Abstract
Purpose
The purpose of this study was to investigate the expression of autophagy-related proteins in relation to androgen receptor (AR) status in estrogen receptor (ER)-negative breast cancers.
Methods
We extracted 334 ER-negative breast cancer samples to construct tissue microarrays (TMAs), which were immunohistochemically stained for autophagy-related proteins (beclin-1, LC3A, LC3B, p62) and for AR and HER-2.
Results
There were 127 AR-positive cases and 207 AR-negative cases, and 140 HER-2-positive cases and 194 HER-2 negative cases. The AR-negative group was associated with tumoral LC3A expression (P<0.001), while the AR-positive group was associated with tumoral BNIP3 expression (P<0.001). Tumoral LC3A was most highly expressed in the AR-negative and HER-2 negative group, while stromal LC3A showed the highest expression in the AR-negative and HER-2-positive group. Tumoral BNIP3 and stromal BNIP3 were highest in the AR-positive and HER-2-negative group. In the AR-positive and HER-2-negative group, stromal p62 positivity was an independent factor that was statistically significant in its association with shorter disease-free survival (DFS) (Hazard ratio: 10.21, 95% CI: 1.130–92.31, P = 0.039). Shorter DFS was associated with tumoral LC3A positivity (Hazard ratio: 10.28, 95% CI: 2.068–51.19, P = 0.004) in the AR-negative and HER-2-positive group.
Conclusion
In ER-negative breast cancers, AR status was associated with expression of different types of autophagy-related proteins. Tumoral LC3A was most highly expressed in AR-negative breast cancers, while tumor BNIP3 was highest in AR-positive breast cancers.
Introduction
Autophagy is defined as the lysosomal degradation of cellular components within cells. There are three types of autophagy: microautophagy, chaperone-mediated autophagy and macroautophagy, which is the most commonly employed type. Autophagy removes dysfunctional or damaged cellular components while recycling cellular components that can be re-used, thereby playing an important homeostatic role within the cell [1]–[4]. Autophagy-related proteins that are used as markers to evaluate activation levels of autophagy include: beclin-1 [5]–[8], a protein known to participate in the nucleation process; LC3A [9]–[11], a protein that participates in the elongation process and thereby forms autophagosomes; P62, a scaffolding protein that transfers ubiquitinated protein to autophagosomes; and BNIP3, which plays a central role in mitophagy, the autophagy process within mitochondria. However, autophagy is not limited to normal cells; it is also reported to play a significant role in cancer cells. In general, the cancer cell adopts specialized metabolic processes through angiogenesis and/or aerobic glycolysis in their usual harsh hypoxic and nutrient-deficient environment. However, in particularly highly aggressive malignant tumors, where stresses are higher for metabolic demand, these specialized metabolic pathways may not be sufficient, so some tumors may adopt an alternative metabolic pathway of autophagy [12], [13]. In such cases, autophagy works to recycle cytoplasmic components to supply extra energy to the cell. Therefore, the autophagy process should be closely associated with metabolism in cancer progression and survival.
The development and natural history of breast cancer is significantly influenced by the status of steroidal hormones, such as those of estrogen. It is common practice to evaluate the status of estrogen receptor (ER) and progesterone receptor (PR) in order to treat and prognosticate breast cancer. In addition to ER/PR, androgen receptor (AR) is another steroidal hormone that influences and is associated with breast cancers, but the relationship is still not clearly understood. In general, androgen receptors are expressed in 70% of all breast cancers [14], with higher rates in apocrine and lobular types [15]. Previous studies have revealed that ER negativity is associated with glycolysis-related proteins such as Glut-1, CAIX and MCT-4 [16], [17]. Therefore, it has been suggested that ER-negative breast cancer has a higher metabolic activity than ER-positive breast cancer. Because the autophagy process should be closely associated with metabolism in cancer progression and survival, it is expected that autophagy activity is more elevated in ER-negative breast cancer than in ER-positive breast cancer. However, AR status and its association with autophagy-related proteins remain unexplored.
The purpose of this study was to investigate how autophagy-related proteins are expressed in relation to AR status in ER-negative breast cancers and to determine the corresponding clinical implications.
Materials and Methods
Patient Selection and Clinicopathologic Evaluation
Formalin-fixed paraffin-embedded tissue samples of patients diagnosed with invasive ductal carcinoma, no specific type, from January 2005 to December 2012 at Severance Hospital were included in this study. The study was approved by the Institutional Review Board (IRB) of Severance Hospital. IRB board waived the need for written informed consent. Those cases that had undergone pre-operative chemotherapy were excluded. Information on ER, AR and HER-2 status was collected from pathology reports. A cut-off value of 1% or more positively stained nuclei was used to define ER and AR positivity [18]. HER-2 staining was analyzed according to the American Society of Clinical Oncology (ASCO)/College of American Pathologists (CAP) guidelines using the following categories: 0 = no immunostaining; 1+ = weak incomplete membranous staining, less than 10% of tumor cells; 2+ = complete membranous staining, either uniform or weak in at least 10% of tumor cells; and 3+ = uniform intense membranous staining in at least 30% of tumor cells [19]. HER-2 immunostaining was considered positive when strong (3+) membranous staining was observed, whereas cases with 0 to 1+were regarded as negative. The cases showing 2+ HER-2 expression were evaluated for HER-2 amplification by fluorescent in situ hybridization (FISH).
All cases were retrospectively reviewed by a breast pathologist (Koo JS), in which histological evaluation was based on hematoxylin and eosin (H&E)–stained slides. The histological grade was assessed using the Nottingham grading system [20]. Tumor staging was based on the 7th American Joint Committee on Cancer (AJCC) criteria. Disease-free survival (DFS) was calculated from the date of the first curative surgery to the date of the first loco-regional or systemic relapse, or death without any type of relapse. Overall survival (OS) was estimated from the date of the first curative operation to the date of the last follow-up or death from any cause. Clinicopathologic parameters evaluated in each breast cancer included patient age at initial diagnosis, lymph node metastasis, tumor recurrence, distant metastasis, and patient survival.
Tissue Microarray
After reviewing H&E–stained slides, the most suitable formalin-fixed, paraffin-embedded (FFPE) tumor tissue samples were retrospectively selected. The most representative tumor region on the FFPE sample was then marked and a 3-mm tissue core sample was extracted using a punch machine and planted onto a 6×5 recipient block. A total of 2 tissue cores were taken for all samples in this TMA construction.
