 2009;2:227–234. doi: 10.1159/000226278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125▪.Schag K, Schönleber J, Teufel M, et al. Food-related impulsivity in obesity and binge eating disorder: a systematic review. Obes Rev. 2013;14:477–495. doi: 10.1111/obr.12017. This review summarizes accumulating data addressing the seriousness of BED in obese patients, and why it is important to screen for and treat this disorder in any weight loss therapy program. [DOI] [PubMed] [Google Scholar]
- 126.de Zwaan M, Herzog W. Diagnostic criteria for eating disorders: what will DSM-5 feature? Nervenarzt. 2011;82:1100–1106. doi: 10.1007/s00115-010-3225-z. [DOI] [PubMed] [Google Scholar]
- 127.National Institute for Clinical Excellence. Eating disorders: core interventions in the treatment and management of anorexia nervosa, bulimia nervosa, related eating disorders (NICE Clinical Guideline No. 9) London, England: 2004. [Google Scholar]
- 128.Grilo CM, Masheb RM, Wilson GT, et al. Cognitive–behavioral therapy, behavioral weight loss, and sequential treatment for obese patients with binge-eating disorder: a randomized controlled trial. J Consult Clin Psychol. 2011;79:675–685. doi: 10.1037/a0025049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Brownley KA, Berkman ND, Sedway JA, et al. Binge eating disorder treatment: a systematic review of randomized controlled trials. Int J Eat Disord. 2007;40:337–348. doi: 10.1002/eat.20370. [DOI] [PubMed] [Google Scholar]
- 130.Diabetes Prevention Program (DPP) Research Group. The diabetes prevention program (DPP): description of lifestyle intervention. Diabetes Care. 2002;25:2165–2171. doi: 10.2337/diacare.25.12.2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.The Look AHEAD Research Group. The Look AHEAD study: a description of the lifestyle intervention and the evidence supporting it. Obesity. 2006;14:737–752. doi: 10.1038/oby.2006.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Bray GA. Why do we need drugs to treat the patient with obesity? Obesity. 2013;21:893–899. doi: 10.1002/oby.20394. [DOI] [PubMed] [Google Scholar]
- 133.Smith BR, Schauer P, Nguyen NT. Surgical approaches to the treatment of obesity: bariatric surgery. Med Clin N Am. 2011;95:1009–1030. doi: 10.1016/j.mcna.2011.06.010. [DOI] [PubMed] [Google Scholar]
- 134.Wing RR, Lang W, Wadden TA, et al. Look AHEAD Research Group. Benefits of modest weight loss in improving cardiovascular risk factors in overweight and obese individuals with type 2 diabetes. Diabetes Care. 2011;34:1481–1486. doi: 10.2337/dc10-2415. [DOI] [PMC free article] [PubMed] [Google Scholar]