Abstract
Physical activity decreases risk for diabetes and cardiovascular disease morbidity and mortality; however, the specific impact of exercise on the diabetic vasculature is unexamined. We hypothesized that an acute, moderate exercise intervention in diabetic and hypertensive rats would induce mitochondrial biogenesis and mitochondrial antioxidant defence to improve vascular resilience. SHHF/Mcc-facp lean (hypertensive) and obese (hypertensive, insulin resistant), as well as Sprague Dawley (SD) control rats were run on a treadmill for 8 days. In aortic lysates from SD rats, we observed a significant increase in subunit proteins from oxidative phosphorylation (OxPhos) complexes I–III, with no changes in the lean or obese SHHF rats. Exercise also increased the expression of mitochondrial antioxidant defence uncoupling protein 3 (UCP3) (p < 0.05) in SHHF lean rats, whereas no changes were observed in the SD or SHHF obese rats with exercise. We evaluated upstream signalling pathways for mitochondrial biogenesis, and only peroxisome proliferators–activated receptor gamma coactivator 1α (PGC-1α) significantly decreased in SHHF lean rats (p < 0.05) with exercise. In these experiments, we demonstrate absent mitochondrial induction with exercise exposure in models of chronic vascular disease. These findings suggest that chronic vascular stress results in decreased sensitivity of vasculature to the adaptive mitochondrial responses normally induced by exercise.
Keywords: Cyclic adenosine monophosphate response element binding protein, mitochondria, exercise, SHHF, diabetes, vasculature
Introduction
The emerging epidemics of diabetes and metabolic syndrome are contributing to a worldwide cardiovascular public health crisis. These epidemics are driven by sedentary behaviour and over-nutrition. Reduction in cardiovascular disease (CVD) risk and mortality associated with physical activity and physical fitness is firmly established.1,2 Low cardiovascular fitness predicted cardiovascular and all-cause mortality in a study of both healthy and diabetic men.3,4 Even occasional physical activity in humans (one or less bouts per week) conferred a hazard ratio of 0.70 compared to no physical activity.5 Despite the strong, demonstrated benefits of exercise, the precise vascular targets conferring these improved outcomes are unknown.
Mitochondrial dysfunction has recently emerged as a risk for vascular disease.6–8 Mitochondrial integrity is essential for calcium-mediated vasodilation and normal contractile function.9 Specifically, ageing, diabetes, hyperlipidaemia and mitochondrial diseases, such as MELAS (mitochondrial myopathy, encephalopathy, lactic acidosis and stroke) syndrome, were associated with increased vascular mitochondrial reactive oxygen species (ROS) generation, vascular stiffness, mitochondrial mutations, fragmentation and abnormal calcium regulation (important for vasomotion and vascular stiffness).6–8,10 In addition, skeletal muscle mitochondrial dysfunction was observed in both type 1 diabetes mellitus (T1D) and type 2 diabetes mellitus (T2D). In T2D, mitochondrial dysfunction was related to insulin resistance and was present in offsprings of people with T2D even prior to diagnosis of diabetes.11 Increase in antioxidant defence, increase in mitochondrial numbers and decreased mitochondrial DNA mutations were observed in response to exercise training in the aorta;12 however, the impact of diabetes on this response has not been reported. Previous studies have found positive vascular metabolic and antioxidant outcomes in rats exposed to short-term and acute exercise regimens. 11,12 Acute exercise interventions (as short as 8 days) led to induction of endothelial nitric oxide synthase (eNOS), catalase and manganese superoxide dismutase (MnSOD) in the aorta of the low-density lipoprotein (LDL) receptor–null mouse.13 Similar effects were seen in young apolipoprotein E (ApoE)–null mice but not older ApoE–null mice, suggesting that chronic vascular disease might blunt the vascular response to exercise.13 In rodents, exercise training increased muscle mitochondrial biogenesis, increased mitochondrial antioxidant defence and decreased mitochondrial DNA damage.14
Mitochondrial content and function are regulated by diverse signals. For example, nitric oxide (NO) regulates mitochondrial biogenesis and is increased in response to exercise-induced eNOS activation. Most important, eNOS-null mice had defects in skeletal muscle mitochondrial biogenesis, 15–18 and subsequent baseline decreases in skeletal muscle oxidative capacity and overall exercise capacity (as measured running time).15 It was recently reported that the transcription factor cyclic adenosine monophosphate response element binding protein (CREB) is an upstream regulator of mitochondrial biogenesis and mitochondrial antioxidant defence in cardiac myocytes.16 We have observed that myocardial CREB can be activated by exercise training.19 Induction of CREB and pivotal targets of CREB [Bcl-2, MnSOD, haem oxygenase-1 (HO-1)] in response to exercise training is also observed in the nervous system.17,18 In addition, acute exercise intervention increases active neuronal CREB and contributes to improved recovery from ischaemia, stroke and memory tasks post seizure.19,20
We hypothesized that a short-term exercise intervention in diabetic and hypertensive models would induce vascular mitochondrial biogenesis and mitochondrial antioxidant defence to improve vascular resilience, potentially via a CREB-mediated mechanism. We examined the impact of a moderate, 8-day exercise intervention on vascular mitochondrial profiles and established regulators of mitochondrial function on rodent models of hypertension and hypertension plus obesity, insulin resistance and glucose intolerance. These experiments revealed an unexpected effect of these disease states on the vascular response to exercise intervention.
Methods
Materials
Antibodies to CREB (source species: rabbit), phosphorylated CREB (pCREB; Ser133; source species: rabbit), ubiquitin (source species: mouse) and anti-mouse and anti-rabbit IgG [alkaline phosphatase (AP)-linked antibodies] were obtained from Cell Signaling. Antibodies to cytochrome c (source species: mouse) and eNOS (source species: mouse) were from BD Biosciences. Antibody to MnSOD (source species: rabbit) was acquired from Upstate Biotechnology, and antibody to uncoupling protein 3 (UCP3; source species: rabbit) was from Calbiochem. Antibodies to voltage-dependent anion-selective channel (VDAC; source species: mouse) and mitochondrial oxidative phosphorylation (OxPhos) complexes I, II, III, IV and V (source species: mouse) were obtained from MitoSciences. Antibodies to 5′ adenosine monophosphate–activated protein kinase (AMPK; source species: goat) and peroxisome proliferators–activated receptor gamma coactivator 1α (PGC-1α; source species: rabbit) and anti-goat IgG (AP-linked antibodies) were from Santa Cruz Biotechnology. Antibody to nitrotyrosine (source species: mouse) and thiobarbituric acid reactive substances (TBARS) assay were from Cayman Chemical, and antibody to calnexin (source species: rabbit) was from Abcam. Protease inhibitor cocktail and antibody to β-actin (source species: mouse) were acquired from Sigma–Aldrich. Mammalian Protein Extraction Reagent (M-PER) was from Thermo Scientific, and immobilon-P polyvinylidene difluoride (PVDF) membrane was from Millipore. CDP-Star Reagent was from New England BioLabs, and protein A sepharose beads were from GE Healthcare.
Animals
We used the SHHF/Mcc-facp (SHHF) rat, a genetic model that has been selectively bred for Spontaneous Hypertension and Heart Failure. Two distinct strains of the SHHF rat were used to examine the impact of an acute exercise intervention in rats with hypertension alone (SHHF lean) and rats with hypertension plus obesity, insulin resistance and glucose intolerance (SHHF obese). Sprague Dawley (SD) rats were used as the control group. Male, 16-weeks old, SD (n = 10), SHHF lean (n = 10) and SHHF obese (n = 10) rats were used for the studies. The use of animals and all of the experimental interventions used in this study received prior approval from the Institutional Animal Care and Use Committee at the University of Colorado at Boulder.
Exercise intervention
SD, SHHF lean and SHHF obese rats were divided into exercise and sedentary groups and subjected to 8 days of treadmill exercise following acclimation. Acclimation took place over the course of 1 week and began by placing rats on a stationary treadmill (0% grade) for 5–15 min on day 1 with progressively increased speed to 10 m/min on treadmill by the end of the week. Exercised animals were run on the treadmill (0% grade) at 15 m/min for 30 min on days 1 and 2 and for 45 min on days 3–8. This particular exercise paradigm was chosen based on previous short-term, moderate exercise interventions in the literature examining antioxidant profiles and eNOS.11,12 The sedentary animals were placed on the non-moving treadmill for the same amount of time each day for acclimation and exercise periods. Animals were anaesthetized 24 h after their last exercise bout by intraperitoneal injection of pentobarbital sodium (35 mg/kg body weight). A blood glucose measurement was made from the tail vein using a glucometer at this time. Blood was taken by cardiac puncture and saved as serum at −80°C until further analysis. The aortas were removed, frozen and also stored at −80°C until analysis, and retroperitoneal fat depots were weighed.
