Abstract
Group-living typically provides benefits to individual group members but also confers costs. To avoid incredulity and betrayal and allow trust and cooperation, individuals must understand the intentions and emotions of their group members. Humans attend to other's eyes and from gaze and pupil-size cues, infer information about the state of mind of the observed. In humans, pupil-size tends to mimic that of the observed. Here we tested whether pupil-mimicry exists in our closest relative, the chimpanzee (P. troglodytes). We conjectured that if pupil-mimicry has adaptive value, e.g. to promote swift communication of inner states and facilitate shared understanding and coordination, pupil-mimicry should emerge within but not across species. Pupillometry data was collected from human and chimpanzee subjects while they observed images of the eyes of both species with dilating/constricting pupils. Both species showed enhanced pupil-mimicry with members of their own species, with effects being strongest in humans and chimpanzee mothers. Pupil-mimicry may be deeply-rooted, but probably gained importance from the point in human evolution where the morphology of our eyes became more prominent. Humans' white sclera surrounding the iris, and the fine muscles around their eyes facilitate non-verbal communication via eye signals.
Introduction
Many daily social decisions are made through quick evaluations of others. The ability to recognize emotions is one of the most important factors involved in regulating social interactions in primates [1].Through face-to-face interactions, humans and chimpanzees learn to recognize characteristics (such as emotional expressions and group membership) that signal safety, and to cooperate with those that seem trustworthy [2]. According to the theory of emotional contagion, another's state may be perceived through synchronization or mimicry [3]. Mimicry is shared among the great apes [4], allows individuals to mirror other's minds [5], [6] and has positive effects on the bond between individuals [7], [8], [9].
Synchronization occurs at different levels, ranging from body postures and facial expressions [10], [11] to physiological states, such as heart-beat rythm [12], [13], neural activity [14], or eye-blinks [15]. Research has also shown mimicry of pupil-size [16], [17], a phenomenon dubbed pupillary contagion that is linked to empathy and involves activity of the amygdala [18], [19], an area densely connected with brain-stem areas that regulate the pupil [20]. This interesting phenomenon has only received little attention in the literature and many questions have remained unanswered about the exact meaning of pupil-size mimicry.
The meaning of pupil-dilation is also diverse and can reflect many inner states including attention, arousal, cognitive effort and interest [21]. Importantly, there is a qualitative difference between the expression and the perception of pupil-size. Large pupils are perceived positively. In the 17th century, Italian women through the use of atropine-containing eyedrops induced pupil dilation to appear more attractive. Also Japanese manga- and Disney illustrators have since long applied this common knowledge “that large pupils are good” successfully in their cartoons (bambi has large pupils, the big bad woolf pin-point pupils). Despite this anecdotal evidence, the positive impression that large pupils evoke has only received very little scientific attention thus far. We here propose that in the context of a social interaction, pupil-dilation may implicitly signal social interest, which would explain why large pupils in an observed induce positive feelings and pupil-mimicry in an observer [16].
The positive effects of mimicry are particularly relevant for members within the same group, as group cohesion and cooperation are important predictors for survival [22]. Previous research has indeed shown that mimicry occurs more often between two members of the same group than between members from different groups [23], [24], [25], even on automatic levels such as heart-beat, which is particularly interesting as it cannot be controlled or regulated [26], [27]. From the literature, it seems that how group is defined is not of much influence. A group setting, whether defined by political attitudes, sport affiliation [24], status [28], familiarity [23], ethnicity [25] or species [29], impacts on the perceived similarity between observer and observed, which enhances mimicry. The current study is the first in investigating within-species advantages on pupil-mimicry and in investigating an autonomic form of synchronization in two different species.
In humans, the eyes are important in understanding another's intentions [30] and capture more attention than any other area of the face [31]. The typical white color of our sclera facilitated non-vocal communication [32] which has recently been demonstrated in a series of experimental studies [33]. Lee et al (2013) suggest that our eye-white served to link expresser and observer (see also [34]). Eye-white appeared after the human and chimpanzee lineages split six million years ago and may have served as a catalyst for new forms of eye communication to emerge, including pupil-mimicry. Chimpanzees have no visible eye-white but do have bright amber-colored irises in which the pupil is clearly visible. Not only in humans, but across different species, pupil-dilation indicates attention and arousal [35]. For that reason, one could also argue that picking up pupillary cues from a conspecific would be beneficial for all species that live in social groups, that cooperate and are active during daytime.
In order to investigate the uniqueness of pupil-mimicry for the human species, we here make a direct comparison with the chimpanzee. If chimpanzees show pupil-mimicry, this would suggest that it existed in our common ancestor and may even be shared by other primates or mammals. Chimpanzees form a perfect comparison group as they are most closely related to us, are known to synchronize on other more explicit levels (laughing, [36]; yawning [28], [29], [37], [38]; tapping with a sound [39] and also mimic familiar others more than unfamiliar others [38]. Moreover, they have similar visual acuity as humans [40], a similar pupil-response [41], they attend to eyes [42], and although less automatically than humans, also follow eye-gaze [43], [44], [45].
In the current study, eighteen human and eight chimpanzee subjects watched four-second video-clips of the eye-region of both species. The pupils in these images either slightly dilated or constricted and with eye-tracking equipment, we measured whether subject's pupils mimicked the pupils they observed. We predicted that chimpanzees would synchronize their pupils with chimpanzees and not with humans. As human eyes are ideally set up for communication, we predicted that humans would show even stronger pupil-mimicry effects than chimpanzees, and specifically with observed human eyes.
