Abstract
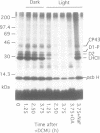
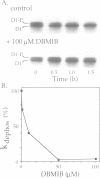
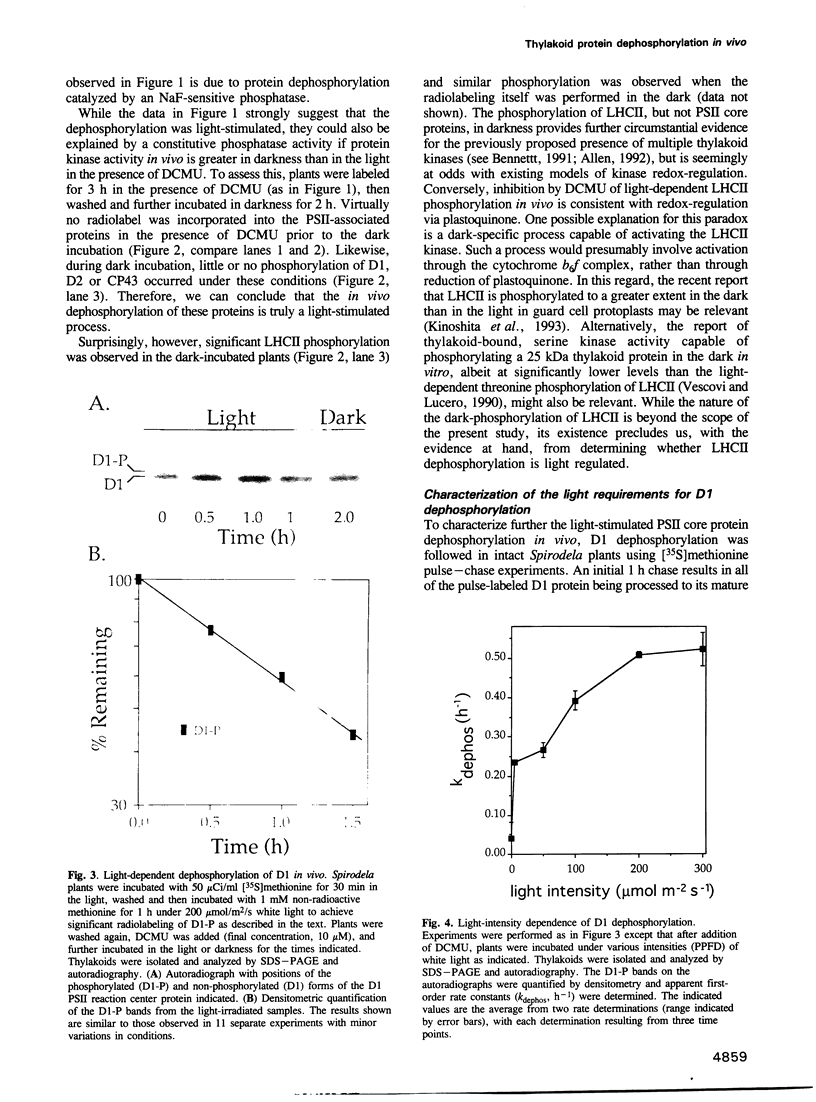
A number of photosystem II (PSII)-associated proteins, including D1, D2, CP43 and LHCII, are phosphorylated post-translationally by a membrane-bound, redox-regulated kinase activity. In vitro studies have demonstrated that these proteins can be dephosphorylated by membrane-bound phosphatase activity, reportedly insensitive to light or redox control. We demonstrate here that the PSII core proteins, D1, D2 and CP43, undergo light-stimulated, linear electron-transport-independent dephosphorylation in vivo. The in vivo dephosphorylation of D1 was characterized further and shown to depend upon light intensity, and to occur throughout the visible light spectrum with characteristics most consistent with light absorption by chlorophyll. PSII core protein dephosphorylation in vivo was stimulated by photosystem I (PSI)-specific far-red light, and inhibited by 2,5-dibromo-3-methyl-6-isopropyl-p-benzoquinone, an inhibitor of plastoquinol oxidation by the cytochrome b6f complex. Based on these findings, we propose that PSI excitation is involved in regulating dephosphorylation of PSII core proteins in vivo.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen J. F. Protein phosphorylation in regulation of photosynthesis. Biochim Biophys Acta. 1992 Jan 22;1098(3):275–335. doi: 10.1016/s0005-2728(09)91014-3. [DOI] [PubMed] [Google Scholar]
- Aro E. M., Kettunen R., Tyystjärvi E. ATP and light regulate D1 protein modification and degradation. Role of D1* in photoinhibition. FEBS Lett. 1992 Feb 3;297(1-2):29–33. doi: 10.1016/0014-5793(92)80320-g. [DOI] [PubMed] [Google Scholar]
- Bennett J. Chloroplast phosphoproteins. Evidence for a thylakoid-bound phosphoprotein phosphatase. Eur J Biochem. 1980 Feb;104(1):85–89. doi: 10.1111/j.1432-1033.1980.tb04403.x. [DOI] [PubMed] [Google Scholar]
- Callahan F. E., Ghirardi M. L., Sopory S. K., Mehta A. M., Edelman M., Mattoo A. K. A novel metabolic form of the 32 kDa-D1 protein in the grana-localized reaction center of photosystem II. J Biol Chem. 1990 Sep 15;265(26):15357–15360. [PubMed] [Google Scholar]
- Canaani O., Barber J., Malkin S. Evidence that phosphorylation and dephosphorylation regulate the distribution of excitation energy between the two photosystems of photosynthesis in vivo: Photoacoustic and fluorimetric study of an intact leaf. Proc Natl Acad Sci U S A. 1984 Mar;81(6):1614–1618. doi: 10.1073/pnas.81.6.1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cyert M. S., Thorner J. Putting it on and taking it off: phosphoprotein phosphatase involvement in cell cycle regulation. Cell. 1989 Jun 16;57(6):891–893. doi: 10.1016/0092-8674(89)90325-5. [DOI] [PubMed] [Google Scholar]
- Elich T. D., Edelman M., Mattoo A. K. Identification, characterization, and resolution of the in vivo phosphorylated form of the D1 photosystem II reaction center protein. J Biol Chem. 1992 Feb 15;267(5):3523–3529. [PubMed] [Google Scholar]
- Gal A., Hauska G., Herrmann R., Ohad I. Interaction between light harvesting chlorophyll-a/b protein (LHCII) kinase and cytochrome b6/f complex. In vitro control of kinase activity. J Biol Chem. 1990 Nov 15;265(32):19742–19749. [PubMed] [Google Scholar]
- Herbert S. K., Fork D. C., Malkin S. Photoacoustic measurements in vivo of energy storage by cyclic electron flow in algae and higher plants. Plant Physiol. 1990 Nov;94(3):926–934. doi: 10.1104/pp.94.3.926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter T. Protein-tyrosine phosphatases: the other side of the coin. Cell. 1989 Sep 22;58(6):1013–1016. doi: 10.1016/0092-8674(89)90496-0. [DOI] [PubMed] [Google Scholar]
- Kieleczawa J., Coughlan S. J., Hind G. Isolation and characterization of an alkaline phosphatase from pea thylakoids. Plant Physiol. 1992 Jul;99(3):1029–1036. doi: 10.1104/pp.99.3.1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinoshita T., Shimazaki Ki., Nishimura M. Phosphorylation and Dephosphorylation of Guard-Cell Proteins from Vicia faba L. in Response to Light and Dark. Plant Physiol. 1993 Jul;102(3):917–923. doi: 10.1104/pp.102.3.917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattoo A. K., Edelman M. Intramembrane translocation and posttranslational palmitoylation of the chloroplast 32-kDa herbicide-binding protein. Proc Natl Acad Sci U S A. 1987 Mar;84(6):1497–1501. doi: 10.1073/pnas.84.6.1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattoo A. K., Hoffman-Falk H., Marder J. B., Edelman M. Regulation of protein metabolism: Coupling of photosynthetic electron transport to in vivo degradation of the rapidly metabolized 32-kilodalton protein of the chloroplast membranes. Proc Natl Acad Sci U S A. 1984 Mar;81(5):1380–1384. doi: 10.1073/pnas.81.5.1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattoo A. K., Marder J. B., Edelman M. Dynamics of the photosystem II reaction center. Cell. 1989 Jan 27;56(2):241–246. doi: 10.1016/0092-8674(89)90897-0. [DOI] [PubMed] [Google Scholar]
- Michel H., Griffin P. R., Shabanowitz J., Hunt D. F., Bennett J. Tandem mass spectrometry identifies sites of three post-translational modifications of spinach light-harvesting chlorophyll protein II. Proteolytic cleavage, acetylation, and phosphorylation. J Biol Chem. 1991 Sep 15;266(26):17584–17591. [PubMed] [Google Scholar]
- Michel H., Hunt D. F., Shabanowitz J., Bennett J. Tandem mass spectrometry reveals that three photosystem II proteins of spinach chloroplasts contain N-acetyl-O-phosphothreonine at their NH2 termini. J Biol Chem. 1988 Jan 25;263(3):1123–1130. [PubMed] [Google Scholar]
- Sun G., Bailey D., Jones M. W., Markwell J. Chloroplast thylakoid protein phosphatase is a membrane surface-associated activity. Plant Physiol. 1989 Jan;89(1):238–243. doi: 10.1104/pp.89.1.238. [DOI] [PMC free article] [PubMed] [Google Scholar]