Serum analyses
Insulin was measured in the serum using the ALPCO Insulin (Rat) ELISA. Serum was analysed and quantified for a panel of 27 cytokines by RayBiotech, Inc., using their Rat Cytokine Array 3 Quantibody multiplex ELISA array kit. Due to sample limitations, a representative sampling (n = 4–6) was analysed from each group.
Preparation of tissue for Western blot analyses and immunoprecipitation
Protein extracts from aortas were prepared as previously described. Briefly, aortas were pulverized to a powder in mammalian cell lysis buffer [M-PER with 150 mM NaCl, 1 mM ethylenediaminetetraacetic acid (EDTA), 1 mM ethylene glycol tetraacetic acid (EGTA), 5 mM Na4P2O7·10H2O, 1 mM Na3VO4, 20 mM NaF, 500 mM okadaic acid and 1% protease inhibitor cocktail] under liquid nitrogen. Once thawed on ice, samples were homogenized using an Ultra-Turrax T8 Homogenizer (IKA). Homogenates were centrifuged at 18,000 × g, 4°C for 10 min, and the protein concentration of the supernatant was analysed by Bradford protein assay.
Western blot analyses
Protein samples (20–30 μg) in Laemmli sample buffer [LSB; boiled together with dithiothreitol (DTT)] were run on sodium dodecyl sulphate (SDS)-12% polyacrylamide gels. The resolved proteins were electrophoretically transferred to PVDF membranes, and equivalence of protein loading was assessed by staining of the membrane-bound proteins by Ponceau S stain. Blots were probed using specific primary antibodies of interest and followed by AP-linked secondary antibodies. Proteins were detected by chemiluminescence using CDP-Star Reagent on film, and densitometric analysis was performed using BioRad QuantityOne software. Due to sample limitations, a representative sample (n = 3–10) was analysed from each group.
CREB–ubiquitin immunoprecipitation
A 100–200 μg protein sample in mammalian lysis buffer (from Western blot tissue preparation) was pre-cleared with sepharose beads conjugated with protein A in buffer 1 [1% IGEPAL and 0.1 mM Na3VO4 in phosphate-buffered saline (PBS)]. After rotating at 4°C for 30 min, sample was centrifuged at 2000 × g for 1 min and the supernatant was removed, and then the supernatant was incubated with CREB antibody (1:250) and rotated at 4°C for 16 h. Protein A sepharose beads in buffer 1 were added and rotated at 4°C for 2 h. After centrifuging at 2000 × g for 1 min, the supernatant was removed and discarded, and the beads were washed four times with buffer 1 and four times with buffer 2 (10 mM Tris pH 7.5, 100 mM NaCl, 1 mM EDTA and 0.1 mM Na3VO4). Immunoprecipitated (IP) CREB proteins were eluted from the beads with LSB and subjected to Western blot analyses probing for ubiquitin, followed by AP-linked secondary antibodies detection by chemiluminescence on film and densitometric analysis. Due to sample limitations, a representative sample (n = 4–9) was analysed from each group.
Statistical analyses
Student’s t-tests were performed in the analysis of Western blots; significance level was set at p < 0.05. One-way analyses of variance (ANOVAs) were performed in the analysis of blood glucose, body weight, fat pad weight, serum insulin and serum cytokines with Tukey’s pairwise comparison; significance level was set at p < 0.05.
Results
Rat characteristics
Table 1 illustrates the characteristics of the SD, SHHF lean and SHHF obese rats. Body weight, glucose, insulin and retroperitoneal fat mass were all significantly higher in the SHHF obese rats compared to both SD and SHHF lean rats at baseline. Insulin levels appeared higher in the SHHF lean compared to SD rats at baseline (sedentary), yet there was no significant difference between the baseline insulin and glucose levels or retroperitoneal fat weight of the SD and SHHF lean rats. SHHF lean rats weighed 16% less than SD rats (p < 0.05). These parameters are consistent with a graded model of cardiometabolic risk from control to isolated hypertension and finally to hypertension, obesity, plus mild hyperglycemia/diabetes. Body weight, glucose, insulin and retroperitoneal weight were not significantly changed by the 8-day exercise intervention in any group.
Table 1.
Metabolic characteristics of Sprague Dawley, SHHF lean and SHHF obese rats, presented as mean ± SEM.
| Metabolic characteristics | Sprague Dawley | SHHF Lean | SHHF Obese | |||
|---|---|---|---|---|---|---|
|
|
|
|
||||
| Sedentary mean ± SEM (n) | Exercised mean ± SEM (n) | Sedentary mean ± SEM (n) | Exercised mean ± SEM (n) | Sedentary mean ± SEM (n) | Exercised mean ± SEM (n) | |
| Body weight (g) | 415 ± 6 (10) | 393 ± 5 (10) | 350 ± 5 (10)a | 336 ± 6 (10)a | 550 ± 7 (10)a,b | 538 ± 6 (10)a,b |
| Glucose (mg/dL) | 97 ± 2 (10) | 99 ± 2 (10) | 103 ± 2 (10) | 104 ± 3 (10) | 235 ± 32 (10)a,b | 181 ± 22 (10) |
| Insulin (ng/mL) | 3.83 ± 0.44 (4) | 4.87 ± 0.88 (7) | 9.13 ± 1.02 (10) | 9.36 ± 0.64 (10) | 65.87 ± 5.18 (8)a,b | 65.97 ± 9.16 (10)a,b |
| Retroperitoneal fat weight (g) | 2.25 ± 0.10 (10) | 1.57 ± 0.13 (10) | 2.10 ± 0.16 (10) | 2.0 ± 0.12 (10) | 23.9 ± 1.4 (10)a,b | 21.7 ± 1.0 (10)a,b |
SEM: standard error of mean; ANOVA: analysis of variance.
ANOVA, Tukey’s pairwise comparison, p < 0.05 versus Sprague Dawley in same group (sedentary or exercised).
ANOVA, Tukey’s pairwise comparison, p < 0.05 versus SHHF lean in same group.
Characterizations of inflammation and oxidant stress
Inflammation and oxidant stress are closely linked to diabetes and CVD. It has also been reported that exercise acutely stimulates and chronically decreases inflammation.12,21–23 Therefore, we performed a cytokine array on serum from sedentary and exercised rats. Of the 27 cytokines assayed, 9 are presented in Table 2; the remaining cytokines from the assay panel [B7-2, β-nerve growth factor (NGF), cytokine-induced neutrophil chemoattractant-1 (CINC-1), CINC-2, CINC-3, ciliary neurotrophic factor (CNTF), granulocyte macrophage colony simulating factor (GM-CSF), interleukin (IL)-1β, IL-2, IL-4, IL-13, lipopolysaccharide- induced CXC chemokine (LIX aka CXCL5), L-selectin, platelet derived growth factor (PDGF)-AA, prolactin R, receptor for advanced glycation end products (RAGE), thymus chemokine-1 (TCK-1) and vascular endothelial growth factor (VEGF)] are presented in the supplementary material. Unexpectedly, neither the SHHF lean nor the obese group had elevated levels of cytokines that indicate systemic inflammation compared to each other or to the SD group at baseline (Table 2). In fact, IL-1α was 74% lower (p < 0.05) and fractalkine, IFNγ and IL-10 were more than 30% lower in the sedentary SHHF obese rats compared to the sedentary SD rats (not significantly different). The 8-day, moderate exercise intervention did not significantly change any of the cytokines in Table 2.
Table 2.
Serum cytokines of Sprague Dawley, SHHF lean and SHHF obese rats, presented as mean ± SEM.
| Cytokine | Sprague Dawley (ng/mL)
|
SHHF lean (ng/mL)
|
SHHF obese (ng/mL)
|
|||
|---|---|---|---|---|---|---|
| Sedentary (n = 4) | Exercised (n = 6) | Sedentary (n = 4) | Exercised (n = 5) | Sedentary (n = 5) | Exercised (n = 5) | |
| Fractalkine | 1.17 ± 0.14 | 1.05 ± 0.04 | 1.15 ± 0.14 | 1.36 ± 0.23 | 0.73 ± 0.05 | 0.77 ± 0.04b |
| ICAM-1 | 1.76 ± 0.19 | 1.39 ± 0.24 | 1.14 ± 0.37 | 1.70 ± 0.32 | 1.74 ± 0.79 | 1.81 ± 0.44 |
| IFNγ | 0.45 ± 0.05 | 0.34 ± 0.04 | 0.38 ± 0.09 | 0.38 ± 0.05 | 0.29 ± 0.06 | 0.25 ± 0.03 |
| IL-1α | 0.54 ± 0.14 | 0.31 ± 0.05 | 0.26 ± 0.06 | 0.16 ± 0.05 | 0.14 ± 0.11a | 0.06 ± 0.03 |
| IL-6 | nd | nd | nd | nd | nd | nd |
| IL-10 | 2.44 ± 0.47 | 1.92 ± 0.14 | 1.85 ± 0.49 | 2.03 ± 0.25 | 1.67 ± 0.54 | 1.48 ± 0.23 |
| MCP-1 | 4.88 ± 0.31 | 3.11 ± 0.27 | 4.02 ± 0.24 | 3.64 ± 0.35 | 5.77 ± 0.67 | 5.10 ± 0.58a |
| TIMP-1 | 5.61 ± 0.57 | 5.96 ± 0.35 | 5.71 ± 0.38 | 6.87 ± 0.25 | 5.92 ± 0.35 | 5.95 ± 0.45 |
| TNFα | 0.75 ± 0.19 | 0.62 ± 0.08 | 0.54 ± 0.18 | 0.56 ± 0.17 | 0.77 ± 0.38 | 0.40 ± 0.15 |
SEM: standard error of mean; IFNγ: interferon γ: IL: interleukin; ICAM: intercellular adhesion molecule 1; MCP-1: monocyte chemotactic protein-1; nd: levels were below the detectability of the assay; TIMP-1: tissue inhibitor of metalloproteinase 1; TNFα: tumour necrosis factor; ANOVA: analysis of variance.