Immunohistochemistry
The antibodies used for immunohistochemistry in this study are shown in Table 1. Briefly, FFPE blocks were sectioned at a thickness of 3 um and then deparaffinized and rehydrated using xylene and alcohol solutions, respectively. Sections were then stained using the VentanaDiscoversy XT automated stainer (Ventana Medical System, Tucson, AZ, USA). Antigen retrieval was achieved by soaking sections in a CC1-buffered solution (Cell Conditioning 1; citrate buffer Ph 6.0, Ventana Medical System). The appropriate positive and negative controls were included together with the study sample for staining.
Table 1. Clone, dilution, and source of antibodies used.
| Antibody | Clone | Dilution | Source |
| Autophagy related | |||
| Beclin-1 | Polyclonal | 1∶100 | Abcam, Cambridge, UK |
| LC3A | EP1528Y | 1∶100 | Abcam, Cambridge, UK |
| LC3B | Polyclonal | 1∶100 | Abcam, Cambridge, UK |
| p62 | SQSTM1 | 1∶100 | Abcam, Cambridge, UK |
| BNIP3 | Ana40 | 1∶100 | Abcam, Cambridge, UK |
Interpretation of Immunohistochemical Results
Interpretations of IHC stains were standardized as the proportion of stained cells multiplied by the intensity of the immunohistochemical staining. The proportion of stained cells was scored with a system ranging from 0 to 2, defined as follows: 0 represented a negative result, 1 represented a section in which less than 30% of cells were positively stained, and 2 represented a section in which more than 30% of cells were positively stained. Immunostaining intensity was scored with a system ranging from 0 to 3, defined as follows: 0 represented a negative result, 1 represented weak, 2 represented moderate, and 3 represented strong. The number obtained after the multiplication of stained cell proportion by immunostaining intensity resulted in the overall interpretation score: 0–1 was defined as negative, 2–6 as positive [21].
Statistical Analysis
Data were statistically processed using SPSS for Windows version 12.0 (SPSS Inc., Chicago, IL). Student’s t test and Fisher’s exact test were used for continuous and categorical variables, respectively. To analyze data with multiple comparisons, a corrected P-value with application of the Bonferroni method for multiple comparisons was used. Statistical significance was assumed when P<0.05. Kaplan-Meier survival curves and log-rank statistics were employed to evaluate time to tumor metastasis and time to survival. Multivariate regression analysis was performed using a Cox proportional hazards model.
Results
Basal Characteristics of Patients According to the AR and HER-2 Status in ER-Negative Breast Cancer
There were 127 AR-positive cases, and 140 HER-2-positive cases. After dividing samples into four groups based on AR and HER-2 status, there were 53 cases in the AR (+)/HER-2 (–) group, 74 cases in the AR (+)/HER-2 (+) group, 66 cases in the AR (–)/HER-2 (+) group and 141 cases in the AR (–)/HER-2 (–) group. When ER negative cancer was divided into groups according to AR and HER-2 status, these groups exhibited a noticeable difference in age at diagnosis and Ki-67 expression levels. AR negative group was associated with older age and higher Ki-67 LI(P = 0.003, and P<0.001, respectively). While within the AR negative group, HER-2positive group was shown to be associated with older age at diagnosis and HER-2 negative group with higher Ki-67 LI(P = 0.030, and P = 0.002, respectively, Table 2).
Table 2. Clinicopathologic characteristics according to the AR and HER-2 status in ER-negative breast cancer.
| AR-positive group, n = 127 | AR-negative group, n = 207 | |||||||||
| Parameters | Sub-category | TotalN = 334 (%) | HER-2−n = 53 (%) | HER-2+n = 74 (%) | P-value | HER-2+n = 66 (%) | HER-2−n = 141 (%) | P-value | P-value* | P-value† |
| Age (years) | 0.936 | 0.030 | 0.001 | 0.003 | ||||||
| ≤35 | 34 (10.2) | 2 (3.8) | 3 (4.1) | 4 (6.1) | 25 (17.7) | |||||
| >35 | 300 (89.8) | 51 (96.2) | 71 (95.6) | 62 (93.9) | 116 (82.3) | |||||
| Histologic grade | 0.729 | 0.074 | 0.078 | 0.067 | ||||||
| I/II | 136 (40.7) | 26 (49.1) | 34 (45.9) | 30 (45.5) | 46 (32.6) | |||||
| III | 198 (59.3) | 27 (50.9) | 40 (54.1) | 36 (54.5) | 95 (67.4) | |||||
| T stage | 0.619 | 0.089 | 0.126 | 0.115 | ||||||
| T1 | 160 (47.9) | 27 (50.9) | 41 (55.4) | 35 (53.0) | 57 (40.4) | |||||
| T2/T3 | 174 (52.1) | 26 (49.1) | 33 (44.6) | 31 (47.0) | 84 (59.6) | |||||
| Lymph node metastasis | 0.839 | 0.354 | 0.594 | 0.373 | ||||||
| No | 245 (73.4) | 40 (75.5) | 57 (77.0) | 50 (75.8) | 98 (69.5) | |||||
| Yes | 89 (26.6) | 13 (24.5) | 17 (23.0) | 16 (24.2) | 43 (30.5) | |||||
| Tumor recurrence | 0.801 | 0.562 | 0.843 | 0.600 | ||||||
| No | 295 (88.3) | 48 (90.6) | 66 (89.2) | 59 (89.4) | 122 (86.5) | |||||
| Yes | 39 (11.7) | 5 (9.4) | 8 (10.8) | 7 (10.6) | 19 (13.5) | |||||
| Patient death | 0.950 | 0.442 | 0.242 | 0.080 | ||||||
| No | 303 (90.7) | 50 (94.3) | 70 (94.6) | 60 (90.9) | 123 (87.2) | |||||
| Yes | 31 (9.3) | 3 (5.7) | 4 (5.4) | 6 (9.1) | 18 (12.8) | |||||
| Ki-67 LI (%) | 0.294 | 0.002 | <0.001 | <0.001 | ||||||
| ≤14 | 79 (23.7) | 22 (41.5) | 24 (32.4) | 18 (27.3) | 15 (10.6) | |||||
| >14 | 31 (58.5) | 31 (58.5) | 50 (67.6) | 48 (72.7) | 126 (89.4) | |||||
*P-value was from comparison among 4 groups.
P-value was from comparison between AR+ and AR– groups by Fisher exact test.
