Abstract
Quinolone has the disadvantage of easily acquired drug resistance. It is important to prescribe it wisely for a high eradication rate. The current study aimed to determine the clinical and bacteriological factors for optimal levofloxacin-containing triple therapies in second-line H. pylori eradication. We enrolled a total of 158 H. pylori-infected patients who failed H. pylori eradication using the 7-day standard triple therapy (proton-pump inhibitor [PPI] twice daily, 500 mg clarithromycin twice daily, and 1 g amoxicillin twice daily). They were prescribed with either a 10-day (group A) or 14-day (group B) levofloxacin-containing triple therapy group (levofloxacin 500 mg once daily, amoxicillin 1 g twice daily, and esomeprazole 40 mg twice daily for 10 days) by their clinicians. Follow-up studies to assess treatment responses were carried out 8 weeks later. The eradication rates attained by groups A and B were 73.6% (95% confidence interval [CI] = 63.9–85.3%) and 90.5% (95% CI = 84.5–98.1%), respectively in the per protocol analysis (P = 0.008 in the per protocol analysis) and 67.1% (95% CI = 56.6–78.5%) and 84.8% (95% CI = 76.8–93.4%), respectively, in the intention-to-treat analysis (P = 0.009). The subgroup analysis revealed that H. pylori eradication rates for group A patients with levofloxacin-susceptible strains were 92.9% (13/14) but it dropped to 12.5% (1/8) when levofloxacin-resistant strains existed. H. pylori was eradicated among all the group B patients with levofloxacin-susceptible strains, but only half of patients with levofloxacin-resistant strains were successfully eradicated. In conclusion, this study confirms the effectiveness of 14-day treatment. Importantly, the results imply that 10-day treatment duration should be optimal if a culture can be performed to confirm the existence of susceptible strains. The duration of H. pylori eradication and levofloxacin resistance were the influencing factors for successful treatment. This study suggests that tailored levofloxacin-containing therapy should be administered only for patients with susceptible strains because it can achieve >90% success rates.
Introduction
The Maastricht IV/Florence-Consensus Report states that after failure of a proton-pump inhibitor (PPI)-clarithromycin-containing treatment, either a bismuth-containing quadruple therapy or levofloxacin-containing triple therapy is recommended in regions of clarithromycin resistance [1]. In areas of high clarithromycin resistance, levofloxacin containing triple therapy is recommended after failure of bismuth-containing quadruple therapy. In both cases, rising rates of levofloxacin resistance should be taken into account.
Avoiding problems due to antibiotic resistance has become an important issue when deciding a second-line therapy for H. pylori infection [2]–[4]. Quinolone has the disadvantage of easily acquired drug resistance [5]. It is therefore an important issue to prescribe it wisely to achieve a high eradication rate. Obviously, a quinolone-containing therapy is an important salvage therapy recommended by both the Maastricht IV/Florence-Consensus Report and the second Asian Pacific Consensus Guidelines [1], [6]. In second-line H. pylori eradication with fluoroquinolone-based triple therapy it has been shown that neither 7 nor 10 days of therapy provides a grade B or better report card [7]. Nevertheless, 10-day fluroquinolone triple therapies are still being used as rescue therapies in many countries [8]–[10]. The other key factor to eradication success is antibiotic susceptibility [11], [12]. The current study aimed to determine the clinical and bacteriological factors for optimal levofloxacin-containing triple therapies in second-line H. pylori eradication. Optimal levofloxacin-containing triple therapies refer to therapeutic regimens that have 90% or greater (grade B level) and probably at 95% or greater (grade A level) to meet the existing practice criteria (13).
Materials and Methods
Ethics Statement
This retrospective study was approved by both the Institutional Review Board and the Ethics Committee of Chang Gung Memorial Hospital (IRB102-5044B). All patients provided their written inform consent before undergoing endoscopic interventions. None of our patients belonged to the minors/children group.
