Abstract
PA-824 is a bicyclic 4-nitroimidazole, currently in phase II clinical trials for the treatment of tuberculosis. Dose fractionation pharmacokinetic-pharmacodynamic studies in mice indicated that the driver of PA-824 in vivo efficacy is the time during which the free drug concentrations in plasma are above the MIC (fT>MIC). In this study, a panel of closely related potent bicyclic 4-nitroimidazoles was profiled in both in vivo PK and efficacy studies. In an established murine TB model, the efficacy of diverse nitroimidazole analogs ranged between 0.5 and 2.3 log CFU reduction compared to untreated controls. Further, a retrospective analysis was performed for a set of seven nitroimidazole analogs to identify the PK parameters that correlate with in vivo efficacy. Our findings show that the in vivo efficacy of bicyclic 4-nitroimidazoles correlated better with lung PK than with plasma PK. Further, nitroimidazole analogs with moderate-to-high volume of distribution and Lung to plasma ratios of >2 showed good efficacy. Among all the PK-PD indices, total lung T>MIC correlated the best with in vivo efficacy (rs = 0.88) followed by lung Cmax/MIC and AUC/MIC. Thus, lung drug distribution studies could potentially be exploited to guide the selection of compounds for efficacy studies, thereby accelerating the drug discovery efforts in finding new nitroimidazole analogs.
Introduction
Every year nearly 8 million new cases of Tuberculosis (TB) are reported globally resulting in 1.4 million deaths [1]. Poor treatment compliance – due to the requirement for prolonged multidrug therapy – as well as the use of inadequate regimens has fueled the emergence of multi-drug-resistant and extensively-drug-resistant (MTD-TB and XDR-TB) TB strains. MDR-TB is resistant to at least isoniazid and rifampicin and XDR-TB is resistant to isoniazid, rifampicin, fluoroquinolones and at least one of the injectables [2]. TB control programs are further complicated in settings where the incidence of co-infection with HIV is high because drug-drug interactions with anti-retroviral therapy are difficult to avoid [2], [3]. Hence there is an urgent need to discover new TB drugs active against drug-resistant forms of TB and compatible with treatment against HIV.
PA-824 [4] and OPC-67683 [5] are two bicyclic 4-nitroimidazoles currently in phase II clinical trials, representing a promising new class of therapeutics for TB [6]. Preclinical testing of PA-824 showed bactericidal activity in various in vitro and in vivo models [7], . PA-824 was shown to be well tolerated in healthy subjects, following oral daily doses for 7 days [9]. These results, combined with the demonstrated activity of PA-824 against drug-sensitive and multidrug-resistant Mtb, supported the progression of this compound and its evaluation as a novel treatment against TB. An early bactericidal activity (EBA) study of PA-824 revealed a lack of dose response between 200 and 1200 mg administered daily for 14 days [10]. Dose-fractionation PK-PD studies in mice showed the PK-PD driver of PA-824 to be the time during which the free drug concentrations in plasma were above the MIC (fT>MIC) [11]. In retrospect, clinical investigators established that fT>MIC was 100% at all doses between 200 and 1200 mg daily. An additional phase II trial between 50 and 200 mg was undertaken to establish the lowest efficacious dose [12]. 200 mg of PA-824 was found to be efficacious and used in combination with other anti-TB drugs [13].
Physico-chemical properties, in vitro potency, in vitro and in vivo pharmacokinetics (PK) are critical determinants for in vivo efficacy. PA-824 is highly lipophilic and exhibits poor aqueous solubility. To overcome the limitation of its low solubility and improve its oral bioavailability, a cyclodextrin formulation was developed and used for in vivo animal efficacy studies [4], [7]. Extensive lead optimization efforts were undertaken to improve aqueous solubility, metabolic stability, in vitro potency and in vivo efficacy of anti-tubercular nitroimidazoles and various analogs were synthesized [5], [14]–[23]. Comprehensive in vivo pharmacology studies are generally resource and time intensive. This is particularly true for TB because of the slow rate of M. tuberculosis growth, lengthy treatment duration and requirement of high containment facility. In this study, a panel of closely related potent bicyclic 4-nitroimidazoles (NI) was profiled both in vitro and in vivo. The data is retrospectively analyzed to identify the PK parameters that correlate with in vivo efficacy of a series of bicyclic 4-nitroimidazoles. The results of this investigation showed that PK properties such as volume of distribution and lung exposure predicts in vivo efficacy of bicyclic 4-nitroimidazoes better than other PK parameters. Thus, in vitro potency and lung PK could be used as surrogate to guide the prioritization of new pre-clinical candidates for lengthy efficacy studies, there by expediting drug discovery.
Materials and Methods
Bacteria, culture conditions and chemicals
M. tuberculosis (Mtb) (H37Rv, ATCC 27294) culture conditions have been described previously [16]. Synthesis of PA-824, Amino-824, Aminoethyl-824, NI-135, NI-147 and NI-136 have been previously reported [16], [20]. Other NI analogs NI-622, NI-644, NI-176, NI-269, NI-182, NI-145, NI-297 and NI-302 have been described in two patents [14], [18] and synthesis of these compounds to be described elsewhere. All solutions were made as 20 mM stocks in DMSO. Hydroxypropyl-β-cyclodextrin, Lecithin, granular was purchased from Acros/Organics, New Jersey, and USA. Minimum Inhibitory concentration (MIC99) against wild type Mtb H37Rv and cofactor F420 deficient (FbiC mutant) [24] was determined by the broth dilution method as described earlier [16].
In vitro physico-chemical properties
In vitro physicochemical and PK parameters like solubility, log P, PAMPA, Caco-2 permeability and mouse plasma protein binding were determined in-house in medium to high throughput format assays. Briefly, solubility was measured using a high throughput equilibrium-solubility (HT-Eq sol) assay using a novel miniaturized shake-flask approach and streamlined HPLC analysis [25]; lipophilicity determination was carried out in 96-well micro titer plates and the diffusion of compounds between two aqueous compartments separated by a thin octanol liquid layer was measured [26]; PAMPA permeability experiments were carried out in 96-well micro titer filter plates at absorption wavelengths between 260 and 290 nm [27]; Caco-2 assay was carried out in a 96-well format, and compound concentrations in each chamber were measured by LC/MS as described previously [28] and plasma protein binding was determined in mouse plasma using an ultra-filtration method [29].
Ethics Statement
All animal experimental protocols (protocol #023/2009 and #025/2009 for PK; protocol #004/2010 for efficacy) involving mice were reviewed and approved by the Institutional Animal Care and Use Committee (IACUC) of Novartis Institute for Tropical Diseases (NITD). The animal research complied with Singapore Animal Veterinary Authority and global Novartis policy on the care and use of animals. Experimental and control animals infected with Mtb were euthanized at the end of the experiment. All procedures during pharmacokinetic experiments were performed under isoflurane anesthesia and all efforts were made to minimize suffering.
In vivo pharmacokinetic (PK) studies
Female CD-1 mice obtained from Biological Resource Center in Singapore were used for in vivo PK studies. Mice were acclimatized before initiation of pharmacokinetic (PK) experiments. Feed and water were given ad libitum. The compounds were formulated at a concentration of 1, 2.5 or 5 mg/mL for a dose of 10 mg/kg (Amino-824, AminoEthyl-824) or 25 mg/kg (PA-824, NI-135, NI-147, NI-136, NI-176, NI-269, NI-182, NI-145, NI-297 and NI-302) or 50 mg/kg (NI-622 and NI-644) given orally and at 1 or 2 mg/mL concentration for a dose of 5 or 10 mg/kg given intravenously. The CM-2 formulations were prepared in 10% w/v hydroxypropyl-β-cyclodextrin and 10% v/v lecithin in water as described earlier [7], [8]. The formulation was centrifuged and the supernatant was collected for intravenous administration. After oral dosing, blood and lung samples from mice were collected at various time points ranging from 0.08 hrs (but 0.02 hrs for i.v dosing) to 48 hrs. Groups of three mice were used for each time point. Blood was centrifuged at 13,000 rpm for 7 min at 4°C, plasma was harvested and stored at –20°C until analysis. Lung tissue samples were excised, dipped in PBS, gently blotted with absorbent paper, dried, weighed and stored at –20°C until further analysis.
For LC/MS/MS analysis, 50 µL of plasma samples were precipitated using 400 µL of acetonitrile:methanol:acetic acid (90∶9.8∶0.2) containing 500 ng/mL of either related compound or warfarin as internal standard. After vortexing and centrifuging the mixture, the supernatant was removed and 5 µL of sample analyzed. Whole lung tissue was homogenized in 2 mL of PBS. 50 µL of lung homogenate was taken and processed as described above for plasma samples. The standard calibration curve was prepared by spiking blank plasma and lung tissue with different concentrations of the compound. In addition, quality control samples with three different concentrations were prepared in respective blank matrix and analyzed together with the unknown samples for validation purposes. Analyte quantitation was performed by LC/MS/MS using optimized conditions for each compound. Liquid chromatography was performed using an Agilent 1100 HPLC system (Santa Clara, CA), with the Agilent Zorbax XDB Phenyl (3.5 µ, 4.6×75 mm) column at an oven temperature of 35°C and 45°C, coupled with a triple quadruple mass spectrometer (Applied Biosystems, Foster City, CA). Instrument control and data acquisition were performed using Applied Biosystems software Analyst 1.4.2. The mobile phases used were A: water-acetic acid (99.8∶0.2, v/v) and B either as: acetonitrile-acetic acid (99.8∶0.2, v/v) or methanol-acetic acid (99.8∶0.2, v/v), using a gradient, with a flow rate of 1.0 mL/min, and a run time of 6 to 8 min. Under these conditions the retention times of various compounds ranged between 3.2 and 6.5 minutes. Multiple reaction monitoring (MRM) was combined with optimized mass spectrometry parameters to maximize detection specificity and sensitivity. Most of the compounds were analyzed using electrospray ionization in the positive mode. The recovery of the compound from both plasma and lung tissue were good and consistent across the concentration range studied. The lower limit of quantification for different compounds ranged between 1 and 49 ng/mL in plasma and 1 and 132 ng/g in lungs. Calibration curve was prepared freshly and analyzed with every set of study samples. Intraday variability was established with triplicate quality control samples at three concentration levels. The results were accepted if relative standard deviation was less than 15%.
Mean values of compound concentrations in plasma and lungs were obtained from three animals at each time point and plotted against time to generate concentration-time profiles. PK parameters were determined using Phoenix WinNonlin, version 6.3 (Pharsight, A Certara company, USA, www.pharsight.com), by non-compartmental modeling using built-in model (200–202) for extravascular and intravenous bolus dosing. The oral bioavailability (F) was calculated as the ratio between the area under the curve (AUCinf) following oral administration and the AUCinf following intravenous administration corrected for dose (F = AUCp.o*dosei.v./AUCi.v*dosep.o).
In vivo mouse efficacy studies
In vivo mouse efficacy studies were determined after intranasal infection of Balb/c mice with 103 cfu Mtb H37Rv. Treatment was initiated 4 weeks after infection. Compounds were orally administered in CM-2 formulation for 4 weeks daily. Bacterial loads were determined at 2 and 4 weeks post treatment [30]. Statistical analysis was done by a one-way analysis of variance, followed by a multiple comparison analysis of variance by a one-way Tukey test (GraphPad Prism, version 5.02, San Diago, California USA, www.graphpad.com). Differences were considered statistically significant at the 95% level of confidence [7].
Calculation of PK-PD parameters
The MIC against Mtb was used to calculate PK-PD indices (Cmax/MIC, AUC/MIC and T>MIC). The Cmax/MIC was defined as the ratio of peak plasma concentration (Cmax) to the MIC, the AUC/MIC was defined as the ratio of the AUC0–24 to the MIC, and the time above MIC (T>MIC) was defined as 24 h period during which the total compound concentration exceeded the MIC. Cmax/MIC and AUC/MIC were calculated as ratios from PK parameter obtained from non-compartmental analysis and MIC. T>MIC were derived from Phoenix WinNonlin software by specifying MIC as therapeutic response and time above therapeutic response was obtained. Using plasma protein binding, unbound concentrations in plasma were calculated, PK parameters were derived from Phoenix WinNonlin and PK-PD indices were defined as fCmax/MIC, fAUC/MIC and % fT>MIC where ‘f’ refers to free concentration. For calculation and plotting of mean concentration-time curve, concentrations indicated as below the lower limit of quantification (LLOQ) were replaced by 0.5*LLOQ. Ignoring the values here would impact some of the PK-PD parameters. This approach has no impact on pharmacokinetic parameter calculations [31].
PK-PD analysis
PK-PD indices were estimated from the plasma and lung drug concentrations, in vitro potency and plasma protein binding. A Spearman’s rank correlation [32], [33] was run to determine the relationship between various PK parameters and mean log CFU reduction using Prism software (GraphPad Prism, version 5.02, San Diago, California USA, www.graphpad.com).
Results
In vitro potency and physico-chemical properties
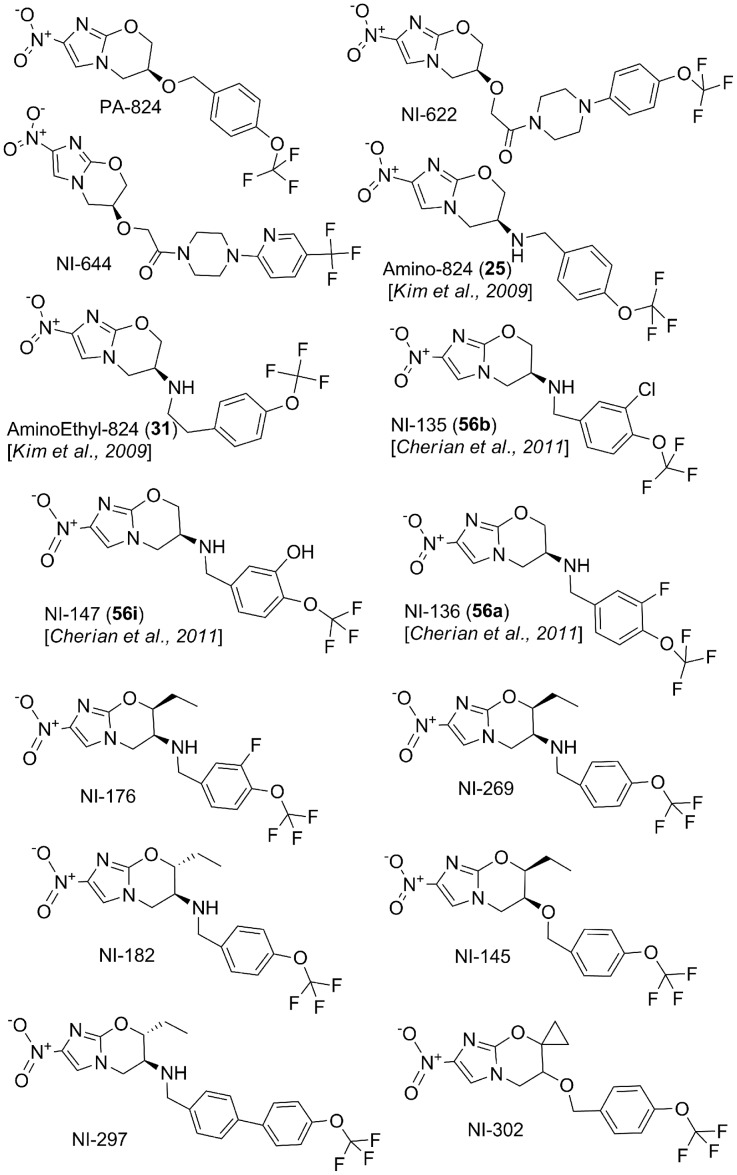
In an effort to improve the solubility and potency of PA-824, diverse structural analogs of PA-824 were synthesized and their in vitro activities reported [14], [16], [18]–[20]. A few potent bicyclic 4-nitroimidazole analogs were selected and characterized in detail (Figure 1). In vitro Mtb potency and physico-chemical properties of these nitroimidazole analogs are summarized in Table 1. All the NI analogs studied showed Mtb specific growth inhibitory activity and no cytotoxicity was observed in THP1 macrophage cell lines (Table 1). F420 deficient (FbiC) mutants were resistant to all these bicyclic 4-nitroimidazoles analogs (Table 1), suggesting a mechanism of action similar to PA-824, involving F420-dependent bio-activation [24]. Modifications on the benzyl ring (NI-135, NI-147, NI-136 and NI-176), and oxazine ring (NI-269, NI-182, NI-145, NI-297, NI-302 and NI-176) showed significant improvement of in vitro potency compared to PA-824. The nitroimidazole (NI) analogs tested in this study displayed a wide range of solubility (<2 to 127 µg/mL). Amino-nitroimidazoles showed improved solubility compared to their respective benzyl ether analogs (Amino-824 vs. PA-824 and NI-269 vs. NI-145). NI-297, a biphenyl derivative of NI-182, had very poor aqueous solubility (<2 µg/mL) due to its high lipophilicity (logP 6). In general, the logP of all the other NI derivatives ranged between 2.4 and 3.8 and all showed high permeability except NI-644, which had moderate permeability in the Caco-2 assay. Overall the compounds exhibited moderate-to-high mouse plasma protein binding (80 to 98%), except for Aminoethyl-824, which showed the lowest binding (45%).
Figure 1. Chemical structures of bicyclic 4-nitroimidazole analogs used in this study.
Table 1. In vitro potency and physicochemical properties for bicyclic 4-nitroimidazole analogs.
| Compound ID | H37Rv MIC99(mg/L) | H37Rv::F420-mutantsMIC99 (mg/L) | CytotoxicityCC50 (mg/L) | SolubilitypH 6.8(µg/mL) | Log P | PAMPA(Log Pecm/sec, %FA) | CaCo-2(Papp, 10−6 cm/sec) | CaCo-2(Efflux ratio) | Mice PPB (%) | ||
| A-B | B-A | B-A/A-B | |||||||||
| PA-824 | 0.30 | >10 | >10 | 13.0 | 2.75 | –4.2, 99 | 27.6 | 20.4 | 0.8 | 90 | |
| NI-622 | 0.18 | >10 | >10 | 28.2 | 2.9 | –5.3, 31 | 9.5 | 12.4 | 1.3 | 97 | |
| NI-644 | 0.09 | >10 | >10 | 14.0 | 2.6 | –4.9, 83 | 2.8 | 13.7 | 4.8 | 90 | |
| Amino-824 | 0.14 | >10 | >10 | 127 | 2.5 | –4.8, 91 | 35.4 | 21.0 | 0.6 | 80 | |
| AminoEthyl-824 | 0.06 | >10 | >10 | 104 | 3.6 | –4.6, 85 | 18.6 | 20.7 | 1.1 | 45 | |
| NI-135 | 0.03 | >10 | >10 | 77.0 | 3 | –4.5, 90 | 23.4 | 15.3 | 0.6 | 91.5 | |
| NI-147 | 0.03 | >10 | nd | 56.0 | 2.4 | –5.2, 43 | nd | nd | |||
| NI-136 | 0.03 | >10 | >10 | 43.0 | 2.6 | –4.7, 83 | 15.4 | 12.6 | 0.8 | 82.7 | |
| NI-176 | 0.03 | >10 | >10 | 8.0 | 3.5 | –3.7, 99 | nd | nd | |||
| NI-269 | 0.03 | >10 | >10 | 12.0 | 3.15 | –3.7, 99 | nd | nd | |||
| NI-182 | 0.02 | >10 | >10 | 32.0 | 3.05 | –3.8, 99 | 18.3 | 13.9 | 0.8 | 91.3 | |
| NI-145 | 0.06 | >10 | >10 | <2.0 | 3.85 | –3.85, 99 | nd | nd | |||
| NI-297 | 0.02 | >10 | >10 | <2.0 | 6 | –4.1, 97 | nd | 98.2 | |||
| NI-302 | 0.03 | >10 | >10 | 26.0 | 3.2 | –3.6, 99 | 45.1 | 18.7 | 0.4 | 95.7 | |
MIC99 = Minimum Inhibitory Concentration required to reduce the bacterial growth by 99%, MIC against both H37Rv (wild type) and F420 deficient (FbiC) mutants were tested. PAMPA = Parallel Artificial Membrane Permeability Assay, CaCo-2 = Permeability using colon carcinoma cell lines, PPB = Plasma Protein Binding, Pe = effective permeability, FA = fraction absorbed, Papp = apparent permeability, A-B = Apical to Basolateral, B-A = Basolateral to Apical, nd = not determined, CC50 = Cytotoxicity against THP1 macrophage cell lines was determined as described previously [76], with Puromycin as positive control (CC50 = 1.4 mg/L).
In vivo plasma PK properties
Each compound was subjected to intravenous and oral mouse PK in CM-2 formulation. The total compound concentration in mouse plasma was measured and free plasma concentrations were calculated using in vitro plasma protein binding (Table 1 and 2). NI-147 with a hydroxyl functional group on the benzyl ring, displayed markedly inferior PK properties (Table 2, Figure S1 in File S1). The poor PK is likely due to glucuronidation of the hydroxyl group as suggested by the presence of an extra peak corresponding to +176 Da in the mass spectrometry analysis. Hence NI-147 was not included in in vivo mouse efficacy studies. The NI analogs displayed a wide range of volume of distribution (Vss = 0.7 to 4.2 L/kg) corresponding to 1.1 to 7 times total body water. NI-135 showed higher Vss (4.2 L/kg), followed by NI-182, NI-297 and NI-269 (2.6 to 3 L/kg). All other analogs showed moderate Vss similar to PA-824, except for NI-644, which showed a low volume of distribution (Vss = 0.7 L/kg). The total systemic clearance was low to moderate (4 to 44 mL/min/kg) corresponding to 5 to 49% of hepatic blood flow. NI-135 and AminoEhtyl-824 showed moderate clearance (41 and 44 mL/min/kg respectively). All other analogs showed clearance similar to PA-824 (10 to 25 mL/min/kg), except for NI-297 and NI-302, which showed very low clearance (5 mL/min/kg). The elimination half-life ranged between 0.7 h and 6.7 h for the NI analogs studied. NI-297, NI-135 and NI-302 showed long half-life (3.7 to 6.7 h). All other analogs showed half-life similar to PA-824 (1.3 to 2.8 h), except for NI-644 and AminoEthyl-824, which showed short half-life (<1 h). Generally, the NI analogs at comparable doses displayed rapid absorption (Tmax, 0.3 to 4 h), except for NI-297, which showed delayed absorption with a Tmax of 8 h, possibly due to its higher lipophilicity and lower solubility. The peak plasma concentration (Cmax) ranged between 1.2 µg/mL and 12.9 µg/mL and exposure (AUC) ranged between 4.8 µg.h/mL and 144 µg.h/mL (Table 2). NI-135 had significantly lower plasma Cmax, exposure and oral bioavailability compared to PA-824, likely due to its three times higher in vivo clearance combined with higher volume of distribution. On the contrary, NI-302 and NI-297 had higher systemic plasma exposure mostly due to decreased in vivo clearance (Table 2, Figure S1 in File S1). At comparable dose, NI-622 and NI-644 showed similar plasma Cmax, exposure and oral bioavailability compared to PA-824. All other NI analogs showed moderate plasma exposure and oral bioavailability (64 to 88%) except for NI-135, NI-297 and NI-302. Despite, NI analogs displayed varied aqueous solubility (<2 to 127 ug/mL), in CM2 formulation all the analogs showed moderate to high oral bioavailability (Table 2). Interestingly, NI-145 and NI-297 had least solubility (<2 µg/mL), in CM-2 formulation both compounds showed good oral bioavailability. The use of CM-2 (lipid coated cyclodextrin complexation) formulation is known to improve solubility and bioavailability [34]. At 25 mg/kg, the free plasma Cmax and AUC parameters ranged from 0.1–0.8 µg/mL and 0.4–5.1 µg.h/mL respectively. NI-644 showed similar free plasma concentration as PA-824, whereas all other NI analogs showed relatively lower free plasma Cmax and exposure (Table S1 in File S1).
Table 2. In vivo pharmacokinetic parameters in plasma for bicyclic 4-nitroimidazole analogs.
| CompoundID | Dose(mg/kg) | p.o. PK parameters | i.v. PK parametersa | ||||||
| Cmax(µg/mL) | AUC24(µg.h/mL) | Tmax(hr) | T1/2(p.o.) | F (%) | Vss(L/kg) | CL(mL/min/kg) | T1/2(i.v) | ||
| PA-824* | 25 | 6.0 | 50.9 | 2 | 2.7 | 100 | 1.6 | 12.1 | 1.6 |
| NI-622* | 50 | 14.8 | 108.4 | 4 | 2.1 | 100 | 1.8 | 14.7 | 1.8 |
| NI-644* | 50 | 16.2 | 89.5 | 1 | 3.6 | 100 | 0.7 | 9.5 | 0.9 |
| Amino-824* | 10 | 1.7 | 6.0 | 0.3 | 2.0 | 74 | 2.3 | 20.5 | 2.0 |
| AminoEthyl-824* | 10 | 1.0 | 2.9 | 1 | 1.7 | 76 | 2.0 | 44.0 | 0.7 |
| NI-135∧ | 25 | 1.2 | 4.8 | 0.5 | 2.9 | 51 | 4.2 | 41.0 | 4.0 |
| NI-147∧ | 25 | 0.04 | 0.02 | 0.1 | 0.2 | 0.3 | 0.4 | 70.8 | 0.3 |
| NI-136∧ | 25 | 2.0 | 10.7 | 0.5 | 2.0 | 64 | 1.8 | 25.1 | 1.3 |
| NI-176∧ | 25 | 2.2 | 13.7 | 1 | 4.3 | 86 | 1.8 | 22.2 | 1.0 |
| NI-269∧ | 25 | 2.8 | 16.0 | 0.3 | 3.8 | 88 | 2.6 | 19.7 | 2.1 |
| NI-182∧ | 25 | 3.5 | 22.5 | 0.5 | 2.0 | 82 | 3.0 | 15.2 | 2.8 |
| NI-145∧ | 25 | 1.8 | 16.2 | 2 | 4.2 | 68 | 1.7 | 17.0 | 2.0 |
| NI-297∧ | 25 | 6.0 | 99.1 | 8 | 4.9 | 100 | 2.6 | 5.0 | 6.7 |
| NI-302∧ | 25 | 12.9 | 144.1 | 4 | 4.1 | 100 | 1.2 | 4.3 | 3.7 |
i.v dosing at either 10 mg/kg* or 5 mg/kg∧.
Cmax = maximum concentration reached in plasma, AUC24 = exposure between 0 to 24 h, Tmax = time to reach maximum concentration, T1/2 = half-life, F = oral bioavailability, Vss = volume of distribution at steady state, CL = total systemic clearance.
In vivo lung PK properties
The primary and the most important site of Mtb infection in patients is lung tissue. To understand the effect of structural changes in the NI molecules on lung PK parameters, we measured total compound concentration in mouse lungs (Table 3). The NI analogs at 25 mg/kg dose showed a wide range of values for lung Cmax (4.2–17.8 µg/g), Tmax (0.08–2 h) and exposure (18.6–233 µg*h/g). All NI analogs displayed near parallel concentration-time profile in plasma and lung tissue, suggesting a rapid equilibrium between these two tissues. Interestingly, the lung-to-plasma partitioning varied from 0.5 to 4.6 for Cmax and 0.4 to 3.9 for AUCs across the series in correlation with the observed volume of distribution (Table 3). NI-135, NI-136, NI-182 and NI-297 showed lung partitioning of 2.5 to 4.6 fold, and are comparable to PA-824. NI-622, NI-644, Amino-824 and NI-302 showed lower lung partitioning (<1) compared to PA-824. Although, NI-135 and NI-136 showed higher lung to plasma ratio (3.6 to 4.6), their absolute lung concentrations were 2.5 to 7.5 fold lower than PA-824. On the contrary, NI-302 showed lower lung partitioning, but its absolute concentrations were comparable to PA-824 (Table 3).
Table 3. In vivo pharmacokinetic parameters in lungs for bicyclic 4-nitroimidazole analogs.
| Compound | Dose(mg/kg) | Lung PK parameters | Lung to Plasma ratio | ||||
| Cmax (µg/g) | AUC24 (µg.h/g) | Tmax (hr) | T1/2 (p.o.) | Cmax | AUC24 | ||
| PA-824 | 25 | 17.8 | 139.9 | 0.3 | 4.8 | 3.0 | 2.7 |
| NI-622 | 50 | 10.2 | 71.1 | 0.5 | 1.8 | 0.7 | 0.7 |
| NI-644 | 50 | 7.5 | 38.1 | 1 | 2.9 | 0.5 | 0.4 |
| Amino-824 | 10 | 1.4 | 4.5 | 0.5 | 1.3 | 0.8 | 0.8 |
| AminoEthyl-824 | 10 | 1.6 | 5.2 | 0.5 | 1.2 | 1.6 | 1.8 |
| NI-135 | 25 | 5.5 | 18.6 | 0.5 | 3.3 | 4.6 | 3.9 |
| NI-147 | 25 | 0.5 | 2.6 | 0.1 | - | - | - |
| NI-136 | 25 | 7.2 | 39.8 | 0.5 | 2.5 | 3.6 | 3.7 |
| NI-176 | 25 | 4.8 | 30.3 | 0.5 | 4.3 | 2.2 | 2.2 |
| NI-269 | 25 | 5.4 | 27.5 | 0.3 | 2.7 | 1.9 | 1.7 |
| NI-182 | 25 | 11.4 | 73.2 | 0.5 | 1.9 | 3.3 | 3.3 |
| NI-145 | 25 | 4.2 | 34.6 | 2 | 4.1 | 2.3 | 2.1 |
| NI-297 | 25 | 16.3 | 233.4 | 8 | 4.6 | 2.7 | 2.4 |
| NI-302 | 25 | 9.7 | 100.3 | 2 | 4.1 | 0.8 | 0.7 |
Established mouse efficacy
Based on the in vitro potency and the in vitro and in vivo PK results, ten compounds were selected for in vivo mouse efficacy studies with 4 weeks of daily oral treatment. The mean lung CFU reductions compared to untreated controls are summarized in Table 4. The efficacy ranged from 0.5 to 1.56 log at 25 mg/kg and 0.6 to 2.3 log at 100 mg/kg compared to vehicle treated animals. At 25 mg/kg, NI-622 and NI-644 were significantly (P<0.05) less efficacious than PA-824, however other NI analogs (NI-135, NI-136, NI-182 and NI-297) showed comparable efficacy to PA-824. At 100 mg/kg, AminoEthyl-824, NI-135, NI-136 and NI-302 showed comparable efficacy to PA-824, however NI-622, NI-644, Amino-824 and NI-182 were significantly (P<0.05) less efficacious than PA-824. For PA-824, NI-135 and NI-136 a dose dependent increase in efficacy was observed, on the contrary, no dose-dependent increase in bactericidal activity was observed for NI-622, NI-644 and NI-182. However, none of the selected bicyclic 4-nitroimidazole analogs showed significantly better efficacy than PA-824 at respective 25 and 100 mg/kg doses.
Table 4. In vivo pharmacodynamics of bicyclic 4-nitroimidazole analogs studied in mice.
| Mean log lung CFU ± SEM | ΔMean log lung CFU reduction ± SEM | ||||
| Dose (mg/kg) | Vehicle control | 25 | 100 | 25 | 100 |
| PA-824 | 6.07±0.12 | 4.67±0.37 | 4.12±0.13 | 1.40±0.37 | 1.95±0.13 |
| 6.24±0.02 | 4.67±0.10 | 3.93±0.09 | 1.57±0.10 | 2.31±0.09 | |
| 6.66±0.14 | 5.20±0.05 | 4.19±0.11 | 1.46±0.05 | 2.47±0.11 | |
| 1.48±0.09$ | 2.30±0.08$ | ||||
| NI-622 | 6.66±0.14 | 5.77±0.08 | 5.85±0.11 | 0.89±0.08* | 0.81±0.11* |
| NI-644 | 6.66±0.14 | 6.18±0.03 | 6.09±0.07 | 0.48±0.03* | 0.57±0.07* |
| Amino-824 | 6.22±0.08 | nd | 5.14±0.08 | nd | 1.08±0.08* |
| AminoEthyl-824 | 6.22±0.08 | nd | 4.53±0.07 | nd | 1.69±0.07ns |
| NI-135 | 6.24±0.02 | 4.76±0.07 | 4.36±0.07 | 1.48±0.07ns | 1.88±0.07ns |
| NI-136 | 6.24±0.02 | 4.93±0.06 | 4.18±0.17 | 1.31±0.06ns | 2.06±0.17ns |
| NI-182 | 6.07±0.12 | 4.84±0.14 | 4.81±0.18 | 1.23±0.14ns | 1.26±0.18* |
| NI-297 | 6.07±0.12 | 4.51±0.11 | nd | 1.56±0.11ns | nd |
| NI-302 | 6.62±0.11 | nd | 4.45±0.22 | nd | 2.17±0.22ns |
The mean log lung CFU’s in five independent in vivo efficacy studies ranged between 6.07 and 6.66 in untreated infected mice.
nd = not determined.
Mean log lung CFU reduction compared to untreated controls. Each data represents mean value ± SEM from 5 animals.
Mean value from three independent experiments (n = 15).
*Significant difference at P<0.05 compared to respective PA-824 doses.
No significant difference at P<0.05 compared to respective PA-824 doses.
Correlation of PK parameters with efficacy
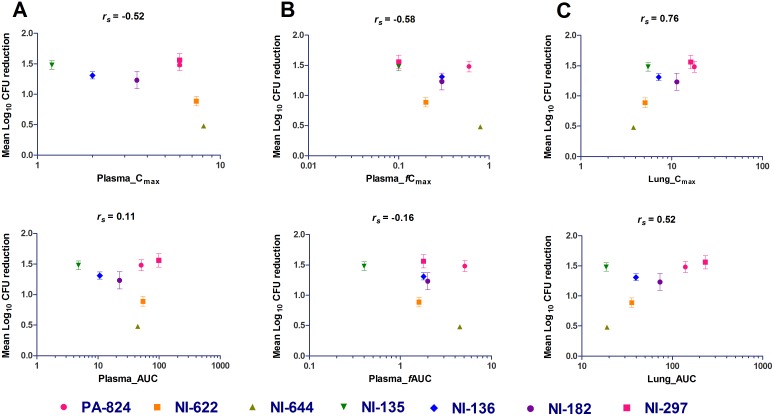
Further, mouse PK and efficacy data was used to understand the relationship of PK parameters with in vivo efficacy for a series of NI analogs. Both PK and efficacy data at 25 mg/kg were available for only 7 compounds. PK parameters were obtained after a single oral dose (Table 2), while efficacy studies were performed at oral daily doses of 25 and 100 mg/kg for 4 weeks (Table 4). The relationship between mean log CFU reduction with PK parameters (Cmax and AUC) was analyzed in both plasma and lungs using the Spearman’s rank correlation (Figure 2, Table S1 in File S1). The free plasma concentrations were obtained by correcting for in vitro mouse plasma protein binding. As shown in Figure 2, with the limited set of compounds, the in vivo efficacy correlated well with lung PK parameters than plasma PK parameters. The Spearman’s rank correlation coefficient for lung Cmax and AUC were 0.76 and 0.52 respectively. Across the NI analogs studied, compounds with higher lung concentration (PA-824, NI-297 and NI-182) tended to achieve higher efficacy (Δlog CFUs ranging from 1.23 to 1.56), likewise compounds with lower lung concentration (NI-644 and NI-622) displayed only marginal efficacy (Δlog CFUs ranging from 0.48 to 0.89) (Table 3 and 4). In general, lung Cmax and exposure showed positive correlation with in vivo efficacy for bicyclic 4-nitroimidazoles.
Figure 2. Correlation of PK parameters (Cmax, AUC) with in vivo efficacy in mice for bicyclic 4-nitroimidazole analogs in total plasma concentration (A), free plasma concentration (B) and total lung concentration (C).
Each data point represents Δ mean log lung CFU reduction compared to untreated controls (mean value ± SEM from 5 animals). rs is the Spearman’s rank correlations coefficient.
Correlation of PK-PD indices with efficacy
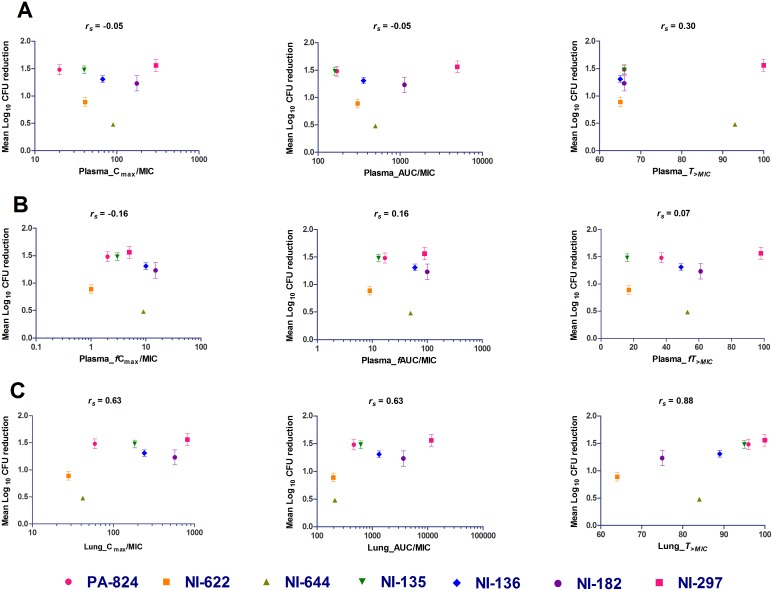
In vitro activity against Mtb is one of the key determinants of in vivo efficacy, hence the relationship between mean log lung CFU reduction with three primary descriptive PK-PD indices (Cmax/MIC, AUC/MIC and T>MIC) was analyzed in both plasma and lungs (Figure 3, Table S2 in File S1). As observed above, over all, the in vivo efficacy seems to have strong positive correlation with lung PK parameters than plasma. Among all the PK-PD indices, total lung T>MIC correlated the best with in vivo efficacy (rs = 0.88) than lung Cmax/MIC (rs = 0.63) and AUC/MIC (rs = 0.63) (Figure 3C, Table S2 in File S1). For all the compounds analyzed, the total lung T>MIC ranged between 64 and 100% resulting in 0.9–1.56 log lung CFU reduction. In this analysis, NI-644 was found to be an outlier, with T>MIC of 84% resulted in only 0.48 log CFU reduction. Overall, these results suggest that in vivo efficacy of bicyclic 4-nitroimidazole analogs correlates better with the time during which the total lung concentrations are above in vitro potency.
Figure 3. Correlation of PK-PD indices (Cmax/MIC, AUC/MIC and T>MIC) with in vivo efficacy in mice for bicyclic 4-nitroimidazole analogs in total plasma concentration (A), free plasma concentration (B) and total lung concentration (C).
Each data point represents Δ Mean log lung CFU reduction compared to untreated controls (mean value ± SEM from 5 animals). rs is the Spearman’s rank correlations coefficient.
Discussion
Understanding pharmacokinetic-pharmacodynamic (PK-PD) relationships in the early drug discovery process is essential to minimize the attrition rate during the pre-clinical and clinical development phases. In murine models of TB, PK-PD relationships have been established for several standard TB drugs, such as rifampicin [35], isoniazid [36], fluoroquinolones (FQ) [37] and TMC207 [38]. Based on the PK-PD findings with rifampicin, further clinical studies are still in progress to optimize the clinical dose [39]–[42]. PA-824, a bicyclic 4-nitroimidazole has demonstrated bactericidal activity in both preclinical and clinical settings [7], [8], [10]. Extensive medicinal chemistry efforts to improve aqueous solubility, metabolic stability, in vitro potency and in vivo efficacy have independently generated several series of NI analogs [5], [14]–[23]. All the bicyclic 4-nitroimidazole analogs analyzed in this study showed cofactor F420 dependent bio-activation (Table 1) suggesting the mechanism of action of these compounds similar to PA-824 [24], [43]. Comprehensive in vivo efficacy studies are generally resource/time intensive and are particularly true for TB. Thus, prioritizing potential lead compounds for in vivo efficacy studies would be useful based on PK parameters. This study is a retrospective analysis of in vivo efficacy with PK for bicyclic 4-nitroimidazole analogs to identify the PK parameters and PK-PD indices that correlate with the in vivo potency. The results of this analysis could potentially be exploited to prioritize new analogs for efficacy studies.
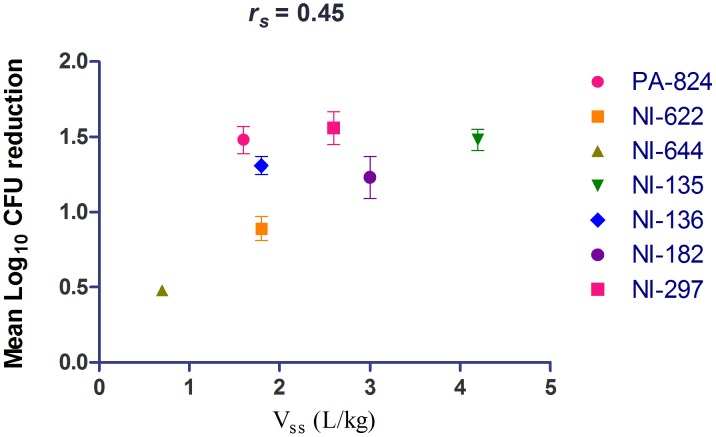
Mtb mainly resides in lung granulomatous structures and hence it is important for a drug to be available at the site of the infection for it to be active. The volume of distribution is a primary PK parameter defined by the physico-chemical properties of the compound that indicates the extent of compound distribution in the body. Azithromycin, a macrolide antibiotic, with very high Vss (33 L/kg) is known to have higher lung concentration than serum (AUC lung/serum = 21) and it correlates well with in vivo activity against respiratory pathogens [44], [45]. Likewise, moxifloxacin displays a high volume of distribution (Vss = 2 to 5 L/kg) resulting in pronounced penetration into tissues (AUC L/P ratio of 3.3) [46], [47] possibly leading to its potent in vivo efficacy against TB [48]. Recently, moxifloxacin has been shown to penetrate and accumulate in granulomatous lesions in TB infected rabbit lungs [49]. TMC207, a diarylquinoline analog, extensively distributes to lungs (AUC L/P ratio of 22) and is efficacious against Mtb [50]. In this study, NI analogs having moderate-to-high volume of distribution (Vss = 1.6 to 4.2 L/kg) and L/P ratio of >2 showed good efficacy in a murine TB model (Δlog CFUs ranging from 1.23 to 1.56) (Table 2, 3 and 4, Figure 4). Interestingly, NI-622 and NI-644 that showed lower lung to plasma ratio displayed only a marginal efficacy (Δlog CFUs ranging from 0.48 to 0.89). Although, NI-135 and NI-136 showed higher lung to plasma ratio (3.6 to 4.6), their absolute lung concentrations were 2.5 to 7.5 fold lower than PA-824. However, both these compounds displayed 10 times better in vitro potency resulting in comparable in vivo efficacy to PA-824. Overall, the relationship between in vivo efficacy of bicyclic 4-nitroimidazoles displayed positive correlation with Vss (rs = 0.45) (Figure 4). Based on these observations, the Vss and lung distribution could give an initial indication about a compound’s potential for in vivo efficacy and thus these two parameters could be used for initial prioritization of compounds during early drug discovery.
Figure 4. Correlation of volume of distribution with in vivo efficacy in mice for bicyclic 4-nitroimidazole analogs.
Each data point represents Δ Mean log lung CFU reduction compared to untreated controls (mean value ± SEM from 5 animals). rs is the Spearman’s rank correlations coefficient.
A thorough dose fractionation study of PA-824 in a murine model showed that the primary PK-PD driver for in vivo efficacy is the duration during which the free concentration are above MIC (fT>MIC) in plasma [11]. In this study lung PK parameters have not been measured. Further, fT>MIC in plasma of 22%, 48% and 77% is required for it to show bacteriostatic, 1-log10 and 1.59 log10 kill respectively. In general, the PK-PD parameter driving efficacy is conserved within a given class of compounds [51], for example, the efficacy of all FQ analogs is driven by AUC/MIC [37], [52], [53], while the efficacy of β-lactams correlates with T>MIC [53]–[55]. These studies are done with thorough dose fractionation of single compound with multiple doses and dosing regimen. During lead optimization program prioritization of promising compounds that show good in vivo efficacy is important to reduce the overall turnaround time. In this retrospective analysis with 7 different bicyclic 4-nitroimidazole analogs, we attempted to correlate in vivo efficacy at 25 mg/kg with PK parameters. On the contrary to what has been observed by Ahmad et al., in this study, with seven bicyclic 4-nitroimidazole analogs having varied lung distribution, in vivo efficacy showed weak correlation with free T>MIC in plasma. However, the total T>MIC in lungs showed positive correlation with in vivo efficacy (rs = 0.88) likely due to their preferential distribution into lungs for some analogs. For all the compounds analyzed, the total lung T>MIC ranged between 64–100% resulting in 0.9–1.56 log lung CFU reduction, hence efficacy studies at lower doses (resulting in T>MIC less than 65%) might be necessary to see a better correlation. Overall, in this study a diverse set of bicyclic 4-nitroimidazoles with Vss ranging from 0.7 L/kg to 4.2 L/kg, lung to plasma ratio ranging from 0.5 to 4.6 showed positive correlation with lung T>MIC than with any other parameters.
The results presented in this study must be interpreted with a couple of limitations in mind. First, single-dose PK parameters determined in healthy mice were assumed to be similar to multiple-dose PK parameters in infected animals and were correlated with efficacy data. This assumption is supported by published preclinical data that has shown the absence of plasma accumulation of PA-824 in mice dosed for 2 months [56]. Further, in clinical studies with PA-824, the PK parameters from a single dose phase I study were similar to a multiple dose phase II study in patients [9], [10], [12], [13]. Another limitation of this study is that total concentrations in lungs rather than the free lung concentrations were used for the PK-PD analysis. It is well accepted that for a given compound unbound drug concentrations in plasma are equivalent to unbound tissue concentrations when active transport is not involved in the drug distribution [57], [58]. Further, it is the unbound concentration of a compound at its target site driving the pharmacological effect [58]–[63]. Nevertheless, whole-tissue concentrations can be of some value in early drug discovery providing a first assessment of partition into the lungs [61]. Techniques like microdialysis in lungs can be applied to assess unbound tissue concentration [64], [65]. In TB patients, Mtb mainly resides in diverse and heterogeneous lesions in lungs. In general, interpretation of PD activity of anti-TB compounds is complicated by differential lung pathophysiology. PK in intrapulmonary compartments like the epithelial lining fluid and alveolar macrophages have also been studied in humans for standard TB drugs like rifampicin [66], isoniazid [67], ethambutol [68], pyrazinamide [69], rifapentine [70], moxifloxacin [71], ofloxacin [72] and linezolid [73]. The concentration in these sites could be the key factor governing the efficacy of anti-TB drugs. However, measurement of compound concentration in lungs by microdialysis, epithelial lining fluid and alveolar macrophages have limitations in sampling, methodology and interpretation of results [61], [74]; and such studies have not been explored in preclinical settings for TB. The total lung concentration may not be equal to the concentration in Mtb lesions, thus warranting lesion PK analysis to improve the predictive power for efficacy. Recently PK in lung lesions of mycobacterium-infected rabbits has been investigated for isoniazid, rifampicin, pyrazinamide and moxifloxacin [49], [75]. Although lesion PK can offer better insights in understanding PK-PD relationships, it is not easily applicable to early drug discovery especially with mouse efficacy model as it doesn’t display spectrum of lesions observed in TB patients or in higher animal models. In addition, similar studies with bicyclic 4-nitroimidazoles may be challenging as they undergo enzymatic transformation in Mtb to multiple stable and unstable metabolites [43].
Our findings show that the efficacy of all bicyclic 4-nitroimidazole analogs is most likely driven by PK parameters in lungs. A simple efficacy surrogate would be useful during the lead optimization to prioritize candidates for lengthy efficacy studies. For this class, efficacy correlated better with concentration in lungs rather than in plasma, consistent with Vss and differential lung: plasma distributions. The results of this analysis potentially be exploited to prioritize new analogs for efficacy studies based on in vitro potency, volume of distribution and lung concentration.
Supporting Information
Figure S1: Plasma concentration time profile for representative bicyclic 4-nitroimidazole analogs following a single 25 mg/kg oral dose in mice. Table S1: Correlation of PK parameters with in vivo efficacy in mice for bicyclic 4-nitroimidazole analogs. Table S2: Correlation of PK-PD indices with in vivo efficacy in mice for bicyclic 4-nitroimidazole analogs.
(DOCX)
Acknowledgments
We would like to thank Véronique Dartois for her support and guidance; Paul Smith, Christian Noble and Prakash Vachaspati for critical feedback on the manuscript; animal pharmacology and bio-analytical team members for their technical help. MG was a PhD student under the guidance of UHM.
Data Availability
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was funded in part by NITD, the Intramural Research Program of the NIAID to CEB, the Bill and Melinda Gates Foundation and the Wellcome Trust. NITD provided support in the form of salaries for authors SBL, JC, SR, AG, JJ, MN, MG, TD, FB and UHM but did not have any additional role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript. The specific roles of these authors are articulated in the ‘author contributions’ section.
References
- 1.WHO (2012) Global Tuberculosis Report. World Health Organization, Geneva, Switzerland. www.who.int/iris/bitstream/10665/75938/1/9789241564502_eng.pdf.
- 2.WHO (2012) Multidrug and extensively drug-resistant TB (M/XDR-TB): 2010 global report on surveillance and response. World Health Organization, Geneva, Switzerland. Report no.: WHO/HTM/TB/2010.3.
- 3. Balganesh TS, Alzari PM, Cole ST (2008) Rising standards for tuberculosis drug development. Trends Pharmacol Sci 29: 576–581 S0165-6147(08)00181-8 [pii]; 10.1016/j.tips.2008.08.001 [doi] [DOI] [PubMed] [Google Scholar]
- 4. Stover CK, Warrener P, VanDevanter DR, Sherman DR, Arain TM, et al. (2000) A small-molecule nitroimidazopyran drug candidate for the treatment of tuberculosis. Nature 405: 962–966 10.1038/35016103 [doi] [DOI] [PubMed] [Google Scholar]
- 5. Matsumoto M, Hashizume H, Tomishige T, Kawasaki M, Tsubouchi H, et al. (2006) OPC-67683, a nitro-dihydro-imidazooxazole derivative with promising action against tuberculosis in vitro and in mice. PLoS Med 3: e466 06-PLME-RA-0146R3 [pii]; 10.1371/journal.pmed.0030466 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kaneko T, Cooper C, Mdluli K (2011) Challenges and opportunities in developing novel drugs for TB. Future Med Chem 3: 1373–1400 10.4155/fmc.11.115 [doi] [DOI] [PubMed] [Google Scholar]
- 7. Lenaerts AJ, Gruppo V, Marietta KS, Johnson CM, Driscoll DK, et al. (2005) Preclinical testing of the nitroimidazopyran PA-824 for activity against Mycobacterium tuberculosis in a series of in vitro and in vivo models. Antimicrob Agents Chemother 49: 2294–2301 49/6/2294 [pii]; 10.1128/AAC.49.6.2294-2301.2005 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Tyagi S, Nuermberger E, Yoshimatsu T, Williams K, Rosenthal I, et al. (2005) Bactericidal activity of the nitroimidazopyran PA-824 in a murine model of tuberculosis. Antimicrob Agents Chemother 49: 2289–2293 49/6/2289 [pii]; 10.1128/AAC.49.6.2289-2293.2005 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ginsberg AM, Laurenzi MW, Rouse DJ, Whitney KD, Spigelman MK (2009) Safety, tolerability, and pharmacokinetics of PA-824 in healthy subjects. Antimicrob Agents Chemother 53: 3720–3725 AAC.00106-09 [pii]; 10.1128/AAC.00106-09 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Diacon AH, Dawson R, Hanekom M, Narunsky K, Maritz SJ, et al. (2010) Early bactericidal activity and pharmacokinetics of PA-824 in smear-positive tuberculosis patients. Antimicrob Agents Chemother 54: 3402–3407 AAC.01354-09 [pii]; 10.1128/AAC.01354-09 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ahmad Z, Peloquin CA, Singh RP, Derendorf H, Tyagi S, et al. (2011) PA-824 exhibits time-dependent activity in a murine model of tuberculosis. Antimicrob Agents Chemother 55: 239–245 AAC.00849-10 [pii]; 10.1128/AAC.00849-10 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Diacon AH, Dawson R, du BJ, Narunsky K, Venter A, et al. (2012) A phase II dose-ranging trial of the early bactericidal activity of PA-824. Antimicrob Agents Chemother. AAC.06125-11 [pii]; 10.1128/AAC.06125-11 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Diacon AH, Dawson R, von Groote-Bidlingmaier F, Symons G, Venter A, et al. (2012) 14-day bactericidal activity of PA-824, bedaquiline, pyrazinamide, and moxifloxacin combinations: a randomised trial. Lancet 380: 986–993 S0140-6736(12)61080-0 [pii]; 10.1016/S0140-6736(12)61080-0 [doi] [DOI] [PubMed] [Google Scholar]
- 14.Barry CE, Cherian, J, Chio, I, Keller, T, Manjunatha UH, et al. (7-21-2011) Organic compounds. US Patent WO 2011/087995. United States patent application.
- 15. Blaser A, Palmer BD, Sutherland HS, Kmentova I, Franzblau SG, et al. (2012) Structure-activity relationships for amide-, carbamate-, and urea-linked analogues of the tuberculosis drug (6S)-2-nitro-6-{[4-(trifluoromethoxy)benzyl]oxy}-6,7-dihydro-5H-imidazo[2,1-b][1, 3]oxazine (PA-824). J Med Chem 55: 312–326 10.1021/jm2012276 [doi] [DOI] [PubMed] [Google Scholar]
- 16. Cherian J, Choi I, Nayyar A, Manjunatha UH, Mukherjee T, et al. (2011) Structure-activity relationships of antitubercular nitroimidazoles. 3. Exploration of the linker and lipophilic tail of ((s)-2-nitro-6,7-dihydro-5H-imidazo[2,1-b][1,3]oxazin-6-yl)-(4-trifluoromethoxybe nzyl)amine (6-amino PA-824). J Med Chem 54: 5639–5659 10.1021/jm1010644 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Denny WA, Palmer BD (2010) The nitroimidazooxazines (PA-824 and analogs): structure-activity relationship and mechanistic studies. Future Med Chem 2: 1295–1304 10.4155/fmc.10.207 [doi] [DOI] [PubMed] [Google Scholar]
- 18.Jiricek J, Patel S, Keller T, Barry CE, Dowd CS, inventors; (5-7-2007) Nitroimidazole compounds. US Patent WO 2007/075872. United States patent application.
- 19. Kim P, Zhang L, Manjunatha UH, Singh R, Patel S, et al. (2009) Structure-activity relationships of antitubercular nitroimidazoles. 1. Structural features associated with aerobic and anaerobic activities of 4- and 5-nitroimidazoles. J Med Chem 52: 1317–1328 10.1021/jm801246z [doi];10.1021/jm801246z [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kim P, Kang S, Boshoff HI, Jiricek J, Collins M, et al. (2009) Structure-activity relationships of antitubercular nitroimidazoles. 2. Determinants of aerobic activity and quantitative structure-activity relationships. J Med Chem 52: 1329–1344 10.1021/jm801374t [doi];10.1021/jm801374t [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kmentova I, Sutherland HS, Palmer BD, Blaser A, Franzblau SG, et al. (2010) Synthesis and Structure-Activity Relationships of Aza- and Diazabiphenyl Analogues of the Antitubercular Drug (6S)-2-Nitro-6-{[4-(trifluoromethoxy)benzyl]oxy}-6,7-dihydro-5H-imidazo[2,1-b][1, 3]oxazine (PA-824). J Med Chem. 10.1021/jm101288t [doi] [DOI] [PubMed] [Google Scholar]
- 22. Palmer BD, Thompson AM, Sutherland HS, Blaser A, Kmentova I, et al. (2010) Synthesis and structure-activity studies of biphenyl analogues of the tuberculosis drug (6S)-2-nitro-6-{[4-(trifluoromethoxy)benzyl]oxy}-6,7-dihydro-5H-imidazo[2,1-b][1, 3]oxazine (PA-824). J Med Chem 53: 282–294 10.1021/jm901207n [doi] [DOI] [PubMed] [Google Scholar]
- 23. Thompson AM, Sutherland HS, Palmer BD, Kmentova I, Blaser A, et al. (2011) Synthesis and structure-activity relationships of varied ether linker analogues of the antitubercular drug (6S)-2-nitro-6-{[4-(trifluoromethoxy)benzyl]oxy}-6,7-dihydro-5h-imidazo[2,1-b][1, 3]oxazine (PA-824). J Med Chem 54: 6563–6585 10.1021/jm200377r [doi] [DOI] [PubMed] [Google Scholar]
- 24. Manjunatha UH, Boshoff H, Dowd CS, Zhang L, Albert TJ, et al. (2006) Identification of a nitroimidazo-oxazine-specific protein involved in PA-824 resistance in Mycobacterium tuberculosis. Proc Natl Acad Sci U S A 103: 431–436 0508392103 [pii]; 10.1073/pnas.0508392103 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zhou L, Yang L, Tilton S, Wang J (2007) Development of a high throughput equilibrium solubility assay using miniaturized shake-flask method in early drug discovery. J Pharm Sci 96: 3052–3071 10.1002/jps.20913 [doi] [DOI] [PubMed] [Google Scholar]
- 26. Wohnsland F, Faller B (2001) High-throughput permeability pH profile and high-throughput alkane/water log P with artificial membranes. J Med Chem 44: 923–930. jm001020e [pii] [DOI] [PubMed] [Google Scholar]
- 27. Avdeef A, Bendels S, Di L, Faller B, Kansy M, et al. (2007) PAMPA–critical factors for better predictions of absorption. J Pharm Sci 96: 2893–2909 10.1002/jps.21068 [doi] [DOI] [PubMed] [Google Scholar]
- 28. Marino AM, Yarde M, Patel H, Chong S, Balimane PV (2005) Validation of the 96 well Caco-2 cell culture model for high throughput permeability assessment of discovery compounds. Int J Pharm 297: 235–241 S0378-5173(05)00186-9 [pii]; 10.1016/j.ijpharm.2005.03.008 [doi] [DOI] [PubMed] [Google Scholar]
- 29. Fung EN, Chen YH, Lau YY (2003) Semi-automatic high-throughput determination of plasma protein binding using a 96-well plate filtrate assembly and fast liquid chromatography-tandem mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci 795: 187–194. S1570023203005646 [pii] [DOI] [PubMed] [Google Scholar]
- 30. Rao SP, Lakshminarayana SB, Kondreddi RR, Herve M, Camacho LR, et al. (2013) Indolcarboxamide is a preclinical candidate for treating multidrug-resistant tuberculosis. Sci Transl Med 5: 214ra168 5/214/214ra168 [pii]; 10.1126/scitranslmed.3007355 [doi] [DOI] [PubMed] [Google Scholar]
- 31. Duijkers IJ, Klipping C, Boerrigter PJ, Machielsen CS, De Bie JJ, et al. (2002) Single dose pharmacokinetics and effects on follicular growth and serum hormones of a long-acting recombinant FSH preparation (FSH-CTP) in healthy pituitary-suppressed females. Hum Reprod 17: 1987–1993. [DOI] [PubMed] [Google Scholar]
- 32. Djukic M, Munz M, Sorgel F, Holzgrabe U, Eiffert H, et al. (2012) Overton’s rule helps to estimate the penetration of anti-infectives into patients’ cerebrospinal fluid. Antimicrob Agents Chemother 56: 979–988 AAC.00437-11 [pii]; 10.1128/AAC.00437-11 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ferl GZ, Zhang X, Wu HM, Kreissl MC, Huang SC (2007) Estimation of the 18F-FDG input function in mice by use of dynamic small-animal PET and minimal blood sample data. J Nucl Med 48: 2037–2045 jnumed.107.041061 [pii]; 10.2967/jnumed.107.041061 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Stella VJ, Rajewski RA (1997) Cyclodextrins: their future in drug formulation and delivery. Pharm Res 14: 556–567. [DOI] [PubMed] [Google Scholar]
- 35. Jayaram R, Gaonkar S, Kaur P, Suresh BL, Mahesh BN, et al. (2003) Pharmacokinetics-pharmacodynamics of rifampin in an aerosol infection model of tuberculosis. Antimicrob Agents Chemother 47: 2118–2124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Jayaram R, Shandil RK, Gaonkar S, Kaur P, Suresh BL, et al. (2004) Isoniazid pharmacokinetics-pharmacodynamics in an aerosol infection model of tuberculosis. Antimicrob Agents Chemother 48: 2951–2957 10.1128/AAC.48.8.2951-2957.2004 [doi];48/8/2951 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Shandil RK, Jayaram R, Kaur P, Gaonkar S, Suresh BL, et al. (2007) Moxifloxacin, ofloxacin, sparfloxacin, and ciprofloxacin against Mycobacterium tuberculosis: evaluation of in vitro and pharmacodynamic indices that best predict in vivo efficacy. Antimicrob Agents Chemother 51: 576–582 AAC.00414-06 [pii]; 10.1128/AAC.00414-06 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Rouan MC, Lounis N, Gevers T, Dillen L, Gilissen R, et al. (2012) Pharmacokinetics and pharmacodynamics of TMC207 and its N-desmethyl metabolite in a murine model of tuberculosis. Antimicrob Agents Chemother 56: 1444–1451 AAC.00720-11 [pii]; 10.1128/AAC.00720-11 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Burman W, Dooley KE, Nuermberger EL (2011) The Rifamycins: Renewed Interest in an Old Drug Class. Chapter 3 Donald RP, Van Helden PD (eds): Antituberculosis Chemotherapy. Prog Respir Res. Basel, Karger. 40. 40: 18–24. [Google Scholar]
- 40. Rosenthal IM, Tasneen R, Peloquin CA, Zhang M, Almeida D, et al. (2012) Dose-ranging comparison of rifampin and rifapentine in two pathologically distinct murine models of tuberculosis. Antimicrob Agents Chemother 56: 4331–4340 AAC.00912-12 [pii]; 10.1128/AAC.00912-12 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Steingart KR, Jotblad S, Robsky K, Deck D, Hopewell PC, et al. (2011) Higher-dose rifampin for the treatment of pulmonary tuberculosis: a systematic review. Int J Tuberc Lung Dis 15: 305–316. [PubMed] [Google Scholar]
- 42. van IJ, Aarnoutse RE, Donald PR, Diacon AH, Dawson R, et al. (2011) Why Do We Use 600 mg of Rifampicin in Tuberculosis Treatment? Clin Infect Dis 52: e194–e199 cir184 [pii]; 10.1093/cid/cir184 [doi] [DOI] [PubMed] [Google Scholar]
- 43. Singh R, Manjunatha U, Boshoff HI, Ha YH, Niyomrattanakit P, et al. (2008) PA-824 kills nonreplicating Mycobacterium tuberculosis by intracellular NO release. Science 322: 1392–1395 322/5906/1392 [pii]; 10.1126/science.1164571 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Obach RS, Lombardo F, Waters NJ (2008) Trend analysis of a database of intravenous pharmacokinetic parameters in humans for 670 drug compounds. Drug Metab Dispos 36: 1385–1405 dmd.108.020479 [pii]; 10.1124/dmd.108.020479 [doi] [DOI] [PubMed] [Google Scholar]
- 45. Veber B, Vallee E, Desmonts JM, Pocidalo JJ, Azoulay-Dupuis E (1993) Correlation between macrolide lung pharmacokinetics and therapeutic efficacy in a mouse model of pneumococcal pneumonia. J Antimicrob Chemother 32: 473–482. [DOI] [PubMed] [Google Scholar]
- 46. Siefert HM, Domdey-Bette A, Henninger K, Hucke F, Kohlsdorfer C, et al. (1999) Pharmacokinetics of the 8-methoxyquinolone, moxifloxacin: a comparison in humans and other mammalian species. J Antimicrob Chemother 43 Suppl B: 69–76. [DOI] [PubMed] [Google Scholar]
- 47. Siefert HM, Kohlsdorfer C, Steinke W, Witt A (1999) Pharmacokinetics of the 8-methoxyquinolone, moxifloxacin: tissue distribution in male rats. J Antimicrob Chemother 43 Suppl B: 61–67. [DOI] [PubMed] [Google Scholar]
- 48. Nuermberger EL, Yoshimatsu T, Tyagi S, O’Brien RJ, Vernon AN, et al. (2004) Moxifloxacin-containing regimen greatly reduces time to culture conversion in murine tuberculosis. Am J Respir Crit Care Med 169: 421–426 10.1164/rccm.200310-1380OC [doi];200310-1380OC [pii] [DOI] [PubMed] [Google Scholar]
- 49. Prideaux B, Dartois V, Staab D, Weiner DM, Goh A, et al. (2011) High-sensitivity MALDI-MRM-MS imaging of moxifloxacin distribution in tuberculosis-infected rabbit lungs and granulomatous lesions. Anal Chem 83: 2112–2118 10.1021/ac1029049 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Andries K, Verhasselt P, Guillemont J, Gohlmann HW, Neefs JM, et al. (2005) A diarylquinoline drug active on the ATP synthase of Mycobacterium tuberculosis. Science 307: 223–227 1106753 [pii]; 10.1126/science.1106753 [doi] [DOI] [PubMed] [Google Scholar]
- 51. Barbour A, Scaglione F, Derendorf H (2010) Class-dependent relevance of tissue distribution in the interpretation of anti-infective pharmacokinetic/pharmacodynamic indices. Int J Antimicrob Agents 35: 431–438 S0924-8579(10)00068-3 [pii]; 10.1016/j.ijantimicag.2010.01.023 [doi] [DOI] [PubMed] [Google Scholar]
- 52. Craig WA (1998) Pharmacokinetic/pharmacodynamic parameters: rationale for antibacterial dosing of mice and men. Clin Infect Dis 26: 1–10. [DOI] [PubMed] [Google Scholar]
- 53. Craig WA (2001) Does the dose matter? Clin Infect Dis 33 Suppl 3S233–S237 CID001113 [pii]; 10.1086/321854 [doi] [DOI] [PubMed] [Google Scholar]
- 54. Andes D, Craig WA (2002) Animal model pharmacokinetics and pharmacodynamics: a critical review. Int J Antimicrob Agents 19: 261–268. S0924857902000225 [pii] [DOI] [PubMed] [Google Scholar]
- 55. Scaglione F, Paraboni L (2006) Influence of pharmacokinetics/pharmacodynamics of antibacterials in their dosing regimen selection. Expert Rev Anti Infect Ther 4: 479–490 10.1586/14787210.4.3.479 [doi] [DOI] [PubMed] [Google Scholar]
- 56. Nuermberger E, Rosenthal I, Tyagi S, Williams KN, Almeida D, et al. (2006) Combination chemotherapy with the nitroimidazopyran PA-824 and first-line drugs in a murine model of tuberculosis. Antimicrob Agents Chemother 50: 2621–2625 50/8/2621 [pii]; 10.1128/AAC.00451-06 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Lin JH (2006) Tissue distribution and pharmacodynamics: a complicated relationship. Curr Drug Metab 7: 39–65. [DOI] [PubMed] [Google Scholar]
- 58. Smith DA, Di L, Kerns EH (2010) The effect of plasma protein binding on in vivo efficacy: misconceptions in drug discovery. Nat Rev Drug Discov 9: 929–939 nrd3287 [pii]; 10.1038/nrd3287 [doi] [DOI] [PubMed] [Google Scholar]
- 59. Gonzalez D, Schmidt S, Derendorf H (2013) Importance of relating efficacy measures to unbound drug concentrations for anti-infective agents. Clin Microbiol Rev 26: 274–288 26/2/274 [pii]; 10.1128/CMR.00092-12 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Liu P, Muller M, Derendorf H (2002) Rational dosing of antibiotics: the use of plasma concentrations versus tissue concentrations. Int J Antimicrob Agents 19: 285–290. S0924857902000249 [pii] [DOI] [PubMed] [Google Scholar]
- 61. Mouton JW, Theuretzbacher U, Craig WA, Tulkens PM, Derendorf H, et al. (2008) Tissue concentrations: do we ever learn? J Antimicrob Chemother 61: 235–237 dkm476 [pii]; 10.1093/jac/dkm476 [doi] [DOI] [PubMed] [Google Scholar]
- 62. Muller M, dela PA, Derendorf H (2004) Issues in pharmacokinetics and pharmacodynamics of anti-infective agents: distribution in tissue. Antimicrob Agents Chemother 48: 1441–1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Theuretzbacher U (2007) Tissue penetration of antibacterial agents: how should this be incorporated into pharmacodynamic analyses? Curr Opin Pharmacol 7: 498–504 S1471-4892(07)00096-3 [pii]; 10.1016/j.coph.2007.05.003 [doi] [DOI] [PubMed] [Google Scholar]
- 64. Brunner M, Derendorf H, Muller M (2005) Microdialysis for in vivo pharmacokinetic/pharmacodynamic characterization of anti-infective drugs. Curr Opin Pharmacol 5: 495–499 S1471-4892(05)00106-2 [pii]; 10.1016/j.coph.2005.04.010 [doi] [DOI] [PubMed] [Google Scholar]
- 65. Chaurasia CS, Muller M, Bashaw ED, Benfeldt E, Bolinder J, et al. (2007) AAPS-FDA workshop white paper: microdialysis principles, application and regulatory perspectives. Pharm Res 24: 1014–1025 10.1007/s11095-006-9206-z [doi] [DOI] [PubMed] [Google Scholar]
- 66. Ziglam HM, Baldwin DR, Daniels I, Andrew JM, Finch RG (2002) Rifampicin concentrations in bronchial mucosa, epithelial lining fluid, alveolar macrophages and serum following a single 600 mg oral dose in patients undergoing fibre-optic bronchoscopy. J Antimicrob Chemother 50: 1011–1015. [DOI] [PubMed] [Google Scholar]
- 67. Conte JE Jr, Golden JA, McQuitty M, Kipps J, Duncan S, et al. (2002) Effects of gender, AIDS, and acetylator status on intrapulmonary concentrations of isoniazid. Antimicrob Agents Chemother 46: 2358–2364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Conte JE Jr, Golden JA, Kipps J, Lin ET, Zurlinden E (2001) Effects of AIDS and gender on steady-state plasma and intrapulmonary ethambutol concentrations. Antimicrob Agents Chemother 45: 2891–2896 10.1128/AAC.45.10.2891-2896.2001 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Conte JE Jr, Golden JA, Duncan S, McKenna E, Zurlinden E (1999) Intrapulmonary concentrations of pyrazinamide. Antimicrob Agents Chemother 43: 1329–1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Conte JE Jr, Golden JA, McQuitty M, Kipps J, Lin ET, et al. (2000) Single-dose intrapulmonary pharmacokinetics of rifapentine in normal subjects. Antimicrob Agents Chemother 44: 985–990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Soman A, Honeybourne D, Andrews J, Jevons G, Wise R (1999) Concentrations of moxifloxacin in serum and pulmonary compartments following a single 400 mg oral dose in patients undergoing fibre-optic bronchoscopy. J Antimicrob Chemother 44: 835–838. [DOI] [PubMed] [Google Scholar]
- 72. Chierakul N, Klomsawat D, Chulavatnatol S, Chindavijak B (2001) Intrapulmonary pharmacokinetics of ofloxacin in drug-resistant tuberculosis. Int J Tuberc Lung Dis 5: 278–282. [PubMed] [Google Scholar]
- 73. Conte JE Jr, Golden JA, Kipps J, Zurlinden E (2002) Intrapulmonary pharmacokinetics of linezolid. Antimicrob Agents Chemother 46: 1475–1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Kiem S, Schentag JJ (2008) Interpretation of antibiotic concentration ratios measured in epithelial lining fluid. Antimicrob Agents Chemother 52: 24–36 AAC.00133-06 [pii]; 10.1128/AAC.00133-06 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Kjellsson MC, Via LE, Goh A, Weiner D, Low KM, et al. (2012) Pharmacokinetic evaluation of the penetration of antituberculosis agents in rabbit pulmonary lesions. Antimicrob Agents Chemother 56: 446–457 AAC.05208-11 [pii]; 10.1128/AAC.05208-11 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Pethe K, Sequeira PC, Agarwalla S, Rhee K, Kuhen K, et al. (2010) A chemical genetic screen in Mycobacterium tuberculosis identifies carbon-source-dependent growth inhibitors devoid of in vivo efficacy. Nat Commun 1: 57 ncomms1060 [pii]; 10.1038/ncomms1060 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1: Plasma concentration time profile for representative bicyclic 4-nitroimidazole analogs following a single 25 mg/kg oral dose in mice. Table S1: Correlation of PK parameters with in vivo efficacy in mice for bicyclic 4-nitroimidazole analogs. Table S2: Correlation of PK-PD indices with in vivo efficacy in mice for bicyclic 4-nitroimidazole analogs.
(DOCX)
Data Availability Statement
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper and its Supporting Information files.