Expression of Autophagy-Related Proteins According to the AR and HER-2 Status in ER-Negative Breast Cancer
Among autophagy-related proteins, LC3A, LC3B, BNIP3 exhibited cytoplasmic expression. In the case of beclin-1 and p62 proteins, these showed cytoplasmic and nuclear expression. Reports in the literature indicate that when these proteins exhibited nuclear expression, they were unrelated with autophagy activity. Therefore, we eliminated those with nuclear expression from our data and only counted those with cytoplasmic expression as positive [22], [23].
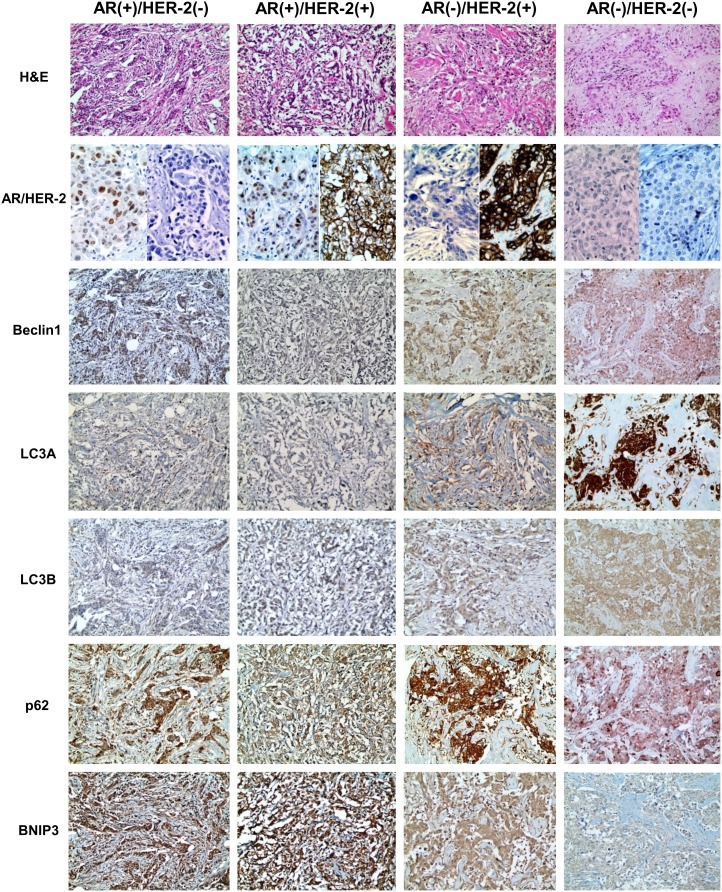
For expression of autophagy-related proteins according to AR and HER-2 status in ER-negative breast cancers, tumoral LC3A expression was highest in the AR (–)/HER-2 (–) group, and lowest in the AR (+)/HER-2 (+) group (P<0.001). Stromal LC3A was highest in the AR (–)/HER-2 (+) group, and lowest in the AR (–)/HER-2 (–) group (P<0.001). Tumoral BNIP3 and stromal BNIP3 had the highest expression in the AR (+)/HER-2 (–) group and lowest in the AR (–)/HER-2 (–) group (P<0.001, and P = 0.009, respectively, Figure 1).
Figure 1. Expression of autophagy-related proteins according to AR and HER-2 status in ER-negative breast cancer.
Tumoral LC3A expression was highest in the AR (–)/HER-2 (–) group, and lowest in the AR (+)/HER-2 (+) group. Stromal LC3A was highest in the AR (–)/HER-2 (+) group, and lowest in the AR (–)/HER-2 (–) group. Tumoral BNIP3 and stromal BNIP3 had the highest expression in the AR (+)/HER-2 (–) group and lowest in the AR (–)/HER-2 (–) group. Expression of Beclin-1, LC3B, and p62 is similar among 4 subgroups.
For expression of autophagy-related proteins according to AR status, tumoral LC3A was expressed most highly in the AR-negative group (P<0.001), while tumoral BNIP3 was most highly expressed in the AR-positive group (P<0.001, and Table 3).
Table 3. Expression of metabolism-related proteins according to the AR and HER-2 status in ER-negative breast cancer.
| Parameters | Total N = 334(%) | AR-positive group | AR-negative group | p-value* | p-value† | ||||
| HER-2−n = 53 (%) | HER-2+n = 74 (%) | p-value | HER-2+n = 66 (%) | HER-2−n = 141 (%) | p-value | ||||
| Beclin-1 (T) | 1.000 | 0.074 | 0.185 | 0.260 | |||||
| Negative | 184 (55.1) | 31 (58.5) | 44 (59.5) | 41 (62.1) | 68 (48.2) | ||||
| Positive | 150 (44.9) | 22 (41.5) | 30 (40.5) | 25 (37.9) | 73 (51.8) | ||||
| LC3A (T) | 0.067 | <0.001 | <0.001 | <0.001 | |||||
| Negative | 272 (81.4) | 47 (88.7) | 72 (97.3) | 60 (90.9) | 93 (66.0) | ||||
| Positive | 62 (18.6) | 6 (11.3) | 2 (2.7) | 6 (9.1) | 48 (34.0) | ||||
| LC3A (S) | 0.205 | <0.001 | <0.001 | 0.602 | |||||
| Negative | 252 (75.4) | 44 (83.0) | 54 (73.0) | 35 (53.0) | 119 (84.4) | ||||
| Positive | 82 (24.6) | 9 (17.0) | 20 (27.0) | 31 (47.0) | 22 (15.6) | ||||
| LC3B (T) | 0.848 | 0.172 | 0.204 | 0.132 | |||||
| Negative | 209 (62.9) | 35 (66.0) | 51 (68.9) | 44 (66.7) | 79 (56.0) | ||||
| Positive | 125 (37.4) | 18 (34.0) | 23 (31.1) | 22 (33.3) | 62 (44.0) | ||||
| p62 (T) | 0.260 | 0.061 | 0.153 | 0.814 | |||||
| Negative | 116 (34.7) | 21 (39.6) | 22 (29.7) | 17 (25.8) | 56 (39.7) | ||||
| Positive | 218 (65.3) | 32 (60.4) | 52 (70.3) | 49 (74.2) | 85 (60.3) | ||||
| p62 (S) | 0.409 | 0.867 | 0.770 | 0.609 | |||||
| Negative | 246 (73.9) | 38 (71.7) | 58 (78.4) | 49 (74.2) | 101 (72.1) | ||||
| Positive | 87 (26.1) | 15 (28.3) | 16 (21.6) | 17 (25.8) | 39 (27.9) | ||||
| BNIP3 (T) | 0.362 | <0.001 | <0.001 | <0.001 | |||||
| Negative | 257 (76.9) | 28 (52.8) | 46 (62.2) | 48 (72.7) | 135 (95.7) | ||||
| Positive | 77 (23.1) | 25 (47.2) | 28 (37.8) | 18 (27.3) | 6 (4.3) | ||||
| BNIP3 (S) | 0.088 | 0.010 | 0.009 | 0.328 | |||||
| Negative | 304 (91.0) | 44 (83.0) | 69 (93.2) | 56 (84.8) | 135 (95.7) | ||||
| Positive | 30 (9.0) | 9 (17.0) | 5 (6.8) | 10 (15.2) | 6 (4.3) | ||||
*P-value was from comparison among 4 groups.