Patients
A total of 158 H. pylori-infected patients who failed H. pylori eradication using the standard triple therapy (PPI twice daily, 500 mg clarithromycin twice daily, and 1 g amoxicillin twice daily) for 7 days were recruited from our registered files. All patients were at least 18 years of age and had received endoscope examinations that showed peptic ulcers or gastritis. The confirmation of H. pylori eradication failure was defined as patients with either one positive 13C-UBT or any two positive of the rapid urease test, histology and culture after first-line eradication therapy. These patients were prescribed a levofloxacin-containing triple therapy group (levofloxacin 500 mg once daily, amoxicillin 1 g twice daily, and esomeprazole 40 mg twice daily for 10 days) for either 10 or 14 days (groups A and B, respectively). According to hospital requirements, all registered patients were followed-up to assess drug compliance and adverse effects after they finished the medication regimens. These patients underwent either an endoscopy or a urea breath test 8 weeks later. We also performed a back-up urea breath test on all participants to avoid any false-negative results. Poor compliance was defined as failure to finish 80% of all medication due to adverse effects [14], [15].
Outcomes
The primary endpoint was the successful eradication of H. pylori. We also analyzed antibiotic susceptibility.
Culture and antimicrobial resistance
One antral gastric and one corpus biopsy specimen were obtained for H. pylori isolation using previously described culture methods [16]. The biopsy specimens were cultured on plates containing Brucella chocolate agar with 7% sheep blood and incubated for 4–5 days under micro-aerobic conditions. The minimal inhibitory concentration (MIC) was determined by the agar dilution test. The H. pylori strains were tested for amoxicillin, levofloxacin, metronidazole and tetracycline susceptibility using the E-test (AB Biodisck, Solna, Sweden). H. pylori strains with MIC values >0.5 µg/mL, >1 µg/mL, >8 µg/mL, and 4 µg/mL were considered to be the resistant breakpoints for amoxicillin, levofloxacin, and tetracycline respectively.
Statistical analysis
The primary outcome variables were the eradication rate, presence of adverse events, and level of patient compliance. Using the SPSS program (Statistical Package for the Social Sciences version 18, Chicago, IL, USA), Chi-square tests with or without Yates’ correction for continuity and Fisher’s exact tests were used when appropriate to compare the major outcomes between groups. Eradication rates were analyzed by both the intent-to-treat (ITT) and per-protocol (PP) approaches. ITT analysis included all assigned patients who had taken at least one dose of the study medication. Patients whose infection status was unknown following treatment were considered treatment failures for the purposes of the ITT analysis. The PP analysis excluded patients with unknown H. pylori status following therapy and those with major protocol violations. A P-value <0.05 was considered statistically significant. To determine the independent factors that affected treatment response, the clinical and bacterial parameters were analyzed by univariate and multivariate analyses.
Results
A total of 158 patients were enrolled (n = 79 per group). Ultimately, 7 and 5 patients were lost during follow-up in groups A and B, respectively, resulting in 72 in the PP study for group A and 74 for group B. The demographic data of the two groups are summarized in Table 1; none of the variables was significantly different between the groups.
Table 1. Demographic data and endoscopic appearances of the two patient groups.
| Characteristics | Group A (n = 72) | Group B (n = 74) | P-value |
| Age (years) (mean ± SD) | 55.5±12.1 | 56.3±13.2 | 0.703 |
| Gender (male/female) | 34/45 | 39/40 | 0.425 |
| Smoking | 7 (9.7%) | 6 (8.1%) | 0.732 |
| Previous history of peptic ulcer | 69 (95.8%) | 72 (97.2%) | 0.627 |
| Endoscopic findings | |||
| Gastric ulcer | 28 (38.9%) | 27 (36.5%) | 0.765 |
| Duodenal ulcer | 11 (15.3%) | 14 (18.9%) | 0.559 |
| Gastric and duodenal ulcer | 6 (8.3%) | 11 (14.8%) | 0.219 |
| Unspecified (including gastritis) | 27 (37.5%) | 22 (29.7%) | 0.320 |
Group A: 10-day esomeprazole/amoxicillin/levofloxacin triple therapy;
Group B: 14-day esomeprazole/amoxicillin/levofloxacin triple therapy.