Method
Participants
Eight chimpanzees (two female and one male juvenile, four female and one male adult) and eighteen humans (seven male) participated in this study. Chimpanzees were 24.63 years old on average (SD = 11.54) and human subjects were on average 25.83 years old (SD = 8.40). At the time of testing, the chimpanzees lived within a social group of fourteen individuals in an enriched environment with a 700-m2 outdoor compound and an attached indoor residence that was illuminated during day-time at the Kyoto University Research Unit in Inuyama, Japan. The outdoor compound was equipped with climbing frames, ropes, small streams, and various species of trees. Access to the outdoor compound was available to them every other day during the day. Daily meals included a wide variety of fresh fruits and vegetables fed throughout the day supplemented with nutritionally balanced biscuits (fed twice daily) and water available ad libitum. The chimpanzees have been familiar with humans since birth and interact with them on a daily basis (especially during feeding and prior to and after the experiments). They have taken part in various cognitive experiments since youth [34], [39], [40]. For the daily experiments, the chimpanzees left the social group voluntarily on the request of experimenters, moved into the experimental booth with the guidance of experimenters, and moved back to the social group after the completion of experiments (approx. 1 hour). The human participants were scientists or students from Kyoto University (fourteen Asian, four Caucasian). Sixteen had experience with the observation of chimpanzees. As the chimpanzees in our sample were very familiar with humans, our aim was to include humans that were also familiar with chimpanzees. Human subjects provided written informed consent prior to the experiment and received full debriefing upon completion of the study. The study with human participants reported here was reviewed and approved by the Human Research Ethics Committee, Primate Research Institute, Kyoto University (H2010-17). The care and use of the chimpanzees adhered to the 3rd edition of the Guide for the Care and Use of Laboratory Primates issued by Primate Research Isntitute, Kyoto University (KUPRI) in 2010, which is compatible with the guidelines issued by the National Institute of Health in the United States of America. The research design was approved by the Animal Welfare and Animal Care Committee of KUPRI and by the Animal Research Committee of Kyoto University (#2011-078). All procedures adhered to the Japanese Act on Welfare and Management of Animals.
Apparatus
We used a binocular eye tracker that allowed for relatively large head movements of participants during tracking (60 Hz; Tobii ×120). The chimpanzees had experience with eye-tracking [43]. A five- and two-point calibration was applied in humans and chimpanzees subjects respectively. A corneal reflection technique was applied. All participants were tested in the same experimental booth, with the experimenter and the participant separated by transparent acrylic panels. The distance between the participants and the movable table on which the eye-tracker and a 17-inch LCD display were mounted was adjusted to the point at which gaze was best recorded (approximately 60 cm). See Figure 1.
Figure 1. Experimental Set-up.
A/B. Stimulus' pupils changed dynamically in size after 600 ms of static presentation. The maximum or minimum was reached after 1000 ms. C. The testing situation was the same for human and chimpanzee subjects. D. Schematic representation of pupil-size mimicry.
Stimulus Materials
We prepared pictures from four chimpanzees and four humans (two males, two females). The human faces were selected from the McArthur set (www.macbrain.org) and the chimpanzee pictures were shot at the chimpanzee sanctuary in Kumamoto, Japan. The individuals depicted in the stimuli were all unfamiliar to the participants. We first measured the average luminance of the pixels in the irises to make sure that it was the same for the human and chimpanzee pictures so that pupillary changes were equally visible in the human and chimpanzee stimuli (Mean luminance chimpanzee iris = 201 vs. Mean human iris = 212). The pictures were then edited in Adobe Photoshop to crop the eye region, remove the pupil and make them life-size. The reason for cropping the pictures was to minimize possible confounding differences in eye-gaze patterns across the two species. In addition, the average luminance of the pixels covering the whole eye region was measured for each stimulus, to be able to include these values in our statistical model. In Adobe After Effects, an artificial pupil was created which started to dilate or contract for 400 ms after 600 ms of static presentation. During the final three seconds of stimulus presentation, the pupil-size was static and smaller (40%) or larger (160%) as compared to the beginning of stimulus presentation. The size and dilation speed were based on measurements in human subjects in extreme lighting conditions (a flashlight in a dark room). We made the pupils in the stimuli start to change after 600 ms of static presentation because we wanted to create the impression in the participant that the change in the pupil-size in the stimulus was a reaction related to the observed watching him/the participant (as in a social interaction). For this same reason, we chose dynamic stimuli and not static pictures where pupils were either large or small. The stimuli were presented against a grey background, which had the same luminance as the average of all stimuli. See Figure 1.
Procedure
After successful calibration, the first trial was presented. A trial started with a fixation cross on which subjects had to focus for 250 ms, followed by a stimulus, i.e., a pair of life-size eyes. Chimpanzees were given two random trials a day, after which they were rewarded with small pieces of fruit. Trials were repeated if subjects got distracted during the presentation of a stimulus [42], [43]. Human subjects completed all trials in one session. The three juvenile chimpanzees in our sample were 11 years old and were never separated from their mothers. As always, also during the current experiment, they were in the same testing booth but did not disturb each other. If the juvenile was involved in our eye-tracking experiment, the mother was performing an unrelated, non-social task on a touch-screen in the same testing booth at a distance of approximately three meter (and vise-versa).
Experimental Design/Statistical Analyses
The experiment had a 2×2×2 design (subject species (2), looking at their own or the other species (2), with the stimulus pupils either dilating or constricting (2)).