ANOVA, Tukey’s pairwise comparison, p < 0.05 versus Sprague Dawley in same group (sedentary or exercise).
ANOVA, Tukey’s pairwise comparison, p < 0.05 versus SHHF Lean in same group.
We next evaluated a measure of nitrative stress when aortic lysates were probed for nitrotyrosine. At baseline, SHHF obese rats showed a significantly lower level of protein tyrosine nitration in the aorta when compared to SHHF lean rats (p < 0.05, Figure 1). Consistent with this, serum TBARS [micromolar malondialdehyde (MDA) equivalents] levels were more than twofold higher in the SHHF obese rats (11.2 ± 0.4 μM sedentary, 11.0 ± 0.9 μM exercise) when compared to SD and SHHF lean rats both at baseline (4.6 ± 0.4 μM and 4.9 ± 0.4 μM, respectively) and after exercise (5.1 ± 0.2 μM and 5.2 ± 0.5 μM, respectively). Potential caveats are that the TBARS (as a measure of MDA) may be overestimated in the SHHF obese rats since serum lipids are elevated in this model,24 and MDA is not solely generated by lipid peroxidation. Our future directions include more comprehensive evaluation of oxidant stress by measuring ROS generation, lipid peroxidation, protein oxidation and antioxidants in the vasculature of diabetic models.
Figure 1.
Differences in nitrotyrosine in aortic lysates between SHHF lean and obese rats at baseline. Protein tyrosine nitration was 14% lower in the SHHF obese compared to SHHF lean rats. SHHF lean: n = 7 and SHHF obese: n = 9 rats. Data are expressed as mean fold change from SHHF lean + SEM. *p < 0.05, Student’s t-test.
SEM: standard error of mean.
Examination of aortic mitochondrial protein expression
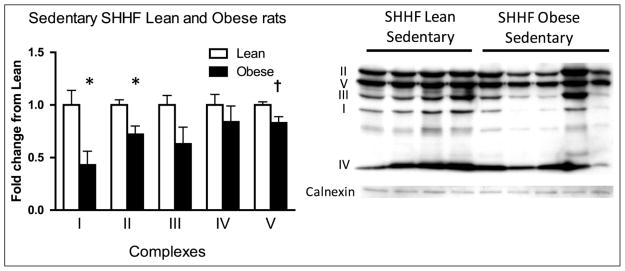
We performed Western blots to examine the components of the mitochondrial OxPhos system in aortic lysates. Complexes I and II were significantly lower in SHHF obese rats compared to SHHF lean rats at baseline (sedentary), while complex V trended lower in the SHHF obese rats (Figure 2).
Figure 2.
Differences in aortic mitochondrial OxPhos complexes between SHHF lean and obese rats at baseline. Blots were probed for representative subunits of the mitochondrial complexes of the electron transport chain. Complexes I and II were significantly lower in the SHHF obese rats compared to the SHHF lean rats. SHHF lean rats: n = 8 and SHHF obese rats: n = 10. Data are expressed as mean fold change from SHHF lean + SEM. *p < 0.05, †p = 0.05–0.10, Student’s t-test.
OxPhos: oxidative phosphorylation; SEM: standard error of mean.
Others have reported increased vascular mitochondrial expression in response to exercise,13,14 so we next examined the impact of a short-term, moderate exercise intervention in the SHHF lean and obese rats. SD rats were included as well to provide representation of a ‘normal’ response to this particular exercise protocol. Complexes I, II and III significantly increased with 8 days of moderate exercise in the SD rats with no significant changes in either the lean or obese SHHF rats (Figure 3).
Figure 3.
Changes in mitochondrial OxPhos complexes after 8 days of exercise in (a) Sprague Dawley rats, (b) SHHF lean rats and (c) SHHF obese rats. Exercise induced a significant increase in complexes I, II and III in the Sprague Dawleys, and no changes were seen in either the SHHF lean or SHHF obese groups with exercise. Sprague Dawleys (sedentary: n = 8, exercise: n = 9), SHHF lean (sedentary: n = 8, exercise: n = 10) and SHHF obese (sedentary: n = 9, exercise: n = 9). Data are expressed as mean fold change from sedentary groups + SEM. *p < 0.05, †p = 0.05–0.10, Student’s t-test.
OxPhos: oxidative phosphorylation; SEM: standard error of mean.
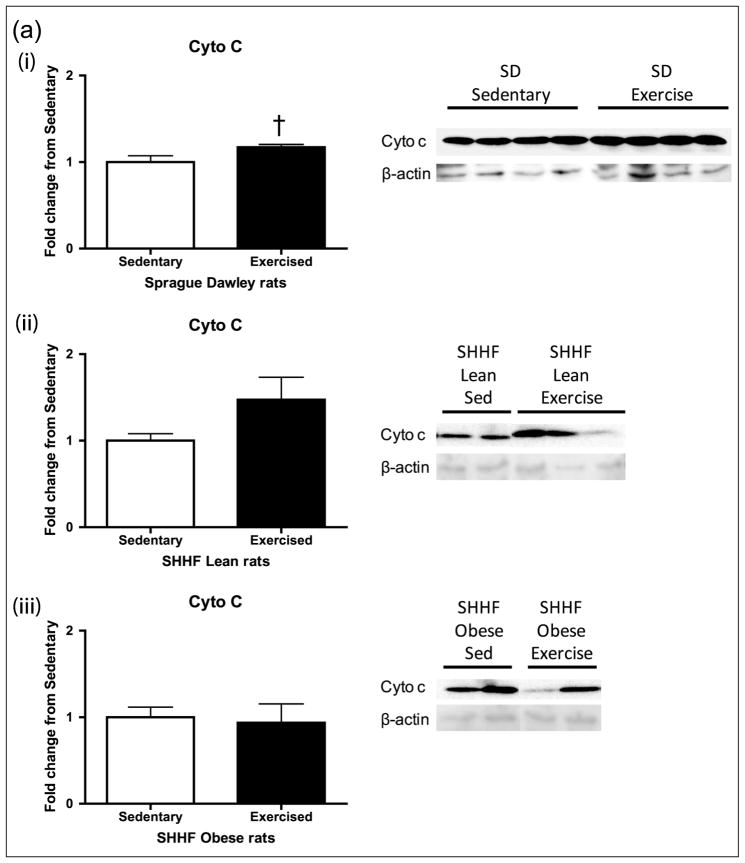
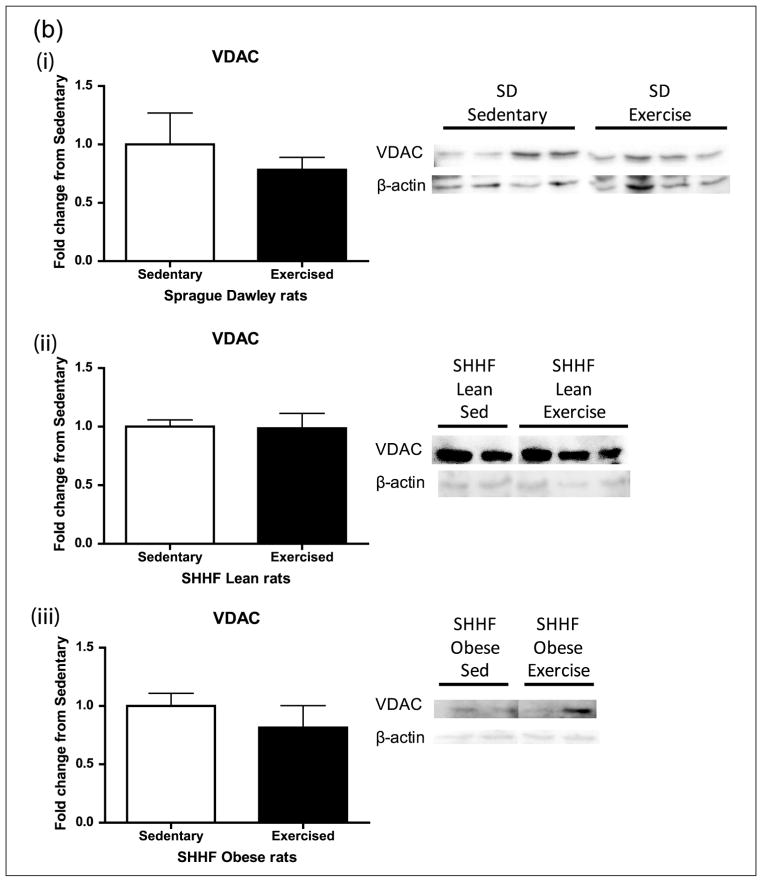
Aortic lysates were analysed for expression of the mitochondrial inner membrane cytochrome c and the outer membrane protein VDAC (Figure 4(a) and (b)). Cytochrome c transfers electrons from complex III to IV. VDAC (Porin1) is a major permeability factor of the mitochondrial outer membrane and is used as a mitochondrial marker, reflecting changes in mitochondria number. While cytochrome c trended higher after exercise in the SD rats (p = 0.075, Figure 4(a)), VDAC levels did not change with exercise in any of the groups (Figure 4(b)).