The eradication rates in groups A and B are detailed in Table 2. They were 73.6% (95% confidence interval [CI] = 63.9–85.3%) and 90.5% (95% CI = 84.5–98.1%), respectively, in the PP analysis (P = 0.008) and 67.1% (95% CI = 56.6–78.5%) and 84.8% (95% CI = 76.8–93.4%), respectively, in the ITT analysis (P = 0.009).
Table 2. Major outcomes of eradication therapy.
| Eradication rate | |||
| Group A | Group B | P-value | |
| Intention-to-treat | 67.1% (53/79) | 84.8% (67/79) | 0.009 |
| Per-protocol | 73.6% (53/72) | 90.5% (67/74) | 0.008 |
| Adverse event | 15.3% (11/72) | 28.4% (21/74) | 0.412 |
| Compliance | 100% (72/72) | 98.6% (73/74) | 0.322 |
Group A: 10-day esomeprazole/amoxicillin/levofloxacin triple therapy;
Group B: 14-day esomeprazole/amoxicillin/levofloxacin triple therapy.
Adverse events and complications
The adverse event rates were 15.3% (11/72) in group A and 28.4% (21/74) in group B (Table 3). These adverse events included abdominal pain, constipation, diarrhea, dizziness, headache, nausea/vomiting, and skin rash; however, these were mild and did not markedly disturb the patients’ daily activities with the exception of one patient who complained of headache after taking the medications and stopped taking them after 1 week. Both groups had good drug compliances (100% in group A vs. 98.6% group B).
Table 3. Adverse events during eradication therapies.
| Adverse event | Group A | Group B | P-value |
| Abdominal pain | 5 | 6 | 0.792 |
| Constipation | 1 | 2 | 0.576 |
| Diarrhea | 0 | 3 | 0.084 |
| Dizziness | 4 | 1 | 0.163 |
| Headache | 4 | 2 | 0.385 |
| Nausea/vomiting | 1 | 2 | 0.576 |
| Skin rash | 0 | 2 | 0.160 |
Group A: 10-day esomeprazole/amoxicillin/levofloxacin triple therapy;
Group B: 14-day esomeprazole/amoxicillin/levofloxacin triple therapy.
Antibiotic resistance
Samples from 60 patients were cultured for H. pylori, and the positive culture rate was 73.3% (44/60). Hence, the antibiotic resistance rates were 2.3% (1/44) for amoxicillin and 31.8% (14/44) for levofloxacin.
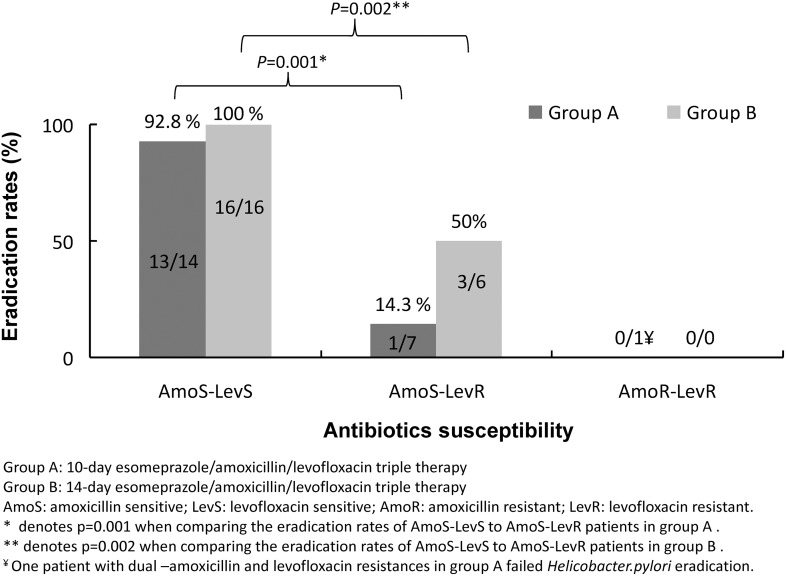
Overall, the H. pylori eradication rates for the levofloxacin-susceptible strains and levofloxacin-resistant strains were 96.6% (29/30) and 28.6% (4/14) in the PP analysis. Among patients with susceptible strains to both amoxicillin and levofloxacin (AmoS-LevS), 13 of the 14 patients (92.9%) in group A and all the 16 patients in group B (100%) were eradicated successfully (Figure 1). There was only one of the seven patients with amoxicillin susceptible but levofloxacin-resistant strains (AmoS-LevR) attained eradication in group A (12.5%, P = 0.001 compared to AmoS-LevS patients). However, three of the six patients with AmoS-LevR straints were eradicated in group B (50%, P = 0.002 compared to AmoS-LevS patients). Overall, there was only one patient with dual resistant to both amoxicillin and levofloxacin and he failed the eradication. In addition, we also observed that more patients in group B with amoxicillin-susceptible strains were successfully eradicated (86.3%) compared to group A (66.7%).