There are multiple ways to analyze pupil-size. While the most common way is to average pupil-size over stimulus presentation time, some researchers prefer to analyze the peak amplitude [46]. Both indices are informative, but effects on the slope of the pupil response cannot be detected when pupil-size is averaged over time or when just one time-point (the peak) is selected. Fortunately, there is a more complete and precise analysis method which allows us to compare the intercept and the steepness and curvature of the slope of participant's pupil size over time. For that purpose, multi-level models have been suggested as the most appropriate analysis for any type of psychophysiological study, including pupillometry [47]. With this statistical method it is possible to include all sampled data-points in the analysis without the necessity to average over trials, time-points or even the two eyes. That way, all variance in the data is maintained, nested data is accounted for, and with the possibility to include fixed and random factors, the statistical model can be set up in a way so that it most optimally explains the variance in the data.
In order to analyze participant's pupil-size over time, we used a four-level regression model (Linear Mixed Model, implemented in SPSS) [47], [48], [49]. The multi-level structure is defined by the repeated measures, i.e., time (level 1), nested in trials (level 2), nested in eyes (level 3), nested in participants (level 4). Time was included as a repeated factor with a First-Order Autoregressive (AR1) covariance structure to control for auto-correlation with regard to time. As we were specifically interested in the effect of the stimulus pupil size on the pupil response of the subject and not in direct comparisons between human and chimpanzee stimuli or subjects, we group-centered the pupil response in order to be able to better interpret the intercept as a function of pupil mimicry. Group-centering does not affect the model fit nor does it impact on the interactions.
We first included the following fixed predictors in the model: Species Subject, Species Stimulus, Pupil-size Stimulus, Linear Term, Quadratic Term, Cubic Term and all their interactions (i.e., the factors of primary interest), but also Luminance of Stimulus, Trial and Stimulus individual (i.e., factors not of primary interest but which could potentially modulate pupil-size). As a common method, and in order to get the most parsimonious model with the best fit, non-significant effects were removed one by one, starting with the higher-order interactions. Via likelihood ratio tests, we verified whether the removal of a non-significant factor improved the fit of the model or not, or significantly decreased model fit. In the latter case, the factor was kept, otherwise it was excluded. This is the most standard model-selection procedure [47], [48], [49]. After specifying the fixed effects, model building proceeded with statistical tests of the variances of the random effects. The following random predictors were included: Subject individual, Subject individual * Stimulus Individual and Linear, Quadratic and Cubic Terms. Below we explain the procedures for the fixed and random effects separately, with all the considerations and tests that were made. The final factors that endured all these tests were kept in the final model that is shown in Table 1.
Table 1. Final model human and chimpanzee subjects.
| Type III Tests of Fixed Effects | |||||||
| Source | Numerator | Denominator df | F | Sig. | |||
| Intercept | 1 | 388.617 | 3.997 | 0.046 | |||
| Lin | 1 | 52.515 | 40.492 | 0.000 | |||
| Pupil-size Stimulus | 1 | 374.441 | 0.621 | 0.431 | |||
| Species Subject | 1 | 337.706 | 2.917 | 0.089 | |||
| Own/Other Species | 1 | 368.324 | 4.346 | 0.038 | |||
| Lin * Pupil-size Stimulus | 1 | 353.329 | 1.102 | 0.294 | |||
| Lin * Species Subject | 1 | 51.723 | 2.693 | 0.107 | |||
| Lin * Own/Other Species | 1 | 344.961 | 0.037 | 0.847 | |||
| Pupil-size Stimulus * Species Subject | 1 | 372.082 | 1.458 | 0.228 | |||
| Pupil-size Stimulus * Own/Other Species | 1 | 371.83 | 0.000 | 0.984 | |||
| Species Subject * Own/Other Species | 1 | 367.83 | 2.618 | 0.107 | |||
| Lin * Pupil-size Stimulus * Species Subject | 1 | 348.939 | 0.464 | 0.496 | |||
| Lin * Pupil-size Stimulus * Own/Other Species | 1 | 335.653 | 2.558 | 0.111 | |||
| Lin * Species Subject * Own/Other Species | 1 | 349.287 | 1.335 | 0.249 | |||
| Pupil-size Stimulus * Species Subject * Own/Other Species | 1 | 369.921 | 5.816 | 0.016 | |||
| Lin * Pupil-size Stimulus * Species Subject * Own/Other Species | 1 | 336.842 | 5.703 | 0.017 | |||
| Quad | 1 | 49.423 | 42.081 | 0.000 | |||
| Quad * Pupil-size Stimulus | 1 | 365.48 | 8.035 | 0.005 | |||
| Quad * Species Subject | 1 | 45.267 | 2.156 | 0.149 | |||
| Quad * Own/Other Species | 1 | 357.35 | 0.585 | 0.445 | |||
| Quad * Pupil-size Stimulus * Species Subject | 1 | 354.967 | 4.796 | 0.029 | |||
| Quad * Pupil-size Stimulus * Own/Other Species | 1 | 349.82 | 13.124 | 0.000 | |||
| Quad * Species Subject * Own/Other Species | 1 | 357.433 | 27.084 | 0.000 | |||
| Cub | 1 | 35.771 | 15.955 | 0.000 | |||
| Cub * Species Subject | 1 | 35.332 | 2.868 | 0.099 | |||
| Cub * Own/Other Species | 1 | 358.649 | 0.236 | 0.627 | |||
| Cub * Species Subject * Own/Other Species | 1 | 356.59 | 12.727 | 0.000 | |||
| Luminance | 1 | 367.921 | 3.902 | 0.049 |
Fixed factors
In order to capture the nonlinear change of pupil size, we included linear, quadratic and cubic terms for the time factor in the model. All these terms were significant. We included Species of Subject (human vs. chimpanzee), Species Stimulus (own vs. different species), Pupil-size Stimulus (dilating vs. constricting) in the model and as there was a four-way interaction with the Linear Term and two three-way interactions between Species Subject * Own/Other Species * Quadratic Term and Species Subject * Own/Other Species * Cubic Term respectively, all lower-order effects had to be kept in the model too.
