Abstract
Background
Lipid-lowering guidelines endorse a low-density lipoprotein cholesterol goal of <100 mg/dL for people with coronary heart disease (CHD). A more stringent threshold of <70 mg/dL is recommended for those with CHD and “very high-risk” conditions such as diabetes mellitus, metabolic syndrome, or cigarette smoking. Whether chronic kidney disease (CKD) confers a similar risk for recurrent CHD events is unknown.
Methods and Results
We evaluated the risk for recurrent CHD events and all-cause mortality among 3,938 participants ≥45 years with CHD in the Reasons for Geographic and Racial Differences in Stroke (REGARDS) study. Chronic kidney disease was defined by estimated glomerular filtration rate <60 mL/min per 1.73 m2 or urinary albumin to creatinine ratio ≥30 mg/g. Participants were categorized by the presence or absence of CKD and any very high-risk condition. Over a median of 4.1 years, the crude incidence (95% CI) of recurrent CHD events were 12.1 (9.0–15.2), 18.9 (15.5–22.3), 35.0 (25.4–44.6), and 34.2 (28.2–40.3) among those without CKD or high-risk conditions; very high-risk conditions alone; and CKD alone and both CKD and very high-risk conditions. After multivariable adjustment, compared with those without CKD or very high-risk conditions, the hazard ratio (95% CI) for recurrent CHD events was 1.45 (1.02–2.05), 2.24 (1.50–3.34), and 2.10 (1.47–2.98) among those with very high-risk conditions alone, CKD alone, and both CKD and very high-risk conditions, respectively. Results were consistent for all-cause mortality.
Conclusions
Chronic kidney disease is associated with risk for recurrent CHD events that approximates or is larger than other established very high-risk conditions.
Lowering serum cholesterol with statin therapy reduces cardiovascular morbidity and mortality among individuals with coronary heart disease (CHD).1,2 Although an low-density lipoprotein cholesterol (LDL-C) level of <100 mg/dL is considered optimal for individuals with established CHD or CHD risk equivalents, the most recent guidelines recommend a more aggressive treatment goal of <70 mg/dL among those considered to be at “very high-risk.” In these guidelines, people with CHD are defined as being at very high-risk if they have multiple major risk factors (especially diabetes mellitus [DM]), several components of the metabolic syndrome (MetSyn), and recent acute coronary syndrome or severe or poorly controlled risk factors, “especially continued cigarette smoking.”3
Chronic kidney disease (CKD) is common among individuals with CHD and is associated with increased risk for recurrent events.4 Among people without CHD, CKD is associated with a magnitude of incremental risk that approximates the risk conferred by having DM.5 Among those with established CHD, the magnitude of the excess coronary risk associated with CKD (as compared with DM, MetSyn, and current smoking) is unknown. Addressing this issue is clinically relevant as patients CKD with a history of CHD continue to be undertreated with evidence-based therapies such as statins.6 Accordingly, we sought to compare the risk for recurrent CHD events and all-cause mortality associated with CKD versus DM, MetSyn, or current cigarette smoking among US adults with a history of CHD participating in the Reasons for Geographic And Racial Differences in Stroke (REGARDS) study.
Methods
Details on the REGARDS study have been published previously.7 In brief, the REGARDS study is a population-based cohort study of stroke incidence and cognitive decline with the incidence of CHD being investigated through an ancillary study. The study enrolled US adults ≥45 years of age from the continental United States. By design, African Americans and residents from the southern US states, commonly referred to as the stroke buckle (coastal North and South Carolina and Georgia) or belt (the remaining parts of these states and Tennessee, Alabama, Mississippi, and Louisiana) were oversampled for enrollment into the REGARDS study. Potentially eligible participants were identified from commercially available lists of US residents and sent an initial mailing, which provided details of the study. This mailing was followed by a telephone call and a subsequent in-home visit, during which time participants were enrolled. Between January 2003 and October 2007, 30,239 African American and white adults were enrolled into the REGARDS study. The current analysis was limited to 5,314 participants with a history of CHD (as defined below) at baseline. Of these participants, we excluded 87 with no follow-up information, 54 with dialysis-dependent renal failure at baseline, and 415 with missing information on renal function, 13 on smoking status, and 31 on components of the MetSyn. In addition, we excluded 776 participants who did not fast before their in-home study visit. After these exclusions, the present analysis included 3,938 REGARDS participants with complete data. The REGARDS study protocol was approved by the Institutional Review Boards at the participating centers, and all participants provided informed consent.