Figure 1. Antibiotic resistance and H. pylori eradication rate.
Factors influencing the efficacy of anti-H. pylori therapies
Univariate analysis showed that duration of H. pylori eradication (P = 0.008), compliance (P = 0.031), amoxicillin resistance (P = 0.046), and levofloxacin resistance (P<0.001) were the clinical factors influencing the efficacy of H. pylori eradication therapy (Table 4). Multivariate analysis revealed that the duration of H. pylori eradication (P<0.014) and levofloxacin resistance (P<0.001) were independent risk factors for eradication failure (Table 5).
Table 4. Univariate analysis of the clinical factors influencing the efficacy of H. pylori eradication.
| Principle parameter | Case no. | Eradication rate | P-value | |
| Age | <60 years | 75/93 | 86.6% | 0.518 |
| ≥60 years | 45/53 | 84.9% | ||
| Sex | Female | 58/76 | 76.3% | 0.053 |
| Male | 62/70 | 88.6% | ||
| Smoking | (−) | 108/133 | 81.2% | 0.318 |
| (+) | 12/13 | 92.3% | ||
| Previous history of peptic ulcer | (−) | 4/5 | 80.0% | 0.895 |
| (+) | 116/141 | 82.3% | ||
| HP eradication (per-protocol) | 10-day | 53/72 | 73.6% | 0.008 |
| 14-day | 67/74 | 90.5% | ||
| Compliance | Good | 120/145 | 82.8% | 0.031 |
| Poor | 0/1 | 0% | ||
| H. pylori culture | ||||
| Amoxicillin susceptible | 43 | 33 (76.7%) | 0.046 | |
| Resistance | 1 | 0 (0%) | ||
| Levofloxacin susceptible | 30 | 29 (96.6%) | <0.001 | |
| Resistance | 14 | 4 (28.5%) | ||
Group A: 10-day esomeprazole/amoxicillin/levofloxacin triple therapy;
Group B: 14-day esomeprazole/amoxicillin/levofloxacin triple therapy.
Table 5. Multivariate analysis of the clinical factors influencing the efficacy of H. pylori eradication.
| Clinical factor | Coefficient | Standard error | Odds ratio (95% CI) | P-value |
| Duration of eradication (10-day vs. 14-day) | 1.25 | 0.81 | 5.79 (1.29±9.50) | 0.014 |
| Levofloxacin (susceptible vs. resistance) | 4.28 | 1.18 | 72.50 (7.22–727.61) | <0.001 |
Discussion
The emergence of H. pylori strains resistant to both clarithromycin and metronidazole necessitates the identification of other rescue therapy options after initial eradication failure. Fluoroquinolones have been candidates for such rescue eradication regimens. One of these fluoroquinolones, levofloxacin, has been recommended as the second-line regimen for people who fail classical first-line therapy containing clarithromycin for H. pylori eradication in the Maastricht-Florence IV consensus report [1]. There are many reasons for this. Firstly, levofloxacin exerts remarkable in vitro activity against H. pylori [17]. Second, there is an in vitro synergistic effect of quinolone antimicrobial agents and PPIs on certain H. pylori strains [18]. Third, an in vitro study showed that levofloxacin retains its activity even when H. pylori strains are resistant to clarithromycin and metronidazole [19]. The mechanisms of action of these three antibiotics are different; therefore clarithromycin and metronidazole resistance will not influence the bactericidal activity of levofloxacin. Finally, this therapy is simple and well tolerated, with a high compliance rate (100% and 98.6% in groups A and B in the present study). Although the incidences of adverse events in groups A and B were 15.3% and 28.4%, respectively, they were mild and tolerable. Only one patient had poor compliance due to adverse events; he did not finish his medication, which resulted in eradication failure.