We had four different human pictures and four different chimpanzee pictures. To test whether stimulus individual had any unforeseen effect on the higher order interactions, we created dummy codes and included them in the model. As the significance of the higher order interactions were unaffected by stimulus individual, we could omit stimulus individual as a fixed predictor from the model.
Random factors
We considered random effects of the overall intercept, overall linear, overall quadratic and overall cubic term and random effects of the intercept, linear, quadratic and cubic terms associated with each stimulus individual. All were significant which means that individual differences were present, i.e., some subjects had a larger pupil response than others or had a steeper increase, a higher peak, or the pattern showed a clearer cubic pattern than was the case for other individual subjects. The variance in the data clustered around these individual patterns and also around each unique stimulus seen by each unique individual. See Table 1 for the final model and the statistics.
Predictions
We had four à priori predictions. We predicted that (i) subjects would have a larger pupil-size when observing pupils that dilate vs. constrict in their own vs. the other species; (ii) subject's pupil-sizes would increase faster, i.e., have a steeper slope in the condition where stimulus' pupils dilate vs. constrict, especially when observing their own species; (iii) subject's pupil-response would show a greater bell-shaped curve or peak when observing stimulus' pupils that dilate vs. constrict, especially when they observe their own species. Of importance is the relative difference in participant's pupil-size/change when stimulus' pupils dilate vs. constrict and not so much changes as compared to baseline. The presentation of any new stimulus to the eye initiates sudden changes in neural activity in the visual cortex which causes initial pupil constriction. The pupils take +-6 seconds to recover which is why participant's pupil size will dilate in all our stimulus conditions.
As pupil-mimicry can have potential benefits in both species, we expected to find these interactions in both, but predicted stronger effects in humans, given their particularly communicative eyes with the most ideal morphology for detecting eye signals (iv). In statistical terms, we expected to find interactions between (i) Own/Other Species Stimulus × Pupil-size Stimulus; (ii) Own/Other Species Stimulus × Pupil-size Stimulus × Linear Term and (iii) Own/Other Species Stimulus × Pupil-size Stimulus × Quadratic Term. We finally predicted that these three interactions would be further qualified by Species Subject (iv). Below we will only report on effects that include the factor Pupil-size Stimulus. All effects are listed in Table 1.
Results
Pupil-Mimicry
There was no interaction between Own/Other Species Stimulus × Pupil-size Stimulus (i) or between Own/Other Species Stimulus × Pupil-size Stimulus × Linear term (ii) (ps >.111). However, these two interactions were both further qualified by Species Subject F = 5.703, p = .017; F = 5.816, p = .016. In line with our prediction, there was a strong interaction between Own/Other Species Stimulus × Pupil-size Stimulus × Quadratic Term F = 13.124, p<.0005 that was not further qualified by Species Subject. See Table 1.
We further investigated these effects in separate models within each species. Human subjects showed a significant interaction between Stimulus Pupil-size and Own/Other Species Stimulus F = 17.399, p<.001, indicating that their pupils synchronized with the pupils as observed in human eyes, but not in chimpanzee eyes. Interestingly, both human and chimpanzee subjects showed an interaction between the Quadratic Term, Stimulus Pupil-size and Own/Other Species Stimulus (humans F = 8.231, p = .004; chimpanzees F = 4.631, p = .034. The interaction demonstrates that both species showed a greater peak in their pupil size when observing a member of their own species with pupils that dilated vs. constricted. See Figure 2A and Table 2.
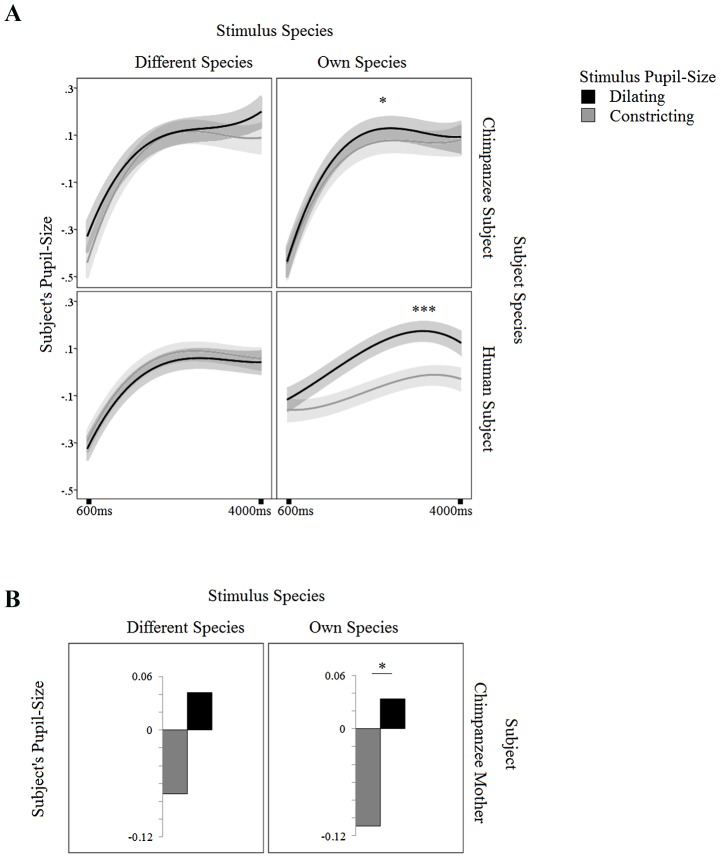
Figure 2. Pupil-Mimicry.