Figure 4.
Mitochondrial membrane proteins (a) cytochrome c and (b) VDAC after 8-day exercise intervention in (i) Sprague Dawleys, (ii) SHHF lean and (iii) SHHF obese rats. When looking at an inner and outer mitochondrial membrane proteins, there was a trend towards (a) exercise increasing cytochrome c levels in the SD group only (p = 0.075) and (b) no changes with exercise in any group when looking at VDAC. Sprague Dawleys (sedentary: n = 4, exercise: n = 4), (ii) SHHF lean (sedentary: n = 8, exercise: n = 9) and (iii) SHHF obese (sedentary: n = 10, exercise: n = 9). Data are expressed as mean fold change from sedentary groups + SEM. †p = 0.075, Student’s t-test.
VDAC: voltage-dependent anion-selective channel; SEM: standard error of mean.
Examination of mitochondrial antioxidant defence
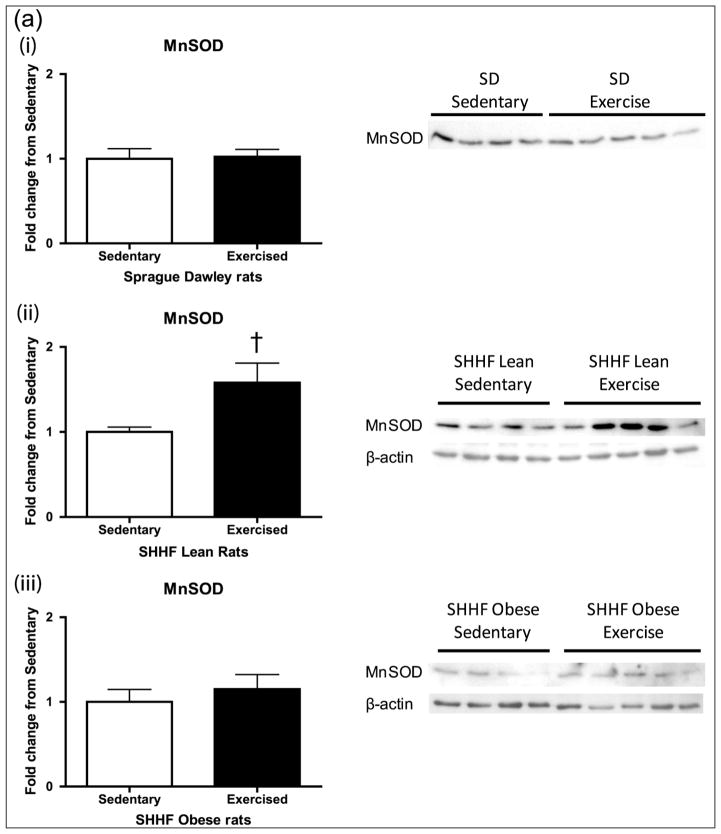
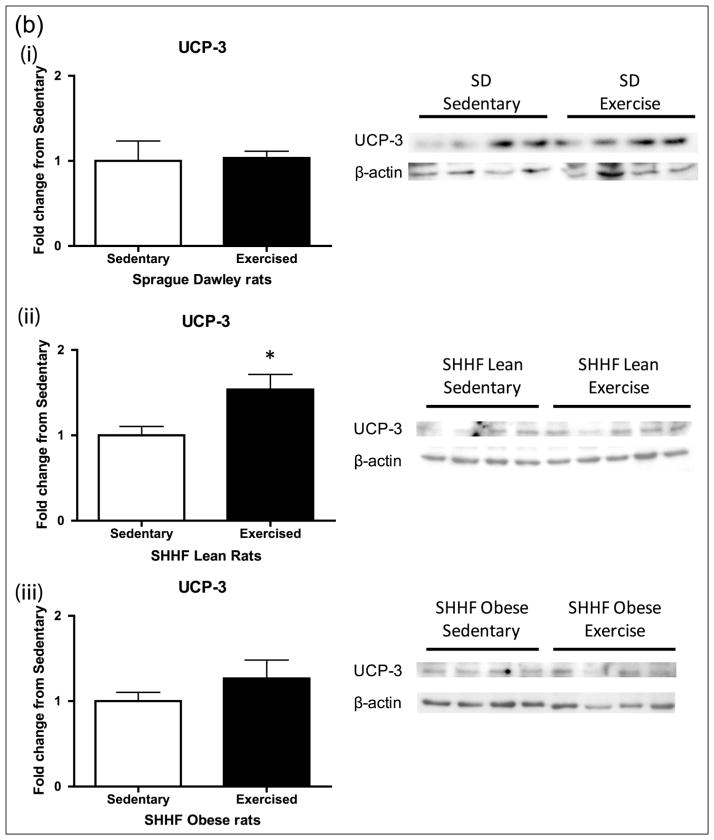
We next examined the impact of 8 days of moderate exercise upon UCP3 and MnSOD, antioxidant proteins important for mitochondrial antioxidant defence (Figure 5(a) and (b)). There was a significant increase in UCP3 (p < 0.05) in the SHHF lean rats with exercise as well as a trend of increase in MnSOD (p = 0.1), but there was no significant change in either protein in the SD and SHHF obese rats.
Figure 5.
Mitochondrial proteins (a) MnSOD and (b) UCP3 after 8-day exercise intervention in (i) SD, (ii) SHHF lean and (iii) SHHF obese rats. Exercise-induced increases in the CREB-dependent mitochondrial antioxidant proteins (a) MnSOD and (b) UCP3 in the SHHF lean rats only. SD (sedentary: n = 8/4, exercise: n = 10/4), SHHF lean (sedentary: n = 8/9, exercise: n = 10/9), SHHF obese (sedentary: n = 9, exercise: n = 9). Data are expressed as mean fold change from sedentary groups + SEM. *p < 0.05, †p = 0.1, Student’s t-test.
SD: Sprague Dawleys; MnSOD: manganese superoxide dismutase; CREB: cyclic adenosine monophosphate response element binding protein; UCP3: uncoupling protein 3; SEM: standard error of mean.