Generally, the eradication rate of a 10-day levofloxacin-containing regimen has been shown to be higher than that of a 7-day treatment course; therefore 10-day regimens have been widely applied as second-line therapies [9], [10]. However, studies using 7–10-day fluoroquinolone triple therapies have not been shown to obtain eradication rates >90%. Three 14-day studies that included fluoroquinolone reported eradication rates >90% were recently published [20]–[22]. The current study results further confirm the efficacy of a 14-day regimen; we attained a 90.5% PP success rate for the eradication of H. pylori infection.
The shortcoming of our previous report was that we did not perform antibiotics susceptibility to that patient cohort (22). In the current study cohort, sixty patients agreed to perform endoscopy for H. pylori culture. The novelty of this study was that we observed that even a 10-day levofloxacin-containing regimen achieved an eradication rate >90% (92.9%) against levofloxacin-susceptible strains, but there was only one patient with resistant strains who achieved eradication. This is a very important observation that suggests the identification of levofloxacin-susceptible strains before prescribing a treatment regimen. Generally, antibiotic resistance remains the key factor that is associated with either success/failure when treating H. pylori. Unfortunately, resistance to quinolone antimicrobial agents is easily acquired [5]. It is not surprising that the numbers of new resistant strains are increasing in most parts of the world due to plasmid-mediated horizontally transferable genes encoding quinolone resistance [23]–[27]. Therefore, we should be very careful and consider quinolone resistance when prescribing levofloxacin-containing regimens, with the end goal of achieving high eradication rates (>90%). By carefully prescribing tailored regimens according to antibiotics susceptibility, this goal can be achieved with 10-day treatment durations, as shown in the current study.
The main limitation of antibiotic sensitivity determination by Epsilometer test (E-test) is pathogen culturing, which is time consuming. However, the successful culture rate of H. pylori from clinical specimens is 70–80% [28], [29], so the use of other biological methods to detect antibiotic resistance in H. pylori is necessary and helpful. Recent breakthroughs in scientific technology allow genotypic resistance to be determined from gastric biopsy specimens with a >93% success rate, compared with the relatively lower 70% success rates of traditional susceptibility tests [30]. Resistance can also be detected by using stool samples, which means that endoscopy can be avoided [31]. Quinolone resistance in H. pylori is caused by point mutations (N87 and D91) in the quinolone-resistance-determining region of the gyrA gene [32]. The presence of a gyrA mutation is predictive of treatment failure with triple therapy for quinolones, such as levofloxacin [33]. In real-world practice, genotypic resistance-guided therapy is not widely commercially available to allow wide clinical application. The good news is that genotypic resistance determination for clarithromycin and levofloxacin is now commercially available (HelicoDR, Hain, Germany) [34].
Furthermore, we observed that more patients in group B with amoxicillin-susceptible strains were successfully eradicated (86.3%) compared to group A (66.7%). One possible explanation for this could be the benefit of extended use of amoxicillin to 14 days, resulting in a better outcome of PPI/amoxicillin dual therapy, which is duration and dose dependent [35]. Hsu and colleagues further explored this hypothesis and found that continuing the amoxicillin through the entire 14 days could possibly result in a Grade A H. pylori eradication result [36]. According to Tanimura and colleagues, the H. pylori eradication rate was 46.7% for 2 weeks’ treatment, increasing to 83.4% for 4 weeks’ treatment, and to 100% with 6 weeks’ treatment [35].
Amoxicillin is bactericidal and has been widely and effectively used for anti-H. pylori therapy worldwide; few strains are resistant to it. The antibiotic resistance rate to amoxicillin in current study was 2.3% (1/44). This patient belonged to dual resistant to both amoxicillin and levofloxacin and failed to be eradicated eventually. However, resistance to amoxicillin detected in a single strain may be asserted without taking great care. The MIC level determined by E-test might be over-estimated and misinterpreted to be resistant phenotype. There were studies, even if they have been published incorrectly, reported that H. pylori strains resistant to amoxicillin have never been confirmed. Only a few well-described strains with MIC verified by a dilution method were actually resistant and had mutations in the gene for penicillin binding proteins [37]. Three substitutions (Ser 414 Arg, Thr556Ser, and Asn 562) are the most common amino acid changes in penicillin binding proteins connected to amoxicillin resistance [38], [39].
Conclusions
This study confirms the effectiveness of 14-day treatment. Importantly, the results imply that 10-day treatment duration should also be optimal if a culture can be performed to confirm the existence of susceptible strains. The duration of H. pylori eradication and levofloxacin resistance were the influencing factors for successful treatment. This study suggests that tailored levofloxacin-containing therapy should be administered only for patients with susceptible strains because it can achieve >90% success rates.
Data Availability
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper and its Supporting Information files.
Funding Statement
The authors have no support or funding to report.
References
- 1. Malfertheiner P, Megraud F, O’Morain CA, Atherton J, Axon AT, et al. (2012) Management of Helicobacter pylori infection–the Maastricht IV/Florence Consensus Report. Gut 61: 646–664. [DOI] [PubMed] [Google Scholar]
- 2. Kiesslich R (2008) Does treatment with levofloxacine improve eradication of Helicobacter pylori ? Z Gastroenterol. 2008; 46: 83–84. [DOI] [PubMed] [Google Scholar]
- 3. Güzelbulut F, Sezikli M, Akkan, Erhan Altunöz M, Günes P, et al. (2011) Application of levofloxacine in the second phase of sequential therapy regimen for Helicobacter pylori eradication: is it a good choice? Minerva Med. 102: 171–176. [PubMed] [Google Scholar]
- 4. Chuah SK, Tsay FW, Hsu PI, Wu DC (2011) A new look at anti-Helicobacter pylori therapy. World J Gastroenterol. 17: 3971–3975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gisbert JP (2012) Rescue Therapy for Helicobacter pylori Infection 2012. Gastroenterol Res Pract. 2012: 974594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Fock KM, Katelaris P, Sugano K, Ang TL, Hunt R, et al. (2009) Second Asia-Pacific Conference. Second Asia-Pacific Consensus Guidelines for Helicobacter pylori infection. J Gastroenterol Hepatol. 24: 1587–1600. [DOI] [PubMed] [Google Scholar]
- 7. Gisbert JP, Morena F (2006) Systematic review and meta-analysis: Levofloxacin-based rescue regimens after Helicobacter pylori treatment failure. Aliment Pharmacol Ther 23: 35–44. [DOI] [PubMed] [Google Scholar]
- 8. Gisbert JP, Bermejo F, Castro-Fernández M, Perez-Aisa A, Fenandez-Bermejo M, et al. (2008) Second-line rescue therapy with levofloxacin after H. pylori treatment failure: a Spanish multicenter study of 300 patients. Am J Gastroenterol. 103: 71–76. [DOI] [PubMed] [Google Scholar]
- 9. Gisbert JP, Pérez-Aisa A, Bermejo F, Castro-Fernandez M, Almela P, et al. (2013) Second-line therapy with levofloxacin after failure of treatment to eradicate helicobacter pylori infection: time trends in a Spanish Multicenter Study of 1000 patients. J Clin Gastroenterol. 2013; 47: 130–135. [DOI] [PubMed] [Google Scholar]
- 10. Bilardi C, Dulbecco P, Zentilin P, Reglioni S, Liritano E, et al. (2004) A 10-day levofloxacin-based therapy in patients with resistant Helicobacter pylori infection: a controlled trial. Clin Gastroenterol Hepatol 2004 2: 997–1002. [DOI] [PubMed] [Google Scholar]
- 11. O’Connor A, Molina-Infante J, Gisbert JP, O’Morain C (2013) Treatment of Helicobacter pylori infection 2013. Helicobacter. 18 Suppl 1: 58–65. [DOI] [PubMed] [Google Scholar]
- 12. Chuah SK, Hsu PI, Chang KC, Chiu YC, Wu KL, et al. (2012) Randomized comparison of two non-bismuth-containing second-line rescue therapies for Helicobacter pylori. Helicobacter. 17: 216–223. [DOI] [PubMed] [Google Scholar]
- 13. Graham DY, Lu H, Yamaoka Y (2007) A report card to grade Helicobacter pylori therapy. Helicobacter 12; 275–8. [DOI] [PubMed] [Google Scholar]
- 14. Kuo CH, Hsu PI, Kuo FC, Wang SS, Hu HM, et al. (2013) Comparison of 10 day bismuth quadruple therapy with high-dose metronidazole or levofloxacin for second-line Helicobacter pylori therapy: a randomized controlled trial. J Antimicrob Chemother. 68: 222–228. [DOI] [PubMed] [Google Scholar]
- 15. Hsu PI, Chen WC, Tsay FW (2014) Ten-day Quadruple therapy comprising proton-pump inhibitor, bismuth, tetracycline, and levofloxacin achieves a high eradication rate for Helicobacter pylori infection after failure of sequential therapy. Helicobacter. 19: 74–79. [DOI] [PubMed] [Google Scholar]
- 16. Hsu PI, Hwang IR, Cittelly D, Lai KH, El-Zimaity HM, et al. (2002) Clinical presentation in relation to diversity within the Helicobacter pylori cag pathogenicity island. Am J Gastroenterol 97: 2231–2238. [DOI] [PubMed] [Google Scholar]
- 17. Sánchez JE, Sáenz NG, Rincón MR, Martin IT, Sánchez EG, et al. (2000) “Susceptibility of Helicobacter pylori to mupirocin, oxazolidionones, quinupristin/dalfopristin and new quinolones,” J Antimicrob Chemother. 46: 283–285. [DOI] [PubMed] [Google Scholar]
- 18. Tanaka M, Isogai E, Isogai H, Hayashi S, Hirose K, et al. (2002) “Synergic effect of quinolone antibacterial agents and proton pump inhibitors on Helicobacter pylori,”. J Antimicrob Chemother 49: 1039–1040. [DOI] [PubMed] [Google Scholar]
- 19. Antos D1, Schneider-Brachert W, Bästlein E, Hänel C, Haferland C, et al. (2006) “7-Day triple therapy of Helicobacter pylori infection with levofloxacin, amoxicillin, and high-dose esomeprazole in patients with known antimicrobial sensitivity,”. Helicobacter 11: 39–45. [DOI] [PubMed] [Google Scholar]
- 20. Chuah SK, Tai WC, Hsu PI, Wu DC, Wu KL, et al. (2012) The efficacy of second-line anti-Helicobacter pylori therapy using an extended 14-day levofloxacin/amoxicillin/proton-pump inhibitor treatment–a pilot study. Helicobacter. 17: 374–381. [DOI] [PubMed] [Google Scholar]
- 21. Miehlke S, Krasz S, Schneider-Brachert W, Kuhlisch E, Berning M, et al. (2011) Randomized days of esomeprazole, moxifloxacin, and amoxicillin for trial on 14 versus 7 second-line or rescue treatment of Helicobacter pylori infection. Helicobacter 16: 420–426. [DOI] [PubMed] [Google Scholar]
- 22. Tai WC, Chiu CH, Liang CM, Chang KC, Kuo CM, et al. (2013) Ten-day versus 14-day Levofloxacin-containing Triple Therapy for Second-line anti-Helicobacter Pylori Eradication in Taiwan. Gastroenterol Res Pract. 2013: 932478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Robicsek A, Jacoby GA, Hooper DC (2006) The worldwide emergence of plasmid-mediated quinolone resistance. Lancet Infect Dis. 6: 629–640. [DOI] [PubMed] [Google Scholar]
- 24. Su P, Li Y, Li H, Zhang J, Lin L, et al. (2013) Antibiotic resistance of Helicobacter pylori isolated in the Southeast Coastal Region of China. Helicobacter. 18: 274–279. [DOI] [PubMed] [Google Scholar]
- 25. Murakami K, Furuta T, Ando T, Nakajima T, Inui Y, et al. (2013) Multi-center randomized controlled study to establish the standard third-line regimen for Helicobacter pylorieradication in Japan. J Gastroenterol 48: 1128–1135. [DOI] [PubMed] [Google Scholar]
- 26. Kobayashi I, Murakami K, Kato M, Kato S, Azuma T, et al. (2007) Changing antimicrobial susceptibility epidemiology of Helicobacter pylori strains in Japan between 2002 and 2005. J Clin Microbiol. 45: 4006–4010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Goh KL, Navaratnam P (2011) High Helicobacter pylori resistance to metronidazole but zero or low resistance to clarithromycin, levofloxacin, and other antibiotics in Malaysia. Helicobacter 16: 241–245. [DOI] [PubMed] [Google Scholar]
- 28. Wu DC, Hsu PI, Tseng HH, Tsay FW, Lai KH, et al. (2011) Helicobacter pylori infection. A randomized, controlled study comparing 2 rescue therapies after failure of standard triple therapies Medicine 90: 180–185. [DOI] [PubMed] [Google Scholar]
- 29. Vakil N, Megraud F (2007) Eradication therapy for Helicobacter pylori. Gastroenterology 133: 985–1001. [DOI] [PubMed] [Google Scholar]
- 30. Liou JM, Chen CC, Chang CY, Chen MJ, Fang YJ, et al. (2013) Efficacy of genotypic resistance-guided sequential therapy in the third-line treatment of refractory Helicobacter pylori infection: a multicentre clinical trial. J Antimicrob Chemother 68: 450–456. [DOI] [PubMed] [Google Scholar]
- 31. Schabereiter-Gurtner C, Hirschl AM, Dragosics B, Hufnagl P, Puz S, et al. (2004) Novel real-time PCR assay for detection of Helicobacter pylori infection and simultaneous clarithromycin susceptibility testing of stool and biopsy specimens. J Clin Microbiol 42: 4512–4518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Garcia M, Raymond J, Garnier M, Cremniter J, Burucoa C (2012) Distribution of spontaneous gyrA mutations in 97 fluoroquinolone-resistant Helicobacter pylori isolates collected in France. Antimicrob Agents Chemother. 56: 550–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Liou JM, Chang CY, Sheng WH, Wang YJ, Chen MJ, et al. (2011) Genotypic resistance in Helicobacter pylori strains correlates with susceptibility test and treatment outcomes after levofloxacin- and clarithromycin-based therapies. Antimicrob Agents Chemother 55: 1123–1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Cambau E, Allerheiligen V, Coulon C, Corbel C, Lascols C, et al. (2009) Evaluation of a new test, genotype HelicoDR, for molecular detection of antibiotic resistance in Helicobacter pylori. J Clin Microbiol. 47: 3600–3607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Tanimura H, Kawano S, Kubo M, Abe T, Goto M, et al. (1998) Does Helicobacter pylori eradication depend on the period of amoxicillin treatment? A retrospective study. J.Gastroenterol. 33: 23–26. [DOI] [PubMed] [Google Scholar]
- 36. Hsu PI, Wu DC, Wu JY, Graham DY (2011) Modified sequential Helicobacter pylori therapy: proton pump inhibitor and amoxicillin for 14 days with clarithromycin and metronidazole added as a quadruple (hybrid) therapy for the final 7 days. Helicobacter. 16: 139–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wu W, Yang Y, Sun G (2012) Recent Insights into Antibiotic Resistance in Helicobacter pylori Eradication. Gastroenterol Res Pract. 2012; 723183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Bonis M, Williams A, Guadagnini S, Werts C, Boneca IG (2012) The effect of bulgecin A on peptidoglycan metabolism and physiology of Helicobacter pylori. Microb Drug Resist. 18: 230–9. [DOI] [PubMed] [Google Scholar]
- 39. Qureshi NN, Morikis D, Schiller NL (2011) Contribution of specific amino acid changes in penicillin binding protein 1 to amoxicillin resistance in clinical Helicobacter pylori isolates. Antimicrob Agents Chemother. 55: 101–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper and its Supporting Information files.