A. In human subjects, a strong effect of mimicry -specifically with the pupils in human eyes- was observed. The chimpanzee subjects also showed pupil-mimicry with their own group, but only in terms of the quadratic effect and not on the average pupil-size. B. The chimpanzee mothers in our sample, as humans, did show an effect on the intercept. Their pupils were larger when they observed chimpanzees with dilating vs. constricting pupils.
Table 2. Final model for humans.
| Type III Tests of Fixed Effects | |||||||
| Source | Numerator | Denom. df | F | Sig. | |||
| Intercept | 1 | 268.941 | 8.579 | 0.004 | |||
| Lin | 1 | 35.650 | 48.510 | 0.000 | |||
| Pupil-size Stimulus | 1 | 253.120 | 0.897 | 0.345 | |||
| Own/Other Species | 1 | 252.455 | 4.434 | 0.036 | |||
| Lin * Pupil-size Stimulus | 1 | 251.578 | 0.215 | 0.643 | |||
| Lin * Own/Other Species | 1 | 254.067 | 6.161 | 0.014 | |||
| Pupil-size Stimulus * Own/Other Species | 1 | 253.405 | 17.399 | 0.000 | |||
| Lin * Pupil-size Stimulus * Own/Other Species | 1 | 251.910 | 3.672 | 0.056 | |||
| Quad | 1 | 33.314 | 49.516 | 0.000 | |||
| Quad * Pupil-size Stimulus | 1 | 252.212 | 0.154 | 0.695 | |||
| Quad * Own/Other Species | 1 | 252.735 | 54.352 | 0.000 | |||
| Quad * Pupil-size Stimulus * Own/Other Species | 1 | 253.086 | 8.231 | 0.004 | |||
| Cub | 1 | 24.220 | 11.512 | 0.002 | |||
| Cub * Own/Other Species | 1 | 246.465 | 65.304 | 0.000 | |||
| Luminance | 1 | 252.461 | 8.389 | 0.004 |
Differences between Chimpanzee Individuals
Eight unique chimpanzee individuals were tested. Inspection of the individual patterns showed that the three mothers in our sample (Ai, Chloe and Pan), showed the strongest pupil-mimicry, especially when viewing their own species. When running the final chimpanzee model with just these three individuals included, a main effect of Stimulus Pupil-size was observed when viewing chimpanzees with pupils that dilated vs. constricted F = 5.455, p = .028. A trend towards a late pupil-mimicry effect was found when these three mothers observed human eyes with pupils that dilated vs. constricted F = 3.128, p = .091. See Figure 2B. See Table 3.
Table 3. Final model for chimpanzees.
| Type III Tests of Fixed Effects | |||||||
| Source | Numerator | Denom. df | F | Sig. | |||
| Intercept | 1 | 14.400 | 0.132 | 0.722 | |||
| Lin | 1 | 8.191 | 41.889 | 0.000 | |||
| Pupil-size Stimulus | 1 | 116.778 | 1.668 | 0.199 | |||
| Own/Other Species | 1 | 116.898 | 0.948 | 0.332 | |||
| Quad | 1 | 21.019 | 32.423 | 0.000 | |||
| Quad * Pupil-size Stimulus | 1 | 108.521 | 4.790 | 0.031 | |||
| Quad * Own/Other Species | 1 | 100.250 | 0.438 | 0.510 | |||
| Quad * Pupil-size Stimulus * Own/Other Species | 1 | 101.117 | 4.631 | 0.034 | |||
| Cub | 1 | 6.096 | 11.280 | 0.015 |
Eye Movements
It is possible that human observers viewed the faces differently than the chimpanzees and that that caused the difference in the pattern of the pupillary response. To rule out this explanation, we analyzed the relative looking time on the eyes, by dividing the absolute looking time on the eye-region by the absolute looking time on the whole computer screen. The statistical model was the same as the pupil-mimicry model described above, but without the three polynomials. We observed a main effect of Species Subject showing that humans attended longer to the eye region of the observed stimuli than chimpanzees (p = .007). Importantly, this was independent of Stimulus Pupil-size or Own/Other Species Stimulus.
Including looking times in the analysis of pupil-size yielded very similar results to the results discussed above. In short, there was no confounding of eye movements on species differences in pupil-mimicry.
Discussion
The findings of the current study demonstrate that both humans and chimpanzees mimic the pupil-size, especially when viewing their own species, suggesting that the sharing of focused attention at close range and the recognition and mimicry of subtle and autonomic signals such as pupil dilation enhances affiliative behavior and social bonding between conspecifics. It has been suggested that the appearance of eye-white was the starting point of linkage between two individuals on the level of eye-signals [33]. However, the current findings show that pupil-mimicry is not uniquely human but is possible in a species that has no clear visible eye-white and has less communicative eyes than humans. Nonetheless, eye-white most probably still helped observers in detecting salient and biologically relevant cues from the eyes and evolved for that exact purpose [50]. This viewpoint supports the somewhat stronger effects of mimicry in the human as compared to the chimpanzee subjects in our study.