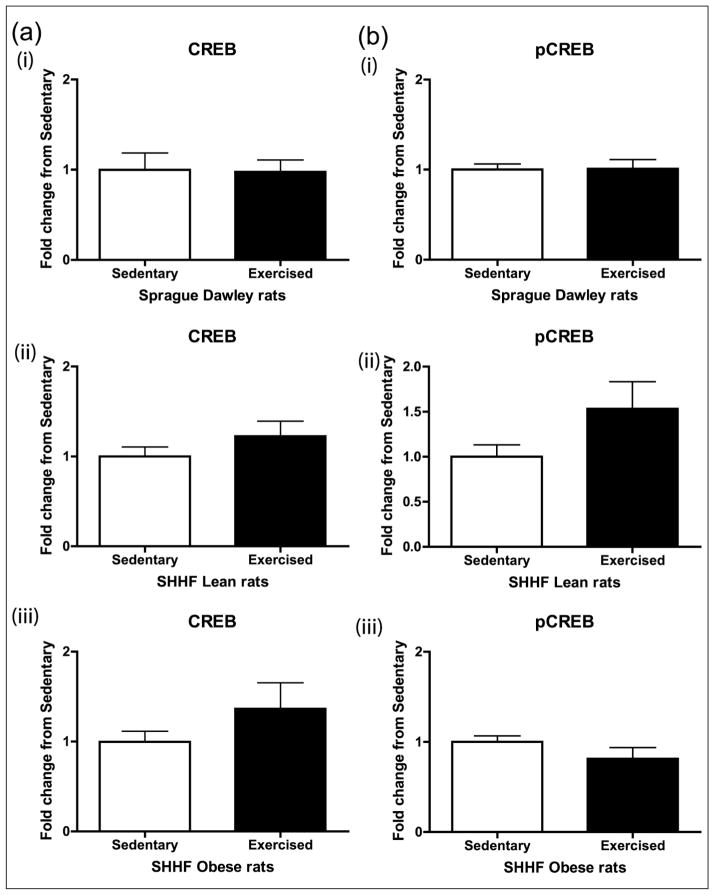
CREB content, activation and ubiquitination
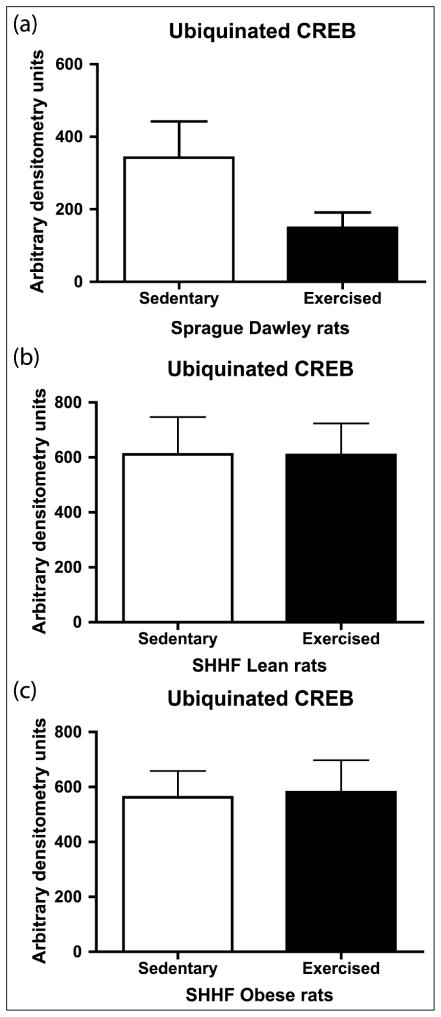
Aortic CREB content was decreased in a spectrum of rodent models of CVD risk (hypertension, obesity, diabetes, metabolic syndrome and dyslipidaemia).25,26 We previously reported that CREB content is decreased in aortic lysates from SHHF lean and obese rats and that the obesity decreased the vascular CREB content more than the SHHF lean phenotype alone.25 Reports in brain and β cells demonstrated induction of CREB protein and phosphorylation with exercise training.17,27 We examined CREB and active Ser133 pCREB in aortic lysates. The 8-day exercise intervention did not significantly change CREB or pCREB activity in the SD, SHHF lean or obese rats (Figure 6). We recently demonstrated in an in vitro model of pulmonary hypertension that vascular smooth muscle cell CREB loss is secondary to ubiquitin-mediated degradation28,29; thus, we examined the impact of an 8-day exercise intervention on CREB ubiquitination in vivo. There appeared to be a decrease in CREB ubiquitination in the SD, although it was not statistically significant (Figure 7).
Figure 6.
Effect of 8-day exercise intervention on (a) CREB and (b) pCREB in (i) Sprague Dawleys, (ii) SHHF lean and (iii) SHHF obese rats. Eight days of moderate exercise did not significantly change CREB or pCREB activity in the SD, SHHF lean or SHHF obese rats: Sprague Dawleys (sedentary: n = 8, exercise: n = 10), SHHF lean rats (sedentary: n = 8, exercise: n = 10) and SHHF obese rats (sedentary: n = 9, exercise: n = 9). Data are expressed as mean fold change from sedentary groups + SEM.
CREB: cyclic adenosine monophosphate response element binding protein; pCREB: phosphorylated CREB; SEM: standard error of mean.
Figure 7.
Effect of 8-day exercise intervention on ubiquitinated CREB in (a) Sprague Dawleys, (b) SHHF lean and (c) SHHF obese rats. 100–200 μg protein samples were IP using rabbit CREB antibodies (1:250) and Protein A sepharose beads. IP-CREB proteins were eluted from the beads with LSB and subjected to Western blot analyses probing for ubiquitin, followed by AP-linked secondary antibody detection by chemiluminescence on film and densitometric analysis. The amount of ubiquitinated CREB was decreased by 56% with exercise in the Sprague Dawley rats although the difference was not statistically significant (p = 0.1255): Sprague Dawleys (sedentary: n = 4, exercise: n = 4), SHHF lean (sedentary: n = 8, exercise: n = 9), SHHF obese (sedentary: n = 9, exercise: n = 8). Data are expressed as mean arbitrary units + SEM.
IP: immunoprecipitated; AP: alkaline phosphatase; CREB: cyclic adenosine monophosphate response element binding protein; SEM: standard error of mean; LSB: Laemmli sample buffer.
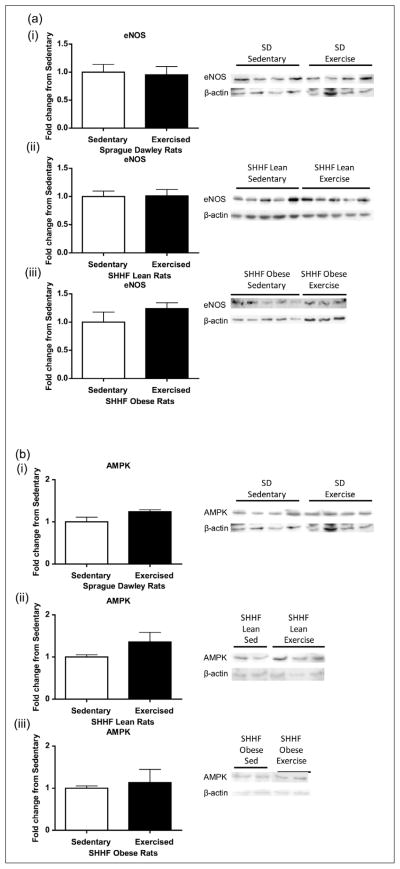
AMPK, PGC-1α and eNOS
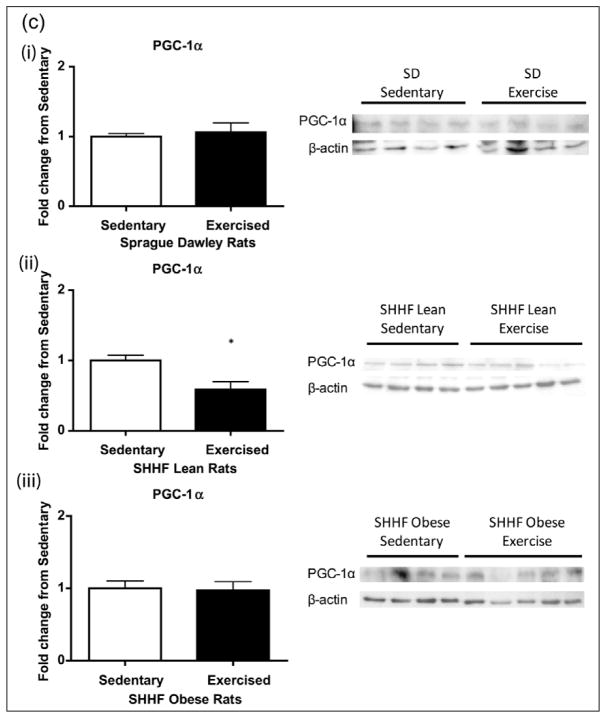
Observed increases in the mitochondrial OxPhos complexes were not explained by CREB content or its activation; we next looked at other classical upstream signalling pathways of the mitochondrial exercise response. Eight days of exercise did not induce any changes in eNOS protein expression in the aortas of SD, SHHF lean or obese rats 24 h post final exercise bout (Figure 8(a)). We also did not see changes in PGC-1α or AMPK expression in the SD or SHHF obese rats (Figure 8(b) and (c)); however, the PGC-1α levels significantly decreased in SHHF lean animals (p < 0.05, Figure 8(c)).
Figure 8.
Effect of 8-day exercise intervention on (a) eNOS, (b) AMPK and (c) PGC-1α in (i) Sprague Dawleys, (ii) SHHF lean and (iii) SHHF obese rats. When looking at other classical mediators of exercise response, only PGC-1α in the SHHF lean rats [(ii) in Figure 8(c)] showed a significant decrease with exercise: Sprague Dawleys (sedentary: n = 4, exercise: n = 4), SHHF lean (sedentary: n = 9/8/9, exercise: n = 10/9/9), SHHF obese (sedentary: n = 5/10/9, exercise: n = 3/9/9) rats. Data are expressed as mean fold change from sedentary groups + SEM. *p < 0.05, Student’s t-test.
SEM: standard error of mean; eNOS: nitric oxide synthase; AMPK: 5′ adenosine monophosphate–activated protein kinase; PGC-1α: peroxisome proliferators–activated receptor gamma coactivator 1α.
Discussion
The mitochondria has a role in a broad spectrum of pathologies. In diabetes, decreased skeletal muscle mitochondrial content and function are present both in patients and their offspring at risk for the disease.30,31 In addition, there are reports of decreased mitochondrial flexibility to nutrient stress in diabetes.32,33 Vascular mitochondrial dysfunction has emerged as a risk for vascular dysfunction in general;6–8 in diabetes, the effect of exercise on vascular mitochondrial adaptation and flexibility has not been examined. In this article, we specifically evaluated the vascular mitochondrial response to exercise exposure. We tested the effects of exercise on the vascular mitochondrial abundance in control, hypertensive and diabetic models to understand the vascular response to exercise intervention in health and disease. Previous studies reported that short-term exercise increases molecular pathways that regulate mitochondrial content and function.11,12,34,35 Specifically, acute exercise resulted in the activation of AMPK and also bolstered mitochondria against doxorubicin-induced damage,11,12 and thus, we elected to conduct our initial studies with a short-term exercise intervention. We present new evidence that there is impaired vascular mitochondrial adaptation to an 8-day exercise intervention in rat models of hypertension and metabolic syndrome with mild diabetes.
Non-disease states are characterized by the ability to maintain homeostasis in response to stresses such as exercise. 14 Previous reports in rat models indicated increased vascular and skeletal muscle mitochondrial numbers with exercise.13,35 We observed a significant induction of mitochondrial OxPhos complexes in the vasculature of SD rats with an 8-day exercise intervention. This observation indicated that the dose of exercise delivered to the SD rats was adequate to elicit the expected response in the SD rat, a standard control for SHHF rats.36–38 The OxPhos machinery itself is organized into supercomplexes, and it may be the stoichiometries of OxPhos components that play a key role in regulating energy production, ROS generation and apoptosis. Higher order supercomplexes, called respirasomes, might allow for the increase in the rate and efficiency of electron transfer. Dynamic mitochondrial adaptation to an exercise stimulus improves mitochondrial content and quality.39–41
Increase in mitochondrial content could be a consequence of increased mitochondrial biogenesis. We evaluated AMPK, PGC1-α, eNOS or CREB established upstream signalling pathways important for mitochondrial biogenesis. Of note, we did not observe the expected increase in AMPK, PGC1-α, eNOS or CREB activity with increased mitochondrial complex expression in the SD animals. Exercise did, however, significantly reduce AMPK activation by 25% in the SHHF obese but not the SHHF lean rats (p = 0.028, data not shown). Potentially, the mitochondrial adaptation may be downstream of the activation of other pathways or signalling agents, such as sirtuin 1 (SIRT1) or Nrf2, both known to promote mitochondrial biogenesis (reviewed in Gurd,42 Houtkooper et al.43 and Suliman et al.44). It has been recently reported that aortic SIRT is activated in response to an acute exhaustive exercise bout without CREB or Akt stimulation.34,35 In addition, H2O2, induced by a bout of exercise, has also been found to directly activate pathways leading to mitochondrial biogenesis and macroautophagy.44,45 The most likely explanation for no change in these targets is that the dose of exercise used in this study acutely stimulated the established AMPK, SIRT, eNOS, CREB and possibly Akt pathways, and that these signalling events returned to baseline over the 24-h from bout to sacrifice. Future experiments in our laboratory will focus on the immediate exercise response to examine signalling and increased dose and duration of exercise training to more strongly perturb these pathways.
A recent metabolomics article suggested that increased antioxidant defence systems correlated with increased fitness in humans.46 The increase in the mitochondrial OxPhos complexes in the SD rats was not accompanied by an expected increase in the mitochondrial antioxidant enzymes MnSOD and UCP3. As with the pathways outlined above, the 24-h window from end of final exercise bout to tissue collection may be enough time to mitigate the acute oxidant burden stimulated by exercise and return enzymes to baseline as a normal homeostatic response. In the SHHF lean rats, increases in the mitochondrial antioxidant enzymes are consistent with exercise- mediated vascular ROS not buffered by endogenous antioxidant defence and suggest that an increase in oxidative stress was not corrected within a 24-h time period. Of note, these same rats had a decrease in PGC-1α with the intervention. Transient decrease in PGC-1α has been observed in the myocardium during exercise adaptation, and it returns to baseline with adaptive training.47 Both of these changes suggest a vascular tissue effect of the exercise intervention in the SHHF lean without full adaptive mitochondrial response at the 8-day interval. Lack of changes in the antioxidant enzymes in the SHHF obese is consistent with impaired ability to mount an antioxidant defence as a result of underlying chronic oxidative stress. Failed compensation for oxidant stress has been reported in rodent models and human subjects with diabetes (reviewed in Sheikh-Ali et al.48 and Codoner-Franch49). The disparate response in antioxidant defence across the three models reflects the complexity of redox balance and cannot be characterized by the current experimental design.
eNOS and CREB are both upstream regulators of mitochondrial biogenesis.15,50–56 We failed to observe any response in eNOS protein in SD or SHHF rats with exercise. We observed a significant decrease in expression of nitrotyrosine, a proxy measurement of the presence of oxidative NO damage and NO synthesis, in the SHHF obese as compared with the SHHF lean aorta lysates. In the SHHF obese rats, this may be indicative of suppressed eNOS activity as previously reported.57 Lower NO synthesis is observed with increased reactive oxygen,57 which may be consistent with a more than twofold higher serum TBARS levels in the SHHF obese rats when compared to SD and SHHF lean rats both at baseline and after exercise. We failed to observe any significant changes in cytokine expression in representative pro- and anti-inflammatory agents, indicating a lack of chronic inflammation in our models with or without exercise. Decreased serum inflammatory cytokines in the SHHF obese rats would not have been predicted based on a recent report in the Zucker fatty model with an analogous leptin receptor mutation.58
Similar to the eNOS results, measures of CREB content, activity and turnover were unchanged 24-h post final exercise bout in all three groups. CREB is a fundamental transcriptional regulator of the homeostatic response to physiological metabolic stress that is downregulated in the vasculature in diabetes.26,29,59 Lack of a CREB response in any of the models was unexpected but is consistent with a recent study examining acute signalling after a bout of exhaustive exercise.34 In the SD rats, this may be due to insufficient length or intensity of the intervention; lack of pCREB or CREB response in the SHHF animals may be due to the impaired physiological response to exercise in disease states, such as impaired activation of eNOS or PGC-1α.
The 8-day exercise intervention used in the present study was chosen based on previous interventions in the literature examining antioxidant profiles and eNOS.60 The same exercise regimen was provided to all; as such, the obese animals had a greater relative workload from the intervention than the other animals. It is of note that the obese animals showed no response to exercise despite a greater amount of work during the exercise, supporting our hypothesis that exercise response is blunted in disease states. Future studies will focus on increased and matched workloads in diabetic models and controls.
In conclusion, exercise is a complex intervention with tissue-specific effects that need to be defined.61 Our results demonstrate an increase in mitochondrial protein expression in healthy rats, absent in models of hypertension and insulin resistance. Overall, the significant aortic mitochondrial response in the SD rats suggests that this is an early adaption to exercise challenge in the ‘healthy’ vasculature. Lack of an antioxidant response in the SD is consistent with this small dose of exercise failing to induce persistent oxidant stress in the vasculature. This ‘exercise resistance’ in the SHHF models is likely mediated by suppression of the activity of molecular mediators of exercise-induced adaptation, which normally lead to enhanced eNOS, AMPK, PGC-1α and CREB activity with exercise. Our findings suggest that chronic vascular stress, either metabolic or hemodynamic, results in decreased sensitivity or resistance of vasculature to the positive adaptive responses normally induced by acute exercise; this supports the need for targeted research on exercise response in the context of disease. For example, to elicit a salutary response to exercise in disease states, the dose or type of exercise may be different. Clinical data suggest that this is true in diabetes, menopause and ageing.62–65
Acknowledgments
Funding
This work was supported by the Department of Veterans Affairs Office of Research and Development (Merit Review JEBR and PAW), National Institutes of Health (HL14985, UL1 RR025780, JEBR) and American Heart Association Grant (0835545N, AJC).
Footnotes
Reprints and permissions: sagepub.co.uk/journalsPermissions.nav
Conflict of interest
None declared.
References
- 1.Williams PT. Physical fitness and activity as separate heart disease risk factors: a meta-analysis. Med Sci Sports Exerc. 2001;33:754–761. doi: 10.1097/00005768-200105000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gill JM, Malkova D. Physical activity, fitness and cardiovascular disease risk in adults: interactions with insulin resistance and obesity. Clin Sci (Lond) 2006;110:409–425. doi: 10.1042/CS20050207. [DOI] [PubMed] [Google Scholar]
- 3.Wei M, Gibbons LW, Kampert JB, et al. Low cardiorespiratory fitness and physical inactivity as predictors of mortality in men with Type 2 diabetes. Ann Intern Med. 2000;132:605–611. doi: 10.7326/0003-4819-132-8-200004180-00002. [DOI] [PubMed] [Google Scholar]
- 4.Wei M, Kampert JB, Barlow CE, et al. Relationship between low cardiorespiratory fitness and mortality in normal-weight, overweight, and obese men. JAMA. 1999;282:1547–1553. doi: 10.1001/jama.282.16.1547. [DOI] [PubMed] [Google Scholar]
- 5.Sundquist K, Qvist J, Sundquist J, et al. Frequent and occasional physical activity in the elderly: a 12-year follow-up study of mortality. Am J Prev Med. 2004;27:22–27. doi: 10.1016/j.amepre.2004.03.011. [DOI] [PubMed] [Google Scholar]
- 6.Davidson SM. Endothelial mitochondria and heart disease. Cardiovasc Res. 2010;88:58–66. doi: 10.1093/cvr/cvq195. [DOI] [PubMed] [Google Scholar]
- 7.Ungvari Z, Sonntag WE, Csiszar A. Mitochondria and aging in the vascular system. J Mol Med (Berl) 2010;88:1021–1027. doi: 10.1007/s00109-010-0667-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sansbury BE, Jones SP, Riggs DW, et al. Bioenergetic function in cardiovascular cells: the importance of the reserve capacity and its biological regulation. Chem Biol Interact. 2010;191:288–295. doi: 10.1016/j.cbi.2010.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Katakam PV, Domoki F, Snipes JA, et al. Impaired mitochondria-dependent vasodilation in cerebral arteries of Zucker obese rats with insulin resistance. Am J Physiol Regul Integr Comp Physiol. 2009;296:R289–R298. doi: 10.1152/ajpregu.90656.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nemes A, Geleijnse ML, Sluiter W, et al. Aortic distensibility alterations in adults with m. 3243A>G MELAS gene mutation. Swiss Med Wkly. 2009;139:117–120. doi: 10.4414/smw.2009.12390. [DOI] [PubMed] [Google Scholar]
- 11.Kavazis AN, Smuder AJ, Min K, et al. Short-term exercise training protects against doxorubicin-induced cardiac mitochondrial damage independent of HSP72. Am J Physiol Heart Circ Physiol. 2012;299:H1515–H1524. doi: 10.1152/ajpheart.00585.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pauli JR, Ropelle ER, Cintra DE, et al. Acute physical exercise reverses S-nitrosation of the insulin receptor, insulin receptor substrate 1 and protein kinase B/Akt in diet-induced obese Wistar rats. J Physiol. 2008;586:659–671. doi: 10.1113/jphysiol.2007.142414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Young CG, Knight CA, Vickers KC, et al. Differential effects of exercise on aortic mitochondria. Am J Physiol Heart Circ Physiol. 2005;288:H1683–H1689. doi: 10.1152/ajpheart.00136.2004. [DOI] [PubMed] [Google Scholar]
- 14.Bo H, Zhang Y, Ji LL. Redefining the role of mitochondria in exercise: a dynamic remodeling. Ann N Y Acad Sci. 2011;1201:121–128. doi: 10.1111/j.1749-6632.2010.05618.x. [DOI] [PubMed] [Google Scholar]
- 15.Momken I, Lechene P, Ventura-Clapier R, et al. Voluntary physical activity alterations in endothelial nitric oxide synthase knockout mice. Am J Physiol Heart Circ Physiol. 2004;287:H914–H920. doi: 10.1152/ajpheart.00651.2003. [DOI] [PubMed] [Google Scholar]
- 16.Watson PA, Birdsey N, Huggins GS, et al. Cardiac-specific overexpression of dominant-negative CREB leads to increased mortality and mitochondrial dysfunction in female mice. Am J Physiol Heart Circ Physiol. 2010 doi: 10.1152/ajpheart.00394.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen MJ, Russo-Neustadt AA. Exercise activates the phosphatidylinositol 3-kinase pathway. Brain Res Mol Brain Res. 2005;135:181–193. doi: 10.1016/j.molbrainres.2004.12.001. [DOI] [PubMed] [Google Scholar]
- 18.Finkbeiner S. CREB couples neurotrophin signals to survival messages. Neuron. 2000;25:11–14. doi: 10.1016/s0896-6273(00)80866-1. [DOI] [PubMed] [Google Scholar]
- 19.Luo CX, Jiang J, Zhou QG, et al. Voluntary exercise-induced neurogenesis in the postischemic dentate gyrus is associated with spatial memory recovery from stroke. J Neurosci Res. 2007;85:1637–1646. doi: 10.1002/jnr.21317. [DOI] [PubMed] [Google Scholar]
- 20.Ploughman M, Granter-Button S, Chernenko G, et al. Exercise intensity influences the temporal profile of growth factors involved in neuronal plasticity following focal ischemia. Brain Res. 2007;1150:207–216. doi: 10.1016/j.brainres.2007.02.065. [DOI] [PubMed] [Google Scholar]
- 21.Rosa JS, Heydari S, Oliver SR, et al. Inflammatory cytokine profiles during exercise in obese, diabetic, and healthy children. J Clin Res Pediatr Endocrinol. 2011;3:115–121. doi: 10.4274/jcrpe.v3i3.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Strasser B, Arvandi M, Siebert U. Resistance training, visceral obesity and inflammatory response: a review of the evidence. Obes Rev. 2012;13:578–591. doi: 10.1111/j.1467-789X.2012.00988.x. [DOI] [PubMed] [Google Scholar]
- 23.Zhang H, Zhang C. Vasoprotection by dietary supplements and exercise: role of TNF alpha signaling. Exp Diabetes Res. 2012;2012:972679, 6. doi: 10.1155/2012/972679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McCune SA, Baker PB, Stills HF., Jr New rat models of obesity and Type II diabetes SHHF/Mcc-cp rat: model of obesity, non-insulin-dependent diabetes, and congestive heart failure. ILAR News. 1990;32:23–27. [Google Scholar]
- 25.Schauer IE, Knaub LA, Lloyd M, et al. CREB downregulation in vascular disease: a common response to cardiovascular risk. Arterioscler Thromb Vasc Biol. 2010;30:733–741. doi: 10.1161/ATVBAHA.109.199133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Watson PA, Nesterova A, Burant CF, et al. Diabetes-related changes in cAMP response element-binding protein content enhance smooth muscle cell proliferation and migration. J Biol Chem. 2001;276:46142–46150. doi: 10.1074/jbc.M104770200. [DOI] [PubMed] [Google Scholar]
- 27.Park S, Hong SM, Sung SR. Exendin-4 and exercise promotes beta-cell function and mass through IRS2 induction in islets of diabetic rats. Life Sci. 2008;82:503–511. doi: 10.1016/j.lfs.2007.12.018. [DOI] [PubMed] [Google Scholar]
- 28.Garat CV, Crossno JT, Jr, Sullivan TM, et al. Thiazolidinediones prevent PDGF-BB-induced CREB depletion in pulmonary artery smooth muscle cells by preventing upregulation of casein kinase 2 alpha’ catalytic subunit. J Cardiovasc Pharmacol. 2010;55:469–480. doi: 10.1097/FJC.0b013e3181d64dbe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Garat CV, Fankell D, Erickson PF, et al. Platelet-derived growth factor BB induces nuclear export and proteasomal degradation of CREB via phosphatidylinositol 3-kinase/Akt signaling in pulmonary artery smooth muscle cells. Mol Cell Biol. 2006;26:4934–4948. doi: 10.1128/MCB.02477-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mootha VK, Lindgren CM, Eriksson KF, et al. PGC-1alpha-responsive genes involved in oxidative phosphorylation are coordinately downregulated in human diabetes. Nat Genet. 2003;34:267–273. doi: 10.1038/ng1180. [DOI] [PubMed] [Google Scholar]
- 31.Patti ME, Butte AJ, Crunkhorn S, et al. Coordinated reduction of genes of oxidative metabolism in humans with insulin resistance and diabetes: potential role of PGC1 and NRF1. Proc Natl Acad Sci U S A. 2003;100:8466–8471. doi: 10.1073/pnas.1032913100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Aucouturier J, Duche P, Timmons BW. Metabolic flexibility and obesity in children and youth. Obes Rev. 2011;12:e44–e53. doi: 10.1111/j.1467-789X.2010.00812.x. [DOI] [PubMed] [Google Scholar]
- 33.Meex RC, Schrauwen-Hinderling VB, Moonen-Kornips E, et al. Restoration of muscle mitochondrial function and metabolic flexibility in Type 2 diabetes by exercise training is paralleled by increased myocellular fat storage and improved insulin sensitivity. Diabetes. 2010;59:572–579. doi: 10.2337/db09-1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cacicedo JM, Gauthier MS, Lebrasseur NK, et al. Acute exercise activates AMPK and eNOS in the mouse aorta. Am J Physiol Heart Circ Physiol. 2011;301:H1255–H1265. doi: 10.1152/ajpheart.01279.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang QJ, McMillin SL, Tanner JM, et al. Endothelial nitric oxide synthase phosphorylation in treadmill-running mice: role of vascular signalling kinases. J Physiol. 2009;587:3911–3920. doi: 10.1113/jphysiol.2009.172916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cittadini A, Napoli R, Monti MG, et al. Metformin prevents the development of chronic heart failure in the SHHF rat model. Diabetes. 2012;61:944–953. doi: 10.2337/db11-1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Saini-Chohan HK, Holmes MG, Chicco AJ, et al. Cardiolipin biosynthesis and remodeling enzymes are altered during development of heart failure. J Lipid Res. 2009;50:1600–1608. doi: 10.1194/jlr.M800561-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sparagna GC, Chicco AJ, Murphy RC, et al. Loss of cardiac tetralinoleoyl cardiolipin in human and experimental heart failure. J Lipid Res. 2007;48:1559–1570. doi: 10.1194/jlr.M600551-JLR200. [DOI] [PubMed] [Google Scholar]
- 39.He C, Bassik MC, Moresi V, et al. Exercise-induced BCL2-regulated autophagy is required for muscle glucose homeostasis. Nature. 2012;481:511–515. doi: 10.1038/nature10758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Romanello V, Sandri M. Mitochondrial biogenesis and fragmentation as regulators of muscle protein degradation. Curr Hypertens Rep. 2010;12:433–439. doi: 10.1007/s11906-010-0157-8. [DOI] [PubMed] [Google Scholar]
- 41.Weber TA, Reichert AS. Impaired quality control of mitochondria: aging from a new perspective. Exp Gerontol. 2010;45:503–511. doi: 10.1016/j.exger.2010.03.018. [DOI] [PubMed] [Google Scholar]
- 42.Gurd BJ. Deacetylation of PGC-1alpha by SIRT1: importance for skeletal muscle function and exercise-induced mitochondrial biogenesis. Appl Physiol Nutr Metab. 2011;36:589–597. doi: 10.1139/h11-070. [DOI] [PubMed] [Google Scholar]
- 43.Houtkooper RH, Pirinen E, Auwerx J. Sirtuins as regulators of metabolism and healthspan. Nat Rev Mol Cell Bio. 2012;13:225–238. doi: 10.1038/nrm3293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Suliman HB, Sweeney TE, Withers CM, et al. Co-regulation of nuclear respiratory factor-1 by NFkappaB and CREB links LPS-induced inflammation to mitochondrial biogenesis. J Cell Sci. 2011;123:2565–2575. doi: 10.1242/jcs.064089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Du J, Teng RJ, Guan T, et al. Role of autophagy in angiogenesis in aortic endothelial cells. Am J Physiol Cell Physiol. 2012;302:C383–C391. doi: 10.1152/ajpcell.00164.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chorell E, Svensson MB, Moritz T, et al. Physical fitness level is reflected by alterations in the human plasma metabolome. Mol Biosyst. 2012;8:1187–1196. doi: 10.1039/c2mb05428k. [DOI] [PubMed] [Google Scholar]
- 47.Watson PA, Reusch JE, McCune SA, et al. Restoration of CREB function is linked to completion and stabilization of adaptive cardiac hypertrophy in response to exercise. Am J Physiol Heart Circ Physiol. 2007;293:H246–H259. doi: 10.1152/ajpheart.00734.2006. [DOI] [PubMed] [Google Scholar]
- 48.Sheikh-Ali M, Chehade JM, Mooradian AD. The antioxidant paradox in diabetes mellitus. Am J Ther. 2011;18:266–278. doi: 10.1097/MJT.0b013e3181b7badf. [DOI] [PubMed] [Google Scholar]
- 49.Codoner-Franch P, Pons-Morales S, Boix-Garcia L, et al. Oxidant/antioxidant status in obese children compared to paediatric patients with Type 1 diabetes mellitus. Pediatr Diabetes. 2010;11:251–257. doi: 10.1111/j.1399-5448.2009.00565.x. [DOI] [PubMed] [Google Scholar]
- 50.Chiueh CC, Andoh T, Chock PB. Induction of thioredoxin and mitochondrial survival proteins mediates preconditioning- induced cardioprotection and neuroprotection. Ann N Y Acad Sci. 2005;1042:403–418. doi: 10.1196/annals.1338.034. [DOI] [PubMed] [Google Scholar]
- 51.Das S, Tosaki A, Bagchi D, et al. Resveratrol-mediated activation of cAMP response element-binding protein through adenosine A3 receptor by Akt-dependent and -independent pathways. J Pharmacol Exp Ther. 2005;314:762–769. doi: 10.1124/jpet.105.084285. [DOI] [PubMed] [Google Scholar]
- 52.Donato AJ, Magerko KA, Lawson BR, et al. SIRT-1 and vascular endothelial dysfunction with ageing in mice and humans. J Physiol. 2011;589:4545–4554. doi: 10.1113/jphysiol.2011.211219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kronke G, Bochkov VN, Huber J, et al. Oxidized phospholipids induce expression of human heme oxygenase-1 involving activation of cAMP-responsive element-binding protein. J Biol Chem. 2003;278:51006–51014. doi: 10.1074/jbc.M304103200. [DOI] [PubMed] [Google Scholar]
- 54.Nisoli E, Clementi E, Paolucci C, et al. Mitochondrial biogenesis in mammals: the role of endogenous nitric oxide. Science. 2003;299:896–899. doi: 10.1126/science.1079368. [DOI] [PubMed] [Google Scholar]
- 55.Nisoli E, Tonello C, Cardile A, et al. Calorie restriction promotes mitochondrial biogenesis by inducing the expression of eNOS. Science. 2005;310:314–317. doi: 10.1126/science.1117728. [DOI] [PubMed] [Google Scholar]
- 56.St-Pierre J, Drori S, Uldry M, et al. Suppression of reactive oxygen species and neurodegeneration by the PGC-1 transcriptional coactivators. Cell. 2006;127:397–408. doi: 10.1016/j.cell.2006.09.024. [DOI] [PubMed] [Google Scholar]
- 57.Erdos B, Snipes JA, Miller AW, et al. Cerebrovascular dysfunction in Zucker obese rats is mediated by oxidative stress and protein kinase C. Diabetes. 2004;53:1352–1359. doi: 10.2337/diabetes.53.5.1352. [DOI] [PubMed] [Google Scholar]
- 58.Miranville A, Herling AW, Biemer-Daub G, et al. Differential adipose tissue inflammatory state in obese nondiabetic Zucker fatty rats compared to obese diabetic Zucker diabetic fatty rats. Horm Metab Res. 2012;44:273–278. doi: 10.1055/s-0032-1304581. [DOI] [PubMed] [Google Scholar]
- 59.Klemm DJ, Watson PA, Frid MG, et al. cAMP response element- binding protein content is a molecular determinant of smooth muscle cell proliferation and migration. J Biol Chem. 2001;276:46132–46141. doi: 10.1074/jbc.M104769200. [DOI] [PubMed] [Google Scholar]
- 60.Meilhac O, Ramachandran S, Chiang K, et al. Role of arterial wall antioxidant defense in beneficial effects of exercise on atherosclerosis in mice. Arterioscler Thromb Vasc Biol. 2001;21:1681–1688. doi: 10.1161/hq1001.097106. [DOI] [PubMed] [Google Scholar]
- 61.Booth FW, Laye MJ. Lack of adequate appreciation of physical exercise’s complexities can pre-empt appropriate design and interpretation in scientific discovery. J Physiol. 2009;587:5527–5539. doi: 10.1113/jphysiol.2009.179507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Earnest CP, Blair SN, Church TS. Does age attenuate aerobic conditioning response in postmenopausal women? Response. Eur J Appl Physiol. 2011;111:1559–1560. doi: 10.1007/s00421-010-1778-y. [DOI] [PubMed] [Google Scholar]
- 63.Johannsen NM, Swift DL, Johnson WD, et al. Effect of different doses of aerobic exercise on total white blood cell (WBC) and WBC subfraction number in postmenopausal women: results from DREW. PLoS One. 2012;7:e31319. doi: 10.1371/journal.pone.0031319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Swift DL, Earnest CP, Blair SN, et al. The effect of different doses of aerobic exercise training on endothelial function in postmenopausal women with elevated blood pressure: results from the DREW study. Br J Sports Med. 2011 doi: 10.1136/bjsports-2011-090025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Swift DL, Johannsen NM, Earnest CP, et al. Effect of exercise training modality on C-reactive protein in Type-2 diabetes. Med Sci Sports Exerc. 2011 doi: 10.1249/MSS.0b013e31824526cc. [DOI] [PMC free article] [PubMed] [Google Scholar]