P-value was from comparison between the AR+ and AR– group by Fisher exact test.
Correlation between Clinicopathologic Parameters and Expression of Autophagy-Related Proteins in ER-Negative Breast Cancer
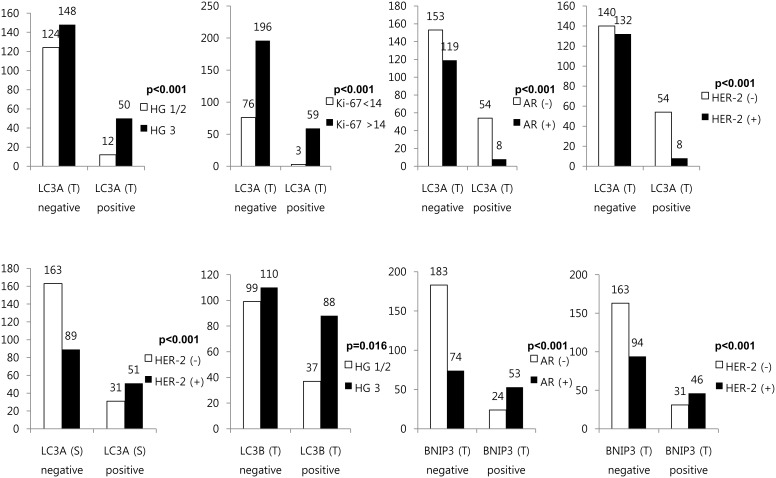
Autophagy-related protein expression according to clinicopathologic parameters was analyzed in ER-negative breast cancer (Figure 2). Tumoral LC3A positivity was significantly associated with higher histologic grade (P<0.001), higher Ki-67 LI (P<0.001), AR negativity (P<0.001), and HER-2 negativity (P<0.001), while stromal LC3A positivity showed a significant relationship with HER-2 positivity (P<0.001). Tumoral LC3B positivity was associated with higher histologic grade (P = 0.016), while tumoral BNIP3 positivity was significantly associated with AR positivity (P<0.001) and HER-2 positivity (P<0.001).
Figure 2. Correlation between clinicopathologic parameters and expression of autophagy-related proteins in ER-negative breast cancer.
In each of the four groups defined by AR and HER-2 status, we analyzed the autophagy-related protein expression according to clinicopathologic parameters. The AR (+)/HER-2 (+) group was the only group that showed a significant association between tumoral p62 positive and higher histologic grade (P = 0.032) as well as higher T stage (P = 0.032, Figure 3).
Figure 3. Correlation between clinicopathologic parameters and expression of autophagy-related proteins in the AR (+)/HER-2 (+) group.
In each of the groups, autophagy-related protein expression with clinicopathologic parameters was assessed according to AR status (Figure 4). Within the AR-negative group, tumoral LC3A positivity was associated with higher histologic grade (P = 0.016), higher Ki-67 LI (P = 0.001), AR negativity (P<0.001) and HER-2 negativity (P<0.001). Stromal LC3A positivity was associated with HER-2 positivity (P<0.001) and tumoral BNIP3 positivity was associated with HER-2 positivity (P<0.001).
Figure 4. Correlation between clinicopathologic parameters and expression of autophagy-related proteins in the AR-negative group.
Impact of Expression of Autophagy-Related Proteins on Prognosis
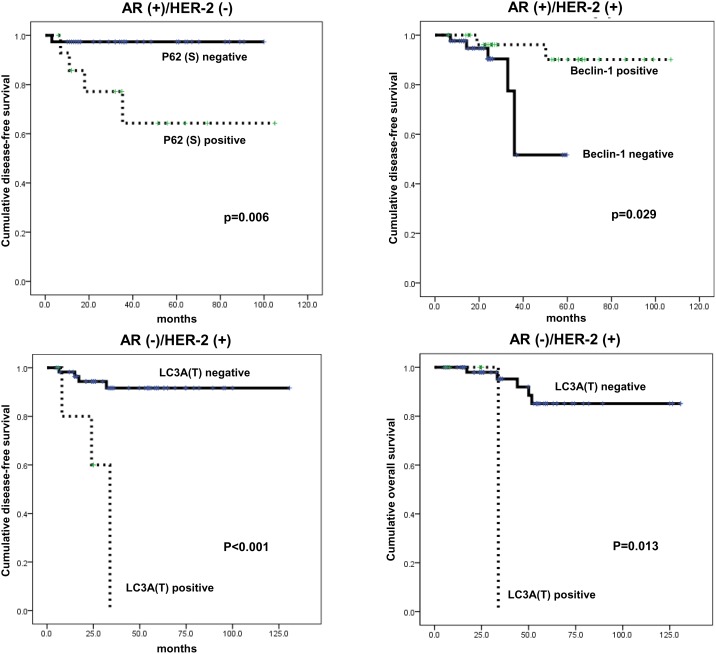
According to univariate analysis of autophagy-related protein expression with prognosis, no factors were significantly associated with prognosis (Table 4). However, amongst the four groups divided based on AR and HER-2 status, the AR (+)/HER-2 (–) group showed shorter disease-free survival (DFS) in association with stromal p62 positivity (P = 0.006), while in the AR (+)/HER-2 (+) group, shorter DFS was associated with tumoral beclin-1 negativity (P = 0.029). In the AR (–)/HER-2 (+) group, shorter DFS and shorter overall survival were significantly associated with tumoral LC3A positivity (P<0.001 and P = 0.013, respectively, Figure 5).