The within-species effect on pupil-mimicry that was observed in the current study suggests that the function of pupil-mimicry may be similar to other forms of mimicry. Mimicry in general grounds positive feelings and impacts on the bond between individuals [9]. Even linkage on the physiological level with a close partner predicts satisfaction about the relationship [13]. Mimicry facilitates cooperation and occurs in mammals and birds, species with complex social networks that care for their young [51]. Previous studies have shown that social relevance is crucial for mimicry to occur [8]. In humans, it has been shown that attractiveness, friendship, and social status can enhance mimicry while social stigma, negative mood, and out-group membership can inhibit mimicry [52]. Social relevance can be directly modulated by eye contact. For example, action mimicry decreases when the gaze of the observed is averted [53], [54]. The relevance of an eye-signal is also dependent on whom is signaling it, whether it is someone familiar or unfamiliar and as we show here, whether the observed belongs to one's own species as compared to another species. From evolutionary perspective, it is more adaptive to establish and maintain social bonds with members of your own species than with members of a different species because there is a higher probability of (future) contact with in-group members. Therefore, mimicry with one's own species is more relevant than mimicry with members of another species. Relevance may also differ between individuals within a group. We here observed that all three chimpanzee mothers synchronized specifically with chimpanzees. For the survival of their young, chimpanzee mothers (and human mothers alike although we did not have them in our sample) strive after stable relationships and seek support and protection from group members, possibly especially with synchronizing partners. It could be that especially the chimpanzee mothers, by great experience learned to make use of pupil-synchronization. How, in terms of neural mechanisms, pupil-synchronization is regulated and how factors such as experience may impact upon it is uncertain but the amygdala is likely to play an important role.
The amygdala responds strongly to socially relevant cues of conspecifics. This area signals when being confronted with an out-group member [55], processes emotions [56] and detects changes in anothers pupil size [57]. The ability to quickly respond to such salient cues has been attributed to an evolutionary old subcortical route for processing of emotional information [59], [60]. Previous research with blind-sight patients has shown that the mimicry of facial expressions is possible via this subcortical route. Patients that could not consciously perceive presented emotional expressions, did unconsciously and very rapidly synchronize their facial expression [61]. In the current study, subjects' pupil-size very rapidly adapted to the observed pupil-size. This new finding, together with previous findings suggesting that the amygdala might regulate pupil-mimicry [18], [19], suggests the involvement of a sub-cortical pathway [60]. Two possible routes to pupil-mimicry might be via the superior culliculi, the pulvinar, and the amygdala, which projects to different brain stem areas that induce pupil mimicry [20], [58], or from the superior colliculus directly to the brainstem nuclei that subsequently project to the amygdala [62]. With the low temporal resolution of fMRI, it is difficult to get direct insight into this ancient route although it might solve part of the puzzle. Studying pupil-mimicry in blind-sight patients could provide further understanding in the neural underpinnings and more specifically about the role of the subcortical route.
Our study has several limitations. First of all, in real life, ambient light changes constantly and therefore the real-life impact of pupil-mimicry remains unknown. That brings us to the second limitation, which is the ecological validity of the set-up of this study. It is possible that chimpanzees show stronger pupil-mimicry during real-world social interactions with familiar others that have direct consequences for them. Studying two individuals at the same time during an interaction is an interesting future avenue [63]. We found not only stronger pupil-mimicry effects in the human sample, but also a greater difference between mimicking ones own vs. the other species in human as compared to chimpanzee subjects. It is possible that stronger effects would have been found in the chimpanzees had they been less familiarized to humans or had they been brought into a competitive setting. Moreover, whether within vs. cross-species effects translate to in-group/out-group differentiation as described in for example the facial mimicry literature, is uncertain. Future studies in humans with different in-group/out-group manipulations can give more insight into this question.
Evolutionary theory implies that the propensity to mimic pupil-size should be especially adaptive within groups. In line with this assumption, pupil-mimicry is shared among humans and chimpanzees and is stronger during interactions with members of one's own species than during interactions with members of the other species. Humans most likely evolved their communicative eyes with clear eye-white and fine musculature precisely because it benefits within-group interactions, survival, and prosperity.
Acknowledgments
We thank statisticians C. Dolan and H. Huizenga for their help with the analyses and C.K.W. de Dreu, A. H. Fischer and S. Shalvi for comments on the manuscript. Thanks are also due to the staff of Language and Intelligence Section and the Center for Human Evolution Modeling Research of the Primate Research Institute, Kyoto University for their support of the study and care of chimpanzees. Special thanks go to F. Kano who was involved in the data collection and with whom we discussed previous versions of this manuscript.
Data Availability
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper.
Funding Statement
This work was supported by the Japan Society for the Promotion of Science (pE10084 and BR120105) and the Dobberke Foundation for Comparative Psychology Koningklijke Nederlandse Akademie van Wetenschappen (UPS/BP/5215) awarded to MEK and JSPS Grants in Aid for Scientific Research to MT (#23220006) and TM (#20002001, #24000001). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Parr LA, Waller BM (2006) Understanding chimpanzee facial expression: insights into the evolution of communication. Soc Cogn Affect Neurosci 1: 221–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Mussweiler T, Ockenfels A (2013) Similarity increases altruistic punishment in humans. Proc Natl Acad Sci U S A 110: 19318–19323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hatfield E, Cacioppo JT, Rapson RL (1993) Emotional contagion. Cur Dir Psychol Sci 2: 96–100. [Google Scholar]
- 4. Haun DB, Call J (2008) Imitation recognition in great apes. Curr Biol 18: R288–290. [DOI] [PubMed] [Google Scholar]
- 5.Carruthers P, Smith PK (1996) Theories of theories of mind. Camebridge, UK: University Press.