Data collection
The REGARDS study baseline data collection included a computer-assisted telephone interview, an in-home examination, and self-administered questionnaires. Of relevance to the current analysis, research staff conducted telephone interviews to collect data on demographics, education, income, cigarette smoking, physical activity, aspirin use, and use of antihypertensive, diabetic, and cholesterol-lowering medications. During the in-home study visit, certified health professionals conducted a physical examination, collected blood and urine samples, and recorded all medications taken by participants in the prior 2 weeks. Based on the average of the 2 blood pressure measurements, hypertension was defined as systolic blood pressure ≥140 mm Hg, diastolic blood pressure ≥90 mm Hg, and/or self-reported use of antihypertensive medication. A history of CHD at baseline was defined as a self-reported history of myocardial infarction (MI) or coronary revascularization procedure or evidence of MI on the study electrocardiogram.8
For the current analysis, very high-risk conditions were defined as current smoking, diabetes, and MetSyn. Participants who responded “yes” to both of the following 2 questions were considered to be current smokers: “Have you smoked 100 or more cigarettes?” and “Do you smoke cigarettes now?” Diabetes was defined as a serum glucose ≥126 mg/dL or self-report of a prior diagnosis of diabetes with current use of insulin or oral hypoglycemic medications. Participants with 3 or more of the following 5 conditions were considered to have MetSyn: (1) systolic blood pressure ≥130 mm Hg, diastolic blood pressure ≥85 mm Hg, or current antihypertensive medication use; (2) high-density lipoprotein cholesterol <40 mg/dL in men and <50 mg/dL in women; (3) serum triglycerides ≥150 mg/dL; (4) fasting plasma glucose ≥100 mg/dL or antidiabetes medication use; and (5) waist circumference >102 cm in men and >88 cm in women.9
Serum creatinine assays were performed at the University of Vermont and calibrated with an isotope dilution mass spectroscopic standard. The Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation was used to calculate estimated glomerular filtration rate (eGFR).10 On the basis of the spot urine collected during the study visit, urinary albumin and creatinine were measured at the Department of Laboratory Medicine and Pathology at the University of Minnesota and expressed as the urinary albumin-to-creatinine ratio (ACR). For the primary analyses, CKD was defined as baseline eGFR <60 mL/min per 1.73 m2 or ACR ≥30 mg/g.11
Outcomes
The primary outcome was the occurrence of a recurrent CHD event, defined as definite or probable MI or CHD death. Secondary outcomes included all-cause mortality and the composite of a CHD event or all-cause mortality. Subsequent to the REGARDS in-home examination, participants were contacted every 6 months by telephone to identify potential events. When a cardiac-related hospitalization was reported, medical records were retrieved, reviewed, and adjudicated by a team of trained experts using a standardized protocol.12 Myocardial infarctions were classified as being definite, probable, or possible based on published guidelines; only definite or probable MIs were included in the present analysis here.12 Deaths due to acute CHD were classified as definite or probable CHD death as recommended by Luepker et al.12 Date of death was confirmed through the social security death index, death certificates, or the National Death Index. Follow-up time was recorded as the number of days from the baseline in-home visit to a participant's confirmed date of death, occurrence of a definite or probable CHD event, or their last REGARDS study telephone contact prior to December 31, 2009, whichever occurred first.
Statistical analyses
REGARDS participants with a baseline history of CHD were categorized into the following 4 mutually exclusive groups: no CKD and no very high-risk conditions; one or more very high-risk conditions without CKD; CKD alone (ie, CKD but no very high-risk conditions); one or more very high-risk conditions and CKD. Baseline participant characteristics of each group were calculated. Cumulative incidence curves were calculated using the Kaplan-Meier method for (1) CHD events, (2) death from any cause, and (3) the pooled outcome of CHD events or death. The crude and adjusted rates for CHD events were calculated by the 4 CKD/very high-risk groups. Using Cox proportional-hazards regression models, the adjusted hazard ratios (HRs) for CHD events were calculated for each group with participants having no CKD and no very high-risk serving as the referent category. Initially, HRs were calculated with adjustment for age, race, gender, and region of residence (stroke belt, stroke buckle, or other). Subsequently, HRs were calculated with additional adjustment for household income, self-rated health, alcohol consumption, physical activity, body mass index, history of MI, history of stroke, statin use, angiotensin-converting-enzyme (ACE) inhibitor, angiotensin receptor blocker (ARB), β-blocker, aspirin and clopidogrel use, and high-sensitivity C-reactive protein (hsCRP) (log transformed). Analyses were repeated for the outcomes of all-cause mortality and the pooled outcome of CHD events or all-cause mortality. Sensitivity analyses were performed using alternative definitions of CKD (ie eGFR <60 mL/min per 1.73 m2, ACR ≥30 mg/g, having both eGFR <60 mL/min per 1.73 m2 and ACR ≥30 mg/g, and eGFR <45 mL/min per 1.73 m2). In addition, separate analyses were performed after stratifying the study population by the presence or absence of CKD and each very high-risk condition alone (eg, no CKD/no DM, CKD alone, DM alone, and CKD with DM). Analyses were conducted using SAS 9.2 (SAS Institute, Cary, NC).