Table 4. Univariate analysis by log-rank test of the impact of metabolism-related protein expression in estrogen receptor-negative breast cancer on disease-free survival and overall survival times.
| Disease-free survival (months) | Overall survival (months) | ||||
| Parameters | 95% CI | P-value | 95% CI | P-value | |
| Beclin-1 (T) | 0.295 | 0.917 | |||
| Negative | 114 (106–123) | 124 (117–131) | |||
| Positive | 97 (92–101) | 119 (113–125) | |||
| LC3A (T) | 0.596 | 0.314 | |||
| Negative | 117 (110–125) | 123 (116–129) | |||
| Positive | 111 (100–122) | 129 (120–137) | |||
| LC3A (S) | 0.537 | 0.796 | |||
| Negative | 117 (109–124) | 125 (119–130) | |||
| Positive | 75 (70–80) | 76 (72–80) | |||
| LC3B (T) | 0.325 | 0.432 | |||
| Negative | 114 (105–123) | 121 (114–129) | |||
| Positive | 116 (108–124) | 121 (115–127) | |||
| p62 (T) | 0.907 | 0.289 | |||
| Negative | 112 (99–125) | 117 (106–128) | |||
| Positive | 117 (111–124) | 126 (120–131) | |||
| p62 (S) | 0.850 | 0.616 | |||
| Negative | 118 (111–125) | 126 (121–132) | |||
| Positive | 91 (83–99) | 111 (100–122) | |||
| BNIP3 (T) | 0.750 | n/a | |||
| Negative | 117 (110–124) | n/a | |||
| Positive | 90 (83–97) | n/a | |||
| BNIP3 (S) | 0.570 | n/a | |||
| Negative | 117 (110–124) | n/a | |||
| Positive | 93 (84–102) | n/a | |||
Figure 5. Impact of expression of autophagy-related proteins on prognosis according AR and HER-2 status in ER-negative breast cancer.
Multivariate Cox analysis revealed that in the AR (+)/HER-2 (–) group, stromal p62 positivity was an independent factor significantly associated with shorter DFS (Hazard ratio: 10.21, 95% CI: 1.130–92.31, P = 0.039, Table 5), while in the AR (–)/HER-2 (+) group, tumoral LC3A positivity was an independent factor significantly associated with shorter DFS (Hazard ratio: 10.28, 95% CI: 2.068–51.19, P = 0.004, Table 6).
Table 5. Multivariate analysis of DFS and OS in the AR (+)/HER-2 (–) group.
| Disease-free survival | Overall survival | ||||||
| Included parameters | Hazard ratio | 95% CI | P-value | Hazard ratio | 95% CI | P-value | |
| T stage | 0.426 | n/a | |||||
| T1 versus T2–3 | 2.693 | 0.235–30.85 | n/a | n/a | |||
| N stage | 0.708 | n/a | |||||
| N0 versus N1–3 | 1.409 | 0.234–8.491 | n/a | n/a | |||
| Histologic grade | 0.608 | n/a | |||||
| I/II versus III | 1.895 | 0.165–21.71 | n/a | n/a | |||
| p62 (S) | 0.039 | n/a | |||||
| Negative vs positive | 10.21 | 1.130–92.31 | n/a | n/a | |||
Table 6. Multivariate analysis of DFS and OS in the AR (–)/HER-2 (+) group.
| Disease-free survival | Overall survival | ||||||
| Included parameters | Hazard ratio | 95% CI | P-value | Hazard ratio | 95% CI | P-value | |
| T stage | 0.159 | 0.820 | |||||
| T1 vs T2–3 | 4.749 | 0.542–41.59 | 1.227 | 0.211–7.149 | |||
| N stage | 0.950 | 0.557 | |||||
| N0 vs N1–3 | 1.054 | 0.207–5.370 | 1.690 | 0.293–9.736 | |||
| Histologic grade | 0.773 | 0.776 | |||||
| I/II vs III | 0.794 | 0.167–3.789 | 0.774 | 0.132–4.522 | |||
| LC3A (T) | 0.004 | 0.086 | |||||
| Negative vs positive | 10.28 | 2.068–51.19 | 10.52 | 0.718–154.3 | |||
Discussion
In the current study, expression of autophagy-related proteins according to AR status in ER-negative breast cancer was evaluated. Tumoral LC3A expression was highest in AR-negative breast cancer, while tumoral BNIP3 was highest in AR-positive breast cancer. In previous studies on invasive breast cancers, expression of autophagy-related proteins such as LC3A, LC3B, and beclin-1 was shown to be associated with ER negativity and PR negativity [24]. Therefore, it was suggested that hormone receptor-negative breast cancers were more closely associated with autophagy activity, and for ER-negative breast cancers, particularly AR-negative breast cancers, there was an association with increased autophagy activity. Although there are no studies that have explored the relationship between AR and autophagy in breast cancer, there have been studies of prostate cancer tumors, which are most representative of AR-positive tumors. While there are reports that AR positivity promotes autophagy in prostate cancer [25], there are reports of AR positivity with lower levels of autophagy activity [26], [27]. The current study revealed that there was an association between AR negativity and LC3A expression in which was that of an inverse relationship. This inverse relationship can be explained through an adapted interpretation of what has been already reported in prostate cancer studies. One study concluded that endoplasmic reticulum chaperone glucose-regulated protein 78/BiP(Grp 78/Bip) is upregulated by AR, which ultimately results in autophagy inhibition [26]. Another study demonstrated that AR increases p62 expression, which in turn inhibits autophagy [28]. Regardless of whether AR promotes or inhibits autophagy in prostate cancer, there is a consistent finding among these different studies that AR does play a role in cancer cell growth. Further in vitro cellular studies of AR and autophagy in breast cancer are required.
Our study is unique because this is the first in literature to analyze the relationship between BNIP3 and AR. Previous studies have done so far as to observe an overexpression of BNIP3 in breast cancers [29]. The relationship between BNIP3 and AR expressions would be explained through the mechanism of autocrine loop of tyrosine kinase receptor/phosphatidylinositol 3′-kinase/protein kinase B. In a AR positive prostate cancer cell study, androgen had activated this tyrosine kinase receptor/phosphatidylinositol 3′-kinase/protein kinase B which increases HIF-1α and HIF-α regulated gene expression [30]. BNIP3 happens to be one such HIF-α regulated gene [31].
In the current study of breast cancers, BNIP3 was highly expressed in the AR-positive group, which corresponded with a previous report of a significant association between BNIP3 expression and AR in prostate cancers [32]. The proposed mechanism for these findings is as follows; in prostate cancer, androgen is bound to AR, which activates HIF-1α through a cascade of several protein kinase systems, continuously increasing expression of the HIF-1α-related gene, one of which is BNIP3. BNIP3 increases mitophagy and prevents oxidative phosphorylation in mitochondria [33], [34]. Therefore, metabolism of tumor cells is shifted from mitochondrial oxidative phosphorylation to oxidative glycolysis, known as the Warburg effect [35]. Although it remains unclear if similar mechanisms are at work between AR and BNIP3 in breast cancer, as shown with prostate cancer, this study demonstrates that the AR-positive group has higher expression of BNIP3 in ER-negative breast cancer, suggesting possible causal mitochondrial dysfunction within the AR-positive group in ER-negative breast cancer.
Within the AR-negative group, there was a significant difference in LC3A and BNIP3 expression between the HER-2-positive and HER-2-negative groups. However, in the AR-positive group, there was no significant difference between HER-2-positive and HER-2-negative groups in autophagy-related protein expression. The ER (–)/AR (–)/HER-2 (+) group can be presumed to be mostly the HER-2 type, and the ER (–)/AR (–)/HER-2 (–) group is thought to be the basal-like/triple negative types. Because HER-2 type and basal-like/triple negative type are both distinct clinicopathologic entities [36], [37], differences in autophagy activity according to HER-2 status in the AR-negative group could be explained. The ER-negative and AR-positive group could be classified as molecular apocrine breast cancer (MABC) according to surrogate immunohistochemical markers in this study. In the literature, MABC is reportedly 20–50% HER-2 overexpressed/amplified [38], [39]. In the current study, 58.3% had HER-2 overexpression/amplification that was similar to the reported value. Although HER-2 status is an important biomarker in breast cancers, the current study revealed that there is no difference in the expression of autophagy-related proteins between the AR (+)/HER-2 (–) group and AR (+)/HER-2 (+) group, which is compatible with results from a previous study indicating that MABC does not exhibit differences in tumor characteristics according to HER-2 status [38].
Our study observed cytoplasmic expression of LC3A, LC3B and BNIP3 and nuclear expression in beclin-1 and p62. Previous reports noted that different expression patterns of LC3A had resulted in different biologic behaviors of tumors; in tumors with diffuse cytoplasmic or perinuclear LC3A expression had an association with ER and PR positivity, whereas those tumors with stone-like pattern of LC3A expression was associated with ER and PR negativity and a worse prognosis [10]. In the current study, there were no cases with stone-like pattern expression of LC3A. Instead, the majority exhibited cytoplasmic and/or perinuclear pattern. This may be due to difference in the antibody used from previous reports. However there are still other studies that support the observation that according to the type of tumor there may be no stone-like pattern expression of LC3A. All in all, LC3A seems to be an area that merits much study [40], [41]. In the case of p62 and beclin1, nuclear expression was observed in addition to cytoplasmic expression. This finding is in agreement with previous reports that observed both nuclear and cytoplasmic expression of p62 [42], [43]. This finding is attributed to the fact that p62 is a major component of the nuclear pore complex that functions as a nucleocytoplasmic transport thereby allowing it to exist both in nucleic and cytoplasmic compartments [22]. Beclin1 is also known to be expressed in both nucleic and cytoplasmic compartments [23]. Although there is no study dealing with the nuclear expression of beclin1, in one study on brain tumors, beclin1 tended to shift towards nucleic expression as the grade of the tumor worsens. This observation signified that beclin1 protein transports between both nucleic and cytoplasmic compartments, but more importantly this shift in expression implied the loss of becline1 gene function [23]. In summary, nucleic expression of beclin1 would imply a suspension of its role in autophagy regulation.
Although there was no association between autophagy-related protein expression and breast cancer prognosis in ER-negative breast cancer, in the AR (+)/HER-2 (–) group, stromal p62 positivity was an independent factor associated with shorter DFS, while in the AR (–)/HER-2 (+) group, tumoral LC3A positivity was an independent factor for shorter DFS. Previous studies have revealed that LC3A expression in ovarian clear cell carcinoma [44], non-small cell lung carcinoma [45], and colorectal adenocarcinoma [46] is associated with poor prognosis, providing a basis for the claim that increased autophagy associates with poor prognosis. With this in mind, the reason that LC3A was associated with poor prognosis only in the AR (–)/HER-2 (+) group is a question that requires further study. Also, it was stromal p62 expression that was associated with prognosis rather than tumoral p62 expression. Expression of p62, LC3A, LC3B, and BNIP3 in stromal cells in breast cancer was reported in a previous study [24]. The association between stromal p62 expression and poor prognosis may be explained by the reverse Warburg effect theory. The reverse Warburg effect is a theory that proposes a metabolic interaction between tumor cells and stromal cells in breast cancer, where the reactive oxygen species produced from tumor cells result in glycolysis, mitochondrial dysfunction, and increased autophagy in stromal cells. In addition, the lactate produced from stromal cell glycolysis is in turn utilized by the tumors in oxidative phosphorylation to produce ATP [47]–[51]. Therefore, breast cancers that produce energy by the reverse Warburg effect have an advantage in tumor growth and maintenance. If the AR (+)/HER-2 (–) group, in which stromal cells express p62, is presumed to produce energy by the reverse Warburg effect, it may be then associated with poor prognosis. The stromal cell that shows increased autophagy activity is called the cancer-associated fibroblast according to the reverse Warburg effect theory, and it is characterized by caveolin-1 loss[49]. Caveolin-1 loss is reported to occur in 5–40% of all breast cancers [52]–[54], and therefore only a portion of breast cancers would be exhibiting the reverse Warburg effect.
In conclusion, there was a significant difference in autophagy-related protein expression according to AR status in ER-negative breast cancer. Tumoral LC3A expression was higher in AR-negative breast cancer, while tumoral BNIP3 was higher in AR-positive breast cancer.
Funding Statement
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF), funded by the Ministry of Education, Science and Technology (2012R1A1A1002886). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Levine B, Klionsky DJ (2004) Development by self-digestion: molecular mechanisms and biological functions of autophagy. Dev Cell 6: 463–477. [DOI] [PubMed] [Google Scholar]
- 2. Mizushima N (2007) Autophagy: process and function. Genes Dev 21: 2861–2873. [DOI] [PubMed] [Google Scholar]
- 3. Mizushima N, Levine B, Cuervo AM, Klionsky DJ (2008) Autophagy fights disease through cellular self-digestion. Nature 451: 1069–1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Yang Z, Klionsky DJ (2010) Eaten alive: a history of macroautophagy. Nat Cell Biol 12: 814–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chen Y, Lu Y, Lu C, Zhang L (2009) Beclin-1 expression is a predictor of clinical outcome in patients with esophageal squamous cell carcinoma and correlated to hypoxia-inducible factor (HIF)-1alpha expression. Pathol Oncol Res 15: 487–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Li BX, Li CY, Peng RQ, Wu XJ, Wang HY, et al. (2009) The expression of beclin 1 is associated with favorable prognosis in stage IIIB colon cancers. Autophagy 5: 303–306. [DOI] [PubMed] [Google Scholar]
- 7. Pirtoli L, Cevenini G, Tini P, Vannini M, Oliveri G, et al. (2009) The prognostic role of Beclin 1 protein expression in high-grade gliomas. Autophagy 5: 930–936. [DOI] [PubMed] [Google Scholar]
- 8. Wan XB, Fan XJ, Chen MY, Xiang J, Huang PY, et al. (2010) Elevated Beclin 1 expression is correlated with HIF-1alpha in predicting poor prognosis of nasopharyngeal carcinoma. Autophagy 6: 395–404. [DOI] [PubMed] [Google Scholar]
- 9. Kabeya Y, Mizushima N, Ueno T, Yamamoto A, Kirisako T, et al. (2000) LC3, a mammalian homologue of yeast Apg8p, is localized in autophagosome membranes after processing. EMBO J 19: 5720–5728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sivridis E, Koukourakis MI, Zois CE, Ledaki I, Ferguson DJ, et al. (2010) LC3A-positive light microscopy detected patterns of autophagy and prognosis in operable breast carcinomas. Am J Pathol 176: 2477–2489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Yoshioka A, Miyata H, Doki Y, Yamasaki M, Sohma I, et al. (2008) LC3, an autophagosome marker, is highly expressed in gastrointestinal cancers. Int J Oncol 33: 461–468. [PubMed] [Google Scholar]
- 12. Degenhardt K, Mathew R, Beaudoin B, Bray K, Anderson D, et al. (2006) Autophagy promotes tumor cell survival and restricts necrosis, inflammation, and tumorigenesis. Cancer Cell 10: 51–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Roy S, Debnath J (2010) Autophagy and tumorigenesis. Semin Immunopathol 32: 383–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kuenen-Boumeester V, Van der Kwast TH, Claassen CC, Look MP, Liem GS, et al. (1996) The clinical significance of androgen receptors in breast cancer and their relation to histological and cell biological parameters. Eur J Cancer 32A: 1560–1565. [DOI] [PubMed] [Google Scholar]
- 15. Riva C, Dainese E, Caprara G, Rocca PC, Massarelli G, et al. (2005) Immunohistochemical study of androgen receptors in breast carcinoma. Evidence of their frequent expression in lobular carcinoma. Virchows Arch 447: 695–700. [DOI] [PubMed] [Google Scholar]
- 16. Choi J, Jung WH, Koo JS (2013) Metabolism-related proteins are differentially expressed according to the molecular subtype of invasive breast cancer defined by surrogate immunohistochemistry. Pathobiology 80: 41–52. [DOI] [PubMed] [Google Scholar]
- 17. Pinheiro C, Sousa B, Albergaria A, Paredes J, Dufloth R, et al. (2011) GLUT1 and CAIX expression profiles in breast cancer correlate with adverse prognostic factors and MCT1 overexpression. Histol Histopathol 26: 1279–1286. [DOI] [PubMed] [Google Scholar]
- 18. Hammond ME, Hayes DF, Dowsett M, Allred DC, Hagerty KL, et al. (2010) American Society of Clinical Oncology/College Of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. J Clin Oncol 28: 2784–2795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wolff AC, Hammond ME, Schwartz JN, Hagerty KL, Allred DC, et al. (2007) American Society of Clinical Oncology/College of American Pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. J Clin Oncol 25: 118–145. [DOI] [PubMed] [Google Scholar]
- 20. Elston CW, Ellis IO (1991) Pathological prognostic factors in breast cancer. I. The value of histological grade in breast cancer: experience from a large study with long-term follow-up. Histopathology 19: 403–410. [DOI] [PubMed] [Google Scholar]
- 21. Won KY, Kim GY, Kim YW, Song JY, Lim SJ (2010) Clinicopathologic correlation of beclin-1 and bcl-2 expression in human breast cancer. Hum Pathol 41: 107–112. [DOI] [PubMed] [Google Scholar]
- 22. Fukuhara T, Sakaguchi N, Katahira J, Yoneda Y, Ogino K, et al. (2006) Functional analysis of nuclear pore complex protein Nup62/p62 using monoclonal antibodies. Hybridoma (Larchmt) 25: 51–59. [DOI] [PubMed] [Google Scholar]
- 23. Miracco C, Cosci E, Oliveri G, Luzi P, Pacenti L, et al. (2007) Protein and mRNA expression of autophagy gene Beclin 1 in human brain tumours. Int J Oncol 30: 429–436. [PubMed] [Google Scholar]
- 24. Choi J, Jung W, Koo JS (2013) Expression of autophagy-related markers beclin-1, light chain 3A, light chain 3B and p62 according to the molecular subtype of breast cancer. Histopathology 62: 275–286. [DOI] [PubMed] [Google Scholar]
- 25. Shi Y, Han JJ, Tennakoon JB, Mehta FF, Merchant FA, et al. (2013) Androgens promote prostate cancer cell growth through induction of autophagy. Mol Endocrinol 27: 280–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bennett HL, Fleming JT, O’Prey J, Ryan KM, Leung HY (2010) Androgens modulate autophagy and cell death via regulation of the endoplasmic reticulum chaperone glucose-regulated protein 78/BiP in prostate cancer cells. Cell Death Dis 1: e72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Jiang Q, Yeh S, Wang X, Xu D, Zhang Q, et al. (2012) Targeting androgen receptor leads to suppression of prostate cancer via induction of autophagy. J Urol 188: 1361–1368. [DOI] [PubMed] [Google Scholar]
- 28. Arner ES, Holmgren A (2006) The thioredoxin system in cancer-introduction to a thematic volume of Seminars in Cancer Biology. Semin Cancer Biol 16: 419. [DOI] [PubMed] [Google Scholar]
- 29. Arner ES, Holmgren A (2006) The thioredoxin system in cancer. Semin Cancer Biol 16: 420–426. [DOI] [PubMed] [Google Scholar]
- 30. Barth RJ Jr (1999) Histologic features predict local recurrence after breast conserving therapy of phyllodes tumors. Breast Cancer Res Treat 57: 291–295. [DOI] [PubMed] [Google Scholar]
- 31. Behrend L, Henderson G, Zwacka RM (2003) Reactive oxygen species in oncogenic transformation. Biochem Soc Trans 31: 1441–1444. [DOI] [PubMed] [Google Scholar]
- 32. Shaida N, Launchbury R, Boddy JL, Jones C, Campo L, et al. (2008) Expression of BNIP3 correlates with hypoxia-inducible factor (HIF)-1alpha, HIF-2alpha and the androgen receptor in prostate cancer and is regulated directly by hypoxia but not androgens in cell lines. Prostate 68: 336–343. [DOI] [PubMed] [Google Scholar]
- 33. Bellot G, Garcia-Medina R, Gounon P, Chiche J, Roux D, et al. (2009) Hypoxia-induced autophagy is mediated through hypoxia-inducible factor induction of BNIP3 and BNIP3L via their BH3 domains. Mol Cell Biol 29: 2570–2581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Zhang H, Bosch-Marce M, Shimoda LA, Tan YS, Baek JH, et al. (2008) Mitochondrial autophagy is an HIF-1-dependent adaptive metabolic response to hypoxia. J Biol Chem 283: 10892–10903. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 35. Warburg O (1956) On the origin of cancer cells. Science 123: 309–314. [DOI] [PubMed] [Google Scholar]
- 36. Sorlie T, Perou CM, Tibshirani R, Aas T, Geisler S, et al. (2001) Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci U S A 98: 10869–10874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Perou CM, Sorlie T, Eisen MB, van de Rijn M, Jeffrey SS, et al. (2000) Molecular portraits of human breast tumours. Nature 406: 747–752. [DOI] [PubMed] [Google Scholar]
- 38. Farmer P, Bonnefoi H, Becette V, Tubiana-Hulin M, Fumoleau P, et al. (2005) Identification of molecular apocrine breast tumours by microarray analysis. Oncogene 24: 4660–4671. [DOI] [PubMed] [Google Scholar]
- 39. Banneau G, Guedj M, MacGrogan G, de Mascarel I, Velasco V, et al. (2010) Molecular apocrine differentiation is a common feature of breast cancer in patients with germline PTEN mutations. Breast Cancer Res 12: R63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Giatromanolaki AN, Charitoudis GS, Bechrakis NE, Kozobolis VP, Koukourakis MI, et al. (2011) Autophagy patterns and prognosis in uveal melanomas. Mod Pathol 24: 1036–1045. [DOI] [PubMed] [Google Scholar]
- 41. Sivridis E, Koukourakis MI, Mendrinos SE, Karpouzis A, Fiska A, et al. (2011) Beclin-1 and LC3A expression in cutaneous malignant melanomas: a biphasic survival pattern for beclin-1. Melanoma Res 21: 188–195. [DOI] [PubMed] [Google Scholar]
- 42.Tavassoli FA, Devilee P (2003) Pathology and genetics of tumors of the breast and female genital organs. In: Kleihues P, Sobin LH, editors. IARC WHO Classification of Tumours. Lyon: International Agency for Research on Cancer (IARC). [Google Scholar]
- 43. Williams AR, Piris J, Wyllie AH (1990) Immunohistochemical demonstration of altered intracellular localization of the C-Myc oncogene product in human colorectal neoplasms. J Pathol 160: 287–293. [DOI] [PubMed] [Google Scholar]
- 44. Spowart JE, Townsend KN, Huwait H, Eshragh S, West NR, et al. (2012) The Autophagy Protein LC3A Correlates with Hypoxia and is a Prognostic Marker of Patient Survival in Clear Cell Ovarian Cancer. J Pathol. [DOI] [PubMed] [Google Scholar]
- 45. Karpathiou G, Sivridis E, Koukourakis MI, Mikroulis D, Bouros D, et al. (2011) Light-chain 3A autophagic activity and prognostic significance in non-small cell lung carcinomas. Chest 140: 127–134. [DOI] [PubMed] [Google Scholar]
- 46. Giatromanolaki A, Koukourakis MI, Harris AL, Polychronidis A, Gatter KC, et al. (2010) Prognostic relevance of light chain 3 (LC3A) autophagy patterns in colorectal adenocarcinomas. J Clin Pathol 63: 867–872. [DOI] [PubMed] [Google Scholar]
- 47. Bonuccelli G, Tsirigos A, Whitaker-Menezes D, Pavlides S, Pestell RG, et al. (2010) Ketones and lactate “fuel” tumor growth and metastasis: Evidence that epithelial cancer cells use oxidative mitochondrial metabolism. Cell Cycle 9: 3506–3514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Martinez-Outschoorn UE, Balliet RM, Rivadeneira DB, Chiavarina B, Pavlides S, et al. (2010) Oxidative stress in cancer associated fibroblasts drives tumor-stroma co-evolution: A new paradigm for understanding tumor metabolism, the field effect and genomic instability in cancer cells. Cell Cycle 9: 3256–3276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Pavlides S, Tsirigos A, Vera I, Flomenberg N, Frank PG, et al. (2010) Loss of stromal caveolin-1 leads to oxidative stress, mimics hypoxia and drives inflammation in the tumor microenvironment, conferring the “reverse Warburg effect”: a transcriptional informatics analysis with validation. Cell Cycle 9: 2201–2219. [DOI] [PubMed] [Google Scholar]
- 50. Pavlides S, Whitaker-Menezes D, Castello-Cros R, Flomenberg N, Witkiewicz AK, et al. (2009) The reverse Warburg effect: aerobic glycolysis in cancer associated fibroblasts and the tumor stroma. Cell Cycle 8: 3984–4001. [DOI] [PubMed] [Google Scholar]
- 51. Witkiewicz AK, Whitaker-Menezes D, Dasgupta A, Philp NJ, Lin Z, et al. (2012) Using the “reverse Warburg effect” to identify high-risk breast cancer patients: stromal MCT4 predicts poor clinical outcome in triple-negative breast cancers. Cell Cycle 11: 1108–1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Koo JS, Park S, Kim SI, Lee S, Park BW (2011) The impact of caveolin protein expression in tumor stroma on prognosis of breast cancer. Tumour Biol 32: 787–799. [DOI] [PubMed] [Google Scholar]
- 53. Sloan EK, Ciocca DR, Pouliot N, Natoli A, Restall C, et al. (2009) Stromal cell expression of caveolin-1 predicts outcome in breast cancer. Am J Pathol 174: 2035–2043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Witkiewicz AK, Dasgupta A, Nguyen KH, Liu C, Kovatich AJ, et al. (2009) Stromal caveolin-1 levels predict early DCIS progression to invasive breast cancer. Cancer Biol Ther 8: 1071–1079. [DOI] [PubMed] [Google Scholar]