- 6. Lipps T (1905) Das Wissen von fremden Ichen. Psychologische Untersuchungen 4: 694–722. [Google Scholar]
- 7. Chartrand TL, Lakin JL (2013) The antecedents and consequences of human behavioral mimicry. Annu Rev Psychol 64: 285–308. [DOI] [PubMed] [Google Scholar]
- 8. Hess U, Fischer A (2013) Emotional mimicry as social regulation. Pers Soc Psychol Rev 17: 142–157. [DOI] [PubMed] [Google Scholar]
- 9. van Baaren RB, Holland RW, Kawakami K, van Knippenberg A (2004) Mimicry and prosocial behavior. Psychol Sci 15: 71–74. [DOI] [PubMed] [Google Scholar]
- 10. Chartrand TL, Bargh JA (1999) The chameleon effect: the perception-behavior link and social interaction. J Pers Soc Psychol 76: 893–910. [DOI] [PubMed] [Google Scholar]
- 11. Dimberg U (1982) Facial reactions to facial expressions. Psychophysiology 19: 643–647. [DOI] [PubMed] [Google Scholar]
- 12. Feldman R, Magori-Cohen R, Galili G, Singer M, Louzoun Y (2011) Mother and infant coordinate heart rhythms through episodes of interaction synchrony. Infant Behav Dev 34: 569–577. [DOI] [PubMed] [Google Scholar]
- 13. Levenson RW, Gottman JM (1983) Marital interaction: physiological linkage and affective exchange. J Pers Soc Psychol 45: 587–597. [DOI] [PubMed] [Google Scholar]
- 14. Anders S, Heinzle J, Weiskopf N, Ethofer T, Haynes JD (2011) Flow of affective information between communicating brains. NeuroImage 54: 439–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Nakano T, Yamamoto Y, Kitajo K, Takahashi T, Kitazawa S (2009) Synchronization of spontaneous eyeblinks while viewing video stories. Proc Biol Sci 276: 3635–3644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hess EH (1975) The role of pupil size in communication. Scientific American 233: : 110–112, 116–119. [DOI] [PubMed] [Google Scholar]
- 17.Simms MS (1967) Pupillary response of male and female subjects to pupillary difference in male and female picture stimuli. Percep Psychophys 2.
- 18. Harrison NA, Gray MA, Critchley HD (2009) Dynamic pupillary exchange engages brain regions encoding social salience. Soc Neurosci 4: 233–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Harrison NA, Singer T, Rotshtein P, Dolan RJ, Critchley HD (2006) Pupillary contagion: central mechanisms engaged in sadness processing. Soc Cogn Affect Neurosci 1: 5–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Moore RY, Bloom FE (1979) Central catecholamine neuron systems: anatomy and physiology of the norepinephrine and epinephrine systems. Annu Rev Neurosci 2: 113–168. [DOI] [PubMed] [Google Scholar]
- 21. Hess EH, Polt JM (1960) Pupil size as related to interest value of visual stimuli. Science 132: 349–350. [DOI] [PubMed] [Google Scholar]
- 22.Bowles SG H (2011) A cooperative species. Human reciprocity and its evolution. Princeton, New Jersey: Princeton Universiy Press.
- 23. Norscia I, Palagi E (2011) Yawn contagion and empathy in Homo sapiens. PLoS ONE 6: e28472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bourgeois P, Hess U (2008) The impact of social context on mimicry. Biol Psychol 77: 343–352. [DOI] [PubMed] [Google Scholar]
- 25. van der Schalk J, Fischer A, Doosje B, Wigboldus D, Hawk S, et al. (2011) Convergent and divergent responses to emotional displays of ingroup and outgroup. Emotion 11: 286–298. [DOI] [PubMed] [Google Scholar]
- 26. Helm JL, Sbarra D, Ferrer E (2012) Assessing cross-partner associations in physiological responses via coupled oscillator models. Emotion 12: 748–762. [DOI] [PubMed] [Google Scholar]
- 27. Reed RG, Randall AK, Post JH, Butler EA (2013) Partner influence and in-phase versus anti-phase physiological linkage in romantic couples. Int J Psychophysiol 88: 309–316. [DOI] [PubMed] [Google Scholar]
- 28. Massen JJ, Vermunt DA, Sterck EH (2012) Male yawning is more contagious than female yawning among chimpanzees (Pan troglodytes). PLoS ONE 7: e40697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Campbell MW, de Waal FB (2014) Chimpanzees empathize with group mates and humans, but not with baboons or unfamiliar chimpanzees. Proc Biol Sci 281: 20140013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Emery NJ (2000) The eyes have it: the neuroethology, function and evolution of social gaze. Neuroscience Biobehavioral Review 24: 581–604. [DOI] [PubMed] [Google Scholar]
- 31. Farroni T, Csibra G, Simion F, Johnson MH (2002) Eye contact detection in humans from birth. Proc Natl Acad Sci U S A 99: 9602–9605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kobayashi H, Kohshima S (1997) Unique morphology of the human eye. Nature 387: 767–768. [DOI] [PubMed] [Google Scholar]
- 33. Lee DH, Susskind JM, Anderson AK (2013) Social transmission of the sensory benefits of eye widening in fear expressions. Psychol Sci 24: 957–965. [DOI] [PubMed] [Google Scholar]
- 34. Tomasello M, Hare B, Lehmann H, Call J (2007) Reliance on head versus eyes in the gaze following of great apes and human infants: the cooperative eye hypothesis. J Hum Evol 52: 314–320. [DOI] [PubMed] [Google Scholar]
- 35. Iriki A, Tanaka M, Iwamura Y (1996) Attention-induced neuronal activity in the monkey somatosensory cortex revealed by pupillometrics. Neurosci Res 25: 173–181. [DOI] [PubMed] [Google Scholar]