Results
Baseline characteristics of the study population stratified by the presence or absence of CKD and other very high-risk conditions are presented in Table I. Those with CKD (with or without very high-risk conditions) were older compared with their counterparts without CKD, while those with any very high-risk condition (with or without CKD) were more often female and black, had higher mean BMI, and were more likely to have a household income <$20,000 and report fair or poor health. Those with both very high-risk conditions and CKD were less likely to participate in physical activity, more likely to have a history of stroke and be taking an ACE inhibitor or ARB, and had higher baseline hsCRP levels.
Table I.
Characteristics of REGARDS study participants with a history of CHD stratified by the presence of CKD and very high-risk conditions
| No CKD | CKD | |||
|---|---|---|---|---|
| Not DM, MetSyn, or smoking (n = 1117) |
DM, MetSyn, or smoking (n = 1511) |
No DM, MetSyn, or smoking (n = 370) |
DM, MetSyn, or smoking (n = 940) |
|
| Age, y | 68.6 (9.2) | 65.6 (8.3) | 74.7 (7.8) | 69.6 (8.8) |
| Women, % | 36.4% | 40.2% | 34.1% | 40.2% |
| Black, % | 26.1% | 35.8% | 27.3% | 39.4% |
| Region of residence, % | ||||
| Stroke belt | 32.8% | 36.7% | 30.0% | 32.1% |
| Stroke buckle | 20.0% | 22.1% | 24.3% | 22.6% |
| Nonbelt | 47.2% | 41.2% | 45.7% | 55.3% |
| Annual household income <$20,000, % | 14.6% | 25.3% | 21.9% | 32.1% |
| Self-rated health, % | ||||
| Excellent | 16.7% | 6.0% | 9.7% | 4.6% |
| Very good | 33.5% | 21.1% | 28.1% | 18.1% |
| Good | 34.8% | 39.9% | 34.6% | 38.6% |
| Fair | 13.2% | 24.5% | 21.9% | 27.9% |
| Poor | 1.9% | 8.6% | 5.7% | 10.9% |
| Alcohol consumption, % | ||||
| None | 58.5% | 63.4% | 63.2% | 69.0% |
| Moderate | 37.6% | 32.4% | 33.5% | 29.1% |
| Heavy | 3.9% | 4.2% | 3.2% | 2.0% |
| Physical activity, % | ||||
| None | 27.0% | 37.8% | 38.2% | 47.5% |
| 1–3 times per week | 35.9% | 32.9% | 30.2% | 29.5% |
| 4+ times per week | 37.2% | 29.3% | 31.6% | 23.0% |
| Body mass index, kg/m2 | 26.9 (4.5) | 30.8 (6.1) | 26.8 (5.8) | 30.9 (6.1) |
| Systolic blood pressure, mm Hg | 125.2 (15.1) | 130.4 (16.7) | 131.4 (19.9) | 134.5 (19.4) |
| Diastolic blood pressure, mm Hg | 74.6 (9.1) | 76.6 (9.8) | 75.2 (10.5) | 76.1 (11.2) |
| LDL-C, mg/dL | 104.7 (34.1) | 102.5 (35.0) | 99.0 (32.2) | 101.0 (34.5) |
| HDL-C, mg/dL | 53.9 (15.4) | 44.2 (13.9) | 53.1 (15.5) | 43.2 (13.4) |
| History of MI, % | 70.6% | 72.5% | 70.9% | 71.0% |
| History of stroke, % | 6.4% | 11.0% | 10.8% | 15.9% |
| Medication use, % | ||||
| Statin | 56.9% | 58.4% | 60.8% | 61.0% |
| ACE inhibitor | 28.2% | 37.4% | 36.0% | 45.1% |
| ARB | 13.2% | 17.9% | 17.8% | 22.9% |
| β-Blocker | 37.6% | 46.9% | 46.2% | 55.9% |
| Aspirin | 68.5% | 71.2% | 68.9% | 69.9% |
| Clopidogrel | 13.9% | 14.9% | 16.2% | 18.9% |
| C-reactive protein, mg/L | 1.5 (1.4–1.6) | 2.6 (2.4–2.7) | 2.5 (2.2–2.8) | 3.3 (3.0–3.6) |
Very high-risk conditions include diabetes, MetSyn, and current smoking. Age and BMI are mean (SD), and C-reactive protein is geometric mean (95% CI). DM, Diabetes mellitus; HDL, high-density lipoprotein cholesterol.
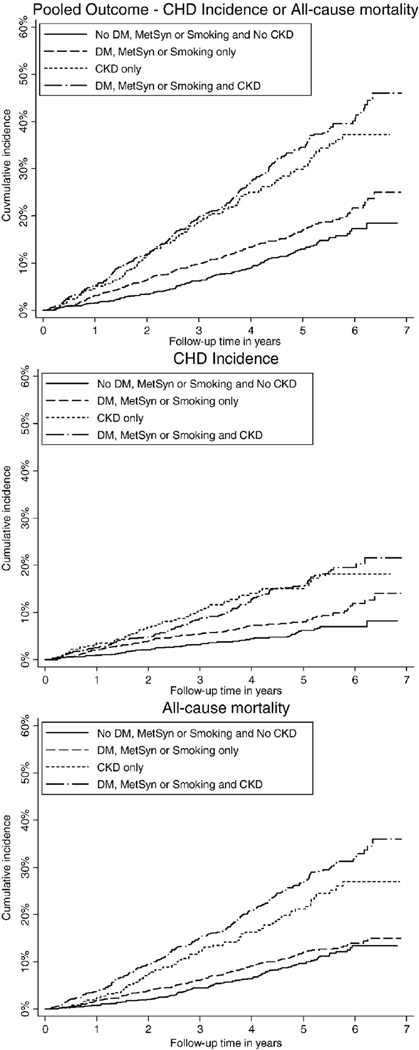
Participants were followed for up to 7 years (median follow-up, 4.1 years). The incidence of recurrent CHD events was highest among individuals with CKD alone and those with both CKD and very high-risk conditions (Figure 1 and Table II). A higher risk of recurrent CHD events for participants with CKD, with or without very high-risk conditions, remained present after age, race, gender, region, and full multivariable adjustment. Compared with those with neither CKD nor any very high-risk conditions, the full multivariable adjusted HR (95% CI) for having a CHD event was 1.45 (1.02–2.05), 2.24 (1.50–3.34), and 2.10 (1.47–2.98) among those with very high-risk conditions alone, CKD alone, and both CKD and any very high-risk conditions, respectively. A higher incidence and adjusted HR for all-cause mortality and the pooled outcome of CHD or all-cause mortality were present for participants with CKD compared with participants with very high-risk conditions.
Figure 1.
Cumulative incidence of recurrent CHD events, all-cause mortality, and CHD or all-cause mortality among study participants with no very high-risk condition and no CKD, any very high-risk condition alone, CKD alone, and both CKD and any very high-risk condition.
Table II.
Incidence rates and HRs for CHD or all-cause mortality by CKD and very high-risk status
| No CKD | CKD | |||
|---|---|---|---|---|
| No DM, MetSyn, or smoking (n = 1117) |
DM, MetSyn, or smoking (n = 1511) |
No DM, MetSyn, or smoking (n = 370) |
DM, MetSyn, or smoking (n = 940) |
|
| Recurrent CHD events | ||||
| No. of events | 58 | 119 | 51 | 124 |
| Crude incidence rate* | 12.1 (9.0–15.2) | 18.9 (15.5–22.3) | 35.0 (25.4–44.6) | 34.2 (28.2–40.3) |
| HR | ||||
| Model 1 | 1 (ref) | 1.71 (1.25–2.35) | 2.60 (1.78–3.82) | 2.91 (2.13–3.98) |
| Model 2 | 1 (ref) | 1.45 (1.02–2.05) | 2.24 (1.50–3.34) | 2.10 (1.47–2.98) |
| All-cause mortality | ||||
| No. of events | 104 | 167 | 77 | 246 |
| Crude incidence rate* | 20.2 (16.3–24.1) | 24.2 (20.5–27.8) | 48.0 (37.3–58.7) | 61.6 (53.9–69.3) |
| HR | ||||
| Model 1 | 1 (ref) | 1.42 (1.10–1.81) | 1.86 (1.38–2.50) | 3.01 (2.39–3.79) |
| Model 2 | 1 (ref) | 1.19 (0.90–1.57) | 1.35 (0.98–1.86) | 2.20 (1.69–2.86) |
| Pooled outcome—recurrent CHD events or all-cause mortality | ||||
| No. of events | 131 | 236 | 105 | 297 |
| Crude incidence rate* | 27.2 (22.6–31.9) | 37.5 (32.7–42.3) | 72.0 (58.2–85.8) | 82.0 (72.7–91.3) |
| HR | ||||
| Model 1 | 1 (ref) | 1.57 (1.27–1.95) | 2.19 (1.69–2.85) | 2.99 (1.69–2.85) |
| Model 2 | 1 (ref) | 1.37 (1.08–1.73) | 1.74 (1.32–2.30) | 2.25 (1.78–2.84) |
Very high-risk conditions include diabetes, MetSyn, and current smoking. DM, Diabetes mellitus.
Crude incidence rates (95% CI) per 1,000 person-years. Model 1 adjusted for age, race, gender, and region of residence (stroke belt, stroke buckle, or other). Model 2 adjusted for age, race, gender, and region of residence (stroke belt, stroke buckle, or other), household income, self-rated health, alcohol consumption, physical activity, body mass index, history of MI, history of stroke, statin use, ACE inhibitor, ARB, β-blocker, aspirin and clopidogrel use, and hsCRP (log transformed).
The number of outcomes among people with and without DM, MetSyn, and current smoking, evaluated separately, and CKD are provided in the online Appendix Supplementary Table I. Crude rates for recurrent CHD, all-cause mortality, and the pooled outcome were highest for participants with CKD alone compared with those of DM, MetSyn, and current smoking, evaluated separately (online Appendix Supplementary Table II).The HRs for outcomes were higher for CKD alone versus each high risk condition, separately, after multivariable adjustment (Table III).
Table III.
Hazard ratios for CHD or all-cause mortality by CKD and diabetes status, MetSyn, and cigarette smoking analyzed separately
| No CKD | CKD | |||
|---|---|---|---|---|
| No DM (n = 2001) |
DM (n = 627) |
No DM (n = 742) |
DM (n = 568) |
|
| Recurrent CHD events | ||||
| Model 1 | 1 (ref) | 1.45 (1.05–2.00) | 1.90 (1.44–2.51) | 2.65 (2.01–3.50) |
| Model 2 | 1 (ref) | 1.30 (0.92–1.84) | 1.59 (1.18–2.13) | 2.07 (1.51–2.83) |
| All-cause mortality | ||||
| Model 1 | 1 (ref) | 1.03 (0.78–1.37) | 1.85 (1.50–2.28) | 2.72 (2.20–3.35) |
| Model 2 | 1 (ref) | 0.96 (0.71–1.31) | 1.43 (1.14–1.79) | 2.14 (1.69–2.71) |
| Pooled outcome—Recurrent CHD events or all-cause mortality | ||||
| Model 1 | 1 (ref) | 1.19 (0.94–1.50) | 1.91 (1.59 – 2.29) | 2.65 (2.20–3.19) |
| Model 2 | 1 (ref) | 1.11 (0.86–1.43) | 1.54 (1.26–1.87) | 2.08 (1.68–2.57) |
|
No MetSyn (n = 1419) |
MetSyn (n = 1209) |
No MetSyn (n = 530) |
MetSyn (n = 780) |
|
| Recurrent CHD events | ||||
| Model 1 | 1 (ref) | 1.44 (1.07–1.94) | 2.29 (1.65–3.17) | 2.55 (1.91–3.41) |
| Model 2 | 1 (ref) | 1.19 (0.85–1.66) | 1.88 (1.34–2.64) | 1.77 (1.27–2.48) |
| All-cause mortality | ||||
| Model 1 | 1 (ref) | 1.07 (0.84–1.36) | 2.01 (1.58–2.56) | 2.42 (1.95–3.00) |
| Model 2 | 1 (ref) | 0.94 (0.72–1.23) | 1.46 (1.13–1.90) | 1.84 (1.43–2.37) |
| Pooled outcome—recurrent CHD events or all-cause mortality | ||||
| Model 1 | 1 (ref) | 1.22 (0.99–1.50) | 2.23 (1.80–2.75) | 2.39 (1.97–2.90) |
| Model 2 | 1 (ref) | 1.10 (0.87–1.39) | 1.72 (1.38–2.16) | 1.83 (1.46–2.30) |
|
Not smoking (n = 2240) |
Current smoking (n = 388) |
Not smoking (n = 1094) |
Current smoking (n = 216) |
|
| Recurrent CHD events | ||||
| Model 1 | 1 (ref) | 2.25 (1.57–3.22) | 2.11 (1.66–2.68) | 3.61 (2.47–5.27) |
| Model 2 | 1 (ref) | 2.08 (1.42–3.06) | 1.75 (1.35–2.27) | 2.58 (1.70–3.92) |
| All-cause mortality | ||||
| Model 1 | 1 (ref) | 2.67 (2.00–3.57) | 2.30 (1.91–2.76) | 3.94 (2.95–5.27) |
| Model 2 | 1 (ref) | 2.06 (1.51–2.81) | 1.84 (1.51–2.26) | 2.56 (1.86–3.52) |
| Pooled outcome—recurrent CHD events or all-cause mortality | ||||
| Model 1 | 1 (ref) | 2.58 (2.01–3.31) | 2.20 (1.87–2.59) | 4.04 (3.14–5.19) |
| Model 2 | 1 (ref) | 2.09 (1.60–2.72) | 1.81 (1.52–2.16) | 2.62 (1.98–3.46) |
Numbers in table are HR (95% CI). Models 1 and 2 adjusted as stated in Table II. DM, Diabetes mellitus.
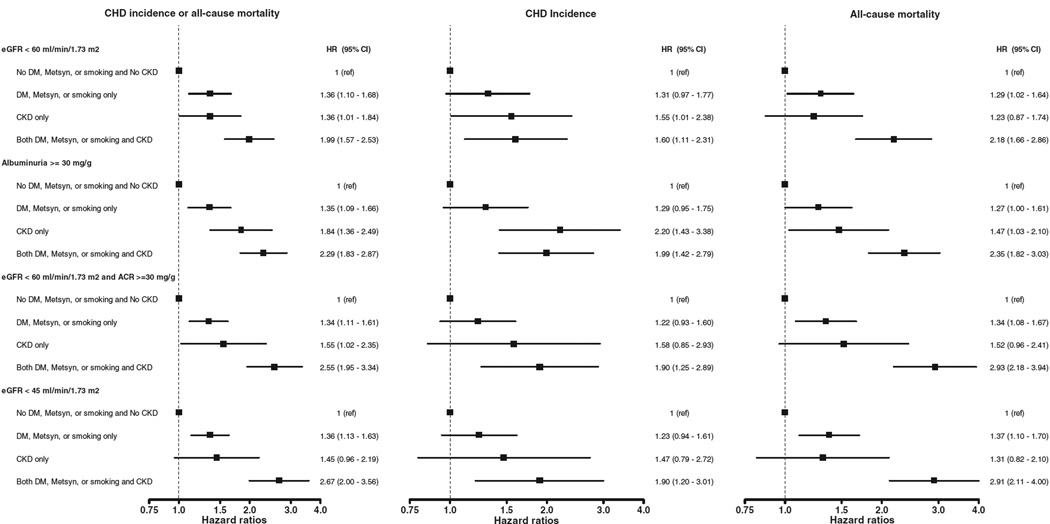
The number of outcomes among people with very high-risk conditions and CKD, using alternative definitions, are provided in the online Appendix Supplementary Table III. The crude rates and adjusted HRs for CHD events or death for participants with very high-risk conditions alone, CKD alone, and both CKD and any high-risk condition using alternative criteria to define CKD are shown in the online Appendix Supplementary Table IV and Figure 2. Regardless of the definition of CKD applied, the incidence rates and HRs for all outcomes was highest for participants with CKD alone and CKD and very high risk conditions. The association between eGFR <60 mL/min per 1.73 m2 and recurrent CHD events, all-cause mortality, and the pooled outcome was similar for individuals with and without ACR ≥30 mg/g (each P value for interaction >.80).
Figure 2.
Forest plots demonstrating HRs for recurrent CHD events, all-cause mortality, and CHD or all-cause mortality among participants groups using different criteria to define CKD. Boxes represent HR, and lines 95% CI.
Discussion
In the present study involving approximately 4,000 US adults with CHD, we found that the crude rate of recurrent CHD events was approximately twofold higher among those with CKD alone compared with individuals with DM, MetSyn, or current smoking but not CKD. The associations between CKD and recurrent CHD events remained large and statistically significant after multivariable adjustment and were consistent when CKD was compared with DM, MetSyn, and current smoking, separately. Moreover, the incidence of recurrent CHD events or all-cause mortality was higher for participants with CKD, using 4 alternate definitions, versus DM, MetSyn, and smoking. In aggregate, these findings suggest that among individuals with CHD, the presence of CKD may be considered to carry a similar, if not higher, risk to other very high-risk conditions that currently warrant aggressive preventive therapy, including lipid-lowering pharmacotherapy.
Previous studies examining whether CKD is a risk equivalent to high-risk conditions have primarily involved people without CHD.13 In a recent study involving more than 1 million individuals, for example, Tonelli et al5 reported that the unadjusted incidence proportion of MI hospitalization among those without prior MI was marginally higher among those with CKD alone (2.8%) versus DM alone (2.4%). Extending earlier reports involving those without CHD, our results suggest that CKD increases the risk for recurrent CHD events or all-cause mortality to a similar or larger extent than DM, MetSyn, or current smoking.
We also found that recurrent CHD risk with CKD alone was higher than either DM or MetSyn but similar to current cigarette smoking. Similar to our results, Weiner et al4 previously reported relative risks for mortality associated with CKD of 1.56 (95% CI, 1.37–1.78) and current smoking of 1.60 (95% CI, 1.9–1.84) among US adults with underlying cardiovascular disease. Our results, combined with earlier findings, suggest that CKD and smoking status may be more important determinants of CHD and mortality risk than either DM or MetSyn among those with CHD.
Although the HRs for recurrent CHD events or all-cause mortality among those with either DM or MetSyn alone were nonsignificant, risk was significantly greater among those with either condition with CKD. These findings suggest that the established associations between DM or MetSyn and cardiac risk may be largely attributable to the high prevalence of underlying CKD with either condition. Further studies with adequate power to detect effect modification between CKD and DM or MetSyn on cardiovascular risk are warranted.
Although people with CKD consistently had a higher risk for CHD and all-cause mortality compared with the other very high-risk conditions, this risk was not uniform for all 4 definitions of CKD tested. In particular, the HRs for events associated with CKD defined by the presence of albuminuria were larger than when CKD was defined by eGFR <45 or <60 mL/min per 1.73 m2. These results are consistent with prior reports documenting strong and positive associations between albuminuria and CHD risk.5 The current findings highlight the importance of measuring urinary albumin when assessing CKD.
Existing recommendations and secondary prevention guidelines do not consider CKD a very high-risk condition warranting aggressive management of coronary risk factors including lipid-lowering pharmacotherapy.3,14 Our data suggest that among individuals with CHD, the presence of CKD confers an incremental risk for adverse events that at least approximates or is larger than these conditions. The public health implications of our findings are further highlighted by the high prevalence of CKD among people with CHD and that statins continue to be prescribed less frequently to people with versus without CKD after MI hospitalization.6 Such “therapeutic nihilism” is particularly concerning as the randomized Study of Heart and Renal Protection (SHARP) demonstrated a significant 25% reduction in atherosclerotic events with cholesterol-lowering pharmacotherapy in CKD patients, a magnitude of risk reduction similar to that observed in non-CKD populations.15,16 It is important to note that our findings do not directly support a guideline LDL-C <70 mg/dL for individuals with both CHD and CKD but rather that such a threshold may be considered among such individuals. The safety of this approach is supported by the SHARP results as the mean LDL-C was lowered to approximately 70 mg/dL among those allocated to statin therapy.
A limitation of our study is the assessment of eGFR and ACR at only a single time point, which might result in the misclassification of CKD. In addition, only 79 REGARDS participants had an eGFR <30 mL/min per 1.73 m2 precluding assessment of recurrent CHD and mortality in this more advanced stage of CKD. As in any observational design, we cannot rule out the possibility of residual confounding on our observed associations between CKD and adverse CHD events. In addition, we did not collect information on certain variables that might augment cardiac risk in CKD, such as serum fibrinogen and homocysteine.
In conclusion, we found that the presence of CKD is associated with a risk for recurrent CHD events or mortality that equals or exceeds the risk associated with DM, MetSyn, or current cigarette smoking. These data suggest strong consideration should be made in future guidelines for including CKD as a very high-risk condition among individuals with CHD. Studies are needed to evaluate the risk reduction benefits of more aggressive approaches to lowering cardiovascular risk in CKD patients with underlying CHD.
Supplementary Material
Acknowledgements
We thank the other investigators, the staff, and the participants of the REGARDS study for their valuable contributions. A full list of participating REGARDS investigators and institutions can be found at http://www.regardsstudy.org.
Sources of Funding: This study was supported by a cooperative agreement U01 NS041588 from the National Institute of Neurological Disorders and Stroke, National Institutes of Health. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Neurological Disorders and Stroke or the National Institutes of Health. Representatives of the funding agency have been involved in the review of the article but not directly involved in the collection, management, analysis, or interpretation of the data. Additional funding was provided by an investigator-initiated grant-in-aid from Amgen Corporation. Amgen did not have any role in the design and conduct of the study; the collection, management, data analysis, or interpretation of the data; or the preparation of the article. The manuscript was sent to Amgen Corporation for review prior to submission for publication.
Footnotes
Disclosures
D.G.W. and P.M. serve as consultants for Amgen Corporation. P.M., M.S., E.L., and D.G.W. have received research support from the Amgen Corporation. No other authors report financial disclosures.
References
- 1.Prevention of cardiovascular events and death with pravastatin in patients with coronary heart disease and a broad range of initial cholesterol levels. The Long-Term Intervention with Pravastatin in Ischaemic Disease (LIPID) Study Group. N Engl J Med. 1998;339(19):1349–1357. doi: 10.1056/NEJM199811053391902. [DOI] [PubMed] [Google Scholar]
- 2.Cannon CP, Braunwald E, McCabe CH, et al. Intensive versus moderate lipid lowering with statins after acute coronary syndromes. N Engl J Med. 2004;350(15):1495–1504. doi: 10.1056/NEJMoa040583. [DOI] [PubMed] [Google Scholar]
- 3.Grundy SM, Cleeman JI, Merz CN, et al. Implications of recent clinical trials for the National Cholesterol Education Program Adult Treatment Panel III guidelines. Circulation. 2004;110(2):227–239. doi: 10.1161/01.CIR.0000133317.49796.0E. [DOI] [PubMed] [Google Scholar]
- 4.Weiner DE, Tighiouart H, Stark PC, et al. Kidney disease as a risk factor for recurrent cardiovascular disease and mortality. Am J Kidney Dis. 2004;44(2):198–206. doi: 10.1053/j.ajkd.2004.04.024. [DOI] [PubMed] [Google Scholar]
- 5.Tonelli M, Muntner P, Lloyd A, et al. Risk of coronary events in people with chronic kidney disease compared with those with diabetes: a population-level cohort study. Lancet. 2012 doi: 10.1016/S0140-6736(12)60572-8. [DOI] [PubMed] [Google Scholar]
- 6.Fox CS, Muntner P, Chen AY, et al. Use of evidence-based therapies in short-term outcomes of ST-segment elevation myocardial infarction and non-ST-segment elevation myocardial infarction in patients with chronic kidney disease: a report from the National Cardiovascular Data Acute Coronary Treatment and Intervention Outcomes Network registry. Circulation. 2010;121(3):357–365. doi: 10.1161/CIRCULATIONAHA.109.865352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Howard VJ, Cushman M, Pulley L, et al. The Reasons for Geographic And Racial Differences in Stroke study: objectives and design. Neuroepidemiology. 2005;25(3):135–143. doi: 10.1159/000086678. [DOI] [PubMed] [Google Scholar]
- 8.Prineas RJCR, Blackburn H. The Minnesota Code Manual of Electrocardiographic Findings. Boston: John Wright PSB; 1982. [Google Scholar]
- 9.Alberti KG, Eckel RH, Grundy SM, et al. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; and International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009;120(16):1640–1645. doi: 10.1161/CIRCULATIONAHA.109.192644. [DOI] [PubMed] [Google Scholar]
- 10.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Levey AS, Coresh J, Balk E, et al. National Kidney Foundation practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Ann Intern Med. 2003;139(2):137–147. doi: 10.7326/0003-4819-139-2-200307150-00013. [DOI] [PubMed] [Google Scholar]
- 12.Luepker RV, Apple FS, Christenson RH, et al. Case definitions for acute coronary heart disease in epidemiology and clinical research studies: a statement from the AHA Council on Epidemiology and Prevention; AHA Statistics Committee; World Heart Federation Council on Epidemiology and Prevention; the European Society of Cardiology Working Group on Epidemiology and Prevention; Centers for Disease Control and Prevention; and the National Heart, Lung, and Blood Institute. Circulation. 2003;108(20):2543–2549. doi: 10.1161/01.CIR.0000100560.46946.EA. [DOI] [PubMed] [Google Scholar]
- 13.Rashidi A, Sehgal AR, Rahman M, et al. The case for chronic kidney disease, diabetes mellitus, and myocardial infarction being equivalent risk factors for cardiovascular mortality in patients older than 65 years. Am J Cardiol. 2008;102(12):1668–1673. doi: 10.1016/j.amjcard.2008.07.060. [DOI] [PubMed] [Google Scholar]
- 14.Smith SC, Jr, Benjamin EJ, Bonow RO, et al. AHA/ACCF Secondary Prevention and Risk Reduction Therapy for Patients with Coronary and other Atherosclerotic Vascular Disease: 2011 update: a guideline from the American Heart Association and American College of Cardiology Foundation. Circulation. 2011;124(22):2458–2473. doi: 10.1161/CIR.0b013e318235eb4d. [DOI] [PubMed] [Google Scholar]
- 15.Baigent C, Landray MJ, Reith C, et al. The effects of lowering LDL cholesterol with simvastatin plus ezetimibe in patients with chronic kidney disease (Study of Heart and Renal Protection): a randomised placebo-controlled trial. Lancet. 2011;377(9784):2181–2192. doi: 10.1016/S0140-6736(11)60739-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baigent C, Keech A, Kearney PM, et al. Efficacy and safety of cholesterol-lowering treatment: prospective meta-analysis of data from 90,056 participants in 14 randomised trials of statins. Lancet. 2005;366(9493):1267–1278. doi: 10.1016/S0140-6736(05)67394-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.