- 36. Davila-Ross M, Allcock B, Thomas C, Bard KA (2011) Aping expressions? Chimpanzees produce distinct laugh types when responding to laughter of others. Emotion 11: 1013–1020. [DOI] [PubMed] [Google Scholar]
- 37. Anderson JR, Myowa-Yamakoshi M, Matsuzawa T (2004) Contagious yawning in chimpanzees. Proc Biol Sci 271 Suppl 6S468–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Campbell MW, de Waal FB (2011) Ingroup-outgroup bias in contagious yawning by chimpanzees supports link to empathy. PLoS ONE 6: e18283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Hattori Y, Tomonaga M, Matsuzawa T (2013) Spontaneous synchronized tapping to an auditory rhythm in a chimpanzee. Sci Rep 3: 1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Matsuzawa T (1990) Form perception and visual acuity in a chimpanzee. Folia Primatol (Basel) 55: 24–32. [DOI] [PubMed] [Google Scholar]
- 41. May PJ, Sun W, Erichsen JT (2008) Defining the pupillary component of the perioculomotor preganglionic population within a unitary primate Edinger-Westphal nucleus. Prog Brain Res 171: 97–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kano F, Tomonaga M (2010) Face scanning in chimpanzees and humans: Continuity and discontinuity. Animal Behaviour 79: 227–235. [Google Scholar]
- 43. Hattori Y, Kano F, Tomonaga M (2010) Differential sensitivity to conspecific and allospecific cues in chimpanzees and humans: a comparative eye-tracking study. Biol Lett 6: 610–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Tomonaga M (2007) Is chimpanzee (Pan troglodytes) spatial attention reflexively triggered by gaze cue? J Comp Psychol 121: 156–170. [DOI] [PubMed] [Google Scholar]
- 45. Tomonaga M, Imura T (2010) Visual search for human gaze direction by a Chimpanzee (Pan troglodytes). PLoS ONE 5: e9131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. van Steenbergen H, Band GP (2013) Pupil dilation in the Simon task as a marker of conflict processing. Front Hum Neurosci 7: 215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Bagiella E, Sloan RP, Heitjan DF (2000) Mixed-effects models in psychophysiology. Psychophysiology 37: 13–20. [PubMed] [Google Scholar]
- 48. Mirman D, Dixon JA, Magnuson JS (2008) Statistical and computational models of the visual world paradigm: Growth curves and individual differences. J Mem Lang 59: 475–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.West BT, Welch KB, Galecki AT (2006) Linear Mixed Models: A Practical Guide Using Statistical Software: Chapman and Hall/CRC.
- 50. Whalen PJ, Kagan J, Cook RG, Davis FC, Kim H, et al. (2004) Human amygdala responsivity to masked fearful eye whites. Science 306: 2061. [DOI] [PubMed] [Google Scholar]
- 51.de Waal FB (2013). The bonobo and the atheist: In search of humanism among the primates. 1 ed. New York: W.W. Norton & Company. pp.141–156.
- 52. van Baaren R, Janssen L, Chartrand TL, Dijksterhuis A (2009) Where is the love? The social aspects of mimicry. Philos Trans R Soc Lond B Biol Sci 364: 2381–2389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Kilner JM, Marchant JL, Frith CD (2006) Modulation of the mirror system by social relevance. Soc Cogn Affect Neurosci 1: 143–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Wang Y, Ramsey R, Hamilton AF (2011) The control of mimicry by eye contact is mediated by medial prefrontal cortex. J Neurosci 31: 12001–12010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Hart AJ, Whalen PJ, Shin LM, McInerney SC, Fischer H, et al. (2000) Differential response in the human amygdala to racial outgroup vs ingroup face stimuli. NeuroReport 11: 2351–2355. [DOI] [PubMed] [Google Scholar]
- 56. Adolphs R, Tranel D, Damasio H, Damasio A (1994) Impaired recognition of emotion in facial expressions following bilateral damage to the human amygdala. Nature 372: 669–672. [DOI] [PubMed] [Google Scholar]
- 57. Demos KE, Kelley WM, Ryan SL, Davis FC, Whalen PJ (2008) Human amygdala sensitivity to the pupil size of others. Cereb Cortex 18: 2729–2734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Gilzenrat MS, Nieuwenhuis S, Jepma M, Cohen JD (2010) Pupil diameter tracks changes in control state predicted by the adaptive gain theory of locus coeruleus function. Cogn Affect Behav Neurosci 10: 252–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Vuilleumier P, Armony JL, Driver J, Dolan RJ (2003) Distinct spatial frequency sensitivities for processing faces and emotional expressions. Nat Neurosci 6: 624–631. [DOI] [PubMed] [Google Scholar]
- 60. Tamietto M, de Gelder B (2010) Neural bases of the non-conscious perception of emotional signals. Nat Rev Neurosci 11: 697–709. [DOI] [PubMed] [Google Scholar]
- 61. Tamietto M, Castelli L, Vighetti S, Perozzo P, Geminiani G, et al. (2009) Unseen facial and bodily expressions trigger fast emotional reactions. Proc Natl Acad Sci U S A 106: 17661–17666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Tamietto M, Cauda F, Corazzini LL, Savazzi S, Marzi CA, et al. (2010) Collicular vision guides nonconscious behavior. J Cogn Neurosci 22: 888–902. [DOI] [PubMed] [Google Scholar]
- 63. Hasson U, Ghazanfar AA, Galantucci B, Garrod S, Keysers C (2012) Brain-to-brain coupling: a mechanism for creating and sharing a social world. Trends Cogn Sci 16: 114–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper.